Abstract
The binding of IL-18 to IL-18Rα induces both pro-inflammatory and protective functions during infection, depending on the context in which it occurs. IL-18 is highly expressed in the liver of wild type (WT) C57BL/6 mice following lethal infection with highly virulent Ixodes Ovatus Ehrlichia (IOE), an obligate intracellular bacterium that causes acute fatal toxic shock-like syndrome. In this study, we found that IOE infection of IL-18Rα-/- mice resulted in significantly less host cell apoptosis, decreased hepatic leukocyte recruitment, enhanced bacterial clearance and prolonged survival compared to infected WT mice, suggesting a pathogenic role of IL-18/IL-18Rα in Ehrlichia-induced toxic shock. Although lack of IL-18R decreases the magnitude of IFN-γ producing type-1 immune response, enhanced resistance of the IL-18Rα-/- mice against Ehrlichia correlated with increased pro-inflammatory cytokines at sites of infection, decreased systemic IL-10 production, increased frequency of protective natural killer T (NKT) cells producing TNF-α and IFN-γ and decreased frequency of pathogenic TNF-α-producing CD8+ T cells. Adoptive transfer of immune wild type CD8+ T cells increased bacterial burden in IL-18Rα-/- mice following IOE infection. Furthermore, rIL-18 treatment of WT mice infected with mildly virulent Ehrlichia muris (EM) impaired bacterial clearance and enhanced liver injury. Finally, lack of IL-18R signal reduced dendritic cells (DCs) maturation and their TNF-α production, suggesting that IL-18 possibly promote the adaptive pathogenic immune responses against Ehrlichia via influencing T cell priming functions of DCs Together, these results suggest that the presence or absence of IL-18R signals governs the pathogenic versus protective immunity in a model of Ehrlichia-induced immunopathology.
Keywords: IL-18, IL-18 receptor alpha, Ehrlichiosis
Introduction
Human monocytotropic ehrlichiosis (HME) is an emerging tick-borne disease caused by Ehrlichia chaffeensis (1, 2), a Gram-negative obligate intracellular bacterium that lacks LPS (3, 4). HME can manifest as an acute mild disease with non-specific flu-like symptoms or as an acute severe multisystem disease that progress to multi-organ failure and fatal toxic shock-like syndrome (1, 2, 5). Doxycycline treatment is frequently ineffective in preventing disease progression when administered late in the course of illness (6). Animal models of HME include WT C57BL/6 mice infected with E. muris, which causes mild, self-limited disease (7, 8), or WT C57BL/6 mice infected with IOE, which causes acute fatal toxic shock like syndrome or mild disease, depending on the dose and route of inoculation (7, 9-11). The pathologic changes in patients with fatal HME as well as in murine models of fatal ehrlichiosis suggested that the severity of HME is closely associated with immune-mediated pathology, as indicated by the severe tissue injury and multi-organ failure that occurs in the absence of large quantities of ehrlichiae in the blood or tissues (1, 2, 5, 7, 12). Protective immunity against Ehrlichia is mediated by Ehrlichia-specific IFN-γ–producing CD4+Th1 cells and IFN-γ–producing natural killer T (NKT) cells are critical for effective bacterial elimination (7, 11, 13-18). However, these cells undergo apoptosis at the end stages of fatal disease (7, 16, 19). On the other hand, Ehrlichia-induced shock in mice is associated with systemic overproduction of pro and anti-inflammatory cytokines and chemokines (e.g., TNF-α, IL-10, MCP-1, and MIP-1) (7, 13, 19). Furthermore, cytotoxic natural killer (NK) and TNF-α-producing CD8+ T cells play pathogenic roles during fatal murine ehrlichiosis as they directly mediate tissue injury and suppress anti-Ehrlichia protective immunity and thus impede effective bacterial clearance (7,16, 19).
IL-18, formerly termed IFN-γ-inducing factor, is a member of the IL-1 superfamily and is initially synthesized as an inactive 24-kDa precursor protein (pro-IL-18) (20). Stimulation and secretion of IL-18 is mediated by a number of inflammatory mediators and cytosolic proteins that regulate the cysteine protease caspase-1 within a multiprotein complex known as the ‘inflammasome’ (21-23). Activation of caspase-1 (also called IL-1-converting enzyme) leads to the cleavage of pro-IL-18 into its mature and biologically active 18-kDa form. A wide range of cells (mainly activated blood and tissue monocytes/macrophages, Kupffer cells, B cells, dendritic cells (DC), epithelial cells and T cells) are capable of producing IL-18 upon stimulation (21-23). IL-18 binds to IL-18Rα, originally described as an IL-1 receptor-related protein because of its homology to the IL-1/Toll receptor family. IL-18Rβ subunit, which is also a member of the IL-1R family, is responsible for signal transduction mediated by the IL-18R complex (23). The binding of IL-18 to the heterodimeric IL-18Rα/β complexes expressed on T lymphocytes, NK cells, macrophages, neutrophils, and endothelial cells induces downstream signals leading to the activation of NF-κB (20-23). IL-18 has pleiotropic functions depending on the context of stimulation, cytokine milieu, and genetic predisposition. Some studies have suggested that IL-18 is a Th1-promoting and pro-inflammatory cytokine that promote the production of IFN-γ from T and NK cells, particularly in the presence of IL-12p70 (23-26), and thus plays a role in the protection against several infectious diseases caused by intracellular bacteria (24-27). Other studies have demonstrated that IL-18 promotes Th2 and increases allergic sensitization (28, 29). IL-18 increases FAS expression on host cells in murine hepatitis model (30) and promotes the secretion of TNF-α, IL-1β, IL-8 and GM-CSF, and as a consequence, enhances expansion, migration and activation of neutrophils during infections (31, 32). In addition, IL-18 has been defined as an important cofactor for enhanced cytotoxic activity and proliferation of CD8+ T and NK cells (33-35).
Several studies have shown that elevated serum levels of IL-18 are associated with poor clinical outcome in severe inflammatory and septic conditions (36-38). Neutralization of IL-18 via caspase-1 intervention or through the administration of IL-18-binding protein has been postulated to be a promising therapeutic approach (38, 39). However, the factors that influence the functional outcomes of IL-18 expression remain poorly defined, and thus, additional studies are required to evaluate its full potential in acute inflammatory and infectious diseases.
Our recent studies have demonstrated a significant association between elevated IL-18 levels and development of fatal ehrlichiosis caused by intraperitoneal infection with high doses of virulent IOE (7). In the present study, we examined the contribution of IL-18 to anti-Ehrlichia protective immunity and the pathogenesis of Ehrlichia-induced tissue injury and toxic shock. Here we show that IL-18/IL-18Rα interaction negatively regulates protective immunity and promoting immunopathology during lethal ehrlichial infection. Furthermore, this study establishes a critical role of IL-18/IL-18R in the maturation of dendritic cells, cytokine production by NKT and NK cells, and induction of pathogenic TNF-α producing- CD8+ T cells following infection with obligate intracellular bacteria that cause toxic shock.
Materials and Methods
Mice and Ehrlichia infection
Female WT C57BL/6 mice and IL-18Rα-/- mice (strain B6.129P2-Il18r1tm1Aki/J), 8-12 weeks old were obtained from the Jackson Laboratories (Bar Harbor, ME). All animals were housed under specific pathogen-free conditions at the Animal Research Center at Meharry Medical College in accordance with the institutional guidelines for animal welfare. Two species of monocytotropic Ehrlichia were used in this study: the highly virulent IOE and the mildly virulent E. muris. Both strains were provided by Dr. Y. Rikihisa (Ohio State University, Columbus, OH). IOE and EM stock were propagated by passage through WT C57BL/6 mice. Single-cell suspensions from the spleens of day 7 infected mice were stored in liquid nitrogen and used as stocks. Mice were infected intraperitoneally (i.p.) with 1 mL of 106 bacteria/mouse of E. muris inoculum (non-lethal infection) or 5 × 103 bacteria/mouse of IOE (lethal infection). Mice were monitored daily for signs of illness and survival. On the indicated days post-infection (p.i.), 3-6 mice/group were sacrificed, and selected organs were harvested for further analysis.
Preparation of host cell-free Ehrlichia
Host cell-free IOE antigens (Ags) were prepared from IOE-infected spleens and livers harvested on day 7 post-infection as previously described (7, 11, 40). Spleen and liver of naive mice were prepared and were used as a negative control in all experiments using cell-free IOE Ags (mock Ag). The E. muris Ag was prepared from E. muris-infected DH82 canine macrophage cell line. Ehrlichiae were harvested when ~90–100% of the cells were infected, and cell-free ehrlichiae were prepared as previously described (3, 4, 7, 11, 13). Cell lysates from uninfected DH82 cells were similarly prepared and used as a negative control (mock Ag) for all experiments using E. muris Ag. The total protein concentration of the resulting bacterial preparations was determined using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL) and was used as the Ag in flow cytometry and the in vitro culture of splenocytes or peritoneal exudates cells.
Isolation of peritoneal exudates cells (PECs) and preparation of culture supernatant from PECs following in vitro antigen stimulation
The peritoneal cavities of infected mice were washed with 10 ml of sterile PBS. The peritoneal wash/lavage was centrifuged at 275 χ g for 5 minutes to separate the cell fraction (PECs). As negative controls, PECs were prepared from uninfected mice injected with 1.5 ml of 3% thioglycolate medium (Difco Laboratories) and resident PECs were harvested at 4 h after the injection. PECs from IOE-infected WT and IL-18Rα-/- mice and uninfected controls were directly processed for flow cytometry and intracellular cytokine staining as described below. In addition, PECs from infected WT, IL-18Rα-/- mice and uninfected controls were cultured at a concentration of (5 χ 104 cells/well) with host cell-free IOE (5×104 bacteria/well) in a 24-well plate. The culture supernatants were harvested at 24 hour, passed through a membrane filter (pore size, 0.22 μm), and the levels of different cytokines in the peritoneal culture supernatant were measured by ELISA.
In vitro stimulation of splenocytes
Spleens were excised on the indicated days p.i. from groups of three to four Ehrlichia (EM or IOE)-inoculated C57BL/6 and IL-18Rα−/− mice. Splenocytes were isolated as previously described (7, 11). Briefly, spleens were homogenized, and RBCs were lysed with 0.84% ammonium chloride treatment. A total of 5 × 106 cells were resuspended in 2 ml DMEM supplemented with 10% FBS and 100 μg/ml penicillin and streptomycin (HyClone) and plated into 12-well tissue culture plates. Splenocytes were cultured with or without cell free- EM or IOE Ags. After 48 hours, the supernatants were collected and cytokine levels were analyzed by ELISA as described below.
Cytokine ELISA
The concentrations of IFN-γ, IL-4, TNF-α, IL-6, IL-10, IL-12, and IL-18 in the sera, or peritoneal exudates cell culture supernatant were measured using Quantikine enzyme linked immunosorbent assays (ELISA) (R&D Systems, Minneapolis, MN). The minimum detection limit of the mouse cytokines were as follows: IFN-γ - 2 pg/mL, IL-4 - 2 pg/ml, TNF-α - 5.1 pg/mL, IL-6 - 1.6 pg/mL, IL-10 - 4.0 pg/mL, IL-12 p40 - 4 pg/mL, and IL-18 - 25.0 pg/mL.
Measurements of bacterial burden by real-time PCR (RT-PCR)
Ehrlichial stock and bacterial burden in different organs were measured using an iCycler IQ multicolor real-time detection system (Bio-Rad, Hercules, CA), as previously described (7, 11). Ehrlichial burdens were determined using two primer sets (Table 1). The first set included primers targeting both E. muris and IOE (EM/IOE) dsb (a thio-disulfide oxidoreductase) gene, eukaryotic housekeeping gene GAPDH and the real-time PCR was performed using specific probes as described previously (11). The second set included primers targeting a different sequence of the dsb gene of Ehrlichia, the same housekeeping GAPDH gene and the real-time PCR was performed using the SYBR Green iQ Supermix (Bio-Rad) as described before (41, 42). The results were normalized to the levels of expression of the GAPDH in the same sample and expressed as copy number per 104 GAPDH (7, 11). PCR analyses were considered negative for ehrlichial DNA if the critical threshold (CT) values exceeded 40 cycles.
Table I.
Real-Time PCR Ehrlichia Genus -specific primers and probe.
| PRIMER SET | SEQUENCE |
|---|---|
| 1. | |
| DSB (Forward, 5’-3’) | CAG GAT GGT AAA GTA CGT GTG A |
| DSB (Reverse, 5’-3’) | TAG CTA ACG CTG CCT GAA CA |
| PROBE (5’-3’) | FAM/AGG GAT TTC CCT ATA CTC GGT GAG GC/BHQ |
| 2. | |
| DSB-321(Forward, 5’-3’) | TTG CAA AAT GAT GTC TGA AGA TAT GAA ACA |
| DSB-671(Reverse, 5’-3’) | GCT GCT CCA CCA ATA AAT GTA TCY CCT A |
Neutralization of IL-18Rα
C57BL/6 WT mice were injected i.p. with 100 μg/mouse of anti-IL-18Rα monoclonal antibody (mAb) (Clone: 112624, R&D Systems) on days 0, 1, 3 and 5 following IOE infection. Control mice were injected with 100 μg/mouse of rat IgG2a isotype control mAb at the indicated time points. Anti-IL-18Rα-treated and isotype control mice were infected with a high lethal dose of IOE (5×103 bacteria/mouse). Of note, IOE-infected mice treated with the isotype control Ab had higher bacterial burdens than IOE-infected untreated WT mice in some experiments, especially when measured by the first dsb primer set.
Flow cytometry and intracellular cytokine staining
Splenocytes were harvested, counted, and resuspended in fluorescence-activated cell sorter staining buffer (Dulbecco's phosphate-buffered saline without Mg2+ or Ca2+ containing 1% heat-inactivated fetal calf serum and 0.09% sodium azide, pH 7.4 - 7.6) at a concentration of 106 cells/well. Fc receptors were blocked with a monoclonal antibody (clone 2.4G2) against mouse cell surface antigens CD16 and CD32 for 15 min. The following fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, peridinin-chlorophyll protein (PerCP-Cy5.5)-, Alexa Fluor- and allophycocyanin-conjugated antibodies (APC) were purchased from BD Biosciences unless indicated otherwise: anti-CD45.2 (clone 69), anti-CD3 (clone 145-2C11), anti-CD11c (clone HL3), anti-F4/80 (clone 6F12), anti-CD4 (clone RM4-4), anti-CD8a (clone 53-6.7), anti-CD11b (clone M1/70), anti-CD4 (clone RM4-5), anti-NK1.1 (clone PK136), anti-CD19 (clone 1D3), anti-TNF-α (clone MP6-XT22), anti-IFN-γ (clone XMG102), anti-CD95 (clone JO2), anti-Ly6G (clone 1A8), anti-I-Ad/Ed (M5/114.15.2), anti-H2Db (KH95), anti-CD40 (3/23), anti-CD80 (16-10A1), and anti-CD86 (GL1). Isotype control mAbs including FITC-, PE-, or APC -conjugated hamster IgG1 (A19-3), rat IgG1 (R3-34), rat IgG2a (R35-95), mouse IgG2a (X39), mouse IgG2b (MPC-11), mouse IgG1 (X40), and rat IgG2b (A95-1) were purchased from Biolegend (San Diego, CA). For intracellular cytokine staining, splenocytes were incubated at 37°C for 4 hours in complete medium with the addition of BD Golgi Plug (BD Biosciences, CA) according to the manufacturer's recommendations. Lymphocytes and granulocytes populations were gated based on forward and side-scatter parameters. 50,000 to 200,000 events were collected using BD-LSR or BD FACS Caliber (BD Immunocytometry Systems, San Jose, CA) flow cytometer, and data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR).
Generation of bone marrow derived dendritic cells and in vitro Ehrlichia stimulation
Immature CD11b+CD11c+ cells were isolated from the bone marrow of naive WT C57BL/6 or IL-18Rα-/- mice. Bone marrow derived dendritic cells (BMDCs) were generated as originally described (43-45) with slight modifications. Briefly, single cell suspension from bone marrow was prepared from mouse femurs and adjusted to 2 χ 106 cells per 10 mL of complete IMDM (Iscove's modified DMEM containing 10% FBS, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol, 100 μg/mL streptomycin sulfate and 100 U/mL penicillin). DC culture medium was supplemented with 20 ηg/mL recombinant granulocyte-macrophages colony stimulating factor, GM-CSF (BD Biosciences, CA). On day 3, 6 mL of fresh GM-CSF-containing medium was added. On day 6, 10 mL of the culture medium was replaced with fresh GM-CSF-containing medium. On day 8, the cultures were examined by FACS analysis and were used for experiments if they contained 70-75% CD11c+ cells. On day 8 of the culture, BMDCs were collected, and 5 χ 105 cells/mL were seeded into 24-well culture plates with complete medium without antibiotics. Cell-free IOE organisms were added to each well at a multiplicity of infection (MOI) of 5:1. The cells were harvested 24 hours following infection, and the expression of different maturation markers (MHC class I and II, and costimulatory molecules including CD80, CD86, CD40) were examined by flow cytometry. In addition, the levels of TNF-α and IL-6 in BMDCs culture supernatant were determined by ELISA as described above.
Histopathology and TUNEL Assays
Liver segments were fixed in 10% neutral buffered formalin, dehydrated in graded alcohols, and embedded in paraffin wax. Sections (3 μm thick) were collected on coated slides and stained with hematoxylin and eosin (H&E). Liver lesions were assessed semi-quantitatively using two main parameters that have been previously shown to be associated with Ehrlichia-induced immunopathology and toxic shock; the number of apoptotic and/or necrotic cells and the number of inflammatory foci. TUNEL staining was performed on liver sections to quantify the number of apoptotic cells as described previously (7, 11, 16)
Adoptive cell transfer
For adoptive transfer of cells, splenocytes were collected from IOE-infected WT mice on day 7 p.i. (5 χ 103 IOE organisms/mouse) or from naive mice. Splenocytes were enriched for the CD8+ population using mouse CD8 microbeads (MACS, Milteny Biotec Inc. Auburn, CA) before injection, resulting in a cell population of 75-85% CD8+ as determined by cell-surface staining and flow cytometry. 106 purified CD8+ cells in 200 ul were i.v. injected into groups of six C57BL/6 or IL-18Rα-/- mice (in two separate experiments) through retro-orbital route 2 hours before inoculation with a high dose of IOE (5 χ 103 organisms/mouse).
Recombinant IL-18 therapy
C57BL/6 mice were infected i.p. with a high dose of E. muris (i.e., non-lethal infection) and were treated with recombinant mouse IL-18 (rIL-18) (MBL International, Woburn, MA). To mimic the in vivo increase in IL-18 production at late stages of lethal IOE infection, multiple IL-18 injections (1 μg/mouse) were administered to E. muris-infected mice on days 3, 4, 5, and 6 p.i. These time points correspond to time points at which a significant elevation of IL-18 in the sera and livers of lethally/IOE-infected WT mice was observed. E. muris-infected mice injected with PBS only or uninfected mice injected with rIL-18 only at the same time points comprised the sham controls.
Statistical analyses
For comparison of mean values for two experimental groups, two-tailed t test was used, and P values were calculated using GraphPad Prism (GraphPad Software, Inc., San Diego, CA, USA). For comparisons of multiple experimental groups, one-way analysis of variance (ANOVA) was used. Post hoc group pair wise comparisons were conducted using the Bonferroni procedure and an overall alpha level of significance of 0.05. P values < 0.001 were considered highly significant (***), P values < 0.01 considered moderately significant (**), and those < 0.05 considered significant (*).
Results
High serum levels of IL-18, IL-12, IFN-γ, and IL-10 correlate with lethality
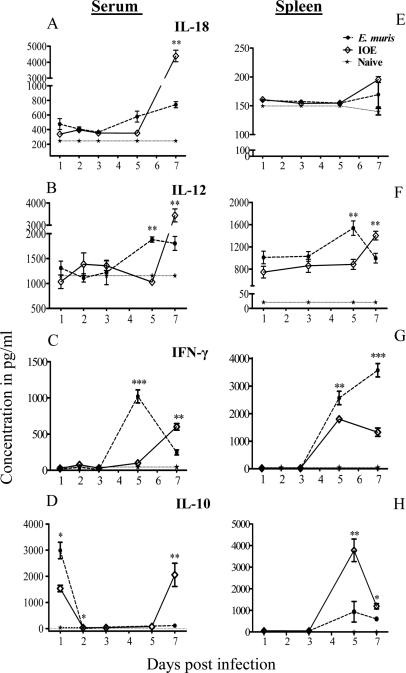
We recently showed that lethal IOE infection induced large amounts of IL-18 production by liver mononuclear cells during the late stages of infection, which correlated with expansion of pathogenic NK and CD8+ T cell responses, liver injury, and progression to fatal disease (16). To determine whether acute lethal/IOE infection alters local and systemic levels of pro-inflammatory/Th1 and Th2 cytokines compared to non-lethal/EM infections, the levels of IL-18, IL-12, IFN-γ, IL-4 and IL-10 in the sera and spleens of infected mice were examined on days 1, 3, 5, and 7 p.i. Consistent with our previous studies, the levels of IL-4 in both sera and spleens of lethally and non-lethally infected mice were negligible and not significantly different than uninfected controls (data not shown). While non lethal infection induced higher serum levels of IL-12, IL-18, and IFN-γ on day 5 p.i, lethal infection induced significantly higher serum levels of IL-18, IL-12, and IFN-γ on day 7 p.i. (Fig. 1A-C). The levels of IL-18 in the spleens of lethally and non-lethally infected mice did not significantly differ from that in uninfected controls during course of infection (Fig. 1E). The levels of IL-12 in the spleens of lethally infected mice on day 5 p.i. were lower than that detected in the spleens of non-lethally infected mice. However, IL-12 increased in the spleens of lethally infected mice on day 7 p.i. (Fig. 1F). Interestingly, regardless of the levels of IL-12 and IL-18 in the sera or spleens, IFN-γ levels in the spleens of lethally infected mice were significantly lower on days 5 and 7 p.i. compared to non-lethally infected mice (Fig. 1G).
FIGURE 1. Lethal Ehrlichia infection induces higher serum levels of IL-18, IL-12, and IL-10 than non-lethal infection.
Serum samples (A-D) and Spleen cell culture (E-H) were prepared as described in the Materials and Methods from C57BL/6 mice following i.p.infection with either a high dose of IOE (lethal) or E. muris (non lethal). IL-18 (A, E), IL-12 (B, F), IFN-γ (C, G), and IL-10 (D, H) were measured in sera or spleen culture supernatant at different time points after infection by ELISA. The data shown represent the mean ± SEM of three mice per group. The data are representative of two independent experiments with similar results. Significant differences between lethally and non-lethally infected mice were assessed. *P <0.05, **P< 0.01. , ***P< 0.001
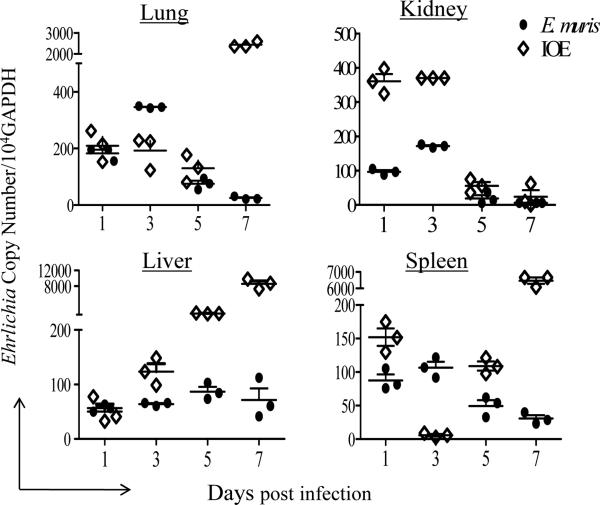
We next examined whether altered systemic and local production of Th1 cytokines during lethal Ehrlichia infection is due to changes in kinetics of IL-10 production. Both lethal and non-lethal infections increased serum levels of IL-10 on day 1 p.i. compared to uninfected controls, with a significantly higher level in non-lethally infected mice. IL-10 dramatically declined in the sera of non-lethally infected mice on days 2, 5, and 7 p.i. (Fig. 1D). On the other hand, the transient decline of serum levels of IL-10 in lethally infected mice on days 2 and 5 p.i. was followed by a substantial increase on day 7 p.i. (Fig. 1D). IL-10 was also significantly higher in the spleens of lethally infected mice than that in non-lethally infected mice on day 5 and 7 p.i. (Fig. 1H). Decreased IFNγ in the spleens and increased systemic and local IL-10 production in lethally/IOE- infected mice was associated with higher bacterial burdens in all organs at late stages of infection compared to non-lethally EM-infected mice (Fig. 2). Collectively, these data suggested that IOE infection induced a late burst in the levels of circulating pro-inflammatory/Th1 and anti-inflammatory cytokines that coincide with lethality and defective bacterial clearance.
FIGURE 2. Lethal Ehrlichia infection is associated with higher bacterial burden and dissemination compared to non-lethal infection.
C57BL/6 mice were infected via the i.p. route with a high dose of IOE (lethal) or E. muris (non-lethal). Different organs (lung, liver, kidney, and spleen) were collected on days 1, 3, 5, and 7 p.i. Organs from uninfected mice were used as negative controls. DNA was extracted from different organs, and bacterial burden determined by RT-PCR. The copy number of IOE/EM was normalized to the housekeeping gene GAPDH. The data shown represent the mean of three mice per group and are representative of three independent experiments with similar results.
Absence of IL-18R enhanced bacterial elimination and prolonged survival following infection with a lethal dose of IOE
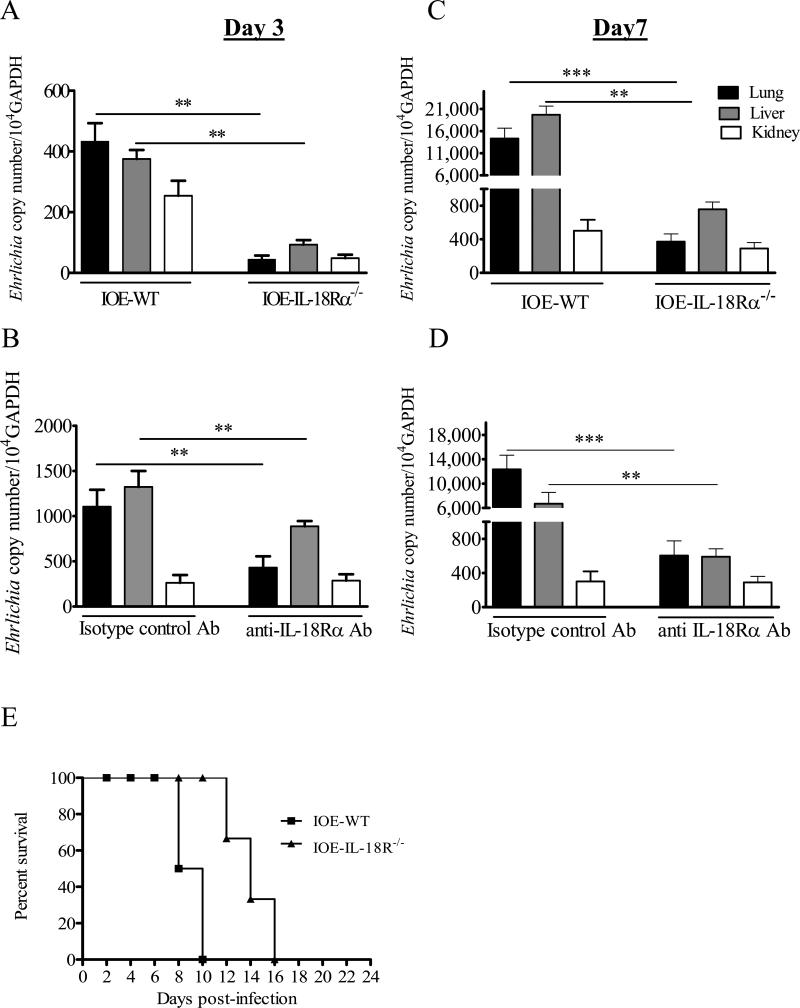
To examine the role of IL-18 in the control of ehrlichial infection, C57BL/6 wild-type and IL-18Rα-/- mice were infected via i.p route with a lethal dose of IOE and the bacterial burden in different organs at early and late stages of infection was determined by real time PCR using two sets of primers. Unexpectedly, we found that IL-18Rα-/- mice had significantly lower bacterial burdens in the liver, lung, and kidney when compared to WT mice on day 3 and 7 p.i. (Fig. 3A and C). Furthermore, while IOE-infected WT mice succumbed to lethal infection on days 8-10 p.i., IOE-infected IL-18Rα-/- survived longer where 100% of mice succumbed to infection on day 16 p.i. (Fig. 3E). To ensure that enhanced resistance to IOE infection in IL-18Rα-/- mice was not due to changes in the immune system or compensatory mechanisms of these knockout animals, WT infected mice were treated with anti-IL-18Rα mAb. Similar to the IL-18Rα-/- mice, WT mice treated with anti-IL-18Rα mAb contained lower bacterial burdens in their lungs, livers and kidneys on days 3 and 7 p.i. (Fig. 3B and D) compared to isotype-sham controls. Using the two primer sets, we consistently detected a significant difference in the bacterial burden between IL-18Rα-/- and anti-IL-18Rα-/- treated mice compared to infected WT and isotype control mice. However, the ehrlichial copy number in organs in all groups of mice was higher when measured by the first primer set than that detected by the second set of primers (data not shown). Taken together, these results demonstrate that IL-18/IL-18Rα interaction contribute to ineffective bacterial elimination and mortality following acute lethal ehrlichial infection.
FIGURE 3. Enhanced resistance to IOE infection in IL-18Rα-/- and anti-IL-18Rα -mAb treated mice compared to infected WT or sham control mice.
C57BL/6 WT and IL-18Rα-/- mice (A and C) or anti-IL-18Rα-/- antibody-treated mice and isotype controls (B and D) were infected via the i.p. route with a high dose of IOE (i.e. lethal infection). On days 3 (A and B) and 7 (C and D) p.i., tissues from lung, liver, and kidney were collected and bacterial burden was determined by real time PCR using the second primer set. The copy number of IOE was normalized to the housekeeping gene GAPDH. Bacterial burden in all organs were lower in IOE-infected IL-18Rα-/- and anti-IL-18Rα mAb treated mice compared to WT and isotype controls on days 3 and 7 p.i. The data represent the mean ± SEM of three mice/ group. The data are representative of three independent experiments with similar results. *P <0.05, **P< 0.01. , ***P< 0.001. E, Survival of WT and IL-18Rα-/- mice over 24 days after i.p. infection with high dose of IOE. The data shown represent one of three independent experiments with a total of 12 mice/group.
IL-18Rα is required for induction of Ehrlichia-induced liver inflammation and tissue injury
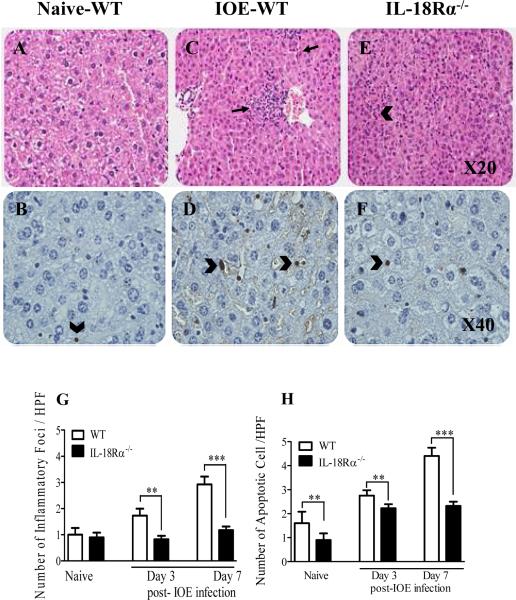
One of the main pathologic features of HME in humans and mice is the development of severe tissue injury followed by multi-organ failure and toxic shock (7, 11-13). Hence, we examined the impact of the IL-18/IL-18Rα interaction on acute liver damage and inflammation following lethal ehrlichial infection on days 3 and 7 p.i. Our data show that the differences in hepatic pathology between IOE-infected C57BL/6 wild-type mice and IL-18Rα-/- mice were minimal on day 3 p.i. (not shown). However, on day 7 p.i., at the peak of the disease, the livers of IOE-infected WT mice showed an increased influx of inflammatory cells, including lymphocytes and macrophages, which was associated with marked apoptosis of hepatic cells (Fig. 4C and D) compared to uninfected mice (Fig. 4A and B). In contrast, IOE-infected IL-18Rα-/- mice developed an attenuated pathology, with significantly less cellular infiltration and fewer apoptotic cells (Fig. 4E and F) in the livers when compared to infected WT controls. Quantitative data on the number of cellular foci determined by H&E staining and the number of apoptotic cells between different groups of mice determined by TUNEL assay are presented in Figure 4G and H, respectively. These results suggest that the absence of IL-18Rα signals confers protection against Ehrlichia-induced immune-mediated pathology. Consistent with the results from IL-18Rα-/- mice, IOE-infected WT mice treated with anti-IL-18Rα monoclonal antibodies exhibited decreased cellular recruitment to the liver, and fewer disruptions in liver architecture on day 7 p.i. when compared to IOE-infected sham control mice (data not shown). Collectively, these data indicate that IL-18/IL-18R interaction contributes to the pathogenesis of lethal Ehrlichia-induced tissue injury and inflammation during fatal disease.
FIGURE 4. Attenuation of liver pathology and cellular infiltration in IOE-infected IL-18Rα-/- mice.
Liver sections from naive (A and B), IOE infected WT mice (C and D), and IOE infected IL-18Rα-/- mice (E and F) harvested on day 7 p.i. are stained with H&E Original magnification x20, or TUNEL x40. H&E staining show that IOE-infected IL-18Rα-/- mice had a lower influx of inflammatory cells (arrows) and a lower number of apoptotic cells (arrowhead) than infected WT and naive mice. TUNEL assays showed substantially decreased number of apoptotic cells (arrowhead) in IL-18Rα-/- mice with ~ 1 to 2 apoptotic cells observed per 40x high power field (HPF) compared with to 5-10 apoptotic cells per 40x HPF in the infected WT mice. Uninfected control mice had only one apoptotic cells/HPF. G and H show the quantitative analysis of the number of inflammatory foci and apoptotic cells determine by H&E and TUNEL assays, respectively, in different groups of mice. The data shown are from a representative mouse from each group (n=4) and are representative of three different experiments. *P <0.05, **P< 0.01. , ***P< 0.001.
Lack of IL-18Rα influences innate immune responses against Ehrlichia
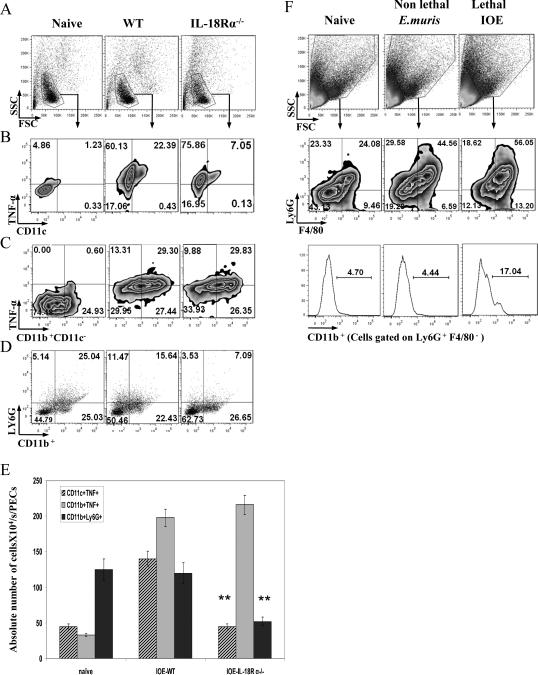
During the initial phase, the anti-ehrlichial immune response is mediated by elements of the innate immune system and involves a complex interaction between cytokines, such TNF-α, and IFN-γ, and innate immune cells (7, 13, 16, 46, 47). We hypothesized that enhanced bacterial elimination in IL-18Rα-/- mice is due to enhanced protective immunity at the sites of infection. To test this hypothesis, we examined effects of IL-18 signals on the expansion and function of phagocytic cells at the primary site of infection, the peritoneum. Our data indicate that the absence of IL-18R signals in IOE-infected KO mice on day 3 post-infection resulted in a decrease in the percentage (Fig. 5B) and absolute number (Fig. 5E) of TNF-α-producing CD11c+ dendritic cells, while it did not influence the percentage or absolute number of TNF-α–producing CD11c-CD11b+ cells (consistent with the phenotype of macrophages) (Fig. 5C and E). Furthermore, the absence of IL-18R signals decreased the percentage and absolute number of Ly6G+ expressing CD11b activation marker, which is consistent with the phenotype of activated neutrophils (48, 49) compared to infected WT mice (Fig. 5D and E). Interestingly, resistance of WT mice to non-lethal infection with E. muris was associated with reduced expansion of activated neutrophils (Ly6G+CD11b+F4/80-) in the spleen on day 3 p.i. when compared to lethal IOE infection (Fig. 5F). These data suggest that the lack of IL-18/IL-18R interactions can shift the immune response against Ehrlichia from a pathogenic to a protective phenotype and that activated neutrophils may play a role in the pathogenesis of Ehrlichia-induced fatal shock.
FIGURE 5. The lack of IL-18/IL-18R interaction influences the frequency and function of innate immune cells in the peritoneum.
Peritoneal exudates were collected from naive, IOE-infected IL-18Rα-/- and WT mice on day 3 p.i. A, Cells were gated based on forward and side scatter and the frequencies of macrophages, dendritic cells and granulocytes were analyzed by flow cytometry. A-D, Flow cytometric analysis of peritoneal cells from uninfected, infected WT, and IL-18Rα-/- mice stained for: CD11C and TNF-α (B); CD11b+ CD11c- and TNF-α (C); and Ly6G+ CD11b+ (D) [CD11b expression was measured to quantify the degree of neutrophil activation in the peritoneal fluid in response to Ehrlichia as previously described]. E, Absolute number of different cell subsets in the peritoneal fluid of naive, IOE-infected WT, and IOE infected IL-18Rα-/- mice on day 3p.i.. F, Spleen cells from naive, lethally (IOE) and non-lethally (E. muris)-infected mice harvested on day 3 p.i. were gated on granulocytes based on side and forward scatter. Dot plot showing gated granulocytes stained for: F4/80 and Ly6G as markers of macrophages and neutrophils, respectively. Histogram show the percentage of activated neutrophils, as marked by the expression of CD11b, an activation marker, on gated Ly6G+ F4/80-neutrophils. The data shown are from a representative mouse from each group (n=4), and the numbers indicate the percentage of cells within each quadrant. The data shown are from one experiment that is representative of three different experiments. **P< 0.01.
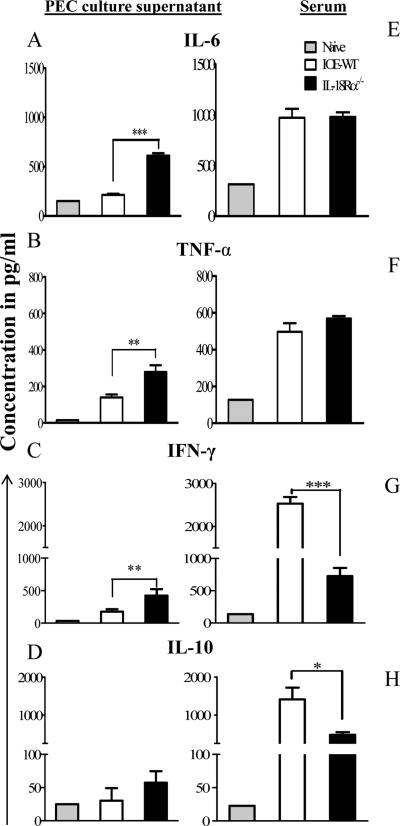
We subsequently analyzed the systemic and local cytokine responses on day 7 post infection in serum and culture supernatant from peritoneal exudates cells and spleens of infected mice following in vitro Ag stimulation. Our data showed that a lack of IL-18Rα resulted in significant increase in levels of IL-6, TNF-α, and IFN-γ in the culture supernatant of peritoneal exudates cells from IOE infected-IL-18Rα-/- mice when compared to WT mice (Fig. 6A-C). While the serum levels of IL-6 and TNF-α were not significantly different between infected WT and IL-18Rα-/- mice (Fig. 6E and F), the serum level of IFN-γ was lower in IL-18Rα-/- compared to WT mice (Fig. 6G). The levels of IFN-γ, IL-6, TNF-α in the culture supernatants of splenocytes from infected IL-18Rα-/- mice were similar to that detected in infected WT mice (cytokine levels in IL-18Rα-/- vs. WT mice are: IFN-γ, 420±35 vs. 380±56; TNF-α, 680±43 vs.590±28; and IL-6, 4200±360 vs.3700±244). The lack of IL-18Rα did not influence the levels of IL-10 in the peritoneum of IL-18Rα-/- mice (Fig. 6D); however, it significantly decreased its serum levels compared to infected WT mice (Fig. 6H). Taken altogether, our data suggest that IL-18/IL-18R interaction is critical for systemic production of IL-10, and suppression of pro-inflammatory and Th1 cytokines at the peripheral site of IOE infection.
FIGURE 6. Altered pro- and anti- inflammatory cytokines in the sera and peritoneum of IL-18Rα-/- mice compared to infected WT or sham control mice.
Peritoneal exudates cells (PEC) harvested from day 7, IOE infected WT and IL-18Rα-/- mice were cultured in vitro in the presence of IOE Ags for 48 hours. Peritoneal cell culture supernatant (A-D) and sera (E-H) collected on day 7 p.i. from all infected mice were tested for IL-6, TNF-α, IFN-γ, and IL-10, using ELISA. The data are expressed as the mean ± SEM for three mice in each group. The data shown are from one experiment and are representative of three different experiments. . *P <0.05, **P< 0.01. ***P< 0.001.
Enhanced resistance to infection in IL-18Rα-/- mice is not linked to elevated type-1 immune responses in the spleen
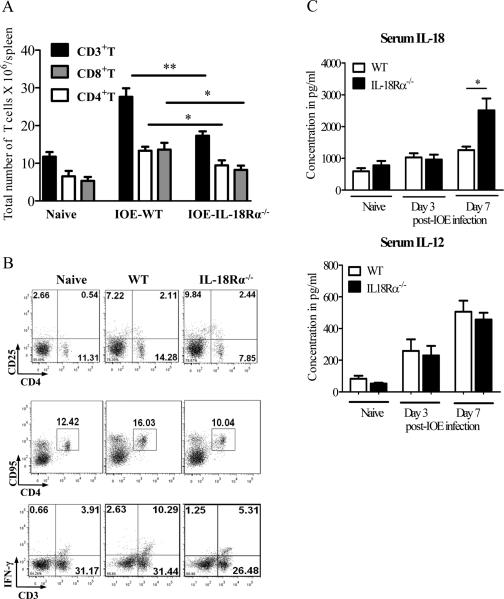
We next examined whether attenuated pathology and enhanced bacterial elimination in IL-18Rα-/- mice on day 7 p.i. was due to an enhanced protective Th1 response in the spleen. At the single cell level, the lack of IL-18Rα in IOE-infected IL-18Rα-/- mice resulted in decreased absolute numbers of CD3+, CD8+ and CD4+ T cells on day 7 p.i. (Fig.7A). In particular, IOE-infected IL-18Rα-/- mice have lower percentage of CD4+CD25- effector T cells compared to IOE-infected WT mice (7.85% ± 2.6% compared to 13.45% ±2.2%, Fig. 7B), indicating that IL-18R signal is critical for activation of CD4+ T cells. However, the expression of apoptotic markers (CD95/ FAS) on CD4+T cells was also lower in IOE-infected IL-18Rα-/- mice compared to infected WT mice (Fig. 7B), suggesting that the decline in the number of effector CD4+ T cells is unlikely to be caused by apoptotic cell death. Surprisingly, attenuated pathology and enhanced resistance in IOE-infected IL-18Rα-/- mice correlated with a decreased percentage (Fig. 7B) and absolute number (data not shown) of Ehrlichia-specific IFN-γ-producing CD3+ type-1 cells in the spleen. Decreased type-1 responses in IOE-infected IL-18Rα-/- mice did not shift the responses towards a Th2 response since the percentage of IL-4-producing Typ-2 cells was minimal and not significantly different than that in naive or infected WT mice (data not shown).
FIGURE 7. IL-18/IL18Rα interaction is essential for the expansion and activation of type-1 cells during Ehrlichia infection.
Splenocytes harvested from naive, IOE-infected IL-18Rα-/- and WT mice on day 7 p.i. were stimulated with IOE Ags. Lymphocyte population was gated based on forward and side scatter and analyzed by flow cytometry. A, IOE infection in IL-18Rα-/- mice resulted in a decrease in the absolute numbers of total CD3+ T cells, CD4+CD3+ cells (CD4+ T cells), and CD8+CD3+ cells (CD8+ T cells) compared to infected WT mice. B, Dot plots showing surface expression of activation marker CD25 and apoptotic marker CD95/FAS, respectively, on CD4+ lymphocytes as well as intracellular IFN-γ production by CD3+ T cells (type-1 T cells). C, Serum cytokine levels of IL-18 and IL-12 in naive, IOE- infected WT and IL-18Rα-/- mice on day 3 and 7 p.i. The data shown are from a representative mouse from each group (n=4), and the numbers indicate the percentage of cells within each quadrant. The data shown are representative of three different experiments. . *P <0.05, **P< 0.01.
Since IL-12 and IL-18 are produced by the same antigen presenting cells and can act cooperatively in the induction and expansion of Th1 responses (20-24), we examined whether decreased type-1 immune response in IL-18Rα-/- mice was due to reduced production of Th1-promoting cytokines, mainly IL-12. Our data showed that on day 3 p.i., there was no significant difference in the levels of IL-12 and IL-18 between the two groups of mice (Fig. 7C). In contrast, on day 7 p.i., we detected an increase in the serum level of IL-18 in IOE-infected IL18Rα-/- mice compared to WT mice, while IL-12 levels were comparable between the two groups of infected mice (Fig. 7C). Thus, decreased frequency of type-1 cells in IL-18Rα-/- mice is less likely due to decreased production of Th1-promoting cytokines.
Enhanced resistance to infection in IL-18Rα-/- mice is linked to altered NK and NKT cell responses
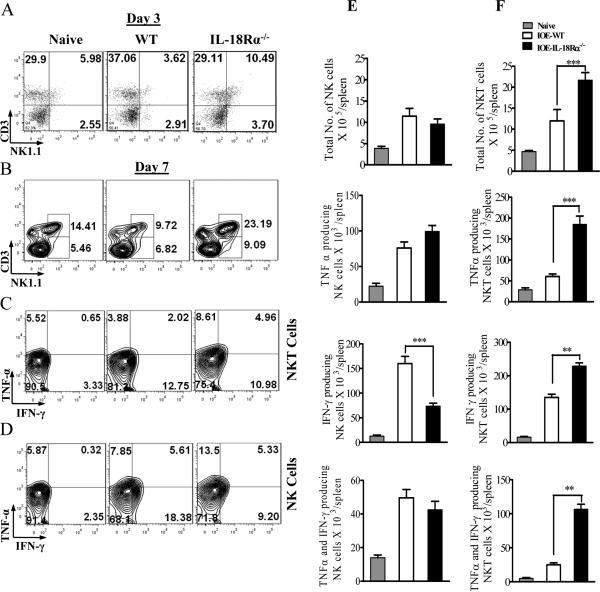
Our previous study indicated that IFN-γ and TNF-α producing NKT cells promote effective bacterial elimination, while NK cells producing IFN-γ inhibit protective anti-Ehrlichia immunity and mediate pathogenic responses and tissue injury during fatal disease (16-18). To further address the effect of IL-18R on NK and NKT responses, we measured the frequency of and cytokine production by these cells in the spleens of WT and IL-18Rα-/- mice during lethal IOE infection. On day 3 p.i., lethal IOE infection in IL-18Rα-/- mice resulted in significant increase in the percentage of NKT cells compared to IOE-infected WT mice (Fig. 8A). A similar increase in the percentage (Fig. 8B) and absolute number (Fig. 8F) of NKT cells in IL-18Rα-/- mice was observed on day 7 p.i. compared to WT mice. Within the NKT cell population, we detected significantly greater percentage and absolute number of NKT cells producing TNF-α only and NKT cells producing TNF-α and IFN-γ (Fig. 8C and F) as well as absolute number of NKT cells producing IFN-γ only (Fig. 5F) in infected IL-18Rα-/- mice compared to infected WT mice. In contrast, the lack of IL-18R signals did not significantly influence the percentage and absolute number of NK cells in the spleen of IL-18Rα-/- mice on days 3 (Fig. 8A) or 7 p.i. (Fig. 8B and E). Within the NK population, IL-18Rα-/- mice have lower percentage and absolute number of IFN-γ, but not TNF-α,-producing NK cells than that detected in IOE-infected WT mice (Fig. 8D and E). These data suggest that IL-18/IL-18R interaction contributes to decreasing frequency of protective NKT cells producing both TNF-α/IFN-γ- NKT, while enhancing the frequency of IFN-γ producing pathogenic NK during fatal ehrlichial infection.
FIGURE 8. Enhanced resistance to Ehrlichia infection in IL-18Rα-/- mice is associated with enhanced expansion of NKT cells and altered function.
WT C57BL/6 and IL-18Rα-/- mice were infected with a high dose of IOE. Splenocytes were harvested from naive, IOE-infected IL-18Rα-/- and WT mice on day 3 (A) and 7 (B-F) p.i., were stimulated in vitro with IOE Ags followed by intracellular cytokine staining. A and B, dot plots showing an increased percentage of CD3+NK1.1+ NKT cells, but not CD3-NK1.1+NK cells, on days 3 and 7 p.i., respectively, in IL-18Rα-/- mice compared to infected WT controls. C and D, cells were gated on NKT and NK cells, respectively, and analyzed for IFN-γ and TNF-α production. E and F show the absolute number of IFN-γ only, TNF-α only and IFN-γ and TNF-α producing NK (E), NKT (F) cells in the spleens of naive, IOE-infected WT and IOE-infected IL-18Rα-/- mice. Data shown are from a representative mouse from each group (n=4), and the numbers indicate the percentage of cells within each quadrant. The data shown are from one experiment that is representative of three different experiments. **P < 0.01. ***P < 0.001.
IL-18Rα is required for induction and expansion of pathogenic TNF-α-producing CD8+ T cells during fatal ehrlichial infection
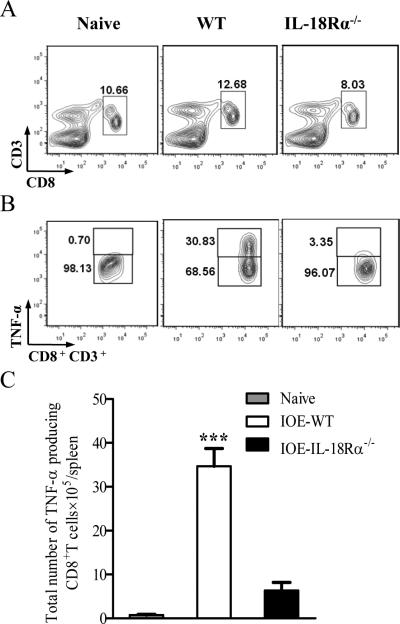
Our previous studies showed that Ehrlichia-induced toxic shock and tissue injury is directly mediated by cytotoxic pathogenic CD8+ T cells producing TNF-α (7, 13, 19). Here, we assessed whether IL-18Rα acts as a limiting factor for the activation and expansion of pathogenic Ehrlichia-specific CD8+ T cells during the peak stage of the disease. Compared to infected WT mice, absence of IL-18Rα in KO mice resulted in decreased percentage (Fig. 9A) and absolute numbers (Fig. 7A) of CD8+ T cells in the spleen, with a significant decrease in the percentage and absolute number of TNF-α producing CD8+T cells on day 7 p.i. (Fig. 9B and C).
FIGURE 9. IL-18/IL18Rα interaction is essential for the induction of pathogenic TNF-α-producing CD8+ T cells during severe Ehrlichia infection.
Spleen cells were harvested from IOE-infected IL-18Rα-/- and WT mice on day 7 p.i., in vitro stimulated with IOE Ags and the frequency of IOE-specific CD8+T cells as well as TNF-α producing CD8+T cells was analyzed by flow cytometry. A, Contour plot shows the percentage of CD3+CD8+ T cells in naive, IOE-infected WT, and IL-18Rα-/- mice. B, CD8+ T cells were gated and analyzed for intracellular TNF-α production, naive mice have less than 1% of TNF-α-producing CD8+ T cells, infected WT mice have a higher percentage of TNF-α-producing CD8+ T cells than IOE infected IL-18Rα-/-. C, The absolute number of TNF-α-producing CD8+ T cells in the three groups of mice. The data shown are from a representative mouse from each group (n=3). The data shown are from one experiment that is representative of three different experiments. ***P < 0.001.
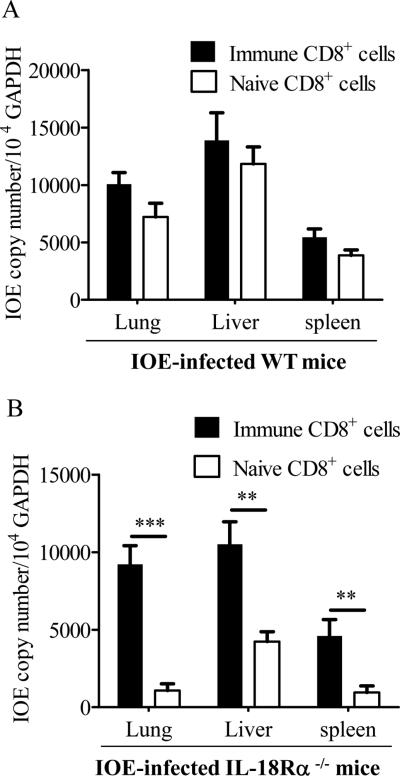
Adoptive transfer of CD8+ T cells from immune wild type mice increased IOE loads in IL-18Rα-/- mice
Based on the above data, we hypothesized that enhanced resistance against IOE in 18Rα-/- mice is due to reduction in the number of pathogenic CD8+T cells, and that adoptive transfer of immune CD8+ cells from wild type mice may reverse the observed resistance and protective immunity against IOE in IL-18Rα-/- infected mice. To test this hypothesis, we purified CD8+T cells from spleens of IOE-infected WT on day 7 p.i., transferred these cells or naïve WT CD8+ cells to WT or 18Rα-/- mice, and then challenged these mice via i.p. route with IOE. Bacterial burden in different organs were determined at 7 days later. IL-18Rα-/- mice treated with naive WT CD8+ splenocytes harbored significantly less IOE in their organs compared to similarly-treated IOE-infected WT mice (Fig. 10 compared panels B to A). WT mice treated with naive or immune CD8+ cells had similar burden of IOE in their organs, suggesting that additional immune CD8+ cells are not sufficient to further inhibit protective immunity and lead to increased bacterial burden (Fig. 10A). In contrast, bacterial burdens in different organs of 18Rα-/- mice that received immune WT CD8+ splenocytes were significantly increased compared with IL-18Rα-/- mice given naïve CD8+T splenocytes (Fig. 10B). Furthermore, bacterial burden in immune CD8+ cell treated IL-18Rα-/- were not statistically different than that in wild type mice receiving naive or immune CD8+ cells (Compare panels B to A), suggesting that the protective responses of IL-18Rα-/- in the control of IOE infection can be inhibited or abrogated by pathogenic immune WT CD8+T cells.
Figure 10. Adoptive transfer of splenocytes from immune wild type mice increases IOE numbers in IL-18Rα-/- mice.
A total of 106 naive splenocytes and immune splenocytes were harvested from WT mice on day 7 post-high dose IOE infection, enriched for CD8+ population and were i.v. injected into groups of 3-7 WT mice (A) or IL-18Rα-/- (B) 2 hours prior to IOE challenge. Bacterial loads in lungs, livers, and spleens on day 7 p.i. are expressed as copy number/104 GAPDH. The data shown are from one experiment that is representative of two different experiments with similar results. **P< 0.01. , ***P< 0.001.
Recombinant IL-18 inhibits bacterial elimination and exacerbates pathology
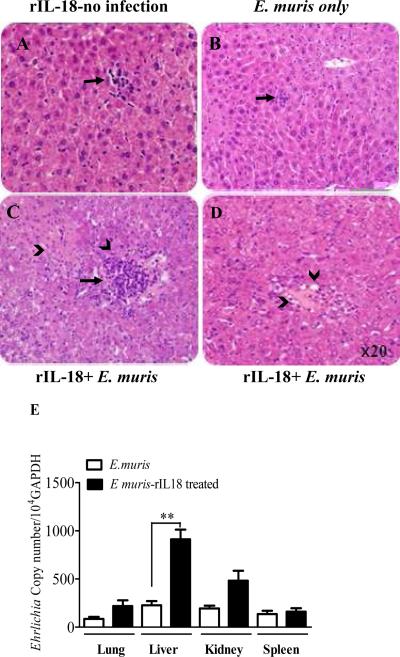
Based on the above data, we hypothesized that the late burst of IL-18 following lethal ehrlichial infection promotes immunopathology and negatively regulates protective immunity. To further test this hypothesis, we examined the effects of administering a high concentration of IL-18 on the host defense following non-lethal E. muris infection. WT mice were infected with a high dose of E. muris and subsequently treated with recombinant IL-18 (rIL-18). Compared to IL-18-treated but uninfected control mice (Fig. 11A) or untreated E. muris-infected mice (Fig. 11B), rIL-18 therapy of E. muris infected mice (Fig. 11C) resulted in an enhanced inflammatory cellular infiltration in the liver, increased numbers of apoptotic cells , and the development of marked fatty changes in the liver (Fig. 11D). In addition, rIL-18 therapy of E. muris-infected mice resulted in a significantly increased ehrlichial burden in different organs on days 7 p.i. (Fig. 11E) compared to untreated E. muris-infected mice. These results support the notion that Ehrlichia-induced inflammation and tissue injury are maintained in the liver under conditions of IL-18 overproduction, which negatively influences protective anti-ehrlichial immunity.
FIGURE 11. Multiple injections of rIL-18 enhance cellular infiltration, increase tissue injury and inhibit protective anti-ehrlichial immunity following non-lethal ehrlichial infection.
C57BL/6 mice were infected with mildly virulent E. muris, and rIL-18 was administered at different time points after infection. On day 7 p.i., mice were sacrificed and liver sections from rIL-18 treated naive (A), untreated E. muris - infected mice (B), and rIL-18 treated EM-infected mice (C, D) were stained with H&E staining. Enhanced inflammatory infiltration and apoptotic cell death in rIL-18-treated E. muris-infected mice and were associated with fatty changes (arrowhead) (D), compared to naive or untreated but E. muris-infected mice. (E) Bacterial burden in the lungs, liver, kidney, and spleen determined by real-time PCR was higher in treated E. muris-infected mice compared to untreated controls on day 7 p.i. The data are shown as the means ± SD of three mice/ group. The data are representative of three independent experiments with similar results. **P < 0.01.
Decreased frequency of CD4+ and CD8+T cells in IL-18Rα-/- mice is associated with decreased dendritic cells maturation
To determine whether the decreased numbers of TNF-α producing CD8+T cells in KO mice are due to activation-induced cell death (AICD), we examined the expression of apoptotic receptor (FAS) on CD8+T cells. Similar to expression levels of FAS/CD95 on CD4+T cells, lack of IL-18R signals in IOE-infected KO mice decreased the expression of FAS on CD8+T cells compared to infected WT mice (data not shown), suggesting that FAS-mediated AICD is unlikely to be responsible for decreased CD8+T cells in KO mice.
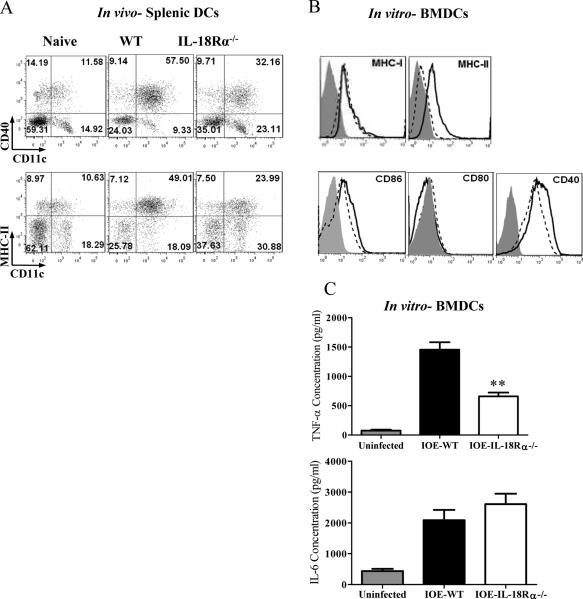
Next, we examined whether decreased absolute number of both CD4+ and CD8+T cells is due to defective priming by APC, mainly DCs. To this end, we analyzed the maturation and pro-inflammatory cytokine production of DCs from WT and IL-18Rα-/- mice following in vivo and in vitro stimulation with IOE. Compared to IOE-infected WT mice, IOE infection in IL-18Rα-/- mice resulted in lower expression levels of MHC class II and CD40 on splenic CD11c+ DCs in vivo (Fig. 12A). Consistent with the in vivo findings, IL-18Rα-deficient BMDCs infected in vitro with cell-free Ehrlichia had lower surface expression of MHC class II, CD86, and CD40 at 24 hours p.i. (Fig. 12B), but not MHC class I and CD80, when compared to IOE-stimulated IL-18Rα-expressing BMDCs from WT mice. As a positive control, LPS-stimulated BMDCs from IL-18Rα-/- mice had comparable levels of maturation markers compared to LPS-stimulated BMDCs from WT mice (not shown), suggesting that defective maturation of BMDCs from IL-18Rα-/- mice is not due to intrinsic defect in TLR4/MyD88 signaling pathways. Interestingly, decreased maturation of IOE-stimulated IL-18Rα-deficient BMDCs was associated with a significant decrease in their production of TNF-α, but not IL-6, when compared to IOE-stimulated BMDCs from WT (Fig. 12C). Thus, our data suggest that IL-18Rα signals promote DC maturation and TNF-α production by these cells, and both of these factors might contribute to the induction of pathogenic CD8+ T cells and subsequent immunopathology during fatal Ehrlichia-induced shock.
FIGURE 12. The lack of IL-18Rα signals causes downregulation of T cell costimulatory functions of dendritic cells during severe ehrlichial infection.
A, Splenocytes were harvested from IOE-infected IL-18Rα-/- and WT mice on day 3 p.i., and the expression levels of CD40 and MHCII costimulatory molecules on CD11c+ dendritic cells were analyzed by flow cytometry. B, Bone marrow derived dendritic cells (BMDCs) were isolated from WT and IL-18Rα-/- mice and were propagated in vitro. Cells were either left unstimulated or infected with IOE at MOI of 5: 1 for 24 hours. Histogram show different maturation markers; MHC-I, MHC-II, CD80, CD86, and CD40 on CD11c+ BMDCs from naive (filled line), WT (solid line) and IL-18Rα-/- (dotted line). C, TNF-α and IL-6 production in culture supernatant of IOE-infected BMDCs from WT and IL-18Rα-/-. Uninfected BMDCs from WT mice was used as negative controls. In vivo experiments showing results from four mice/ group, and similar results were obtained in three independent experiments. In vitro data are representative from one of three different experiments with similar results.
Discussion
Acquired immune responses against several pathogens are known to be regulated by innate immunity involving cytokines. Protection against lethal systemic infection with LPS-lacking Ehrlichia requires a balance between several protective (mediated by CD4+Th1 cells and IFN-γ producing NKT cells) and pathogenic (mediated by cytotoxic, IFN-γ and TNF-α producing NK and CD8+T cells) components of innate and adaptive immune systems. We identified here an unexpected pathogenic role of IL-18Rα in promoting Ehrlichia-induced toxic shock. Lack of IL-18/IL-18R interaction in knockout or antibody-treated mice resulted in enhanced protective immunity against Ehrlichia, as revealed by decreased bacterial burden and prolonged survival. Enhanced resistance in IL-18Rα-/- mice was associated with an elevated levels of pro-inflammatory and Th1 cytokines (TNF-α, IL-6, IFN-γ) only at the sites of infection (peritoneum), decreased systemic IL-10 production, and increased numbers of protective NKT cells producing both TNF-α and IFN-γ More intriguing, IL-18Rα deficiency resulted in abrogation of multiple pathogenic components of Ehrlichia-induced toxic shock, including decreased leukocyte infiltration into livers and host cell apoptosis and necrosis, and decreased numbers of pathogenic TNF-α-producing CD8+ T cells. Consistent with the pathogenic role of IL-18 in our model, systemic administration of recombinant IL-18 to WT mice infected with mildly virulent Ehrlichia muris resulted in increased host cell apoptosis and ineffective bacterial elimination in different organs compared to untreated infected controls. Thus, it is clear that IL-18 signaling plays negative and detrimental regulatory roles in Ehrlichia-induced toxic shock.
Several studies have suggested a role of IL-18 in promoting IFN-γ production, which enhances elimination of intracellular pathogens (22-26), while others suggested a role for IL-18 in mediating autoimmune and inflammatory disorders (36-39). Our data demonstrated a significant correlation between enhanced clearances of ehrlichiae, decreased IFN-γ production by type-1 T cells (with no bias towards Th2 response) and decreased IL-10 production in IL-18Rα-/- mice compared to WT controls. These results are unexpected since IFN-γ is a hallmark of protective immunity against Ehrlichia. However, the enhanced bacterial elimination in IL-18Rα-/- mice could be attributed, in part, to lack of immunosuppressive effect of IL-10 on microbicidal functions of macrophages. Studies have shown that IL-6 contributes to host defense against various pathogens including S. penumoniae, Escherichia coli, Listeria monocytogenes and Borrelia burgdorferi (50-53). Thus, it is also possible that the local levels of IFN-γ, in addition to the levels of other inflammatory cytokines such as TNF-α and IL-6 are optimal for effective elimination of bacteria at local sites of infection. Alternatively, other anti-Ehrlichia effector molecules, such as NKT cells producing both IFN-γ and TNF-α may be compartmentalized to the sites of infection in peripheral organs and thus compensate for the lack of IFN-γ production by Type-1 T cells. This conclusion is supported by our data in Fig. 8, showing expansion of NKT cells producing both IFN-γ and TNF–α in IOE-infected IL-18Rα-/- mice compared to infected WT mice. NKT cells are critical for the initiation and regulation of immune responses during infection (54-56). Indeed, our previous studies demonstrated that NKT cells play a protective role in immune responses following non-lethal ehrlichial infections via IFN-γ production (16-18). This study provide evidence suggesting that that IL-18/IL-18Rα interaction influence the frequency and functions of NKT cells following systemic and severe Ehrlichia infection, and that the absence of IL-18R signals leads to restoration of splenic NKT cells. Interestingly, we previously demonstrated that doxycycline treatment of lethally infected mice resulted in expansion of NKT cells following lethal IOE infection of WT mice (16). Thus, the lack of IL-18R signals could indirectly influenced the response of NKT cells via enhancing protective immunity and bacterial clearance. These data are consistent with other studies showing that IL-18 influences the frequency and function of the invariant NKT cell population (20-24).
The present study suggest that neutrophils are unlikely to mediate ehrlichial elimination, but possibly contribute to tissue injury following systemic ehrlichial infection as evidenced by: 1) decreased number of neutrophils in IL-18Rα-/- mice compared to WT mice following lethal infection with IOE; and 2) decreased expansion of neutrophils in non lethally infected mice compared to lethally infected mice. In support of this conclusion, studies have shown that IL-18 delays apoptosis of neutrophils and enhances neutrophils recruitments to peripheral organs during stressful and inflammatory conditions such as a burn wounds and liver inflammation, and this can lead to prolonged release of harmful enzymes and multiple organ dysfunction (57, 58).
Systemic IL-10 production was decreased in IOE-infected IL-18Rα-/- mice (Fig. 6). We previously demonstrated that IL-10 overproduction is associated with increased bacterial burden, immunopathology, and mortality following lethal ehrlichial infection (7, 13,16). However, the direct correlation between decreased levels of both IFN-γ and IL-10 in IL-18Rα-/- mice is paradoxical in the context of known regulatory function of IL-10 on Th1 responses during infections with other intracellular pathogens. It is possible that during the course of systemic and severe Ehrlichia infection, IL-18 secreted by APC induces immune cells to produce IL-10, which in turn inhibits further production of Th1-promoting cytokines such as IL-12 and IL-18 by APC or IFN-γ production by T cells. Although the source of IL-10 is not yet well defined, we argue that NK cells could be a major source. This conclusion is consistent with a recent study suggesting that during acute infection with diverse rapidly disseminating pathogens, IL-12 production by DCs stimulates IL-10 production by NK cells as an innate, negative feedback loop in which IL-12 limits its own production (59). Further support to this conclusion are the following findings: 1) Fig. 1 shows a positive and negative feedback loops between increased systemic production of IL-12 and IL-18 in IOE-infected WT mice and an increased IL-10 production at late stages of the disease, which coincide with decreased level of IFN-γ in the spleens; 2) Lack of IL-18R signals in infected IL-18Rα-/- mice resulted in decreased systemic IL-10 production and enhanced IFN-γ α and TNF-α production by NKT cells (Fig. 8). Interestingly, while infected IL-18Rα-/- mice had lower frequency of splenic type-1 T cells and lower serum level of IFN-γ than WT mice, these mice produced higher or similar concentrations of IFN-γ in the peritoneum and spleens (Fig. 6), respectively, when compared to that produced by the corresponding cells from WT mice. These data suggest that during the course of systemic ehrlichial infection, this IL-10/Th1 negative feedback loop may be compartmentalized and effective at the sites of infection in response to inflammation and tissue damage; 3) Recently, we showed that depletion of NK cells following IOE infection results in abrogation of tissue injury and enhanced bacterial elimination, which correlated with decreased systemic IL-10 production (7, 13, 16), suggesting that IL-10 could be derived from NK cells.
Studies have shown that IL-18 contributes to enhanced cytotoxicity of NK and CD8+T cells, particularly when combined with IL-10 (60-62), which in turn drives apoptosis of hepatocytes in vivo via a FAS-dependent mechanism (29, 33, 61, 63). We did not directly measure the cytotoxic functions of NK and CD8+T cells against Ehrlichia in this study. However, decreased IFN-γ and TNF-α production by NK and CD8+T cells, respectively (Fig 8 and 9), abrogation of tissue injury, and decreased FAS expression on CD4+T cells (Fig. 7B) and CD8+ T cells (data not shown) in IOE-infected IL-18Rα-/- mice compared to infected WT mice suggested that IL-18/IL-18R interaction may play a synergistic role with IL-10 in promoting pathogenic cytotoxic functions of NK and CD8+T cells following severe Ehrlichia infection.
We have previously shown that TNF-α producing CD8+T cells play a pathogenic role in fatal ehrlichiosis. Data in Fig 9 suggested that the enhanced bacterial elimination in IOE-infected IL-18Rα-/- mice could be due to defective induction and/or expansion of pathogenic CD8+T cells, or other cellular responses that influence the outcome of infection. Adoptive transfer of splenocytes enriched for CD8+ T cells from immune WT mice increased IOE numbers in different organs of IL-18Rα-/- mice to numbers similar to those in infected WT mice. These data suggested that pathogenic CD8+T cells are sufficient to inhibit the protective anti-Ehrlichia immune responses of IL-18Rα-/- mice. The mechanism (s) by which IL-18/IL-18R interaction mediate induction and/or expansion of pathogenic TNF–α producing CD8+T cells is not yet known. However, decreased number of CD8+T cells in IL-18Rα-/- mice (Fig. 7A) could be due to: 1) activation induced cell death (AICD), which possibly occur via FAS-mediated mechanisms as suggested before (16, 17, 19); 2) migration of cells to peripheral sites of infection; or 3) defective induction and expansion of naïve and effector T cells, respectively. Our data support the third possibility for the following reasons. First, the expression of FAS on CD8+ T cells was decreased in IL-18Rα-/- compared to infected WT mice. Second, the absence of IL-18R signals resulted in marked attenuation of leukocyte accumulation in the liver, which could be due to the effect of IL-18 on production of several chemokines as suggested by other studies (22-25). Thus, migration of T cells to liver is unlikely to account for the observed decreased in T cells. Third, IOE infection of IL-18Rα-/- mice resulted in decreased splenic DCs, decreased maturation of BMDCs as well as in vivo and in vitro production of TNF-α by DCs. In the context of known functions of DCs (64-70), these results suggest that absence of IL-18R signals in IL-18Rα-/-mice influences the T cell priming functions of DCs, which in turn influences the induction of Ehrlichia-specific CD8+ T cells. DCs are known to effectively prime CD8+T cells against intracellular bacteria that reside within endosomes and cannot access the endogenous MHC class I pathway via cross-presentation pathways (64-70). Ehrlichia (IOE) reside within phagosomes, do not escape into the cytosol, and is not known if they secrete proteins into the cytoplasm that access the endogenous pathway of Ag presentation (71, 72). Furthermore, lethal ehrlichial infection induce marked host cell apoptosis and necrosis; which are pathologic events that support cross-presentation of exogenous pathogen-derived Ag to CD8+T cells (64-68). Furthermore, IOE-stimulated BMDCs from IL-18R-/- mice has lower expression of MHC class II, but not MHC class I, compared to BMDCs from WT mice (Fig. 12). Taken all together, we envisage that IL-18/1L-18R interaction is critical for maturation of infected DCs, which in turn prime pathogenic CD8+T cells, most likely via cross-presentation pathway.
The data presented in this study is novel because it unravels a previously unidentified pathogenic role of IL-18R in the immunopathogenesis of infection-induced inflammatory diseases and toxic shock-like syndrome. As we demonstrated, targeting the IL-18/IL-18R interaction would not only enhance protective immunity, but it would also abrogate the immune-mediated pathology that causes multi-organ failure following infections with intracellular pathogens. Although further studies are needed to dissect the IL-18-dependent molecular and cellular interactions, our data suggest the fascinating proposition that targeting the IL-18/IL-18R interaction could represent an effective treatment for LPS-lacking Gram negative, intracellular bacterial infection-induced toxic shock, mainly in patients who do not respond to antibiotic treatment.
Acknowledgments
Flow cytometry experiments were performed in the Vanderbilt Medical Center Flow Cytometry Shared Resource, which is supported by the Vanderbilt Ingram Cancer Center (P30 CA68485) and the Vanderbilt Digestive Disease Research Center (DK058404). We would like to thank Dr. Luc Van Kaer (Vanderbilt University) for critical reading of the manuscript.
Source of Support:
This work was supported by a grant from the National Center for Research Resources, 5g12RR003032, and National Institute of General and Medical Science 1SC3GM089641-01 to N. Ismail.
Special abbreviations
- p.i.
post-infection
- i.d.
intradermal
- i.p.
intraperitoneal
- PECs
peritoneal exudate cells
REFERENCES
- 1.Olano JP, Walker DH. Human ehrlichioses. Med. Clin. North Am. 2002;86:375–392. doi: 10.1016/s0025-7125(03)00093-2. [DOI] [PubMed] [Google Scholar]
- 2.Walker DH, Dumler JS. Human monocytic and granulocytic ehrlichioses. Discovery and diagnosis of emerging tick-borne infections and the critical role of the pathologist. Arch. Pathol. Lab. Med. 1997;121:785–791. [PubMed] [Google Scholar]
- 3.Lin M, Rikihisa Y. Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival. Infect Immun. 2003;71:5324–31. doi: 10.1128/IAI.71.9.5324-5331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang H, Lin M, Wang X, Kikuchi T, Mottaz H, Norbeck A, Rikihisa Y. Proteomic analysis of and immune responses to Ehrlichia chaffeensis lipoproteins. Infect Immun. 2008;76:3405–14. doi: 10.1128/IAI.00056-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fichtenbaum CJ, Peterson LR, Weil GJ. Ehrlichiosis presenting as a life-threatening illness with features of the toxic shock syndrome. Am. J. Med. 1993;95:351–357. doi: 10.1016/0002-9343(93)90302-6. [DOI] [PubMed] [Google Scholar]
- 6.Hamburg BJ, Storch GA, Micek ST, Kollef MH. The importance of early treatment with doxycycline in human ehrlichiosis. Medicine (Baltimore) 2008;87:53–60. doi: 10.1097/MD.0b013e318168da1d. [DOI] [PubMed] [Google Scholar]
- 7.Ismail N, Soong L, McBride JW, Valbuena G, Olano JP, Feng HM, Walker DH. Overproduction of TNF-α by CD8+ type 1 cells and down-regulation of IFN-γ production by CD4+ Th1 cells contribute to toxic shock-like syndrome in an animal model of fatal monocytotropic ehrlichiosis. J. Immunol. 2004;172:1786–1800. doi: 10.4049/jimmunol.172.3.1786. [DOI] [PubMed] [Google Scholar]
- 8.Olano JP, Wen G, Feng HM, McBride JW, Walker DH. Histologic, serologic, and molecular analysis of persistent ehrlichiosis in a murine model. Am. J. Pathol. 2004;165:997–1006. doi: 10.1016/S0002-9440(10)63361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shibata S, Kawahara M, Rikihisa Y, Fujita H, Watanabe Y, Suto C, Ito T. New Ehrlichia species closely related to Ehrlichia chaffeensis isolated from Ixodes ovatus ticks in Japan. J. Clin. Microbiol. 2000;38:1331–1338. doi: 10.1128/jcm.38.4.1331-1338.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sotomayor E, Popov V, Feng HM, Walker DH, Olano JP. Animal model of fatal human monocytotropic ehrlichiosis. Am. J. of Pathology. 2001;158:757–769. doi: 10.1016/S0002-9440(10)64018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevenson HL, Jordan JM, Peerwani Z, Wang HQ, Walker DH, Ismail N. An intradermal environment promotes a protective type-1 response against lethal systemic monocytotropic ehrlichial infection. Infect. Immun. 2006;74:4856–4864. doi: 10.1128/IAI.00246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sehdev AE, Dumler JS. Hepatic pathology in human monocytic ehrlichiosis. Ehrlichia chaffeensis infection. Am. J. Clin. Pathol. 2003;119:859–865. doi: 10.1309/F7EA-B5P7-3217-16LJ. [DOI] [PubMed] [Google Scholar]
- 13.Ismail N, Stevenson HL, Walker DH. Role of tumor necrosis factor α (TNF-α) and interleukin-10 in the pathogenesis of severe murine monocytotropic ehrlichiosis: increased resistance of TNF receptor p55- and p75-deficient mice to fatal ehrlichial infection. Infect. Immun. 2006;74:1846–1856. doi: 10.1128/IAI.74.3.1846-1856.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bitsaktsis C, Huntington J, Winslow GM. Production of IFN-γ by CD4 T cells is essential for resolving ehrlichia infection. J. Immunol. 2004;172:6894–6901. doi: 10.4049/jimmunol.172.11.6894. [DOI] [PubMed] [Google Scholar]
- 15.Ganta RR, Wilkerson MJ, Cheng C, Rokey AM, Chapes SK. Persistent Ehrlichia chaffeensis infection occurs in the absence of functional major histocompatibility complex class II genes. Infect. Immun. 2002;70:380–388. doi: 10.1128/IAI.70.1.380-388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevenson HL, Estes DM, Thirumalapura RN, Walker DH, Ismail N. Natural Killer Cells Promote Tissue Injury and Systemic Inflammatory Responses during Fatal Ehrlichia-Induced Toxic Shock-like Syndrome. Am. J. Pathology. 2010;177:766–776. doi: 10.2353/ajpath.2010.091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevenson HL, Crossley EC, Thirumalapura N, Walker DH, Ismail N. Regulatory roles of CD1d-restricted NKT cells in the induction of toxic shock-like syndrome in an animal model of fatal ehrlichiosis. Infect. Immun. 2008;76:1434–1444. doi: 10.1128/IAI.01242-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker DB, Beutler, Teyton L, Savage PB, Bendelac A. Both exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 19.Ismail N, Crossley EC, Stevenson HL, Walker DH. Relative importance of T-cell subsets in monocytotropic ehrlichiosis: a novel effector mechanism involved in Ehrlichia-induced immunopathology in murine ehrlichiosis. Infect. Immun. 2007;75:4608–4620. doi: 10.1128/IAI.00198-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reddy P. Interleukin-18: recent advances. Curr. Opin. Hematol. 2004;11:405–410. doi: 10.1097/01.moh.0000141926.95319.42. [DOI] [PubMed] [Google Scholar]
- 21.Broz P, von Moltke J, Jones JW, Vance RE, Monack DM. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe. 2010;8:471–483. doi: 10.1016/j.chom.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fremond CM, Togbe D, Doz E, Rose S, Vasseur V, Maillet I, Jacobs M, Ryffel B, Quesniaux VF. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J. Immunol. 2007;179:1178–1189. doi: 10.4049/jimmunol.179.2.1178. [DOI] [PubMed] [Google Scholar]
- 24.Pedra JH, Sutterwala FS, Sukumaran B, Ogura Y, Qian F, Montgomery RR, Flavell RA, Fikrig E. ASC/PYCARD and caspase-1 regulate the IL-18/IFN-gamma axis during Anaplasma phagocytophilum infection. J. Immunol. 2007;179:4783–4791. doi: 10.4049/jimmunol.179.7.4783. [DOI] [PubMed] [Google Scholar]
- 25.Humann J, Lenz LL. Activation of naive NK cells in response to Listeria monocytogenes requires IL-18 and contact with infected dendritic cells. J. Immunol. 2010;184:5172–8. doi: 10.4049/jimmunol.0903759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haring JS, Harty JT. Interleukin-18-related genes are induced during the contraction phase but do not play major roles in regulating the dynamics or function of the T-cell response to Listeria monocytogenes infection. Infect. Immun. 2009;77:1894–903. doi: 10.1128/IAI.01315-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamauchi K, Choi IJ, Lu H, Ogiwara H, Graham DY, Yamaoka Y. Regulation of IL-18 in Helicobacter pylori infection. J. Immunol. 2008;180:1207–1216. doi: 10.4049/jimmunol.180.2.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smeltz RB, Chen J, Hu-Li J, Shevach EM. Regulation of Interleukin (IL)-18 Receptor Chain Expression on CD4T Cells during T Helper (Th)1/Th2 Differentiation: Critical Down regulatory Role of IL-4. J. Exp. Med. 2001;194:143–154. doi: 10.1084/jem.194.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wild JS, Sigounas A, Sur N, Siddiqui MS, Alam M, Kurimoto R, Sur S. IFN-gamma-inducing factor (IL-18) increases allergic sensitization, serum IgE, Th2 cytokines, and airway eosinophilia in a mouse model of allergic asthma. J. Immunol. 2000;164:2701–10. doi: 10.4049/jimmunol.164.5.2701. [DOI] [PubMed] [Google Scholar]
- 30.Raffaella F, Russell C, Cattley JG, Flores S, Brown H, Qi M, Yin S, Hill D, Scully S, Chen C, Brankow D, Lewis J, Baikalov C, Yamane H, Meng T, Martin F, Hu S, Boone T, Senaldi G. IL-18-Binding Protein Protects Against Lipopolysaccharide- Induced Lethality and Prevents the Development of FAS/FAS Ligand-Mediated Models of Liver Disease in Mice. J. Immunol. 2001;167:5913–5920. doi: 10.4049/jimmunol.167.10.5913. [DOI] [PubMed] [Google Scholar]
- 31.Netea MG, Fantuzzi G, Kullberg BJ, Stuyt RJ, Pulido EJ, McIntyre RC, Jr, Joosten LA, Van der Meer JW, Dinarello CA. Neutralization of IL-18 reduces neutrophil tissue accumulation and protects mice against lethal Escherichia coli and Salmonella typhimurium endotoxemia. J. Immunol. 164:2644–9. doi: 10.4049/jimmunol.164.5.2644. [DOI] [PubMed] [Google Scholar]
- 32.Shadab NR, Xiaoling LI, Irshad H, Kirby IB, Mashkoor AC. Inhibition of IL-18 reduces myeloperoxidase activity and prevents edema in intestine following alcohol and burn injury. J. Leukoc. Biol. 2005;77:719–728. doi: 10.1189/jlb.0704396. [DOI] [PubMed] [Google Scholar]
- 33.Brady J, Carotta S, Thong RP, Chan CJ, Hayakawa Y, Smyth MJ, Nutt SL. The interactions of multiple cytokines control NK cell maturation. J. Immunol. 2010;185:6679–6688. doi: 10.4049/jimmunol.0903354. [DOI] [PubMed] [Google Scholar]
- 34.Tsutsui H, Nakanishi K, Matsui K, Higashino K, Okamura H, Miyazawa Y, Kaneda K. IFN-gamma-inducing factor up-regulates Fas ligand-mediated cytotoxic activity of murine natural killer cell clones. J. Immunol. 1996;157:3967–3973. [PubMed] [Google Scholar]
- 35.Freeman CM, Han MK, Martinez FJ, Murray S, Liu LX, Chensue SW, Polak TJ, Sonstein J, Todt JC, Ames TM, Arenberg DA, Meldrum CA, Getty C, McCloskey L, Curtis JL. Cytotoxic potential of lung CD8+ T cells increases with chronic obstructive pulmonary disease severity and with in vitro stimulation by IL-18 or IL-15. J. Immunol. 2010;184:6504–6513. doi: 10.4049/jimmunol.1000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamkanfi M, Sarkar A, Walle Vande L., Vitari AC, Amer AO, Wewers MD, Tracey KJ, Kanneganti TD, Dixit VM. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J. Immunol. 2010;185:4385–4392. doi: 10.4049/jimmunol.1000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hochholzer P, Lipford GB, Wagner H, Pfeffer K, Heeg K. Role of Interleukin-18 (IL-18) during Lethal Shock: Decreased Lipopolysaccharide Sensitivity but Normal Superantigen Reaction in IL-18-Deficient Mice. Infect. Immun. 2000;68:3502–3508. doi: 10.1128/iai.68.6.3502-3508.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamanaka K, Tanaka M, Tsutsui H, Kupper TS, Asahi K, Okamura H, Nakanishi K, Suzuki M, Kayagaki N, Black RA, Miller DK, Nakashima K, Shimizu M, Mizutani H. Skin-Specific Caspase-1-Transgenic Mice Show Cutaneous Apoptosis and Pre-Endotoxin Shock Condition with a High Serum Level of IL-18. J. Immunol. 2000;165:997–1003. doi: 10.4049/jimmunol.165.2.997. [DOI] [PubMed] [Google Scholar]
- 39.Kim SH, Azam T, Yoon DY, Reznikov LL, Novick D, Rubinstein M, Dinarello CA. Site-specific mutations in the mature form of human IL-18 with enhanced biological activity and decreased neutralization by IL-18 binding protein. Proc. Natl. Acad. Sci. U S A. 2001;98:3304–3309. doi: 10.1073/pnas.051634098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiss E, Coolbaugh JC, Williams JC. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975;30:456–63. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doyle CK, Labruna MB, Breitschwerdt EB, Tang YW, Corstvet RE, Hegarty BC, Bloch KC, Li P, Walker DH, McBride JW. Detection of medically important Ehrlichia by quantitative multicolor TaqMan real-time polymerase chain reaction of the dsb gene. J. Mol. Diagn. 2005;7:504–510. doi: 10.1016/S1525-1578(10)60581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eshoo MW, Crowder CD, Matthews HE, Meng S, Sefers SE, Sampath R, Stratton CW, Blyn LB, Ecker DJ, Tang YW. Detection and identification of Ehrlichia species in blood by use of PCR and electrospray ionization mass spectrometry. J. Clin. Microbiol. 2010;48:472–8. doi: 10.1128/JCM.01669-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 45.Fang R, Ismail N, Soong L, Popov VL, Whitworth T, Bouyer DH, Walker DH. Differential interaction of dendritic cells with Rickettsia conorii: impact on host susceptibility to murine spotted fever rickettsiosis. Infect Immun. 2007;75:3112–3123. doi: 10.1128/IAI.00007-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bitsaktsis C, Winslow G. Fatal recall responses mediated by CD8 T cells during intracellular bacterial challenge infection. J. Immunol. 2006;177:4644–4651. doi: 10.4049/jimmunol.177.7.4644. [DOI] [PubMed] [Google Scholar]
- 47.Thirumalapura NR, Crossley EC, Walker DH, Ismail N. Persistent infection contributes to heterologous protective immunity against fatal ehrlichiosis. Infect. Immun. 2009;77:5682–5689. doi: 10.1128/IAI.00720-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 49.De Clerck LS, De Gendt CM, Bridts CH, Van Osselaer N, Stevens WJ. Expression of neutrophil activation markers and neutrophil adhesion to chondrocytes in rheumatoid arthritis patients: relationship with disease activity. Res Immunol. 1995;146:81–7. doi: 10.1016/0923-2494(96)80241-0. [DOI] [PubMed] [Google Scholar]
- 50.Anguita J, Rincón M, Samanta S, Barthold SW, Flavell RA, Fikrig E. Borrelia burgdorferi-infected, interleukin-6-deficient mice have decreased Th2 responses and increased Lyme arthritis. J. Infect. Dis. 1998;178:1512–1515. doi: 10.1086/314448. [DOI] [PubMed] [Google Scholar]
- 51.Van der Poll T, Keogh CV, Guirao X, Buurman WA, Kopf M, Lowry SF. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J. Infect. Dis. 1997;176:439–444. doi: 10.1086/514062. [DOI] [PubMed] [Google Scholar]
- 52.Dalrymple SA, Lucian LA, Slattery R, McNeil T, Aud DM, Fuchino S, Lee F, Murray R. Interleukin-6-deficient mice are highly susceptible to Listeria monocytogenes infection: correlation with inefficient neutrophilia. Infect. Immun. 1995;63:2262–2268. doi: 10.1128/iai.63.6.2262-2268.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dalrymple SA, Slattery R, Aud DM, Krishna M, Lucian LA, Murray R. Interleukin-6 is required for a protective immune response to systemic Escherichia coli infection. Infect. Immun. 1996;64:3231–3235. doi: 10.1128/iai.64.8.3231-3235.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brigl M, Bry1 L, Kent SC, Gumperz1 JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nature Immunology. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 55.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The Regulatory Role of Vα14 NKT Cells in Innate and Acquired Immune Response. Annu. Rev. Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 56.Lind SM, Kuylenstierna C, Moll M, Jordo ED, Winqvist O, Lundeberg L, Karlsson MA, Linder MT, Johansson C, Scheyniu1 A, Sandberg JK, Karlsson MCI. IL-18 skews the invariant NKT-cell population via autoreactive activation in atopic eczema. Eur. J. Immunol. 2009;39:2293–2301. doi: 10.1002/eji.200839195. [DOI] [PubMed] [Google Scholar]
- 57.Akhtar S, Li X, Chaudry IH, Choudhry MA. Neutrophil chemokines and their role in IL-18-mediated increase in neutrophil O2- production and intestinal edema following alcohol intoxication and burn injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;297:340–347. doi: 10.1152/ajpgi.00044.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kimura K, Sekiguchi S, Hayashi S, Hayashi Y, Hishima T, Nagaki M, Kohara M. Role of interleukin-18 in intrahepatic inflammatory cell recruitment in acute liver injury. J. Leukoc. Bio. 2010 doi: 10.1189/jlb.0710412. doi:10.1189/jlb.0710412. [DOI] [PubMed] [Google Scholar]
- 59.Perona-Wright G, Mohrs K, Szaba FM, Kummer LW, Madan R, Karp CL, Johnson LL, Smiley ST, Mohrs M. Systemic but not local infections elicit immunosuppressive IL-10 production by natural killer cells. Cell Host Microbe. 2009;6:503–12. doi: 10.1016/j.chom.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cai G, Kastelein RA, Hunter CA. IL-10 enhances NK cell proliferation, cytotoxicity and production of IFN-gamma when combined with IL-18. Eur. J. Immunol. 1999;29:2658–2665. doi: 10.1002/(SICI)1521-4141(199909)29:09<2658::AID-IMMU2658>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 61.Suk K, Kim S, Kim YH, Kim KA, Chang I, Yagita H, Shong M, Lee MS. IFN-γ/TNF-α Synergism as the Final Effector in Autoimmune Diabetes: A Key Role for STAT1/IFN Regulatory Factor-1 Pathway in Pancreatic β Cell Death. J. Immunol. 2001;166:4481–448. doi: 10.4049/jimmunol.166.7.4481. [DOI] [PubMed] [Google Scholar]
- 62.Micallef MJ, Tanimoto T, Torigoe K, Nishida Y, Kohno K, Ikegami H, Kurimoto M. Simultaneous exposure to interleukin-18 and interleukin-10 in vitro synergistically augments murine spleen natural killer cell activity. Cancer Immunol. Immunother. 1999;48:109–17. doi: 10.1007/s002620050554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hyodo Y, Matsui K, Hayashi N, Tsutsui H, Kashiwamura S, Yamauchi H, Hiroishi K, Takeda K, Tagawa Y, Iwakura Y, Kayagaki N, Kurimoto M, Okamura H, Hada T, Yagita H, Akira S, Nakanishi K, Higashino K. IL-18 Up-Regulates Perforin-Mediated NK Activity Without Increasing Perforin Messenger RNA Expression by Binding to Constitutively Expressed IL-18 Receptor. J. Immunol. 1999;162:1662–1668. [PubMed] [Google Scholar]
- 64.Domínguez PM, Ardavín C. Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol. Rev. 2010;234:90–104. doi: 10.1111/j.0105-2896.2009.00876.x. [DOI] [PubMed] [Google Scholar]
- 65.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 66.Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, Jenkins MK. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 67.den Haan JM, Lehar SM, Bevan MJ. CD8+ but not CD8- dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Montoya CJ, Jie HB, Al-Harthi L, Mulder C, Patino PJ, Rugeles MT, Krieg AM, Landay AL, Wilson SB. Activation of plasmacytoid dendritic cells with TLR9 agonists initiates invariant NKT cell mediated cross-talk with myeloid dendritic cells. J. Immunol. 2006;177:1028–1039. doi: 10.4049/jimmunol.177.2.1028. [DOI] [PubMed] [Google Scholar]
- 69.Ferguson TA, Herndon J, Elzey B, Griffith TS, Schoenberger S, Green DR. Uptake of apoptotic antigen-coupled cells by lymphoid dendritic cells and cross-priming of CD8+ T cells produce active immune unresponsiveness. J. Immunol. 2002;168:5589–5595. doi: 10.4049/jimmunol.168.11.5589. [DOI] [PubMed] [Google Scholar]
- 70.Sabrina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 71.Wakeel A, Kuriakose JA, McBride JW. An Ehrlichia chaffeensis tandem repeat protein interacts with multiple host targets involved in cell signaling, transcriptional regulation, and vesicle trafficking. Infect Immun. 2009;77:1734–45. doi: 10.1128/IAI.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rikihisa Y. Anaplasma phagocytophilum and Ehrlichia chaffeensis: subversive manipulators of host cells. Nat Rev Microbiol. 2010;8:328–39. doi: 10.1038/nrmicro2318. [DOI] [PubMed] [Google Scholar]