Abstract
Angiogenesis, the growth of new blood vessels, plays a critical role in progression of tumor growth and metastasis, making it an attractive target for both cancer imaging and therapy. Several molecular markers, including those that are involved in the angiogenesis signaling pathway and those unique to tumor angiogenic vessels, have been identified and can be used as targets for molecular imaging of cancer. With the introduction of ultrasound contrast agents that can be targeted to those molecular markers, targeted contrast-enhanced ultrasound (molecular ultrasound) imaging has become an attractive imaging modality to non-invasively assess tumor angiogenesis at the molecular level. The advantages of molecular ultrasound imaging such as high temporal and spatial resolution, non-invasiveness, real-time imaging, relatively low cost, lack of ionizing irradiation and wide availability among the imaging community will further expand its roles in cancer imaging and drug development both in preclinical research and future clinical applications.
Introduction to Tumor Angiogenesis
Angiogenesis is the development of new vasculature from pre-existing blood vessels and/or circulating endothelial stem cells [1]. This process is required for pre- and postnatal development and for tissue repair [2,3]. It is well established that angiogenesis is also one of the key aspects in the growth and metastasis of solid tumors [4,5,6]. Typically, tumor-associated angiogenesis goes through two phases, an avascular and a vascular phase that are separated by the “angiogenic switch” (Figure 1A). The avascular phase of tumors corresponds to small and occult lesions that stay dormant and subsist on diffusion of nutrients from the host microvasculature. After reaching a certain size (usually around 1–2 mm [7]), a small subset of dormant tumors enter the vascular phase in which exponential tumor growth ensues. Angiogenesis is a complex multistep process regulated by many factors. At the onset of angiogenesis, a number of pro-angiogenic growth factors (e.g., vascular endothelial growth factors, platelet-derived growth factor, fibroblast growth factors) and proteolytic enzymes (e.g., matrix metalloproteinases, cathepsin cysteine proteases, plasmin) are secreted into the interstitium. This leads to the degradation of basal membrane surrounding the pre-existing vasculature, along with proliferation and migration of smooth muscle and endothelial cells (Figure 1A). All these events finally lead to the alignment and organization of endothelial cells to form new vessels and a vascular network within the tumor [1].
Figure 1.
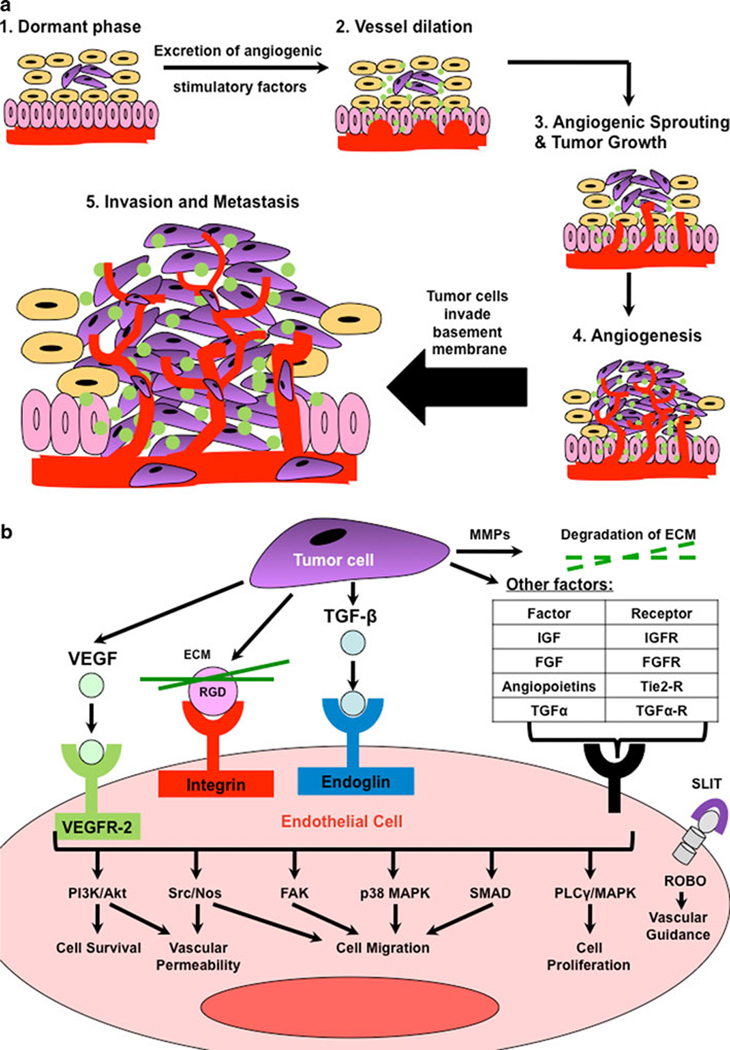
Tumor angiogenesis is a complex multi-step and multi-signalling process. (a) When a dormant tumor (step 1) reaches critical size (usually ~1–2 mm) and receives intracellular signals from the tumor microenvironment (e.g., hypoxia), the tumor cells (purple; yellow cells: normal epithelial cells; pink vertical cells: stroma/basement membrane; red line: blood vessel) begin to excrete growth factors (step 2), cytokines, and other signaling molecules (green). These factors can influence the stromal cells to also produce these factors, which signal (see b) blood vessels to dilate, and grow towards the tumor cells. Microvessels are sprouted (step 3) within the tumor, which survives and proliferates due to the new delivery of nutrients, growth factors, and oxygen. Eventually, a complex vascular network has been created within the tumor (step 4) which promotes (step 5) invasion beyond the basement membrane and metastasis to other tissues. Schematic is adapted from [1]. (b) Several growth factors are involved in promoting tumor angiogenesis (in a). The most characterized players include ligand-receptor complexes of VEGF-VEGFR-2, RGD (from ECM matrix)-integrin, and TGF-β-endoglin. These, along with other factor-receptor (shown in table) or protein-protein (SLIT and ROBO) binding complexes, result in activation and cross-talk among downstream signaling pathways to promote increased endothelial cell survival, vascular permeability, cell migration, cell proliferation, and vascular guidance. Adapted from Cell Signaling Technologies [79].
Advances in knowledge of tumor angiogenesis have resulted in the identification of several molecules involved in tumor angiogenic signaling. These molecules have been exploited for their use as targets for molecular imaging and quantification of tumor angiogenesis. Furthermore, discovery of these molecules has lead to realization of the concept that tumor vessels can be selectively targeted for therapy. The development of anti-angiogenic therapy (e.g., an antibody or small molecule inhibitor of new vessel formation [6,8], or anti-vascular therapy (e.g., a small molecule inhibitor of new vessel formation as well as destructor of pre-existing tumor microvessels [8]) has been one of the most promising avenues for cancer therapeutics in the last few years.
Molecular Markers of Tumor Angiogenesis
There are numerous proteins/enzymes involved in the angiogenic signal transduction pathway (Figure 1B) such as vascular endothelial growth factor receptor type 2 (VEGFR-2), integrins, and endoglin (to mention only a few), and, several studies have demonstrated that these molecules are over-expressed on tumor angiogenic vessels compared with normal vessels [9,10,11]. Among the best-characterized angiogenic signal transduction pathways is the avenue modulated by vascular endothelial growth factor (VEGF) and its receptors (VEGFRs) [12,13]. The VEGF family is composed of 7 members with a common VEGF homology domain, and, amongst them, VEGFA-plays an important role in tumor angiogenesis. VEGF-A is a homodimeric, disulfide-bound glycoprotein existing in several isoforms with different numbers of amino acid residues. VEGF-A binds to two receptor tyrosine kinases, VEGFR-1 and VEGFR-2 [14], and of these two receptors, VEGFR-2 acts as a direct signal transducer of tumor angiogenesis. Activation of VEGFR-2 triggers multiple signaling networks that result in endothelial cell survival, mitogenesis, migration, differentiation, and vascular permeability (Figure 1B). Tumor-associated endothelial cells over-express VEGFR-2 and its expression has been associated with tumor progression and poor prognosis in several tumors, including colorectal, gastric, and pancreatic carcinomas; angiosarcoma; breast, prostate and lung cancers, malignant gliomas and melanomas [15], [16]. The expression of VEGFR-2 in tumor vascular endothelial cells in much higher compared with endothelial cells of normal tissue [14] which makes it an attractive molecular target for angiogenic molecular imaging and cancer therapy.
Another signaling pathway involved in the angiogenic response involves the composition of the extracellular matrix (ECM) and a family of transmembrane proteins, the integrins (Figure 1B). Integrins are expressed both on endothelial cells for modulation of cell migration and survival during angiogenesis, as well as on cancer cells for invasion and movement across blood vessels for metastasis. Integrins consist of two non-covalently bound chains, the α (alpha) and β (beta) subunits. In mammals, 18 α and 8 β subunits assemble into 24 different types of receptors with varying functions. Integrin αvβ3 in particular has received a lot of attention as it is highly expressed on tumor-associated endothelium, and almost absent on normal vessels making it very useful as a molecular target of angiogenesis [17,18]. Integrin αvβ3 plays a role in tumor invasion and metastasis by recruiting and activating proteases such as matrix metalloproteinases (MMPs). Expression of MMPs is associated with the removal of the ECM barrier to allow cancer cells and endothelial cells to invade the basement membrane. A number of MMPs are also specifically involved in angiogenesis, including MMPs 1, 2, 3, 9, and 14 [19]. Several ECM molecules, such as fibronectin and tenascin, are also markers of angiogenesis. Spliced variants of fibronectin (the extra B domain, ED-B) [20] and tenascin (tenascin-c) [21,22] are highly expressed around tumor neovasculature. ED-B of fibronectin, inserted in fibironectin by alternative splicing, is specifically expressed in neovessels of tumors [20]. A spliced variant of tenascin known as tenascin-c which interacts with several ECM molecules, is highly expressed in the tumor neovasculature of lung cancers and modulates cell migration, proliferation and cellular signaling [21,22].
Identification of other key players in angiogenic signaling is emerging. Transforming growth factor β (TGF-β) signaling plays a role in several biological processes, including embryonic development, carcinogenesis, wound healing and angiogenesis [11]. In normal cells, the TGF-β pathway restricts cell growth, differentiation and cell death. In contrast, in malignant cells, various components of the TGF-β signaling pathway become mutated thereby exploiting the ability of TGF-β to modulate growth promoting processes, including cell invasion and angiogenesis. TGF-β signaling is mediated by TGF-β binding to TGF-β receptors, of which, there are three classes: Types I and II are heterodimeric receptors whereas type III are homodimeric receptors. Endoglin is a TGF-β type III receptor which has been shown to participate in signaling angiogenesis. Endoglin is predominantly expressed on proliferating endothelial cells [23], and inhibition of its expression has been shown to restore the growth suppressing signals of the TGF-β signaling pathway [24]. Thus, endoglin is an attractive molecular target of angiogenesis since it is over-expressed on tumor-associated endothelium [11].
Other molecular targets of tumor angiogenesis can be identified either by immunohistology or by differential gene expression analysis on isolated tumor endothelial cells [25, Orgaz, 2008 #535]; several of these include prostate-specific membrane antigen (PSMA), ephrin (Eph) ligands and receptors, and magic roundabout-4 (Robo-4), among others [26]. PSMA is a type II transmembrane glycoprotein over-expressed in prostate cancer. It is also expressed on the neovascular endothelium of most solid tumor types [27]. Invasion studies with PSMA-null cells showed that PSMA regulates cell invasion by controlling signaling from integrins [28]. Robo-4, a new member of the roundabout receptor family [29], is exclusively expressed in endothelial cells [30]. Transfection of Robo4 into endothelial cells has been shown to increase the number of filopodial extention from the cell suggesting its role in endothelial cell migration [31]. The ephrin (Eph) ligands and its receptors are also involved in the process of angiogenesis. The ephrins bind to two families of trans-membrane receptor tyrosine kinases, EphA and EphB. Several ephrins and Eph receptors have been shown to be present on tumor vascular endothelium in ovarian cancer [32]. Amongst them, EphB2 in particular is thought to play an important role in promoting tumor recruitment of blood vessels and tumor cell intravasation into the blood circulation, contributing to both tumor growth and metastasis [33,34].
The advantage of identifying new proteins that are highly specific for tumor-endothelium is not only that they are attractive targets for drug development but also for distinguishing the process of angiogenesis from inflammation. Angiogenesis also plays a role in inflammation, where the aforementioned molecular targets (i.e., VEGFR-2, integrin αVβ3, etc.) can also be over-expressed on angiogenic vessels associated with inflammation; therefore, it is important to have the ability to differentiate inflammation from cancer. This is especially important considering the relationship between cancer and inflammation – cancer formation has been shown to induce an inflammatory response (i.e., the immune system recognizes the cancer cells as foreign and initiates an immune response) [35,36] and inflammation is associated with an increased risk of cancer (e.g., conditions such as hepatitis or pancreatitis involve production of free radicals that can lead to cellular damage and/or transformation [37]). Since patients with pre-existing inflammatory conditions are at an elevated risk for developing cancer, they may undergo more frequent screening in the future (e.g., via molecular imaging) for cancer; therefore, it is important to be able to distinguish the cancer tissue in a background of inflamed tissue by using tumor-specific contrast agents (i.e., targeted to tumor-specific molecular markers). In depth understanding of the cellular and molecular basis of tumor angiogenesis over the past two decades, and continuing research to identify new molecular targets of angiogenesis has resulted in the identification of several promising molecules over-expressed on tumor endothelium. These molecules can be used for cancer imaging, staging, therapeutic treatment, and monitoring.
Molecular Imaging of Tumor Angiogenesis
Molecular imaging – the in vivo visualization and quantification of molecular markers involved in biological/cellular processes [38,39] – can be performed with various imaging modalities including, positron emission tomography (PET), single-photon emission computed tomography (SPECT), magnetic resonance imaging (MRI), magnetic resonance spectroscopy (MRS), ultrasound (US), and optical imaging (Figure 2). Spectroscopic techniques, including MRS [40] and optical spectroscopy methods such as Raman spectroscopy, [41,42] infrared spectroscopy,[42] or Fourier Transform infrared spectroscopy [43], among others [44], can measure molecular markers directly by detecting changing energies associated with molecular bonds. These methods involve applying a pulse of energy (e.g., magnetic field or photon) to excite molecules to a different energy state, and measuring the difference in energy states during relaxation following the energy pulse; thus, different energies can be measured for specific molecules. Other modalities require the use of contrast agents (Figure 2), constructed of a label for readout by an imaging modality (e.g., radiolabel for PET or SPECT imaging) and a ligand that will bind to the targeting molecule of interest. Ligands for molecular targeting can be small molecules, peptides, oligonucleotides, proteins, antibody fragments or antibodies. More simplistic contrast agents can involve direct conjugation or an insertion of a small linking molecule between an imaging label and ligand, while more sophisticated contrast agents can involve multiple labels and/or using a biocompatible nano- or micro-sized particles (e.g., carbon nanotube, liposome, microbubble; see Figure 2).
Figure 2.
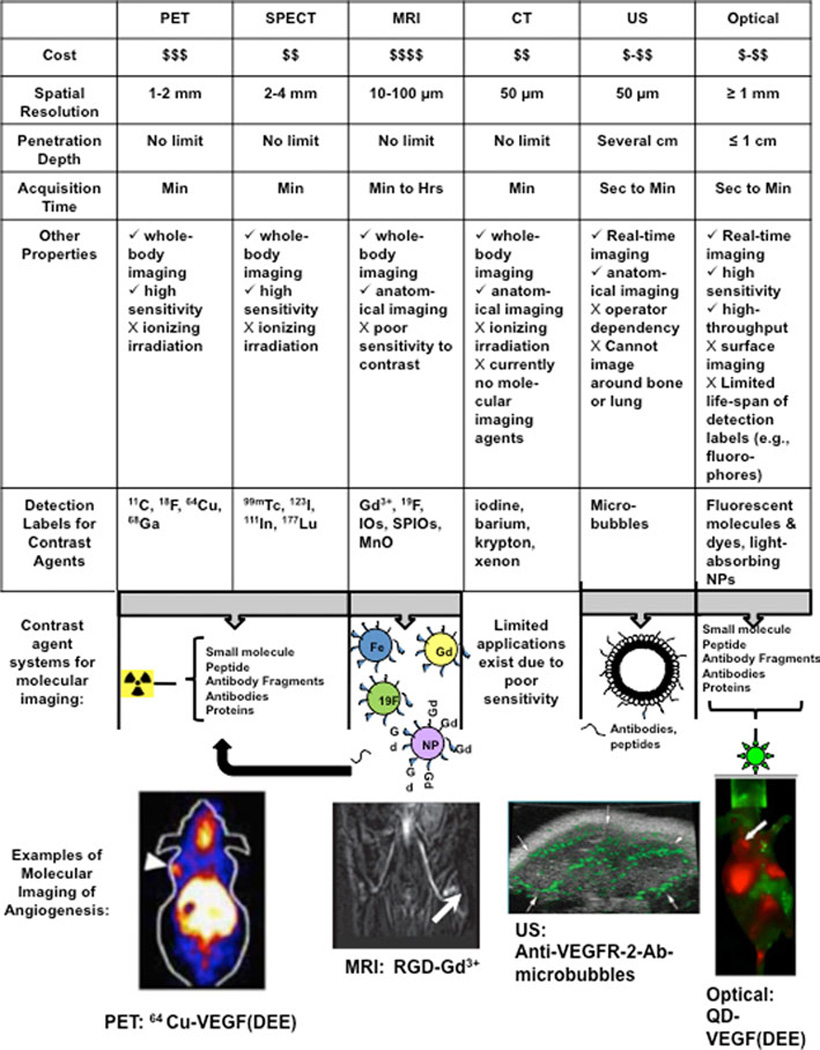
Advantages and disadvantages of various molecular imaging modalities, including positron emission tomography (PET), single photon emission computed tomography (SPECT), magnetic resonance imaging (MRI), computed tomography (CT), ultrasound (US), and optical imaging (adapted from Willmann et al. 2008, [47]). Values are listed for small animal imaging systems. Basic contrast agent design includes a binding ligand such as a small molecule, peptide, antibody/antibody fragment, or protein to bind to the protein target of interest, as well as a label for readout by the different imaging modalities. Readout labels are shown as 1) radiolabels for PET or SPECT (radioisotopes listed separately); 2) Gadolinium (Gd, Gd3+)-loaded nanoparticles (nanoparticles are approximately 10 nm to 200 nm; see review Nune et al, [80]) or liposomes (~200 nm lipid-shelled vesicle; see review: Blanco et al, [81]), iron oxide (IO) nanoparticles (NPs), 19F-loaded nanoparticles for MRI, or not shown: superparamagnetic iron oxide (SPIO) NPs, or monocrystalline oxide (MnO) (see review on molecular MRI contrast agents: [82]) for use with MRI; 3) microbubbles (e.g., ~1–4 µm phospholipid-shelled bubbles filled with gas core; see Figure 3) for use with US imaging; or, 4) fluorescent dyes or molecules (e.g., green fluorescent protein (GFP)) or optical-absorbing nanoparticles (e.g., quantum dots (QDs) are 1–10 nm nanocrystals; see Bentolila et al: [83]) for use with optical imaging. Examples of molecular imaging of angiogenesis in small-animals are provided for each modality: 1) PET imaging of VEGFR-2 expression in 4T1 breast subcutaneous tumors (shoulder; arrow) with a 64Cu-labelled VEGF peptide sequence binding specifically to VEGFR-2 (VEGF(DEE);reprinted with permission from Wang et al. [84]; 2) T1-weighted MRI imaging of integrin αvβ3 expression in M21 melanoma (white arrow) with Gadolinium-loaded dendrimers targeted with cyclic RGD (reprinted with permission from Barrett and Choyke [85]); 3) VEGFR-2-targeted microbubbles (conjugation of biotinylated anti-VEGFR-2 antibody (Ab) to streptavidin-coated microbubbles (see Figure 3)) and molecular ultrasound in C6 rat glioma subcutaneous xenografts (arrows: tumor borders) in mice. Green targeted contrast signal was overlaid on gray Brightness-mode (B-mode) image (reprinted with permission: Willmann et al: [74]); and, 4) near-infrared optical imaging of quantum dots conjugated to VEGF(DEE) (same peptide as in PET image; reprinted with permission from Chen et al. [86] in U87MG glioma subcutaneous tumors in mice (shoulder; arrow).
Molecular imaging strategies of tumor angiogenesis can be divided into 2 categories: 1) imaging environments or factors that contribute to angiogenesis (e.g., tumor hypoxia induces VEGF production, which in turn induces angiogenesis; in this example, one can image hypoxia or VEGF); and, 2) imaging angiogenic vessels by targeting specific markers on tumor-associated endothelial cells. Several contrast agents for imaging tumor endothelial markers with various imaging modalities (Figure 2) have been developed preclinically, and the most common targets are the most characterized to date – integrins and VEGFR-2 (Table 1). For integrins, the RGD amino acid sequence (arginine-glycine-aspartic acid) to which integrins bind has been used as the binding ligand for 1) radiolabelled contrast agents for use with PET and SPECT; 2) attachment to Gadolinium-loaded or magnetic nanoparticles for imaging with MRI; 3) for attachment to microbubbles for imaging with ultrasound (discussed further below); and, 4) fluorescently-labeled contrast agents for detection by optical imaging (Table 1). Currently, the only RGD-targeted imaging agent for approved clinical use is the PET radiotracer, 18F-galacto-RGD radiotracer [45]. Contrast agents targeted to VEGF receptors can either be labeled with ligands that bind these receptors or by an antibody or a peptide that directly binds to VEGFR-2 (Table 1, Figure 2).
Table 1.
Examples of molecular imaging modalities and contrast agents targeted to the angiogenic markers integrins and VEGFR2 (Adapted from [70] [71])
| Molecular Target/Event |
PET | SPECT | MRI/MRS | US | Optical |
|---|---|---|---|---|---|
| Integrins (Tumor angiogenesis) | 64Cu-RGD (SWNT); 64Cu-RGD (QD); 64Cu-RGD (SPIO); 64Cu-knottin peptides [72] | 111In-perfluorocarbon NP-RGD | RGD peptide- Gd containing paramagnetic and fluorescent liposomes; RGD peptide-SPIOs | Knottin-RGD conjugated MBs; RGD MBs; Anti-β3 Ab-MB; [61]Echista tin-coated MBs; β3-targeted perfluorocarbon NP | RGD-QD705; RGD-Rhodamine/PE-liposomes; Cy5.5-knottin peptides; Raman: SWNT-RGD [73] |
| Vascular endothelial growth factor (VEGF) receptor (VEGFR) (Tumor angiogenesis) | 89Zr-Avastin; 64Cu-DOTA-VEGF; 64Cu-DOTA-VEGF (peptide) | 111In-Avastin; 125I-VEGF165, (125I or 99Tc)-VEGF121,111In-hnTf-VEGF | Anti-VEGFR2 Ab-MB; [74] KDR peptide-conjugated MBs[60] | VEGF-Cy5.5; VEGF-QD |
Strategies for choosing the specific angiogenic marker to target and the imaging modality are dependent upon the contrast agent type and properties (e.g., biodistribution, kinetics), as well as the application. For example, the molecular marker, PSMA, a marker of prostate cancer, has been shown to be expressed on both prostate cancer cells and prostate cancer-associated endothelial cells [46]. Therefore, this target is not optimal for imaging with a small molecule or antibody that can leak out of tumor microvessels and also bind to prostate cancer cells; in this case, the quantified signal would not accurately represent a measure of tumor angiogenesis, but rather, the tumor as a whole. However, if a larger particle, such as a microbubble (i.e., lipid-shelled, gas-filled bubbles that are ~1–4 µm in diameter and used as a contrast agent with ultrasound imaging), that remains in the vasculature and only binds to endothelial-specific PSMA, the associated quantified signal of bound contrast agent could directly measure PSMA expression levels in prostate cancer endothelium. Application-specific choices of imaging modalities and contrast agents are strongly driven by the advantages and disadvantages of the modality (Figure 2). In addition to the routine use of molecular imaging for diagnosing and staging of cancer (currently performed mostly with PET and SPECT), the in vivo identification and quantification of tumor molecular profiles, including angiogenic markers, with molecular imaging can be used to decide on target-specific chemotherapy on a patient-specific basis (personalized medicine), and for monitoring the direct effect of therapy on the targeted molecule. The direct monitoring of therapeutic treatment – for example, monitoring VEGFR-2 expression following a therapy targeted to VEGFR-2 and resulting in downregulation of VEGFR-2 expression – often occur much sooner than other changes, such as tumor cell death or changes in tumor size. Therefore, measurement of these therapeutic effects before overt morphological changes can provide an earlier assessment, and may avoid exposing non-responding patients to unnecessary possible side effects from a therapy they may not benefit from.
In summary, molecular imaging has many applications, and can involve frequent imaging of a patient, which can dictate the choice molecular imaging strategy. PET and SPECT imaging both involve the use of ionizing radiation, which is impractical for frequent imaging (e.g., in a screening setting) as the cumulative irradiation dose may harm the patient. MRI does not involve ionizing irradiation exposure; however, this modality is expensive, and sensitive quantification of molecularly targeted contrast agents often involves high-doses that can be toxic [47]. Compared to these modalities, optical imaging is inexpensive and highly quantitative; however, it has limited applications due to its lack of depth penetration in tissue (i.e., limited to surface imaging - for example, skin cancer, esophagus or colon with optical endoscopy, or bladder cancer imaging with optical cystoscopy [48,49,50], and availability to clinicians. Ultrasound imaging with molecularly-targeted contrast microbubbles is of particular interest from this point of view, since this modality is relatively inexpensive, offers real-time contrast imaging, allows relatively deep tissue penetration [51], and does not involve ionizing irradiation, and is widely available and portable (Figure 2). These advantages make molecular ultrasound imaging ideal for protocols involving frequent imaging such as early detection strategies through screening (e.g., for cancer in high risk patients) and therapeutic monitoring. In the following section we focus on use of molecular ultrasound for imaging and quantification of angiogenic markers and monitoring anti-cancer therapies.
Principles of Molecular Ultrasound Imaging
Contrast-enhanced ultrasound imaging is based on the reception, analysis and display of acoustic signals produced by reflection or backscatter of sound (echo) with use of contrast agents. Microbubbles, the most commonly used contrast agent, are gas-liquid emulsions consisting of a gaseous core (e.g., perfluorocarbon, sulfur hexafluoride, or nitrogen) that is enclosed by a shell made up by biocompatible materials (albumin, galactose, lipids, polymers) (Figure 3). The gaseous core of the microbubbles causes a very high echogenic response following insonification with ultrasound, resulting in a high contrast-to-tissue background ratio. Owing to their micron size (usually ranging in size between 1–4 µm diameter), these microbubbles stay within the vascular compartment, and do not leak out into the extra-vascular space. Thus, microbubbles are highly suitable for imaging angiogenic markers that are overexpressed on tumor vascular endothelial cells.
Figure 3.
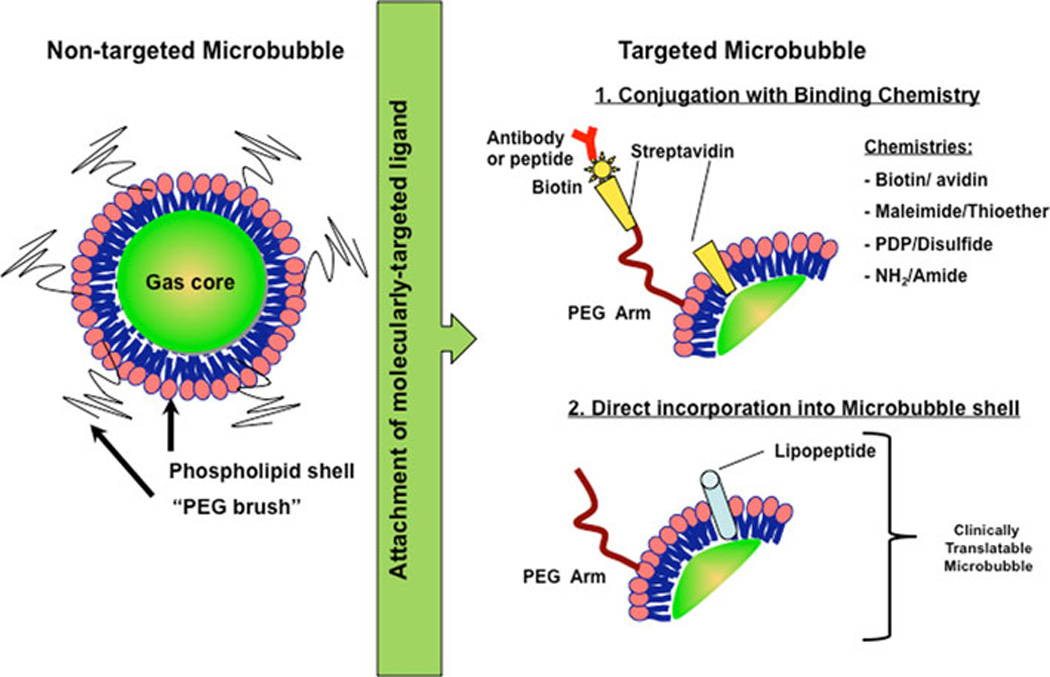
Design considerations for molecularly-targeted microbubbles (adapted from [59]). Most non-targeted microbubbles (left) are 1–4 µm microspheres consisting of a phospholipid monolayer shell and filled with a heavy gas such as perfluorobutane or perfluorocarbon. Additional coatings of biocompatible polymers (e.g., polyethylene glycol (PEG)) or proteins (e.g., albumin) are added for physical stability, to escape immune surveillance, to prevent microbubble aggregation, to provide spacing between lipid shell and binding ligand, and as platform for attaching binding ligands to molecular targets. Ligands (such as antibodies or peptides) for binding proteins expressed on endothelial cell surfaces can be attached by several approaches, including 1) chemical conjugation using biotin/streptavidin, biotin/avidin, maleimide/thioether, 2-(Pridylthio)propionyl (PDP)/disulfide, or amine (NH2)/amide attachment systems via the PEG arm or lipid shell components; or, 2) direct incorporation of peptides conjugated to lipids (to form lipopeptides) during the manufacturing process, which is needed for clinical translation of molecularly-targeted microbubbles (see text).
Microbubbles have been engineered for safe clinical applications (e.g., identification and characterization of focal liver lesions [52,53]. Following intravenous administration, microbubbles do not coalesce to form emboli, but dissolve leaving remnants that are easily metabolized or excreted. Further, biodistribution studies revealed that microbubbles have low circulation residence times as they are rapidly removed by the reticuloendothelial system (RES) [54,55,56]. To increase the circulation time of the microbubbles in serum, additional coatings, such as polyethylene glycol (PEG) polymer arms are added onto the microbubble shell. Additionally these coatings help stabilize the microbubble by providing additional stearic protection, preventing aggregation and help escape immune surveillance by the body [57](Figure 3).
To make ultrasound a molecular imaging tool, contrast microbubbles can be functionalized with ligands such as antibodies or peptides that bind molecular marker of interest with high affinity. These binding ligands can either be coupled to the microbubble using a chemical conjugation system (e.g., biotin-streptavidin [58]), or they can be integrated into the microbubble shell directly during the production process or after manufacturing (Figure 3). Furthermore, the attachment of ligands can be on the PEG arm which acts as a spacer between lipid shell and the site of binding in the tissue (For details see [59]). The nature of the binding chemistry is important for potential clinical translation of targeted contrast microbubbles. For example, streptavidin can cause immunogenic and allergic reactions in patients and, therefore, cannot be used in clinically translatable microbubbles. Alternative binding strategies of ligands onto the microbubbles shell (e.g., maleimide/thioether, amine (NH2)/amide attachment) or direct insertion of a lipid-associated molecule (Figure 3) are being explored as potential alternative strategies for designing clinically translatable microbubbles [60].
With the advent of microbubbles, ultrasound technology has undergone technical advances as traditional ultrasound imaging techniques were inadequate to selectively isolate imaging signal from microbubbles. New imaging techniques exploit the unique behavior of a microbubble in an ultrasonic field for the purposes of detection as well as quantification of signal. Upon insonification with low ultrasound transmit frequencies, microbubbles gives rise to nonlinear echoes. These echoes are readily distinguishable from the surrounding issue. Today, most contrast ultrasound systems are equipped for detection of these nonlinear echoes. Moderns ultrasound imaging approaches for enhancing microbubble-enhanced ultrasound imaging signal and for quantification of microbubble-enhanced imaging signal are reviewed elsewhere [59].
Assessment of Tumor Angiogenesis with Molecular Ultrasound Imaging
Several preclinical studies have validated the use of targeted microbubbles for detection of tumor angiogenesis in mouse models of human cancers (Tables 1 and 2). Molecular ultrasound performed using microbubbles targeting makers such as VEGFR-2, integrin αVβ3, and endoglin (Tables 2) showed high accumulation in tumor tissue which translated to higher signals compared to non targeted microbubbles. Further, results from these studies correlated with data obtained by ex vivo analysis such as immunohistochemistry and immunoblotting. Earlier studies generated targeted microbubbles primarily by attaching monoclonal antibodies against markers of angiogenesis using streptavidin-biotin conjugation chemistry. Subsequently, several research studies worked towards enhancing the ultrasound signal due to microbubbles and making it feasible for clinical translation by several modifications. One such modification is to use small peptide sequences that bind the molecular marker with high affinities to target microbubbles. The use of peptides could enhance microbubble-enhanced ultrasound imaging signal compared to monoclonal antibodies due to their smaller size allowing for more microbubbles to bind in the same physical space, and/or that, the high binding affinity of peptides could increase the adhering efficiency of microbubbles at the region of interest. This idea was demonstrated by Willmann et al.[61], who showed that an RGD-targeted microbubble (constructed by incorporating the RGD sequence in a knottin peptide – a compact peptide consisting of 20–60 amino acids with a core of at least 3 disulfide bonds that are interwoven into a “knotted” conformation [62]; the RGD-knottin peptide was then bound to the microbubble by streptavidin-biotin conjugation for proof-of-principle studies) resulted in 2-fold higher in vivo signal than an anti-integrin αvβ3 antibody-targeting microbubble in human ovarian tumor xenografts in mice. An important consideration for designing clinically translatable microbubbles conjugated with peptides for binding molecular targets is the microbubble and peptide stability. For example, the knottin peptide configuration provides stability against protease degradation compared with the short, linear RGD sequence [62]. In addition to peptide stability, increased microbubble stability can enhance signal by providing an increased efficiency of binding. For example, Jun et al [63] demonstrated that microbubbles conjugated to a cyclic form RGD peptide, (arginine-glycine-aspartate-D-tyrosine-lysine) was more stable in circulation (circulation time was greater than 1 hour) compared to control microbubbles targeted to AGD peptide (biotin-alanine-glycine-apartate). Increased circulation time can provide sufficient time for more microbubbles to attach the target, thus, providing high targeted signal and improved signal-to-background ratio.
Table 2.
Summary of published studies on the use of targeted microbubbles for molecular ultrasound imaging of tumor angiogenesis.
| Animal Model | Molecular Target | Binding Ligand | Ligand- Microbubble Conjugation System |
Reference |
|---|---|---|---|---|
| Anti-VEGF antibody (B20), | Subcutaneous human colon cancer xenografts in mice | Human KDR/VEGFR2 | Heterodimeric KDR-targeted peptide | Pysz et al, 2010[60] |
| Subcutaneous human ovarian cancer xenografts in mice | αvβ3 integrin | Biotinylated knottin peptide | Streptavidin-biotin | Willmann et al 2010 [61] |
| Subcutaneous human ovarian cancer xenografts in mice | VEGFR2 and αvβ3 integrin | Biotinylated anti-VEGFR2 and anti-αvβ3 integrin antibodies | Streptavidin-biotin | Willmann et al 2008 [64] |
| Subcutaneous mouse angiosarcoma and rat malignant glioma xenografts in mice | VEGFR2 | Biotinylated anti-VEGFR2 antibody | Streptavidin-biotin | Willmann et al 2008 [74] |
| Subcutaneous murine breast cancer tumors in mice | VEGFR2 | Biotinylated anti-VEGFR2 antibody | Streptavidin-biotin | Lee et al 2008 [75] |
| Subcutaneous human melanoma xenografts in mice | VEGFR2 | Biotinylated anti-VEGFR2 antibody | Streptavidin-biotin | Rychak et al 2007 [76] |
| Subcutaneous murine breast cancer xenografts in mice | VEGFR2 | Biotinylated anti-VEGFR2 antibody | Streptavidin-biotin | Lyshchik et al 2007 [77] |
| Subcutaneous human prostate cancer xenografts and murine clone C tumors in mice | Tumor endothelial cell (target was not identified) | Biotinylated RRL containing peptide | Streptavidin-biotin | Weller et al 2005 [78] |
Note.- Vascular endothelial growth factor receptor 2 (VEGFR2), RGD peptide (arginine-glycine-aspartic acid), RRL peptide (arginine, arginine, leucine)
Another approach to increase microbubble-enhanced ultrasound imaging signal for detection of tumor angiogenesis is the concept of dual- or multi-targeting microbubbles. Here, two or more ligands that bind to different angiogenic markers are attached to the surface of microbubbles which may increase the likelihood of microbubbles to actually attach to angiogenic vessels. This concept was shown by Willmann et al [64] exploring the use of dual-targeted microbubbles carrying antibodies targeted to both VEGFR2 and integrin αvβ3. In this study, dual-targeted microbubbles accumulated more to tumor vessels of human ovarian cancer xenografts in mice than single-targeted microbubbles targeting either VEGFR2 or integrin αvβ3. This increased imaging signal at sites of tumor angiogenesis produced by using dual-targeted microbubbles may be useful in cases such as the early detection of cancer when tumors are too small to cause detectable morphologic changes but large enough to induce tumor angiogenesis [64].
Regarding moving targeted contrast microbubbles into the clinic, several steps are necessary in order to formulate a clinically translatable microbubble and rigorously test its use prior to clinical translation. An exemplary study by Pysz et al [60] demonstrates these steps for the use of a novel, clinically-translatable microbubble targeted to human kinase insert domain receptor (KDR is the human protein analogous of murine VEGFR-2): The first step in designing a clinically translatable microbubble was to identify peptides that bind to the target of interest with high affinity, and then to conjugate it to the microbubbles in a manner that avoids immunogenic chemistries (see above). Two peptides were identified by phage display and were found to bind to human KDR with high affinity [65]. These two individual peptides were then linked by a hydrophilic spacer to form a heteropeptide (further increasing the binding affinity of that hetropeptide to KDR to be 0.2–0.5 nM [66]. The heteropeptide was then connected to a lipid (separated by PEG to provide steric separation from the microbubble shell) to form a heterolipopeptide, which could be incorporated directly into the microbubble shell during manufacturing (BR-55 microbubbles; Bracco Research, Geneva) [60,67].
The next step for translating peptide-targeted microbubbles involves testing the microbubbles in a clinically relevant animal model. However, this can be challenging if the binding peptide binds to the human target with high affinity but may not recognize the respective counterpart in the animal model. Testing cross-reactivity of a novel binding peptide between e.g. human and mouse receptors can be performed in cell culture experiments by testing binding affinity to isolated mouse VEGFR-2 protein ([67]) or to cells expressing VEGFR-2 [60]. Pysz et al [60] demonstrated cross-reactivity of KDR-targeted microbubbles to adhere to mouse VEGFR-2-positive and human KDR-positive vascular endothelial cells under shear flow conditions in cell culture. Non-targeted microbubbles did not bind to any cell type, thus, demonstrating one level of target specificity. In addition to positive confirmation of binding, another level of specificity to the VEGFR-2 was further tested by pre-treating the cells with an anti-mouse VEGFR-2 antibody to block the VEGFR-2 receptor from binding the KDR-microbubbles. This experiments showed that mouse VEGFR-2-positive cells failed to bind human KDR-targeted microbubble when blocked with anti-mouse VEGFR2 antibody indicating that KDR-microbubbles can cross-react with and bind specifically to mouse VEGFR-2 [60].
Following proof of binding peptide cross-reactivity between human and e.g. mouse receptors, in vivo imaging studies in animal models of tumor angiogenesis can be performed. Pysz et al. [60] tested KDR-targeted microbubbles in a mouse subcutaneous tumor model for human colon cancer with a dedicated small-animal ultrasound imaging system. Similar to the cell culture studies, the specific binding of KDR-targeted microbubble was demonstrated first by comparison to non-targeted microbubbles, and second, by pre-administration of a mouse anti-VEGFR-2 antibody to block the receptor from binding to KDR-targeted microbubbles. These experiments demonstrated higher in vivo imaging signals with KDR-targeted microbubbles compared to non-targeted microbubbles and showed that the in vivo imaging signal using KDR-targeted microbubbles could be blocked following intravenous administration of blocking anti-VEGFR2 antibodies Pysz, 2010 #171}, confirming in vivo binding specificity of human KDR-targeted microbubbles for murine VEGFR-2.
According to the aforementioned example, the path towards clinical translation of molecular ultrasound and molecularly-targeted microbubbles involves 1) designing a safe microbubble that is targeted to a relevant target for human cancer and specific for the human protein of interest; 2) testing its binding ability to bind to both the human target and a similar, homologous rodent target with high specificity in vitro or in cell culture; and, 3) to test its binding ability to the rodent target with high specificity in a human relevant application such as cancer imaging. Additionally, ex vivo quantification of molecular levels with immunoblotting and/or immunostaining could confirm and correlate ultrasound signal representing target expression to actual molecular marker expression on vessels.
Prior to full clinical use of the peptide-targeted microbubbles, its safety and toxicity needs to be tested. Several non-targeted microbubbles have been approved for clinical use in cardiology and radiology applications [55]; thus, the initial building blocks to clinical translation are already established. Additional testing of molecularly-targeted microbubbles needs to include biodistribution analyses. Biodistribution studies performed by Willmann et al, on lipid shell perfluorocabon-filled microbubbles targeted to VEGFR2 was analyzed in vivo in living mice by using dynamic micro-PET [54]. Anti-VEGFR-2 antibodies were radiolabelled by conjugating radiofluorination agent N-succinimidyl-4-[18F]fluorobenzoate to the antibodies as a tracer for in vivo assessment of targeted microbubble biodistribution using micro-PET imaging. Imaging revealed accumulation in the liver and spleen indicating that the microbubbles are cleared by the reticuloendothelial system and that 50% of targeted microbubbles cleared from the blood pool after ~3.5 minutes and ~95% were cleared from the blood circulation after 30 minutes [54]. Toxicity studies in several animal models and first in human dose escalation studies are the final steps for further moving a clinically-translatable targeted contrast microbubble into clinical applications.
Monitoring Anti-angiogenic Therapy with Molecular Ultrasound Imaging
Monitoring anti-angiogenic therapy with molecular ultrasound can be a direct measure of the molecular markers targeted by the therapeutic drug. Moreover, changes in angiogenic markers from conventional cancer therapies can also be measured with molecular ultrasound. A few preclinical studies have tested this idea in small animal models of human cancers (Table 3). Korpanty et al [68] used VEGFR-2- or endoglin-targeted microbubbles to measure therapeutic response of subcutaneous or orthotopic pancreatic cancer tumor-bearing mice that were treated with anti-vascular endothelial growth factor (VEGF) monoclonal antibodies (100 µg twice weekly of bevacizumab; Genentech) and/or gemcitabine (chemotherapeutic agent that inhibits DNA replication; 2 mg twice weekly; Eli Lilly). They detected decreasing accumulation of microbubbles labeled with either anti-VEGF-VEGFR-2 complex, VEGFR-2 or endoglin antibodies compared to control microbubbles (IgG labeled) after tumor- suppressive therapy. This result correlated with ex vivo expression analysis of the marker expression levels. Pysz et al. [60] reported the ability of KDR-targeted microbubbles to monitor anti-VEGF therapy (5 mg/kg B20-4.1.1.1, an anti-VEGF antibody targeting both human and mouse VEGF; Genentech) in human LS174T colon cancer xenografts in mice with molecular ultrasound (Figure 4). Comparison of molecular ultrasound imaging signal with KDR-targeted microbubbles in mice receiving anti-angiogenic treatment or placebo (saline) observed a significant decrease (~ 40%) in treated mice (compared to pretreatment baseline signal) as early as 24 hours after initiation of anti-angiogenic therapy (Figure 4). In contrast, no difference in molecular ultrasound KDR-targeted imaging signal was observed in non-treated mice. Furthermore, KDR-associated molecular ultrasound signal was observed prior to any changes in tumor size; thus, demonstrating the advantage of early assessment of anti-angiogenic therapy prior to overt morphological-anatomical changes become visible in tumors.
Table 3.
Summary of studies on the use molecular for monitoring anti-angiogenic therapy.
| Anti cancer therapy |
Type of Cancer | Ligand | Ligand-microbubble Conjugation System |
Reference |
|---|---|---|---|---|
| Anti-VEGF antibody | Subcutaneous human colon cancer xenografts in mice | Human KDR/VEGFR2 | Heterodimeric KDR-targeted peptide integrated in microbubble shell | Pysz et al, 2010 [60] |
| Anti-VEGF antibody and/or gemcitabine | Subcutaneous and orthotopic human pancreatic cancer xenografts in mice | Biotinylated anti-Endoglin, anti-VEGFR2, anti-VEGF-VEGFR complex antibodies | Streptavidin-biotin | Korpanty et al, 2007 [68]. |
Figure 4.
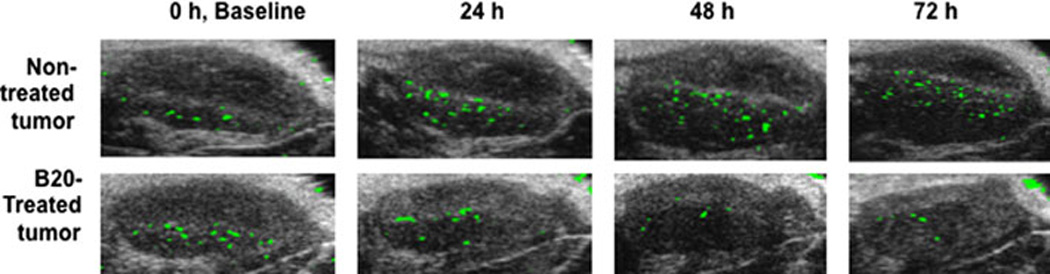
Molecular ultrasound allows longitudinal monitoring of anti-angiogenic therapy. Ultrasound images represent subcutaneous human colon cancer xenografts in nude mice imaged with a novel, human KDR-targeted contrast microbubble [60]. Imaging signal from microbubbles attached to VEGFR2 is shown as green signal overlaid on B-mode image. Note, that the in vivo molecular imaging signal measured in tumor of non-treated mouse increases over time; whereas imaging signal in mouse treated with an anti-angiogenic therapy (B20 anti-VEGF-antibody) substantially deceases as early as 24 hours after initiation of therapy. Of note is also, that the molecular ultrasound imaging signal decreases before overt morphological changes in treated tumor become visible (for more details please refer to [60]).
Future Outlook of Molecular Ultrasound Imaging
The potential of molecular ultrasound imaging with various angiogenic molecular targets has been firmly established with preclinical research applications in the areas of cancer detection and therapeutic monitoring. However, clinical translation of molecular ultrasound imaging requires several improvements including design of biocompatible molecularly-targeted microbubbles, improvements in targeted microbubble quantification, and improvements in instrumentation for sensitive and enhanced detection. First, microbubbles must be conjugated to molecular targeting moieties without the use of strept(avidin)/biotin conjugation chemistries, since those chemistries are immunogenic [69]. Several alternate strategies that allow covalent binding of ligands (e.g., KDR-binding lipopeptide inserted into microbubble shell during manufacturing, [60,67]) instead of antibodies to the microbubble shell have to be explored.
Second, improvements and standardization of targeted microbubble quantification needs to be performed for clinical translation. Imaging techniques that selectively detect only the adhered microbubbles as opposed to floating microbubbles could aid in better quantification of signal. Finally, ultrasound devices currently use a two-dimensional imaging approach allowing only a limited assessment of the diseased tissue under consideration. New device technology has resulted in transducer access to a variety of tissues, including endoscopic ultrasound, intravascular ultrasound, transvaginal ultrasound, and transcranial ultrasound, among others. Current on-going advancements in three-dimensional ultrasound imaging techniques will also enable to extent the limited field-of-view of current to-dimensional transducers and allows a more accurate quantification of molecular marker expression without relying on finding e.g. the same two-dimensional imaging plane during longitudinal monitoring of therapy. Three-dimensional ultrasound imaging is also important for reliable tumor detection and assessment of angiogenic treatment in a heterogeneous tissue, such as a cancer.
In conclusion, with rapid advances being made using molecular ultrasound in preclinical research, its translation into clinics is eminent. Once established in a clinical setting, molecular ultrasound may serve as a powerful tool for screening, diagnosing and treatment monitoring of cancer.
References
- 1.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 2.Red-Horse K, Ferrara N. Imaging tumor angiogenesis. J Clin Invest. 2006;116:2585–2587. doi: 10.1172/JCI30058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 4.Algire GH, Chalkley HW, Legallais FY, Park HD. Vascular reactions of normal and malignant tumors in vivo. I. Vascular reactions of mice to wounds and to normal and neoplastic transplants. J Natl Cancer Inst. 1945;6:73–85. [Google Scholar]
- 5.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 7.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 8.Abdelrahim M, Konduri S, Basha R, Philip PA, Baker CH. Angiogenesis: an update and potential drug approaches (review) Int J Oncol. 2010;36:5–18. [PubMed] [Google Scholar]
- 9.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 10.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 11.Bernabeu C, Lopez-Novoa JM, Quintanilla M. The emerging role of TGF-beta superfamily coreceptors in cancer. Biochim Biophys Acta. 2009;1792:954–973. doi: 10.1016/j.bbadis.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol. 2002;29:10–14. doi: 10.1053/sonc.2002.37264. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara N. The role of VEGF in the regulation of physiological and pathological angiogenesis. EXS. 2005:209–231. doi: 10.1007/3-7643-7311-3_15. [DOI] [PubMed] [Google Scholar]
- 14.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 15.Brekken RA, Thorpe PE. VEGF-VEGF receptor complexes as markers of tumor vascular endothelium. J Control Release. 2001;74:173–181. doi: 10.1016/s0168-3659(01)00333-9. [DOI] [PubMed] [Google Scholar]
- 16.Paz K, Zhu Z. Development of angiogenesis inhibitors to vascular endothelial growth factor receptor 2. Current status and future perspective. Front Biosci. 2005;10:1415–1439. doi: 10.2741/1629. [DOI] [PubMed] [Google Scholar]
- 17.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai W, Chen X. Anti-angiogenic cancer therapy based on integrin alphavbeta3 antagonism. Anticancer Agents Med Chem. 2006;6:407–428. doi: 10.2174/187152006778226530. [DOI] [PubMed] [Google Scholar]
- 19.Stollman TH, Ruers TJ, Oyen WJ, Boerman OC. New targeted probes for radioimaging of angiogenesis. Methods. 2009;48:188–192. doi: 10.1016/j.ymeth.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Castellani P, Viale G, Dorcaratto A, Nicolo G, Kaczmarek J, et al. The fibronectin isoform containing the ED-B oncofetal domain: a marker of angiogenesis. Int J Cancer. 1994;59:612–618. doi: 10.1002/ijc.2910590507. [DOI] [PubMed] [Google Scholar]
- 21.Carnemolla B, Castellani P, Ponassi M, Borsi L, Urbini S, et al. Identification of a glioblastoma-associated tenascin-C isoform by a high affinity recombinant antibody. Am J Pathol. 1999;154:1345–1352. doi: 10.1016/S0002-9440(10)65388-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silacci M, Brack SS, Spath N, Buck A, Hillinger S, et al. Human monoclonal antibodies to domain C of tenascin-C selectively target solid tumors in vivo. Protein Eng Des Sel. 2006;19:471–478. doi: 10.1093/protein/gzl033. [DOI] [PubMed] [Google Scholar]
- 23.Fonsatti E, Altomonte M, Arslan P, Maio M. Endoglin (CD105): a target for anti angiogenetic cancer therapy. Curr Drug Targets. 2003;4:291–296. doi: 10.2174/1389450033491073. [DOI] [PubMed] [Google Scholar]
- 24.Ma X, Labinaz M, Goldstein J, Miller H, Keon WJ, et al. Endoglin is overexpressed after arterial injury and is required for transforming growth factor-beta-induced inhibition of smooth muscle cell migration. Arterioscler Thromb Vasc Biol. 2000;20:2546–2552. doi: 10.1161/01.atv.20.12.2546. [DOI] [PubMed] [Google Scholar]
- 25.Kolonin MG. Tissue-specific targeting based on markers expressed outside endothelial cells. Adv Genet. 2009;67:61–102. doi: 10.1016/S0065-2660(09)67003-6. [DOI] [PubMed] [Google Scholar]
- 26.Hardwick JS, Yang Y, Zhang C, Shi B, McFall R, et al. Identification of biomarkers for tumor endothelial cell proliferation through gene expression profiling. Mol Cancer Ther. 2005;4:413–425. doi: 10.1158/1535-7163.MCT-04-0209. [DOI] [PubMed] [Google Scholar]
- 27.Chang SS, O'Keefe DS, Bacich DJ, Reuter VE, Heston WD, et al. Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clin Cancer Res. 1999;5:2674–2681. [PubMed] [Google Scholar]
- 28.Conway RE, Petrovic N, Li Z, Heston W, Wu D, et al. Prostate-specific membrane antigen regulates angiogenesis by modulating integrin signal transduction. Mol Cell Biol. 2006;26:5310–5324. doi: 10.1128/MCB.00084-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huminiecki L, Gorn M, Suchting S, Poulsom R, Bicknell R. Magic roundabout is a new member of the roundabout receptor family that is endothelial specific and expressed at sites of active angiogenesis. Genomics. 2002;79:547–552. doi: 10.1006/geno.2002.6745. [DOI] [PubMed] [Google Scholar]
- 30.Seth P, Lin Y, Hanai J, Shivalingappa V, Duyao MP, et al. Magic roundabout, a tumor endothelial marker: expression and signaling. Biochem Biophys Res Commun. 2005;332:533–541. doi: 10.1016/j.bbrc.2005.03.250. [DOI] [PubMed] [Google Scholar]
- 31.Sheldon H, Andre M, Legg JA, Heal P, Herbert JM, et al. Active involvement of Robo1 and Robo4 in filopodia formation and endothelial cell motility mediated via WASP and other actin nucleation-promoting factors. FASEB J. 2009;23:513–522. doi: 10.1096/fj.07-098269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herath NI, Spanevello MD, Sabesan S, Newton T, Cummings M, et al. Over-expression of Eph and ephrin genes in advanced ovarian cancer: ephrin gene expression correlates with shortened survival. BMC Cancer. 2006;6:144. doi: 10.1186/1471-2407-6-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, Zhuang G, Frieden L, Debinski W. Eph receptors and Ephrins in cancer: common themes and controversies. Cancer Res. 2008;68:10031–10033. doi: 10.1158/0008-5472.CAN-08-3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Q, Suo Z, Kristensen GB, Baekelandt M, Nesland JM. The prognostic impact of EphB2/B4 expression on patients with advanced ovarian carcinoma. Gynecol Oncol. 2006;102:15–21. doi: 10.1016/j.ygyno.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 35.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 36.Porta C, Larghi P, Rimoldi M, Totaro MG, Allavena P, et al. Cellular and molecular pathways linking inflammation and cancer. Immunobiology. 2009;214:761–777. doi: 10.1016/j.imbio.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 37.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 38.Mankoff DA. A definition of molecular imaging. J Nucl Med. 2007;48:18N–21N. [PubMed] [Google Scholar]
- 39.Peterson TE, Manning HC. Molecular imaging: 18F-FDG PET and a whole lot more. J Nucl Med Technol. 2009;37:151–161. doi: 10.2967/jnmt.109.062729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burtscher IM, Holtas S. Proton MR spectroscopy in clinical routine. J Magn Reson Imaging. 2001;13:560–567. doi: 10.1002/jmri.1079. [DOI] [PubMed] [Google Scholar]
- 41.Robert B. Resonance Raman spectroscopy. Photosynth Res. 2009;101:147–155. doi: 10.1007/s11120-009-9440-4. [DOI] [PubMed] [Google Scholar]
- 42.Krafft C, Steiner G, Beleites C, Salzer R. Disease recognition by infrared and Raman spectroscopy. J Biophotonics. 2009;2:13–28. doi: 10.1002/jbio.200810024. [DOI] [PubMed] [Google Scholar]
- 43.Sahu RK, Mordechai S. Fourier transform infrared spectroscopy in cancer detection. Future Oncol. 2005;1:635–647. doi: 10.2217/14796694.1.5.635. [DOI] [PubMed] [Google Scholar]
- 44.Ellis DI, Dunn WB, Griffin JL, Allwood JW, Goodacre R. Metabolic fingerprinting as a diagnostic tool. Pharmacogenomics. 2007;8:1243–1266. doi: 10.2217/14622416.8.9.1243. [DOI] [PubMed] [Google Scholar]
- 45.Schnell O, Krebs B, Carlsen J, Miederer I, Goetz C, et al. Imaging of integrin alpha(v)beta(3) expression in patients with malignant glioma by [18F] Galacto-RGD positron emission tomography. Neuro Oncol. 2009;11:861–870. doi: 10.1215/15228517-2009-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dijkgraaf I, Boerman OC. Radionuclide imaging of tumor angiogenesis. Cancer Biother Radiopharm. 2009;24:637–647. doi: 10.1089/cbr.2009.0694. [DOI] [PubMed] [Google Scholar]
- 47.Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS. Molecular imaging in drug development. Nat Rev Drug Discov. 2008;7:591–607. doi: 10.1038/nrd2290. [DOI] [PubMed] [Google Scholar]
- 48.Contag CH. In vivo pathology: seeing with molecular specificity and cellular resolution in the living body. Annu Rev Pathol. 2007;2:277–305. doi: 10.1146/annurev.pathol.2.010506.091930. [DOI] [PubMed] [Google Scholar]
- 49.Wang TD, Friedland S, Sahbaie P, Soetikno R, Hsiung PL, et al. Functional imaging of colonic mucosa with a fibered confocal microscope for real-time in vivo pathology. Clin Gastroenterol Hepatol. 2007;5:1300–1305. doi: 10.1016/j.cgh.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim HL. Optical imaging in oncology. Urol Oncol. 2009;27:298–300. doi: 10.1016/j.urolonc.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 51.Ritchie WA, Thompson W. A clinical assessment of real time ultrasound. Ir J Med Sci. 1979;148:251–254. [PubMed] [Google Scholar]
- 52.Wilson SR, Jang HJ, Kim TK, Iijima H, Kamiyama N, et al. Real-time temporal maximum-intensity-projection imaging of hepatic lesions with contrast-enhanced sonography. AJR Am J Roentgenol. 2008;190:691–695. doi: 10.2214/AJR.07.3116. [DOI] [PubMed] [Google Scholar]
- 53.Celli N, Gaiani S, Piscaglia F, Zironi G, Camaggi V, et al. Characterization of liver lesions by real-time contrast-enhanced ultrasonography. Eur J Gastroenterol Hepatol. 2007;19:3–14. doi: 10.1097/01.meg.0000250585.53608.3c. [DOI] [PubMed] [Google Scholar]
- 54.Willmann JK, Cheng Z, Davis C, Lutz AM, Schipper ML, et al. Targeted microbubbles for imaging tumor angiogenesis: assessment of whole-body biodistribution with dynamic micro-PET in mice. Radiology. 2008;249:212–219. doi: 10.1148/radiol.2491072050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weskott HP. Emerging roles for contrast-enhanced ultrasound. Clin Hemorheol Microcirc. 2008;40:51–71. [PubMed] [Google Scholar]
- 56.Perkins AC, Frier M, Hindle AJ, Blackshaw PE, Bailey SE, et al. Human biodistribution of an ultrasound contrast agent (Quantison) by radiolabelling and gamma scintigraphy. Br J Radiol. 1997;70:603–611. doi: 10.1259/bjr.70.834.9227254. [DOI] [PubMed] [Google Scholar]
- 57.Klibanov AL. Preparation of targeted microbubbles: ultrasound contrast agents for molecular imaging. Med Biol Eng Comput. 2009 doi: 10.1007/s11517-009-0498-0. [DOI] [PubMed] [Google Scholar]
- 58.Klibanov AL. Ligand-carrying gas-filled microbubbles: ultrasound contrast agents for targeted molecular imaging. Bioconjug Chem. 2005;16:9–17. doi: 10.1021/bc049898y. [DOI] [PubMed] [Google Scholar]
- 59.Deshpande N, Needles A, Willmann JK. Molecular Ultrasound Imaging: Current Status and Future Directions. Clinical Radiology. 2010 doi: 10.1016/j.crad.2010.02.013. 2010 (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pysz MA, Foygel K, Rosenberg J, Gambhir SS, Schneider M, Willmann JK. Monitoring anti- angiogenic therapy with molecular ultrasound and a new clinically translatable contrast agent (BR55) Radiology. 2010 doi: 10.1148/radiol.10091858. 2010 (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Willmann JK, Kimura RH, Deshpande N, Lutz AM, Cochran JR, et al. Targeted contrast-enhanced ultrasound imaging of tumor angiogenesis with contrast microbubbles conjugated to integrin-binding knottin peptides. J Nucl Med. 2010;51:433–440. doi: 10.2967/jnumed.109.068007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kimura RH, Cheng Z, Gambhir SS, Cochran JR. Engineered knottin peptides: a new class of agents for imaging integrin expression in living subjects. Cancer Res. 2009;69:2435–2442. doi: 10.1158/0008-5472.CAN-08-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jun HY, Park SH, Kim HS, Yoon KH. Long residence time of ultrasound microbubbles targeted to integrin in murine tumor model. Acad Radiol. 2010;17:54–60. doi: 10.1016/j.acra.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 64.Willmann JK, Lutz AM, Paulmurugan R, Patel MR, Chu P, et al. Dual-targeted contrast agent for US assessment of tumor angiogenesis in vivo. Radiology. 2008;248:936–944. doi: 10.1148/radiol.2483072231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pillai R, Marinelli ER, Swenson RE. A flexible method for preparation of peptide homo-and heterodimers functionalized with affinity probes, chelating ligands, and latent conjugating groups. Biopolymers. 2006;84:576–585. doi: 10.1002/bip.20570. [DOI] [PubMed] [Google Scholar]
- 66.Pillai R, Marinelli ER, Fan H, Nanjappan P, Song B, et al. A Phospholipid-PEG2000 Conjugate of a Vascular Endothelial Growth Factor Receptor 2 (VEGFR2)-Targeting Heterodimer Peptide for Contrast-Enhanced Ultrasound Imaging of Angiogenesis. Bioconjug Chem. 2010 doi: 10.1021/bc9005688. [DOI] [PubMed] [Google Scholar]
- 67.Pochon S, Tardy I, Bussat P, Bettinger T, Brochot J, et al. BR55: a lipopeptide-based VEGFR2-targeted ultrasound contrast agent for molecular imaging of angiogenesis. Invest Radiol. 2010;45:89–95. doi: 10.1097/RLI.0b013e3181c5927c. [DOI] [PubMed] [Google Scholar]
- 68.Korpanty G, Carbon JG, Grayburn PA, Fleming JB, Brekken RA. Monitoring response to anticancer therapy by targeting microbubbles to tumor vasculature. Clin Cancer Res. 2007;13:323–330. doi: 10.1158/1078-0432.CCR-06-1313. [DOI] [PubMed] [Google Scholar]
- 69.Marshall D, Pedley RB, Boden JA, Boden R, Melton RG, et al. Polyethylene glycol modification of a galactosylated streptavidin clearing agent: effects on immunogenicity and clearance of a biotinylated anti-tumour antibody. Br J Cancer. 1996;73:565–572. doi: 10.1038/bjc.1996.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis. J Nucl Med. 2008;49 Suppl 2:113S–128S. doi: 10.2967/jnumed.107.045922. [DOI] [PubMed] [Google Scholar]
- 71.Cai W, Gambhir SS, Chen X. Chapter 7. Molecular imaging of tumor vasculature. Methods Enzymol. 2008;445:141–176. doi: 10.1016/S0076-6879(08)03007-3. [DOI] [PubMed] [Google Scholar]
- 72.Miao Z, Ren G, Liu H, Kimura RH, Jiang L, et al. An engineered knottin peptide labeled with 18F for PET imaging of integrin expression. Bioconjug Chem. 2009;20:2342–2347. doi: 10.1021/bc900361g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zavaleta C, de la Zerda A, Liu Z, Keren S, Cheng Z, et al. Noninvasive Raman spectroscopy in living mice for evaluation of tumor targeting with carbon nanotubes. Nano Lett. 2008;8:2800–2805. doi: 10.1021/nl801362a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Willmann JK, Paulmurugan R, Chen K, Gheysens O, Rodriguez-Porcel M, et al. US imaging of tumor angiogenesis with microbubbles targeted to vascular endothelial growth factor receptor type 2 in mice. Radiology. 2008;246:508–518. doi: 10.1148/radiol.2462070536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee DJ, Lyshchik A, Huamani J, Hallahan DE, Fleischer AC. Relationship between retention of a vascular endothelial growth factor receptor 2 (VEGFR2)-targeted ultrasonographic contrast agent and the level of VEGFR2 expression in an in vivo breast cancer model. J Ultrasound Med. 2008;27:855–866. doi: 10.7863/jum.2008.27.6.855. [DOI] [PubMed] [Google Scholar]
- 76.Rychak JJ, Graba J, Cheung AM, Mystry BS, Lindner JR, et al. Microultrasound molecular imaging of vascular endothelial growth factor receptor 2 in a mouse model of tumor angiogenesis. Mol Imaging. 2007;6:289–296. [PubMed] [Google Scholar]
- 77.Lyshchik A, Fleischer AC, Huamani J, Hallahan DE, Brissova M, et al. Molecular imaging of vascular endothelial growth factor receptor 2 expression using targeted contrast-enhanced high-frequency ultrasonography. J Ultrasound Med. 2007;26:1575–1586. doi: 10.7863/jum.2007.26.11.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weller GE, Wong MK, Modzelewski RA, Lu E, Klibanov AL, et al. Ultrasonic imaging o tumor angiogenesis using contrast microbubbles targeted via the tumor-binding peptide arginine-arginine-leucine. Cancer Res. 2005;65:533–539. [PubMed] [Google Scholar]
- 79.Website. Angiogenesis. Cell Signaling Technologies. 2010
- 80.Nune SK, Gunda P, Thallapally PK, Lin YY, Forrest ML, et al. Nanoparticles for biomedical imaging. Expert Opin Drug Deliv. 2009;6:1175–1194. doi: 10.1517/17425240903229031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blanco E, Kessinger CW, Sumer BD, Gao J. Multifunctional micellar nanomedicine for cancer therapy. Exp Biol Med (Maywood) 2009;234:123–131. doi: 10.3181/0808-MR-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sosnovik DE, Weissleder R. Emerging concepts in molecular MRI. Curr Opin Biotechnol. 2007;18:4–10. doi: 10.1016/j.copbio.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 83.Bentolila LA, Ebenstein Y, Weiss S. Quantum dots for in vivo small-animal imaging. J Nucl Med. 2009;50:493–496. doi: 10.2967/jnumed.108.053561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang H, Cai W, Chen K, Li ZB, Kashefi A, et al. A new PET tracer specific for vascular endothelial growth factor receptor 2. Eur J Nucl Med Mol Imaging. 2007;34:2001–2010. doi: 10.1007/s00259-007-0524-0. [DOI] [PubMed] [Google Scholar]
- 85.Barrett T, Choyke PL. Imaging of Angiogenesis Angiogenesis An Integrative Approach from Science to Medicine: Springer. 2008. p. 332. [Google Scholar]
- 86.Chen K, Li ZB, Wang H, Cai W, Chen X. Dual-modality optical and positron emission tomography imaging of vascular endothelial growth factor receptor on tumor vasculature using quantum dots. Eur J Nucl Med Mol Imaging. 2008;35:2235–2244. doi: 10.1007/s00259-008-0860-8. [DOI] [PubMed] [Google Scholar]


