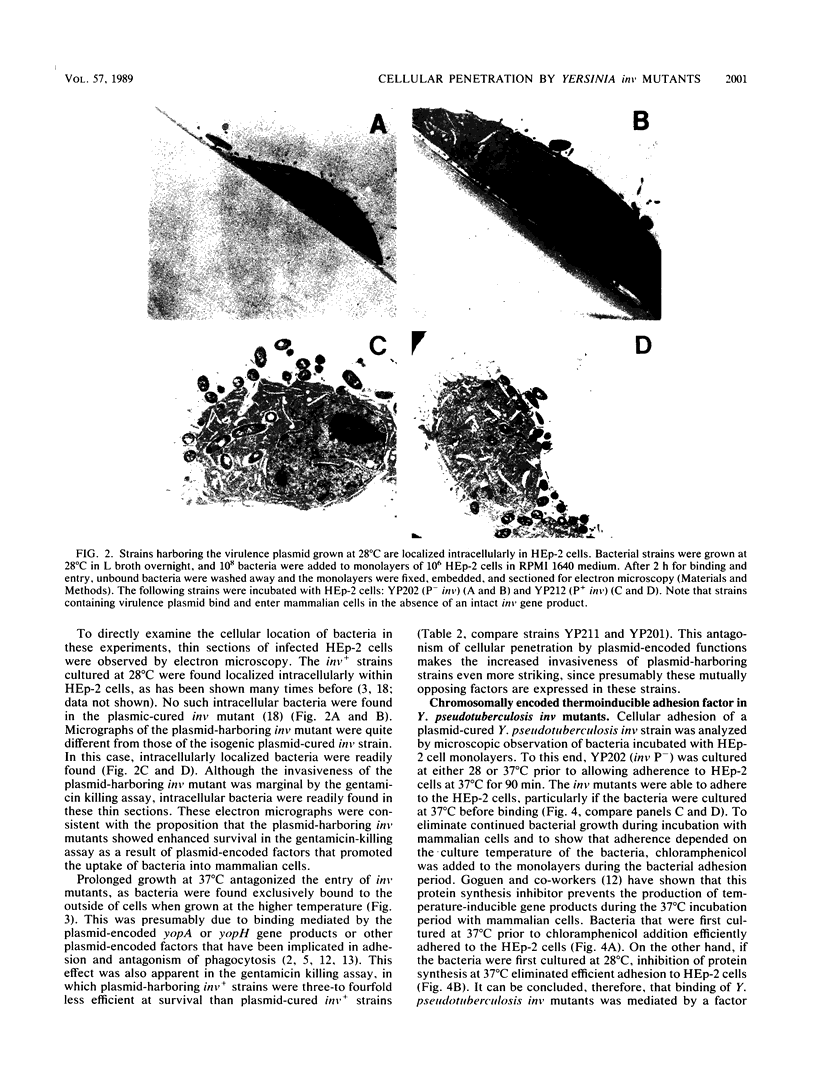
Abstract
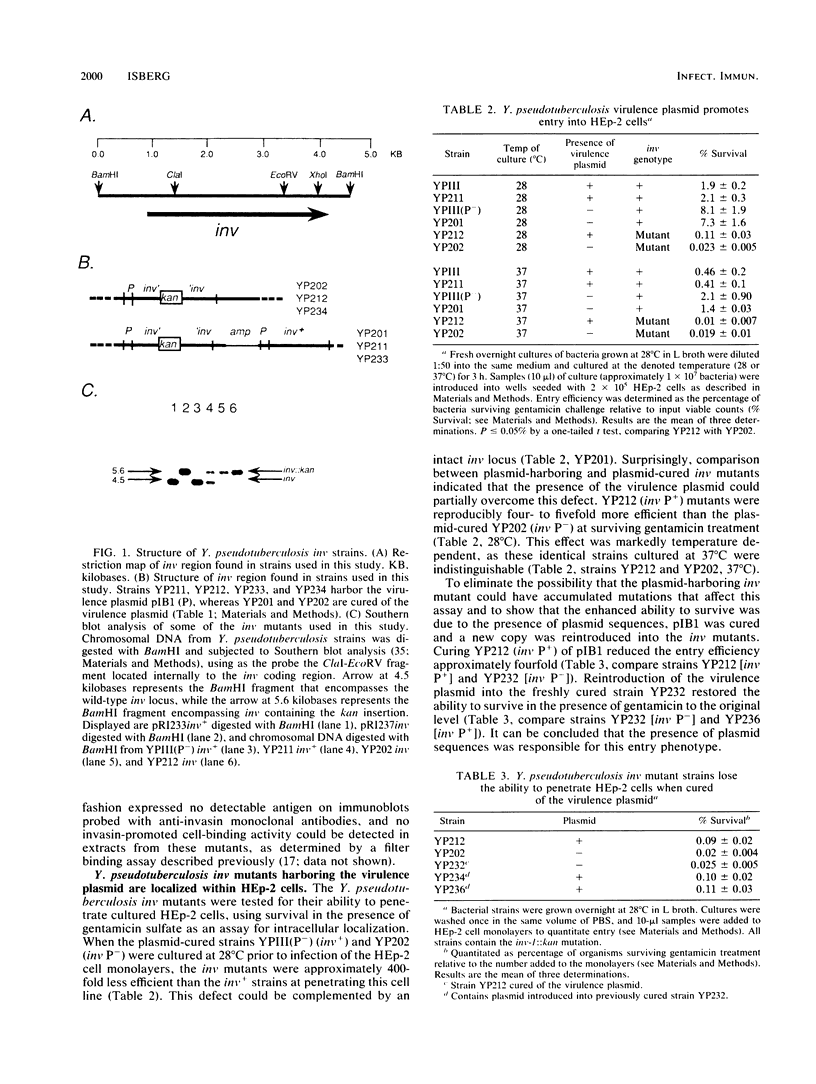
Yersinia pseudotuberculosis inv mutants were analyzed for their ability to bind and penetrate mammalian cell lines. Strains defective for the production of invasin and cured of the Yersinia virulence plasmid pIB1 were extremely defective for entry into the HEp-2 cell line. inv strains harboring the virulence plasmid partially overcame this defect, indicating that the virulence plasmid mediates an invasin-independent pathway for low-level entry into cultured cells. Plasmid-cured inv mutants were able to attach efficiently to mammalian cells after bacterial culture at 37 degrees C but not after culture at a lower temperature. The enhanced cellular binding of inv mutants grown at 37 degrees C did not result in efficient cellular penetration, indicating that invasin-mediated entry is the primary chromosomally encoded pathway responsible for Y. pseudotuberculosis penetration into both HEp-2 and Chinese hamster ovary cells under the assay conditions described here.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bovallius A., Nilsson G. Ingestion and survival of Y. pseudotuberculosis in HeLa cells. Can J Microbiol. 1975 Dec;21(12):1997–2007. doi: 10.1139/m75-287. [DOI] [PubMed] [Google Scholar]
- Bölin I., Norlander L., Wolf-Watz H. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect Immun. 1982 Aug;37(2):506–512. doi: 10.1128/iai.37.2.506-512.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölin I., Wolf-Watz H. Molecular cloning of the temperature-inducible outer membrane protein 1 of Yersinia pseudotuberculosis. Infect Immun. 1984 Jan;43(1):72–78. doi: 10.1128/iai.43.1.72-78.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. B. Pathogenecity of Yersinia enterocolitica for mice. Infect Immun. 1975 Jan;11(1):164–170. doi: 10.1128/iai.11.1.164-170.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnetzky W. T., Shuford W. W. Survival and growth of Yersinia pestis within macrophages and an effect of the loss of the 47-megadalton plasmid on growth in macrophages. Infect Immun. 1985 Jan;47(1):234–241. doi: 10.1128/iai.47.1.234-241.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerc P., Sansonetti P. J. Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect Immun. 1987 Nov;55(11):2681–2688. doi: 10.1128/iai.55.11.2681-2688.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenish J. A., Schiemann D. A. HeLa cell infection by Yersinia enterocolitica: evidence for lack of intracellular multiplication and development of a new procedure for quantitative expression of infectivity. Infect Immun. 1981 Apr;32(1):48–55. doi: 10.1128/iai.32.1.48-55.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields P. I., Swanson R. V., Haidaris C. G., Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Gumbiner B., Falkow S. Penetration of Salmonella through a polarized Madin-Darby canine kidney epithelial cell monolayer. J Cell Biol. 1988 Jul;107(1):221–230. doi: 10.1083/jcb.107.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal S. B., Hale T. L., Sansonetti P. J. Invasive enteric pathogens. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S702–S707. doi: 10.1093/clinids/5.supplement_4.s702. [DOI] [PubMed] [Google Scholar]
- Gemski P., Lazere J. R., Casey T., Wohlhieter J. A. Presence of a virulence-associated plasmid in Yersinia pseudotuberculosis. Infect Immun. 1980 Jun;28(3):1044–1047. doi: 10.1128/iai.28.3.1044-1047.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goguen J. D., Walker W. S., Hatch T. P., Yother J. Plasmid-determined cytotoxicity in Yersinia pestis and Yersinia pseudotuberculosis. Infect Immun. 1986 Mar;51(3):788–794. doi: 10.1128/iai.51.3.788-794.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGUCHI K., SMITH J. L. Studies on the nutrition and physiology of Pasteurella pestis. VI. A differential plating medium for the estimation of the mutation rate to avirulence. J Bacteriol. 1961 Apr;81:605–608. doi: 10.1128/jb.81.4.605-608.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R., Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985 Sep 19;317(6034):262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Leong J. M. Cultured mammalian cells attach to the invasin protein of Yersinia pseudotuberculosis. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6682–6686. doi: 10.1073/pnas.85.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R., Voorhis D. L., Falkow S. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987 Aug 28;50(5):769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Makino S., Sasakawa C., Kamata K., Kurata T., Yoshikawa M. A genetic determinant required for continuous reinfection of adjacent cells on large plasmid in S. flexneri 2a. Cell. 1986 Aug 15;46(4):551–555. doi: 10.1016/0092-8674(86)90880-9. [DOI] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli A. T., Baudry B., d'Hauteville H., Hale T. L., Sansonetti P. J. Cloning of plasmid DNA sequences involved in invasion of HeLa cells by Shigella flexneri. Infect Immun. 1985 Jul;49(1):164–171. doi: 10.1128/iai.49.1.164-171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAbee D. D., Grinnell F. Fibronectin-mediated binding and phagocytosis of polystyrene latex beads by baby hamster kidney cells. J Cell Biol. 1983 Nov;97(5 Pt 1):1515–1523. doi: 10.1083/jcb.97.5.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Falkow S. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect Immun. 1988 May;56(5):1242–1248. doi: 10.1128/iai.56.5.1242-1248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Farmer J. J., 3rd, Hill W. E., Falkow S. The ail locus is found uniquely in Yersinia enterocolitica serotypes commonly associated with disease. Infect Immun. 1989 Jan;57(1):121–131. doi: 10.1128/iai.57.1.121-131.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988 Jun;170(6):2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Blank H. F., Kingsbury D. T., Falkow S. Genetic analysis of essential plasmid determinants of pathogenicity in Yersinia pestis. J Infect Dis. 1983 Aug;148(2):297–304. doi: 10.1093/infdis/148.2.297. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Wolf-Watz H., Bolin I., Beeder A. B., Falkow S. Characterization of common virulence plasmids in Yersinia species and their role in the expression of outer membrane proteins. Infect Immun. 1984 Jan;43(1):108–114. doi: 10.1128/iai.43.1.108-114.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson M., Harkness T. K. Intracellular Pasteurella pseudotuberculosis: Multiplication in Cultured Spleen and Kidney Cells. Infect Immun. 1970 Nov;2(5):631–639. doi: 10.1128/iai.2.5.631-639.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins-Browne R. M., Tzipori S., Gonis G., Hayes J., Withers M., Prpic J. K. The pathogenesis of Yersinia enterocolitica infection in gnotobiotic piglets. J Med Microbiol. 1985 Jun;19(3):297–308. doi: 10.1099/00222615-19-3-297. [DOI] [PubMed] [Google Scholar]
- Rosqvist R., Bölin I., Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988 Aug;56(8):2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosqvist R., Skurnik M., Wolf-Watz H. Increased virulence of Yersinia pseudotuberculosis by two independent mutations. Nature. 1988 Aug 11;334(6182):522–524. doi: 10.1038/334522a0. [DOI] [PubMed] [Google Scholar]
- Sansonetti P. J., Ryter A., Clerc P., Maurelli A. T., Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986 Feb;51(2):461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet M., Mazigh D., Berche P. Growth of Yersinia pseudotuberculosis in mouse spleen despite loss of a virulence plasmid of mol. wt 47 X 10(6). J Med Microbiol. 1984 Dec;18(3):371–375. doi: 10.1099/00222615-18-3-371. [DOI] [PubMed] [Google Scholar]
- Small P. L., Isberg R. R., Falkow S. Comparison of the ability of enteroinvasive Escherichia coli, Salmonella typhimurium, Yersinia pseudotuberculosis, and Yersinia enterocolitica to enter and replicate within HEp-2 cells. Infect Immun. 1987 Jul;55(7):1674–1679. doi: 10.1128/iai.55.7.1674-1679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Sprinz H. Electron-Microscope Studies of Experimental Salmonella Infection in the Preconditioned Guinea Pig: II. Response of the Intestinal Mucosa to the Invasion by Salmonella typhimurium. Am J Pathol. 1967 Jul;51(1):137–161. [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Une T. Studies on the pathogenicity of Yersinia enterocolitica. III. Comparative studies between Y. enterocolitica and Y. pseudotuberculosis. Microbiol Immunol. 1977;21(9):505–516. doi: 10.1111/j.1348-0421.1977.tb00316.x. [DOI] [PubMed] [Google Scholar]
- Van de Water L., Destree A. T., Hynes R. O. Fibronectin binds to some bacteria but does not promote their uptake by phagocytic cells. Science. 1983 Apr 8;220(4593):201–204. doi: 10.1126/science.6338594. [DOI] [PubMed] [Google Scholar]
- Zaleska M., Lounatmaa K., Nurminen M., Wahlström E., Mäkelä P. H. A novel virulence-associated cell surface structure composed of 47-kd protein subunits in Yersinia enterocolitica. EMBO J. 1985 Apr;4(4):1013–1018. doi: 10.1002/j.1460-2075.1985.tb03732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]






