Abstract
Decreased cognitive control over prepotent responses has been hypothesized to contribute to ethanol-induced behavioral disinhibition. However, the effects of ethanol on specific cognitive domains associated with decision making have not been extensively studied. We examined the impact of acute ethanol administration on cognitive performance of nonhuman primates. Studies were conducted using 0.2, 0.5, and 1 g/kg intravenous ethanol in rhesus macaques performing touch screen–based tasks examining stimulus discrimination, stimulus reversal, and stimulus response performance. The impact on attentional processing was also evaluated. Ethanol reduced the accuracy of reversal performance marginally at 0.2 g/kg and significantly at 0.5 g/kg. This effect was selective given an absence of impairment on the stimulus discrimination and stimulus response tasks at these doses. Performance on stimulus discrimination was impaired at 1.0 g/kg, which prevented determination of reversal performance. Analysis of post-error response times demonstrated that error processing was impaired at both 0.2 and 0.5 g/kg. Ethanol also increased the number of omissions and delayed responses on an attentional task, suggesting more frequent attentional lapses. These data demonstrate that cognitive function mediated by specific prefrontal cortical brain regions is particularly sensitive to ethanol and suggest specific cognitive mechanisms that may underlie harmful decisions made at low doses of ethanol.
Keywords: alcohol, anterior cingulate cortex, attention, cognition, cognitive flexibility, orbitofrontal cortex, stimulus discrimination, stimulus reversal
Introduction
The impact of ethanol consumption on society is not limited to motor impairment resulting from inebriation following excessive intake but also results from poor decision making and risk taking associated with lower levels of ethanol intake. Despite the extensive consequences of poor decision making, the investigation of the impact of ethanol on cognitive function has been relatively limited compared with the research into impact on motor function (Schweizer and Vogel-Sprott 2008). Developing a better understanding of the cognitive impact of ethanol is important because the dangerous behavioral effects of ethanol, such as aggression, risk taking, and impulsivity, are thought to result from decreased cognitive control over behavior (Linnoila et al. 1994; Hoaken et al. 1998; Giancola 2000; Curtin and Fairchild 2003; Hernandez et al. 2007).
An important element of cognitive control is the ability to inhibit responses which are predominant and automatic, either through practice or because they are innate (Miller and Cohen 2001; Aron 2007). Such prepotent responses need to be inhibited to permit adaptation to changing circumstances, such as altered reward contingencies. Control subjects under the influence of ethanol and abstinent alcoholic subjects both demonstrate disruptions of inhibitory control on a stop signal response task, which is frequently used in humans to determine the ability to inhibit planned movements (de Wit et al. 2000; Easdon and Vogel-Sprott 2000; Dougherty et al. 2008). Although abstinent alcoholic subjects appear to exhibit impaired inhibition on other, more cognitive tasks (Noel et al. 2007; Fortier et al. 2008), it is not clear whether acute ethanol has the same impact (Richards et al. 1999).
Cognitive inhibition is an inferred process that can be assessed in stimulus reversal tasks, wherein established responding according to a previously learned association has to be suppressed in order to be replaced by the new association. The orbitofrontal cortex in human and nonhuman primates is essential for performance on reversal tasks (Butter 1969; Fellows and Farah 2003; Clarke et al. 2007; Murray and Izquierdo 2007). No previous studies have been conducted on the acute effect of ethanol on reversal performance. Therefore, in the present study, we used a touch screen-based stimulus reversal task for nonhuman primates to investigate the impact of ethanol on cognitive control over prepotent responding.
Another important aspect of cognitive control over behavior is the ability to monitor performance and process errors (Botvinick 2007). A substantial literature encompassing error-related negativity, a negative deflection in the electroencephalogram (EEG) following errors, and functional imaging studies (Carter et al. 1998; Ridderinkhof et al. 2004) implicate the anterior cingulate cortex in error monitoring. A behavioral method for inferring reaction to errors is to compare response times on trials following errors to those following correct responses. In both humans and nonhuman primates, trials following errors show increased response times (Rabbitt 1966; Li et al. 2006; Liu et al. 2009), and it has been reported that error processing in humans is disrupted by acute ethanol (Ridderinkhof et al. 2002; Schweizer et al. 2004). Given the importance of error processing in regions such as the anterior cingulate in guiding behavior, we evaluated post-error response times in order to evaluate the impact of acute ethanol.
Based on the critical role of sustained attention on higher cognitive functions involved in learning and memory (Sarter et al. 2001), we also examined the impact of acute ethanol administration on sustained attention in order to determine its potential contribution to any impairments on other cognitive tasks used in this study.
Nonhuman primates offer particular advantages for evaluating mechanisms of impact of ethanol on cognition (Grant and Bennett 2003). There is a close homology of cortical structure and function between monkeys and humans (Croxson et al. 2005; Rudebeck et al. 2008), and executive function and cognitive control are largely cortex-dependent phenomena. Consequently, monkeys readily learn complex task structures similar to human studies and reveal similar underlying neuroanatomical substrates (e.g., Li et al. 2006; Roiser et al. 2006; Liu et al. 2009; Jedema et al. 2010). Importantly, given the applicability of imaging methodologies in humans and nonhuman primates, a common set of results from cognitive and/or imaging approaches lends unique clinical relevance to more invasive procedures possible only with nonhuman primates. Although studies with nonhuman primates would permit assessment over a wider range of ethanol concentrations than humans, in this study, we specifically focus on the impact of low-to-moderate doses of ethanol in order to avoid confounding the results with more general impairments in motor control and ataxia typically associated with higher ethanol concentrations (Mello 1971; Katner et al. 2004). In the present work, we evaluate the impact of ethanol in rhesus monkeys on: 1) stimulus response, 2) stimulus discrimination, 3) stimulus reversal, 4) error processing, and 5) sustained attention.
Materials and Methods
Subjects
Subjects were a total of 7 adult male rhesus macaques (age 7–8 years) weighing 10.8 ± 0.6 kg (range 8.9–13.4 kg). Five subjects participated in all tasks. One subject participated only in the stimulus response, and the stimulus discrimination–reversal (SD/Rev) tasks (described below) and another subject participated only in the attention (9-Choice Serial Reaction Time Task [9-CSRT]) task and blood sampling procedure. Subjects were fed sufficient monkey chow biscuits (Labdiet 5038l PMI Richmond) to maintain a healthy body weight (BW) and received fruit treats daily. Water intake of the subjects was regulated (25 mL/kg/day) from Monday to Friday, with ad lib access to water during the weekends.
Subjects had experience working on a touch screen in a sound-attenuated chamber for water rewards delivered via a sipper tube as previously described (Liu et al. 2009; Jedema et al. 2010). While consumption was not specifically measured, there was no apparent spillage associated with particular doses of ethanol. Subjects were trained for approximately 2 months on both the SD/Rev and 9-CSRT tasks to stable performance prior to the start of the experiment (Supplementary Fig. S1). Subjects were first trained and tested on the SD/Rev task and subsequently trained and tested on the 9-CSRT task. At the end of most SD/Rev sessions, subjects completed a stimulus response task. Only one testing session was performed each day between 8:00 AM and 2:00 PM. In addition to days in which subjects were not tested, there was at least one vehicle session between ethanol sessions. All subjects were from the same cohort and were involved in a previous cognitive/imaging study (Jedema et al. 2010), but they had not been exposed to any drugs other than necessary for routine clinical care, surgical procedures, and imaging procedures. All procedures were in accordance with the USPHS Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh.
Ethanol Infusion
Ethanol (0.2 g/mL) or saline vehicle was slowly administered intravenously via a previously implanted vascular access port. The vascular access port was located mid scapula and connected with a subcutaneous catheter to the right internal jugular vein. The infusion rate (range 0.87–3.45 mL/min) was adjusted according to BW such that the entire dose of ethanol (0.2 or 0.5 g/kg BW) for each subject was infused over a 10-min period. The 1.0 g/kg dose of ethanol was infused over 20 min. The infusion paradigm was chosen to provide experimental control over plasma levels and was based on previous studies (Bradberry 2002).
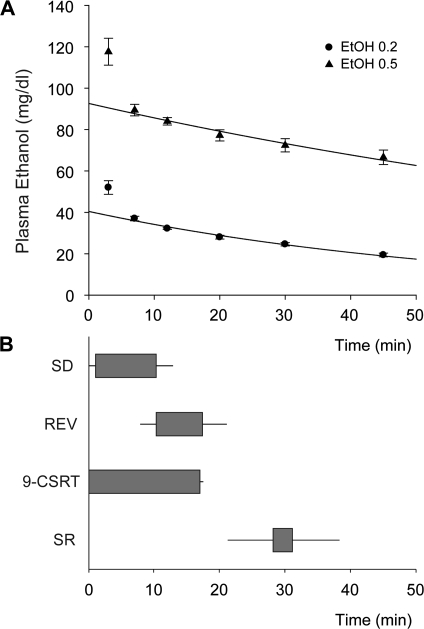
During ethanol infusion, subjects interacted with the touch screen in a modified version of the simple stimulus response task in order to maintain engagement and prevent frustration from a lack of environmental interaction. Immediately after the infusion period had ended, subjects started on the 9-CSRT task or the SD/Rev task. In a subset of sessions, a stimulus response followed the SD/Rev task. The timing of the different cognitive tasks in relation to blood ethanol levels following infusion is shown in Figure 1.
Figure 1.
Blood ethanol levels determined following 0.2 and 0.5 g/kg infusion. (A) Three minutes following the end of the slow intravenous infusion, levels of blood ethanol were 52 (±3) mg/dl and 118 (±7) mg/dl levels following the low (0.2 g/kg) and high dose (0.5 g/kg), respectively. Assuming that the higher levels at the initial timepoint reflected ongoing distribution of ethanol, we determined that the subsequent decay of blood ethanol levels could be very well described by a first-order elimination process (R2 = 0.99 and 0.97 for 0.2 and 0.5 g/kg dose, respectively). (B) The horizontal gray boxes indicate the average start and end time following the end of the ethanol infusion of the stimulus discrimination and reversal trials included in the data analysis. The horizontal lines represent the standard error of the mean. By default, reversal performance was assessed after criterion on the stimulus discrimination trials was achieved. The start and end times of the 9-CSRT sessions largely overlapped with those on the SD/Rev task. Following a subset of SD/Rev sessions, performance on a stimulus response task was assessed.
Subjects participated in 32–34 experimental sessions, 16–17 of which were saline sessions, 8–9 sessions at the 0.2 g/kg dose of EtOH, 4–5 sessions at the 0.5 g/kg dose, and a single session at the 1.0 g/kg dose. The examination of the 1.0 g/kg dose was discontinued after a single session because subjects were too impaired for cognitive function to be assessed: 1) the majority of subjects (4/6) were unable to reach criterion on the stimulus discrimination trials and, consequently, performance on the reversal trials could not be determined. 2) One of the 2 subjects that reached criterion on the discrimination trials had fewer than 20 trials remaining in the task. 3) Performance on the stimulus response task could not be determined in all subjects due to lack of participation. Because of the missing values at the 1.0 g/kg dose, and the inability to compare discrimination and reversal performance, the limited data at this dose are excluded from the main statistical analyses, but when available, they are included in the figures for illustration purposes only. In a previous study, similar nonspecific deterioration of behavior by higher ethanol concentrations has been observed (Melia and Ehlers 1989).
In order to relate the effect of ethanol on cognitive function to actual blood ethanol concentrations, we determined plasma ethanol levels following the infusion paradigm in 5 subjects participating in the 9-CSRT task. One subject was not used because of difficulty withdrawing blood via the port/catheter. Blood samples (2 mL) were obtained from the vascular access port at 6 timepoints (3, 7, 12, 20, 30, and 45 min following ethanol infusion) in a single session at the 0.2 and 0.5 g/kg dose of ethanol. In order to avoid contamination from the infusion fluid, a 10-mL saline flush immediately followed the infusion. Blood ethanol concentrations were determined using headspace gas chromatography at the Special Chemistry Laboratory at Presbyterian Hospital (UPMC, Pittsburgh).
Cognitive Tasks and Data Analysis
Stimulus Discrimination with Reversal
This task was adapted from one previously used by Wallis and Miller (2003). Each session consisted of 200 trials (intertrial interval 2 s), which were initiated by touch of a 2.5 × 2.5-cm blue stimulus in the middle of the screen. Immediately thereafter, subjects were presented with a choice between 2 visual stimuli from a set of 3 stimuli associated with a low, medium, and high amount of water reward (0.02, 0.05, or 0.10 mL/kg, respectively). There was no time limit to make this choice. Upon reaching criterion performance on the discrimination trials (90% choice of the higher reward stimulus over the previous 30 trials), the reward contingencies of the high and the low reward were reversed and subsequent trials were defined as reversal trials. If the criterion was reached after reversal of the first set of visual stimuli, the session continued with new discrimination trials and a novel set of 3 stimuli. Data were collected and analyzed using E-prime (Psychology Software Tools). Stimuli (200 × 200 pixels; ∼61 mm square) were selected from a picture library of 204 readily distinguishable stimuli that were organized in triplets. The identity and order in which the triplets were used was the same for all subjects and the stimuli were randomly presented on the left or right side of the screen.
To evaluate performance, we analyzed the average accuracy over the first 20 discrimination and reversal trials presented for each stimulus set in order to focus on the subject's adaptation to a new stimulus set or the reversal of the reward contingencies. The fundamental difference between discrimination and reversal trials for a given stimulus set is that on the reversal trials, subjects have to inhibit the response to the stimulus previously associated with the relatively higher reward. The performance accuracy for each subject was averaged per treatment condition (saline, ethanol 0.2, or ethanol 0.5) and trial type (discrimination or reversal). Discrimination performance was only analyzed if reversal performance was also available for the same stimulus set to assure the same number of trials for each trial type. Main effects of treatment and/or trial type and interaction were determined using repeated measures analysis of variance (ANOVA) designs (with Huynh–Feldt correction if indicated) and α = 0.05 (Sigmastat 3.5 or SPSS v 17; SPSS, Inc.). Corrections for multiple comparisons were made according to the Holm–Sidak method of adjusting critical thresholds.
Response Time Analysis for Error Processing
The response times on trials after an error is made are typically greater than on trials after a correct response (Rabbitt 1966; Botvinick et al. 2001; Li et al. 2008; Liu et al. 2009). This post-error slowing is a commonly employed metric for error processing. Analysis of response times across all discrimination and reversal trials was confined to response times of less than 5 s, to prevent a small number of extreme response times that exceeded this threshold (0.7% of all trials) from having a disproportionate impact on the average response time. Post-error slowing was quantified as the difference between response times following incorrect and correct trials in each session of the SD/Rev task.
The 9-Choice Serial Reaction Time Task
Each session consisted of 200 trials in which 9 green squares were presented on the screen in a 3 × 3 array. After a variable delay of 2–4 s, one square changed to 1 of 5 different shades of green (of varying contrast) for 2 s. Touching the target square yielded a water reward of 0.075 mL/kg. Incorrect responses resulted in no reward and touching the screen prior to the appearance of the stimulus resulted in a 2-s timeout. The timeout was not specifically indicated by a change on the screen—it merely postponed the start of the next trial by 2 s. Failure to respond during the 2-s period of stimulus presentation was defined as an omission. This touch screen-based task is conceptually similar to the 5-choice task typically used in rodents for evaluating sustained attention and impulsivity (Robbins 2002). The five different shades of stimuli were used to determine whether ethanol had a differential impact on different stimuli. Performance accuracy was defined as the percentage of correct responses of all 200 trials. Premature responses (i.e., response prior to the appearance of the stimulus) were analyzed for trials following correct choices only to avoid confounding this measure of impulsivity (Robbins 2002) with increased delay of reward resulting from incorrect or omitted responses.
Ex-Gaussian Response Time Analysis
There is a growing interest in the analysis of intraindividual variability of response times. This is because of the demonstrated link between attentional lapses and increased variability in response times due to occasional long response times in clinical populations with attentional deficits (Leth-Steensen et al. 2000; Hervey et al. 2006). Ex-Gaussian analysis deconvolves response time distributions into a Gaussian component (with conventional parameters of mean [mu] and standard deviation [sigma]), and a measure related to skewness due to late responses called tau. Response time distributions from all trials of the 9-CSRT sessions from individual subjects were fitted with ex-Gaussian functions as described by Lacouture (Lacouture and Cousineau 2008).
Stimulus Response
At the end of the SD/Rev task, subjects performed a stimulus response task consisting of 50 trials (intertrial interval 2 s) of presentation of a randomly placed blue stimulus of varying size (square; 50–100 pixels). Touching the stimulus within 3 s yielded a small water reward (0.075 mL/kg). This task is used during the initial touch screen training of our subjects and subsequently used to encourage fine motor control rather than swiping at the screen. A modified version of this task (100 trials starting 6 s apart, reward size 0.035 mL/kg) was used to keep subjects engaged during the ethanol infusion.
Results
Three minutes after the end of the slow intravenous infusion, levels of blood ethanol were 52 (±3) mg/dl and 118 (±7) mg/dl levels following the low (0.2 g/kg) and high dose (0.5 g/kg), respectively (Fig. 1). Assuming that the higher levels at the first timepoint reflected ongoing distribution of ethanol throughout the body, we determined that the subsequent decay of blood ethanol levels could be very well described by a first-order elimination process (R2 = 0.99 and 0.97 for 0.2 and 0.5 g/kg dose, respectively). Blood ethanol levels exceeded the legal driving limit for humans in the United States only in the first 3 samples following the highest dose of ethanol.
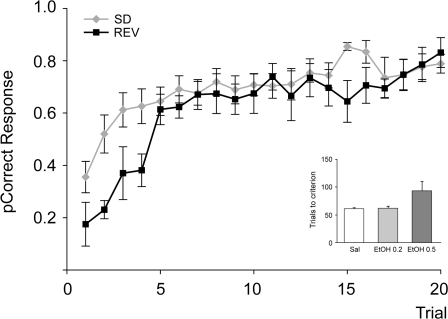
Subjects performing the SD/Rev task quickly learned to select the more advantageous stimulus on the screen following the start of a new stimulus set or the reversal of the reward contingencies (Fig. 2). Criterion (90% correct) was reached after 61 ± 2, 62 ± 4, and 93 ± 17 discrimination trials on saline, 0.2 g/kg, and 0.5 g/kg ethanol sessions, respectively (Fig. 2 inset; main effect of ethanol P = 0.044; post hoc comparisons corrected for multiple comparisons were not significant). At the 1.0 g/kg dose, most subjects failed to reach criterion. In order to obtain insight into the subject's adaptation to a new stimulus set or the reversal of the reward contingencies, we focused our analysis on the response accuracy during the first 20 trials of each trial type. Even if there was a modest increase in the number of stimulus discrimination trials to reach criterion due to the impact of ethanol, sufficient trials remained in each session to assess the performance accuracy on the first 20 reversal trials. Two-way repeated-measures analysis of the average performance accuracy indicated a significant main effect of ethanol treatment (P = 0.005) and trial type (P = 0.007; Fig. 3). One-way repeated-measures ANOVA indicated that the impact of ethanol on performance accuracy was not significant during the discrimination trials (P = 0.205) but was during the reversal trials (P < 0.001). Post hoc comparisons revealed that the performance accuracy on reversal trials was impaired by ethanol (0.2 EtOH vs. saline P = 0.059; 0.5 EtOH vs. saline P < 0.001), with performance deteriorating to chance levels at the 0.5 g/kg dose of ethanol.
Figure 2.
Probability of accurate response on the first 20 discrimination and reversal trials. The probability of a correct response on discrimination and reversal trials provides an indication of the time course of improvement of performance accuracy following the start of a new stimulus set and following stimulus reversal. Inset shows the number of trials to reach criterion performance on the discrimination trials at the 0.2 and 0.5 g/kg doses. At the 1.0 g/kg dose, most subjects failed to reach criterion.
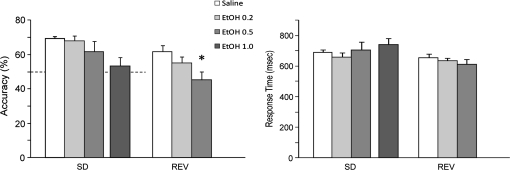
Figure 3.
The impact of ethanol on performance accuracy and response times on the stimulus discrimination and reversal task. (A) The analysis of performance accuracy demonstrated a main effect of trial type (P = 0.007) and ethanol treatment (P = 0.005). One-way repeated-measures ANOVA indicated no main effect of ethanol on discrimination learning trials (P = 0.205), but a significant main effect on reversal trials (P < 0.001). Post hoc comparisons demonstrated a marginal effect at the 0.2 g/kg dose (P = 0.059) and a significant reduction to chance levels at the 0.5 g/kg dose (P < 0.001) compared to saline. The impact of a single session with the 1.0 g/kg dose is not included in the main statistical analysis. (B) The analysis of response times indicated a main effect of trial type (P = 0.016) but no effect of treatment nor an interaction. Similarly, one-way repeated-measures ANOVA of response times on either discrimination or reversal trials indicated no significant impact of ethanol (P = 0.467 and 0.243, respectively). *P < 0.05 versus saline.
Analysis of mean response times indicated a significant effect of trial type (P = 0.016) but no other main effects or interaction. Similarly, one-way repeated-measures ANOVA indicated that the impact of ethanol on response time was not significant during the discrimination learning trials (P = 0.467) or reversal trials (P = 0.243; Fig. 3b).
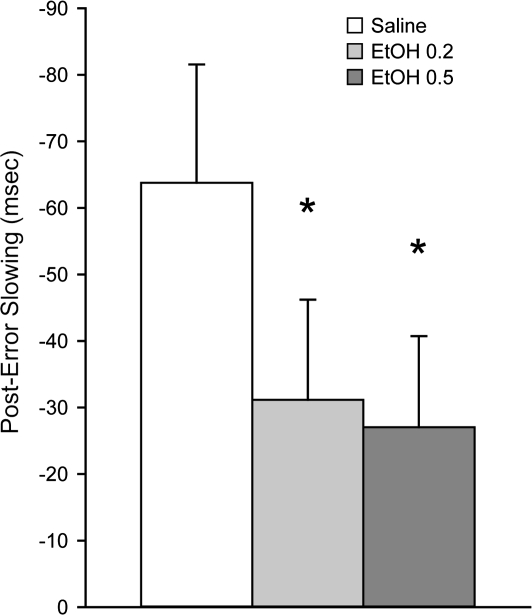
Post-error slowing of response times was reduced by ethanol on both discrimination and reversal trials (SD saline −78 ±23, Rev saline −67 ± 14, SD EtOH −45 ± 21, Rev EtOH −39 ± 15 ms for post-error slowing during saline or ethanol sessions collapsed across the 0.2 and 0.5 g/kg doses, respectively; main effect of treatment P = 0.007, main effect of trial type P = 0.577). Because of the lack of effect of trial type and due to the varying number of trials for each trial type with associated greater variability in response times, data were collapsed across both types of trials (main effect of ethanol treatment on collapsed data P = 0.006; Fig. 4). Post hoc comparisons indicated that a reduction in post-error slowing was evident at both the 0.2 and the 0.5 g/kg dose of ethanol (P = 0.007 and 0.003, respectively).
Figure 4.
The impact of ethanol on error monitoring. Post-error slowing, defined as the difference in response times following correct and incorrect trials, was reduced during ethanol sessions (P = 0.006). The decrease was evident both on discrimination and reversal trials (P = 0.048 and 0.038, respectively), but the data were collapsed across both types of trials due to the limited number of trials and associated greater variability in response times during reversal trials. Post hoc comparisons indicated significant impairments at both doses of ethanol compared to saline (P = 0.007 and 0.003 for 0.2 and 0.5 g/kg, respectively). *P < 0.05 versus saline.
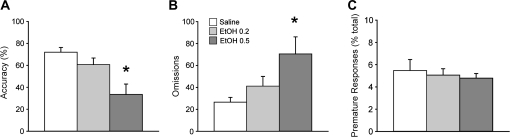
Response accuracy on the 9-CSRT varied as a function of the shade of the stimulus and ethanol treatment (main effect of shade P < 0.001 and main effect of ethanol treatment P < 0.001) without a significant interaction (shade by treatment interaction P = 0.247). The decrease in response accuracy resulting from ethanol (Fig. 5A) was predominantly caused by an increase in omissions (P = 0.02; Fig. 5B), which occurred across all shades of stimuli (main effect of shade P < 0.001, main effect of ethanol treatment P = 0.033, shade by treatment interaction P = 0.188; Supplementary Fig. S2). The percentage of premature responses, a measure of impulsivity (Evenden 1999; Robbins 2002), was not affected by ethanol treatment (P = 0.626, Fig. 5C).
Figure 5.
The impact of ethanol on accuracy, omissions, and premature responses on the 9-choice stimulus reaction time task. (A) Accuracy was reduced during ethanol sessions (P < 0.001). (B) Increases in the number of omissions (P = 0.02) were primarily responsible for the ethanol-induced impairment of accuracy, suggesting more frequent lapses in attention. Post hoc comparisons indicated that the number of omissions at the 0.5 g/kg dose was significantly higher than under saline conditions (P = 0.007). (C) The percentage of premature responses was not affected (P = 0.626). *P < 0.05 versus saline.
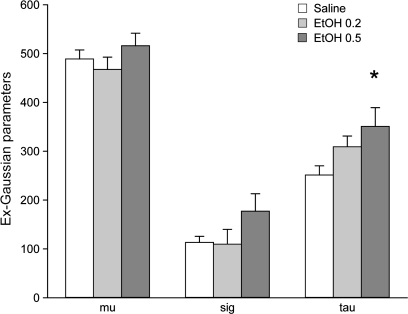
Ex-Gaussian response time analysis revealed an increase in the tau component (representing the right side “skewness” of the response time distribution), consistent with an increase in attentional lapses (Fig. 6). Post hoc analysis indicated that tau, at the 0.5 g/kg dose, was significantly different from saline (P = 0.005). In contrast, mu, the mean of the normal component of the response time distribution, was not affected by ethanol. A similar analysis could not be conducted for the response times on the SD/Rev task due to the multiple factors affecting response times such as trial type and the impact of post-error slowing. However, increased attentional lapses on the SD/Rev task are suggested by the increased duration required to complete the session (main effect of treatment P = 0.037; total time per session: 19 ± 3, 21 ± 4, 29 ± 6 min for saline, 0.2, and 0.5 g/kg ethanol sessions, respectively). Post hoc comparisons revealed that the time to complete the session was increased from saline, only at the 0.5 g/kg ethanol dose (P = 0.036) but not at the lower ethanol dose (P = 0.55). This increased time for task completion is due to an increase in latency to initiate trials by touching the blue square and is not an effect on response time once the trial had been initiated.
Figure 6.
The impact of ethanol on ex-Gaussian components of the response time distribution on the 9-choice stimulus reaction time task. Ethanol administration caused no significant changes in the mean (mu) and standard deviation (sigma) of the Gaussian component of the response time distribution, but a significant increase in the tau parameter (P = 0.017), reflecting an increase in skewness of the response time distribution due to more late responses. Post hoc comparisons demonstrated that the tau value was significantly increased from saline at the 0.5 g/kg dose (P = 0.005). *P < 0.05 versus saline.
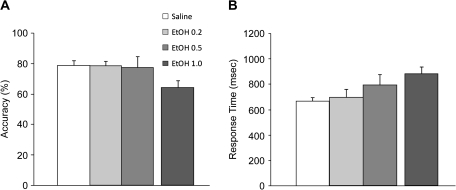
Performance accuracy and response times on the stimulus response task performed at the end of the discrimination–reversal session were not affected by ethanol treatment (main effect of treatment on accuracy P = 0.768 and response time P = 0.138; Fig. 7).
Figure 7.
The impact of ethanol on accuracy and response times on a simple stimulus response task. The average accuracy (panel A) and the response time (panel B) on a simple stimulus response task were not significantly affected by the ethanol administration. The impact of a single session with the 1.0 g/kg dose is not included in the main statistical analysis.
As described in the Materials and Methods section, behavior during the single session at the 1.0 g/kg dose was highly impaired and excluded from general statistical analysis. However, limited data were obtained and its analysis shows that this dose of ethanol impairs performance and influences response times on tasks not affected at lower concentrations. Including the 1.0 g/kg dose, a main effect of ethanol on discrimination trials of the SD/Rev task was observed (P = 0.042), although its impact on the response times of these trials was not significant (P = 0.405). Post hoc comparisons revealed that only the accuracy at the 1.0 g/kg dose was different from saline (P = 0.011). Due to the limited number of data points and the large increase in average and variability of response times, the post-error slowing analysis was not meaningful. The impact of ethanol on the accuracy of the stimulus response task was not significant (P = 0.145), although there was a significant slowing of the response times (P = 0.008) when the 1.0 g/kg was included. Post hoc comparisons revealed that only the response time at the 1.0 g/kg dose was different from saline (P = 0.002). The impact of the 1.0 g/kg on the 9-CSRT was not assessed.
Discussion
The present data demonstrate a selective impact of low to moderate doses of ethanol on particular cognitive domains. Whereas performance on stimulus response and stimulus discrimination tasks was unimpaired at either 0.2 or 0.5 g/kg ethanol, stimulus reversal trial performance was marginally impaired at the 0.2 g/kg dose and significantly impaired at the 0.5 g/kg dose. Post-error slowing, indicative of error processing, was significantly diminished at both the 0.2 and 0.5 g/kg doses. Omissions and late responses on the 9-CSRT task were increased at the 0.5 g/kg dose, consistent with impaired attentional performance. Overall, these results suggest a domain-selective pattern of impairments: no impairments on stimulus response and discrimination at either the 0.2 or the 0.5 g/kg dose, impaired cognitive control at doses of ethanol near the current legal driving limit in the United States, and significantly impaired error processing at plasma levels well below the legal driving limit. It is difficult to be certain that the impact of specific doses of ethanol on cognition is equivalent across species, but impairment of inhibitory control of humans on a stop task has been reported at similarly low ethanol concentrations (de Wit et al. 2000; Fillmore 2003).
Lesions of the orbitofrontal cortex selectively impair reversal performance in a similar manner to the impairments induced by the 0.2 and 0.5 g/kg dose of ethanol, in that performance on reversal trials is impaired while stimulus discrimination learning remains intact (Fellows and Farah 2003; Clarke et al. 2004; Clarke et al. 2005; Clarke et al. 2007; Murray and Izquierdo 2007; Rudebeck et al. 2008). Therefore, the present data suggest that the orbitofrontal cortex may be particularly sensitive to the impact of ethanol. Previous studies have established that chronic ethanol abuse impairs reversal performance and reduces orbitofrontal cortex activity (Volkow et al. 1993; Volkow and Fowler 2000; Noel et al. 2001; Noel et al. 2007; Fortier et al. 2008). Recent studies also indicated decreased gammaγ-aminobutyric acid (GABAA) receptor subunit densities in frontal cortical areas after chronic ethanol self-administration in nonhuman primates (Hemby et al. 2006). Impaired cognitive inhibition following chronic ethanol abuse has been correlated with a higher incidence of relapse (Noel et al. 2002), suggesting that a failure of cognitive inhibition may contribute to more frequent relapsing behavior. The present observations extend the findings of impaired reversal performance in chronic alcohol abuse patients by demonstrating that acute administration of ethanol at low doses also disrupts reversal performance. This suggests that the selective sensitivity of orbitofrontal cortex to acute ethanol may contribute to impaired inhibition of prepotent responding and increased impulsivity that presumably underlies poor decisions associated with subintoxicating levels of ethanol (de Wit et al. 2000; Dougherty et al. 2008).
A brain region actively involved with adaptive control and monitoring of behavioral choices is the anterior cingulate cortex (Ridderinkhof et al. 2004; Rushworth and Behrens 2008). Ethanol impairs human performance on the Stroop task, a classic anterior cingulate task (Rose and Duka 2008). Furthermore, EEG recordings suggest that ethanol preferentially reduces the error or mismatch negativity signals associated with the anterior cingulate and believed to reflect error monitoring/processing (Jaaskelainen et al. 1996; Ridderinkhof et al. 2002). Here, we demonstrate a significant ethanol-induced decrease in post-error slowing, a commonly used behavioral metric for error processing (Ridderinkhof et al. 2004), which is consistent with the EEG observations of reduced error processing. The ethanol-induced reduction in post-error slowing was evident on both discrimination and reversal trials of the SD/Rev task. Consistent with a reduction in error-related negativity at blood alcohol concentrations of 40 and 100 mg/dl in humans (Ridderinkhof et al. 2002), we observed similar effects on post-error slowing at similar plasma levels in the monkey. Given that ethanol impaired the performance accuracy on reversal trials only, the presence of decreased error monitoring function on discrimination trials is consistent with distinct substrates for error monitoring function and discrimination learning.
It has been suggested that ethanol may degrade sensory processing and that impairment of error monitoring is a consequence of the greater difficulty in sensory processing (Yeung et al. 2007). However, the present observations demonstrate specific impairment of higher cognitive functions such as error processing at low doses of ethanol, independent of a general impairment of sensory stimulus processing because performance accuracy on discrimination trials was not affected by ethanol up to 0.5 g/kg. Similarly, on the 9-CSRT, the number of omissions increased with ethanol dose across all stimulus shades, further suggesting that low doses of ethanol do not impair sensory processing (Supplementary Fig. S2). Furthermore, the response times on the present tasks were not affected by ethanol up to 0.5 g/kg (discussed below), which differs from the increased response times observed when sensory processing is degraded by reducing the contrast of the visual stimuli (Yeung et al. 2007). At the highest dose of ethanol (1.0 g/kg), however, discrimination learning was impaired and this may have been a consequence of impaired sensory processing as reported previously (Melia and Ehlers 1989; Yeung et al. 2007). Thus, our data support a preferential disruption of higher cognitive functions such as error monitoring in response to low doses of ethanol, consistent with the interpretations offered by other studies in humans (Jaaskelainen et al. 1996; Ridderinkhof et al. 2002) in addition to a more general impairment of sensory processing that occurs at higher doses of ethanol.
The present SD/Rev task likely involves a working memory component because the relationship between three different stimuli and reward levels had to be kept in memory while choosing between the 2 stimuli presented on the screen on any given trial. The observation that there was no general decline in performance accuracy on the stimulus discrimination trials suggests that low doses of ethanol do not impair the capacity of working memory to keep these stimuli in mind, consistent with previous observations in human (Saults et al. 2007) and nonhuman primates (Mello 1971).
On the 9-CSRT, the decrease in performance accuracy following ethanol was in large part due to an increase in omissions, suggesting more frequent lapses of attention. This was further supported by our ex-Gaussian analysis of the response time distribution that revealed an elevated tau value that reflects the skewness of the right hand side of the distribution. A similar altered response time distribution and elevated tau value is observed in clinical populations with attentional deficits (Leth-Steensen et al. 2000; Castellanos et al. 2005; Hervey et al. 2006). An ethanol-induced decrease in attention was also hypothesized to underlie impaired performance of nonhuman primates on a delayed-match-to-sample task (Fuster et al. 1982). More frequent attentional lapses in the presence of ethanol are also suggested by the increased time required to complete the self-paced SD/Rev task due to delays in initiating trials but not in reaction times on trials once they had been initiated. Brief lapses of attention may also have disrupted the sustained high performance required to reach criterion (90% over 30 trials) and may have led to the slight ethanol-induced increase in the number of discrimination trials to reach criterion on the SD/Rev task without affecting the performance accuracy over the first 20 discrimination trials.
As described above, the anterior cingulate is critical for error processing and performance monitoring in general, but it also plays a pivotal role in sustained attention (Sarter et al. 2006). Therefore, it is plausible that both the increased frequency of attentional lapses on the 9-CSRT and the decreased post-error slowing observed on the SD/Rev task reflect an impairment of anterior cingulate function induced by ethanol. Alternatively, an increase in attentional lapses between trials (resulting in a prolonged intertrial interval) could result in further decay of already weakened performance monitoring in the anterior cingulate and further decrease its influence on behavior on subsequent trials.
It is, however, unlikely that impaired sustained attention contributed to the selective impairment in accuracy on reversal trials due to fundamental differences between the SD/Rev and the 9-CSRT tasks. First, all trials on the SD/Rev task were self-initiated (i.e., when the subject chooses to pay attention) and the choice could be made immediately after the initiation of the trial, making it likely that the subject is paying attention. Second, the choice on the SD/Rev task requires a response between 2 stimuli without time constraints, whereas the opportunity to respond on the 9-CSRT is limited in duration and optional. Most importantly, any impairment on the SD/Rev task resulting from lapses in attention would be expected to equally impair the performance accuracy on discrimination and reversal trials. The observation that subjects continued to perform well on the discrimination trials of the SD/Rev task suggests that while simple stimulus associations remain intact, low doses of ethanol impair the ability to inhibit prepotent responding after the reward contingencies have been reversed.
We observed no evidence for any ethanol-associated increase in premature responses on the 9-CSRT. This is consistent with the limited impact of ethanol on response times during the SD/Rev and the stimulus response tasks. Higher doses (0.6–0.8 g/kg) of oral ethanol in humans have been reported to contribute to faster response initiation, but this effect was not observed at lower doses such as used in the present study (Dougherty et al. 2008). Thus, the impact of moderate doses of ethanol in humans appears to be selective to the impairment of inhibition of prepotent responses, resulting in impulsive responding (de Wit et al. 2000; Dougherty et al. 2008). Our observations demonstrate a similar selectivity in nonhuman primates resulting in selectively impaired performance on the reversal trials, where prepotent responses have to be inhibited, but not on discrimination trials or on trials of the 9-CSRT, where this requirement is absent.
Ethanol distributes rapidly throughout the body, based on our observations that plasma levels stabilize relatively quickly and follow first-order elimination kinetics within 7 min following the end of the slow intravenous infusion. This observation is consistent with the relatively stable plasma ethanol levels within 5–10 min following similar, slow infusion protocols for monkeys reported previously (Fuster et al. 1982; Kalhorn et al. 1986; Bennett and DePetrillo 2004). The plasma levels of ethanol attained after the 0.2 and 0.5 g/kg intravenous dose approximate plasma levels observed after intragastric doses of 1 and 2 g/kg, respectively (Ando et al. 1987; Katner et al. 2004). Given the rapid distribution of intravenous ethanol, it is unlikely any differential impact on reversal versus stimulus discrimination is an artifact of ethanol pharmacokinetics. We cannot completely eliminate the possibility that the lack of impact of ethanol on the stimulus response task is a result of decreasing blood ethanol levels because it was always assessed after the performance on the reversal task. Testing on the stimulus response task typically occurred after approximately 20–30 min, at which time plasma ethanol levels had decreased approximately 20% from levels seen at the 7-min timepoint at which the distributional phase appeared complete. Based on the fact that the ethanol levels at the 20- to 30-min timepoint on the 0.5 g/kg dose were still markedly higher than those achieved at any time on the 0.2 dose at which decreased post-error slowing was observed, we believe that the lack of impact of ethanol on this stimulus response task is more likely a reflection of the relatively limited impact of low doses of ethanol on tasks involving simple and explicit stimuli, involving automatic processing rather than focused attention and cognitive control (Casbon et al. 2003). This is further supported by the observation that ethanol increased the premotor component, but not the response execution component, of reaction time in humans performing an omitted response reaction time task (Hernandez et al. 2006; Hernandez et al. 2007). This notion is further supported by studies in macaques which demonstrated ethanol-induced motor impairment on a bimanual motor task only at higher doses of ethanol than those used in the present study (Katner et al. 2004).
The present data demonstrate the feasibility of examining the impact of ethanol on cognitive function in nonhuman primates in a similar manner to clinical assessment in humans. A major advantage of a nonhuman primate model compared to human studies is the increased control of the subject population (Grant and Bennett 2003) and the ability to repeatedly evaluate the same subjects leading to more powerful experimental design. A particularly relevant future study will be to examine cognitive consequences of adolescent ethanol exposure, for which the present data provide a comparative standard for ethanol's impact on adults (Barron et al. 2005; Pian et al. 2008).
In conclusion, our data demonstrate that low doses of ethanol selectively impair cognitive control and choice outcome monitoring functions associated with specific prefrontal cortical regions. This suggests that the substantial cost to society of choice behavior associated with moderate levels of intoxication is due in part to selective patterns of cognitive impairment outside of sensorimotor dysfunction typically associated with acute intoxication.
Supplementary Material
Supplementary material can be found at:http://www.cercor.oxfordjournals.org/
Funding
National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism (AA014646) and the Office of Research and Development Medical Research Service, Department of Veterans Affairs.
Supplementary Material
Acknowledgments
Conflict of Interest: None declared.
References
- Ando K, Johanson CE, Schuster CR. The effects of ethanol on eye tracking in rhesus monkeys and humans. Pharmacol Biochem Behav. 1987;26:103–109. doi: 10.1016/0091-3057(87)90541-7. [DOI] [PubMed] [Google Scholar]
- Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13:214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Barron S, White A, Swartzwelder HS, Bell RL, Rodd ZA, Slawecki CJ, Ehlers CL, Levin ED, Rezvani AH, Spear LP. Adolescent vulnerabilities to chronic alcohol or nicotine exposure: findings from rodent models. Alcohol Clin Exp Res. 2005;29:1720–1725. doi: 10.1097/01.alc.0000179220.79356.e5. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, DePetrillo PB. Sex differences in total body water in adolescent rhesus macaques estimated by ethanol dilution. J Med Primatol. 2004;33:163–166. doi: 10.1111/j.1600-0684.2004.00065.x. [DOI] [PubMed] [Google Scholar]
- Botvinick MM. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci. 2007;7:356–366. doi: 10.3758/cabn.7.4.356. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bradberry CW. Dose-dependent effect of ethanol on extracellular dopamine in mesolimbic striatum of awake rhesus monkeys: comparison with cocaine across individuals. Psychopharmacology. 2002;165:67–76. doi: 10.1007/s00213-002-1233-9. [DOI] [PubMed] [Google Scholar]
- Butter CM. Perseveration in distinction and in discrimination reversal tasks following selective frontal ablations in Macaca mulatta. Physiol Behav. 1969;4:163–171. [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Casbon TS, Curtin JJ, Lang AR, Patrick CJ. Deleterious effects of alcohol intoxication: diminished cognitive control and its behavioral consequences. J Abnorm Psychol. 2003;112:476–487. doi: 10.1037/0021-843x.112.3.476. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJ, Scheres A, Di Martino A, Hyde C, Walters JR. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biol Psychiatry. 2005;57:1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Crofts HS, Dalley JW, Robbins TW, Roberts AC. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J Neurosci. 2005;25:532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Johansen-Berg H, Behrens TE, Robson MD, Pinsk MA, Gross CG, Richter W, Richter MC, Kastner S, Rushworth MF. Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. J Neurosci. 2005;25:8854–8866. doi: 10.1523/JNEUROSCI.1311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JJ, Fairchild BA. Alcohol and cognitive control: implications for regulation of behavior during response conflict. J Abnorm Psychol. 2003;112:424–436. doi: 10.1037/0021-843x.112.3.424. [DOI] [PubMed] [Google Scholar]
- de Wit H, Crean J, Richards JB. Effects of d-amphetamine and ethanol on a measure of behavioral inhibition in humans. Behav Neurosci. 2000a;114:830–837. doi: 10.1037//0735-7044.114.4.830. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Marsh-Richard DM, Hatzis ES, Nouvion SO, Mathias CW. A test of alcohol dose effects on multiple behavioral measures of impulsivity. Drug Alcohol Depend. 2008;96:111–120. doi: 10.1016/j.drugalcdep.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easdon CM, Vogel-Sprott M. Alcohol and behavioral control: impaired response inhibition and flexibility in social drinkers. Exp Clin Psychopharmacol. 2000;8:387–394. doi: 10.1037//1064-1297.8.3.387. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Fillmore MT. Drug abuse as a problem of impaired control: current approaches and findings. Behav Cogn Neurosci Rev. 2003;2:179–197. doi: 10.1177/1534582303257007. [DOI] [PubMed] [Google Scholar]
- Fortier CB, Steffen EM, Lafleche G, Venne JR, Disterhoft JF, McGlinchey RE. Delay discrimination and reversal eyeblink classical conditioning in abstinent chronic alcoholics. Neuropsychology. 2008;22:196–208. doi: 10.1037/0894-4105.22.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM, Willey TJ, Riley DM, Ashford JW. Effects of ethanol on visual evoked responses in monkeys performing a memory task. Electroencephalogr Clin Neurophysiol. 1982;53:621–633. doi: 10.1016/0013-4694(82)90138-9. [DOI] [PubMed] [Google Scholar]
- Giancola PR. Executive functioning: a conceptual framework for alcohol-related aggression. Exp Clin Psychopharmacol. 2000;8:576–597. doi: 10.1037//1064-1297.8.4.576. [DOI] [PubMed] [Google Scholar]
- Grant KA, Bennett AJ. Advances in nonhuman primate alcohol abuse and alcoholism research. Pharmacol Ther. 2003;100:235–255. doi: 10.1016/j.pharmthera.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Hemby SE, O'Connor JA, Acosta G, Floyd D, Anderson N, McCool BA, Friedman D, Grant KA. Ethanol-induced regulation of GABA-A subunit mRNAs in prefrontal fields of cynomolgus monkeys. Alcohol Clin Exp Res. 2006;30:1978–1985. doi: 10.1111/j.1530-0277.2006.00254.x. [DOI] [PubMed] [Google Scholar]
- Hernandez OH, Vogel-Sprott M, Huchin-Ramirez TC, Ake-Estrada F. Acute dose of alcohol affects cognitive components of reaction time to an omitted stimulus: differences among sensory systems. Psychopharmacology. 2006;184:75–81. doi: 10.1007/s00213-005-0237-7. [DOI] [PubMed] [Google Scholar]
- Hernandez OH, Vogel-Sprott M, Ke-Aznar VI. Alcohol impairs the cognitive component of reaction time to an omitted stimulus: a replication and an extension. J Stud Alcohol Drugs. 2007;68:276–281. doi: 10.15288/jsad.2007.68.276. [DOI] [PubMed] [Google Scholar]
- Hervey AS, Epstein JN, Curry JF, Tonev S, Eugene Arnold L, Keith Conners C, Hinshaw SP, Swanson JM, Hechtman L. Reaction time distribution analysis of neuropsychological performance in an ADHD sample. Child Neuropsychol. 2006;12:125–140. doi: 10.1080/09297040500499081. [DOI] [PubMed] [Google Scholar]
- Hoaken PN, Giancola PR, Pihl RO. Executive cognitive functions as mediators of alcohol-related aggression. Alcohol Alcohol. 1998;33:47–54. doi: 10.1093/oxfordjournals.alcalc.a008347. [DOI] [PubMed] [Google Scholar]
- Jaaskelainen IP, Pekkonen E, Hirvonen J, Sillanaukee P, Naatanen R. Mismatch negativity subcomponents and ethyl alcohol. Biol Psychol. 1996;43:13–25. doi: 10.1016/0301-0511(95)05174-0. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Gianaros PJ, Greer PJ, Kerr DD, Liu S, Higley JD, Suomi SJ, Olsen AS, Porter JN, Lopresti BJ, et al. Cognitive impact of genetic variation of the serotonin transporter in primates is associated with differences in brain morphology rather than serotonin neurotransmission. Mol Psychiatry. 2010;15:512–522. doi: 10.1038/mp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalhorn TF, Bowden DM, Slattery JT. Pharmacokinetics of ethanol in pigtailed macaques: intersubject variability and effect of subchronic administration. Pharmacol Biochem Behav. 1986;24:485–489. doi: 10.1016/0091-3057(86)90545-9. [DOI] [PubMed] [Google Scholar]
- Katner SN, Flynn CT, Von Huben SN, Kirsten AJ, Davis SA, Lay CC, Cole M, Roberts AJ, Fox HS, Taffe MA. Controlled and behaviorally relevant levels of oral ethanol intake in rhesus macaques using a flavorant-fade procedure. Alcohol Clin Exp Res. 2004;28:873–883. doi: 10.1097/01.alc.0000128895.99379.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacouture Y, Cousineau D. How to use MATLAB to fit the ex-Gaussian and other probability functions to a distribution of response times. Tutor Quant Methods Psychol. 2008;4:35–45. [Google Scholar]
- Leth-Steensen C, Elbaz ZK, Douglas VI. Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychologica. 2000;104:167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Li CS, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J Neurosci. 2006;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Huang C, Yan P, Paliwal P, Constable RT, Sinha R. Neural correlates of post-error slowing during a stop signal task: a functional magnetic resonance imaging study. J Cogn Neurosci. 2008;20:1021–1029. doi: 10.1162/jocn.2008.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnoila M, Virkkunen M, George T, Eckardt M, Higley JD, Nielsen D, Goldman D. Serotonin, violent behavior and alcohol. Experientia. 1994;71(Suppl):155–163. doi: 10.1007/978-3-0348-7330-7_16. [DOI] [PubMed] [Google Scholar]
- Liu S, Heitz RP, Bradberry CW. A touch screen based Stop Signal Response Task in rhesus monkeys for studying impulsivity associated with chronic cocaine self-administration. J Neurosci Methods. 2009;177:67–72. doi: 10.1016/j.jneumeth.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melia KF, Ehlers CL. Signal detection analysis of ethanol effects on a complex conditional discrimination. Pharmacol Biochem Behav. 1989;33:581–584. doi: 10.1016/0091-3057(89)90391-2. [DOI] [PubMed] [Google Scholar]
- Mello NK. Alcohol effects on delayed matching to sample performance by rhesus monkey. Physiol Behav. 1971;7:77–101. doi: 10.1016/0031-9384(71)90239-3. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Murray EA, Izquierdo A. Orbitofrontal cortex and amygdala contributions to affect and action in primates. Ann N Y Acad Sci. 2007;1121:273–296. doi: 10.1196/annals.1401.021. [DOI] [PubMed] [Google Scholar]
- Noel X, Paternot J, Van der Linden M, Sferrazza R, Verhas M, Hanak C, Kornreich C, Martin P, De Mol J, Pelc I, et al. Correlation between inhibition, working memory and delimited frontal area blood flow measure by 99mTc-Bicisate SPECT in alcohol-dependent patients. Alcohol Alcohol. 2001;36:556–563. doi: 10.1093/alcalc/36.6.556. [DOI] [PubMed] [Google Scholar]
- Noel X, Sferrazza R, Van Der Linden M, Paternot J, Verhas M, Hanak C, Pelc I, Verbanck P. Contribution of frontal cerebral blood flow measured by (99m)Tc-Bicisate spect and executive function deficits to predicting treatment outcome in alcohol-dependent patients. Alcohol Alcohol. 2002;37:347–354. doi: 10.1093/alcalc/37.4.347. [DOI] [PubMed] [Google Scholar]
- Noel X, Van der Linden M, d'Acremont M, Bechara A, Dan B, Hanak C, Verbanck P. Alcohol cues increase cognitive impulsivity in individuals with alcoholism. Psychopharmacology. 2007;192:291–298. doi: 10.1007/s00213-006-0695-6. [DOI] [PubMed] [Google Scholar]
- Pian JP, Criado JR, Walker BM, Ehlers CL. Differential effects of acute alcohol on EEG and sedative responses in adolescent and adult Wistar rats. Brain Res. 2008;1194:28–36. doi: 10.1016/j.brainres.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt PM. Errors and error correction in choice-response tasks. J Exp Psychol. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, de Wit H. Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav. 1999;71:121–143. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, de Vlugt Y, Bramlage A, Spaan M, Elton M, Snel J, Band GP. Alcohol consumption impairs detection of performance errors in mediofrontal cortex. Science. 2002;298:2209–2211. doi: 10.1126/science.1076929. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Rogers RD, Cook LJ, Sahakian BJ. The effect of polymorphism at the serotonin transporter gene on decision-making, memory and executive function in ecstasy users and controls. Psychopharmacology. 2006;188:213–227. doi: 10.1007/s00213-006-0495-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AK, Duka T. Effects of alcohol on inhibitory processes. Behav Pharmacol. 2008;19:284–291. doi: 10.1097/FBP.0b013e328308f1b2. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Bannerman DM, Rushworth MF. The contribution of distinct subregions of the ventromedial frontal cortex to emotion, social behavior, and decision making. Cogn Affect Behav Neurosci. 2008;8:485–497. doi: 10.3758/CABN.8.4.485. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Behrens TEJ. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat Neurosci. 2008;11:389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Res Rev. 2006;51:145–160. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev. 2001;35:146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Saults JS, Cowan N, Sher KJ, Moreno MV. Differential effects of alcohol on working memory: distinguishing multiple processes. Exp Clin Psychopharmacol. 2007;15:576–587. doi: 10.1037/1064-1297.15.6.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer TA, Jolicoeur P, Vogel-Sprott M, Dixon MJ. Fast, but error-prone, responses during acute alcohol intoxication: effects of stimulus-response mapping complexity. Alcohol Clin Exp Res. 2004;28:643–649. doi: 10.1097/01.alc.0000121652.84754.30. [DOI] [PubMed] [Google Scholar]
- Schweizer TA, Vogel-Sprott M. Alcohol-impaired speed and accuracy of cognitive functions: a review of acute tolerance and recovery of cognitive performance. Exp Clin Psychopharmacol. 2008;16:240–250. doi: 10.1037/1064-1297.16.3.240. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Hitzemann R, Fowler JS, Wolf AP, Pappas N, Biegon A, Dewey SL. Decreased cerebral response to inhibitory neurotransmission in alcoholics. Am J Psychiatry. 1993;150:417–422. doi: 10.1176/ajp.150.3.417. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur J Neurosci. 2003;18:2069–2081. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- Yeung N, Ralph J, Nieuwenhuis S. Drink alcohol and dim the lights: the impact of cognitive deficits on medial frontal cortex function. Cogn Affect Behav Neurosci. 2007;7:347–355. doi: 10.3758/cabn.7.4.347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.