Abstract
Inositol 1,4,5-trisphosphate receptors (IP3Rs) are a family of intracellular Ca2+ channels that exist as homo- or heterotetramers. In order to determine whether the N-terminal ligand-binding domain is in close physical proximity to the C-terminal pore domain, we prepared microsomal membranes from COS-7 cells expressing recombinant type I and type III IP3R isoforms. Trypsin digestion followed by cross-linking and co-immunoprecipitation of peptide fragments suggested an inter-subunit N- and C-terminal interaction in both homo- and heterotetramers. This observation was further supported by the ability of in vitro translated C-terminal peptides to interact specifically with an N-terminal fusion protein. Using a 45Ca2+ flux assay, we provide functional evidence that the ligand-binding domain of one subunit can gate the pore domain of an adjacent subunit. We conclude that common structural motifs are shared between the type I and type III IP3Rs and propose that the gating mechanism of IP3R Ca2+ channels involves the association of the N-terminus of one subunit with the C-terminus of an adjacent subunit in both homo- and heterotetrameric complexes.
Keywords: Ca2+ channel/inositol 1,4,5-trisphosphate/IP3 receptor/trypsin digestion
Introduction
Inositol 1,4,5-trisphosphate receptors (IP3Rs) are intracellular Ca2+ channels that are activated by the second messenger inositol 1,4,5-trisphosphate (IP3) (Berridge and Irvine, 1989). Structurally, each channel is composed of four subunits, which form a single ion-conducting pore (for review see Patel et al., 1999). The N-terminus of each subunit contains the IP3-binding domain, which is separated from the C-terminal channel domain by a large intervening regulatory region (Joseph, 1996). Each subunit is encoded by one of three genes (types I, II and III IP3R) (Furuichi et al., 1989; Mignery et al., 1990; Sudhof et al., 1991; Blondel et al., 1993). Individual cell types can express multiple isoforms, which can be present as either homo- or heterotetramers (Taylor et al., 1999). The C-terminus of the protein has six membrane-spanning segments, and the primary structural determinant for tetramer formation resides within transmembrane- spanning regions 5 and 6 for both homo- and heterotetramers (Joseph et al., 1997). This is also the region that comprises the ion conduction pathway (Ramos-Franco et al., 1999; Boehning and Joseph, 2000).
Through progressive deletions of the N-terminus of the mouse type I IP3R, it has been found that the minimal IP3-binding core was encompassed by amino acids 224–578 (Yoshikawa et al., 1996). Ten amino acids contribute to ligand binding; in particular, Arg265, Lys508 and Arg511 are absolutely required for binding IP3 (Yoshikawa et al., 1996). Therefore, the IP3-binding region makes up a relatively large portion of the N-terminus, which folds to form a pocket of positively charged residues (Yoshikawa et al., 1996). The binding of IP3 is thought to cause a large conformational change in the channel, which is transferred to the C-terminus to gate the channel (Mignery and Sudhof, 1990; Miyawaki et al., 1991).
We have shown previously that limited tryptic cleavage of the cerebellar (type I) IP3R in microsomal membranes leaves the ligand-binding domain tightly associated with the C-terminal transmembrane region (Joseph et al., 1995). It was concluded that the ligand-binding domain may be near the pore-forming domain. Subsequent work by Yoshikawa et al. (1999b) showed that the receptor was cleaved by trypsin into five major domains, all of which remain tightly associated with each other. Hence, the association of N- and C-terminal regions may not necessarily reflect direct interaction between these two domains. Previous studies have not been able to distinguish between intra- and inter-subunit interactions. In addition, it is not known whether the structural organization observed for the type I IP3R is also conserved in other IP3R isoforms.
In order to address these issues, we have examined the N- and C-terminal interactions between homo- and hetero tetrameric type I and III IP3R isoforms. We used epitope-tagged type I and type III IP3Rs expressed in COS-7 cells and subjected these receptors to limited tryptic digestion. Co-immunoprecipitation and cross-linking experiments were used to demonstrate a direct inter-subunit interaction between N- and C-terminal domains in both homo- and heterotetrameric IP3Rs. Further evidence for an inter-subunit interaction was supported by glutathione S-trans ferase (GST) fusion protein experiments. Finally, our studies using mutated IP3R channels in a 45Ca2+ flux assay suggest that ligand binding to an IP3R subunit can gate a neighboring channel subunit. We propose that the subunits of an IP3R tetramer arrange themselves in a head-to-tail manner, bringing the ligand-binding domain of one subunit near to the channel pore of an adjacent subunit.
Results
Limited trypsin digestion of recombinant type I and type III IP3Rs
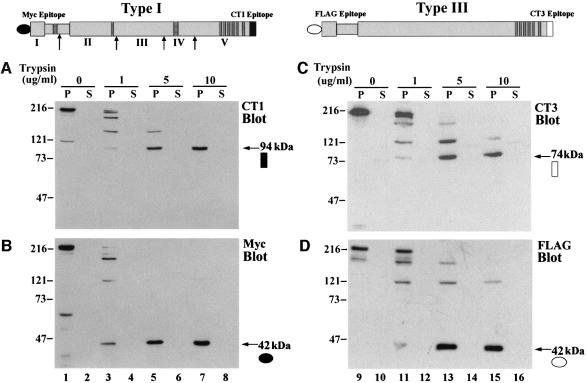
The domain organization of the IP3R, the location of antibody epitopes and the boundaries of the tryptic fragments of the mouse cerebellar (type I) IP3R identified by Yoshikawa et al. (1999b) are depicted at the top of Figure 1. In order to facilitate detection of the N-terminus of recombinant IP3Rs, a Myc epitope was inserted at the N-terminus of the type I receptor and a FLAG epitope at the N-terminus of the type III receptor. This also distinguished recombinant IP3Rs from endogenous IP3Rs found in COS-7 cells, which contain primarily type II and type III IP3Rs (Wojcikiewicz, 1995). Dose-dependent cleavage by trypsin of microsomal membranes prepared from COS cells expressing recombinant Myc-tagged type I IP3R is shown in Figure 1A. An antibody that recognizes the C-terminal sequence of this isoform detected a 94 kDa fragment as the sole cleavage product remaining when the trypsin concentration was increased to 10 µg/ml (Figure 1A, lane 7). A fragment this size has been observed in trypsin digestion experiments of the type I IP3R in native cerebellar microsomes (Joseph et al., 1995) and this fragment is the same size as domain V (Yoshikawa et al., 1999b), which encompasses the C-terminal portion of the IP3R including the transmembrane domains. Analysis of the N-terminal fragments with an anti-Myc antibody revealed a 42 kDa protected fragment (Figure 1B, lane 7). As expected, separation of membrane and soluble fractions revealed that the 94 kDa fragment was present exclusively in the pellet fraction. However, the N-terminal 42 kDa fragment was also found predominantly in the pellet fraction. Similar findings using cerebellar microsomes have been interpreted as indicating that distinct regions of the IP3R may be associated by non-covalent interactions (Joseph et al., 1995; Yoshikawa et al., 1999a,b). Figure 1 also shows the cleavage pattern observed when COS cell microsomes expressing FLAG-tagged type III IP3R were treated with trypsin. A C-terminal antibody that specifically recognizes the type III IP3R isoform detected a membrane-associated fragment of 74 kDa (Figure 1C, lane 15). The FLAG antibody detected a 42 kDa N-terminal fragment that was also membrane associated (Figure 1D, lane 15). The similar size of the N-terminal fragments and their membrane association after trypsin cleavage suggest that both isoforms share a comparable N-terminal domain structure.
Fig. 1. Peptide fragments generated by limited tryptic digestion of recombinant type I and type III IP3Rs. (Above) Schematic representations of the IP3R expression constructs are illustrated. The indented region represents the ligand-binding pocket, striped bars represent sites of alternative splicing and dark gray bars represent the membrane-spanning regions of the pore domain. N-terminal antibody epitopes are designated by closed (type I) or open (type III) circles, and C-terminal (endogenous) epitopes by closed (type I) or open (type III) squares. Sites that are cleaved by trypsin in the mouse type I receptor are indicated by arrows and the resulting five fragments by Roman numerals I–V (Yoshikawa et al., 1999b). (A–D) Microsomes prepared from cells overexpressing recombinant type I or type III IP3Rs were digested with 0, 1, 5 or 10 µg/ml trypsin. The vesicles were then pelleted and both the pellet (P) and supernatant (S) fractions were subjected to SDS–PAGE (see Materials and methods). (A and B) The type I receptor probed with isoform-specific C-terminal (CT1) and N-terminal (Myc) antibodies, respectively. (C and D) Type III receptor probed with an isoform-specific C-terminal antibody (CT3) and an N-terminal FLAG antibody. The N- and C-terminal protease-resistant fragments are highlighted by an arrow and the epitope is designated as given in the diagram. The molecular weights of the fragments (in kilodaltons) are given.
Co-precipitation of N-terminal fragments by C-terminal antibodies
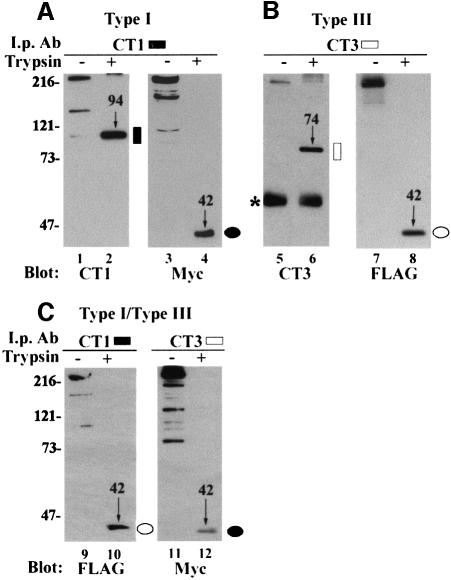
Immunoprecipitation of trypsin-digested type I IP3Rs after solubilization by Triton X-100 showed that the N-terminal Myc-tagged 42 kDa peptide was co-precipitated with the C-terminal 94 kDa peptide (Figure 2, lane 4). This confirms our previous observations on cerebellar microsomes (Joseph et al., 1995). Similarly, the N-terminus of the type III receptor was co-precipitated by a C-terminal type III-specific antibody (Figure 2, lane 8). Hetero-oligomers of type I and type III IP3Rs can be formed when COS cells are co-transfected with both isoforms (Joseph et al., 2000). Figure 2C shows experiments in which microsomes were prepared from doubly transfected cells and treated with trypsin prior to immunoprecipitation with either the CT1 or CT3 antibody. Immunoblotting of the CT1 immunoprecipitates with a FLAG antibody showed the presence of hetero-oligomeric type III IP3Rs in the samples that were not treated with protease (Figure 2C, lane 9). After trypsin treatment, the N-terminal FLAG-tagged fragment of the type III IP3R could be immunoprecipitated by the trypsin-cleaved C-terminus of the type I receptor (Figure 2C, lane 10). Similarly, the Myc-tagged N-terminal fragment of the type I IP3R could be immunoprecipitated by the CT3 antibody (Figure 2C, lane 12). Although the CT3 antibody will also immunoprecipitate the endogenous type III IP3R present in COS cells, we have previously shown that recombinant IP3Rs do not associate with the endogenous receptor population under these experimental conditions (Joseph et al., 2000). Therefore, when analyzing the co-precipitation of recombinant Myc-tagged type I IP3Rs and FLAG-tagged type III IP3Rs, the presence of endogenous type III IP3Rs does not affect the interpretation of the results.
Fig. 2. C-terminal isoform-specific antibodies co-precipitate homo- and heterotypic N-terminal fragments. COS cells were transfected with type I (A), type III (B) and both type I and type III IP3Rs (C). Microsomes prepared from these cells were either mock digested (odd-numbered lanes) or subjected to digestion with 20 µg/ml trypsin (even-numbered lanes) and immunoprecipitated with CT1 (lanes 1–4 and 9–10) or CT3 (lanes 5–8 and 11–12). The antibody used as probe is indicated below each panel. The Myc and FLAG immunoblots illustrate co-precipitating N-terminal peptides. (C) illustrates co-precipitation of heterotypic N-terminal fragments by CT1 or CT3. C- or N-terminal peptide fragments are indicated by molecular weight (in kilodaltons) and an arrow, as well as the schematic epitope designation. An asterisk indicates the IgG band.
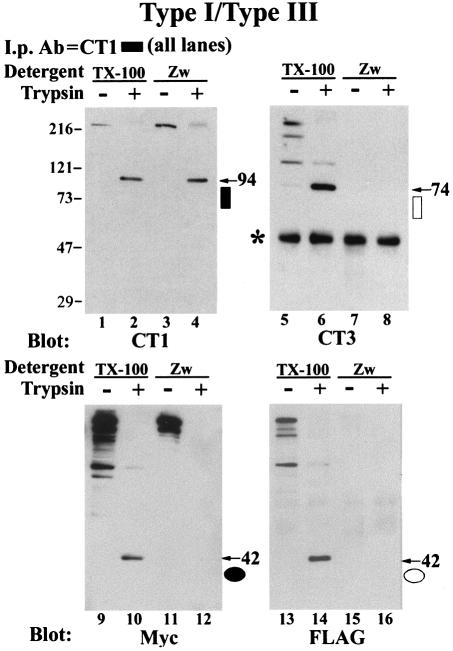
Since tetramers (including all five peptide fragments generated by trypsin digestion) remain associated in the presence of Triton X-100 (Yoshikawa et al., 1999b), the co-immunoprecipitation results do not demonstrate a direct association between N- and C-termini. In order to investigate nearest-neighbor relationships between the cleaved fragments, we chose to use a chemical cross-linking approach followed by immunoprecipitation under conditions where non-covalent interactions between subunits are disrupted. The detergent Zwittergent 3-14 disrupts homotetrameric associations between IP3R subunits (Mignery and Sudhof, 1990; Mignery et al., 1990). In Figure 3, COS cells were co-transfected with type I and type III IP3Rs, digested with trypsin and immunoprecipitated with CT1 in the presence of Zwittergent. Immuno precipitation in the presence of Zwittergent disrupted the co-precipitation of full-length type III hetero-oligomers by CT1 (Figure 3, compare lanes 5 and 7, 13 and 15). Zwittergent also disrupted co-precipitation by CT1 of the type III 74 kDa C-terminal fragment (compare lanes 6 and 8), as well as co-precipitation of N-terminal peptide fragments of both the type I (lane 12) and type III (lane 16) IP3Rs. The disruption of inter-domain interactions by Zwittergent demonstrates that tryptic IP3R peptides remain associated by non-covalent interactions (Joseph et al., 1995; Yoshikawa et al., 1999b).
Fig. 3. Zwittergent disrupts co-precipitation of full-length receptors and peptide fragments. Microsomes prepared from type I/type III co-transfected COS-7 cells were digested with trypsin as described in Figure 2. Microsomes were pelleted and resuspended in solubilization buffer containing 1% Triton X-100 (TX-100) or 1% Zwittergent 3-14 (Zw) and then immunoprecipitated with antibody CT1. Immunoblots were then sequentially probed with CT1, CT3, Myc and FLAG. Molecular weights of C- and N-terminal fragments are indicated by an arrow showing their weight in kilodaltons and the symbol used to designate the epitope in Figure 1. An asterisk indicates the IgG band.
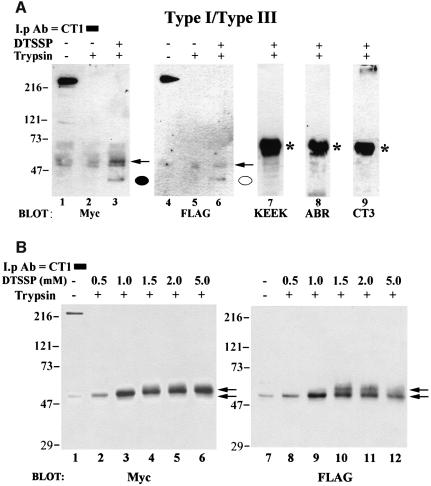
Having established that Zwittergent was effective in disrupting non-covalent associations between tetramers and trypsin-digested fragments, we treated microsomes prepared from doubly transfected COS cells with trypsin, followed by treatment of the membranes with or without the membrane-impermeant, thiol-cleavable, cross-linking agent dithiobis(sulfosuccinimidylpropionate) (DTSSP) before exposure to Zwittergent. Zwittergent-solubilized microsomes were then immunoprecipitated with CT1. As shown in Figure 4A, the Myc-reactive N-terminal fragment was immunoprecipitated with the C-terminal (CT1 reactive) fragment in a Zwittergent-containing buffer only when the trypsinized samples had been previously treated with DTSSP (compare lanes 2 and 3). The CT1 antibody also co-immunoprecipitated the 42 kDa N-terminal FLAG-reactive fragment of the type III IP3R in the presence of Zwittergent when the microsomal vesicles had previously been exposed to DTSSP (Figure 4A, compare lanes 5 and 6). Owing to the high exposure necessary to detect the co-precipitating FLAG-reactive fragment, it was evident that a small portion of hetero-oligomeric full-length type III IP3R was co-precipitated in the presence of Zwittergent, a result that has been shown previously for homotetrameric type I IP3Rs (Mignery et al., 1990). However, co-precipitating N-terminal fragments were never observed in the presence of Zwittergent and the absence of cross-linker treatment (Figure 4A, lanes 2 and 5). It is possible that DTSSP simply cross-linked together all of the peptide fragments in a trypsin-digested tetramer. However, when the immunoblots were screened with antibodies raised against region II (KEEK, Figure 4A, lane 7) and region IV (ABR, Figure 4A, lane 8) of the type I receptor and the type III C-terminus (CT3, Figure 4A, lane 9), no DTSSP-sensitive immunoreactive bands were observed. As expected, strong IP3R immunoreactivity was observed with the ABR and KEEK antibodies in parallel samples that had not been treated with trypsin (data not shown). When the trypsin-digested samples were subjected to increasing concentrations of DTSSP, a dose-dependent increase in both Myc- and FLAG-immunoreactive bands was observed (Figure 4B). High DTSSP concentrations resulted in a shift in mobility of the 42 kDa N-terminal fragments by ∼8 kDa, resulting in a 50 kDa band (Figure 4B, lower arrows), which is consistent with the covalent association of the reduced DTSSP molecule with the ∼27 lysine residues in the N-terminal fragment (complete reactivity would result in an additional 8.2 kDa). This interpretation is supported by observation that the Myc- and FLAG-reactive bands shift to even higher mobilities at DTSSP concentrations of >1.0 mM (Figure 4B, upper arrows). As shown in Figure 4A (lanes 3 and 6, indicated by arrows), the 50 kDa band can also be seen in conjunction with the 42 kDa band at low DTSSP concentrations and high exposure times. A band of similar molecular weight to the 50 kDa Myc- and FLAG-reactive bands was present in lanes that had not been treated with DTSSP (Figure 4A, lanes 1 and 2; Figure 4B, lane 1). This band was determined to be non-specific and possibly derived from IgG based on the fact that (i) it was present when the immunoblots were screened with a goat anti-mouse antibody (GAM) alone and (ii) this GAM-reactive band was insensitive to increasing concentrations of DTSSP (data not shown). As shown in Figure 4A, no DTSSP-sensitive bands were observed when the blots in Figure 4B were probed with KEEK, ABR or CT3 antibodies (data not shown). Taken together, the above results suggest that DTSSP specifically cross-linked the C-terminus of the type I receptor to the N-terminus of both the type I and type III IP3R. We conclude that the C-terminus of the type I isoform must be within 12 Å (spacer arm length of DTSSP) of the N-terminus type I and type III isoform in trypsin-digested homo- and heterotetrameric complexes.
Fig. 4. Cross-linking of N- and C-termini by DTSSP. (A) Co-transfected microsomes were subjected to trypsin digestion (lanes 2, 3 and 5–9) or mock digested (lanes 1 and 4) and pelleted. Microsomes in lane 3 and lanes 6–9 were then treated with 0.5 mM DTSSP for 30 min and the reaction was terminated by the addition of 20 mM Tris pH 7.5 as described in Materials and methods. All samples were then solubilized by the addition of 1% Zwittergent, immunoprecipitated with CT1 and subjected to SDS–PAGE. Lanes 1–3 were probed with an anti-Myc antibody and the same immunoblot was stripped and re-probed with a FLAG antibody (lanes 4–6). Cross-linked N-terminal peptides co-precipitating with CT1 are designated by a closed (type I) or open (type III) circle. No DTSSP-specific bands in trypsin treated samples were observed when the same immunoblot was probed with antibodies raised against amino acids 401–414 (KEEK, lane 7) and 1883–1902 (ABR, lane 8) of the type I receptor. Similarly, no immunoreactive bands were observed when the immunoblot was probed with an antibody to the type III C-terminus (CT3, lane 9). (B) Samples were treated as in (A), except that increasing concentrations (0.5–5.0 mM) of cross-linker were used in lanes 2–6 and lanes 8–12. Increasing the DTSSP concentration was accompanied by a shift in mobility of the 42 kDa N-terminal fragment in a dose-dependent manner (indicated by arrows, see text for details). As in (A), when the blot in (B) was stripped and reprobed with KEEK, ABR or CT3 antibodies, no immunoreactive reactive bands were observed in trypsin-treated samples (data not shown). *IgG band.
In vitro association of the type I ligand domain with transmembrane domain constructs
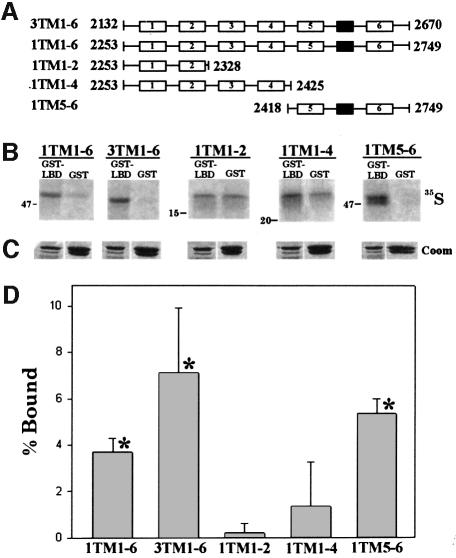
In order to confirm the above results using an alternative approach, the type I ligand-binding domain was expressed as a GST fusion protein (GST–LBD1) and the affinity for various in vitro translated C-terminal transmembrane domain constructs was investigated. Transmembrane domain constructs were first translated in the presence of microsomal membranes (Joseph et al., 1997), which were then lysed in a Triton X-100-containing buffer as outlined in Materials and methods. Binding to GST alone (4-fold excess) was used as a control to monitor non-specific binding. As shown in the representative autoradiogram in Figure 5B and quantitatively in Figure 5D, the type I and type III transmembrane domain constructs that contain all six membrane-spanning segments specifically interacted with GST–LBD1, whereas peptides comprising membrane-spanning regions 1–2 or 1–4 of the type I IP3R did not. However, transmembrane regions 5–6 of the type I IP3R specifically bound to the GST–LBD1 fusion protein. The association of GST–LBD with in vitro translated peptides was not sensitive to the addition of 10 µM IP3 (data not shown). This suggests that the C-terminal determinant for association with the N-terminus probably resides between amino acids 2418 and 2749 of the type I receptor, presumably within the highly conserved exposed cytoplasmic loops immediately preceding transmembrane domain 5 or following transmembrane domain 6 (Figure 5A). This is also the region that encompasses the ion conduction pathway (Ramos-Franco et al., 1999; Boehning and Joseph, 2000).
Fig. 5. Recombinant type I IP3R ligand-binding domain interacts specifically with in vitro translated type I and type III C-terminal transmembrane regions. C-terminal type I and type III in vitro translated transmembrane domain constructs representing transmembrane domains 1–6 of the type III IP3R and transmembrane domains 1–6, 1–2, 1–4 and 5–6 of the type I IP3R are schematically diagrammed in (A) (numbered open boxes denote a transmembrane region, and a closed box represents the putative pore loop). Amino acid boundaries (rat sequence) of these peptides are also indicated. The ligand-binding domain encompassing amino acids 1–605 of the type I receptor (SI– splice variant) was expressed as a GST fusion protein (GST–LBD1) in E.coli. Either 20 µg of GST or 5 µg of GST–LBD1 were immobilized by the batch method on GST–Sepharose in solubilization buffer and the affinity for the in vitro translated peptides was assayed (see Materials and methods). Immobilized proteins were quenched in SDS–PAGE sample buffer and run on a single 15% SDS–polyacrylamide gel. The gel was stained with Coomassie Blue to confirm equal loading (C) and autoradiographed (B). All lanes in (B) are identical exposures from the same gel (for clarity, input lanes have been omitted). Note that in (B) the molecular weight markers are different for each 35S-labeled peptide (for further details of the transmembrane domain constructs, see Joseph et al., 1997). Radiolabeled bands were quantified by densitometry and specific binding [% Bound; (D)] was calculated as outlined in Materials and methods. The data in (D) are pooled from at least three separate experiments. *Significant specific binding (P <0.001).
Inter-subunit gating by IP3 in tetrameric IP3Rs
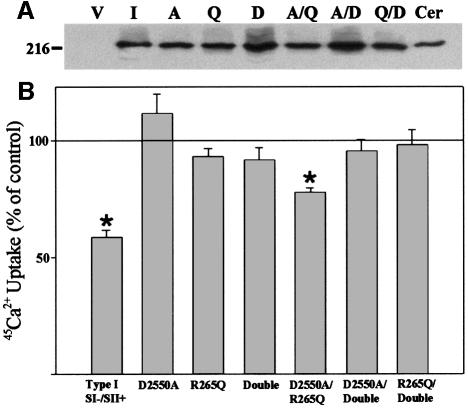
The association of the N-terminus with the C-terminus could be either intra- or inter-subunit in tetrameric IP3Rs. The evidence from the cross-linking experiments suggests that association between subunits is inter-subunit, since the type III N-terminus was found to be near the type I C-terminus. This raises the possibility that the ligand-binding domain of one subunit may be able to gate the ion permeation pathway of an adjacent subunit. A prediction that can be made from such a model is that permeation-defective and ligand-binding-defective mutants may be completely inactive when expressed alone, but when co-expressed may complement each other and partially rescue channel function. To test this hypothesis, we used a 45Ca2+ flux assay that measures the functional properties of recombinant IP3R channels. In this assay, microsomes are prepared from COS-7 cells co-transfected with both the 2b isoform of the sarco/endoplasmic reticulum ATPase (SERCA 2b) and recombinant IP3Rs. Under these conditions, the endogenous IP3Rs segregate into a vesicle population lacking Ca2+ pumps, whereas the recombinant SERCA 2b and recombinant IP3Rs are found in the same vesicle population. This assay allows recombinant IP3Rs to be measured in the absence of the background activity of the endogenous receptor population (Boehning and Joseph, 2000). The validity of this assay was supported by the observation that the mutation D2550A within the putative channel pore of the type I IP3R abolishes IP3-sensitive 45Ca2+ fluxes (Boehning and Joseph, 2000; see Figure 6). The mutation R265Q in the ligand-binding domain abolishes 3H-labeled IP3 binding (data not shown; Yoshikawa et al., 1996) and eliminates 45Ca2+ flux in response to IP3 in the microsome flux assay (Figure 6). When D2550A and R265Q are co-expressed, a partial rescue of IP3-sensitive 45Ca2+ flux was observed (54% of wild type). This reconstitution of channel function was not observed when either R265Q or D2550A was co-expressed with an IP3R subunit that contained both mutations (‘Double’ in Figure 6). None of these observations was related to impaired expression of any of the mutants (Figure 6A). These results, when combined with our cross-linking and in vitro binding data, are consistent with our hypothesis that one subunit is capable of gating an adjacent subunit in a tetramer (Figure 7).
Fig. 6. Effects on 45Ca2+ flux of co-expressing IP3R constructs defective in ligand binding and/or ion permeation. COS cells were transfected with wild-type or mutant IP3Rs that were defective in ion permeation (D2550A; Boehning and Joseph, 2000), ligand binding (R265Q; Yoshikawa et al., 1996) or both (Double). (A) Immunoblot of 20 µg of COS cell lysate prepared from cells expressing each IP3R construct. Lane V, vector pcDNA3.1; lane I, type I wild type; lane A, D2550A; lane Q, R265Q; lane D, double; lane Cer, 20 µg of cerebellar microsomes as a positive control. All mutations were generated in the type I receptor and the immunoblot was probed with CT1. Expression levels of each construct were not significantly different between multiple experiments (A; data not shown) 45Ca2+ flux in microsomal vesicles expressing recombinant IP3Rs was measured exactly as described previously at a [Ca2+]free of 1.0 µM (Boehning and Joseph, 2000). Data in (B) are plotted as the percentage inhibition of ATP-driven 45Ca2+ uptake by 1.0 µM IP3 (see Materials and methods). No response to IP3 is indicated by a line at 100%. *Significantly different from control (P <0.001).
Fig. 7. Model for the inter-subunit association of tetrameric subunits. Each subunit of the tetramer is schematically illustrated by an N-terminal ligand domain (circle) and a C-terminal pore (box). The type I receptor is represented by filled symbols and the type III receptor by open symbols. The results shown in Figures 4 and 5 support the direct association between N- and C-termini, and those in Figure 6 support the gating of one subunit by an adjacent subunit.
Discussion
Very little is known about the ultrastructural arrangement of homo- and heterotetrameric IP3R proteins or how the binding of IP3 at the N-terminus gates the C-terminal ion permeation pathway. In this paper we provide evidence that the N-terminal ligand-binding domain and the C-terminal pore domain of IP3R channels are in close ultrastructural proximity in homo- and heterotetramers. This was demonstrated directly by cross-linking the N-terminal ligand-binding domain peptides to homologous or heterologous C-terminal fragments of trypsin-digested IP3Rs. It is possible that trypsin digestion may affect the native tertiary structure of the IP3R protein, resulting in anomalous subunit interactions. This is unlikely, however, since trypsin-digested IP3R channels retain the ability to release Ca2+ in response to IP3 (Yoshikawa et al., 1999b). Further evidence to support direct N- and C-terminal interaction was provided by in vitro binding assays, which demonstrated that a fusion protein encompassing the ligand-binding domain of the type I receptor interacted with peptides encompassing the transmembrane domains of type I and III IP3Rs. By using several different transmembrane domain constructs, it was concluded that amino acids 2418–2749 (encompassing transmembrane regions 5 and 6) were sufficient for the interaction with the ligand-binding domain. Since this region comprises the ion conduction pore of the channel (Ramos-Franco et al., 1999; Boehning and Joseph, 2000), it provides a direct link between the IP3-binding domain and the channel pore.
In a tetramer, the interaction between N- and C-termini could occur in two possible orientations. In an intra-subunit interaction, the N-terminus of one subunit would interact with its own C-terminus. Alternatively, the N-terminus of one subunit could interact with the C-terminus of an adjacent subunit in an inter-subunit manner (Figure 7). The results of the cross-linking experiments are consistent with an inter-subunit interaction. Functional evidence for an inter-subunit orientation is illustrated in Figure 6, where an inactive ligand-binding mutant, R265Q (Yoshikawa et al., 1996), and permeation mutant D2550A (Boehning and Joseph, 2000) were partially able to reconstitute Ca2+ release activity when co-expressed. Our interpretation of this last observation is that the tetramers containing both mutant isoforms are arranged as shown in Figure 7 such that the ligand-binding domain of one subunit (D2550A) is capable of gating the permeation pathway of an adjacent subunit (R265Q). An alternative possibility is that a conformational change resulting from IP3 binding to one subunit can partially open the channel via conformational transitions that do not necessarily require direct interaction between N- and C-termini. Although it is difficult to distinguish between these models based on the 45Ca2+ flux assays alone, we believe that the biochemical evidence favors our interpretation of the flux data. Several studies have shown that activation of the IP3R by IP3 is highly cooperative, and it has been proposed that the binding of least three molecules of IP3 is required to open the channel (Dufour et al., 1997; Mak et al., 1998). The only possible head-to-tail arrangements of the mutant subunits that would place a functional ligand-binding domain adjacent to a permeation-competent pore are tetramers that contain one or two IP3-binding sites. Thus, in our model, the binding of a single molecule of IP3 to a tetramer could partially open the channel. Experiments to evaluate the gating behavior of D2550A/R265Q tetramers at the single-channel level will be necessary to validate this hypothesis.
The observation that D2550A/R265Q tetramers form functional channels also suggests that four negatively charged residues are not necessary at position 2550 of the type I IP3R. Similar observations have been noted in voltage-gated Ca2+ channels (Kim et al., 1993; Tang et al., 1993; Yang et al., 1993). For example, mutations at each position in the conserved ring of glutamates (E413, E731, E1140 and E1441) all have divergent effects on ionic selectivity due to different affinities for Ca2+ ions (for review see Hofmann et al., 1999). A more detailed biophysical analysis of D2550A/R265Q channel properties is required to elucidate the effects of these mutations on ionic selectivity, conductance and gating kinetics.
The ability of one IP3R subunit to be gated by a heterologous subunit has profound implications for the gating of this ion channel. While homotetrameric type I and type III IP3Rs have very similar ionic conductance, selectivity and permeation properties (Mak et al., 2000), they differ significantly in their modulation by IP3, Ca2+, ATP and phosphorylation (for review see Joseph, 1996). It is reasonable to expect that the regulatory properties of one isoform would exert an influence on other isoforms within a heterotetrameric channel. Indeed, it has been shown that heterotetrameric IP3R channels exhibit subunit dominance with regard to regulation by Ca2+ and ATP (Miyakawa et al., 1999). The direct association of the ligand-binding domain with the pore domain of a heterologous subunit provides a mechanistic link allowing differential regulation of an IP3R channel composed of multiple isoforms.
Several other tetrameric ion channels assemble with their N- and C-termini in close apposition. The most studied example is the calmodulin-sensitive interaction of the N- and C-terminus of cyclic-nucleotide-gated channels (Gordon et al., 1997; Varnum and Zagotta, 1997; He et al., 2000). Ruiz and Karpen (1997, 1999) have shown directly that doubly liganded subunits of cGMP-gated channels are active, and they have incorporated an association between adjacent subunits into a kinetic model of their data. Other examples in the literature include the N- and C-terminal interactions in voltage-gated potassium channels (Schulteis et al., 1996; Tucker and Ashcroft, 1999; Yao et al., 2000), the interaction between the N-terminus and R domain of the cystic fibrosis transmembrane regulator (Naren et al., 1999), and the interaction of the N-terminus with the central domain of ryanodine receptors (El-Hayek et al., 1999; Yamamoto et al., 2000). In each case, this interaction was determined to be important for the gating of the ion channel. These observations suggest that a functional association between N- and C-termini may represent a common structural feature found in many tetrameric ion channels.
The ryanodine receptor shares sequence homology with the IP3R, particularly within the transmembrane domain and the N-terminus (Furuichi et al., 1989; Mignery et al., 1990; Taylor and Traynor, 1995). Susceptibility to the genetic disorders malignant hyperthermia and central core disease (MH/CCD) is linked to mutations primarily within the N-terminus and central domain of the ryanodine receptor, both of which are conserved with IP3Rs (for review see Loke and MacLennan, 1998). Recent work using N-terminal and central domain peptides comprising mutational ‘hot spots’ in MH/CCD have shown that several of these peptides are capable of increasing the sensitivity of the ryanodine receptor to activating stimuli (El-Hayek et al., 1999; Yamamoto et al., 2000). It was concluded that this effect was due to the disruption of an inhibitory inter-domain interaction (El-Hayek et al., 1999; Yamamoto et al., 2000). These results are consistent with the in vivo hypersensitivity of the ryanodine receptor to agonists in MH/CCD and provides evidence that these regions are important for channel gating. Yamamoto et al. (2000) showed that an N-terminal peptide (referred to as DP3) of the skeletal muscle ryanodine receptor (RyR1) was capable of reversing the effects of activating central domain peptides. The authors concluded that this region (among others) was important for maintaining the closed state of the channel by interacting directly with the central domain. Since the DP3 region is 29% homologous to the corresponding domain of the type I IP3R, this region will be an important target in further studies on the mechanism of IP3R gating.
In conclusion, the data presented here provide strong evidence that the N- and C-termini of IP3R channels are tightly associated in an inter-subunit manner. We propose that this arrangement is important for the gating of the channel. Bringing the IP3-binding domain near to the gate of the channel allows efficient transduction of the activating stimulus. This may explain why trypsinized IP3Rs retain the ability to release Ca2+ in response to IP3 (Yoshikawa et al., 1999b). Furthermore, this arrangement allows for intimate regulation of IP3R function based upon the subunit composition of heterotetramers. Future work involving mutagenesis will be necessary in order to determine the molecular mechanism of gating in IP3R Ca2+ channels.
Materials and methods
Materials
Taq DNA polymerase, shrimp alkaline phosphatase, T4 DNA ligase, dNTPs, modified sequencing grade trypsin, chymostatin, tosyl-l-phenylalanine chloromethyl ketone (TPCK) and complete protease inhibitor cocktail were from Roche Molecular Biochemicals (Indianapolis, IN). Pfu polymerase was purchased from Stratagene (Madison, WI). Oligonucleotides were synthesized by the Kimmel Cancer Center Nucleic Acid Facility (Thomas Jefferson University, Philadelphia, PA) or by Gibco (Gaithersburg, MD). TransIT-LT1 cationic lipid transfection reagent was from Pan Vera Corporation (Madison, WI) and Protogel stabilized acrylamide solution from National Diagnostics (Atlanta, GA). Horseradish peroxidase (HRP)-conjugated donkey anti-rabbit antibody and 45Ca2+ were purchased from Amersham (Arlington Heights, IL). HRP-conjugated goat anti-mouse antibody was purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). SuperSignal Pico and SuperSignal Dura chemiluminescent substrates and DTSSP were obtained from Pierce (Rockford, IL). IP3 was purchased from Calbiochem. The trans-[35S]methionine/cysteine mixture was purchased from ICN Radiochemicals (Irvine, CA). Rabbit reticulocyte lysate was obtained from Promega (Madison, WI) and soybean trypsin inhibitor, protein A–Sepharose and heparin–agarose from Sigma (St Louis, MO). All other chemicals and reagents were purchased from Fisher Scientific (Springfield, NJ) at the highest quality available.
Expression constructs
Myc-I. The cDNA encoding the IP3R type I SI–/SII+/SIII+ splice variant in pCMV3 was the kind gift of Dr Thomas Südhof (University of Texas Southwestern Medical Center). 5′ non-coding sequences were removed and a Kozak consensus sequence inserted exactly as described previously, with the exception that the forward primer encoded a Myc epitope (MEQKLISEEDL) immediately preceding the IP3R start codon (ImycFb: 5′-CGGGGTACCGCCACCATGGAACAAAAACTCATCTCAGAAG AGGATCTGATGTCTGACAAAATGTCTAGT-3) (Kaznacheyeva et al., 1998; Boehning and Joseph, 2000).
FLAG-III. The insertion of a FLAG epitope at the N-terminus of the full-length rat type III IP3R has been described previously (Joseph et al., 2000).
GST-LBD1. Amino acids 1–605 of the rat type I (SII–) sequence were amplified by Pfu polymerase. The forward primer (LigFb: 5′-CGC GGATCCGACATGTCTGACAAAATGTCT-3′) encoded a BamHI site and the reverse primer (LigRb: 5′-CGGGAATTCGCTGACAAA CGTGTCAATCTC-3′) an EcoRI site to facilitate cloning into the plasmid pGEX-2TK (Amersham Pharmacia Biotech, Piscataway, NJ). This plasmid encodes GST immediately preceding the coding sequence, and therefore will express the type I LBD as a C-terminal fusion protein with GST.
Pore domain constructs. Pore domain constructs encoding the type I IP3R transmembrane regions 1–2 (1TM1–2), 1–4 (1TM1–4), 5–6 (1TM5–6), 1–6 (1TM1–6) and type III IP3R transmembrane regions 1–6 (3TM1–6) in pCITE-2a have been described elsewhere (Joseph et al., 1997).
D2550A. The generation of the loss-of-function pore mutation aspartic acid 2550 (rat) to alanine has been described previously (Boehning and Joseph, 2000).
R265Q. The IP3-binding-defective point mutation arginine 265 to glutamic acid (Yoshikawa et al., 1996) was accomplished using the single overlap extension (SOE) mutagenesis method (Horton, 1995). Two PCRs were carried out. The first reaction used a wild-type forward primer flanking an endogenous BsaBI site (T1BsaBIF: 5′-CTA CAAAGGATTTGCATCCTTGGCTG-3′) and a reverse primer that encoded the desired mutation (R265QR: 5′-TCTGCCGGTTGTCTG CAGGAAGACGTC-3′). The second amplification was accomplished using a primer complementary to R265QR (R265QF: 5′-CACGTC TTCCTGCAGACAACCGGCAGA-3′) and a primer that flanked an endogenous 3′ KpnI site (LigRb, see above). The PCR fragments generated were gel purified (Qiagen, Chatsworth, CA) and were used in combination as a template for PCR amplification using T1BsaBIF and LigRb. The resultant 1950 bp fragment was then gel purified and cloned into BsaBI- and KpnI-digested type I IP3R cDNA in pCMV to generate the mutated full-length IP3R. The mutation was confirmed by sequencing and [3H]IP3 binding assays (data not shown).
D2550A–R265Q double. Site-directed mutagenesis of aspartic acid 2550 to alanine was accomplished using R265Q as the template for PCR using the Stratagene QuickChange site-directed mutagenesis kit according to the manufacturer’s instructions. The forward primer was D2550AF (5′-GGCGGAGTAGGAGCTGTGCTCAGGAAG-3′) and the reverse primer (D2550AR) was complementary to the forward primer.
Expression and purification of GST–LBD
GST–LBD1 was propagated in Escherichia coli strain DH5α and induced with 0.1 mM isopropyl β-d-thiogalactopyranoside (IPTG) at 37°C for 3 h. After induction, bacteria were processed for purification of fusion proteins by the batch method as described by the manufacturer (Amersham Pharmacia Biotech). Purified fusion protein was affinity purified on a heparin–agarose column and eluted in 1 ml fractions with a 0.5 M NaCl gradient. Fractions enriched in full-length fusion protein (as determined by SDS–PAGE) were then pooled and concentrated to ∼2.5 ml (Ultrafree-15 centrifugal filter device; Millipore Corporation, Bedford, MA). The fusion protein was then desalted on a PD-10 column (Amersham Pharmacia Biotech) and concentrated to ∼2 mg/ml. Typical yields from 1.2 l of culture were 1 mg of fusion protein with a specific IP3-binding capacity of ∼10 pmol/mg protein measured with 9.6 nM [3H]IP3 (data not shown).
In vitro translation of transmembrane domains
Run-off transcription and in vitro translation of the transmembrane domain constructs in the presence of canine pancreatic microsomes were carried out as described previously (Joseph et al., 1997).
Cell culture and transfection
Cell culture, transfection and expression of recombinant IP3Rs in COS-7 cells have been described elsewhere (Boehning and Joseph, 2000).
Trypsin digestion of recombinant IP3Rs
Microsomes were prepared from COS-7 cells expressing epitope-tagged type I and/or type III IP3Rs as described previously with the exception that the final centrifugation was performed at 100 000 g for 60 min at 4°C (Bronfman et al., 1998). Assays were typically performed with 50 µg of microsomes and 20 µg/ml trypsin unless otherwise noted. Digestions were performed in 120 mM KCl, 20 mM Tris–HCl pH 7.8, 1 mM EDTA, 1 mM dithiothreitol (DTT), 30 µg/ml chymostatin and 100 µg/ml TPCK for 10 min at room temperature. Reactions were terminated by the addition of a 10-fold excess of soybean trypsin inhibitor and 1 mM phenylmethylsulfonyl fluoride (PMSF). Each reaction was then spun at 100 000 g for 1 h at 4°C to pellet microsomal membranes. Pellets were either quenched with SDS–PAGE sample buffer directly or resuspended in 150 mM NaCl, 50 mM Tris–HCl pH 7.8, 1 mM EDTA, 1% Triton X-100, 0.5 mM PMSF and 1× complete protease inhibitor cocktail (Roche Molecular Biochemicals) for immunoprecipitation assays. Protein in the supernatant fractions was precipitated by incubation with StrataClean resin (Stratagene, La Jolla, CA) for 10 min at room temperature. The resin was then directly quenched in SDS sample buffer. Samples were run on 8% SDS–polyacrylamide gels (unless otherwise noted) and electrotransferred to nitrocellulose membranes prior to immunoblotting.
Co-precipitation assays
Trypsin-digested microsomes were processed as above and lysed in Triton X-100-containing resuspension buffer. Samples were then immunoprecipitated with protein A–Sepharose and either CT1 or CT3 polyclonal antibodies specific for the type I or type III IP3R, respectively (see below). Immunoprecipitations were carried out for 15 h, after which the beads were washed and quenched directly in SDS–PAGE sample buffer. In some cases, the immunoprecipitation was carried out after disruption of IP3R tetramers using 1% Zwittergent to lyse microsomal membranes.
Cross-linking of peptide fragments
Samples were processed to the pellet stage as above and then resuspended in PBS containing protease inhibitors. Microsomal vesicles were then exposed to varying concentrations of DTSSP for 30 min at room temperature. Reactions were terminated by the addition of 20 mM Tris–HCl pH 7.5 for 15 min at room temperature. Zwittergent was then added to the microsomes at a final concentration of 1% to lyse the vesicles and disrupt any non-covalent interactions. Insoluble material was removed by centrifugation and supernatants were processed for immunoprecipitation as described above.
Antibodies and western blotting
CT1 and CT3 isoform-specific polyclonal antibodies directed against the C-terminus of the type I and type III receptor have been described previously (Joseph et al., 1995, 1997). Monoclonal antibodies generated against the Myc epitope were purchased from the Cell Center of the University of Pennsylvania (Philadelphia, PA). Monoclonal antibodies generated against the FLAG epitope were purchased from Sigma. Polyclonal antibodies raised against amino acids 1883–1902 of the type I IP3R were purchased from Affinity BioReagents (Golden, CO). Polyclonal antibodies directed towards residues 401–414 of the type I IP3R have been described previously (Joseph et al., 1995).
GST–LBD affinity assays
Glutathione–agarose and either 5 µg of GST–LBD or 20 µg of GST were added to varying amounts of in vitro translated transmembrane domain constructs in Triton X-100 solubilization buffer (see above). This mixture was incubated for 30 min at room temperature with rotation and washed with solubilization buffer before quenching the glutathione–agarose beads directly with SDS–PAGE quench buffer. Samples were run on a 15% SDS–polyacrylamide gel together with 1/10 of input transmembrane domain protein. Gels were stained with Coomassie Blue to confirm equal fusion protein loading; they were then dried and autoradiographed. All samples were run on the same gel to standardize exposure times. Radioactive bands corresponding to the transmembrane domains were quantified by densitometry and the percentage specific binding was expressed as [(Rspe) – (Rnon)/10(input)] × 100, where Rspe refers to transmembrane domain constructs pulled down by GST–LBD, Rnon is the non-specific interaction with GST alone, and input is 1/10 the input of in vitro translated protein. Similar results were obtained if the bands were excised from the gel and counted in scintillant (data not shown).
45Ca2+ flux measurements
45Ca2+ flux assays were performed exactly as described previously (Boehning and Joseph, 2000). Briefly, microsomal vesicles were prepared from COS-7 cells transiently transfected with various IP3 receptor constructs in conjunction with the 2b isoform of the human sarcoplasmic reticulum ATPase (hSERCA 2b). The vesicles were then assayed for 45Ca2+ uptake in the presence of potassium oxalate and MgATP. The inclusion of IP3 in the assay buffer caused a reduction in the initial rate of uptake only in those vesicles that were prepared from cells expressing both recombinant IP3Rs and hSERCA 2b, allowing an indirect measurement of recombinant channel activity in isolation from endogenous IP3Rs (Boehning and Joseph, 2000). The data in Figure 6 are expressed as the percentage of the initial rate of uptake in the presence of IP3 versus the initial rate of uptake in the absence of IP3.
Acknowledgments
Acknowledgements
We would like to thank Dr Christopher Nicchitta (Duke University Medical Center) for the kind gift of canine pancreatic microsomes, and Dr Jonathan Lytton (University of Calgary, Alberta, Canada) and Dr David H.MacLennan (University of Toronto, Ontario, Canada) for the SERCA 2b cDNA. The authors also wish to thank Dr György Hajnóczky and Dr J.Kevin Foskett for comments on this manuscript. This work was supported by RO1-DK34804 (S.K.J.) and a pre-doctoral fellowship from training grant T32-AA07463 (D.B.) from the National Institutes of Health (USA).
References
- Berridge M.J. and Irvine,R.F. (1989) Inositol phosphates and cell signaling. Nature, 341, 197–205. [DOI] [PubMed] [Google Scholar]
- Blondel O., Takeda,J., Janssen,H., Seino,S. and Bell,G. (1993) Sequence and functional characterization of a third inositol trisphosphate receptor subtype, IP3R-3, expressed in pancreatic islets, kidney, gastrointestinal tract and other tissues. J. Biol. Chem., 268, 11356–11363. [PubMed] [Google Scholar]
- Boehning D. and Joseph,S.K. (2000) Functional properties of recombinant type I and type III inositol 1,4,5-trisphosphate receptor isoforms expressed in COS-7 cells. J. Biol. Chem., 275, 21492–21499. [DOI] [PubMed] [Google Scholar]
- Bronfman M., Loyola,G. and Koenig,C.S. (1998) Isolation of intact organelles by differential centrifugation of digitonin-treated hepatocytes using a table Eppendorf centrifuge. Anal. Biochem., 255, 252–256. [DOI] [PubMed] [Google Scholar]
- Dufour J., Arias,I.M. and Turner,T.J. (1997) Inositol 1,4,5-trisphosphate and calcium regulate the calcium channel function of the hepatic inositol 1,4,5-trisphosphate receptor. J. Biol. Chem., 272, 2675–2681. [DOI] [PubMed] [Google Scholar]
- El-Hayek R., Saiki,Y., Yamamoto,T. and Ikemoto,N. (1999) A postulated role of the near amino-terminal domain of the ryanodine receptor in the regulation of the sarcoplasmic reticulum Ca2+ channel. J. Biol. Chem., 274, 33341–33347. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Yoshikawa,S., Miyawaki,A., Wada,K., Maeda,N. and Mikoshiba,K. (1989) Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature, 342, 32–38. [DOI] [PubMed] [Google Scholar]
- Gordon S.E., Varnum,M.D. and Zagotta,W.N. (1997) Direct interaction between amino- and carboxy-terminal domains of cyclic nucleotide-gated channels. Neuron, 19, 431–441. [DOI] [PubMed] [Google Scholar]
- He Y., Ruiz,M. and Karpen,J.W. (2000) Constraining the subunit order of rod cyclic nucleotide-gated channels reveals a diagonal arrangement of like subunits. Proc. Natl Acad. Sci. USA, 97, 895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann F., Lacinová,L. and Klugbauer,N. (1999) Voltage-dependent calcium channels: from structure to function. Rev. Physiol. Biochem. Pharmacol., 139, 33–87. [DOI] [PubMed] [Google Scholar]
- Horton R.M. (1995) PCR-mediated recombination and mutagenesis. SOEing together tailor-made genes. Mol. Biotechnol., 3, 93–99. [DOI] [PubMed] [Google Scholar]
- Joseph S.K. (1996) The inositol trisphosphate receptor family. Cell Signal., 8, 1–7. [DOI] [PubMed] [Google Scholar]
- Joseph S.K., Pierson,S. and Samanta,S. (1995) Trypsin digestion of the inositol trisphosphate receptor: implications for the conformation and domain organization of the protein. Biochem. J., 307, 859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S.K., Boehning,D., Pierson,S. and Nicchitta,C.V. (1997) Membrane insertion, glycosylation and oligomerization of inositol trisphosphate receptors in a cell-free translation system. J. Biol. Chem., 272, 1579–1588. [DOI] [PubMed] [Google Scholar]
- Joseph S.K., Boehning,D. and Lin,C. (1999) Inositol 1,4,5-trisphosphate receptors: molecular aspects. In Putney,J.W. (ed.), Calcium Signaling. CRC Press, New York, NY, pp. 203–226. [Google Scholar]
- Joseph S.K., Bokkala,S., Boehning,D. and Zeigler,S. (2000) Factors determining the composition of inositol trisphosphate receptor hetero-oligomers expressed in COS cells. J. Biol. Chem., 275, 16084–16090. [DOI] [PubMed] [Google Scholar]
- Kaznacheyeva E., Lupu,V.D. and Bezprozvanny,I. (1998) Single-channel properties of inositol (1,4,5)-trisphosphate receptor heterologously expressed in HEK-293 cells. J. Gen. Physiol., 111, 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.S., Morii,T., Sun,L.X., Imoto,K. and Mori,Y. (1993) Structural determinants of ion selectivity in brain calcium channel. FEBS Lett., 318, 145–148. [DOI] [PubMed] [Google Scholar]
- Loke J. and MacLennan,D.H. (1998) Malignant hyperthermia and central core disease: disorders of Ca2+ release channels. Am. J. Med., 104, 470–486. [DOI] [PubMed] [Google Scholar]
- Mak D.D., McBride,S. and Foskett,J.K. (1998) Inositol 1,4,5-trisphos phate activation of inositol trisphosphate receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc. Natl Acad. Sci. USA, 95, 15821–15825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.D., McBride,S., Raghuram,V., Yue,Y., Joseph,S.K. and Foskett,J.K. (2000) Single-channel properties in endoplasmic reticulum membrane of recombinant type 3 inositol trisphosphate receptor. J. Gen. Physiol., 115, 241–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignery G.A. and Sudhof,T.C. (1990) The ligand binding site and transduction mechanism in the inositol-1,4,5-triphosphate receptor. EMBO J., 9, 3893–3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignery G.A., Newton,C.L., Archer,B.T.,III and Sudhof,T.C. (1990) Structure and expression of the rat inositol 1,4,5-trisphosphate receptor. J. Biol. Chem., 265, 12679–12685. [PubMed] [Google Scholar]
- Miyakawa T., Maeda,A., Yamazawa,T., Hirose,K., Kurosaki,T. and Lino,M. (1999) Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO J., 18, 1303–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki A., Furuichi,T., Ryou,Y., Yoshikawa,S., Nakagawa,T., Saitoh,T. and Mikoshiba,K. (1991) Structure–function relationships of the mouse inositol 1,4,5-trisphosphate receptor. Proc. Natl Acad. Sci. USA, 88, 4911–4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naren A.P., Cormet-Boyaka,E., Fu,J., Villain,J., Blalock,J.E., Quick,M.W. and Kirk,K.L. (1999) CFTR chloride channel regulation by an interdomain interaction. Science, 286, 544–548. [DOI] [PubMed] [Google Scholar]
- Patel S., Joseph,S.K. and Thomas,A.P. (1999) Molecular properties of inositol 1,4,5 trisphosphate receptors. Cell Calcium, 25, 247–264. [DOI] [PubMed] [Google Scholar]
- Ramos-Franco J., Galvan,D., Mignery,G.A. and Fill,M. (1999) Location of the permeation pathway in the recombinant type I inositol 1,4,5-trisphosphate receptor. J. Gen. Physiol., 114, 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz M. and Karpen,J.W. (1997) Single cyclic nucleotide-gated channels locked in different ligand-bound states. Nature, 389, 389–392. [DOI] [PubMed] [Google Scholar]
- Ruiz M. and Karpen,J.W. (1999) Opening mechanism of a cyclic nucleotide-gated channel based on analysis of single channels locked in each liganded state. J. Gen. Physiol., 113, 873–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis C.T., Nagaya,N. and Papazian,D.M. (1996) Inter-subunit interaction between amino- and carboxy-terminal cysteine residues in tetrameric Shaker K+ channels. Biochemistry, 35, 12133–12140. [DOI] [PubMed] [Google Scholar]
- Sudhof T.C., Newton,C.L., Archer,B.T.,III, Ushkaryov,Y.A. and Mignery,G.A. (1991) Structure of a novel InsP3 receptor. EMBO J., 10, 3199–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S., Mikala,G., Bahinski,A., Yatani,A., Varadi,G. and Schwartz,A. (1993) Molecular localization of ion selectivity sites within the pore of a human L-type cardiac calcium channel. J. Biol. Chem., 268, 13026–13029. [PubMed] [Google Scholar]
- Taylor C.W. and Traynor,D. (1995) Calcium and inositol trisphosphate receptors. J. Membr. Biol., 145, 109–118. [DOI] [PubMed] [Google Scholar]
- Taylor C.W., Genazzani,A.A. and Morris,S.A. (1999) Expression of inositol trisphosphate receptors. Cell Calcium, 26, 237–251. [DOI] [PubMed] [Google Scholar]
- Tucker S.J. and Ashcroft,F.M. (1999) Mapping of the physical interaction between the intracellular domains of an inwardly rectifying potassium channel, Kir6.2. J. Biol. Chem., 274, 33393–33397. [DOI] [PubMed] [Google Scholar]
- Varnum M.D. and Zagotta,W.N. (1997) Interdomain interactions underlying activation of cyclic nucleotide-gated channels. Science, 278, 110–113. [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz R.J.H. (1995) Type I, II and III inositol 1,4,5-trisphos phate receptors are unequally susceptible to down-regulation and are expressed in markedly different proportions in different cell types. J. Biol. Chem., 270, 11678–11683. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., El-Hayek,R. and Ikemoto,N. (2000) Postulated role of interdomain interaction within the ryanodine receptor in Ca2+ channel regulation. J. Biol. Chem., 275, 11618–11625. [DOI] [PubMed] [Google Scholar]
- Yang J., Ellinor,P.T., Sather,W.A., Zhang,J.F. and Tsien,R.W. (1993) Molecular determinants of Ca2+ selectivity and ion permeation in L-type Ca2+ channels. Nature, 366, 158–161. [DOI] [PubMed] [Google Scholar]
- Yao X., Liu,W., Tian,S., Rafi,H., Segal,A.S. and Desir,G.V. (2000) Close association of the N terminus of Kv1.3 with the pore region. J. Biol. Chem., 275, 10859–10863. [DOI] [PubMed] [Google Scholar]
- Yoshikawa F., Morita,M., Monkawa,T., Michikawa,T., Furuichi,T. and Mikoshiba,K. (1996) Mutational analysis of the ligand-binding site of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem., 271, 18277–18284. [DOI] [PubMed] [Google Scholar]
- Yoshikawa F., Iwasaki,H., Michikawa,T., Furuichi,T. and Mikoshiba,K. (1999a) Cooperative formation of the ligand-binding site of the inositol 1,4,5-trisphophate receptor by two separable domains. J. Biol. Chem., 274, 328–334. [DOI] [PubMed] [Google Scholar]
- Yoshikawa F., Iwasaki,H., Michikawa,T., Furuichi,T. and Mikoshiba,K. (1999b) Trypsinized cerebellar inositol 1,4,5-trisphosphate receptor: structural and functional coupling of cleaved ligand binding and channel domains. J. Biol. Chem., 274, 316–327. [DOI] [PubMed] [Google Scholar]