Abstract
Background
The liver is a major organ that is susceptible to injury following blunt and/or penetrating trauma to the abdomen. No specific non-operative treatment exists for traumatic hepatic injury (THI). Adrenomedullin (AM), a vasoactive peptide, combined with its binding protein (AMBP-1) is beneficial in various disease conditions. In this study, we propose to determine whether human AM combined with human AMBP-1 provides benefit in a model of THI in the rat.
Methods
Male adult rats were subjected to trauma-hemorrhage by resection of approximately 50% of total liver tissues and allowed bleeding for 15 min. Immediately thereafter, human AM (48 μg/kg BW) plus human AMBP-1 (160 μg/kg BW) was given intravenously over 30 min in 1 ml normal saline. After 4 h, the rats were euthanized, blood was collected, and tissue injury indicators were assessed. A 10-day survival study was also conducted.
Results
At 4 h after THI, plasma AMBP-1 levels were markedly decreased. Plasma levels of liver injury indicators (i.e., AST, ALT and LDH) were significantly increased after THI. Likewise, lactate, creatinine and TNF-α levels were significantly increased following THI. Administration of human AM/AMBP-1 after THI produced significant decreases of 64%, 23% and 19% of plasma AST, ALT and LDH levels, respectively. Similarly, plasma levels of lactate, creatinine and TNF-α were also decreased by 42%, 28% and 46% following human AM/AMBP-1 treatment, respectively. In a 10-day survival study, while vehicle treatment produced 41% survival, human AM/AMBP-1 treatment improved the survival rate to 81%.
Conclusions
Administration of human AM/AMBP-1 significantly attenuated tissue injury and inflammation, and improved survival following THI. Thus, human AM/AMBP-1 can be developed as a novel treatment for victims with uncontrolled traumatic hemorrhage.
Keywords: traumatic hepatic injury, hemorrhage, adrenomedullin, adrenomedullin binding protein, survival
Introduction
Trauma-related deaths account for nearly 200,000 in the United States alone 1. The liver, with a relatively fixed position in the abdomen, is the largest solid abdominal organ and is rather prone to traumatic injury. Blunt abdominal trauma and penetrating trauma are the primary causes for injury to the liver. Hemorrhagic shock secondary to hepatic injury is a consequence of serious traumatic injuries. It is well accepted that bleeding control, surgical interventions, maintenance of tissue oxygenation with either blood or other means of fluid resuscitation, and normothermia are some of the basic supportive measures that can be employed to circumvent the complications associated with uncontrolled hemorrhage 2,3.
Infusion of large amounts of fluids in an attempt to stabilize the blood pressure and achieve circulatory hemostasis has been the first line of defense against hemorrhagic shock. Resuscitation with vigorous fluid administration, though useful in providing adequate perfusion to vital organs, can increase blood pressure and in turn cause further bleeding making its effect rather counterintuitive 4–6. In fact, these resuscitation strategies are often associated with multi-organ dysfunction, increased hospital stay, and mortality 7,8. Nonetheless, recent advances in trauma management, including surgical and radiological techniques, have improved the outcome of liver injury secondary to blunt abdominal trauma. However, despite these advances, the management of uncontrolled hemorrhage to the liver has remained stagnant.
Adrenomedullin (AM) is a 52-amino acid peptide, with potent vasoactive properties, originally isolated from a human pheochromocytoma in 1993 9. AM is widely distributed in the body, and predominantly found in the endocrine and neuroendocrine systems 10. Circulating levels of AM are increased in patients with sepsis and systemic inflammatory response syndrome, and following major surgery, hemorrhagic and cardiogenic shock, or ischemia-reperfusion injury 11. This suggests that AM plays an important role in the control of systemic and local circulation, as well as cardiovascular and fluid regulation, regulation of growth and differentiation, and secretions of other hormones 12. AM is regulated by a specific binding protein, Adrenomedullin binding protein-1 (AMBP-1), which was reported to be identical to human complement factor H 13,14. Our recent studies show that AMBP-1 augments the biological activity of AM and produces significant beneficial effects under various pathophysiological conditions 15–18. However, it is unknown whether human AM combined with AMBP-1 will provide any beneficial effects during resuscitation of uncontrolled traumatic hemorrhage induced by severe liver injury.
To date, most models of uncontrolled hemorrhage have used lesions of major blood vessels. These models are relevant to some cases of penetrating trauma, but not necessarily blunt hepatic trauma. There have been only a few studies that examined uncontrolled hepatic hemorrhage and their treatment strategies 19. We have, therefore, used a model of traumatic hepatic injury (THI) that closely mimics a clinical trauma scenario and examined whether the use of human AM/AMBP-1 provide favorable benefits in THI in rats.
Materials and Methods
Experimental Animals
Male Sprague-Dawley rats (250–300g), purchased from Charles River Laboratories (Wilmington, MA) were used for this study. The rats were housed in a temperature controlled room and on a 12-h light/dark cycle. The rats were fed a standard Purina rat chow diet and allowed water ad libitum. Animal experimentation was carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources). This project was approved by the Institutional Animal Care and Use Committee (IACUC) of the Feinstein Institute for Medical Research.
Animal Model of Traumatic Hepatic Injury (THI)
A model of uncontrolled THI was performed as previously described, with some modifications 19. Briefly, rats were anesthetized with isoflurane inhalation and a midline laparotomy incision was made, and the liver segments were isolated from the bowel. Approximately 50% of the liver was resected, corresponding to 7–8 g of total liver tissues. The cut edges of the liver were allowed to bleed freely for 15 min, with warm moist gauze covering the abdominal incision. This produced a mean reduction of ~45% in the circulating blood volume, corresponding to a mean arterial blood pressure of ~50 mmHg. Afterwards, free intraperitoneal blood was removed with pre-weighed sterile gauze pads. Clotted blood was meticulously removed and added to the total amount of free blood collected to determine the overall amount of blood lost. The abdominal incision was closed in layers and the animals were returned to their cages and allowed food and water ad libitum. After 4 h following THI, the animals were euthanized, and blood was collected for analyses. Sham operated animals underwent a midline laparotomy, with no liver resection.
Administration of AM/AMBP-1
Immediately following 15 min of uncontrolled hemorrhage, human AM (48 μg/kg BW, Phoenix Pharmaceuticals, Belmont, CA) plus AMBP-1 (160 μg/kg BW), 20 was slowly infused over 30 min in a volume of 1 ml normal saline. The THI rats with vehicle treatment received low dose of human albumin for a period of 30 min. The dosage of AM/AMBP-1 was similar to that was used recently in a rat model of gut ischemia and reperfusion injury 21.
A blood pressure analyzer (Digi-Med, Louisville, KY) was used to monitor the heart rate and blood pressure throughout the experiments. Briefly, the transducer was inserted into the femoral artery prior to THI and remained in place for 75 min. Specific values were recorded in 5 min intervals starting at time 0. The mean arterial pressure was ~50 mmHg at 15 min following THI, which corresponded to the beginning of the infusion of AM/AMBP-1. The pressure slightly decreased during the course of the 30 min infusion. Blood pressure increased to ~60 mmHg by 75 min after THI.
Upon liver resection, the free intraperitoneal blood was removed with a pre-weighed sterile gauze pad and the clotted blood was also collected. The blood loss was calculated by the amount of pooled blood in the abdomen, combined with the blood that had already been clotted. The average blood loss from the Vehicle group was 8.2 ± 0.3 g and that of the AM/AMBP-1 treatment group was 8.1 ± 0.3 g. There was no statistical difference in the amount of blood lost between the vehicle and the treatment groups (P=0.79). The animals did not receive heparin or any other anti-coagulant. We avoided resuscitating the animals as to mimic a wartime scenario where a penetrating abdominal injury from a missile, or a blast injury, would cause uncontrolled hepatic hemorrhage.
Measurement of Plasma AM Levels
Plasma AM levels were assayed using a radioimmunoassay (RIA) kit specific for AM according to the protocols provided by the manufacturer (Peninsula Labs, Belmont, CA). Briefly, 1.5 ml blood was collected into a polypropylene tube containing 1mg/ml EDTA and 500 KIU/ml aprotinin at 4 h after reperfusion, and plasma was separated immediately. The plasma was then used for AM extraction by C18 Sep-Column. RIA was performed as described previously 22 and AM levels were calculated against known standards.
Determination of plasma levels of AMBP-1
Two microliters of plasma was fractionated on a 4–12% Bis-Tris gel and then transferred to a 0.2-μm nitrocellulose membrane. Nitrocellulose blots were blocked in TBST (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% Tween-20) containing 5% milk for 1 hour. Blots were then incubated with goat anti-human complement factor H polyclonal anitibodies (1:5,000, Quidel Corp, San Diego, CA) overnight at 4°C. The blots were then washed, incubated with horseradish peroxidase-linked anti-goat immunoglublin G for 1 h at room temperature, and then washed and detected with chemiluminescent peroxidase substrate (ECL, Amersham Biosciences, Piscataway, NJ) as described previously 22. The membranes were exposed briefly to X-ray film and the band densities were determined using a Bio-Rad Image System (Hercules, CA). The levels of AMBP-1 were determined from the band densities.
Determination of serum levels of organ injury markers
Blood samples were centrifuged for 15 min at 2000 g to collect serum, and stored at −80°C for determination of serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), lactate and creatinine. The levels were measured using commercially available assay kits according to manufacturer’s specifications (Pointe Scientific, Canton, MI).
Determination of serum levels of TNF-α
The concentration of TNF-α in the serum was measured using a commercially obtained enzyme-linked immunosorbent assay (ELISA) kit specific for rat TNF-α (BD Biosciences, San Jose, CA).
Survival study
A 10-day survival study was conducted to determine if human AM combined with AMBP-1 can beneficially affect survival in an animal model of THI. Briefly, in an additional group of animals, THI was induced and immediately after 15 min of uncontrolled hemorrhage, human AM/AMBP-1 (48 μg/kg BW/160 μg/kg BW) or vehicle (very low dose of human albumin) was infused for a period of 30 min as described above. After treatment, the midline incision was closed and the animals were returned to their cages and allowed food and water ad libitum. The animals were monitored for 10 days to record survival. All surviving animals were euthanized on Day 10.
Statistical analysis
All data are expressed as means ± SEM and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls (SNK) method for multiple group analyses and Student’s t-test for two-group analysis. The survival rate was estimated by Kaplan-Meier method and compared by the log rank test. Differences in values were considered significant when P<0.05.
Results
Alterations in plasma AM and AMBP-1 levels after THI
We have previously shown that plasma AM levels are significantly elevated following sepsis, hemorrhagic shock and ischemia/reperfusion injuries in rats15 while AMBP-1 is significantly decreased. Therefore, to determine whether plasma AM and AMBP-1 levels were altered in THI, sham and THI rats were analyzed for such measurements. As shown in Fig. 1A, rats subjected to THI had an increase in plasma AM levels as compared to sham operated animals. However, there was no statistically significant difference in plasma AM levels between the two groups. In contrast, as shown in Fig. 1B, rats subjected to THI had a 71% decrease in serum AMBP-1 as compared to sham operated animals (P < 0.05). These data provided the basis for examining the effect of combined treatment of human AM and human AMBP-1 in a rat model of THI.
Figure 1. Alterations in serum levels of AM and AMBP-1 4h after THI.
A. Plasma samples from sham and THI rats were assessed for AM using a specific RIA kit. Results are shown as pg/ml calculated from known standards. B. Plasma samples were subjected to Western blotting using human anti-AMBP-1 antibody. Results are shown as arbitrary densitometric units (1.6 ± 0.05 sham vs. 0.47 ± 0.2 vehicle). Data are presented as means ± SE (n=4) and compared by Student’s t- test: *P < 0.05 versus Sham group.
Human AM/AMBP-1 attenuated organ injury after THI
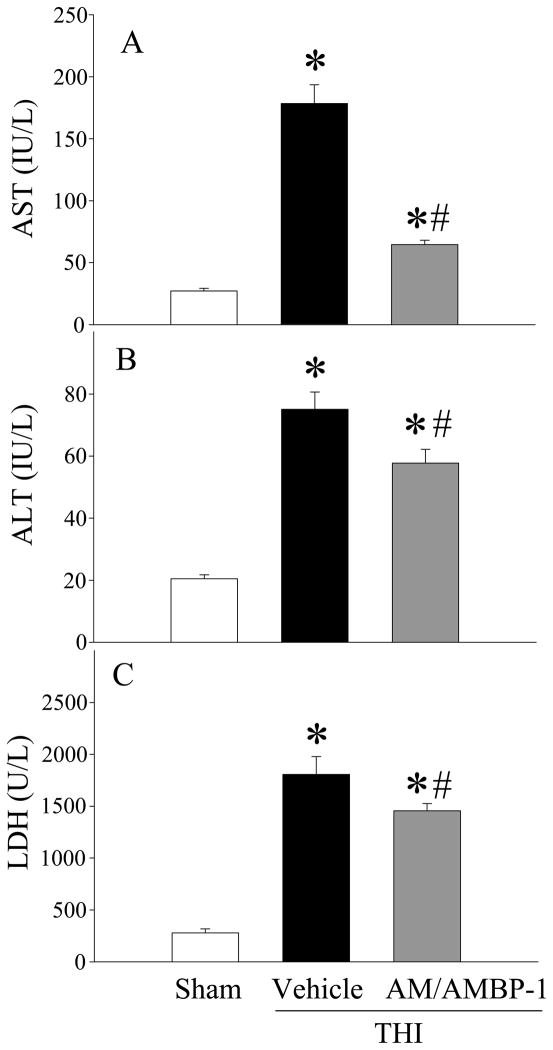
To examine whether combination of human AM and human AMBP-1 produce benefits in THI, serum samples were analyzed for injury indicators. Serum levels of AST in vehicle treated THI animals were significantly increased as compared to sham animals (178 ± 37 vs. 27 ± 6 U/L). Similarly, serum levels of ALT and LDH were also significantly increased after THI as compared to sham animals (75 ± 14 vs. 21 ± 3 U/L and 1806 ± 424 vs. 279 ± 94 U/L), respectively. Treatment with human AM/AMBP-1 significantly reduced AST, ALT, and LDH levels in the serum by 64%, 23% and 19% as compared to vehicle treated THI animals (64 ± 3 U/L, 57 ± 4 U/L, 1455 ± 70 U/L), respectively (Figs. 2A–C; P<0.05). Likewise, serum lactate and creatinine levels were increased after THI as compared to sham animals (38 ± 8 vs. 9.4 ± 2.4 mg/dL and 2.3 ± 0.5 vs. 0.6 ± 0.3 mg/dL), respectively. Human AM/AMBP-1 treatment reduced these levels by 42% and 28%, (27 ± 8 mg/dL and 1.3 ± 0.2 mg/dL), respectively (Figs. 3A–3B; P<0.05).
Figure 2. Alterations in serum levels of liver injury markers in THI rats 4h after human AM/AMBP-1 treatment.
Serum samples from sham or THI rats [administration of human AM/AMBP-1 or human albumin (vehicle)] were measured for (A) aspartate aminotransferase (AST), (B) alanine aminotransferase (ALT), and (C) lactate dehydrogenase (LDH) using commercially available assay kits. Data are presented as means ± SE (n=7–8) and compared by one-way analysis of variance (ANOVA) and Student–Newman–Keuls method: *P < 0.05 versus Sham group, #P < 0.05 versus Vehicle group.
Figure 3. Alterations in serum levels of organ injury indicators in THI rats 4h after human AM/AMBP-1 treatment.
Serum samples from sham or THI rats [administration of human AM/AMBP-1 or human albumin (vehicle)] were measured for (A) lactate and (B) creatinine using commercially available assay kits. Data are presented as means ± SE (n=7–8) and compared by one-way analysis of variance (ANOVA) and Student–Newman–Keuls method: *P < 0.05 versus Sham group, #P < 0.05 versus Vehicle group.
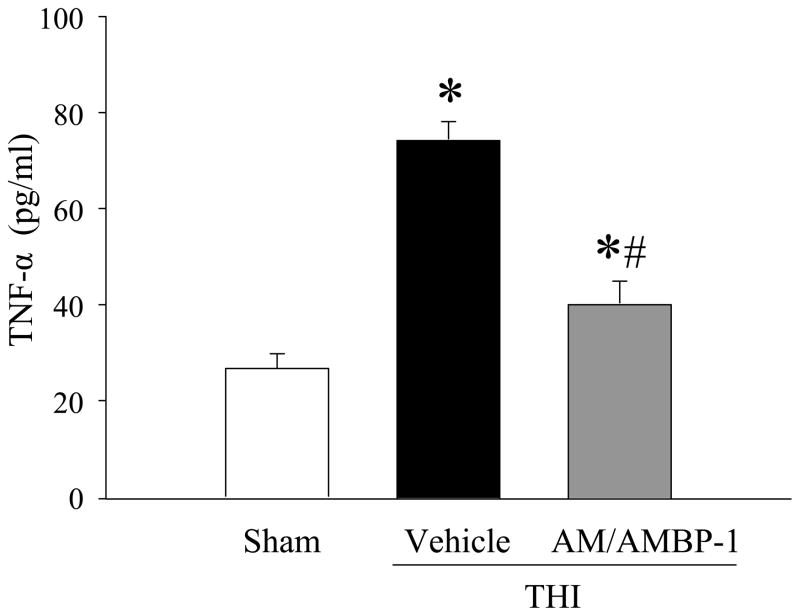
Human AM/AMBP-1 inhibited serum TNF-α after THI
To examine whether AM/AMBP-1 treatment is effective in reducing pro-inflammatory cytokines, serum TNF-α levels were measured. As indicated in Fig. 4, serum TNF-α levels were significantly increased following THI as compared to sham animals (74.5 ± 0.5 vs. 26.7 ± 3.1 pg/mL). Treatment with human AM/AMBP-1 decreased these levels by 46% (40.4 ± 4.4 pg/ml; P< 0.05).
Figure 4. Alterations in serum TNF-α in THI rats 4h after human AM/AMBP-1 treatment.
Serum samples from sham or THI rats [administration of human AM/AMBP-1 or human albumin (vehicle)] were measured for TNF-α using commercially obtained ELISA kit specific for rat TNF-α. Data presented as means ± SE (n=7–8) and compared by one-way analysis of variance (ANOVA) and Student–Newman–Keuls method: *P < 0.05 versus Sham group, #P < 0.05 versus Vehicle group.
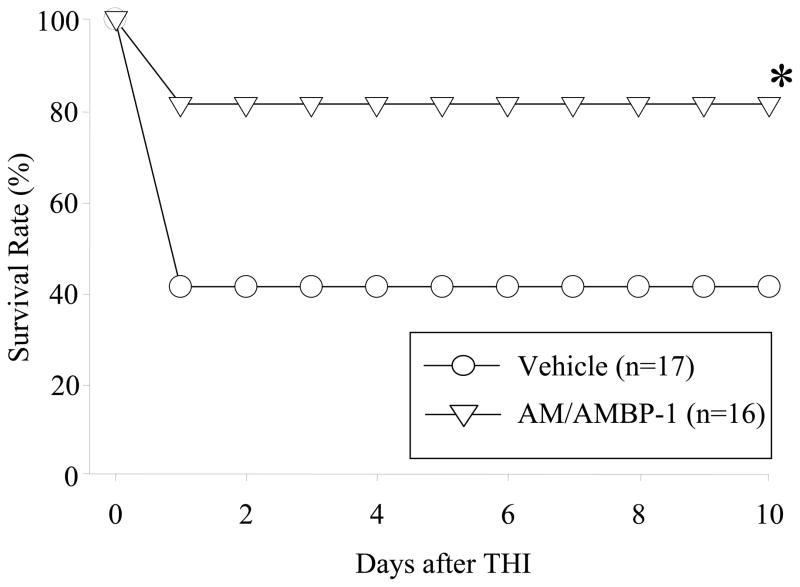
Human AM/AMBP-1 improved survival after THI
To further determine whether AM/AMBP-1 could alter survival rate in THI, a 10-day survival study was conducted in THI rats treated with either human AM/AMBP-1 or vehicle. As shown in Fig. 5, the survival rate in the vehicle group following THI was 41% at day 1, and remained the same for the duration of the study. Treatment with human AM/AMBP-1, however, increased the survival rate to 81% after day 1, and subsequently remained the same for 10 days (P < 0.05). Those animals that died both in the vehicle and treatment groups died within the first 24 h after THI.
Figure 5. Alterations in the survival rate in THI after human AM/AMBP-1 treatment.
THI rats treated with human AM/AMBP-1 or human albumin (vehicle) were observed for 10 days (n=16–17/group). The survival rate was estimated by the Kaplan–Meier method and compared by using the log-rank test. *P < 0.05 versus Vehicle group.
Discussion
Resuscitation of patients in uncontrolled hemorrhagic shock is one of the most challenging aspects of trauma care. Currently employed treatment of fluid management continues to be the first line therapy for controlled hemorrhagic shock; however its role in uncontrolled hemorrhagic shock is controversial 2. Resuscitation of hypotensive victims is based on the rationale that adequate perfusion of vital organs should be restored immediate to the injury. However, one major drawback of increasing organ perfusion is that it increases blood pressure, which, in the case of uncontrolled hemorrhage, may increase bleeding and have adverse consequences that could outweigh the potential benefits 7. Recent advances in trauma management, including surgical and radiological techniques, have improved the outcome of liver injury secondary to blunt abdominal trauma. Thus, despite the advances in trauma care, the management of uncontrolled hemorrhage to the liver has remained elusive.
The use of combined treatment of AM/AMBP-1 as a therapeutic intervention for various disease conditions in experimental animals has been reported 15–18. However, these studies were conducted with rat AM and human AMBP-1. To develop AM/AMBP-1 as therapy for organ injury in humans, it was important to verify the use of human AM instead of rat AM in the preclinical studies. Human AM is a 52-amino acid peptide that has a carboxy-terminal amidated residue and a 6-residue ring structure formed by an intramolecular disulfide bridge. Rat AM is similar to human AM with 50 amino acid residues, with two amino acid deletions and six substitutions from human AM 23. In the present study, therefore, we sought to determine the use of human AM and human AMBP-1 during resuscitation of uncontrolled hemorrhage induced by THI in rats.
Adrenomedullin levels have been previously shown to increase in a variety of disease conditions and surgical states, such as ischemia/reperfusion and hemorrhagic shock 11,24. We have also shown that the decreased level of AMBP-1 leads to reduced vascular responsiveness in AM, thus contributing to the vascular collapse after hemorrhagic shock and severe sepsis 17,24. Consistent with our previous findings, we observed a slight, but not significant, increase in circulating AM levels at 4 h in our THI model. It is possible that AM levels have peaked prior to the 4 h time point and future studies are needed to confirm this notion. Interestingly, plasma AMBP-1 showed a significant reduction in these animals following THI. These data indicated the deficiency of AMBP-1 protein following THI and provided the basis for a combined intervention of human AM with human AMBP-1.
Our results indicated that human AM/AMBP-1 treatment significantly reduced circulating levels of organ injury markers, liver enzymes (AST and ALT), and LDH, lactate and creatinine and a significant reduction was also observed in the circulating TNF-α levels. Our survival study showed that human AM/AMBP-1 treatment significantly improved the survival from 41% to 81%, which was mostly seen on day 1. Organ injury markers have been used as measures to determine the degree of damage caused by models of organ injury such as sepsis, hemorrhagic shock, and ischemia/reperfusion injury17,21,22,25. These markers provide a way to compare the severity of damage to that is seen in clinical scenarios. The significant increases in liver injury markers, lactate, and creatinine indicate that our model induces a significant amount of organ damage that may be seen in the clinical settings. Subsequent treatment with AM/AMBP-1 reduces these parameters indicating healing and amelioration of injury.
Our preliminary results indicated that human AM/AMBP-1 at the dosage used in this study did not cause any tissue injury as evidenced by the similarities of the circulating levels of lactate in treated and non-treated sham animals (11.4 ± 2.1 mg/dL vs. 11.9 ± 0.9 mg/dL), respectively. Therefore, since administration of human AM/AMBP-1 did not cause any tissue injury, we eliminated the group of sham animals that was treated with human AM/AMBP-1 from our current study to conserve animal resources.
The main feature of this study is that the model was very severe and significant mortality was observed within 24 h time period. In a model of uncontrolled hemorrhage, source control is one of the most, if not crucial, determinant for survival. Our model of THI causes uncontrolled hemorrhage, which may or may not spontaneously resolve on its own. The high rate of mortality observed in the vehicle-treated animals in our model of THI is probably due to the inability of the animals to control the source of hemorrhage, either by clotting or tamponade. If early source control is achieved, it is reasonable to assume that the animals will live. Due to the inherent nature of our model, AM/AMBP-1 appears to have an early effect on our model of THI. If animals were able to control the bleeding early, it is highly unlikely that there would have been high mortality in the vehicle group. Although AM/AMBP-1 might exert its effect throughout our THI model, the most dramatic effects are seen early during the survival study. AM/AMBP-1 may help enhance the clotting mechanisms, or simply accelerate healing in severely hemorrhaged animals. The exact mechanism is unknown.
It is also possible that the severity seen in this model is in fact due to the elimination of resuscitation following THI. However, in our study, we have chosen not to resuscitate the animals as to mimic a wartime scenario in which blunt trauma victims in the battlefield may not have a large supply of resuscitation fluid available or possess the time for adequate administration immediately following injury. As shown in our survival studies with human AM/AMBP-1 treatment, those victims may benefit from such therapy when given immediate to the insult. Future studies are warranted to determine any delayed effect of AM/AMBP-1 in this model of uncontrolled hemorrhage.
We have chosen to administer a low dose of human albumin for our Vehicle group animals to control for any potential changes that may occur simply due to administration of human protein into rats. Our treatment consisted of human AM combined with human AMBP-1, which is a protein compound. Clinically, the most widely used form of resuscitation is either Ringer’s Lactate or normal saline. However, in our study, we avoided the use of any resuscitation as to mimic the uncontrolled hemorrhage scenarios normally observed in the battlefield. Our Vehicle group did not serve as a resuscitative group and rather this group was included as control for human protein administration into rats.
In a prior study, we examined the effect of exogenous administration of AM alone, AMBP-1 alone, and the combined treatment of AM and AMBP-1 in a controlled hemorrhage model 16. Our results showed that the administration of AM or AMBP-1 alone had no statistically beneficial effect in this model. In contrast, the combined treatment restored and maintained cardiovascular stability. Furthermore, the combined treatment has been beneficial in a number of disease conditions 15,17,18,21,22,24. Therefore, in the current study, we chose to use a combined treatment of human AM and human AMBP-1 so as to limit the animals used in the study.
Previously we have shown that administration of AM/AMBP-1 restored cardiovascular stability and reduced cardiac TNF-α levels at 4 h after severe hemorrhagic shock and crystalloid resuscitation 16. Furthermore, our survival study indicated a significant 59% mortality within 24 h following THI. Based on both previous studies and the current survival data, we have chosen to use the 4 hour endpoint expecting that significant inflammation and damage will occur only after 4 h following THI. In fact, even as early as 4 h time point, the measurements of all injury markers assessed in this study were increased to 250–550% from the sham levels. Therefore, administration of human AM/AMBP-1 as immediate to injury was beneficial possibly because the treatment restored the integrity of the organs that was originally comprised by the uncontrolled hemorrhage. However, various time points can be considered in future studies.
The actual mechanism of how human AM/AMBP-1 exerts its effects on THI remains to be determined. The binding between AM and AMBP-1 has important physiological consequences. The presence of a binding protein can alter the biological function of a potent factor and determines its inhibitory or stimulatory capabilities. In the case of AM, AMBP-1 may not change the affinity of AM to its receptors, but rather it may bind to cell surface adhesion molecules and bring AM near to its receptors and raise the efficacy of AM 14. As a result, AMBP-1 may effectively increase AM’s potency without modifying its receptor or its binding capacities. Another possibility is that since AMBP-1 is known to prevent degradation of AM 26, AM/AMBP-1 binding can make AM more functionally effective.
The possible mechanism responsible for the beneficial effects of human AM/AMBP-1 could be related to the anti-inflammatory properties of these agents. Previous studies have shown that circulating levels of TNF-α are elevated after hemorrhage and resuscitation. We have shown that combined treatment of AM/AMBP-1 decreases plasma and cardiac TNF-α levels after hemorrhage and resuscitation 16. It has been reported that proinflammatory cytokines such as TNF-α play an important role in regulating organ blood flow 27. Thus, downregulation of TNF-α by human AM/AMBP-1 treatment could improve organ blood flow.
Our previous studies demonstrated that vascular responsiveness to AM is compromised in various disease conditions including hemorrhage with resuscitation and this hypo-responsiveness is due to the decrease in AM binding protein, AMBP-1. In the current study, we observed a significant 71% decrease in AMBP-1. This suggests that as in the case of controlled hemorrhage, the decrease in AMBP-1 could cause hyporesponsiveness to AM in our model of uncontrolled hemorrhage. Therefore, the administration of AM/AMBP-1 in this model of uncontrolled hemorrhage could have been able to improve cardiovascular responses. In addition, studies have shown that downregulation of vascular endothelial constitutive nitric oxide synthase (ecNOS) contributes to vascular hyporesponsiveness in sepsis 28. In this regard, we have previously shown that acetylcholine induced vascular relaxation was significantly reduced in late sepsis, i.e., 20 h in a rat model of cecal ligation and puncture (CLP) 29. Gene and protein expression of ecNOS in aortic and pulmonary tissues were downregulated in late sepsis. Administration of AM/AMBP-1 prevented the reduction of acetylcholine induced vascular relaxation and attenuated the decrease in ecNOS suggesting that the preservation of ecNOS by AM/AMBP-1 treatment prevents the reduction of this vascular relaxation in late sepsis.
It is well recognized that increased production of liver injury markers (AST/ALT) and other organ injury markers such as lactate, creatinine and proinflammatory cytokine, TNF-α, as observed in our model of THI leads to increased apoptosis and cell death. In this regard, we have shown that AM/AMBP-1 administration attenuates apoptosis in various injury models such as sepsis, and gut and hepatic ischemia/reperfusion injuries18,21,22. Therefore, a number of factors can contribute to the survival advantage seen with combined AM/AMBP-1 treatment in our model of THI. Future studies are warranted to understand the exact mechanism of this benefit in THI.
In the present study, we show that treatment with human AM combined with human AMBP-1 attenuates organ injury and increases survival in an animal model of THI. In a previous study using the gut ischemia/reperfusion injury in rats, we have demonstrated that human AM and human AMBP-1 administered at varying doses reduced pro-inflammatory cytokines, attenuated organ injury, and improved survival rate in a dose dependent manner. We chose the most efficient dose of human AM and human AMBP-1 (i.e., 48/160 μg/kg BW) from this previous study for the current experiments in THI. Previously we have shown that exogenous administration of either AM or AMBP-1 alone did not produce the beneficial effect of AM/AMBP-1 in models of organ injury. Therefore, it is unlikely the increase in production of AM or the consumption/depletion of the AMBP-1 receptors that play important role in the benefits shown with combined treatment of AM/AMBP-1.
In future studies, we will examine the optimal dosage and timing of delivery after THI to determine the optimal amount and conditions for infusion. We may also consider using a resuscitation strategy with either normal saline or lactated ringers, in combination with AM/AMBP-1 treatment. Nonetheless, our studies suggest that human AM/AMBP-1 can be potentially developed as a novel and effective treatment to reduce morbidity and mortality for uncontrolled hepatic hemorrhage following blunt abdominal trauma.
Acknowledgments
This study was supported by the National Institutes of Health grants, R01 HL076179 and R01 GM057468 (P.W.)
Footnotes
Presented at the 39th Society of Critical Care Medicine’s Critical Care Congress, Miami Beach, Florida; January 9-13, 2010.
References
- 1.Matthes G, Stengel D, Seifert J, et al. Blunt liver injuries in polytrauma: results from a cohort study with the regular use of whole-body helical computed tomography. World J Surg. 2003;27:1124–1130. doi: 10.1007/s00268-003-6981-0. [DOI] [PubMed] [Google Scholar]
- 2.Spahn DR, Cerny V, Coats TJ, et al. Management of bleeding following major trauma: a European guideline. Crit Care. 2007;11:R17. doi: 10.1186/cc5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.West MA, Shapiro MB, Nathens AB, et al. Inflammation and the host response to injury, a large-scale collaborative project: Patient-oriented research core-standard operating procedures for clinical care. IV. Guidelines for transfusion in the trauma patient. J Trauma. 2006;61:436–439. doi: 10.1097/01.ta.0000232517.83039.c4. [DOI] [PubMed] [Google Scholar]
- 4.Asensio JA, Demetriades D, Chahwan S, et al. Approach to the management of complex hepatic injuries. J Trauma. 2000;48:66–69. doi: 10.1097/00005373-200001000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Mohr AM, Lavery RF, Barone A, et al. Angiographic embolization for liver injuries: low mortality, high morbidity. J Trauma. 2003;55:1077–1081. doi: 10.1097/01.TA.0000100219.02085.AB. discussion 1081-1072. [DOI] [PubMed] [Google Scholar]
- 6.Polanco P, Leon S, Pineda J, et al. Hepatic resection in the management of complex injury to the liver. J Trauma. 2008;65:1264–1269. doi: 10.1097/TA.0b013e3181904749. discussion 1269–1270. [DOI] [PubMed] [Google Scholar]
- 7.Cotton BA, Guy JS, Morris JA, Jr, et al. The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock. 2006;26:115–121. doi: 10.1097/01.shk.0000209564.84822.f2. [DOI] [PubMed] [Google Scholar]
- 8.Shaftan GW, Chiu CJ, Dennis C, et al. Fundamentals of physiologic control of arterial hemorrhage. Surgery. 1965;58:851–856. [PubMed] [Google Scholar]
- 9.Kitamura K, Kangawa K, Kawamoto M, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 10.Pearson LJ, Rait C, Nicholls MG, et al. Regulation of adrenomedullin release from human endothelial cells by sex steroids and angiotensin-II. J Endocrinol. 2006;191:171–177. doi: 10.1677/joe.1.06815. [DOI] [PubMed] [Google Scholar]
- 11.Fujioka S. Increased plasma concentration of adrenomedullin during and after major surgery. Surg Today. 2001;31:575–579. doi: 10.1007/s005950170089. [DOI] [PubMed] [Google Scholar]
- 12.Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev. 2000;21:138–167. doi: 10.1210/edrv.21.2.0396. [DOI] [PubMed] [Google Scholar]
- 13.Elsasser TH, Kahl S, Martinez A, et al. Adrenomedullin binding protein in the plasma of multiple species: characterization by radioligand blotting. Endocrinology. 1999;140:4908–4911. doi: 10.1210/endo.140.10.7157. [DOI] [PubMed] [Google Scholar]
- 14.Pio R, Martinez A, Unsworth EJ, et al. Complement factor H is a serum-binding protein for adrenomedullin, and the resulting complex modulates the bioactivities of both partners. J Biol Chem. 2001;276:12292–12300. doi: 10.1074/jbc.M007822200. [DOI] [PubMed] [Google Scholar]
- 15.Carrizo GJ, Wu R, Cui X, et al. Adrenomedullin and adrenomedullin-binding protein-1 downregulate inflammatory cytokines and attenuate tissue injury after gut ischemia-reperfusion. Surgery. 2007;141:245–253. doi: 10.1016/j.surg.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Wu R, Dong W, Zhou M, et al. A novel approach to maintaining cardiovascular stability after hemorrhagic shock: beneficial effects of adrenomedullin and its binding protein. Surgery. 2005;137:200–208. doi: 10.1016/j.surg.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Yang S, Zhou M, Chaudry IH, et al. Novel approach to prevent the transition from the hyperdynamic phase to the hypodynamic phase of sepsis: role of adrenomedullin and adrenomedullin binding protein-1. Ann Surg. 2002;236:625–633. doi: 10.1097/00000658-200211000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou M, Simms HH, Wang P. Adrenomedullin and adrenomedullin binding protein-1 attenuate vascular endothelial cell apoptosis in sepsis. Ann Surg. 2004;240:321–330. doi: 10.1097/01.sla.0000133253.45591.5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuoka T, Wisner DH. Resuscitation of uncontrolled liver hemorrhage: effects on bleeding, oxygen delivery, and oxygen consumption. J Trauma. 1996;41:439–445. doi: 10.1097/00005373-199609000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Qiang X, Wu R, Ji Y, et al. Purification and characterization of human adrenomedullin binding protein-1. Mol Med. 2008;14:443–450. doi: 10.2119/2008-00015.Qiang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang F, Wu R, Zhou M, et al. Human adrenomedullin combined with human adrenomedullin binding protein-1 is protective in gut ischemia and reperfusion injury in the rat. Regul Pept. 2009;152:82–87. doi: 10.1016/j.regpep.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J, Wu R, Qiang X, et al. Human adrenomedullin and its binding protein attenuate organ injury and reduce mortality after hepatic ischemia-reperfusion. Ann Surg. 2009;249:310–317. doi: 10.1097/SLA.0b013e3181961d43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakata J, Shimokubo T, Kitamura K, et al. Molecular cloning and biological activities of rat adrenomedullin, a hypotensive peptide. Biochem Biophys Res Commun. 1993;195:921–927. doi: 10.1006/bbrc.1993.2132. [DOI] [PubMed] [Google Scholar]
- 24.Wu R, Cui X, Dong W, et al. Mechanisms responsible for vascular hyporesponsiveness to adrenomedullin after hemorrhage: the central role of adrenomedullin binding protein-1. Ann Surg. 2005;242:115–123. doi: 10.1097/01.sla.0000167849.10599.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu R, Dong W, Qiang X, et al. Human vasoactive hormone adrenomedullin and its binding protein rescue experimental animals from shock. Peptides. 2008;29:1223–1230. doi: 10.1016/j.peptides.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez A, Oh HR, Unsworth EJ, et al. Matrix metalloproteinase-2 cleavage of adrenomedullin produces a vasoconstrictor out of a vasodilator. Biochem J. 2004;383:413–418. doi: 10.1042/BJ20040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mookerjee RP, Sen S, Davies NA, et al. Tumour necrosis factor alpha is an important mediator of portal and systemic haemodynamic derangements in alcoholic hepatitis. Gut. 2003;52:1182–1187. doi: 10.1136/gut.52.8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou M, Wang P, Chaudry IH. Endothelial nitric oxide synthase is downregulated during hyperdynamic sepsis. Biochim Biophys Acta. 1997;1335:182–190. doi: 10.1016/s0304-4165(96)00139-0. [DOI] [PubMed] [Google Scholar]
- 29.Zhou M, Maitra SR, Wang P. Adrenomedullin and adrenomedullin binding protein-1protect endothelium-dependent vascular relaxation in sepsis. Mol Med. 2007;13:488–494. doi: 10.2119/2007-00113.Zhou. [DOI] [PMC free article] [PubMed] [Google Scholar]