Abstract
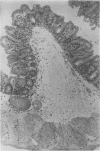
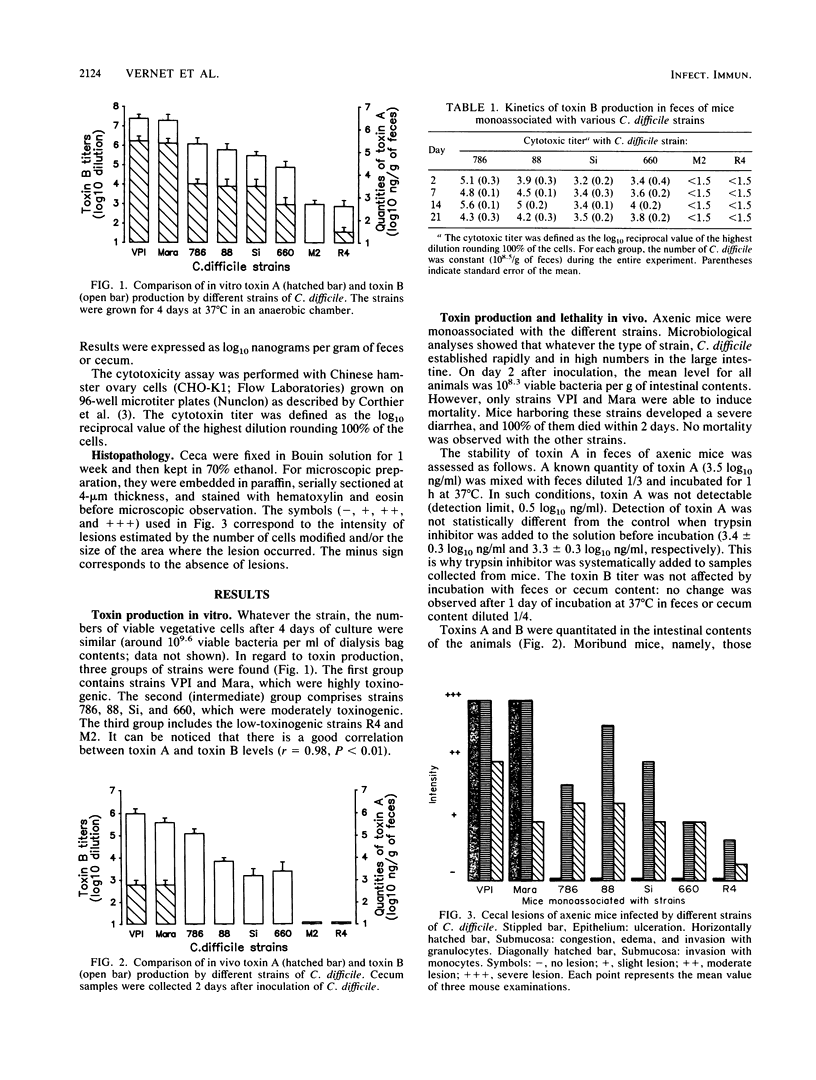
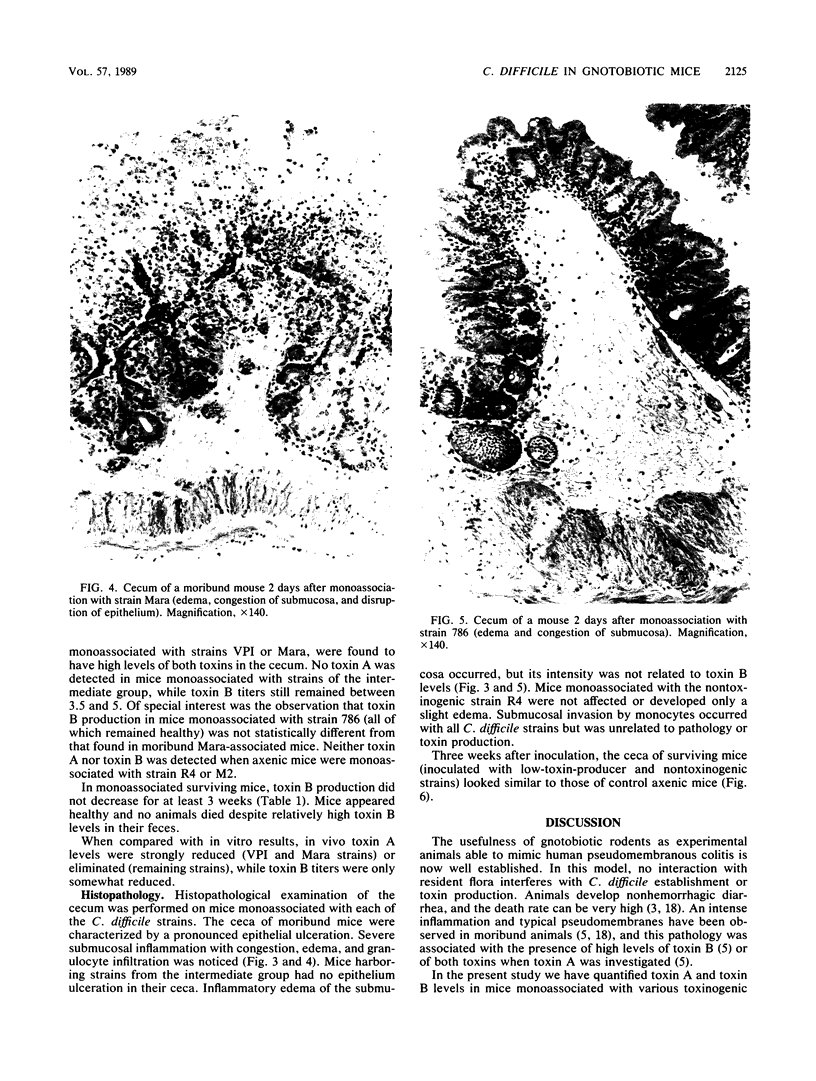
Various Clostridium difficile strains were studied with respect to their pathogenicity in monoassociated mice in relation to levels of toxin A and toxin B in vivo and in vitro. Two strains which were the most potent toxin producers in vitro induced mortality (100%); mice monoassociated with these strains were found to have high levels of both toxins in their ceca and an intense cecal epithelial ulceration together with a severe inflammatory process. No mortality was observed with the other strains. Strains which were moderately toxinogenic in vitro induced inflammation of the cecum but no ulceration, and no toxin A was found. Inflammation intensity was not related to toxin B levels. After 3 weeks, ceca returned to normal in spite of a chronic cytotoxin production. When compared with in vitro results, which showed a good correlation between the levels of the two toxins, toxin A amounts in vivo were found to be lowered relative to toxin B levels. The lack of detectable toxin A levels in animals infected with all but the two most highly toxinogenic strains prevented death. This work points out the importance of investigation of toxin A for the understanding of C. difficile pathogenicity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett J. G., Chang T. W., Gurwith M., Gorbach S. L., Onderdonk A. B. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978 Mar 9;298(10):531–534. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- Borriello S. P., Ketley J. M., Mitchell T. J., Barclay F. E., Welch A. R., Price A. B., Stephen J. Clostridium difficile--a spectrum of virulence and analysis of putative virulence determinants in the hamster model of antibiotic-associated colitis. J Med Microbiol. 1987 Aug;24(1):53–64. doi: 10.1099/00222615-24-1-53. [DOI] [PubMed] [Google Scholar]
- Corthier G., Dubos F., Raibaud P. Modulation of cytotoxin production by Clostridium difficile in the intestinal tracts of gnotobiotic mice inoculated with various human intestinal bacteria. Appl Environ Microbiol. 1985 Jan;49(1):250–252. doi: 10.1128/aem.49.1.250-252.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthier G., Muller M. C. Emergence in gnotobiotic mice of nontoxinogenic clones of Clostridium difficile from a toxinogenic one. Infect Immun. 1988 Jun;56(6):1500–1504. doi: 10.1128/iai.56.6.1500-1504.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Johnson W. J., Balish E., Wilkins T. Pseudomembranous colitis in Clostridium difficile-monoassociated rats. Infect Immun. 1983 Mar;39(3):1368–1376. doi: 10.1128/iai.39.3.1368-1376.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine O., Ducluzeau R., Raibaud P., Chabanet C., Popoff M. R., Badoual J., Gabilan J. C., Andremont A. Comparaison entre le nombre et la nature des Clostridium fécaux et d'autres facteurs de risque impliqués dans la pathologie intestinale des nouveau-nés. Ann Inst Pasteur Microbiol. 1986 Jul-Aug;137B(1):61–75. [PubMed] [Google Scholar]
- George R. H., Symonds J. M., Dimock F., Brown J. D., Arabi Y., Shinagawa N., Keighley M. R., Alexander-Williams J., Burdon D. W. Identification of Clostridium difficile as a cause of pseudomembranous colitis. Br Med J. 1978 Mar 18;1(6114):695–695. doi: 10.1136/bmj.1.6114.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivan H. C., Clark G. F., Smith D. F., Wilkins T. D. Cell surface binding site for Clostridium difficile enterotoxin: evidence for a glycoconjugate containing the sequence Gal alpha 1-3Gal beta 1-4GlcNAc. Infect Immun. 1986 Sep;53(3):573–581. doi: 10.1128/iai.53.3.573-581.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson H. E., Price A. B., Honour P., Borriello S. P. Clostridium difficile and the aetiology of pseudomembranous colitis. Lancet. 1978 May 20;1(8073):1063–1066. doi: 10.1016/s0140-6736(78)90912-1. [DOI] [PubMed] [Google Scholar]
- Libby J. M., Donta S. T., Wilkins T. D. Clostridium difficile toxin A in infants. J Infect Dis. 1983 Sep;148(3):606–606. doi: 10.1093/infdis/148.3.606. [DOI] [PubMed] [Google Scholar]
- Lyerly D. M., Saum K. E., MacDonald D. K., Wilkins T. D. Effects of Clostridium difficile toxins given intragastrically to animals. Infect Immun. 1985 Feb;47(2):349–352. doi: 10.1128/iai.47.2.349-352.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Sullivan N. M., Wilkins T. D. Enzyme-linked immunosorbent assay for Clostridium difficile toxin A. J Clin Microbiol. 1983 Jan;17(1):72–78. doi: 10.1128/jcm.17.1.72-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahe S., Corthier G., Dubos F. Effect of various diets on toxin production by two strains of Clostridium difficile in gnotobiotic mice. Infect Immun. 1987 Aug;55(8):1801–1805. doi: 10.1128/iai.55.8.1801-1805.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Mikawa M., Nakashio S., Takabatake M., Okado I., Yamakawa K., Serikawa T., Okumura S., Nishida S. Isolation of Clostridium difficile from the feces and the antibody in sera of young and elderly adults. Microbiol Immunol. 1981;25(4):345–351. doi: 10.1111/j.1348-0421.1981.tb00036.x. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Cisneros R. L., Bartlett J. G. Clostridium difficile in gnotobiotic mice. Infect Immun. 1980 Apr;28(1):277–282. doi: 10.1128/iai.28.1.277-282.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raibaud P., Dickinson A. B., Sacquet E., Charlier H., Mocquot G. La microflore du tube digestif du rat. I. Techniques d'étude et milieux de culture proposés. Ann Inst Pasteur (Paris) 1966 Apr;110(4):568–590. [PubMed] [Google Scholar]
- Taylor N. S., Thorne G. M., Bartlett J. G. Comparison of two toxins produced by Clostridium difficile. Infect Immun. 1981 Dec;34(3):1036–1043. doi: 10.1128/iai.34.3.1036-1043.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K. H., Sheagren J. N., Freter R., Weatherbee L., Lyerly D. Gnotobiotic models for study of the microbial ecology of Clostridium difficile and Escherichia coli. J Infect Dis. 1986 Mar;153(3):547–551. doi: 10.1093/infdis/153.3.547. [DOI] [PubMed] [Google Scholar]