Abstract
Lipoxin A4 is a lipid mediator that elicits anti-inflammatory and pro-resolution actions via its receptor, formyl peptide receptor 2 (FPR2/ALX). In this study, we aimed to investigate the expression and potential role of lipoxin A4 and FPR2/ALX in the regulation of inflammation associated with cyclical remodeling of the human endometrium across the menstrual cycle and during early pregnancy. Using quantitative RT-PCR analysis, we found that FPR2/ALX expression is upregulated during the menstrual phase of the cycle and in decidua tissue from the first trimester of pregnancy. We localized the site of expression of FPR2/ALX in menstrual phase endometrium and first-trimester decidua tissue to glandular epithelial cells and cells within the stromal compartment, including cells lining the blood vessels and immune cells. Measurement of serum lipoxin A4 by ELISA revealed no difference in its levels across the menstrual cycle but an elevation in early pregnancy (P<0.001). We found that lipoxin A4 was regulated by human chorionic gonadotrophin (hCG) during early pregnancy, because treatment of human decidua tissue with hCG increased lipoxin A4 release (P<0.01). Finally, we have shown that lipoxin A4 can suppress phorbol myristate acetate-induced expression of the inflammatory cytokines interleukin 6 and 8 in human endometrium and decidua tissue. These results demonstrate for the first time that lipoxin A4 and its receptor FPR2/ALX can regulate inflammatory events in the human endometrium and decidua of early pregnancy.
Introduction
Inflammation occurs during important physiological events throughout the female reproductive tract, such as ovulation, menstruation, embryo implantation, and the initiation of labor (Jabbour et al. 2009). In the endometrium, inflammatory leukocytes are believed to be important in the tissue breakdown and remodeling essential for menstruation and the generation of a receptive endometrium during early pregnancy. Chronic unregulated inflammation in the endometrium has been linked with several pathologies including heavy menstrual blood loss (Smith et al. 2007), endometrial cancer (Wallace et al. 2010), endometriosis (Ryan et al. 1995, Arici et al. 1997), and recurrent miscarriage (von Wolff et al. 2000, Laird et al. 2003). Thus, the inflammation associated with menstruation, implantation, and the establishment of pregnancy must be kept under tight control in order to ensure reproductive success.
Inflammation can be controlled by the local production of endogenous anti-inflammatory mediators, such as the steroid hormone cortisol (Chapman et al. 2009), the cytokine interleukin 10 (IL10; Thaxton & Sharma 2010), the protein annexin A1 (Perretti & D'Acquisto 2009), and lipid mediators including the resolvins, protectins, and lipoxins (Serhan et al. 2008).
The lipoxins (which include the naturally occurring lipoxin A4 and lipoxin B4, and the aspirin-triggered lipoxins 15-epimeric lipoxin A4 and 15-epimeric lipoxin B4) are synthesized from arachidonic acid by the action of the lipoxygenase enzymes (Chiang et al. 2006). Lipoxin A4 has been shown to bind to and activate the G protein-coupled receptor formyl peptide receptor 2 (FPR2/ALX), a member of the FPR family (Ye et al. 2009). Lipoxin A4 is a dual acting mediator and activates specific cellular pathways via FPR2/ALX to elicit both anti-inflammatory and pro-resolution effects (Serhan et al. 2008).
The lipoxins act as anti-inflammatory mediators by inhibiting neutrophil infiltration (Takano et al. 1997, 1998, Hachicha et al. 1999) and transmigration (Colgan et al. 1993, Kucharzik et al. 2003) at sites of inflammation, in part via the induction of nitric oxide production to suppress leukocyte–endothelial interactions (Paul-Clark et al. 2004). Lipoxins can also inhibit the production of pro-inflammatory cytokines and chemokines (Wu et al. 2005, 2008, Bonnans et al. 2007), via the inhibition of transcription factors such as nuclear factor-κB and activator protein 1 (Gewirtz et al. 2002, Jozsef et al. 2002, Sodin-Semrl et al. 2004, Wu et al. 2008).
In this study, we aimed to determine the expression and potential role of lipoxin A4 and FPR2/ALX in regulating inflammation in the human endometrium and decidua of early pregnancy. We show that FPR2/ALX is temporally regulated across the menstrual cycle and during early pregnancy and is expressed in glandular, vascular, and stromal cells. Furthermore, we show that serum lipoxin A4 expression is elevated during early pregnancy and is regulated in first-trimester decidua tissue by human chorionic gonadotrophin (hCG). Finally, we show that lipoxin A4 acts as an anti-inflammatory mediator in human endometrium and decidua tissue by counteracting phorbol ester-mediated induction of pro-inflammatory cytokine expression.
Results
Expression and cellular localization of FPR2/ALX in the human endometrium
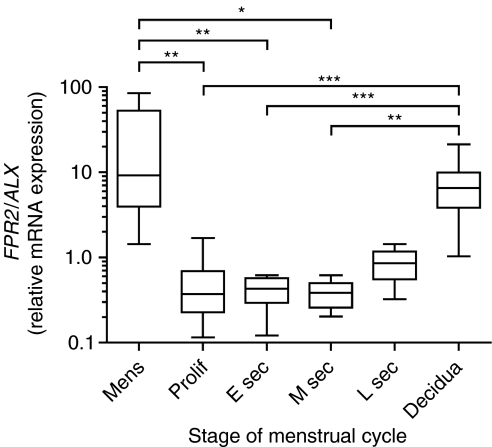
The temporal expression pattern of FPR2/ALX mRNA expression across the menstrual cycle and during early pregnancy was investigated using quantitative RT-PCR analysis. Expression of FPR2/ALX (Fig. 1) was significantly higher in the menstrual phase of the cycle when compared with proliferative (P<0.01), early- (P<0.01), and mid-secretory (P<0.05) phase endometrium. In first-trimester decidua tissue, FPR2/ALX expression was significantly higher than in proliferative (P<0.001), early- (P<0.001), and mid-secretory (P<0.01) phase endometrium.
Figure 1.
Temporal expression of FPR2/ALX in the human endometrium and first-trimester decidua. mRNA expression levels in human endometrium across the menstrual cycle (n=34; consisting of n=5 menstrual, n=10 proliferative, n=7 early secretory, n=6 mid-secretory, and n=6 late secretory phase tissues) and first-trimester decidua tissue (7–12 weeks of gestation; n=27) are shown for FPR2/ALX. mRNA levels are expressed relative to a standard endometrial cDNA sample. Boxes represent data lying within the 5th to the 95th percentile and whiskers represent the minimum and maximum values. *, **, and ***Represent significance at P<0.05, P<0.01, and P<0.001, respectively, as determined by ANOVA with Tukey's post hoc test.
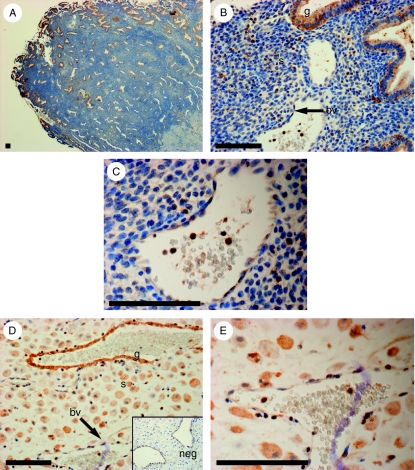
As the mRNA expression of FPR2/ALX was higher in menstrual phase endometrium and first-trimester decidua, we localized the site of expression of FPR2/ALX protein in these tissues by immunohistochemistry. In menstrual phase endometrium (Fig. 2A, B and C), FPR2/ALX expression was strongest in the functional layer (Fig. 2A), where it localized to glandular epithelial cells and distinct cells in the stromal compartment (Fig. 2B) along with cells lining the blood vessels and immune cells within the vasculature (Fig. 2C). Similarly, in first-trimester decidua tissue (Fig. 2D and E), FPR2/ALX expression localized to the glandular epithelium and decidualized stromal cells (Fig. 2D), cells lining the blood vessels, and immune cells within the vasculature (Fig. 2E). Tissue sections incubated with primary antibody pre-absorbed with an excess of FPR2/ALX protein were used as a negative control (Fig. 2D; inset).
Figure 2.
Localization of FPR2/ALX protein in menstrual endometrium and first-trimester decidua. Localization of FPR2/ALX protein in menstrual endometrium (A, B and C; n=5) and during early pregnancy (D and E; n=5) is shown. Expression was detected in the glandular epithelium (g), distinct cells in the stroma (s), and cells lining the blood vessels (bv) in both tissues. FPR2/ALX was also detected in immune cells within the vasculature (C and E). Decidualized stromal cells showed expression of FPR2/ALX (D). A representative sample is shown for each tissue. Negative control (neg) is shown for decidua tissue (D, insert). Scale bar=100 μm.
Serum levels of lipoxin A4 across the menstrual cycle and during early pregnancy
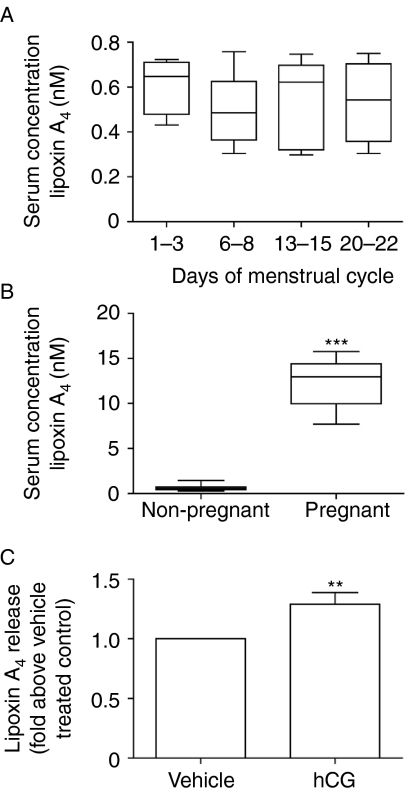
The endometrial expression pattern of FPR2/ALX suggested a role for this receptor in regulating inflammation associated with menstruation and early pregnancy. Therefore, we investigated the levels of its ligand, lipoxin A4, in the blood of women across the menstrual cycle and in early pregnancy. There was no significant variation in serum levels of lipoxin A4 across the menstrual cycle (Fig. 3A). However, serum levels of lipoxin A4 were significantly elevated in samples from women in the first trimester of pregnancy, when compared with levels in non-pregnant women (Fig. 3B, P<0.001).
Figure 3.
Lipoxin A4 serum levels across the menstrual cycle and during the first trimester of pregnancy and regulation of its release by hCG. Lipoxin A4 levels in serum samples taken from a group of women on the days indicated across the menstrual cycle (A; n=5 for each day of the menstrual cycle analyzed). The average level of lipoxin A4 in this group of non-pregnant women (non-pregnant) was compared with serum levels measured in a group of women during the first trimester of pregnancy (pregnant; n=10 from gestational ages 7–12 weeks) (B). Boxes represent data lying within the 5th to the 95th percentile and whiskers represent the minimum and maximum values. First-trimester decidua tissue (n=6 from gestational ages 7–12 weeks) treated with 1 IU of hCG for 8 h showed a significant increase in lipoxin A4 release into the culture medium, when compared with vehicle-treated control tissue. Data are mean±s.e.m. ** and ***Represent significance at P<0.01 and P<0.001, respectively, as determined by t test.
Regulation of lipoxin A4 release by hCG
As we observed an increase in serum levels of lipoxin A4 during early pregnancy, we investigated whether lipoxin release could be regulated by hCG. Treatment of decidua tissue explants with 1 IU hCG for 8 h caused a significant increase in lipoxin A4 release when compared with vehicle-treated decidua tissue (P<0.01, Fig. 3C).
Lipoxin A4 reduces the expression of inflammatory mediators in human endometrium and decidua tissue
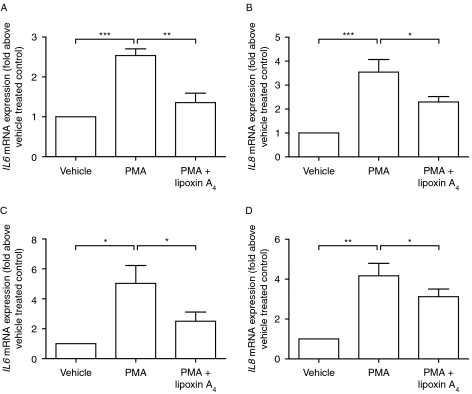
Lipoxin A4 via FPR2/ALX has been shown to mediate anti-inflammatory actions (Bonnans et al. 2007). To investigate whether lipoxin A4 acts as an anti-inflammatory mediator in the endometrium, endometrial tissue explants were cultured with the inflammatory stimulus phorbol myristate acetate (PMA), either alone or in combination with lipoxin A4. Treatment with PMA alone increased the expression of the inflammatory cytokines IL6 (P<0.001, Fig. 4A) and IL8 (P<0.001, Fig. 4B). Co-treatment of tissues with PMA and lipoxin A4 significantly reduced expression of IL6 (P<0.01, Fig. 4A) and IL8 (P<0.05, Fig. 4B). Similarly, to assess the anti-inflammatory actions of lipoxin A4 during early pregnancy, decidua tissue was cultured with PMA, alone or in combination with lipoxin A4. PMA significantly increased the expression of IL6 (P<0.05, Fig. 4C) and IL8 (P<0.01, Fig. 4D) in decidua tissue explants. Co-treatment of decidua tissue with PMA and lipoxin A4 significantly suppressed PMA-induced expression of IL6 and IL8 (P<0.05).
Figure 4.
Anti-inflammatory action of lipoxin A4 in endometrium and decidua tissue. Cultured endometrial tissue consisting of four individual tissues taken across the menstrual cycle (one proliferative, two early secretory, and one late secretory samples) stimulated with the inflammatory stimulus PMA showed a significant increase in the expression of the inflammatory cytokines IL6 (A) and IL8 (B). The expression of these markers was significantly reduced when tissue was cultured with PMA in combination with lipoxin A4. PMA also stimulated an increase in the expression of IL6 (C) and IL8 (D) in cultured decidua tissue (n=6 from gestational ages 7–12 weeks), which was significantly attenuated by the presence of lipoxin A4. Data are mean±s.e.m. *, **, and ***Represent significance at P<0.05, P<0.01, and P<0.001, respectively, as determined by t test.
Discussion
Endometrial events, such as menstruation and embryo implantation, are associated with inflammatory processes that must be tightly regulated to ensure reproductive success. This is achieved by a delicate balance in the production of pro- and anti-inflammatory mediators to ensure proper tissue function and homeostasis. Lipoxin A4 is a recently described anti-inflammatory and pro-resolution lipid mediator, which elicits its actions via the G protein-coupled receptor FPR2/ALX. In this study, we investigated the expression and role of the lipoxin A4-FPR2/ALX system in the human endometrium.
We found that expression of FPR2/ALX is temporally regulated across the human menstrual cycle. The elevation in expression of FPR2/ALX observed in the endometrium during menstruation suggests a role for lipoxin A4 in regulating the inflammation associated with endometrial remodeling and repair. Prior to the onset of menstruation, there is an influx of leukocytes into the endometrium (Salamonsen & Lathbury 2000), facilitated by the expression of pro-inflammatory chemoattractant chemokines and cytokines (Arici et al. 1998, Jolicoeur et al. 1998) and an increase in vascular permeability (Colditz 1990). These inflammatory leukocytes are thought to contribute to tissue breakdown and vascular remodeling during menstruation (Zhang et al. 1998, Sivridis et al. 2001), via the production of proteolytic enzymes such as the matrix metalloproteinases (Salamonsen & Woolley 1999). Regeneration of the endometrium begins soon after the onset of menstruation (Ludwig & Spornitz 1991). The mechanisms of endometrial repair have been likened to those of the wound healing process and involve angiogenesis (Jabbour et al. 2006) and inflammatory processes such as the removal of tissue debris by macrophages (Salamonsen & Lathbury 2000). While we observed no significant fluctuation in serum levels of lipoxin A4 across the menstrual cycle, our data showing that expression of FPR2/ALX is elevated at menstruation indicates a potential role for this receptor in regulating the inflammatory events involved in endometrial tissue breakdown and regeneration at a local level. This may be achieved by local production of lipoxin A4 in the endometrium, or other anti-inflammatory ligands that can activate FPR2/ALX such as annexin A1 (Perretti & D'Acquisto 2009).
Lipoxin A4 has previously been demonstrated to elicit anti-inflammatory actions in a variety of cell types, including epithelial cells (Bonnans et al. 2007), endothelial cells (Wu et al. 2008), and myometrial tissue explants (Maldonado-Perez et al. 2011), where it facilitates tissue remodeling events by reducing the expression of inflammatory chemokines and cytokines. In order to confirm that lipoxin A4 could regulate inflammatory events in the endometrium, we treated endometrial tissue with the general inflammatory stimulus PMA. This elevated the expression of the pro-inflammatory cytokines IL6 and IL8. Co-treatment of endometrial tissue with PMA and lipoxin A4 suppressed PMA-mediated induction of IL6 and IL8 expression. These data confirm that lipoxin A4 has the capacity to mediate anti-inflammatory actions in the endometrium.
In addition to its anti-inflammatory effects, the lipoxin A4-FPR2/ALX system can also elicit pro-resolution actions in order to regulate tissue homeostasis (Serhan et al. 2008). The resolution of inflammation involves the removal of leukocytes and debris from the site of inflammation and was once thought to be a passive process caused by the dispersal of chemotatic gradients (Serhan 2004). However, it is now recognized that the resolution of inflammation is an active process involving the activation of distinct biochemical pathways that can be mediated by the lipoxins (Serhan et al. 2008). To promote resolution, the lipoxins accelerate the clearance of neutrophils from inflamed tissues via the promotion of neutrophil apoptosis (El Kebir et al. 2007), monocyte migration (Maddox & Serhan 1996), and the non-phlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages (Godson et al. 2000). Our observation that FPR2/ALX is expressed on glandular epithelial, stromal, vascular, and immune cells in the endometrium during menstruation suggests that pro-resolution lipoxin A4-FPR2/ALX signaling may contribute to controlling the removal of tissue debris after menstruation. This is essential for wound healing and tissue regeneration in the endometrium.
Inflammatory processes are also important during early pregnancy, for both the establishment of a receptive endometrium and in embryo–endometrial cross talk. Decidualization of the stroma occurs in the human endometrium in preparation for embryo implantation and trophoblast invasion. As well as differentiation of the stromal cells, this involves inflammatory events such as infiltration of leukocytes, modification of the extra-cellular matrix, and an increase in vascular permeability (Hess et al. 2007). The developing embryo also releases many pro-inflammatory cytokines, and it is thought that this induces inflammatory pathways in the endometrium to further enhance its receptivity (Dimitriadis et al. 2005). Dysregulated inflammatory responses have been associated with recurrent miscarriage (von Wolff et al. 2000, Laird et al. 2003); therefore, these inflammatory pathways need to be tightly regulated to ensure reproductive competence.
We found elevated expression of FPR2/ALX in the decidua of early pregnancy compared with non-pregnant endometrium across the menstrual cycle. In addition, serum lipoxin A4 levels were also significantly increased during the first trimester of pregnancy compared with levels in non-pregnant patients, indicating potential fetal–maternal cross talk in the regulation of the inflammatory response during early pregnancy. This led us to investigate the potential of hCG to regulate lipoxin A4 release. hCG is released from the developing blastocyst during early pregnancy and maintains progesterone production from the corpus luteum and directly regulates gene expression in the endometrium to facilitate the establishment and maintenance of pregnancy (Srisuparp et al. 2001, Licht et al. 2007). We observed an increase in lipoxin A4 release from cultured decidua tissue treated with hCG. Whether circulating levels of lipoxin A4 correlate with higher levels seen at the maternal–fetal interface during pregnancy is unclear. However, our data strongly suggest that decidual tissue, in response to embryonic hCG, is capable of secreting lipoxin A4 which in turn may contribute to the higher levels seen in early pregnancy.
To confirm that lipoxin A4 could regulate inflammatory events in the decidua of early pregnancy; we treated decidua tissue from pregnant women with PMA. Similar to our observations for treatment of non-pregnant endometrium, we found that PMA enhanced the expression of IL6 and IL8 in decidua tissue explants. This was suppressed by co-treatment of decidua tissue with PMA and lipoxin A4. Our data showing that lipoxin A4 can moderate the expression of IL6 and IL8 in the decidua suggest a mechanism whereby lipoxin A4 may regulate physiological inflammation during early pregnancy, by controlling the inflammatory processes required for decidualization and the generation of a receptive endometrium.
In summary, we demonstrate for the first time expression of the lipoxin A4 receptor FPR2/ALX in the human endometrium and decidua from the first trimester of pregnancy and show its temporal regulation across the menstrual cycle. We highlight that lipoxin A4 serum levels are elevated during early pregnancy and that its release from the decidua can be regulated by hCG. Furthermore, we show that lipoxin A4 has an anti-inflammatory action in human endometrium and decidua tissue. We believe these data demonstrate that lipoxin A4 signaling via FPR2/ALX represents a novel mechanism to control the inflammation associated with menstruation and the establishment of pregnancy.
Materials and Methods
Reagents
DMEM/F-12 GlutaMAX culture medium was obtained from Invitrogen. Lipoxin A4 (used at a final concentration of 500 nM) was purchased from Calbiochem (Nottingham, UK). PMA; used at a final concentration of 100 nM and recombinant (hCG; used at a final concentration of 1 IU) were purchased from Sigma. FPR2/ALX polyclonal antibody and blocking peptide were obtained from MBL International (Woburn, MA, USA).
Tissue and serum collection
Endometrial tissue was collected from 34 women aged 21–39 years (consisting of n=5 menstrual, n=10 proliferative, n=7 early secretory, n=6 mid secretory and n=6 late secretory phase tissues) with no underlying endometrial pathology and regular menstrual cycles (25–35 days) who had not received any hormonal preparation for 3 months preceding biopsy collection. The phase of the menstrual cycle for each tissue was confirmed by histological assessment by a pathologist. Biopsies were dated according to stated last menstrual period and confirmed with hormone analysis for circulating estradiol and progesterone levels; they were consistent with both the stated last menstrual period and the histological assessment of the stage of the menstrual cycle. First-trimester decidua tissue (7–12 weeks gestation; n=27) was collected from women undergoing elective surgical termination of pregnancy, with gestation confirmed by ultra-sound scan. Blood samples were obtained from two groups of women. The first group included healthy non-pregnant women who attended four visits during a single menstrual cycle (days 1–3 (menstrual), days 6–8 (follicular), days 13–15 (peri-ovulatory), and days 20–22 (luteal)). The second group included women undergoing elective surgical termination during the first trimester of pregnancy (7–12 weeks gestation). Blood samples were taken from the antecubital fossa and transported to the laboratory on ice, where serum was separated by centrifugation and stored at −20 °C. Ethical approval was obtained from Lothian Ethics Research Committee, and written informed consent was obtained before tissue or blood collection.
Tissue culture and treatment
Non-pregnant endometrial tissue and decidua tissue for explant studies were chopped finely and maintained in serum-free DMEM/F-12 medium. For each individual patient sample, tissue was divided into six roughly equal portions that were then treated with the three treatments each in duplicate. Tissue was incubated overnight in serum-free DMEM/F-12 culture medium supplemented with 100 IU penicillin and 100 μg streptomycin, before treatment with 1 IU hCG, 100 nM PMA, or 100 nM PMA+500 nM lipoxin A4. Tissue explants were incubated for up to 8 h at 37 °C and 5% CO2 before tissue was collected for RNA extraction and RT-PCR analysis, or conditioned medium was collected for ELISA.
Taqman quantitative PCR
Total RNA was extracted from tissue using the RNeasy mini kit and RNase-free DNase set from Qiagen, according to the manufacturer's guidelines. RNA samples were quantified and reverse transcribed using the SuperScript VILO cDNA synthesis kit from Invitrogen. PCR reactions were carried out in duplicate using an Applied Biosystems ABI 7500 system, UK. Primer and FAM-labeled probe sequences were as follows (5′–3′): FPR2/ALX, forward GCCATCTGCTATGGGCTCAT and reverse CGTAAGGGACGGCTGGATTT; probe, CAGCCAAGATCCACAAAAAGGGCATG; IL6, forward GCCGCCCCACACAGACA and reverse CCGTCGAGGATGTACCGAAT; probe, CCACTCACCTCTTCAGAACGAATTCACAAAC; IL8, forward CTGGCCGTGGCTCTCTT and reverse TAGCACTCCTTGGCAAAACTG; probe, CCTTCCTGATTTCTGCAGCTCTGTGTGAA. The expression of analyzed genes was normalized for RNA loading using 18S ribosomal RNA primers and probe (Applied Biosystems, Warrington, UK). Results were calculated relative to a standard included in all reactions (endometrial tissue cDNA). Tissue expression levels are shown as relative to the endometrial tissue cDNA standard, and experimental data are expressed as fold change compared with control.
Immunohistochemistry
Localization of FPR2/ALX was performed by immunohistochemistry in 5 μm paraffin-embedded sections using the Vision Biosystems Bond Immunostaining Robot under normal operating conditions (Leica Microsystems, Wetzlar, Germany). Immunostaining was performed across the menstrual cycle/decidua on tissue sections from five different patient samples per stage of the cycle/decidua following antigen retrieval in 0.01 M sodium citrate buffer (pH 6), using primary antibody specific for FPR2/ALX (1:200; 5 μg/ml). Control tissue was incubated with primary antibody pre-absorbed with a ten times excess FPR2/ALX protein (50 μg/ml) overnight at 4 °C.
Lipoxin A4 ELISA
Lipoxin A4 levels were measured in longitudinal serum samples taken at four points across a single menstrual cycle from the same group of five women, a second group of ten patients during the first trimester of pregnancy, and in conditioned medium from decidua tissue (n=6 different patients from gestational ages 7–12 weeks) stimulated with 1 IU hCG, using an ELISA kit according to the manufacturer's instructions (Neogen Corporation, Lexington, KY, USA). Lipoxin A4 was extracted from serum samples using C18 Sep-Pak columns (Waters, Elstree, UK) before analysis. Each sample was assayed in duplicate.
Statistical analysis
Data are shown as mean±s.e.m. and were analyzed by t test or one-way ANOVA with Tukey's post hoc test as described in the figure legend (GraphPad Prism, San Diego, CA, USA). All tissue samples were treated in duplicate at the same time to provide two pools for each treatment condition. These were then averaged to a mean value prior to statistical analysis between and among treatment groups. Statistical analysis was performed on the untransformed data and presented as fold increase above vehicle control. All samples within an experiment or set of experiments were subject to the same pattern of analysis.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by Medical Research Council core funding to H N Jabbour (grant number: U1276.00.004.00002.01).
Acknowledgements
We thank Ms Sharon McPherson and Ms Catherine Murray for patient recruitment and assistance with tissue collection; Ms Sheila McPherson and Mr Mike Millar for assistance with histology; Ms Vivien Grant for technical support; and Mr Ted Pinner for assistance with producing the figures.
References
- Arici A, Oral E, Attar E, Tazuke SI, Olive DL. Monocyte chemotactic protein-1 concentration in peritoneal fluid of women with endometriosis and its modulation of expression in mesothelial cells. Fertility and Sterility. 1997;67:1065–1072. doi: 10.1016/S0015-0282(97)81440-9. [DOI] [PubMed] [Google Scholar]
- Arici A, Seli E, Senturk LM, Gutierrez LS, Oral E, Taylor HS. Interleukin-8 in the human endometrium. Journal of Clinical Endocrinology and Metabolism. 1998;83:1783–1787. doi: 10.1210/jc.83.5.1783. [DOI] [PubMed] [Google Scholar]
- Bonnans C, Gras D, Chavis C, Mainprice B, Vachier I, Godard P, Chanez P. Synthesis and anti-inflammatory effect of lipoxins in human airway epithelial cells. Biomedicine & Pharmacotherapy. 2007;61:261–267. doi: 10.1016/j.biopha.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Chapman KE, Coutinho AE, Gray M, Gilmour JS, Savill JS, Seckl JR. The role and regulation of 11beta-hydroxysteroid dehydrogenase type 1 in the inflammatory response. Molecular and Cellular Endocrinology. 2009;301:123–131. doi: 10.1016/j.mce.2008.09.031. [DOI] [PubMed] [Google Scholar]
- Chiang N, Serhan CN, Dahlen SE, Drazen JM, Hay DW, Rovati GE, Shimizu T, Yokomizo T, Brink C. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacological Reviews. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- Colditz IG. Effect of exogenous prostaglandin E2 and actinomycin D on plasma leakage induced by neutrophil-activating peptide-1/interleukin-8. Immunology and Cell Biology. 1990;68:397–403. doi: 10.1038/icb.1990.53. [DOI] [PubMed] [Google Scholar]
- Colgan SP, Serhan CN, Parkos CA, Delp-Archer C, Madara JL. Lipoxin A4 modulates transmigration of human neutrophils across intestinal epithelial monolayers. Journal of Clinical Investigation. 1993;92:75–82. doi: 10.1172/JCI116601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriadis E, White CA, Jones RL, Salamonsen LA. Cytokines, chemokines and growth factors in endometrium related to implantation. Human Reproduction Update. 2005;11:613–630. doi: 10.1093/humupd/dmi023. [DOI] [PubMed] [Google Scholar]
- El Kebir D, Jozsef L, Khreiss T, Pan W, Petasis NA, Serhan CN, Filep JG. Aspirin-triggered lipoxins override the apoptosis-delaying action of serum amyloid A in human neutrophils: a novel mechanism for resolution of inflammation. Journal of Immunology. 2007;179:616–622. doi: 10.4049/jimmunol.179.1.616. [DOI] [PubMed] [Google Scholar]
- Gewirtz AT, Neish AS, Madara JL. Mechanisms of active intestinal inflammation and potential down-regulation via lipoxins. Advances in Experimental Medicine and Biology. 2002;507:229–236. doi: 10.1007/978-1-4615-0193-0_35. [DOI] [PubMed] [Google Scholar]
- Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. Journal of Immunology. 2000;164:1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- Hachicha M, Pouliot M, Petasis NA, Serhan CN. Lipoxin (LX)A4 and aspirin-triggered 15-epi-LXA4 inhibit tumor necrosis factor 1alpha-initiated neutrophil responses and trafficking: regulators of a cytokine-chemokine axis. Journal of Experimental Medicine. 1999;189:1923–1930. doi: 10.1084/jem.189.12.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess AP, Hamilton AE, Talbi S, Dosiou C, Nyegaard M, Nayak N, Genbecev-Krtolica O, Mavrogianis P, Ferrer K, Kruessel J, et al. Decidual stromal cell response to paracrine signals from the trophoblast: amplification of immune and angiogenic modulators. Biology of Reproduction. 2007;76:102–117. doi: 10.1095/biolreprod.106.054791. [DOI] [PubMed] [Google Scholar]
- Jabbour HN, Kelly RW, Fraser HM, Critchley HO. Endocrine regulation of menstruation. Endocrine Reviews. 2006;27:17–46. doi: 10.1210/er.2004-0021. [DOI] [PubMed] [Google Scholar]
- Jabbour HN, Sales KJ, Catalano RD, Norman JE. Inflammatory pathways in female reproductive health and disease. Reproduction. 2009;138:903–919. doi: 10.1530/REP-09-0247. [DOI] [PubMed] [Google Scholar]
- Jolicoeur C, Boutouil M, Drouin R, Paradis I, Lemay A, Akoum A. Increased expression of monocyte chemotactic protein-1 in the endometrium of women with endometriosis. American Journal of Pathology. 1998;152:125–133. [PMC free article] [PubMed] [Google Scholar]
- Jozsef L, Zouki C, Petasis NA, Serhan CN, Filep JG. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 inhibit peroxynitrite formation, NF-kappa B and AP-1 activation, and IL-8 gene expression in human leukocytes. PNAS. 2002;99:13266–13271. doi: 10.1073/pnas.202296999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharzik T, Gewirtz AT, Merlin D, Madara JL, Williams IR. Lateral membrane LXA4 receptors mediate LXA4's anti-inflammatory actions on intestinal epithelium. American Journal of Physiology. Cell Physiology. 2003;284:C888–C896. doi: 10.1152/ajpcell.00507.2001. [DOI] [PubMed] [Google Scholar]
- Laird SM, Tuckerman EM, Cork BA, Linjawi S, Blakemore AI, Li TC. A review of immune cells and molecules in women with recurrent miscarriage. Human Reproduction Update. 2003;9:163–174. doi: 10.1093/humupd/dmg013. [DOI] [PubMed] [Google Scholar]
- Licht P, Fluhr H, Neuwinger J, Wallwiener D, Wildt L. Is human chorionic gonadotropin directly involved in the regulation of human implantation? Molecular and Cellular Endocrinology. 2007;269:85–92. doi: 10.1016/j.mce.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Ludwig H, Spornitz UM. Microarchitecture of the human endometrium by scanning electron microscopy: menstrual desquamation and remodeling. Annals of the New York Academy of Sciences. 1991;622:28–46. doi: 10.1111/j.1749-6632.1991.tb37848.x. [DOI] [PubMed] [Google Scholar]
- Maddox JF, Serhan CN. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. Journal of Experimental Medicine. 1996;183:137–146. doi: 10.1084/jem.183.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Perez D, Golightly E, Denison FC, Jabbour HN, Norman JE. A role for lipoxin A4 as anti-inflammatory and proresolution mediator in human parturition. FASEB Journal. 2011;25:569–575. doi: 10.1096/fj.10-170340. [DOI] [PubMed] [Google Scholar]
- Paul-Clark MJ, Van Cao T, Moradi-Bidhendi N, Cooper D, Gilroy DW. 15-Epi-lipoxinA4-med iated induction of nitric oxide explains how aspirin inhibits acute inflammation. Journal of Experimental Medicine. 2004;200:69–78. doi: 10.1084/jem.20040566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti M, D'Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nature Reviews. Immunology. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- Ryan IP, Tseng JF, Schriock ED, Khorram O, Landers DV, Taylor RN. Interleukin-8 concentrations are elevated in peritoneal fluid of women with endometriosis. Fertility and Sterility. 1995;63:929–932. [PubMed] [Google Scholar]
- Salamonsen LA, Woolley DE. Menstruation: induction by matrix metalloproteinases and inflammatory cells. Journal of Reproductive Immunology. 1999;44:1–27. doi: 10.1016/S0165-0378(99)00002-9. [DOI] [PubMed] [Google Scholar]
- Salamonsen LA, Lathbury LJ. Endometrial leukocytes and menstruation. Human Reproduction Update. 2000;6:16–27. doi: 10.1093/humupd/6.1.16. [DOI] [PubMed] [Google Scholar]
- Serhan CN. A search for endogenous mechanisms of anti-inflammation uncovers novel chemical mediators: missing links to resolution. Histochemistry and Cell Biology. 2004;122:305–321. doi: 10.1007/s00418-004-0695-8. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nature Reviews. Immunology. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivridis E, Giatromanolaki A, Agnantis N, Anastasiadis P. Mast cell distribution and density in the normal uterus – metachromatic staining using lectins. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2001;98:109–113. doi: 10.1016/S0301-2115(00)00564-9. [DOI] [PubMed] [Google Scholar]
- Smith OP, Jabbour HN, Critchley HO. Cyclooxygenase enzyme expression and E series prostaglandin receptor signalling are enhanced in heavy menstruation. Human Reproduction. 2007;22:1450–1456. doi: 10.1093/humrep/del503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodin-Semrl S, Spagnolo A, Mikus R, Barbaro B, Varga J, Fiore S. Opposing regulation of interleukin-8 and NF-kappaB responses by lipoxin A4 and serum amyloid A via the common lipoxin A receptor. International Journal of Immunopathology and Pharmacology. 2004;17:145–156. doi: 10.1177/039463200401700206. [DOI] [PubMed] [Google Scholar]
- Srisuparp S, Strakova Z, Fazleabas AT. The role of chorionic gonadotropin (CG) in blastocyst implantation. Archives of Medical Research. 2001;32:627–634. doi: 10.1016/S0188-4409(01)00330-7. [DOI] [PubMed] [Google Scholar]
- Takano T, Fiore S, Maddox JF, Brady HR, Petasis NA, Serhan CN. Aspirin-triggered 15-epi-lipoxin A4 (LXA4) and LXA4 stable analogues are potent inhibitors of acute inflammation: evidence for anti-inflammatory receptors. Journal of Experimental Medicine. 1997;185:1693–1704. doi: 10.1084/jem.185.9.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Clish CB, Gronert K, Petasis N, Serhan CN. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. Journal of Clinical Investigation. 1998;101:819–826. doi: 10.1172/JCI1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaxton JE, Sharma S. Interleukin-10: a multi-faceted agent of pregnancy. American Journal of Reproductive Immunology. 2010;63:482–491. doi: 10.1111/j.1600-0897.2010.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace AE, Gibson DA, Saunders PT, Jabbour HN. Inflammatory events in endometrial adenocarcinoma. Journal of Endocrinology. 2010;206:141–157. doi: 10.1677/JOE-10-0072. [DOI] [PubMed] [Google Scholar]
- von Wolff M, Thaler CJ, Strowitzki T, Broome J, Stolz W, Tabibzadeh S. Regulated expression of cytokines in human endometrium throughout the menstrual cycle: dysregulation in habitual abortion. Molecular Human Reproduction. 2000;6:627–634. doi: 10.1093/molehr/6.7.627. [DOI] [PubMed] [Google Scholar]
- Wu SH, Lu C, Dong L, Zhou GP, He ZG, Chen ZQ. Lipoxin A4 inhibits TNF-alpha-induced production of interleukins and proliferation of rat mesangial cells. Kidney International. 2005;68:35–46. doi: 10.1111/j.1523-1755.2005.00379.x. [DOI] [PubMed] [Google Scholar]
- Wu SH, Liao PY, Dong L, Chen ZQ. Signal pathway involved in inhibition by lipoxin A4 of production of interleukins induced in endothelial cells by lipopolysaccharide. Inflammation Research. 2008;57:430–437. doi: 10.1007/s00011-008-7147-1. [DOI] [PubMed] [Google Scholar]
- Ye RD, Boulay F, Wang JM, Dahlgren C, Gerard C, Parmentier M, Serhan CN, Murphy PM. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacological Reviews. 2009;61:119–161. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Nie G, Jian W, Woolley DE, Salamonsen LA. Mast cell regulation of human endometrial matrix metalloproteinases: a mechanism underlying menstruation. Biology of Reproduction. 1998;59:693–703. doi: 10.1095/biolreprod59.3.693. [DOI] [PubMed] [Google Scholar]