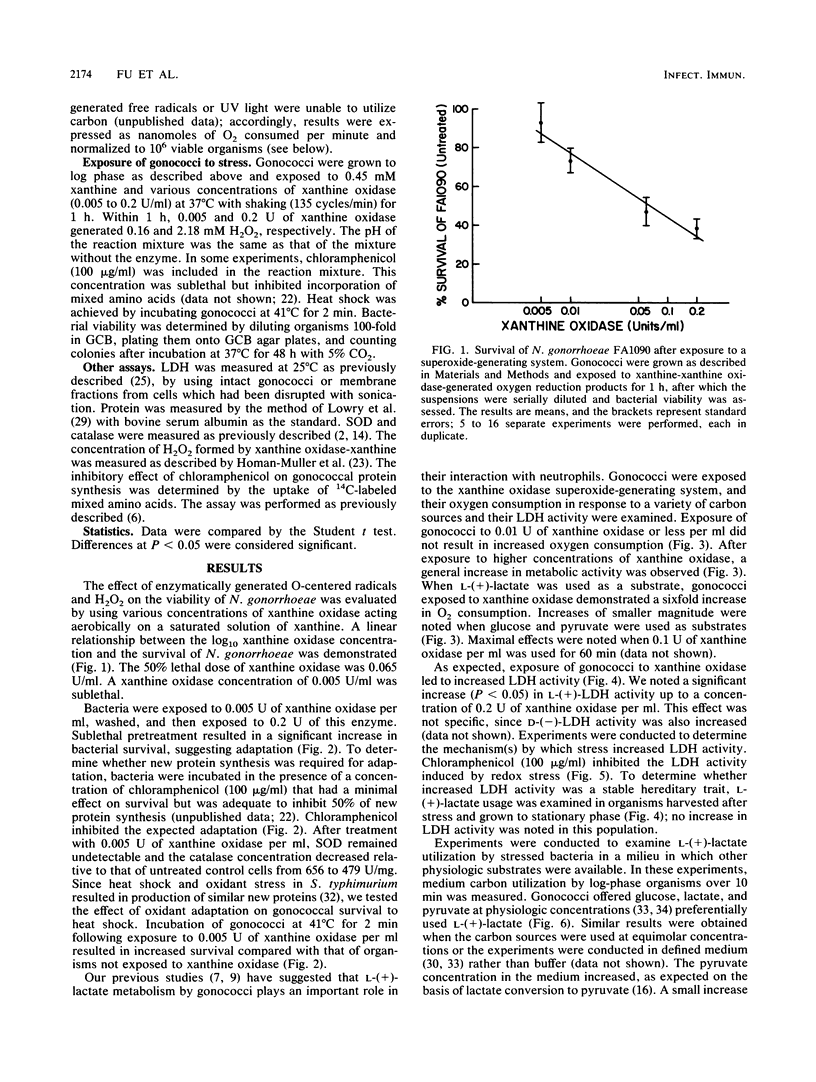
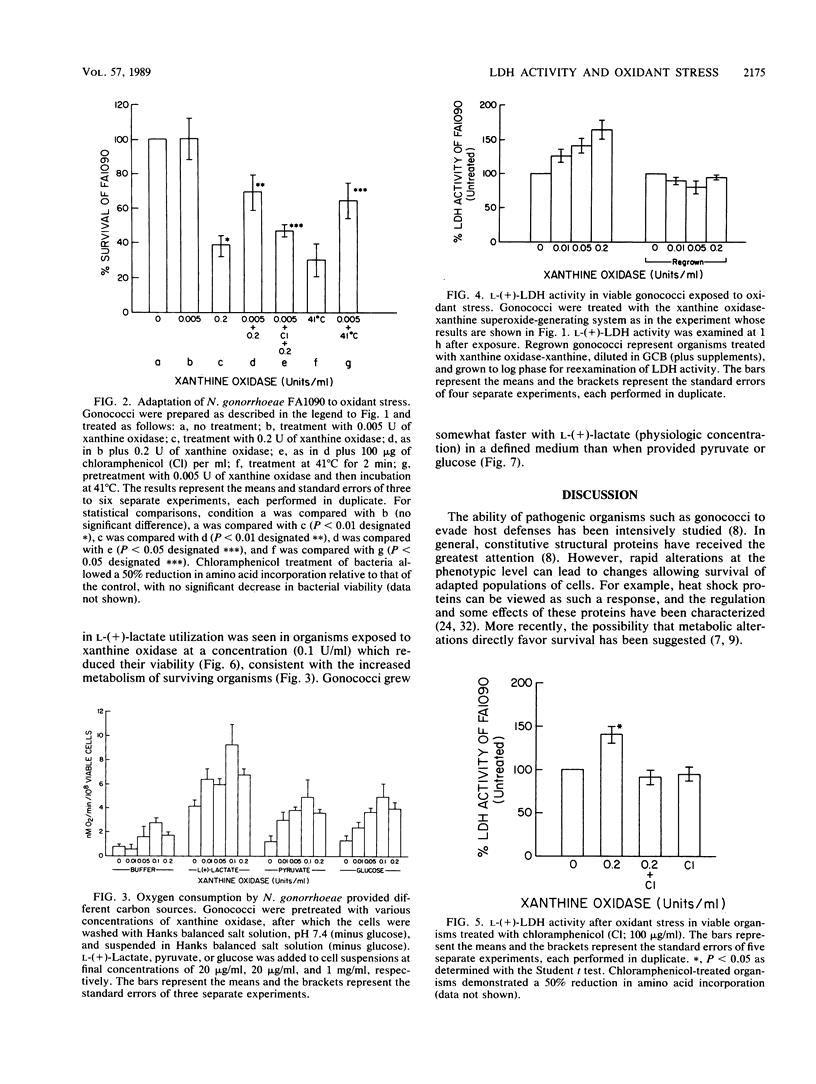
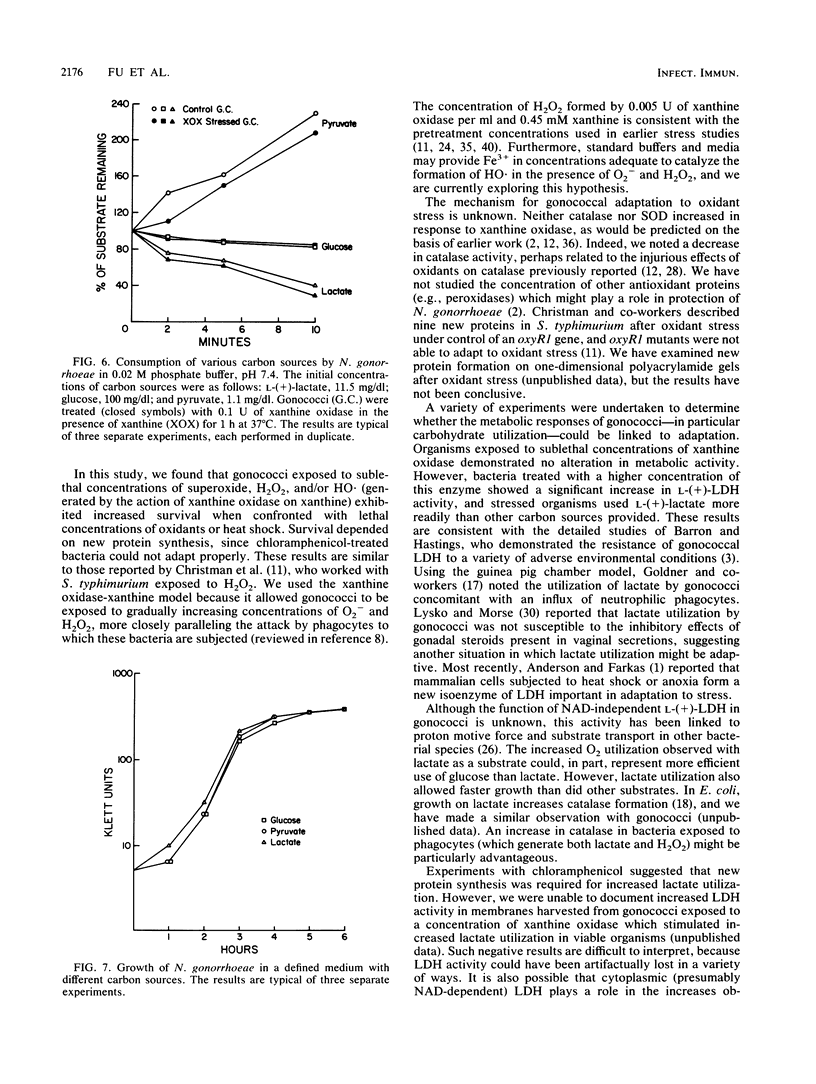
Abstract
Neisseria gonorrhoeae, an obligate human pathogen, is subjected to oxidant stress when attacked by O2 reduction products formed by neutrophils. In this study, exposure of gonococci to sublethal concentrations of superoxide and hydrogen peroxide (and related O-centered radicals) resulted in phenotypic resistance to oxidant stress. Adaptation required new protein formation but was not related to increases in superoxide dismutase or catalase. We have previously demonstrated that gonococci use phagocyte-derived L-(+)-lactate. Oxidant stress of greater magnitude than that required for adaptation led to a generalized increase in bacterial metabolism, particularly in L-(+)- and D-(-)-lactate utilization and lactate dehydrogenase activity. Increased lactate utilization required new protein synthesis. These results suggest the possibility that lactate metabolism is of importance to N. gonorrhoeae subjected to oxidant stress. Use of lct mutant organisms unable to use L-(+)-lactate should allow examination of this hypothesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson G. R., Farkas B. K. The major anoxic stress response protein p34 is a distinct lactate dehydrogenase. Biochemistry. 1988 Mar 22;27(6):2187–2193. doi: 10.1021/bi00406a056. [DOI] [PubMed] [Google Scholar]
- Archibald F. S., Duong M. N. Superoxide dismutase and oxygen toxicity defenses in the genus Neisseria. Infect Immun. 1986 Feb;51(2):631–641. doi: 10.1128/iai.51.2.631-641.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barstow D. A., Clarke A. R., Chia W. N., Wigley D., Sharman A. F., Holbrook J. J., Atkinson T., Minton N. P. Cloning, expression and complete nucleotide sequence of the Bacillus stearothermophilus L-lactate dehydrogenase gene. Gene. 1986;46(1):47–55. doi: 10.1016/0378-1119(86)90165-4. [DOI] [PubMed] [Google Scholar]
- Beaman B. L., Black C. M., Doughty F., Beaman L. Role of superoxide dismutase and catalase as determinants of pathogenicity of Nocardia asteroides: importance in resistance to microbicidal activities of human polymorphonuclear neutrophils. Infect Immun. 1985 Jan;47(1):135–141. doi: 10.1128/iai.47.1.135-141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britigan B. E., Chai Y., Cohen M. S. Effects of human serum on the growth and metabolism of Neisseria gonorrhoeae: an alternative view of serum. Infect Immun. 1985 Dec;50(3):738–744. doi: 10.1128/iai.50.3.738-744.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britigan B. E., Cohen M. S. Effects of human serum on bacterial competition with neutrophils for molecular oxygen. Infect Immun. 1986 Jun;52(3):657–663. doi: 10.1128/iai.52.3.657-663.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britigan B. E., Cohen M. S., Sparling P. F. Gonococcal infection: a model of molecular pathogenesis. N Engl J Med. 1985 Jun 27;312(26):1683–1694. doi: 10.1056/NEJM198506273122606. [DOI] [PubMed] [Google Scholar]
- Britigan B. E., Klapper D., Svendsen T., Cohen M. S. Phagocyte-derived lactate stimulates oxygen consumption by Neisseria gonorrhoeae. An unrecognized aspect of the oxygen metabolism of phagocytosis. J Clin Invest. 1988 Feb;81(2):318–324. doi: 10.1172/JCI113323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlioz A., Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986 Mar;5(3):623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman M. F., Morgan R. W., Jacobson F. S., Ames B. N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985 Jul;41(3):753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- Cohen M. S., Chai Y., Britigan B. E., McKenna W., Adams J., Svendsen T., Bean K., Hassett D. J., Sparling P. F. Role of extracellular iron in the action of the quinone antibiotic streptonigrin: mechanisms of killing and resistance of Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1987 Oct;31(10):1507–1513. doi: 10.1128/aac.31.10.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Halbrook J. Inducible repair of oxidative DNA damage in Escherichia coli. Nature. 1983 Aug 4;304(5925):466–468. doi: 10.1038/304466a0. [DOI] [PubMed] [Google Scholar]
- Elstner E. F., Heupel A. Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal Biochem. 1976 Feb;70(2):616–620. doi: 10.1016/0003-2697(76)90488-7. [DOI] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Garvie E. I. Bacterial lactate dehydrogenases. Microbiol Rev. 1980 Mar;44(1):106–139. doi: 10.1128/mr.44.1.106-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldner M., Penn C. W., Sanyal S. C., Veale D. R., Smith H. Phenotypically determined resistance of Neisseria gonorrhoeae to normal human serum: environmental factors in subcutaneous chambers in guinea pigs. J Gen Microbiol. 1979 Sep;114(1):169–177. doi: 10.1099/00221287-114-1-169. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Enzymatic defenses against the toxicity of oxygen and of streptonigrin in Escherichia coli. J Bacteriol. 1977 Mar;129(3):1574–1583. doi: 10.1128/jb.129.3.1574-1583.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Physiological function of superoxide dismutase in glucose-limited chemostat cultures of Escherichia coli. J Bacteriol. 1977 May;130(2):805–811. doi: 10.1128/jb.130.2.805-811.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Regulation of the synthesis of catalase and peroxidase in Escherichia coli. J Biol Chem. 1978 Sep 25;253(18):6445–6420. [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Superoxide, hydrogen peroxide, and oxygen tolerance of oxygen-sensitive mutants of Escherichia coli. Rev Infect Dis. 1979 Mar-Apr;1(2):357–369. doi: 10.1093/clinids/1.2.357. [DOI] [PubMed] [Google Scholar]
- Hebeler B. H., Morse S. A. Physiology and metabolism of pathogenic neisseria: tricarboxylic acid cycle activity in Neisseria gonorrhoeae. J Bacteriol. 1976 Oct;128(1):192–201. doi: 10.1128/jb.128.1.192-201.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan-Müller J. W., Weening R. S., Roos D. Production of hydrogen peroxide by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):198–207. [PubMed] [Google Scholar]
- Jenkins D. E., Schultz J. E., Matin A. Starvation-induced cross protection against heat or H2O2 challenge in Escherichia coli. J Bacteriol. 1988 Sep;170(9):3910–3914. doi: 10.1128/jb.170.9.3910-3914.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H., Gotschlich E. C. Isolation and characterization of the outer membrane of Neisseria gonorrhoeae. J Bacteriol. 1974 Jul;119(1):250–257. doi: 10.1128/jb.119.1.250-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. Transport studies in bacterial membrane vesicles. Science. 1974 Dec 6;186(4167):882–892. doi: 10.1126/science.186.4167.882. [DOI] [PubMed] [Google Scholar]
- Kono Y., Fridovich I. Superoxide radical inhibits catalase. J Biol Chem. 1982 May 25;257(10):5751–5754. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lysko P. G., Morse S. A. Effects of steroid hormones on Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1980 Aug;18(2):281–288. doi: 10.1128/aac.18.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. J., Gennis R. B. The purification and characterization of the cytochrome d terminal oxidase complex of the Escherichia coli aerobic respiratory chain. J Biol Chem. 1983 Aug 10;258(15):9159–9165. [PubMed] [Google Scholar]
- Morgan R. W., Christman M. F., Jacobson F. S., Storz G., Ames B. N. Hydrogen peroxide-inducible proteins in Salmonella typhimurium overlap with heat shock and other stress proteins. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8059–8063. doi: 10.1073/pnas.83.21.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Fitzgerald T. J. Effect of progesterone on Neisseria gonorrhoeae. Infect Immun. 1974 Dec;10(6):1370–1377. doi: 10.1128/iai.10.6.1370-1377.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Stein S., Hines J. Glucose metabolism in Neisseria gonorrhoeae. J Bacteriol. 1974 Nov;120(2):702–714. doi: 10.1128/jb.120.2.702-714.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy P., Dowds B. C., McConnell D. J., Devine K. M. Oxidative stress and growth temperature in Bacillus subtilis. J Bacteriol. 1987 Dec;169(12):5766–5770. doi: 10.1128/jb.169.12.5766-5770.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrod P., Morse S. A. Absence of superoxide dismutase in some strains of Neisseria gonorrhoeae. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1287–1294. doi: 10.1016/0006-291x(79)91176-8. [DOI] [PubMed] [Google Scholar]
- Pascal M. C., Puig J., Lepelletier M. Etude génétique d'une mutation affectant l'activité L-actate-déshydrogénase chez escherichia coli K 12. C R Acad Sci Hebd Seances Acad Sci D. 1969 Jan 27;268(4):737–739. [PubMed] [Google Scholar]
- Root R. K., Cohen M. S. The microbicidal mechanisms of human neutrophils and eosinophils. Rev Infect Dis. 1981 May-Jun;3(3):565–598. doi: 10.1093/clinids/3.3.565. [DOI] [PubMed] [Google Scholar]
- Rosen H., Rakita R. M., Waltersdorph A. M., Klebanoff S. J. Myeloperoxidase-mediated damage to the succinate oxidase system of Escherichia coli. Evidence for selective inactivation of the dehydrogenase component. J Biol Chem. 1987 Nov 5;262(31):15004–15010. [PubMed] [Google Scholar]
- Winquist L., Rannug U., Rannug A., Ramel C. Protection from toxic and mutagenic effects of H2O2 by catalase induction in Salmonella typhimurium. Mutat Res. 1984 Nov-Dec;141(3-4):145–147. doi: 10.1016/0165-7992(84)90087-3. [DOI] [PubMed] [Google Scholar]


