Abstract
Activation of the intrinsic apoptotic pathway represents a major mechanism for breast cancer regression resulting from anti-estrogen therapy. The BH3-only protein BIK is inducible by estrogen-starvation and anti-estrogen treatment and plays an important role in anti-estrogen induced apoptosis of breast cancer cells. BIK is predominantly localized to the endoplasmic reticulum where it regulates BAX/BAK-dependent release of Ca2+ from the endoplasmic reticulum stores and cooperates with other BH3-only proteins such as NOXA to cause rapid release of cytochrome c from mitochondria and activate apoptosis. BIK is also known to inactivate BCL-2 through complex formation. Previously, we demonstrated that apoptosis triggered by BIK in estrogen-starved human breast cancer cells is suppressed by GRP78, a major endoplasmic reticulum chaperone. Here we described the isolation of a novel clonal human breast cancer cell line (MCF-7/BUS-10) resistant to long-term estrogen deprivation. These cells exhibit elevated level of GRP78, which protects them from estrogen starvation-induced apoptosis. Our studies revealed that overexpression of GRP78 suppresses apoptosis induced by BIK and NOXA, either alone or in combination. Surprisingly, the interaction of GRP78 with BIK does not require its BH3 domain, which has been implicated in all previous BIK protein interactions. We further showed GRP78 and BCL-2 form independent complex with BIK and that increased expression of GRP78 decreases BIK binding to BCL-2. Our findings provide the first evidence that GRP78 can decrease BCL-2 sequestration by BIK at the endoplasmic reticulum, thus uncovering a potential new mechanism whereby GRP78 confers endocrine resistance in breast cancer.
Keywords: Apoptosis, Chaperone Chaperonin, Endoplasmic Reticulum (ER), Estrogen, Protein Domains, BCL-2, BH3-only Protein, BIK, GRP78/BiP, Endocrine Resistance
Introduction
Anti-estrogen therapy represents a major advance in the treatment of estrogen receptor-positive breast cancer that can produce significant clinical responses and delay of progression. However, their efficacy is limited by intrinsic and acquired therapeutic resistance (1, 2). To overcome this limitation, it is important to understand the resistance mechanisms and identify new therapeutic targets. Estrogen is required for the proliferation of estrogen receptor-positive breast cancer cells (3). When subjected to estrogen starvation, exposure to anti-estrogens, or treatment with aromatase inhibitors, significant apoptosis of breast cancer cells was observed. A key regulator of apoptosis is the B-cell lymphoma (BCL)2-2 family of proteins which has been reported to localize to the membranes of various organelles (4). Pro-survival members of the BCL-2 family such as BCL-2, share three or four of the conserved domains known as BCL-2 homology (BH) regions. BCL-2 and BCL-XL lower the Ca2+ store in the endoplasmic reticulum and antagonize the pro-apoptotic function of BAX to promote cell survival (5). Pro-apoptotic members such as BAX and BAK share two or three BH domains. Upon activation, BAX translocates to mitochondria and initiates the release of cytochrome c into the cytosol (6). A third group of apoptotic regulators, referred to as BH3-only proteins, share only the 9 amino acid BH3 region. In their active conformation, BH3-only proteins can induce BAX/BAK activation and initiate apoptosis. They can also bind directly to BCL-2 or BCL-XL through the BH3 domain and inhibit their pro-survival activities.
BCL-2-interacting killer (BIK), the founding member of the BH3-only proteins, is a pro-apoptotic tumor suppressor in several human tissues and has also been used as a therapeutic target for anti-cancer drugs (7). BIK plays a critical role in promotion of anti-estrogen-induced cell death in human breast cancer cells (8). Utilizing MCF-7/BUS cells that have been vigorously characterized as highly dependent on estrogen for growth, BIK mRNA and protein were strongly induced by estrogen-starvation or anti-estrogen treatment while the other BCL-2 family proteins, such as BCL-2, BCL-XL, and BAX were not affected (8–10). Conversely, knockdown of BIK by siRNA significantly inhibited apoptosis caused by anti-estrogen treatment. BIK is predominantly localized to the outer membrane of the endoplasmic reticulum and mediates apoptosis through the mitochondrial pathway (11). Although BIK does not interact directly with pro-apoptotic BAX and BAK, it regulates BAX/BAK-dependent release of Ca2+ from the endoplasmic reticulum stores, and cooperates with other BH3-only proteins such as NOXA, to cause rapid release of cytochrome c from mitochondria and activate caspases (12). BIK is also known to form complex with BCL-2 at the endoplasmic reticulum and modulate BCL-2 activity; when in excess, endoplasmic reticulum-localized BCL-2 is able to protect against BIK-induced apoptosis (13). The discovery that BIK is a key mediator of estrogen-starvation and anti-estrogen induced apoptosis implies that inhibition of BIK expression or activity at the endoplasmic reticulum may represent a novel mechanism for the development of endocrine resistance in human breast cancer.
Toward understanding how BIK function is regulated at the endoplasmic reticulum, a search for novel interactive protein partners of BIK revealed that endogenous BIK selectively forms complex with the glucose-regulated protein GRP78 but not other endoplasmic reticulum chaperones (14). GRP78, also referred to as BiP/HSPA5, is a central regulator of endoplasmic reticulum function due to its role in protein folding and assembly, targeting misfolded proteins for degradation, endoplasmic reticulum Ca2+ binding and controlling the activation of transmembrane endoplasmic reticulum stress inducers (15–17). GRP78 is induced by a wide variety of physiologic and pathologic stress (18) and is up-regulated in many types of cancer including breast cancer (15, 19, 20). Whereas GRP78 is predominantly an endoplasmic reticulum lumen protein, a subfraction can exist as a transmembrane protein, and either directly or indirectly interact with proteins localized at the outer endoplasmic reticulum membrane (21, 22). GRP78 overexpression blocked BIK-induced apoptosis, suppressed estrogen starvation-induced BAX activation, mitochondrial permeability transition, and consequent apoptosis (14). Conversely, knockdown of endogenous GRP78 by siRNA sensitized human breast cancer cells to estrogen starvation-induced apoptosis. These studies provide the first evidence that GRP78 confers resistance to estrogen starvation-induced apoptosis in human breast cancer cells via a novel mechanism mediated by BIK.
Establishment of anti-estrogen-resistant clonal cell lines offers important cell model systems to study the mechanism for resistance to endocrine therapy (23). Here we described the isolation of a novel clonal human breast cancer cell line (MCF-7/BUS-10) resistant to long-term estrogen deprivation. These cells exhibit an elevated level of GRP78, which protects them from estrogen starvation-induced apoptosis. To further understand the functional relationship between GRP78 and BIK, we showed that BIK acts cooperatively with NOXA to induce apoptosis. However, overexpression of GRP78 suppresses apoptosis induced by both BH3-only proteins, either alone or in combination. Surprisingly, the interaction of GRP78 with BIK does not require its BH3 domain, which has been implicated in all previous BIK protein interactions. GRP78 and BCL-2 form an independent complex with BIK and the increased expression of GRP78 can decrease BIK binding to BCL-2. Our findings provide the first evidence that GRP78 can regulate the amount of BCL-2 sequestered by BIK at the endoplasmic reticulum, thus uncovering a potential new mechanism whereby GRP78 confers endocrine resistance in breast cancer.
EXPERIMENTAL PROCEDURES
Cell Culture and Treatment
The estrogen-dependent human breast cancer cell line MCF-7/BUS was kindly provided by A. M. Soto (Tufts University) and has been described (24). MCF-7/BUS, human breast adenocarcinoma cell line MCF-7, and human embryonic kidney 293T cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and antibiotics. Estrogen-starvation was performed as described (8). Briefly, the cells were washed three times with phenol red-free DMEM and incubated in the washing medium at 37 °C for 60 min; the cells were then cultured in phenol red-free DMEM supplemented with 5% charcoal/dextran-stripped fetal bovine serum for 48 h.
Establishment of the MCF-7/BUS-10-resistant Clone
The MCF-7/BUS-10 cells were cloned from wild-type MCF-7/BUS cells through 6 cycles of estrogen starvation. Briefly, MCF-7/BUS cells were cultured in estrogen-free medium (phenol red-free DMEM supplemented with 5% charcoal-dextran-stripped fetal bovine serum) for 5–10 days. The surviving cells (about 10%) were then cultured in regular DMEM with 5% fetal bovine serum for 2–3 days to allow expansion of the remaining cells. This process was repeated six times, and individual clones that survived this treatment were isolated and expanded. The resistant cells were maintained in estrogen-free medium for at least two months prior to characterization of growth rates and protein expression levels.
Expression Vectors
The plasmids FLAG-BIK-b5TM and FLAG-BIK were kindly provided by G. C. Shore (McGill University) (25). For FLAG-BIK-b5TM, the C terminus of BIK was modified, which targets the protein to the membrane of the endoplasmic reticulum (25). The truncated FLAG-BIKΔBH3 was constructed from FLAG-BIK using QuikChange Site-directed mutagenesis kit (Stratagene). The mutants, FLAG-BIKΔ28, Δ56, and Δ71 were constructed by PCR-directed mutagenesis using FLAG-BIK as template. The plasmid FLAG-BIMEL expressing FLAG-tagged full-length BIM was kindly provided by J. Nicholas (Johns Hopkins University School of Medicine) (26). The expression plasmid for GRP78 is FLAG-GRP78, where a FLAG epitope was attached to the 5′ terminus. Construction of FLAG-GRP78 was as described (27). The expression plasmid for NOXA is His-NOXA, where a His epitope was attached to the 5′ terminus. Full-length cDNA of Noxa were prepared from GFP-Noxa (28) using BamHI and EcoRI digestion and cloned into the BamHI and EcoRI sites of pcDNA3/His to construct His-Noxa. The expression plasmid for BCL-2 is pcDNA3-BCL-2. All newly constructed expression plasmids were verified by DNA sequencing.
Transient Transfection
The cells were seeded onto the plate 1 day prior to transfection with the indicated plasmids using BioT transfection reagent (Bioland Scientific). Empty vector was added to adjust the total amount of plasmids to be the same. For protein expression analysis, pro-caspase inhibitor zVAD-FMK was added 6 h after transfection to inhibit apoptosis. Additionally, the proteasome inhibitor MG115 was added to medium 36 h after transfection to stabilize expression of FLAG-BIKΔ28, Δ56, and Δ71. Cell lysates were prepared 24 or 48 h later from the transfected cells, and were subjected to Western blot, or co-immunoprecipitation. For apoptosis assay, cells were collected 24 h after transfection.
Small Interfering RNA
The siRNA against Grp78 is 5′-ggagcgcauugauacuagadTdT-3′ and 5′-ucuaguaucaaugcgcuccdTdT-3′ as described (29). The control siRNA is Silencer Negative Control #3 siRNA (Ambion) composed of a 19 bp scrambled sequence without significant homology to any known genes. After the seeded cells reached 60% confluency, the cells were transfected with control siRNA or siRNA against Grp78 using Lipofectamine 2000 transfection reagent (Invitrogen) for 48 h.
Apoptosis Detection and Cell Viability Assay
For apoptosis detection, cells were collected and resuspended at the concentration of 1 × 106 cells/ml in the binding buffer (10 mm HEPES/NaOH (pH 7.4), 140 mm NaCl, 2.5 mm CaCl2), and then incubated with phycoerythrin-conjugated Annexin V and 7-amino actinomycin (7-AAD) for 15 min at room temperature. Cell apoptosis was analyzed by FACSDiva (BD Biosciences).
Cell proliferation and survival were assayed by CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega) according to the manufacturer's instruction or Trypan Blue (Sigma) staining. Briefly, 20 μl of CellTiter 96® AQueous One Solution Reagent was added into each well of the 96-well assay plate containing the cell samples in 100 μl of culture medium. The plates were incubated for 2 h at 37 °C in a humidified, 5% CO2 atmosphere, and the absorbance at 490 nm were read by the Emax Absorbance Microplate Reader (Molecular Devices Corp.). The data were processed by SoftMax Pro Software (Molecular Devices Corp.). For Trypan Blue staining, the cells were collected and stained with Trypan Blue for 1 min prior to detection.
Glutathione S-transferase Pull-down Assays
Glutathione S-transferase (GST)-BIK was constructed as previously (14). GST-GRP78 (19–654 aa) and GST-GRP78/P45 (19–400 aa) were constructed similarly. These recombinant proteins did not contain the GRP78 signal peptide which spans 1–18 aa. The GST fusion proteins (GST-BIK, GST-GRP78, and GST-GRP78/P45) were purified as described previously (30). Purified human GRP78Δ-H (19–650 aa), a secreted form of GRP78 devoid of the KDEL endoplasmic reticulum retention sequence and contains a His tag for facilitation of purification (27), was kindly provided by P. Gill (University of Southern California). For BIK-GRP78 in vitro binding assay, 5 μg of GST-BIK or GST protein bound to glutathione-Sepharose beads (Sigma-Aldrich) were incubated to 0.5 μg of purified GRP78Δ-H on a rotating shaker at 4 °C overnight. The beads were collected and washed three times with extraction buffer. For GRP78 binding domain detection assay, 5 μg of GST-GRP78, GST-GRP78/P45, or GST bound-glutathione-Sepharose beads were incubated with 500 μg of total protein extract from 293T cells transfected with FLAG-BIK by rotation for 16 h at 4 °C. After collection by centrifugation at 2,000 rpm for 5 min, the beads were washed with extraction buffer three times. The bound proteins were resuspended in SDS-PAGE sample loading buffer and subjected to SDS-PAGE and Western blots.
Co-immunoprecipitation and Western Blot Analysis
The co-immunoprecipitation assays were performed as described (22). Briefly, cells were lysed in RIPA buffer supplemented with protease inhibitor mixture (Roche) for 5 min on ice. After centrifugation at 13,000 rpm for 10 min at 4 °C, the supernatant was collected and used for determining the total protein concentration. One mg of total protein extract was incubated with 50 μl of protein G-agarose on a rotating shaker at 4 °C for 1 h, and then incubated with anti-FLAG M2, anti-NBK(BIK), or anti-BCL-2 antibody at 4 °C overnight. After washing in RIPA buffer three times, the beads were resuspended in SDS-PAGE sample loading buffer and boiled for 5 min. The immunoprecipitates were subjected to Western blot analysis.
The proteins in immunoprecipitates or cell lysates were detected by Western blots as described (16). The source and dilution of the primary antibodies are listed below: goat anti-NBK (BIK N-19, Santa Cruz Biotechnology) (1:1000), rabbit anti-BIM (22–40, EMD Chemicals) (1:1000), mouse anti-FLAG M2 (Santa Cruz Biotechnology) (1:1000), mouse anti-β-actin (Sigma-Aldrich) (1:4000), mouse anti-GRP78 (provided by P. Gill) (1:2000), rabbit anti-Bcl-2 (1:1000), or mouse anti-His antibody (1:1000). Respective HRP-conjugated secondary antibodies (Santa Cruz Biotechnology) (1:1000) were used. The protein signals were detected with the ECL reagent (Roche) or Supersignal chemiluminescence reagent (Pierce) and quantitated by Quantity One software. Some protein bands were visualized and quantitated by Li-Cor Odyssey. The experiments were repeated three times or more and ANOVA was applied for the statistical analysis.
RESULTS
Knockdown of GRP78 Expression Sensitizes Long-term Estrogen Deprivation Breast Cancer Cells to Apoptosis
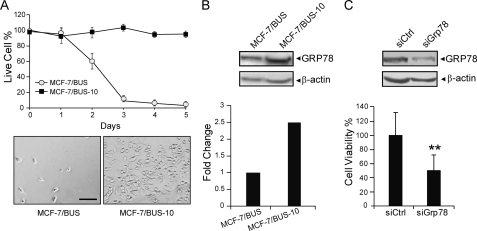
The endocrine resistant MCF-7/BUS-10 cell line was cloned from the estrogen starvation sensitive MCF-7/BUS cells following multiple cycles of estrogen deprivation and was able to survive under long-term maintenance in estrogen-free medium. Compared with the parental MCF-7/BUS cells that started to lose viability after 2 days of estrogen starvation and were not able to survive past 5 days, MCF-7/BUS-10 cells maintained full viability under estrogen-starvation condition, with normal cell morphology (Fig. 1A). Thus, these cells are resistant to long-term estrogen deprivation. GRP78 protein level was elevated in the resistant MCF-7/BUS-10 cells compared with the sensitive MCF-7/BUS cells (Fig. 1B). To test whether GRP78 functionally contributes to the survival of the MCF-7/BUS-10 cells under estrogen-starvation condition, we utilized siRNA to specifically knockdown GRP78 in these cells and tested its effect on survival. Western blot analysis confirmed that compared with control siRNA, transient transfection of siGrp78 reduced the expression level of GRP78 by about 60% in the resistant MCF-7/BUS-10 cells (Fig. 1C). For viability assay, 24 h following transfection of the MCF-7/BUS-10 cells with siRNA, the cells were exposed to estrogen starvation for 48 h prior to determination of cell viability. Comparing the cells transfected with control siRNA, cells transfected with siGrp78 showed significant reduction in cell viability (50%, p < 0.01) (Fig. 1C). These results revealed GRP78 overexpression in long-term estrogen deprivation resistant cells and the down-regulation of GRP78 sensitizes these cells to estrogen starvation-induced apoptosis.
FIGURE 1.
GRP78 confers viability to MCF-7/BUS-10-resistant cells. A, MCF-7/BUS-10 cells (closed ■) and parental cells (open ○) were cultured in estrogen-free medium. At the indicated time points, cell survival was assayed by Trypan Blue staining. Shown below are representative images of MCF-7/BUS and MCF-7/BUS-10-resistant cells at day 5 in estrogen-free medium. Bar, 50 μm. B, cells were grown in estrogen-free medium containing caspase inhibitor for 48 h, and the cell lysates were subjected to Western blots. The fold change of GRP78 protein level normalized against β-actin is plotted. C, MCF-7/BUS-10 cells were transfected with control siRNA (siCtrl) or siGrp78. For protein expression level measurement, cells were maintained in the regular DMEM medium supplemented with FBS for 48 h. Cell lysates from the transfected cells were subjected to Western blots for detection of GRP78 and β-actin levels. To measure cell viability, the transfected cells were cultured in regular DMEM medium supplemented with FBS, then transferred to estrogen-free starvation medium for 48 h and subjected to CellTiter 96@ Aqueous One Solution Cell Proliferation Assay. The standard error is shown. **, p < 0.01.
Overexpression of GRP78 Suppresses Apoptosis Induced by BIK and NOXA
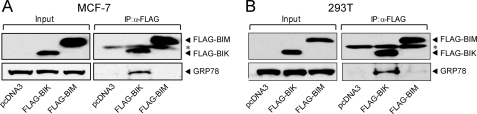
First we established that GRP78 specifically binds to BIK, but not the other BH3-only protein family member such as BIM. Thus, in co-immunoprecipitation assays, GRP78 formed complex with ectopically expressed FLAG-BIK but not with FLAG-BIM, in both MCF-7 (Fig. 2A) and 293T cells (Fig. 2B). To understand the protective mechanisms of GRP78, we examined its activity toward BIK, which induces apoptosis in breast cancer cell subjected to estrogen starvation (8), and NOXA, which acts in concert with BIK (31). The 293T cells were co-transfected with FLAG-GRP78, His-NOXA and FLAG-BIK-b5TM, which targets BIK to the endoplasmic reticulum. The empty vector pcDNA3 was used as negative control. The expression level of the ectopically expressed proteins could be identified with high sensitivity and specificity through the use of specific antibody against each epitope; whereas both the endogenous protein as well as the ectopically expressed protein would be detected with specific antibodies against the target protein. Because these experiments required simultaneous transient transfection of multiple expression vectors, 293T cells were chosen as recipient cells due to their high transfection efficiency.
FIGURE 2.
Specific interaction between GRP78 and BIK. A, MCF-7 cells were transiently transfected with pcDNA3, FLAG-BIK, and FLAG-BIM in the presence of zVAD-fmk, followed by co-immunoprecipitation with anti-FLAG antibody. The protein levels of GRP78, FLAG-BIK, and FLAG-BIM were detected by Western blots using anti-GRP78 and anti-FLAG antibodies. The positions of these proteins were indicated. *, mouse IgG light chain. B, same experiment in A was performed in 293T cells.
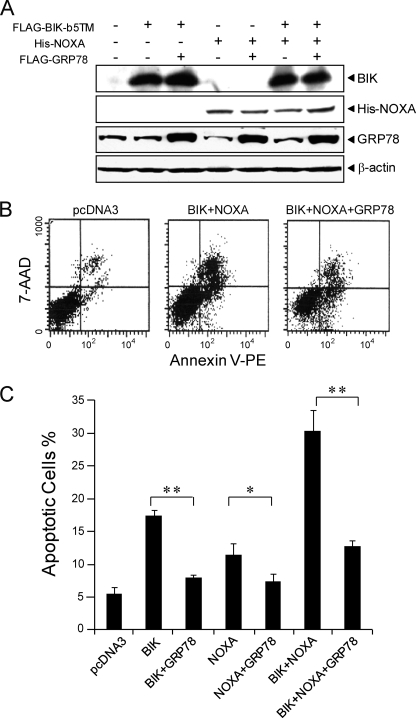
First we optimized the transfection conditions such that BIK, NOXA, and GRP78 could be co-expressed. Western blot analysis showed that there were negligible amounts of endogenous BIK and NOXA, such that the level of BIK or NOXA was mostly from ectopic protein expression (Fig. 3A and data not shown). For GRP78, a basal level was detected for the endogenous protein, and upon transfection with FLAG-GRP78, an elevated amount of total GRP78 was observed (3.5-fold). To determine whether BIK and NOXA acted cooperatively in inducing apoptosis, and whether the combined effect of BIK and NOXA could be suppressed by GRP78, the cells were transfected with expression vectors for BIK, NOXA, and GRP78, either alone or in combination and subjected to Annexin V/7-AAD assay. In this assay, fluorochrome-conjugated Annexin V can bind with the membrane phospholipid phosphatidylserine (PS) which was exposed to external cellular environment in apoptotic cells. 7-AAD was used to distinguish viable cells from nonviable ones. As shown in Fig. 3B, Annexin V-positive cells increased significantly in the BIK/NOXA-overexpressing cells compared with the cells transfected with the same amount of pcDNA3 plasmids while GRP78 overexpression decreased the number of Annexin V-positive cells. Quantitative analysis identified that about 17 and 11% of 293T cells overexpressing BIK and NOXA, respectively, underwent apoptotic death, as compared with 5% for cells transfected with pcDNA3 (Fig. 3C). Co-expression of GRP78 with either BIK or NOXA significantly suppressed the amount of apoptosis (7% for both, p ≤ 0.05). Our results further indicated that BIK and NOXA have an additive effect on inducing apoptosis. In the cells co-transfected with BIK and NOXA, 30% of cells underwent apoptosis while simultaneous overexpression of GRP78 significantly reduced apoptosis to 13% (p < 0.01).
FIGURE 3.
Overexpression of GRP78 suppressed apoptosis induced by BIK and NOXA. A, 293T cells were transfected with empty vector pcDNA3, vector expressing FLAG-BIK-b5TM, His-NOXA, and FLAG-GRP78, either alone or in combination as indicated, in the presence of zVAD-fmk. Cell lysates were collected 24 h post transfection and subjected to Western blots to probe for levels of BIK, His-NOXA, GRP78, and β-actin with the antibodies indicated on the left. B, 293T cells were transfected with the indicated plasmids for 24 h. The cells were stained by PE-Annexin V and 7-AAD and analyzed by flow cytometry. Results are representative from three independent experiments. C, quantitation of the percentage of apoptotic cells (Annexin V-positive) under each condition. The standard deviation is shown. *, p < 0.05; **, p < 0.01.
Down-regulation of Endogenous GRP78 Sensitizes Cells to Apoptosis Induced by BIK and NOXA
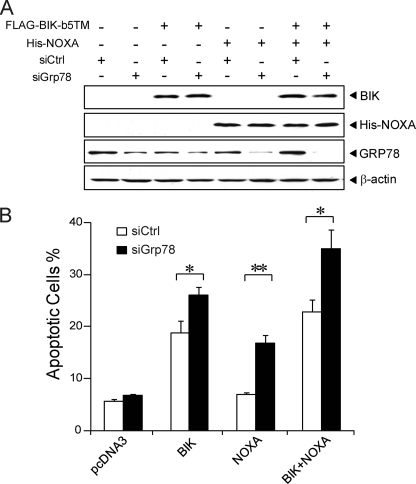
If GRP78 is required for resistance against BIK- and NOXA-induced apoptosis, knockdown of GRP78 expression is expected to sensitize cells to apoptosis induced by BIK and NOXA. To test this, 293T cells were transfected with control siRNA (siCtrl) or siGrp78 for 24 h and then transiently transfected with BIK and NOXA, either alone or in combination for another 24 h. Western blot analysis confirmed overexpression of BIK and NOXA and siGrp78 reduced endogenous GRP78 protein level to varying degrees while having no effect on β-actin level (Fig. 4A). Thus, in cells transfected with the vectors pcDNA3 or BIK, GRP78 knockdown was about 50%; whereas in cells transfected with NOXA, or NOXA in combination with BIK, the knockdown of GRP78 was near complete. For cell viability assay, the transfected cells were cultured for another 24 h prior to subjection to the Annexin V/7-AAD assay and analyzed by flow cytometry. Our results showed that about 18% of 293T cells overexpressing BIK underwent apoptosis. Knockdown of GRP78 in the same cells resulted in 26% of the cells undergoing apoptosis (p < 0.05). About 8% of the cells transfected with NOXA underwent apoptosis and apoptotic cells increased to 16% with GRP78 knockdown (p < 0.01). In the cells co-transfected with BIK and NOXA, 22% of cells underwent apoptosis while GRP78 knockdown increased apoptosis to 34% (p < 0.05) (Fig. 4B). Collectively, the overexpression and knockdown studies showed that GRP78 confers resistance to apoptosis induced by BIK and NOXA.
FIGURE 4.
Knockdown of GRP78 sensitizes 293T cells to BIK- and NOXA-induced apoptosis. A, 293T cells were transfected with siGrp78 or control siRNA (siCtrl) for 24 h and subsequently transfected with FLAG-BIK-b5TM and His-NOXA for 24 h in the presence of zVAD-fmk. The levels of GRP78, BIK, His-NOXA, and β-actin as determined by Western blots are shown. B, percent of apoptotic cells was analyzed by flow cytometry using PE-Annexin V and 7-AAD staining. The experiment was repeated three times. The standard deviation is shown. *, p < 0.05; **, p < 0.01.
Amino Portion of GRP78 Is Sufficient for Interaction with BIK
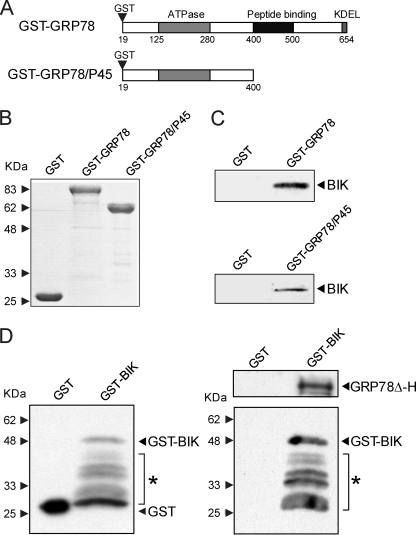
The BH3-only pro-apoptotic protein BIK was localized to the outer surface of the endoplasmic reticulum. Among the endoplasmic reticulum chaperones, BIK was discovered to selectively interact with GRP78 by co-immunoprecipitation assay (14). Two independent studies reported that a subfraction of GRP78 can exist as a transmembrane protein with its carboxyl portion located inside the endoplasmic reticulum lumen (21, 22). This suggested that the amino portion of the subfraction of GRP78 could be exposed to the cytosol and interacts directly or indirectly with cytosolic proteins.
To determine the interactive domain of GRP78 with BIK, we constructed full-length GST-GRP78 and a truncated N-terminal portion GST-GRP78/P45 (Fig. 5A). The yield and purity of the GST-proteins were determined by Coomassie Blue staining (Fig. 5B). In pull-down assays using GST protein-linked beads and BIK-overexpressing 293T cell lysates, full-length GST-GRP78 and GST-GRP78/P45, but not the GST protein itself, were able to bind BIK from the cell extract (Fig. 5C). Thus, the amino 400 aa of GRP78 is sufficient to interact with BIK. To determine whether the binding between GRP78 and BIK is direct or requires other interacting proteins, GST or GST-BIK was used in pull-down assays with purified GRP78Δ-H. We observed that GST-BIK was able to pull-down GRP78 whereas GST alone did not (Fig. 5D). These results suggested that GRP78 and BIK directly interact with each other via the amino portion of GRP78.
FIGURE 5.
BIK binds amino portion of GRP78. A, schematic diagrams of GST-GRP78 and GST-GRP78/P45. The locations of the GST insert and the GRP78 functional domains are indicated. B, Coomassie Blue staining of GST, GST-GRP78, and GST-GRP78/P45 resolved by SDS-PAGE. C, lysates of 293T cells transfected with FLAG-BIK were incubated with GST-GRP78, GST-GRP78/P45, or GST-linked beads. The bound proteins were resolved by SDS-PAGE and probed for BIK by Western blots. D, input proteins GST and GST-BIK were probed for GST by Western blots (left). GST-BIK or GST-linked beads were incubated with purified GRP78Δ-H overnight. The bound proteins were resolved by SDS-PAGE and probed for GRP78 and BIK by Western blots (right). The molecular size markers are shown. The positions of GST-BIK and GST are indicated. * indicates partially degraded GST-BIK.
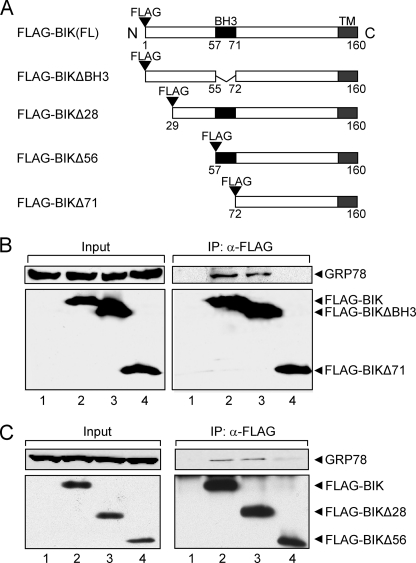
BIK Binding with GRP78 Does Not Require Its BH3 Domain
BIK shares the BH3 domain and the transmembrane domain with other BCL-2 family proteins. Mutational analyses showed that the BH3 domain of BIK is crucial for pro-apoptotic activity of BIK (32, 33), and mutations of the BH3 domain of BIK strongly reduced its ability of binding with other BCL-2 family proteins (34). To determine the interactive domain between GRP78 and BIK, we initially used full-length FLAG-BIK (1–160 aa), FLAG-BIKΔBH3 devoid of the BH3 domain and BIKΔ71 (72 to 160 aa) (Fig. 6A). We also constructed three other BIK mutants, including FLAG-BIK (1–56 aa); FLAG-BIK (1–71 aa), and FLAG-BIK (1–106 aa), however, these three peptides could not be expressed even in the presence of proteasome inhibitor (data not shown). 293T cells were transiently transfected with pcDNA3, FLAG-BIK, FLAG-BIKΔBH3, or FLAG-BIKΔ71 plasmid for 48 h. Western blot analysis confirmed the expression of FLAG-BIK, FLAG-BIKΔBH3, FLAG-BIKΔ71, and GRP78 in 293T cells. Co-immunoprecipitation using the anti-FLAG antibody showed that the BH3 domain-truncated BIK can bind to GRP78 as well as the full-length BIK, while the truncated BIKΔ71 devoid of the amino portion was not able to bind GRP78 (Fig. 6B). Thus, despite the importance of the BH3 domain in binding to other proteins of the BCL-2 family, the BH3 domain is not required for the interaction between BIK and GRP78.
FIGURE 6.
GRP78 binds to amino portion of BIK independent of its BH3 domain. A, schematic diagrams of full-length FLAG-BIK (1–160 aa), FLAG-BIKΔBH3, FLAG-BIKΔ28, Δ56, and Δ71 mutants. The locations of the FLAG tag, the BH3 and transmembrane (TM) domains are indicated. B and C, 293T cells were transfected with the following plasmids. Panel B: lane 1, pcDNA3; lane 2, FLAG-BIK; lane 3, FLAG-BIKΔBH3; and lane 4, FLAG-BIKΔ71. Panel C: lane 1, pcDNA3; lane 2, FLAG-BIK; lane 3, FLAG-BIKΔ28; and lane 4, FLAG-BIKΔ56. All transfections were performed in the presence of zVAD-fmk to avoid BIK-induced apoptosis. Additionally, MG115 was added to stabilize expression of FLAG-BIKΔ28, Δ56 and Δ71. After 48 h, cell lysates (1 mg, B; 500 μg, C) were subjected to co-immunoprecipitation using anti-FLAG antibody. Input represented 20 μg (B) or 25 μg (C) of total lysate and 25% (B) or 30% (C) of the immunoprecipitate was applied to SDS-PAGE. The protein levels of GRP78 and BIK were analyzed by Western blots using anti-GRP78 and anti-FLAG antibodies, respectively.
To dissect further which part of the amino domain of BIK mediates the interaction with GRP78, co-immunoprecipitation experiments were performed with FLAG-BIKΔ28 and FLAG-BIKΔ56, which could be expressed in the presence of the proteasome inhibitor MG115. Our results showed that compared with full-length BIK, the ability to form complex with GRP78 was fully retained in BIKΔ28 but diminished to about 10% in BIKΔ56 (Fig. 6C). These suggest that the region between 29 and 56 aa of BIK is the primary binding domain for GRP78.
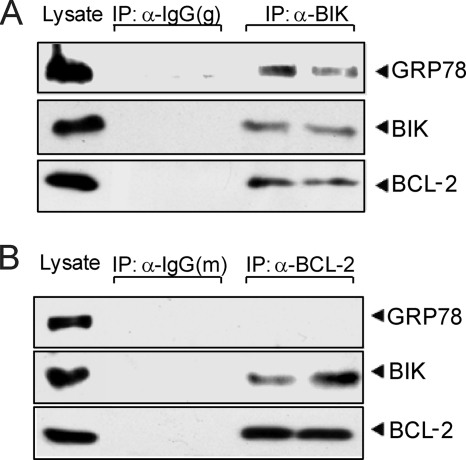
GRP78 and BCL-2 Form Separate Complex with BIK
It was reported that BIK can form complex with BCL-2 via the BH3 domain, and that endoplasmic reticulum-localized BCL-2 can protect against BIK-induced apoptosis (35). To investigate the possibility that BIK, GRP78, and BCL-2 are in the same complex, we co-transfected 293T cells with FLAG-GRP78, FLAG-BIK, and BCL-2 expression plasmids for 24 h. The cell lysate was subjected to co-immunoprecipitation using anti-BIK antibody or anti-BCL-2 antibody. Goat normal IgG or mouse normal IgG served as negative control. BCL-2 and GRP78 were pulled down by anti-BIK antibody, showing that BIK formed complex with BCL-2 and GRP78 (Fig. 7A). However, no GRP78 was detected using anti-BCL-2 antibody while BIK and BCL-2 were found in the same immunoprecipitates (Fig. 7B). Thus, these results suggested that GRP78 and BCL-2 form separate complex with BIK.
FIGURE 7.
GRP78 and BCL-2 form independent complex with BIK. A, cell lysates from 293T cells transiently transfected with FLAG-GRP78, FLAG-BIK-b5TM, and BCL-2 in the presence of zVAD-fmk for 48 h were immunoprecipitated with anti-BIK or goat normal IgG. Input (20 μg of total lysate) together with 25% of the immunoprecipitated products were applied to SDS-PAGE and subjected to Western blots for detection of GRP78, BIK, and BCL-2. The experiments were done in duplicate. B, same lysates from A were subjected to co-immunoprecipitation with anti-BCL-2 or mouse normal IgG. The bound GRP78, BIK, and BCL-2 levels were analyzed by Western blots. The experiments were done in duplicate.
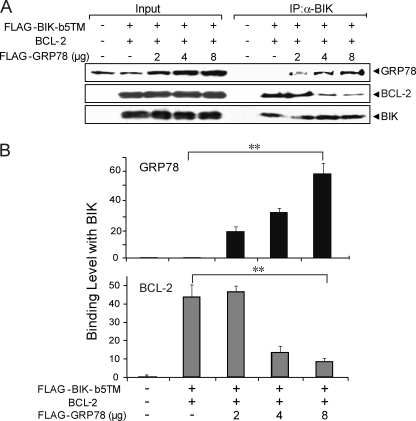
GRP78 Overexpression Decreases BCL-2 Binding to BIK
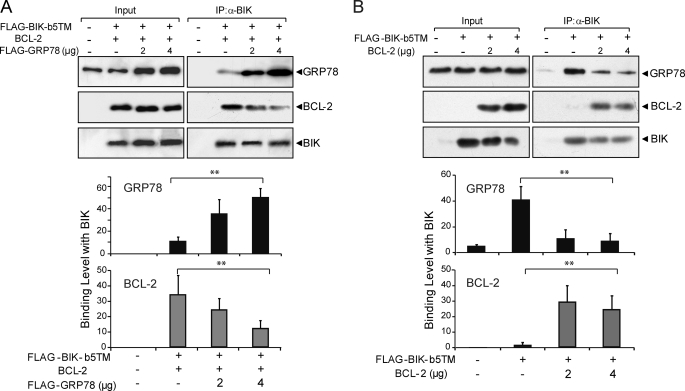
Because BIK forms separate complex with BCL-2 and GRP78, this raises the question whether GRP78 binding to BIK can compete for BIK binding to BCL-2, therefore decreasing the amount of BIK/BCL-2 complex, enabling free BCL-2 to execute its potent anti-apoptotic function. To address this, 293T cells were co-transfected with BIK, BCL-2 and increasing amounts of GRP78 expression vector. The amounts of BIK, BCL-2, and GRP78 were determined by Western blots (Fig. 8A). As expected, GRP78 level increased in a dosage-dependent manner correlating with the amount of transfected GRP78 expression plasmid. BIK and BCL-2 were co-expressed. To analyze for the BIK complex composition, lysates from the transfected cells were subjected to co-immunoprecipitation using the anti-BIK antibody. With increasing expression of GRP78, the binding of GRP78 with BIK increased accordingly (Fig. 7A). In contrast, the binding between BIK and BCL-2 decreased, showing that overexpression of GRP78 reduced the binding between BCL-2 and BIK. Quantitation of the Western blot results was summarized in Fig. 8B, showing an inverse relationship between GRP78 and BCL-2 binding to BIK.
FIGURE 8.
Overexpression of GRP78 reduces BCL-2 binding to BIK. Cell lysates from 293T cells transiently co-transfected with FLAG-BIK-b5TM, BCL-2 and different amounts of FLAG-GRP78 in the presence of zVAD-fmk for 48 h were immunoprecipitated with the anti-BIK antibody. A, input (2% of total lysate) was applied in parallel with 25% of the immunoprecipitated products to SDS-PAGE. The protein levels of GRP78, BIK, and BCL-2 were analyzed by Western blots. B, Western blots were quantitated and plotted. The experiment was repeated three times. ANOVA was applied for statistical analysis. The standard deviation is shown. **, p < 0.01.
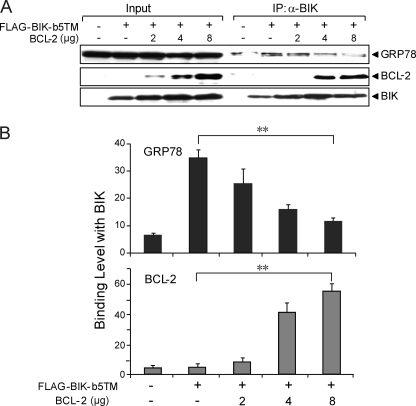
In testing whether the competition is reciprocal between GRP78 and BCL-2, 293T cells were co-transfected with BIK and increasing amounts of BCL-2 expression vector from 2 to 8 μg. The expression levels of BIK, GRP78 and BCL-2 were confirmed by Western blots (Fig. 9A). With increasing expression of BCL-2 in cells, the binding of BCL-2 with BIK increased accordingly whereas the binding between BIK and GRP78 decreased. The GRP78 and BCL-2 binding levels to BIK were summarized in Fig. 9B, showing that increased expression of BCL-2 decreased GRP78 binding to BIK.
FIGURE 9.
Overexpression of BCL-2 reduces GRP78 binding with BIK. A, cell lysates from 293T cells that were transiently co-transfected with FLAG-BIK-b5TM and increasing amount of BCL-2 for 48 h in the presence of zVAD-fmk were subjected to co-immunoprecipitation using anti-BIK antibody. The input (2% of total lysate) was applied in parallel with 25% of the immunoprecipitated products to SDS-PAGE. The protein levels of GRP78, BIK, and BCL-2 were analyzed by Western blots. B, Western blots were quantitated and plotted. The experiment was repeated three times. ANOVA was applied for statistical analysis. The standard deviation is shown. **, p < 0.01.
Competition between GRP78 and BCL-2 Binding with BIK in Human Breast Cancer Cells
To determine if the ability of GRP78 to decrease BIK binding to BCL-2 also occurs in human breast cancer cells, MCF-7 cells were co-transfected with BIK, BCL-2 and increasing amounts of GRP78 expression plasmids. The transfected cells were collected and subjected to co-immunoprecipitation using anti-BIK antibody and Western blots. The results showed that with the increasing expression of GRP78, the binding between BIK and BCL-2 decreased (Fig. 10A). Similarly, increased binding of BCL-2 to BIK was able to decrease BIK binding to GRP78 (Fig. 10B). These studies demonstrated competition between GRP78 and BCL-2 binding to BIK also exists in human breast cancer cells.
FIGURE 10.
Competition between GRP78 and BCL-2 for BIK binding in human breast cancer cells. A, cell lysates from MCF-7 cells transiently transfected with FLAG-BIK-b5TM, Bcl-2, and increasing amount of GRP78 for 48 h in the presence of zVAD-fmk were immunoprecipitated with the anti-BIK antibody. The input (2% of total lysate) was applied in parallel with 25% of the immunoprecipitated products to SDS-PAGE. The protein levels of GRP78, BIK, and BCL-2 were analyzed by Western blots. Plot of quantitation of the Western blots is shown below. B, same as A except that the MCF-7 cells were transiently transfected with FLAG-BIK-b5TM and increasing amount of BCL-2. **, p < 0.01.
DISCUSSION
Evidence is accumulating that induction of apoptosis is an important component of breast cancer regression resulting from anti-estrogen therapy (36). Despite recent advances on the signaling pathways that determine endocrine resistance, the definition of specific resistance factors and the molecular processes that lead to therapy resistance is incomplete and clinical recurrence remains a major challenge (1). The BH3-only protein BIK, which is inducible by estrogen starvation and Faslodex treatment, has been suggested to play a critical role in the anti-estrogen-induced apoptosis of breast cancer cells (8, 36). Previously we demonstrated apoptosis triggered by BIK in estrogen-starved human breast cancer cells could be suppressed by GRP78, which formed complex with BIK (14). Nonetheless, the molecular mechanism is not well understood. In the present study, we made several discoveries on how GRP78 may enable cells to escape from intrinsic apoptosis mediated by BIK.
First, we isolated a clonal human breast cancer cell line MCF-7/BUS-10 that is resistant to long-term estrogen starvation. These cells were derived from the parental MCF-7/BUS which showed high sensitivity to estrogen deprivation. Interestingly, upon exposure of MCF-7/BUS cells to estrogen starvation, while the level of BIK was induced, the level of GRP78 decreased during the first 48 h, corresponding with onset of apoptosis (14). However, upon long-term estrogen starvation, the GRP78 level was restored and even showed increase as exemplified by MCF-7/BUS-10. The loss of cell viability following GRP78 knockdown in the resistant cells further demonstrated that GRP78 contributes to the survival of these cells under estrogen deprivation condition.
How might GRP78 confer resistance to apoptosis under these adverse culture conditions? Our studies have uncovered new functional interactions between GRP78, BIK, NOXA, and BCL-2. Here we demonstrate that BIK is a stronger inducer of apoptosis than NOXA, and when expressed simultaneously, a combinative effect was observed. This is in agreement with the previous observations that overexpression of NOXA did not induce cytochrome c release but co-expression of NOXA and BIK can induce conformational activation of BAX and then initiate mitochondrial cytochrome c release (30). The possible mechanism underlying the additive apoptosis-inducing by BIK and NOXA is that NOXA can complex with MCL-1 liberating BAK from the binding of MCL-1 and BIK can activate BAX (37). There is another possibility that the additive effect of apoptosis induction is due to BIK binding with BCL-2 at the endoplasmic reticulum and NOXA can engage MCL-1. GRP78 is able to suppress both BIK and NOXA-mediated apoptosis, either individually or in combination. Therefore, under conditions where GRP78 is induced and expressed at high level such as under long-term estrogen deprivation condition, it could negate apoptosis resulting from BIK induction, even if it is assisted by another BH3-only protein such as NOXA.
Previous studies established that BH3-only proteins such as BIK rely on their BH3 domain to interact with other members of the BCL-2 protein family (38). In studying the interactive domains between GRP78 and BIK, we discovered that the BH3 domain of BIK is dispensable for its binding to GRP78. Rather, the region between 29 to 56 aa adjacent to the BH3-domain is required for binding to GRP78. On the other hand, GRP78 binding to BIK occurs at its amino portion. This is consistent with previous findings that a subfraction of GRP78 may exist in a transmembrane configuration with amino portion exposed to the cytosol (21, 22). Furthermore, we determined that purified GRP78 protein can bind to purified BIK, implying that the protein interaction is direct, rather than mediated by other proteins within a multi-protein complex.
Through co-immunoprecipitation assays, we demonstrated here that BIK not only forms complex with BCL-2, it also forms complex with GRP78. Interestingly, GRP78 and BCL-2 form separate complex with BIK. This raises the interesting question whether they compete for binding to BIK. Our results showed that increasing amounts of GRP78 expressed in cells leads to reduction of BCL-2 binding to BIK, and reciprocally, increasing amounts of BCL-2 decreases GRP78 binding to BIK. This competition was observed in different cell types, including human breast cancer cells. Because GRP78 and BCL-2 bind BIK at different domains, the reduction in binding cannot be due to direct site competition. In addition to titrating away of BIK, it is possible that the binding between GRP78 and BIK may change the conformation of BIK so it becomes unfavorable to the binding of BCL-2.
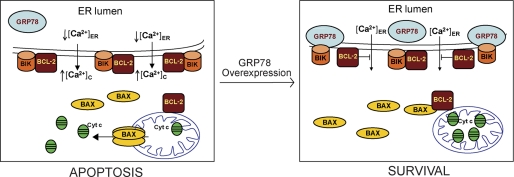
How does this impact apoptosis resulting from estrogen deprivation? As summarized in Fig. 11, we hypothesized that when the breast cancer cells were exposed to estrogen-starvation or anti-estrogen treatment the expression of BIK was elevated, promoting the formation of BIK/BCL-2 heterodimers at the outer surface of the endoplasmic reticulum. High ratio of BIK:BCL-2 changes the set of proteins BCL-2 interacts with the endoplasmic reticulum, leading to Ca2+ release and initiation of the process of apoptosis including BAX translocation to mitochondria and release of cytochrome c to the cytosol. However, when GRP78 was expressed in high level under long-term estrogen deprivation condition, GRP78 binds to and sequesters BIK through complex formation. With reduced binding to BIK, BCL-2 is able to suppress endoplasmic reticulum Ca2+ release, thereby suppressing apoptosis. This mechanistic explanation is consistent with a recent report strongly implicating BCL-2 in the survival pathway activated by GRP78 overexpression (39). While further studies are required to define more precisely the role of GRP78, BIK, NOXA, and BCL-2 in affecting anti-estrogen responsiveness, the issues are particularly complex in clinical settings which need to take into consideration the time of treatment and sensitive versus residual tumors (40, 41). In the simplified cell experimental system, our studies provide the first evidence that GRP78 can negate the apoptotic activities of BIK and NOXA and alter the amount of BCL-2 binding to BIK as a previously unanticipated anti-apoptotic mechanism for GRP78, they also provide proof-of-principle that suppression of GRP78 may offer a novel therapeutic approach to combat resistance to long-term estrogen starvation in breast cancer. Furthermore, considering that the apoptotic function of BIK has also been implicated in response to chemotherapeutics etoposide, doxorubicin, pamidronate, and the proteasome inhibitor bortezomib, the mechanisms uncovered here may also apply in these other systems (42, 43).
FIGURE 11.
Model for GRP78 overexpression in inhibiting apoptosis through reduction of BCL-2 binding to BIK. The endoplasmic reticulum, as a major intracellular Ca2+ store, regulates apoptosis by sensitizing mitochondria to the death stimuli through Ca2+ release to the cytosol. This process is blocked by BCL-2 as part of its anti-apoptotic function. BIK is primarily localized to the endoplasmic reticulum. Induction of BIK, such as under estrogen-starvation condition, leads to binding and inactivation of BCL-2 through complex formation. This triggers Ca2+ release from the endoplasmic reticulum and initiates the apoptotic process including BAX translocation to mitochondria and release of cytochrome c to the cytosol. When GRP78 is overexpressed under stress conditions such as long-term estrogen starvation, GRP78 binds to and sequesters BIK through complex formation. With reduced binding to BIK, BCL-2 is able to suppress endoplasmic reticulum Ca2+ release, thereby suppressing apoptosis. This promotes cell survival and resistance to therapy.
Acknowledgments
We thank Drs. Gordon Shore for the gift of BIK expression plasmids, John Nicholas for BIM expression plasmid, Jianze Li for the NOXA expression plasmid, Parkash Gill for purified GRP78 protein, Cheng Ji for the antibody against BIM, and Ana Soto for MCF-7/BUS cell line. We thank Brenda Lee and Minal Patel for technical assistance. The FACS analysis was performed in the USC Norris Cancer Center Flow Cytometry Core Facility supported in part by Grant P30CA014089 from the National Cancer Institute.
This work was supported, in whole or in part, by National Institutes of Health Grants CA027607 and CA111700 and Department of Defense Grant W81XWH-07-1-0480.
- BCL
- B-cell lymphoma
- aa
- amino acids
- GRP
- glucose-regulated protein
- BH
- BCL-2 homology
- BIK
- BCL-2-interacting killer
- 7-AAD
- 7-amino actinomycin.
REFERENCES
- 1. Musgrove E. A., Sutherland R. L. (2009) Nat. Rev. Cancer 9, 631–643 [DOI] [PubMed] [Google Scholar]
- 2. Tripathy D. (2009) J. Clin. Oncol. 27, 2580–2582 [DOI] [PubMed] [Google Scholar]
- 3. Thiantanawat A., Long B. J., Brodie A. M. (2003) Cancer Res. 63, 8037–8050 [PubMed] [Google Scholar]
- 4. Youle R. J., Strasser A. (2008) Nat. Rev. Mol. Cell Biol. 9, 47–59 [DOI] [PubMed] [Google Scholar]
- 5. Teles A. V., Ureshino R. P., Dorta D. J., Lopes G. S., Hsu Y. T., Smaili S. S. (2008) Neurosci. Lett. 442, 96–99 [DOI] [PubMed] [Google Scholar]
- 6. Hsu Y. T., Wolter K. G., Youle R. J. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 3668–3672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chinnadurai G., Vijayalingam S., Rashmi R. (2008) Oncogene 27, Suppl. 1, S20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hur J., Chesnes J., Coser K. R., Lee R. S., Geck P., Isselbacher K. J., Shioda T. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 2351–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coser K. R., Chesnes J., Hur J., Ray S., Isselbacher K. J., Shioda T. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 13994–13999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hur J., Bell D. W., Dean K. L., Coser K. R., Hilario P. C., Okimoto R. A., Tobey E. M., Smith S. L., Isselbacher K. J., Shioda T. (2006) Cancer Res. 66, 10153–10161 [DOI] [PubMed] [Google Scholar]
- 11. Mathai J. P., Germain M., Marcellus R. C., Shore G. C. (2002) Oncogene 21, 2534–2544 [DOI] [PubMed] [Google Scholar]
- 12. Mathai J. P., Germain M., Shore G. C. (2005) J. Biol. Chem. 280, 23829–23836 [DOI] [PubMed] [Google Scholar]
- 13. Breckenridge D. G., Germain M., Mathai J. P., Nguyen M., Shore G. C. (2003) Oncogene 22, 8608–8618 [DOI] [PubMed] [Google Scholar]
- 14. Fu Y., Li J., Lee A. S. (2007) Cancer Res. 67, 3734–3740 [DOI] [PubMed] [Google Scholar]
- 15. Lee A. S. (2007) Cancer Res. 67, 3496–3499 [DOI] [PubMed] [Google Scholar]
- 16. Ni M., Zhou H., Wey S., Baumeister P., Lee A. S. (2009) PLoS One 4, e6868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang M., Ye R., Barron E., Baumeister P., Mao C., Luo S., Fu Y., Luo B., Dubeau L., Hinton D. R., Lee A. S. (2010) Cell Death Differ. 17, 488–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pfaffenbach K. T., Lee A. S. (2011) Curr. Opin. Cell Biol. 23, 150–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gazit G., Lu J., Lee A. S. (1999) Breast Cancer Res. Treat. 54, 135–146 [DOI] [PubMed] [Google Scholar]
- 20. Lee E., Nichols P., Spicer D., Groshen S., Yu M. C., Lee A. S. (2006) Cancer Res. 66, 7849–7853 [DOI] [PubMed] [Google Scholar]
- 21. Rao R. V., Peel A., Logvinova A., del Rio G., Hermel E., Yokota T., Goldsmith P. C., Ellerby L. M., Ellerby H. M., Bredesen D. E. (2002) FEBS Lett. 514, 122–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reddy R. K., Mao C., Baumeister P., Austin R. C., Kaufman R. J., Lee A. S. (2003) J. Biol. Chem. 278, 20915–20924 [DOI] [PubMed] [Google Scholar]
- 23. Coser K. R., Wittner B. S., Rosenthal N. F., Collins S. C., Melas A., Smith S. L., Mahoney C. J., Shioda K., Isselbacher K. J., Ramaswamy S., Shioda T. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 14536–14541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Soto A. M., Sonnenschein C., Chung K. L., Fernandez M. F., Olea N., Serrano F. O. (1995) Environ. Health Perspect. 103, Suppl .7, 113–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Germain M., Mathai J. P., Shore G. C. (2002) J. Biol. Chem. 277, 18053–18060 [DOI] [PubMed] [Google Scholar]
- 26. Choi Y. B., Nicholas J. (2010) PLoS Pathog. 6, e1001031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Y., Liu R., Ni M., Gill P., Lee A. S. (2010) J. Biol. Chem. 285, 15065–15075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li J., Lee B., Lee A. S. (2006) J. Biol. Chem. 281, 7260–7270 [DOI] [PubMed] [Google Scholar]
- 29. Tsutsumi S., Namba T., Tanaka K. I., Arai Y., Ishihara T., Aburaya M., Mima S., Hoshino T., Mizushima T. (2006) Oncogene 25, 1018–1029 [DOI] [PubMed] [Google Scholar]
- 30. Wu F., Lee A. S. (1998) Nucleic Acids Res. 26, 4837–4845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Germain M., Mathai J. P., McBride H. M., Shore G. C. (2005) EMBO J. 24, 1546–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boyd J. M., Gallo G. J., Elangovan B., Houghton A. B., Malstrom S., Avery B. J., Ebb R. G., Subramanian T., Chittenden T., Lutz R. J. (1995) Oncogene 11, 1921–1928 [PubMed] [Google Scholar]
- 33. Chittenden T., Flemington C., Houghton A. B., Ebb R. G., Gallo G. J., Elangovan B., Chinnadurai G., Lutz R. J. (1995) EMBO J. 14, 5589–5596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gillissen B., Essmann F., Graupner V., Stärck L., Radetzki S., Dörken B., Schulze-Osthoff K., Daniel P. T. (2003) EMBO J. 22, 3580–3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Breckenridge D. G., Stojanovic M., Marcellus R. C., Shore G. C. (2003) J. Cell Biol. 160, 1115–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Riggins R. B., Bouton A. H., Liu M. C., Clarke R. (2005) Vitam. Horm. 71, 201–237 [DOI] [PubMed] [Google Scholar]
- 37. Willis S. N., Chen L., Dewson G., Wei A., Naik E., Fletcher J. I., Adams J. M., Huang D. C. (2005) Genes Dev. 19, 1294–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Willis S. N., Adams J. M. (2005) Curr. Opin. Cell Biol. 17, 617–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Penas C., Font-Nieves M., Fores J., Petegnief V., Planas A., Navarro X., Casas C. (2011) Cell Death Differ., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Daidone M. G., Luisi A., Martelli G., Benini E., Veneroni S., Tomasic G., De Palo G., Silvestrini R. (2000) Br. J. Cancer 82, 270–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cameron D. A., Keen J. C., Dixon J. M., Bellamy C., Hanby A., Anderson T. J., Miller W. R. (2000) Eur. J. Cancer 36, 845–851 [DOI] [PubMed] [Google Scholar]
- 42. Oppermann M., Geilen C. C., Fecker L. F., Gillissen B., Daniel P. T., Eberle J. (2005) Oncogene 24, 7369–7380 [DOI] [PubMed] [Google Scholar]
- 43. Nikrad M., Johnson T., Puthalalath H., Coultas L., Adams J., Kraft A. S. (2005) Mol. Cancer Ther. 4, 443–449 [DOI] [PubMed] [Google Scholar]