Abstract
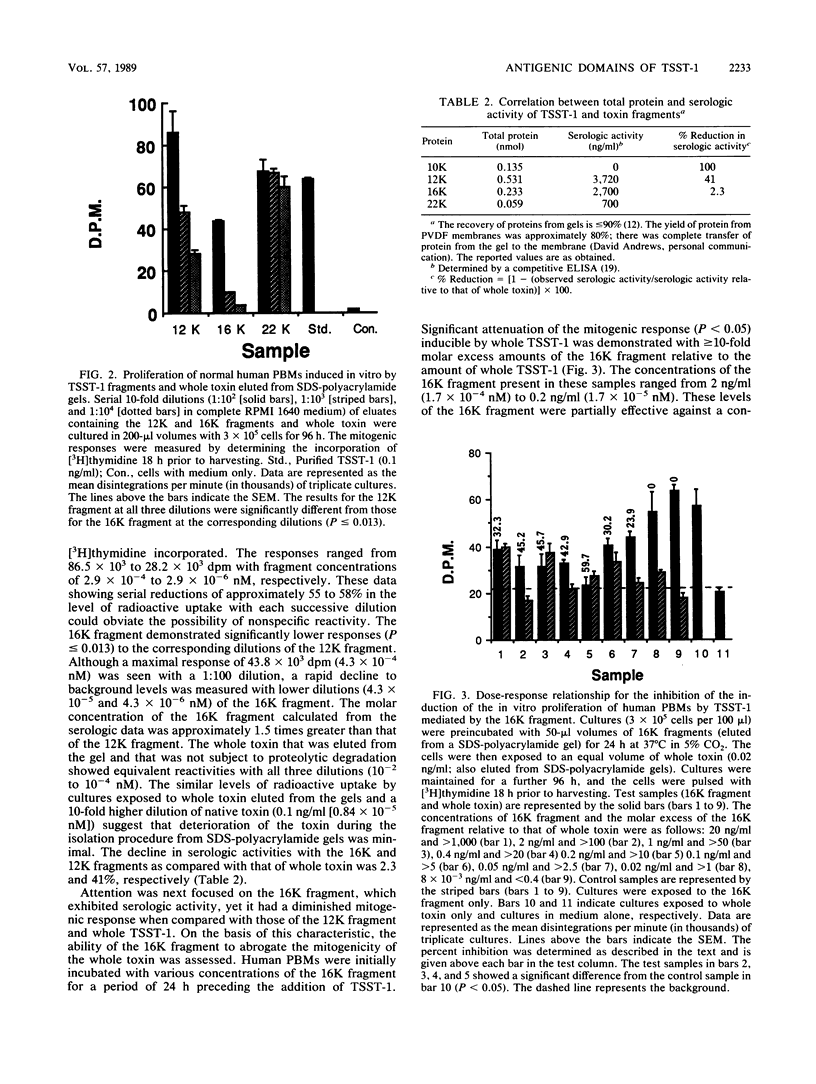
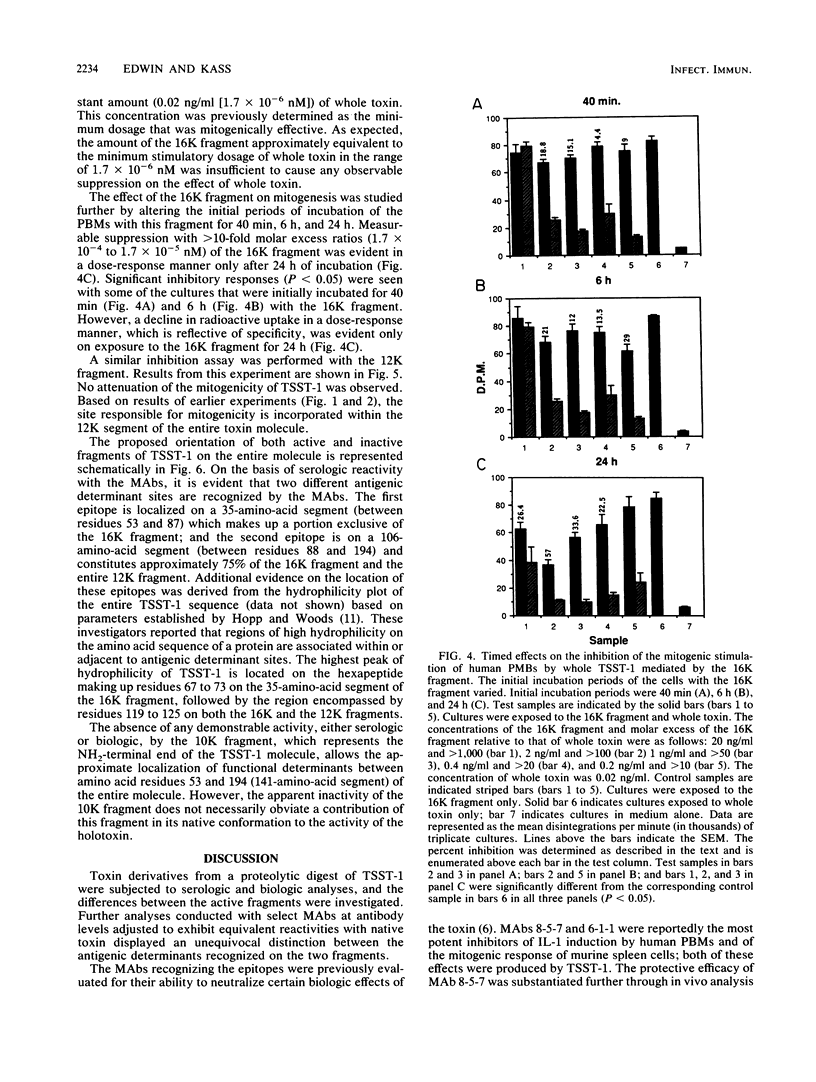
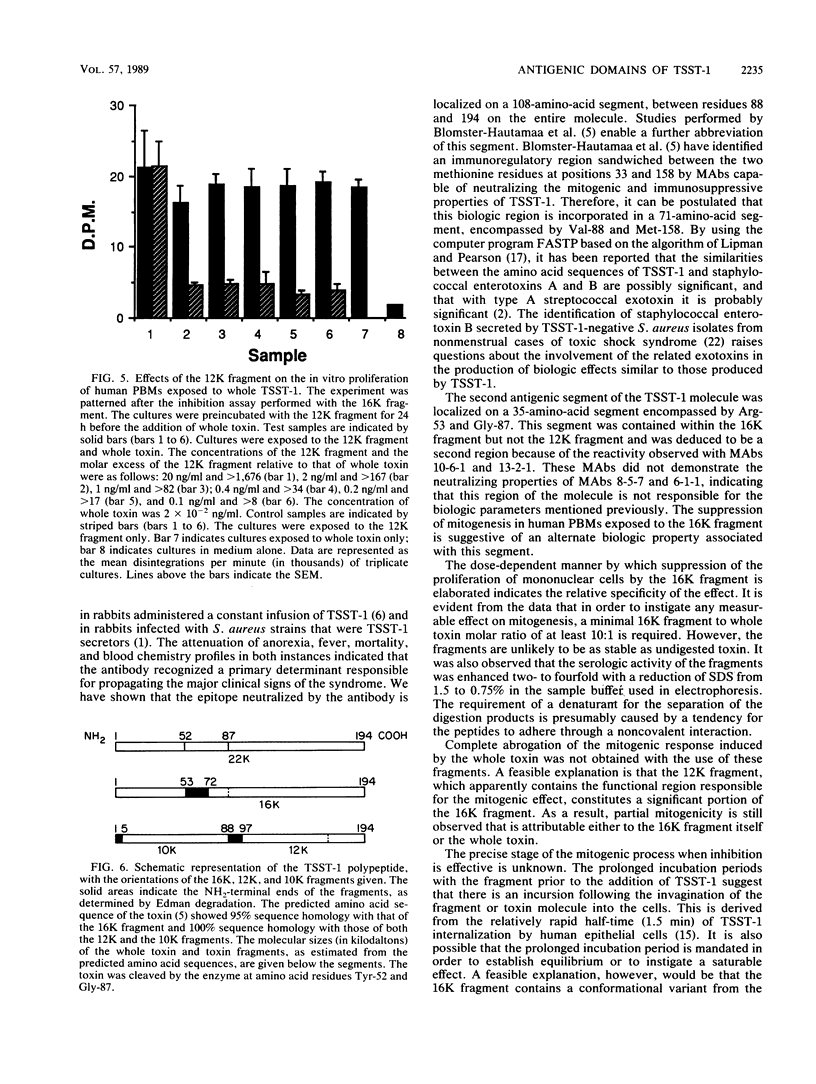
When toxic shock syndrome toxin 1 was subjected to papain hydrolysis, two serologically active fragments of 16.3 kilodaltons (16K fragment) and 12.4 kilodaltons (12K fragment) were generated, whereas a third fragment of 9.7 kilodaltons (10K fragment) was inactive. The biologic activities of the fragments were evaluated in vitro by determining their ability to promote nonspecific proliferation of human peripheral blood mononuclear cells. The 12K fragment was significantly (P less than or equal to 0.013) more stimulatory than the 16K fragment. When human peripheral blood mononuclear cells were preincubated for a period of 24 h with various concentrations of the 16K fragment, followed by incubation with a constant amount (2 x 10(-2) ng/ml) of whole toxin, the level of DNA synthesis induced by the holotoxin was reduced by approximately 60% when compared with that of controls exposed to whole toxin alone. The 12K fragment did not demonstrate a similar blocking effect. Immunoblots of the toxic shock syndrome toxin 1 digest, which were exposed to monoclonal antibodies (MAbs) developed against native toxin, depicted the presence of two different antigenic regions (epitopes). One MAb, 8-5-7, which has been shown previously to inhibit the biologic activity of the holotoxin in vitro and in vivo, reacted primarily with the 12K fragment. A second MAb, 10-6-1, that did not neutralize interleukin-1 production reacted primarily with the 16K fragment. On the basis of the differential mitogenic responses and the identification of heterologous epitopes, it was concluded that the functional region of the holotoxin can be partitioned into at least two functional segments encompassed between amino acid residues 53 and 87 and between amino acid residues 88 and 194 on the polypeptide chain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Best G. K., Scott D. F., Kling J. M., Thompson M. R., Adinolfi L. E., Bonventre P. F. Protection of rabbits in an infection model of toxic shock syndrome (TSS) by a TSS toxin-1-specific monoclonal antibody. Infect Immun. 1988 Apr;56(4):998–999. doi: 10.1128/iai.56.4.998-999.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley M. J., Mekalanos J. J. Nucleotide sequence of the type A staphylococcal enterotoxin gene. J Bacteriol. 1988 Jan;170(1):34–41. doi: 10.1128/jb.170.1.34-41.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Blomster-Hautamaa D. A., Kreiswirth B. N., Kornblum J. S., Novick R. P., Schlievert P. M. The nucleotide and partial amino acid sequence of toxic shock syndrome toxin-1. J Biol Chem. 1986 Nov 25;261(33):15783–15786. [PubMed] [Google Scholar]
- Blomster-Hautamaa D. A., Novick R. P., Schlievert P. M. Localization of biologic functions of toxic shock syndrome toxin-1 by use of monoclonal antibodies and cyanogen bromide-generated toxin fragments. J Immunol. 1986 Dec 1;137(11):3572–3576. [PubMed] [Google Scholar]
- Bonventre P. F., Thompson M. R., Adinolfi L. E., Gillis Z. A., Parsonnet J. Neutralization of toxic shock syndrome toxin-1 by monoclonal antibodies in vitro and in vivo. Infect Immun. 1988 Jan;56(1):135–141. doi: 10.1128/iai.56.1.135-141.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvano S. E., Quimby F. W., Antonacci A. C., Reiser R. F., Bergdoll M. S., Dineen P. Analysis of the mitogenic effects of toxic shock toxin on human peripheral blood mononuclear cells in vitro. Clin Immunol Immunopathol. 1984 Oct;33(1):99–110. doi: 10.1016/0090-1229(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Edwin C., Parsonnet J., Kass E. H. Structure-activity relationship of toxic-shock-syndrome toxin-1: derivation and characterization of immunologically and biologically active fragments. J Infect Dis. 1988 Dec;158(6):1287–1295. doi: 10.1093/infdis/158.6.1287. [DOI] [PubMed] [Google Scholar]
- Hirsch M. L., Kass E. H. An annotated bibliography of toxic shock syndrome. Rev Infect Dis. 1986 Jan-Feb;8 (Suppl 1):S1–104. [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Ikejima T., Dinarello C. A., Gill D. M., Wolff S. M. Induction of human interleukin-1 by a product of Staphylococcus aureus associated with toxic shock syndrome. J Clin Invest. 1984 May;73(5):1312–1320. doi: 10.1172/JCI111334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiswirth B. N., Löfdahl S., Betley M. J., O'Reilly M., Schlievert P. M., Bergdoll M. S., Novick R. P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983 Oct 20;305(5936):709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- Kushnaryov V. M., MacDonald H. S., Reiser R., Bergdoll M. S. Staphylococcal toxic shock toxin specifically binds to cultured human epithelial cells and is rapidly internalized. Infect Immun. 1984 Sep;45(3):566–571. doi: 10.1128/iai.45.3.566-571.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Gillis Z. A., Pier G. B. Induction of interleukin-1 by strains of Staphylococcus aureus from patients with nonmenstrual toxic shock syndrome. J Infect Dis. 1986 Jul;154(1):55–63. doi: 10.1093/infdis/154.1.55. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Mills J. T., Gillis Z. A., Pier G. B. Competitive, enzyme-linked immunosorbent assay for toxic shock syndrome toxin 1. J Clin Microbiol. 1985 Jul;22(1):26–31. doi: 10.1128/jcm.22.1.26-31.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poindexter N. J., Schlievert P. M. Toxic-shock-syndrome toxin 1-induced proliferation of lymphocytes: comparison of the mitogenic response of human, murine, and rabbit lymphocytes. J Infect Dis. 1985 Jan;151(1):65–72. doi: 10.1093/infdis/151.1.65. [DOI] [PubMed] [Google Scholar]
- Schlievert P. M. Alteration of immune function by staphylococcal pyrogenic exotoxin type C: possible role in toxic-shock syndrome. J Infect Dis. 1983 Mar;147(3):391–398. doi: 10.1093/infdis/147.3.391. [DOI] [PubMed] [Google Scholar]
- Schlievert P. M., Shands K. N., Dan B. B., Schmid G. P., Nishimura R. D. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J Infect Dis. 1981 Apr;143(4):509–516. doi: 10.1093/infdis/143.4.509. [DOI] [PubMed] [Google Scholar]
- Schlievert P. M. Staphylococcal enterotoxin B and toxic-shock syndrome toxin-1 are significantly associated with non-menstrual TSS. Lancet. 1986 May 17;1(8490):1149–1150. doi: 10.1016/s0140-6736(86)91859-3. [DOI] [PubMed] [Google Scholar]
- Steward M. W., Lew A. M. The importance of antibody affinity in the performance of immunoassays for antibody. J Immunol Methods. 1985 Apr 22;78(2):173–190. doi: 10.1016/0022-1759(85)90074-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heyningen V., Brock D. J., Van Heyningen S. A simple method for ranking the affinities of monoclonal antibodies. J Immunol Methods. 1983 Aug 26;62(2):147–153. doi: 10.1016/0022-1759(83)90000-5. [DOI] [PubMed] [Google Scholar]



