Abstract
OBJECTIVE
To investigate the mechanisms by which macrophage scavenger receptor BI (SR-BI) regulates macrophage cholesterol homeostasis and protects against atherosclerosis.
METHODS and RESULTS
The expression and function of SR-BI, was investigated in cultured mouse bone marrow-derived macrophages (BMM). SR-BI, the other scavenger receptors SRA and CD36 and the ATP-binding cassette transporters ABCA1 and ABCG1 were each distinctly regulated during BMM differentiation. SR-BI levels increased transiently to significant levels during culture. SR-BI expression in BMM was reversibly down-regulated by lipid loading with modified LDL; SR-BI was shown to be present both on the cell surface as well as intracellularly. BMM exhibited selective HDL CE uptake, however, this was not dependent on SR-BI or another potential candidate glycosylphosphatidylinositol anchored high density lipoprotein binding protein 1 (GPIHBP1). SR-BI played a significant role in facilitating bidirectional cholesterol flux in non lipid-loaded cells. SR-BI expression enhanced both cell cholesterol efflux and cholesterol influx from HDL, but did not lead to altered cellular cholesterol mass. SR-BI-dependent efflux occurred to larger HDL particles but not to smaller HDL3. Following cholesterol loading, ABCA1 and ABCG1 were up-regulated and served as the major contributors to cholesterol efflux, while SR-BI expression was down-regulated.
CONCLUSION
Our results suggest that SR-BI plays a significant role in macrophage cholesterol flux that may partly account for its effects on atherogenesis.
Keywords: SR-BI, ABCA1, ABCG1, cholesterol efflux, macrophage, HDL
1. Introduction
Class B Scavenger Receptor Type 1 (SR-BI) is a CD36-related cell surface glycoprotein involved in lipid metabolism 1, 2. SR-BI is recognized mainly as an HDL receptor, which mediates selective uptake of HDL cholesteryl esters (CE) into cells. SR-BI is most abundantly expressed in liver and steroidogenic tissues, where it functions to deliver HDL CE for cholesterol excretion and for steroid hormone synthesis, respectively 3, 4. SR-BI can also facilitate cholesterol efflux from cells including macrophages 5. In addition to its roles in cholesterol metabolism, other important functions for SR-BI have been proposed, such as platelet aggregation 6, oxidative stress 7, endothelial nitric oxide synthase activation 8 and apoptosis 9. Hepatic SR-BI plays a pivotal role in HDL cholesterol clearance from plasma and consequently plasma HDL cholesterol levels 10. Hepatic SR-BI expression is also an important positive regulator of the rate of macrophage-to-feces reverse cholesterol transport (RCT) 11. In line with this concept, SR-BI has been shown to play an anti-atherogenic role in mice 12, 13, 14
Studies have shown that liver specific SR-BI null mice develop less atherosclerosis than that from whole body SR-BI null mice, suggesting an atheroprotective function of SR-BI in peripheral tissues 15. SR-BI is detected in cultured human monocyte-derived macrophages and atherosclerotic lesions 16. An athero-protective role of macrophage SR-BI has been suggested from studies performed using bone marrow transplantation. Inactivation of macrophage SR-BI promotes advanced atherosclerotic lesion development in apolipoprotein E-deficient mice 17, as well as in LDL receptor-deficient mice 18, 19. However, macrophage SR-BI may have dual effects on atherogenesis in that it is reported to promote early lesion development while inhibiting more advanced lesions 19. The mechanisms by which macrophage SR-BI modulate atherosclerosis are unclear.
Macrophage foam cell formation with CE accumulation plays a key role in the formation of atherosclerotic lesions. Cholesterol efflux from macrophages, mediated by the ATP-binding cassette transporters A1 (ABCA1) and G1 (ABCG1) plays a key role in preventing foam cell formation. On the other hand, the role of SR-BI in macrophage cholesterol efflux is uncertain. In bone marrow transplantation studies, one study reported that SR-BI plays a significant role in efflux from peritoneal macrophages (MPM) 19, while a second study failed to show an SR-BI effect on efflux 17. Another study in which cholesterol efflux to human serum was measured, indicated a minimal role for macrophage SR-BI in efflux 20. Previous studies have demonstrated that SR-BI stimulates the bidirectional flux of free cholesterol between HDL and SR-BI-expressing cells rather than efflux alone 21. To investigate potential functions of SR-BI in macrophage lipid metabolism, the regulation of SR-BI expression and other cholesterol transporters as well as their roles in cholesterol transport were studied during the differentiation of bone marrow-derived macrophages (BMM). Our results suggest that SR-BI plays a significant role in macrophage cholesterol flux that may contribute to the protective effects in atherogenesis.
2. Materials and Methods
2.1. Animals
SR-BI- deficient mice (SR-BI-null) were obtained from M. Krieger 4. SR-BI homozygous (SR-BI−/−) and wild type (WT) mice (both 1:1 mixed C57BL/6 × 129 backgrounds) were bred from a common mating pair of SR-BI heterozygous (SR-BI+/−) mice. ABCG1- deficient mice (ABCG1-null) from Deltagen were backcrossed to a C57BL/6 background (>99.9% C57BL/6 background). For all animal experiments, 8- to 12-week-old male mice, weighing 20–25 g, were used. All animal experiments were approved by the Veterans Affairs Medical Center, Institutional Animal Care and Use Committee.
2.2. Bone marrow macrophage (BMM) preparation
Murine BMM were obtained and cultured by standard procedures 22. Isolated cells were suspended and cultured in RPMI 1640 containing 50 IU of penicillin G per ml, 50 μg of streptomycin per ml, 2 mM glutamine, 10% fetal bovine serum, and 15% (vol/vol) L-cell conditioned medium (LCM).
2.3. Flow Cytometry
Flow cytometric analysis of BMM cell-surface marker expression was carried out as described by Yona et al. 22. Attached cells were dissociated by incubation at room temperature with an enzyme-free cell dissociation buffer (Gibco) for 25 min. Trypan blue positivity was less than 5% for all samples. Cells were stained with rat anti-mouse CD11b (Mac1, Invitrogen), or F4/80 (AbD Serotec) or control rat IgG, followed by FITC-conjugated chicken anti-rat IgG antibody (Molecular Probes). Flow cytometry was performed using a FACScalibur cytometer (Becton Dickinson, Cowley, U.K.).
2.4. Western blotting
Total cell proteins (10 μg) were separated on a 4%–20% polyacrylamide gradient gel, transferred to PVDF membranes and immunoblotted with rabbit anti-mouse SR-BI (Novus, NB400-104), rat anti-mouse CD36 23, goat anti-mouse SRA (R&D Systems, AF1797), mouse anti-human ABCA1 (gift from M. Hayden), rabbit anti-mouse ABCG1 (Novus, NB400-132), or mouse anti-β-actin (Sigma, A5441). Immunoblots were visualized by the AmershamTM ECLTM Western Blotting Detection Reagents (GE Healthcare).
2.5. Cellular lipid and protein determinations
Lipids were extracted as described previously 24 and aliquots were assayed for total and free cholesterol content using a colorimetric kit (Wako). Cell protein was determined using BCATM Protein Assay Kit (Thermo Scientific).
2.6. Biotinylation of cell surface SR-BI
Biotinylation of cell surface proteins was carried out using EZ-Link Sulfo-NHS-LC-Biotin (Thermo Scientific) as described previously 25 followed by isolation using Streptavidin Iron Oxide Particles (Sigma). The supernatant (intracellular proteins) and the pellet (surface proteins) were analyzed by SDS-PAGE and immunoblotting using the rabbit anti-mouse SR-BI (Novus, NB400-104) antibody and quantified by densitometry.
2.7. Isolation and labeling of lipoproteins
HDL (d = 1.063–1.21 g/mL) was isolated from C57BL/6 mouse plasma or human plasma by density gradient ultracentrifugation 26. Human HDL was subfractionated to obtain HDL2b (d = 1.09–1.11 g/mL) and HDL3 (d = 1.13 – 1.18 g/mL). Protein concentrations were determined by the method of Lowry et al. 27. HDL2b was radiolabeled by the iodine monochloride method and HDL-associated CE was traced with non-hydrolyzable [1,2(n)-3H]cholesteryl oleoyl ether (Amersham Biosciences)25. Lipoproteins were analyzed by SDS-PAGE and non-denaturing gradient gel electrophoresis. LDL (d=1.019–1.063 g/mL) was isolated from human plasma 26. Acetylated LDL (acLDL) was prepared as described previously 28.
2.8. HDL cell binding, association and selective uptake
HDL cell association, selective CE uptake and 4ºC binding assays were performed as described previously 25. BMM were incubated with 125I/[3H]CEt labeled HDL2b (10 μg/mL) in serum-free medium containing 0.5% fatty acid-free BSA for 4 h at 37ºC. Cell association of 125I-HDL protein uptake was determined. The non-iodide, trichloroacetic acid-soluble 125I in cell medium corresponding to degraded 125I-apolipoprotein was assayed. Selective lipid uptake is defined as 3H - (125I cell-associated + 125I degraded) and represents the uptake of cholesteryl ester that cannot be accounted for by the internalization of intact particles. SR-BI-specific values were calculated as the difference between the values for WT and SR-BI-null cells.
2.9. RT-PCR
PCR amplification of mouse glycosylphosphatidylinositol anchored high density lipoprotein binding protein 1 (GPIHBP1) was carried out using the following gene specific primers: forward primer: tactaagtggacagccagggagtg, reverse primer: gagcagctctgtgtctgattgcag, (GenBank accession no. NM 026730, nt. 56-266, 211bp). GAPDH was used as a control house keeping gene.
2.10. Cholesterol flux determination
Cellular cholesterol efflux was determined as described 29. Briefly, cells were labeled with 0.2 μCi/mL [3H] cholesterol (35–50 Ci/mmol, Amersham Biosciences) for 48 h, washed, and equilibrated in serum-free medium containing 0.2% fatty acid-free BSA for 16 h. Cells were then incubated for 5 h at 37ºC in 0.2% fatty acid-free BSA medium without or with mouse HDL, human HDL2b or HDL3 (40 μg/mL), human apoA-I (10 μg/mL) for 5 h. Efflux was calculated as the percentage of counts in the medium relative to total counts. For some experiments, counts were converted into ng cholesterol efflux per mg cell protein using the determined [3H] specific activity of cellular cholesterol.
Cholesterol influx was determined as described 30. Briefly, HDL2b was labeled with [3H]cholesterol (20 μCi/mg HDL protein) during an overnight incubation at 4°C. The radiolabeled HDL in serum-free medium containing 0.2% fatty acid-free BSA was incubated with cells at day 5 of culture. After incubation and washing, cells were lysed in 0.1N NaOH and [3H] cholesterol present in the cells determined. Counts were converted into ng cholesterol influx per mg cell protein using the [3H] specific activity of the cholesterol in the radiolabeled HDL.
2.11. Statistical analysis
Statistics and best-fit curves were calculated with Graphpad’s Prism software. Data were expressed as means ± SEM. Results were analyzed by Student's t-test.
3. Results
3.1 Expression of cholesterol transporters during BMM differentiation in culture
Bone marrow derived macrophages have been widely used as a convenient and useful model cell system to study macrophage function 31, 32. We confirmed the differentiation of isolated bone marrow cells into macrophages by staining with two markers of macrophage differentiation, Mac1 and F4/80. Freshly isolated bone marrow cells from WT, SR-BI-null and ABCG1-null mice (day 0) were largely positive for Mac1 (about 50–70% of cells) and to a lesser extent for F4/80 (about 10–20% of cells) (Supplemental Fig.1). During culture in LCM, cells attached and the expression of both markers increased so that by day 5 virtually all attached cells expressed both Mac1 (>99%) and F4/80 (98–99%) and were classified as BMM. Similar results were seen for each of the three genotypes.
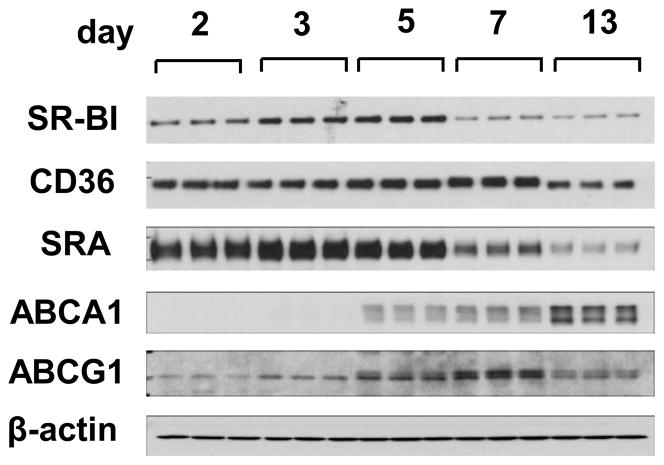
The regulation of SR-BI and other key cholesterol transporters was examined during BMM differentiation. The scavenger receptors SR-BI, CD36, SRA and ATP-binding cassette transporters ABCA1, ABCG1 each showed different protein expression profiles during macrophage differentiation (Fig. 1). SR-BI levels showed an up-regulation during the early stages of culture (2–5 days) with a maximal level of expression at day 5. After day 5, SR-BI levels decreased. CD36 expression increased moderately during culture, declining only at day 13. SRA was highly expressed until day 5 before declining. Interestingly, the closely related ABCA1 and ABCG1 were regulated differently from one another during differentiation. ABCA1 expression reached a maximum at day 13, whereas ABCG1, the other major transporter involved in cholesterol efflux, reached a maximum by day 7 before declining.
Fig. 1.
Gene expression of scavenger receptors and ATP-binding cassette transporters during BMM differentiation and lipid loading.
The expression of scavenger receptors SR-BI, CD36, SRA and ATP-binding cassette transporters ABCA1 and ABCG1 was determined by Western blot analysis (10 μg cell protein/lane) at the indicated days in culture. The figure is representative of three independent experiments.
To assess whether SR-BI regulation during BMM differentiation was reversible, cell culture medium was changed on day 16 to 2% lipoprotein-deficient serum (LPDS)-containing medium and cultured for a further 24 h before SR-BI analysis on day 17. As shown in Figure 2A, SR-BI was markedly up-regulated by LPDS, indicating that the down-regulation of SR-BI during culture was reversible and not the result of irreversible differentiation-induced gene suppression. Cholesterol loading of day 5 cells for 24 h using the SRA ligand acLDL markedly decreased SR-BI expression in a dose dependent manner (Fig. 2B). The down-regulation of SR-BI by acLDL was associated with an increase in cellular total cholesterol, free cholesterol, and particularly esterified cholesterol (Fig. 2D). In contrast to SR-BI, ABCA1 and ABCG1 were markedly up-regulated by acLDL (Fig. 2B,C), consistent with their known LXR-mediated regulation by oxysterols 33.
Fig. 2.
Expression of cholesterol transporters during lipid loading and depletion.
A) Cell culture medium was changed on day 16 to 2% LPDS-containing medium and cells were then cultured for a further 24 h before SR-BI analysis by Western blotting on day 17. B,C) BMM on day 5 were incubated for 24 h in 0.5% BSA medium without or with acLDL at indicated concentrations followed by analysis of SR-BI and ABCA1 (B) and ABCG1 in triplicate wells for each condition (C). D) BMM were incubated for 24 h in L-cell conditioned medium (Full), 0.5% BSA medium or 0.5% BSA medium containing the indicated concentrations of acLDL and cellular lipid content then determined. Values shown are the mean and S.D of triplicate determinations. The figure is representative of three independent experiments.
3.2 SR-BI-independent selective cholesteryl ester uptake in BMM
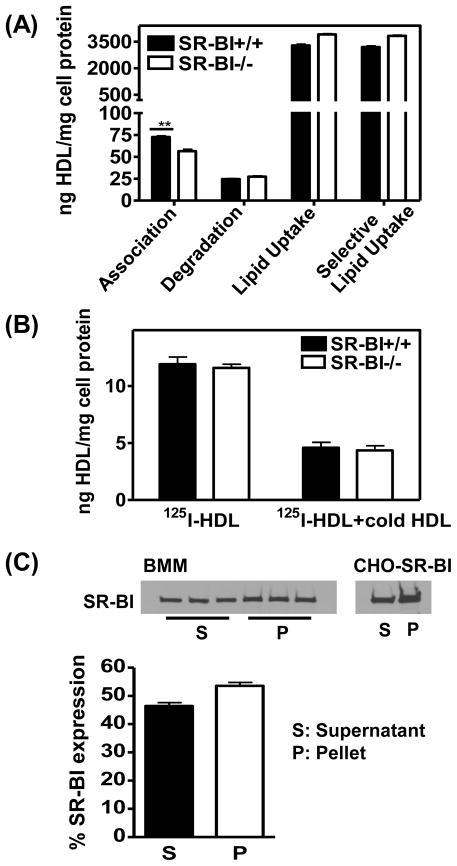
The ability of SR-BI to mediate selective lipid uptake into BMM was investigated using 125I/[3H]CEt-labeled HDL. BMM cells were used on day 5 of culture, at which time SR-BI expression had reached a maximum. Human HDL2b protein cell association was slightly but significantly reduced in SR-BI-null cells compared to WT cells (Fig. 3A). Relatively small amounts of protein degradation were observed in both cell types. Compared to protein uptake, high rates of cellular CE uptake were shown. Interestingly, no difference in CE uptake or the calculated selective CE uptake was found between SR-BI-null and WT cells. Similar results were obtained using mouse HDL (Supplemental Fig. 2) and human HDL3 (data not shown) that showed also no evidence of SR-BI-dependent HDL CE uptake in BMM between day 5 and 9.
Fig. 3.
HDL binding and selective lipid uptake in BMM is independent of SR-BI.
A) Cells at day 5 were incubated with 125I/[3H]CEt labeled HDL2b (10 μg/mL) for 4 h at 37°C. HDL cell association, degradation, CE uptake and selective CE uptake were determined. Values shown were the mean and S.D of triplicate determinations. The figure is representative of three independent experiments. B) Cells were incubated with 125I-labeled HDL2b (10 μg/mL) in the absence or presence of “cold” unlabeled HDL2b (500 μg/mL) for 2 h at 4°C, and HDL cell binding was determined. Values shown were the mean and S.D of triplicate determinations. C) Intact BMM cells at day 5 were biotinylated and SR-BI expression in supernatant (S) (intracellular proteins) and Streptavidin pull-down fractions (P) (surface proteins) determined. As a control, CHO cells over-expressing SR-BI were analyzed in parallel. The figure is representative of two independent experiments.
To address the question why SR-BI does not play a role in CE uptake in BMM, HDL binding to cells was analyzed at 4ºC. As shown in Figure 3B, no difference in HDL binding was observed between SR-BI-null and WT cells. The cell surface expression of SR-BI also was analyzed by cell surface protein biotinylation, SR-BI was distributed approximately equally between the cell surface and intracellular compartments (Fig. 3C). These results indicate that the inability of SR-BI to enhance HDL binding and selective uptake of CE is not due to a lack of SR-BI at the cell surface of BMM.
A candidate molecule for mediating selective lipid uptake is GPIHBP1, which is able to mediate selective lipid uptake from HDL in transfected CHO cells 34. RT-PCR analysis showed that whereas GPIHBP1 mRNA was expressed in heart and liver, no GPIHBP1 mRNA was detected in either BMM or MPM (Supplemental Fig. 3); strongly indicating that GPIHBP1 was also not responsible for selective lipid uptake in these cells.
3.3 Role of SR-BI and other transporters in cholesterol flux
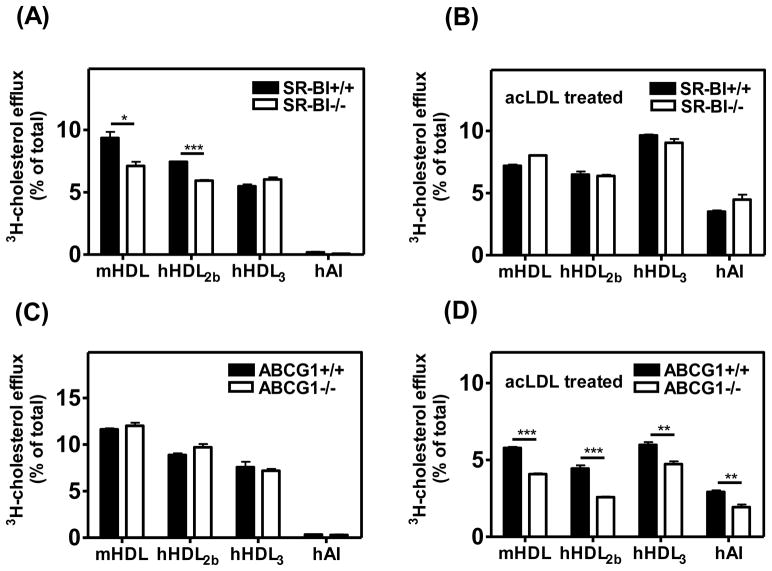
Cellular cholesterol efflux to mouse HDL, human HDL2b or HDL3 or apoA-I was determined in BMM from WT, SR-BI-null, and ABCG1-null mice. The size of mouse HDL and HDL2b was shown to be larger than that of HDL3 (Supplemental Fig. 4). In the absence of lipid loading, cholesterol efflux to the larger mouse HDL and human HDL2b was more efficient than to the smaller human HDL3. Mouse HDL and human HDL2b induced significantly higher cholesterol efflux from SR-BI WT BMM than from SR-BI-null BMM (Fig. 4A), showing that SR-BI played a significant role in cholesterol efflux to these particles. Interestingly, no SR-BI-dependent cholesterol efflux to human HDL3 was observed. There was also a substantial level of cholesterol efflux that was independent of SR-BI, likely by a process designated as aqueous diffusion 20. Very low levels of cholesterol efflux to apoA-I were observed in the absence of cholesterol loading (Fig. 4A, C), consistent with the observed low expression of ABCA1. In non lipid-loaded cells, cholesterol efflux to various HDL particles in ABCG1-null BMM was similar to that in WT BMM (Fig. 4C), indicating that ABCG1 did not play a significant role in cholesterol efflux in the absence of lipid loading. Cholesterol efflux to HDL on day 5 was also compared to efflux on day 3 and day 7 (Supplemental Fig. 5A). In line with the expression level of SR-BI in BMM during differentiation, maximal SR-BI-dependent efflux was seen in day 5 BMM, although the correlation between SR-BI expression and efflux was relatively weak. One possible explanation for this might be differences in the intracellular distribution of SR-BI at the different days in culture. No ABCG1- dependent cholesterol efflux to HDL was observed in non lipid-loaded cells on any of the indicated days in culture (Supplemental Fig. 5B).
Fig. 4.
Role of SR-BI in cholesterol efflux.
BMM from SR-BI WT, SR-BI-null, ABCG1 WT, and ABCG1-null mice were radiolabeled at day 3 for 48 h with [3H]cholesterol (0.2μCi/mL) in the absence (A, C) or presence (B, D) of 100 μg/mL acLDL. Cells were then incubated with mouse HDL (40μg/mL), human HDL2b (40μg/mL), human HDL3 (40μg/mL) or apoAI (10μg/mL) for 5 h to elicit removal of cellular cholesterol. Free cholesterol efflux was calculated as [3H]cholesterol in medium/[3H]cholesterol in medium plus cells. Values shown were the mean and S.D of triplicate determinations. The figure is representative of two independent experiments.
In lipid-loaded cells, SR-BI did not play a significant role in cholesterol efflux as indicated by similar efflux values to various HDLs from BMM with or without SR-BI (Fig. 4B). In contrast, cholesterol efflux to apoA-I in lipid-loaded cells was dramatically increased compared to non lipid-loaded cells (Fig. 4B, D), suggesting a marked increase in efflux by ABCA1, recognized as the major contributor to apoA-I-mediated cholesterol efflux in macrophages and known to be up-regulated by lipid loading. After lipid loading, cholesterol efflux to mouse HDL, HDL2b or HDL3 was significantly higher in WT BMM than that in BMM lacking ABCG1, indicating a significant contribution of this transporter to cellular cholesterol efflux (Fig. 4D). Unlike efflux facilitated by SR-BI, ABCG1 promoted cholesterol efflux to both large and small HDL particles. This data is consistent with previous study that ABCG1 facilitated cholesterol efflux to large and small rHDL particles 35.
To determine whether SR-BI-mediated cholesterol efflux results in a net efflux of cellular cholesterol mass, total cholesterol and free cholesterol levels in BMM were measured after incubation with HDL. HDL treatment for 8 h did not alter cellular cholesterol content, and there was no significant difference in cholesterol content between SR-BI WT and SR-BI-null BMM (Supplemental Fig. 6). These results suggest that SR-BI-induced cholesterol efflux to HDL does not produce a net change in cholesterol mass in macrophages under these conditions.
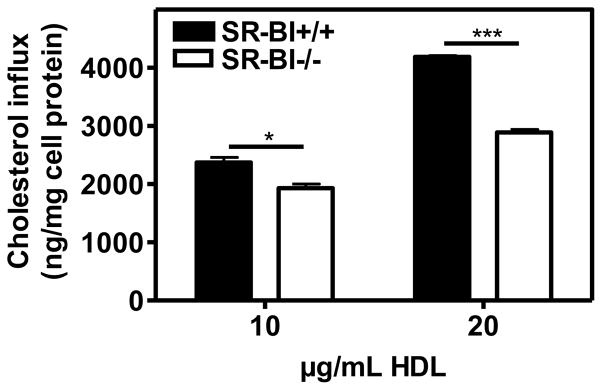
Since SR-BI mediated efflux is part of a bidirectional process, we investigated HDL cholesterol influx into BMM. The role of SR-BI in cholesterol influx was examined by incubating BMM with [3H]cholesterol-labeled HDL for 3 h (Fig. 5). HDL induced significantly higher cholesterol influx to SR-BI WT BMM than to SR-BI-null BMM, indicating that SR-BI expression enhances cholesterol influx. SR-BI is known to mediate bidirectional flux in transfected COS-7 cells through both a low- and high-affinity component 21; we therefore measured [3H]cholesterol flux between cells and HDL at concentrations up to 500 μg/mL HDL. We observed significantly more influx (Supplemental Fig. 7A) and efflux (Supplemental Fig. 7B) in SR-BI WT than SR-BI-null BMM, especially at higher concentrations of HDL. Interestingly, the quantity and the concentration-dependence of influx and efflux were comparable, consistent with a bidirectional process.
Fig. 5.
Function of SR-BI in cholesterol influx.
BMM cells from SR-BI WT and SR-BI-null mice were incubated on day 5 with 10 or 20 μg/mL [3H]cholesterol-labeled HDL2b for 3 h at 37°C. Cholesterol uptake was determined. Cholesterol influx was expressed as cell-associated ng cholesterol per mg cell protein. Values shown were the mean and S.D of triplicate determinations.
4. Discussion
The major finding of the present study is that SR-BI in cultured bone marrow macrophages plays a significant role in facilitating bidirectional cholesterol flux under non lipid-loaded conditions. We show for the first time that SR-BI is transiently induced during BMM differentiation in culture and although not active in promoting HDL CE selective uptake, does mediate bidirectional cholesterol flux between macrophages and HDL. Under these conditions, there were very low levels of ABCA1- or ABCG1-mediated efflux to either apoA-I or HDL. Our results suggest that SR-BI plays a significant role in macrophage cholesterol exchange, a function that may contribute to its effects on atherogenesis.
Our studies show that scavenger receptors SR-BI, CD36 and SRA and the transporters ABCA1 and ABCG1 are distinctly regulated during macrophage differentiation. SR-BI is up-regulated during BMM culture with maximal level expression at day 5. At this stage, CD36 and SRA are highly expressed, whereas the expression of ABCA1 and ABCG1 is relative low. These results indicate a potential role of SR-BI in maintaining macrophage cholesterol homeostasis in non lipid-loaded cells. Further, our results showed that the reduced SR-BI expression even at later stages of cell culture is highly reversible. This could be relevant in the case of macrophage foam cells that could eliminate their lipid load during atherosclerotic lesion regression 36. Under such regression conditions in vivo, SR-BI has been shown to be up-regulated in macrophage foam cells within atherosclerotic lesions 36 and therefore would be able to re-exert its function in such cells. On the other hand, lipid-loading of BMM with acLDL markedly decreased SR-BI expression. This is consistent with a previous study of human monocyte-derived macrophages and MPM that demonstrated that sterol loading using acLDL resulted in decreased SR-BI protein and mRNA levels 37. These results are in contrast with another report that modified lipoproteins such as oxidized LDL and acLDL induced a 5-fold increase in SR-BI mRNA and protein expression in cultured human monocyte-derived macrophages 16. The explanation for these contrasting results is unclear. As we observed, down-regulation of SR-BI by acLDL is associated with an increase in cellular total cholesterol, free cholesterol, and particularly esterified cholesterol. A previous study by Yu et al. indicated that such sterol-associated changes of SR-BI expression in macrophages are not regulated by LXR or through a SREBP dependent pathway 37. The mechanisms involved in SR-BI regulation in macrophages remain to be clarified.
SR-BI plays a key role in mediating selective uptake of HDL CE into liver cells and adrenal cells 3, 4. However, selective CE uptake in BMM was shown to be independent of SR-BI, as previously reported in MPM 38. We and others have reported that SR-BI expression at the cell surface can differ markedly in response to insulin in both hepatocytes 39 and adipocytes 40, raising the possibility that SR-BI was absent from or expressed at very low levels at the macrophage cell surface. However, our determination that SR-BI is localized to a significant extent on the cell surface of BMM indicates that this is not the case. The reason for the apparent inability of SR-BI to mediate selective lipid uptake in macrophages may be that macrophage SR-BI does not bind to HDL (Fig. 3B). Further work is needed to explain why in macrophages SR-BI is accessible for cell-surface biotinylation but does not enhance HDL binding.
The mechanism of HDL CE uptake in macrophages is also poorly understood. It has been proposed that LXR activation increases HDL3 CE uptake through the up-regulation of secreted proteins namely, apoE and LPL, that interact with proteoglycans, and this regulation requires membrane raft domain integrity and may involve caveolae 41. A potential candidate receptor for mediating HDL CE selective uptake is the GPIHBP1. GPIHBP1 has been reported to bind HDL with high affinity and to mediate selective HDL CE uptake in CHO cells expressing GPIHBP1 34. However, we have demonstrated that GPIHBP1 expression is not detectable in BMM or MPM, indicating that GPIHBP1 is probably not responsible for mediating the selective uptake of HDL CE in macrophages.
The fact that whole body SR-BI-null mice develop more arterial lesions than liver-specific SR-BI-null mice 15, suggests that expression of SR-BI in peripheral tissues may fulfill an atheroprotective function. This is supported by bone marrow transplantation experiments which indicate an athero-protective effect of SR-BI in macrophages 17, 18. To understand this phenomenon, we investigated the contribution of macrophage SR-BI in promoting cholesterol efflux. SR-BI was shown to play a significant role in HDL mediated cholesterol efflux in BMM cells not loaded with cholesterol. Our results also showed that under these conditions large HDL particles, such as mouse HDL and human HDL2b promote efflux from BMM more efficiently than that of smaller human HDL3. Moreover, SR-BI-dependent cholesterol efflux was evident to mouse HDL and human HDL2b, but not to HDL3. These findings are consistent with previous studies showing that SR-BI-mediated efflux was positively correlated with the phospholipid content of particles 30. Since SR-BI - mediated cholesterol efflux to various subfractions of HDL differs, the role of SR-BI in mediating cholesterol efflux could be under-estimated when using whole serum 20, 42 or heterogenous HDL 17 as cholesterol acceptors. This may explain in part the discrepancy among previous studies 17, 19, 20 regarding the role of SR-BI mediated cholesterol efflux in macrophages.
In macrophages not loaded with cholesterol very low levels of cholesterol efflux to apoA-I were observed, indicating that under such conditions ABCA1, which is generally recognized as the major contributor to apoA-I-mediated cholesterol efflux macrophages, does not play a significant role in cholesterol efflux. To assess the contributions of ABCG1 to cholesterol efflux, BMM from WT and ABCG1-null mice were studied. Under non lipid-loaded conditions, efflux to HDL acceptors was similar in WT and ABCG1-null cells, showing a lack of ABCG1 dependent cholesterol efflux. This is in line with the low levels of ABCG1 expression under these conditions. In contrast, under lipid-loaded conditions when ABCG1 is markedly up-regulated, cholesterol efflux to HDL is mediated predominantly by this receptor. This suggests that ABCG1 and SR-BI might contribute to cholesterol regulation at different stages of macrophage foam cell formation. Interestingly, SR-BI was previously shown to inhibit ABCG1-mediated efflux in a transfected cell system 43. This was proposed to be due to SR-BI mediating selective CE uptake from the acceptor particles. However, an inhibitory effect of SR-BI on ABCG1-mediated efflux was not evident in cholesterol-loaded macrophages. Our findings would also not support such a mechanism in macrophages since macrophage SR-BI does not appear to bind HDL or promote selective CE uptake.
Our studies demonstrate that although SR-BI stimulates cellular cholesterol efflux from macrophages, this is not accompanied by any detectable reduction in cellular cholesterol mass. This can be accounted for by a simultaneous increase in SR-BI-stimulated cholesterol influx from HDL, as shown in other cell types 21. The inability of SR-BI to promote net efflux of cholesterol from macrophages in vivo 42 is consistent with these findings. However, there is increasing evidence for a role of SR-BI in macrophage function. Specific deletion of macrophage SR-BI was shown to increase atherosclerosis in several studies 17–19. Most recently, the combined deletion of SR-BI and ABCA1 in macrophages was found to markedly increase tissue lipid accumulation and atherosclerosis compared to the single deletion of ABCA1 44. The mechanism(s) responsible for such effects are unclear but may be related to SR-BI presence in plasma membranes. The consequent stimulation of bidirectional cholesterol exchange might play a protective role in several ways. For example, increased flux might alter the lipid composition or organization of plasma membranes, especially lipid rafts. SR-BI-mediated flux might also promote the net efflux of minor lipids, such as oxidized sterols, which may contribute to foam cell formation. Alternatively, increased lipid flux or changes in membrane organization may regulate signaling by SR-BI or other membrane receptors. The differential roles of SR-BI, ABCG1 and ABCA1 in cholesterol flux in lipid loaded or non-loaded macrophages are summarized in Table 1.
Table 1.
Summary of differential roles of SR-BI, ABCG1 and ABCA1 in macrophage cholesterol flux
| Ligand | Basal BMM | Lipid loaded BMM | |||
|---|---|---|---|---|---|
| Influx | Efflux | Influx | Efflux | ||
| SR-BI | HDL | + | + | − | − |
| ABCG1 | HDL | − | − | − | + |
| ABCA1 | apoA-I | − | − | − | + |
In conclusion, in the absence of lipid loading, SR-BI contributes significantly to macrophage cholesterol bi-directional flux. Thus, SR-BI may play a significant role in macrophage cholesterol regulation and function, thereby contributing to its effects on atherosclerosis.
Supplementary Material
Acknowledgments
The authors would like to thank Xuebing Wang, Xin Shi, Kathy Forrest and Susan Bridges for excellent technical support. The ABCA1 antibody was generously provided by Dr Michael Hayden. This work was supported by a National Institutes of Health Program Project Grant (PO1HL086670) to D. R. van der Westhuyzen.
Abbreviations
- ABCA1
ATP-binding cassette transporter A1
- ABCG1
ATP-binding cassette transporter G1
- acLDL
acetylated low density lipoprotein
- apoA-I
apolipoprotein A-I
- BMM
bone marrow-derived macrophage
- CE
cholesteryl ester
- GPIHBP1
glycosylphosphatidylinositol anchored high density lipoprotein binding protein 1
- HDL
high density lipoprotein
- LCM
L-cell conditioned medium
- LDL
low density lipoprotein
- LPDS
lipoprotein-deficient serum
- MPM
mouse peritoneal macrophage
- SRA
class A scavenger receptor
- SR-BI
class B scavenger receptor type 1
Footnotes
Conflict of interest
There are no conflicts of interest or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ailing Ji, Email: ailing.ji@uky.edu.
Jason M. Meyer, Email: jmmeye3@uky.edu.
Lei Cai, Email: lcai8@uky.edu.
Akinwunmi Akinmusire, Email: asakin2@uky.edu.
Maria C. de Beer, Email: mdebeer@uky.edu.
Nancy R. Webb, Email: nrwebb1@uky.edu.
References
- 1.Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 2.Rigotti A, Miettinen HE, Krieger M. The role of the high-density lipoprotein receptor SR-BI in the lipid metabolism of endocrine and other tissues. Endocr Rev. 2003;24:357–387. doi: 10.1210/er.2001-0037. [DOI] [PubMed] [Google Scholar]
- 3.Cai L, Ji A, de Beer FC, Tannock LR, van der Westhuyzen DR. SR-BI protects against endotoxemia in mice through its roles in glucocorticoid production and hepatic clearance. J Clin Invest. 2008;118:364–375. doi: 10.1172/JCI31539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rigotti A, Trigatti BL, Penman M, Rayburn H, Herz J, Krieger M. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc Natl Acad Sci U S A. 1997;94:12610–12615. doi: 10.1073/pnas.94.23.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji Y, Jian B, Wang N, Sun Y, Moya ML, Phillips MC, Rothblat GH, Swaney JB, Tall AR. Scavenger receptor BI promotes high density lipoprotein-mediated cellular cholesterol efflux. J Biol Chem. 1997;272:20982–20985. doi: 10.1074/jbc.272.34.20982. [DOI] [PubMed] [Google Scholar]
- 6.Dole VS, Matuskova J, Vasile E, Yesilaltay A, Bergmeier W, Bernimoulin M, Wagner DD, Krieger M. Thrombocytopenia and platelet abnormalities in high-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:1111–1116. doi: 10.1161/ATVBAHA.108.162347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Eck M, Hoekstra M, Hildebrand RB, Yaong Y, Stengel D, Kruijt JK, Sattler W, Tietge UJ, Ninio E, Van Berkel TJ, Pratico D. Increased oxidative stress in scavenger receptor BI knockout mice with dysfunctional HDL. Arterioscler Thromb Vasc Biol. 2007;27:2413–2419. doi: 10.1161/ATVBAHA.107.145474. [DOI] [PubMed] [Google Scholar]
- 8.Yuhanna IS, Zhu Y, Cox BE, Hahner LD, Osborne-Lawrence S, Lu P, Marcel YL, Anderson RG, Mendelsohn ME, Hobbs HH, Shaul PW. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat Med. 2001;7:853–857. doi: 10.1038/89986. [DOI] [PubMed] [Google Scholar]
- 9.Li XA, Guo L, Dressman JL, Asmis R, Smart EJ. A novel ligand-independent apoptotic pathway induced by scavenger receptor class B, type I and suppressed by endothelial nitric-oxide synthase and high density lipoprotein. J Biol Chem. 2005;280:19087–19096. doi: 10.1074/jbc.M500944200. [DOI] [PubMed] [Google Scholar]
- 10.Varban ML, Rinninger F, Wang N, Fairchild-Huntress V, Dunmore JH, Fang Q, Gosselin ML, Dixon KL, Deeds JD, Acton SL, Tall AR, Huszar D. Targeted mutation reveals a central role for SR-BI in hepatic selective uptake of high density lipoprotein cholesterol. Proc Natl Acad Sci U S A. 1998;95:4619–4624. doi: 10.1073/pnas.95.8.4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Da Silva JR, Reilly M, Billheimer JT, Rothblat GH, Rader DJ. Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J Clin Invest. 2005;115:2870–2874. doi: 10.1172/JCI25327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun A, Trigatti BL, Post MJ, Sato K, Simons M, Edelberg JM, Rosenberg RD, Schrenzel M, Krieger M. Loss of SR-BI expression leads to the early onset of occlusive atherosclerotic coronary artery disease, spontaneous myocardial infarctions, severe cardiac dysfunction, and premature death in apolipoprotein E-deficient mice. Circ Res. 2002;90:270–276. doi: 10.1161/hh0302.104462. [DOI] [PubMed] [Google Scholar]
- 13.Huszar D, Varban ML, Rinninger F, Feeley R, Arai T, Fairchild-Huntress V, Donovan MJ, Tall AR. Increased LDL cholesterol and atherosclerosis in LDL receptor-deficient mice with attenuated expression of scavenger receptor B1. Arterioscler Thromb Vasc Biol. 2000;20:1068–1073. doi: 10.1161/01.atv.20.4.1068. [DOI] [PubMed] [Google Scholar]
- 14.Kozarsky KF, Donahee MH, Glick JM, Krieger M, Rader DJ. Gene transfer and hepatic overexpression of the HDL receptor SR-BI reduces atherosclerosis in the cholesterol-fed LDL receptor-deficient mouse. Arterioscler Thromb Vasc Biol. 2000;20:721–727. doi: 10.1161/01.atv.20.3.721. [DOI] [PubMed] [Google Scholar]
- 15.Huby T, Doucet C, Dachet C, Ouzilleau B, Ueda Y, Afzal V, Rubin E, Chapman MJ, Lesnik P. Knockdown expression and hepatic deficiency reveal an atheroprotective role for SR-BI in liver and peripheral tissues. J Clin Invest. 2006;116:2767–2776. doi: 10.1172/JCI26893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirano K, Yamashita S, Nakagawa Y, Ohya T, Matsuura F, Tsukamoto K, Okamoto Y, Matsuyama A, Matsumoto K, Miyagawa J, Matsuzawa Y. Expression of human scavenger receptor class B type I in cultured human monocyte-derived macrophages and atherosclerotic lesions. Circ Res. 1999;85:108–116. doi: 10.1161/01.res.85.1.108. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Yancey PG, Su YR, Babaev VR, Zhang Y, Fazio S, Linton MF. Inactivation of macrophage scavenger receptor class B type I promotes atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 2003;108:2258–2263. doi: 10.1161/01.CIR.0000093189.97429.9D. [DOI] [PubMed] [Google Scholar]
- 18.Covey SD, Krieger M, Wang W, Penman M, Trigatti BL. Scavenger receptor class B type I-mediated protection against atherosclerosis in LDL receptor-negative mice involves its expression in bone marrow-derived cells. Arterioscler Thromb Vasc Biol. 2003;23:1589–1594. doi: 10.1161/01.ATV.0000083343.19940.A0. [DOI] [PubMed] [Google Scholar]
- 19.Van Eck M, Bos IS, Hildebrand RB, Van Rij BT, Van Berkel TJ. Dual role for scavenger receptor class B, type I on bone marrow-derived cells in atherosclerotic lesion development. Am J Pathol. 2004;165:785–794. doi: 10.1016/S0002-9440(10)63341-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adorni MP, Zimetti F, Billheimer JT, Wang N, Rader DJ, Phillips MC, Rothblat GH. The roles of different pathways in the release of cholesterol from macrophages. J Lipid Res. 2007;48:2453–2462. doi: 10.1194/jlr.M700274-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.de la Llera-Moya M, Rothblat GH, Connelly MA, Kellner-Weibel G, Sakr SW, Phillips MC, Williams DL. Scavenger receptor BI (SR-BI) mediates free cholesterol flux independently of HDL tethering to the cell surface. J Lipid Res. 1999;40:575–580. [PubMed] [Google Scholar]
- 22.Yona S, Heinsbroek SE, Peiser L, Gordon S, Perretti M, Flower RJ. Impaired phagocytic mechanism in annexin 1 null macrophages. Br J Pharmacol. 2006;148:469–477. doi: 10.1038/sj.bjp.0706730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Villiers WJ, Cai L, Webb NR, de Beer MC, van der Westhuyzen DR, de Beer FC. CD36 does not play a direct role in HDL or LDL metabolism. J Lipid Res. 2001;42:1231–1238. [PubMed] [Google Scholar]
- 24.Carr TP, Andresen CJ, Rudel LL. Enzymatic determination of triglyceride free cholesterol, and total cholesterol in tissue lipid extracts. Clin Biochem. 1993;26:39–42. doi: 10.1016/0009-9120(93)90015-x. [DOI] [PubMed] [Google Scholar]
- 25.Eckhardt ER, Cai L, Sun B, Webb NR, van der Westhuyzen DR. High density lipoprotein uptake by scavenger receptor SR-BII. J Biol Chem. 2004;279:14372–14381. doi: 10.1074/jbc.M313793200. [DOI] [PubMed] [Google Scholar]
- 26.Coetzee GA, Strachan AF, van der Westhuyzen DR, Hoppe HC, Jeenah MS, de Beer FC. Serum amyloid A-containing human high density lipoprotein 3. Density, size, and apolipoprotein composition. J Biol Chem. 1986;261:9644–9651. [PubMed] [Google Scholar]
- 27.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 28.Traenkel-Conrat H. Methods for investigating the essential groups for enzymatic activity. Methods Enzymol. 1957;4:247–269. [Google Scholar]
- 29.van der Westhuyzen DR, Cai L, de Beer MC, de Beer FC. Serum amyloid A promotes cholesterol efflux mediated by scavenger receptor B-I. J Biol Chem. 2005;280:35890–35895. doi: 10.1074/jbc.M505685200. [DOI] [PubMed] [Google Scholar]
- 30.Yancey PG, de la Llera-Moya M, Swarnakar S, Monzo P, Klein SM, Connelly MA, Johnson WJ, Williams DL, Rothblat GH. High density lipoprotein phospholipid composition is a major determinant of the bi-directional flux and net movement of cellular free cholesterol mediated by scavenger receptor BI. J Biol Chem. 2000;275:36596–36604. doi: 10.1074/jbc.M006924200. [DOI] [PubMed] [Google Scholar]
- 31.Hirsch S, Austyn JM, Gordon S. Expression of the macrophage-specific antigen F4/80 during differentiation of mouse bone marrow cells in culture. J Exp Med. 1981;154:713–725. doi: 10.1084/jem.154.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray PJ, Wynn TA. Obstacles and opportunities for understanding macrophage polarization. J Leukoc Biol. 2011 doi: 10.1189/jlb.0710409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venkateswaran A, Laffitte BA, Joseph SB, Mak PA, Wilpitz DC, Edwards PA, Tontonoz P. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc Natl Acad Sci U S A. 2000;97:12097–12102. doi: 10.1073/pnas.200367697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ioka RX, Kang MJ, Kamiyama S, Kim DH, Magoori K, Kamataki A, Ito Y, Takei YA, Sasaki M, Suzuki T, Sasano H, Takahashi S, Sakai J, Fujino T, Yamamoto TT. Expression cloning and characterization of a novel glycosylphosphatidylinositol-anchored high density lipoprotein-binding protein, GPI-HBP1. J Biol Chem. 2003;278:7344–7349. doi: 10.1074/jbc.M211932200. [DOI] [PubMed] [Google Scholar]
- 35.Favari E, Calabresi L, Adorni MP, Jessup W, Simonelli S, Franceschini G, Bernini F. Small discoidal pre-beta1 HDL particles are efficient acceptors of cell cholesterol via ABCA1 and ABCG1. Biochemistry. 2009;48:11067–11074. doi: 10.1021/bi901564g. [DOI] [PubMed] [Google Scholar]
- 36.Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, Randolph GJ, Fisher EA. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc Natl Acad Sci U S A. 2006;103:3781–3786. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu L, Cao G, Repa J, Stangl H. Sterol regulation of scavenger receptor class B type I in macrophages. J Lipid Res. 2004;45:889–899. doi: 10.1194/jlr.M300461-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Brundert M, Heeren J, Bahar-Bayansar M, Ewert A, Moore KJ, Rinninger F. Selective uptake of HDL cholesteryl esters and cholesterol efflux from mouse peritoneal macrophages independent of SR-BI. J Lipid Res. 2006;47:2408–2421. doi: 10.1194/jlr.M600136-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Shetty S, Eckhardt ER, Post SR, van der Westhuyzen DR. Phosphatidylinositol-3-kinase regulates scavenger receptor class B type I subcellular localization and selective lipid uptake in hepatocytes. Arterioscler Thromb Vasc Biol. 2006;26:2125–2131. doi: 10.1161/01.ATV.0000233335.26362.37. [DOI] [PubMed] [Google Scholar]
- 40.Yvan-Charvet L, Bobard A, Bossard P, Massiera F, Rousset X, Ailhaud G, Teboul M, Ferre P, Dagher G, Quignard-Boulange A. In vivo evidence for a role of adipose tissue SR-BI in the nutritional and hormonal regulation of adiposity and cholesterol homeostasis. Arterioscler Thromb Vasc Biol. 2007;27:1340–1345. doi: 10.1161/ATVBAHA.106.136382. [DOI] [PubMed] [Google Scholar]
- 41.Bultel S, Helin L, Clavey V, Chinetti-Gbaguidi G, Rigamonti E, Colin M, Fruchart JC, Staels B, Lestavel S. Liver X receptor activation induces the uptake of cholesteryl esters from high density lipoproteins in primary human macrophages. Arterioscler Thromb Vasc Biol. 2008;28:2288–2295. doi: 10.1161/ATVBAHA.108.175042. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, Rothblat GH, Tall AR, Rader DJ. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest. 2007;117:2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yvan-Charvet L, Pagler TA, Wang N, Senokuchi T, Brundert M, Li H, Rinninger F, Tall AR. SR-BI inhibits ABCG1-stimulated net cholesterol efflux from cells to plasma HDL. J Lipid Res. 2008;49:107–114. doi: 10.1194/jlr.M700200-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Zhao Y, Pennings M, Hildebrand RB, Ye D, Calpe-Berdiel L, Out R, Kjerrulf M, Hurt-Camejo E, Groen AK, Hoekstra M, Jessup W, Chimini G, Van Berkel TJ, Van Eck M. Enhanced Foam Cell Formation, Atherosclerotic Lesion Development, and Inflammation by Combined Deletion of ABCA1 and SR-BI in Bone Marrow-Derived Cells in LDL Receptor Knockout Mice on Western-Type Diet. Circ Res. 2010 doi: 10.1161/CIRCRESAHA.110.226282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.