INTRODUCTION
Regenerative dentistry holds promise for the restoration of dental tissues damaged by caries, trauma, or clinical errors. Regenerative endodontic procedures can be defined as biologically based procedures designed to replace damaged structures, including dentin and root structures, as well as cells of the pulp-dentin complex. These regenerative endodontic techniques are based on the basic tissue engineering principles already described in literature and include a triad of cells, growth factors, and scaffolds (1–3). All three components are necessary for appropriate tissue regeneration.
One of the most detrimental iatrogenic mishaps that can occur during endodontic treatment is a perforation. The materials currently available for perforation repair are less than ideal. Mineral trioxide aggregate (MTA) is the current material of choice for perforation repair and has demonstrated good potential for clinical success (4–6). However, MTA does not degrade to allow for replacement with natural tissues. Published reports have demonstrated that dental pulp stem cells (DPSCs) can differentiate to odontoblast-like cells capable of secreting a dentin matrix at the site of injury (7). When DPSCs were transplanted into immuno-compromised mice, they generated a dentin-like structure lined with human odontoblast-like cells that surrounded a pulp-like interstitial tissue (8).
Dentin matrix protein 1 (DMP1) is a noncollagenous extracellular matrix protein that has been implicated to have a regulatory function during mineralized matrix formation (9–13). It is a key regulatory protein that is required for normal growth and development of bone, cartilage and dentin. DMP1 has been shown to function as a signaling molecule that can transform pulp stem cells to differentiate into odontoblast-like cells (13,14). The aim of this study was to utilize biomimetic based regenerative techniques and materials to repair simulated perforations. Our hypothesis was that DPSCs impregnated within a collagen scaffold can differentiate into odontoblastoid cells (or odontoblast-like cells) that secrete the matrix for reparative dentin bridge in a simulated perforation site, in the presence of DMP1.
MATERIALS AND METHODS
Preparation of Dentin wafers
Eighteen, freshly extracted human molar teeth were cleaned with 5.25% NaOCl then decoronated using a grinding disc (#6918B; Brasseler USA, Savannah, GA). Any excess coronal pulp tissue was removed using NaOCl and a pair of cotton forceps. The teeth were then shaved down to roughly the level of the floor of the pulp chamber, both apically and coronally, to create a 2.5mm thick dentin wafer. Utilizing a #557 bur, one perforation was made in each tooth through the entire thickness of the pulpal floor, at an area approximately over the center of the furcation. The perforation was as wide as the #557 bur (1.0mm). After the wafers were autoclaved, they were randomly divided into 6 groups of 3 samples in each and stored in sterile packaging until ready for use.
Expression of Recombinant DMP1
DMP1 was expressed as a recombinant protein in E.coli according to a published protocol (15).
Implantation in immuno-compromised mice
Eighteen immuno-compromised mice (Nu-Nu strain; Charles River Laboratory, Wilmington, MA) were anesthetized using 80–100 mg/kg Ketamine and 10 mg/kg Xylazine. DPSCs were obtained from Dr. Songtao Shi at the University of Southern California. DPSCs were cultured in DMEM/F12 (Cellgro; Mediatech Inc., Manassas, VA) with 10% FBS (Cellgro) and 1% antibiotic/antimycotic solution (Invitrogen, Carlsbad, CA). Collagraft Bone Graft Matrix Strip (Zimmer, Warsaw, IN) was used as the collagen scaffold. The scaffold was impregnated with DMP1, DPSCs or both as published previously (16). One dentin wafer was placed subcutaneously in the dorsal subcutaneous layer of each mouse. All procedures were performed in accordance with specifications of an approved small animal protocol (University of Illinois at Chicago, ACC Protocol No. 06-039). The groups were divided as follows:
Empty
Grey MTA (Pro Root®, Dentsply, Tulsa Dental OK, USA) (MTA)
Collagen Scaffold Alone (SC)
Collagen Scaffold Impregnated with DMP1 (SC+DMP1)
Collagen Scaffold Impregnated with DPSCs (SC+DPSC)
Collagen Scaffold Impregnated with DMP1 and DPSCs (SC+ DMP1+DPSC)
The mice were sacrificed after 12 weeks; samples were removed and immediately stored in 10% formalin.
Faxitron imaging
Fixed samples were subjected to imaging under x-rays to look at the degree of radio opacity. Imaging was performed by a noninvasive high-magnification X-ray analysis to characterize the mineralized matrix using a Faxitron model MX-20 digital radiography machine (Faxitron Corporation, Lincolnshire, IL USA). The imaging conditions were maintained the same for all samples. The radiographic densities of the matrix formed within the perforations under different experiments were calculated from the images using Image J software. One image from each of the triplicate experiments was quantified as percentage opacity with respect to the opacity of the surrounding dentin and the data represented as mean percentage opacity +/− s.e.m for each experimental group. Statistical significance was calculated with respect to the control group (EMPTY) using students t-test.
Histological analysis
After fixation, the dentin wafers were prepared for histological examination. Briefly, the wafers were demineralized in EDTA, embedded in paraffin and 5μm sections were obtained. The sections were stained with hematoxylin and eosin (H&E) and Masson's trichrome stains. Analysis was done using Axio Vision 4.6 computer software and pictures were taken using an AxioCam MRc by Zeiss®. One representative slide from each of the triplicate experiments stained with Massons's trichrome stain was imaged under 5 × magnification. Under this magnification, the entire perforation area could be viewed. The area of the perforation and the area covered by neo-vascularization were then measured for each of the representative slides and percentage of vascularized area was calculated. The data is represented as mean percent vascularized area for each experimental condition +/− s.e.m. Statistical significance was calculated with respect to the control group (EMPTY) using students t-test.
Immunohistochemical Analysis
Immunostaining was performed using anti-dentin sialoprotein (DSP) antibody according to published methods (17,18). After staining, the slides were mounted using mounting media containing propidium iodide (Vector Labs) and imaged using a Zeiss® Axio Observer D1 microscope. When required fluorescence micrographs were obtained using the appropriate filter sets in the Zeiss Axio Observer D1 microscope equipped with Axiovision imaging software.
RESULTS
Evaluation of Mineralized matrix formation using Faxitron imaging
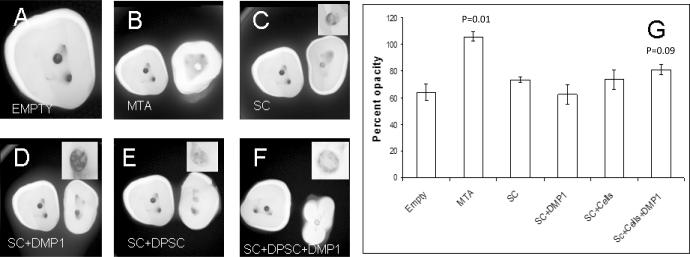
High-resolution digital radiography was used to observe radiodensity of the newly formed tissue with respect to the surrounding dentin. Figures 1A to 1F show comparative images of the different groups. Figures 1B to 1F contain the control sample group corresponding to an empty implant placed alongside (to the left) for comparison with the rest of the groups as indicated. As can be seen from the images, the control group showed the least radio opacity. MTA showed the highest opacity followed by the scaffold comprised of DPSCs and DMP1. Although the group with the triad of scaffold, DPSCs, and DMP1 demonstrated the highest radio opacity of all experimental groups (except MTA), the difference between treatment groups was not statistically significant. The quantification provided in Figure 1G shows the mean opacity for each of the experimental groups.
Figure 1. High resolution digital radiography of de-novo formed tissue after 12 weeks of implantation.
A–F represents faxitron images of the different groups as labeled. B–F contain the control sample (A) to the left of the experimental sample. (G) Is the quantitation of the radioopacity of 3 representative images (one from each repeated experimental group) and represents mean percentage opacity of the perforation with respect to the surrounding dentin. Data represented as mean +/− s.e.m. p represents the p-value obtained from students t-test. SC represents collagen scaffold
Histological Evaluation to demonstrate synthesis of connective tissue matrix by differentiating DPSCs
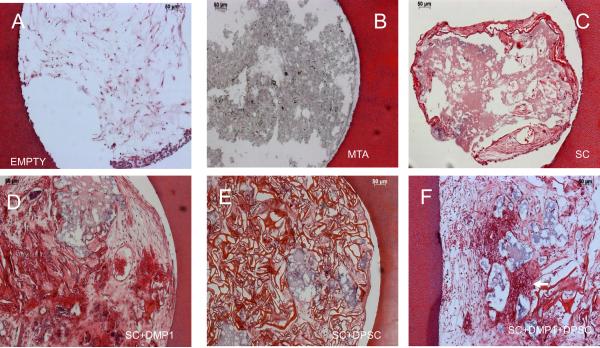
Figures 2 and 3 show representative images of the two types of histological stains used to determine cellular architecture and connective tissue formation. Images in Figs 2A to 2F represent the six groups used depicting the cellular architecture by H&E staining. As can be seen from Figure 2A, the empty scaffold was populated by native mouse tissue and showed loose connective tissue architecture with some cells that are most probably fibroblasts. As expected MTA (Figure 2B) remained stable and did not permit invasion of cells, formation of a matrix or neovascularization. The perforation filled with empty collagen scaffold showed signs of degradation (Figure 2C). However, it had permitted invasion of cells from the surrounding vicinity. Scaffold impregnated with DMP1 showed degradation to a lesser extent. Migratory cells from the surrounding tissue populated the scaffold. The cellular nature of the new tissue was prominent in group 2D which contained DMP1, with the presence of numerous blood vessels (arrow in Figure 2D). Scaffold seeded with DPSC alone (Figure 2E) also showed degradation to a lesser extent, but did not contain as many neo-vascular networks as the group represented in Figure 2D. The most striking observation was in the tissue regenerated with the triad comprising DMP1, DPSCs and the collagen scaffold. The tissue contained abundant cells which might implicate the proliferative capacity of DPSCs and the presence of neovascularization could be attributed to the pro-angiogenic activity of DMP1.
Figure 2. Histological staining by H&E to evaluate cellular architecture.
Images A–F represents the different experimental groups as labeled. The arrows in the images point to blood vessels. Note the highly cellular architecture in groups E & F.
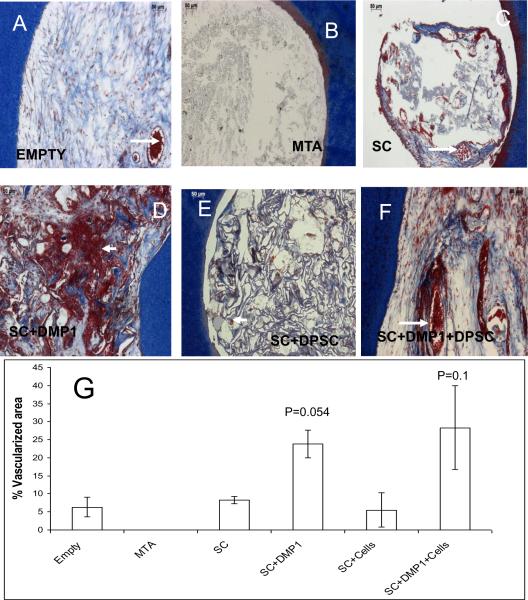
Figure 3. Histological staining by Masson's trichrome to evaluate connective tissue deposition and neovascularization.
Images A–F represents the different experimental groups as labeled. Arrows point to red blood corpuscles indicating presence of blood vessels. (G) Is a graphical representation of the percentage of perforation area covered by the presence of blood vessels. Data represented as mean +/− s.e.m. p represents the p-value obtained from students t-test.
Panel in Figure 3A–F shows Masson's trichrome staining of serial sections from the various groups. The pattern of cellular architecture remains the same as observed with H&E staining. This stain enabled easy visualization of blood vessels that were characterized by the presence of red blood corpuscles (arrows). Lower magnification images (not shown) of the stained sections were used to quantify the extent of neovascularization. Figure 3G shows the result of the quantification of the number of neovascularized regions in the newly regenerated tissue.
Immunohistochemical analysis to demonstrate differentiation of DPSCs to odontoblast-like cells
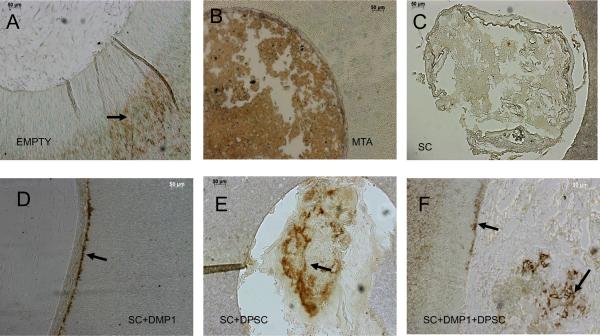
DSP is a marker for differentiation of mesenchymal cells to odontoblast-like cells. DSP immuno-staining was performed to asses the differentiation state of the cells in the scaffold. As expected the cells or the matrix filling the empty perforation did not show any positive staining for DSP (Figure 4A). As the samples were de-mineralized prior to embedding, the collagen dentin matrix is exposed and positive DSP staining can be observed in the dentin matrix (arrow in Figure 4A). Positive staining was observed in the MTA filled perforation (Figure 4B). However, as can be seen from the nature of the staining observed, it is non-specific binding of the reagent to the MTA compound. Scaffolds containing DMP1 did not show positive staining within the matrix. However, the cells that were adjacent to the dentin matrix had secreted DSP into the dentin matrix. This was observed as a ring of positive staining in the innermost region of the dentin matrix (arrow in Figure 4D). The ECM of the scaffold was thicker close to the periphery and the presence of cells was verified by fluorescent nuclear staining. Scaffold containing DPSCs showed the presence of positive staining within the scaffold (arrow in Figure 4E), but lacked the staining in the dentin matrix. Finally, scaffold comprised of both DPSC and DMP1 showed the presence of positive DSP staining both inside the scaffold (from the differentiated cells within) and also in the periphery (Figure 4F).
Figure 4. Immunohistochemical analysis using anti-DSP antibody to identify odontoblast-like cells.
Images A–F represents the different experimental groups as labeled. Arrows point to positive DSP staining. Note the presence of DSP positive cells in group F.
DISCUSSION
The ideal form of root canal therapy would be to regenerate missing or damaged tissues. A current research trend is the exploration of various methods to regenerate dentin, periodontal, and pulpal tissues instead of using repair materials that do not degrade to allow natural tissue formation. These potential approaches include root-canal revascularization, postnatal (adult) stem cell therapy, pulp implant, scaffold implant, three-dimensional cell printing, injectable scaffolds, and gene therapy.
Bone Morphogenetic Proteins (BMPs) have been shown to induce the differentiation of pulp stem cells into odontoblast-like cells (19). Published studies demonstrate that ADAM 28 and Heme Oxygenase-1 function as signaling molecules and facilitate the proliferation and odontoblastic differentiation of human dental pulp stem cells while Wnt6 promotes differentiation without significant effects on proliferation (20–22). In this study we have used DMP1 as a signaling molecule as it is highly expressed in pulp stem cells. Several studies have shown that DMP1 can cause pulp stem cells to differentiate into odontoblast-like cells (9, 13, 14). Mineral deposition was increased 10-fold in the presence of DMP1 (23, 24). The model that we used consisted of cavity preparation in a tooth slice organ culture model that has been previously published (25,26). In the current study, the triad containing DPSCs and DMP1 showed high radiodensity in the newly formed tissue, implying mineralized matrix formation.
In order to ascertain that the DPSCs had differentiated into functional odontoblast-like cells, dentin sialoprotein was used as the marker of choice. DSP is uniquely expressed by differentiating and fully differentiated mature odontoblasts (16). It is likely that DSP actively participates in the conversion of predentin to dentin (27–29) thus it was used in this study for the purpose of identification of functional odontoblasts. In group one, no staining was observed indicating absence of dentin formation. In group two, MTA was the only material visible in all samples. Immunohistochemical evaluation revealed the presence of non-specific binding of DSP antibody to aggregates of MTA. This could be attributed to the lack of odontoblast like cells at the implantation site; furthermore, MTA is not capable of differentiating ectopic mesenchymal cells to odontoblasts. However, MTA has been used successfully for partial pulpotomies on immature permanent teeth (30,31). When the infected coronal part of the pulp in those teeth is removed, stem cells from the apical papilla (SCAP) residing in the apical papilla give rise to primary odontoblasts to complete the root formation (32). Without vital pulp tissue, HERS will not signal the pulp stem cells to differentiate into odontoblasts or odontoblast-like cells. Interestingly, groups with DMP1 impregnated on collagen scaffold showed strong DSP signals. The pattern of localization was at the periphery of newly regenerated dentin. The possible origin of the mesenchymal cells in this group is currently unknown. One possibility that we envisage is that DMP1 acted as a chemo-attractant for stem cells in the surrounding vasculature. In the triad group strong DSP signals were observed in de-novo differentiated odontoblasts inside the scaffold and also at the periphery. This implies that DMP1 functions as a morphogen in the transformation of DPSCs to odontoblast-like cells during dentin regeneration.
Histological evaluation using H&E and Trichrome Mason were particularly interesting. Groups containing DMP1 and DPSCs showed high cellular activity, particularly the triad containing DMP1, DPSC and the scaffold and the group containing DMP1 and scaffold were able to promote angiogenesis (also see supplementary Figure 3). Angiogenesis is an important feature necessary for tissue regeneration. This allows growth factors and nutrients to reach the injured site. In our earlier published study characterized after 6 weeks of implantation the triad of DPSCs, collagen scaffold, and DMP1 induced formation of an organized vascular matrix similar to that of pulpal tissue (16). Currently, the signaling mechanism by which DMP1 mediates stem cell differentiation into odontoblast-like cells is unclear. Further research in this area is required.
Together, the results from this study demonstrated that DPSCs impregnated within a collagen scaffold in the presence of DMP1 can differentiate into odontoblast-like cells capable of secreting a highly cellular and vascularized collagenous matrix (Figure 3). This initial extracellular matrix is required for mineralized matrix formation. During dentin formation the collagenous predentin matrix is first formed before the deposition of calcified particles. Thus, we conclude that endodontic perforations could be repaired using a triad consisting of DPSC cells, DMP1 signaling molecule, delivered to the perforation site using a collagen scaffold.
Supplementary Material
A and B represent identical images shown in Figure 4D and 4F overlaid with a fluorescent image with propidium iodide stained nuclei showing the presence of cells at the interface between the scaffold and the dentin matrix (A and B) and within the scaffold (B).
A and B represent Masson's Trichrome staining of the sections from the labeled groups indicating the presence of blood vessels. Note the presence of numerous red blood corpuscles.
ACKNOWLEDGEMENTS
This research was supported in part by a Research Grant from the American Association of Endodontists Foundation and NIH grant DE 11657. We would like to thank Dr. Michael Albazzaz, Dr. Rebecca Prescott and Dr. Lora Chow for their help in conducting this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors deny any conflicts of interest
REFERENCES
- 1.Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: a review of current status and a call for action. J Endod. 2007;33:377–90. doi: 10.1016/j.joen.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Huang GT, Sonoyama W, Liu Y, Liu H, Wang S, Shi S. The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod. 2008;34:645–51. doi: 10.1016/j.joen.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hargreaves KM, Geisler T, Henry M, Wang Y. Regeneration potential of the young permanent tooth: what does the future hold? J Endod. 2008;34(7 Suppl):S51–6. doi: 10.1016/j.joen.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 4.Main C, Mirzayan N, Shabahang S, Torabinejad M. Repair of root perforations using mineral trioxide aggregate: a long-term study. J Endod. 2004;30:80–3. doi: 10.1097/00004770-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999;25:197–05. doi: 10.1016/S0099-2399(99)80142-3. [DOI] [PubMed] [Google Scholar]
- 6.Mente J, Hage N, Pfefferle T, Koch MJ, Geletneky B, Dreyhaupt J, Martin N, Staehle HJ. Treatment Outcome of Mineral Trioxide Aggregate: Repair of Root Perforations. J Endod. 2010;36:208–13. doi: 10.1016/j.joen.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Kitasako Y, Shibata S, Pereira PN, Tagami J. Short-term dentin bridging of mechanically-exposed pulps capped with adhesive resin systems. Oper Dent. 2000;25:155–62. [PubMed] [Google Scholar]
- 8.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–30. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narayanan K, Srinivas R, Ramachandran A, Hao J, Quinn B, George A. Differentiation of embryonic mesenchymal cells to odontoblast-like cells by overexpression of dentin matrix protein 1. Proc Natl Acad Sci U S A. 2001;98:4516–21. doi: 10.1073/pnas.081075198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He G, Dahl T, Veis A, George A. Nucleation of apatite crystals in vitro by self-assembled dentin matrix protein 1. Nat Mater. 2003;2:552–58. doi: 10.1038/nmat945. [DOI] [PubMed] [Google Scholar]
- 11.Gajjeraman S, Narayanan K, Hao J, Qin C, George A. Matrix macromolecules in hard tissues control the nucleation and hierarchical assembly of hydroxyapatite. J Biol Chem. 2007;282:1193–04. doi: 10.1074/jbc.M604732200. [DOI] [PubMed] [Google Scholar]
- 12.Massa LF, Ramachandran A, George A, Arana-Chavez VE. Developmental appearance of dentin matrix protein 1 during the early dentinogenesis in rat molars as identified by high-resolution immunocytochemistry. Histochem Cell Biol. 2005;124:197–05. doi: 10.1007/s00418-005-0009-9. [DOI] [PubMed] [Google Scholar]
- 13.Ye L, MacDougall M, Zhang S, Xie Y, Zhang J, Li Z, et al. Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. J Biol Chem. 2004;279:19141–48. doi: 10.1074/jbc.M400490200. [DOI] [PubMed] [Google Scholar]
- 14.Narayanan K, Ramachandran R, Jianjun H, He G, Park KW, Cho M, George A. Dual functional roles of DMP1. J Biol Chem. 2003;278:17500–08. doi: 10.1074/jbc.M212700200. [DOI] [PubMed] [Google Scholar]
- 15.Srinivasan R, Chen B, Gorski JP, George A. Recombinant expression and characterization of dentin matrix protein 1. Connect Tissue Res. 1999;40:251–58. doi: 10.3109/03008209909000703. [DOI] [PubMed] [Google Scholar]
- 16.Prescott RS, Alsanea R, Fayad MI, Johnson BR, Wenckus CS, Hao J, et al. In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, and dentin matrix protein 1 after subcutaneous transplantation in mice. J Endod. 2008;34:421–26. doi: 10.1016/j.joen.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hao J, Zou B, Narayanan K, George A. Differential expression patterns of the dentin matrix proteins during mineralized tissue formation. Bone. 2004;34:921–32. doi: 10.1016/j.bone.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 18.Hao J, Narayanan K, Ramachandran A, He G, Almushayt A, Evans C, et al. Odontoblast cells immortalized by telomerase produce mineralized dentin-like tissue both in vitro and in vivo. J Biol Chem. 2002;277:19976–81. doi: 10.1074/jbc.M112223200. [DOI] [PubMed] [Google Scholar]
- 19.Iohara K, Nakashima M, Ito M, Ishikawa M, Nakasima A, Akamine A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J Dent Res. 2004;83:590–95. doi: 10.1177/154405910408300802. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Z, Liu H, Wang D. ADAM28 manipulates proliferation, differentiation, and apoptosis of human dental pulp stem cells. J Endod. 2011;37:332–9. doi: 10.1016/j.joen.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 21.Wang C, Ren L, Peng L, Xu P, Dong G, Ye L. Effect of Wnt6 on Human Dental Papilla Cells In Vitro. J Endod. 2010;36:238–43. doi: 10.1016/j.joen.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Kim S-J, Min K-S, Ryu H-W, Lee H-J, Kim E-C. The Role of Heme Oxygenase-1 in the Proliferation and Odontoblastic Differentiation of Human Dental Pulp Cells. J Endod. 2010;36:1326–31. doi: 10.1016/j.joen.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 23.He G, Dahl T, Veis A, George A. Dentin matrix protein 1 initiates hydroxyapatite formation in vitro. Connect Tissue Res. 2003;44:240–45. [PubMed] [Google Scholar]
- 24.He G, Dahl T, Veis A, George A. Nucleation of apatite crystals in vitro by self-assembled dentin matrix protein 1. Nat Mater. 2003;8:552–58. doi: 10.1038/nmat945. [DOI] [PubMed] [Google Scholar]
- 25.Murray PE, About I, Lumley PJ, Smith G, Franquin JC, Smith AJ. Postoperative pulpal and repair responses. J Am Dent Assoc. 2000;131:321–29. doi: 10.14219/jada.archive.2000.0175. [DOI] [PubMed] [Google Scholar]
- 26.Murray PE, Lumley PJ, Ross HF, Smith AJ. Tooth slice organ culture for cytotoxicity assessment of dental materials. Biomaterials. 2000;21:1711–21. doi: 10.1016/s0142-9612(00)00056-9. [DOI] [PubMed] [Google Scholar]
- 27.Linde A, Goldberg M. Dentinogenesis. Crit Rev Oral Biol Med. 1993;4:679–728. doi: 10.1177/10454411930040050301. [DOI] [PubMed] [Google Scholar]
- 28.Begue-Kirn C, Ruch JV, Ridall AL, Butler WT. Comparative analysis of mouse DSP and DPP expression in odontoblasts, preameloblasts, and experimentally induced odontoblast-like cells. Eur J Oral Sci. 1998;106:254–59. doi: 10.1111/j.1600-0722.1998.tb02184.x. [DOI] [PubMed] [Google Scholar]
- 29.Andelin WE, Shabahang S, Wright K, Torabinejad M. Identification of hard tissue after experimental pulp capping using dentin sialoprotein (DSP) as a marker. J Endod. 2003;29:646–50. doi: 10.1097/00004770-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 30.El-Meligy OA, Avery DR. Comparison of mineral trioxide aggregate and calcium hydroxide as pulpotomy agents in young permanent teeth (apexogenesis) Pediatr Dent. 2006;28:399–04. [PubMed] [Google Scholar]
- 31.Barrieshi-Nusair KM, Qudeimat MA. A prospective clinical study of mineral trioxide aggregate for partial pulpotomy in cariously exposed permanent teeth. J Endod. 2006;32:731–35. doi: 10.1016/j.joen.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, et al. Characterization of the Apical Papilla and Its Residing Stem Cells from Human Immature Permanent Teeth: A Pilot Study. J Endod. 2008;34:166–71. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A and B represent identical images shown in Figure 4D and 4F overlaid with a fluorescent image with propidium iodide stained nuclei showing the presence of cells at the interface between the scaffold and the dentin matrix (A and B) and within the scaffold (B).
A and B represent Masson's Trichrome staining of the sections from the labeled groups indicating the presence of blood vessels. Note the presence of numerous red blood corpuscles.