Abstract
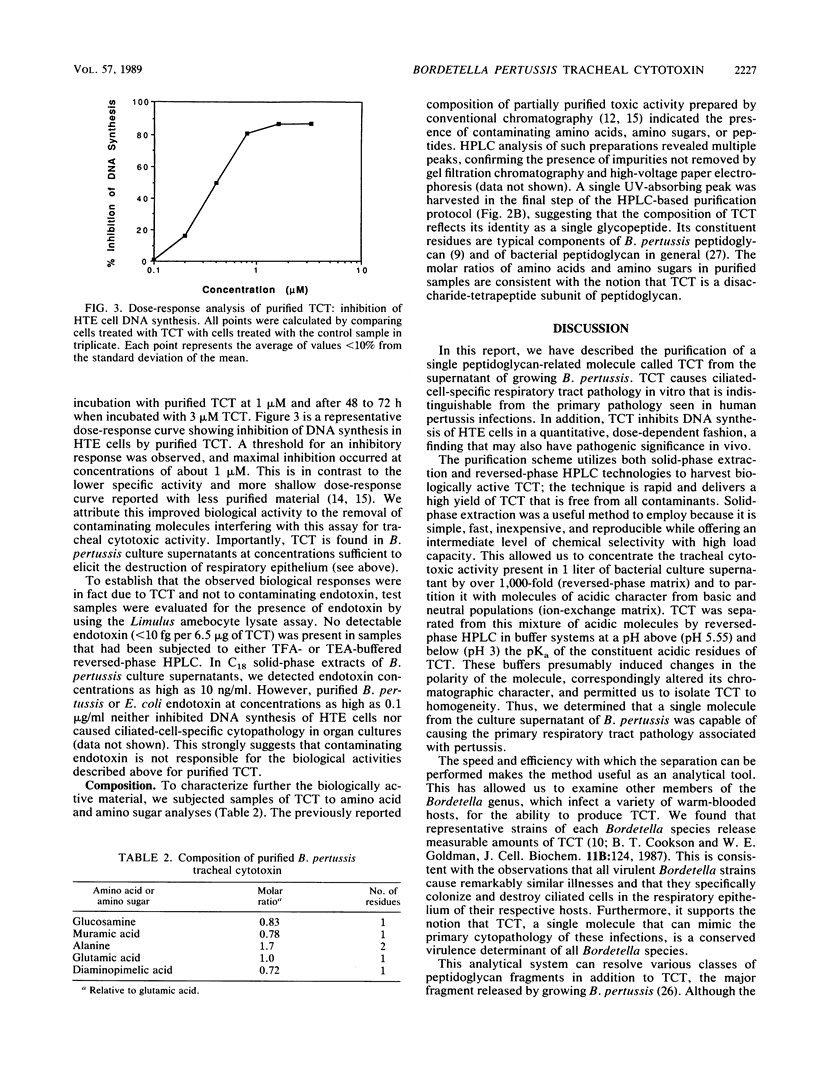
Specific destruction of ciliated epithelial cells lining the large airways is the primary respiratory tract cytopathology associated with human Bordetella pertussis infections. We have purified a single low-molecular-weight glycopeptide, tracheal cytotoxin (TCT), that appears to cause this pathology. By using a combination of solid-phase extraction and reversed-phase high-pressure liquid chromatography, about 700 nmol of biologically active peptide can be isolated from 1 liter of B. pertussis culture supernatant (approximately 60% yield). TCT at concentrations of 1 microM destroyed the ciliated cell population when incubated with respiratory epithelium in vitro. This concentration of TCT is similar to the concentrations found in the culture supernatant of growing B. pertussis. Purified TCT also inhibited DNA synthesis of hamster trachea epithelial cells in a quantitative, dose-dependent fashion. Endotoxin was not detected in the purified material, and neither B. pertussis nor Escherichia coli endotoxin could duplicate the biological activities of TCT. Amino acid and amino sugar analyses of purified TCT revealed the presence of glucosamine, muramic acid, alanine, glutamic acid, and diaminopimelic acid in molar ratios of 1:1:2:1:1. This suggests that TCT, the released ciliostatic principle of B. pertussis, is a disaccharide tetrapeptide subunit of peptidoglycan.
Full text
PDF

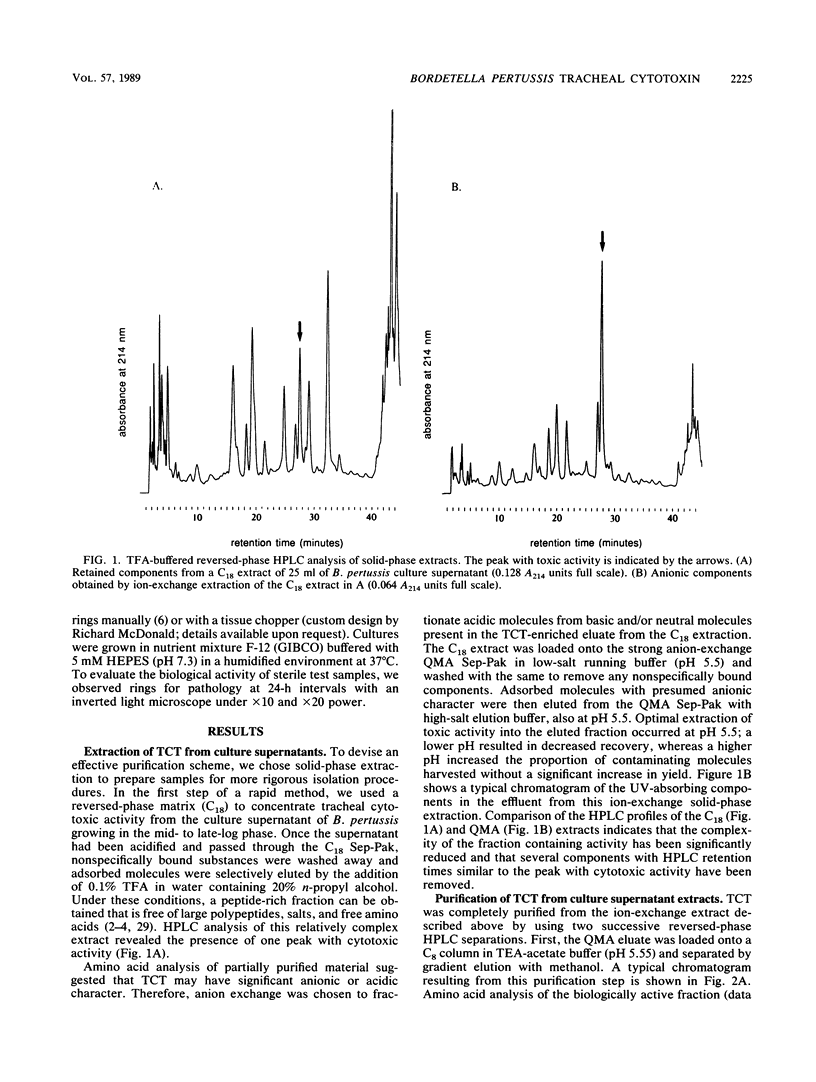


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Lederer E. Muramyl peptides: immunomodulators, sleep factors, and vitamins. Med Res Rev. 1984 Apr-Jun;4(2):111–152. doi: 10.1002/med.2610040202. [DOI] [PubMed] [Google Scholar]
- Bennett H. P., Browne C. A., Solomon S. Purification of the two major forms of rat pituitary corticotropin using only reversed-phase liquid chromatography. Biochemistry. 1981 Aug 4;20(16):4530–4538. doi: 10.1021/bi00519a004. [DOI] [PubMed] [Google Scholar]
- Bennett H. P., Hudson A. M., Kelly L., McMartin C., Purdon G. E. A rapid method, using octadecasilyl-silica, for the extraction of certain peptides from tissues. Biochem J. 1978 Dec 1;175(3):1139–1141. doi: 10.1042/bj1751139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhlen P., Castillo F., Ling N., Guillemin R. Purification of peptides: an efficient procedure for the separation of peptides from amino acids and salt. Int J Pept Protein Res. 1980 Oct;16(4):306–310. doi: 10.1111/j.1399-3011.1980.tb02591.x. [DOI] [PubMed] [Google Scholar]
- Chedid L. Muramyl peptides as possible endogenous immunopharmacological mediators. Microbiol Immunol. 1983;27(9):723–732. doi: 10.1111/j.1348-0421.1983.tb00636.x. [DOI] [PubMed] [Google Scholar]
- Collier A. M., Peterson L. P., Baseman J. B. Pathogenesis of infection with Bordetella pertussis in hamster tracheal organ culture. J Infect Dis. 1977 Aug;136 (Suppl):S196–S203. doi: 10.1093/infdis/136.supplement.s196. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J. Analysis of Neisseria gonorrhoeae peptidoglycan by reverse-phase, high-pressure liquid chromatography. J Bacteriol. 1985 Jul;163(1):69–74. doi: 10.1128/jb.163.1.69-74.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkening W. J., Nogami W., Martin S. A., Rosenthal R. S. Structure of Bordetella pertussis peptidoglycan. J Bacteriol. 1987 Sep;169(9):4223–4227. doi: 10.1128/jb.169.9.4223-4227.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry-Weeks C. R., Cookson B. T., Goldman W. E., Rimler R. B., Porter S. B., Curtiss R., 3rd Dermonecrotic toxin and tracheal cytotoxin, putative virulence factors of Bordetella avium. Infect Immun. 1988 Jul;56(7):1698–1707. doi: 10.1128/iai.56.7.1698-1707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman W. E., Baseman J. B. Selective isolation and culture of a proliferating epithelial cell population from the hamster trachea. In Vitro. 1980 Apr;16(4):313–319. doi: 10.1007/BF02618337. [DOI] [PubMed] [Google Scholar]
- Goldman W. E., Herwaldt L. A. Bordetella pertussis tracheal cytotoxin. Dev Biol Stand. 1985;61:103–111. [PubMed] [Google Scholar]
- Goldman W. E., Klapper D. G., Baseman J. B. Detection, isolation, and analysis of a released Bordetella pertussis product toxic to cultured tracheal cells. Infect Immun. 1982 May;36(2):782–794. doi: 10.1128/iai.36.2.782-794.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett E., Wolff J. Soluble adenylate cyclase from the culture medium of Bordetella pertussis: purification and characterization. J Bacteriol. 1976 Aug;127(2):890–898. doi: 10.1128/jb.127.2.890-898.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Tsujimoto M., Koga T., Nagao S., Tanaka A., Kawata S. Chemical structure and biological activity relationship of bacterial cell walls and muramyl peptides. Fed Proc. 1986 Oct;45(11):2534–2540. [PubMed] [Google Scholar]
- Lane B. P., Gordon R. Regeneration of rat tracheal epithelium after mechanical injury. I. The relationship between mitotic activity and cellular differentiation. Proc Soc Exp Biol Med. 1974 Apr;145(4):1139–1144. doi: 10.3181/00379727-145-37968. [DOI] [PubMed] [Google Scholar]
- Martin S. A., Rosenthal R. S., Biemann K. Fast atom bombardment mass spectrometry and tandem mass spectrometry of biologically active peptidoglycan monomers from Neisseria gonorrhoeae. J Biol Chem. 1987 Jun 5;262(16):7514–7522. [PubMed] [Google Scholar]
- Matrisian L. M., Larsen B. R., Finch J. S., Magun B. E. Further purification of epidermal growth factor by high-performance liquid chromatography. Anal Biochem. 1982 Sep 15;125(2):339–351. doi: 10.1016/0003-2697(82)90015-x. [DOI] [PubMed] [Google Scholar]
- Melly M. A., McGee Z. A., Rosenthal R. S. Ability of monomeric peptidoglycan fragments from Neisseria gonorrhoeae to damage human fallopian-tube mucosa. J Infect Dis. 1984 Mar;149(3):378–386. doi: 10.1093/infdis/149.3.378. [DOI] [PubMed] [Google Scholar]
- Muse K. E., Collier A. M., Baseman J. B. Scanning electron microscopic study of hamster tracheal organ cultures infected with Bordetella pertussis. J Infect Dis. 1977 Dec;136(6):768–777. doi: 10.1093/infdis/136.6.768. [DOI] [PubMed] [Google Scholar]
- Olson L. C. Pertussis. Medicine (Baltimore) 1975 Nov;54(6):427–469. doi: 10.1097/00005792-197511000-00001. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. S., Nogami W., Cookson B. T., Goldman W. E., Folkening W. J. Major fragment of soluble peptidoglycan released from growing Bordetella pertussis is tracheal cytotoxin. Infect Immun. 1987 Sep;55(9):2117–2120. doi: 10.1128/iai.55.9.2117-2120.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S. Release of soluble peptidoglycan from growing gonococci: hexaminidase and amidase activities. Infect Immun. 1979 Jun;24(3):869–878. doi: 10.1128/iai.24.3.869-878.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. K., Rosenthal R. S. Release of soluble peptidoglycan from growing conococci: demonstration of anhydro-muramyl-containing fragments. Infect Immun. 1980 Sep;29(3):914–925. doi: 10.1128/iai.29.3.914-925.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. I., Keith A. B., Edwardson J. A., Biggins J. A., McDermott J. R. Characterization of corticotropin-like immunoreactive peptides in rat brain using high performance liquid chromatography. Neurosci Lett. 1982 May 28;30(2):133–138. doi: 10.1016/0304-3940(82)90285-3. [DOI] [PubMed] [Google Scholar]
- Smith C. J., Coote J. G., Parton R. R-plasmid-mediated chromosome mobilization in Bordetella pertussis. J Gen Microbiol. 1986 Oct;132(10):2685–2692. doi: 10.1099/00221287-132-10-2685. [DOI] [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L. Virulence factors of Bordetella pertussis. Annu Rev Microbiol. 1986;40:661–686. doi: 10.1146/annurev.mi.40.100186.003305. [DOI] [PubMed] [Google Scholar]


