Abstract
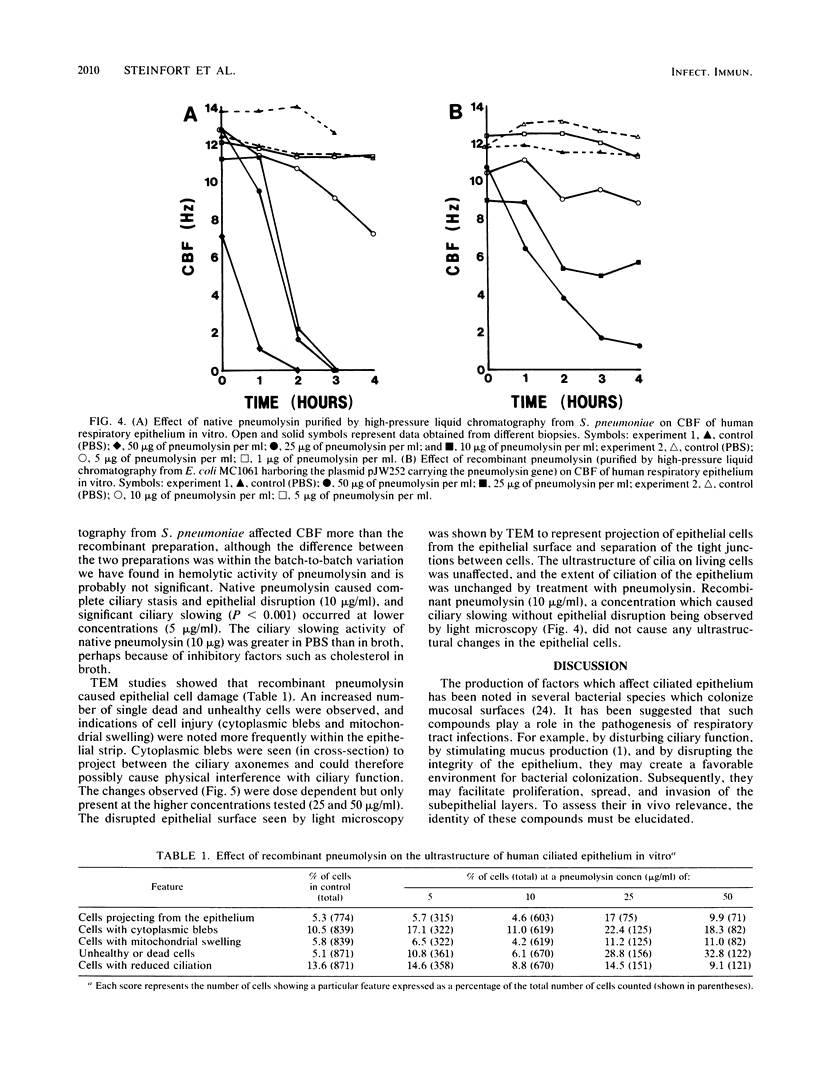
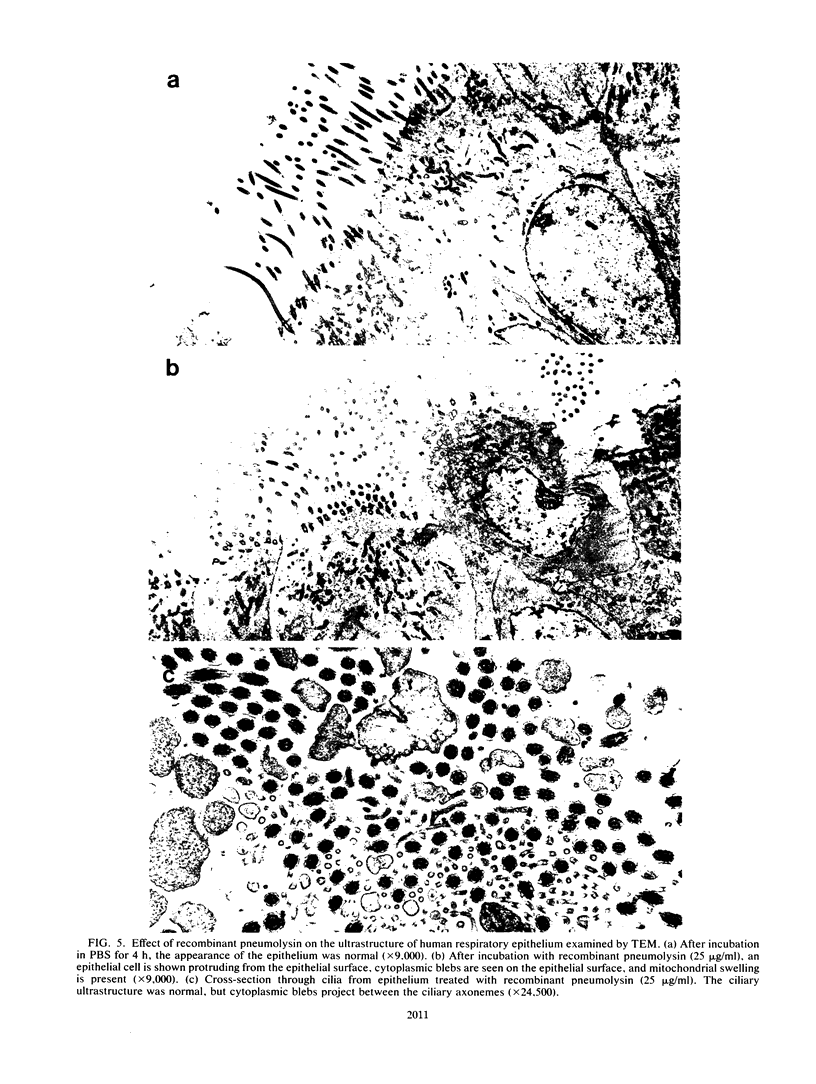
A total of 11 of 15 Streptococcus pneumoniae culture filtrates and all five bacterial autolysates produced by cell death in the stationary phase caused slowed ciliary beating and disruption of the surface integrity of human respiratory epithelium in organ culture. This effect was inhibited by cholesterol and was heat labile and reduced by standing at room temperature but was stable at -40 degrees C. The activity was detected at the late stationary phase of culture and was associated with the presence of hemolytic activity. Gel filtration of a concentrated culture filtrate and autolysate both yielded a single fraction of approximately 50 kilodaltons which slowed ciliary beating and were the only fractions with hemolytic activity. Rabbit antiserum to pneumolysin, a sulfhydryl-activated hemolytic cytotoxin released by S. pneumoniae during autolysis, neutralized the effect of the culture filtrate on respiratory epithelium. Both native and recombinant pneumolysin caused ciliary slowing and epithelial disruption. Electron microscopy showed a toxic effect of pneumolysin on epithelial cells: cytoplasmic blebs, mitochondrial swelling, cellular extrusion, and cell death, but no change in ciliary ultrastructure. Recombinant pneumolysin (10 micrograms/ml) caused ciliary slowing in the absence of changes in cell ultrastructure. Release of pneumolysin in the respiratory tract during infection may perturb host defenses, allowing bacterial proliferation and spread.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler K. B., Hendley D. D., Davis G. S. Bacteria associated with obstructive pulmonary disease elaborate extracellular products that stimulate mucin secretion by explants of guinea pig airways. Am J Pathol. 1986 Dec;125(3):501–514. [PMC free article] [PubMed] [Google Scholar]
- Alouf J. E. Streptococcal toxins (streptolysin O, streptolysin S, erythrogenic toxin). Pharmacol Ther. 1980;11(3):661–717. doi: 10.1016/0163-7258(80)90045-5. [DOI] [PubMed] [Google Scholar]
- Austrian R. The pneumococcus at Hopkins: early portents of future developments. Johns Hopkins Med J. 1979 Jun;144(6):192–201. [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Complement activation and attack on autologous cell membranes induced by streptolysin-O. Infect Immun. 1985 Jun;48(3):713–719. doi: 10.1128/iai.48.3.713-719.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J., Sziegoleit A. Mechanism of membrane damage by streptolysin-O. Infect Immun. 1985 Jan;47(1):52–60. doi: 10.1128/iai.47.1.52-60.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Rowan-Kelly B., Paton J. C. Inhibition of in vitro human lymphocyte response by the pneumococcal toxin pneumolysin. Infect Immun. 1984 Nov;46(2):585–589. doi: 10.1128/iai.46.2.585-589.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg C. R., Melly M. A., Hellerqvist C. G., Coniglio J. G., McGee Z. A. Toxic activity of purified lipopolysaccharide of Neisseria gonorrhoeae for human fallopian tube mucosa. J Infect Dis. 1981 Mar;143(3):432–439. doi: 10.1093/infdis/143.3.432. [DOI] [PubMed] [Google Scholar]
- Hingley S. T., Hastie A. T., Kueppers F., Higgins M. L., Weinbaum G., Shryock T. Effect of ciliostatic factors from Pseudomonas aeruginosa on rabbit respiratory cilia. Infect Immun. 1986 Jan;51(1):254–262. doi: 10.1128/iai.51.1.254-262.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. P., Inzana T. J. Loss of ciliary activity in organ cultures of rat trachea treated with lipo-oligosaccharide from Haemophilus influenzae. J Med Microbiol. 1986 Nov;22(3):265–268. doi: 10.1099/00222615-22-3-265. [DOI] [PubMed] [Google Scholar]
- Johnson M. K., Boese-Marrazzo D., Pierce W. A., Jr Effects of pneumolysin on human polymorphonuclear leukocytes and platelets. Infect Immun. 1981 Oct;34(1):171–176. doi: 10.1128/iai.34.1.171-176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanclerski K., Möllby R. Production and purification of Streptococcus pneumoniae hemolysin (pneumolysin). J Clin Microbiol. 1987 Feb;25(2):222–225. doi: 10.1128/jcm.25.2.222-225.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell T. J., Walker J. A., Saunders F. K., Andrew P. W., Boulnois G. J. Expression of the pneumolysin gene in Escherichia coli: rapid purification and biological properties. Biochim Biophys Acta. 1989 Jan 23;1007(1):67–72. doi: 10.1016/0167-4781(89)90131-0. [DOI] [PubMed] [Google Scholar]
- Paton J. C., Ferrante A. Inhibition of human polymorphonuclear leukocyte respiratory burst, bactericidal activity, and migration by pneumolysin. Infect Immun. 1983 Sep;41(3):1212–1216. doi: 10.1128/iai.41.3.1212-1216.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton J. C., Lock R. A., Hansman D. J. Effect of immunization with pneumolysin on survival time of mice challenged with Streptococcus pneumoniae. Infect Immun. 1983 May;40(2):548–552. doi: 10.1128/iai.40.2.548-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton J. C., Rowan-Kelly B., Ferrante A. Activation of human complement by the pneumococcal toxin pneumolysin. Infect Immun. 1984 Mar;43(3):1085–1087. doi: 10.1128/iai.43.3.1085-1087.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutland J., Cole P. J. Non-invasive sampling of nasal cilia for measurement of beat frequency and study of ultrastructure. Lancet. 1980 Sep 13;2(8194):564–565. doi: 10.1016/s0140-6736(80)91995-9. [DOI] [PubMed] [Google Scholar]
- Walker J. A., Allen R. L., Falmagne P., Johnson M. K., Boulnois G. J. Molecular cloning, characterization, and complete nucleotide sequence of the gene for pneumolysin, the sulfhydryl-activated toxin of Streptococcus pneumoniae. Infect Immun. 1987 May;55(5):1184–1189. doi: 10.1128/iai.55.5.1184-1189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R., Alton E., Rutman A., Higgins P., Al Nakib W., Geddes D. M., Tyrrell D. A., Cole P. J. Upper respiratory tract viral infection and mucociliary clearance. Eur J Respir Dis. 1987 May;70(5):272–279. [PubMed] [Google Scholar]
- Wilson R., Pitt T., Taylor G., Watson D., MacDermot J., Sykes D., Roberts D., Cole P. Pyocyanin and 1-hydroxyphenazine produced by Pseudomonas aeruginosa inhibit the beating of human respiratory cilia in vitro. J Clin Invest. 1987 Jan;79(1):221–229. doi: 10.1172/JCI112787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. Secondary ciliary dysfunction. Clin Sci (Lond) 1988 Aug;75(2):113–120. doi: 10.1042/cs0750113. [DOI] [PubMed] [Google Scholar]




