Abstract
Repair of the central nervous system (CNS) constitutes the integral part in treating neurologic diseases and plays a crucial role in restoring CNS architecture and function. Distinct strategies have been developed to reconstruct the damaged neural tissue with most of them tested preclinically in animal models. In this review, we discuss cell replacement based repair strategy and rewiring of the CNS. We review the major challenges of CNS repair by focusing on neurodegeneration and the pathological obstacles which impede neural cell growth after CNS injury. Finally, we discuss recent progress in developing human monoclonal IgMs that regulate CNS homeostasis and promote neural regeneration.
Introduction
Repairing the central nervous system (CNS) implies restoring tissue architecture of neural networks both morphologically and functionally. Most neurologic diseases share common characteristics including injury to neural cells, immune responses to the damage and consequential neurodegeneration. Each disease develops in the genetic background of an individual, thus emphasizing the relationship between the environment and the host. As a result, each patient may present with distinct pathological and clinical characteristics. Ideally individualized therapy should be designed for each patient. Thus CNS repair, which is the key for reconstructing damaged neural networks, is not an isolated event, but requires the combination of removing the etiological factors, modulating the inflammatory response, protecting neural cells from degeneration, and rebuilding the network connections.
CNS injury can develop in different pathological conditions ranging from infection, malignancy, trauma, ischemia and idiopathic degeneration. In this review, three categories of lesions are taken as examples for CNS repair: traumatic injury of the spinal cord (SCI), ischemia such as focal ischemic stroke, and degenerative disorders such as Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS) and multiple sclerosis (MS). In SCI, contusion of the spinal cord induces direct damage to neural cells and the vasculature that is followed with hemorrhage and secondary damage to spared neural cells after the lesion[1]. Consequently a reactive glial scar may develop to impede regenerating axons from traversing to rebuild neural circuitry[2]. In many cases of cerebral ischemia, a center of necrotic brain region is usually surrounded by the penumbra containing partially injured brain tissue[3]. Neurodegenerative disorders are often associated with progressive CNS atrophy and neural cell death[4]. Symptoms for each of these diseases are the result of breaking neural network connections from loss of neural cells and disruption of neural transmission. Accordingly, approaches for CNS repair are strategized to replace the neural cells lost during the disease process and to induce neural growth, especially axonal outgrowth and its subsequent myelination by oligodendrocytes. The goal is to rebuild neural network connections and restore functions.
CNS repair is mostly studied in preclinical animal models. A variety of cell replacement based repair strategies have been developed and different growth promotion therapies have been tested. Both approaches have been extensively reviewed elsewhere[5–7]. Nevertheless there is still a gap for newly generated neural cells, either exogenously transplanted or born from endogenous resources, to integrate into the neural network and compensate the damaged neural function. In this review, we discuss these issues, but focus much more on neurodegeneration and obstacles impeding axonal rewiring that are the major challenges in repairing CNS tissue. Finally, we review recent progress on development of human natural IgMs to promote neural regeneration.
Strategies In CNS Repair
Cell replacement-based CNS repair
Generally, cell-based repair includes transplantation of exogenous cells and/or induction of endogenous CNS structures to proliferate, migrate and differentiate in order to replace the lost neural cells and/or provide support for the spared neural tissue.
Embryonic stem (ES) cells
ES cells are derived from the inner cell mass of the pre-implantation blastocyst, that can be self-renewed and is pluripotent. In SCI, both neurons in the gray matter and oligodendrocytes ensheathing the axon fibers need replenishment to repair the damaged intraspinal cord circuitry and enhance functional recovery. Mouse ES cells, pre-differentiated into the neural phenotype before transplantation into the injured spinal cord of a rat, have been shown to survive and differentiate into both neurons and glia including mature oligodendrocytes, and have contributed some extent to functional recovery[8]. It has been reported that undifferentiated ES cells transplanted into experimental stroke animal models resulted in tumorigenesis[9]. In the PD model, ES cells transplanted at a low density, were able to proliferate and differentiate into dompaminergic (DA) neurons and showed functional recovery[10]. It appears that ES cells at high density facilitate tumorigenesis, that contain heterogeneous structures[9]. In the PD model, the lower density may allow the grafted ES cells to contact the host cells and facilitate differentiation. Due to the potential risk of tumorigenesis, undifferentiated ES cells are less attractive for direct transplantation before differentiation. Accordingly, protocols have been developed to differentiate these cells into specific neural lineages[11].
Adult neural stem cells
Adult neurogenesis was first demonstrated in brains of cancer patients injected with BrdU for prognostic purposes. Brdu-labeled new neurons were found in the dentate gyrus (DG) of the hippocampus and subventricular zone (SVZ)[12]. Now it has been established that both the SVZ aligning the ventricle and subgranular zone (SGZ) of the hippocampal DG maintain self-renewable neural stem/progenitor cells (NSC) in the CNS, that can proliferate in the presence of growth factors, such as basic fibroblast growth factor (bFGF) or epidermal growth factor (EGF), and differentiate into both neurons and glial cells in vitro. When transplanted into a rat stroke model, hippocampal NSCs were found to differentiate and integrate into the hippocampus with improved learning and memory[13]. NSCs can be genetically engineered to facilitate specific regeneration. Nurr1-engineered adult NSCs from SVZ were able to differentiate into DA neurons in vivo and reversed the behavior deficits in a PD rat model[14]. In experimental SCI, transplanted NSCs tended to differentiate into astrocytes, and only a small number of them become neurons or oligodendrocytes[7]. Thus predifferentiation of NSCs into specific neuronal lineages such as motor neurons or combination of NSCs with neurotrophins have been used to induce directional growth[15]. However, the limited resource of NSCs and restricted range of differentiation are the major disadvantages for them to be transferred to human trials.
Adult non-neural stem cells
A variety of adult stem cells from distinct origins such as bone marrow[16] and umbilical cord[17] have been tested to repair CNS lesions. Bone-marrow derived cells have even been tried in human patients suffering from SCI[18]. It was expected that the transplanted non-neural stem cell could potentially transdifferentiate into neurons and glia. However, it has become evident that the transplanted non-neural cells might not function by replacing neural loss, even though these stem cells were potentially capable to transdifferentiate into some neural lineages[19]. The underlying mechanisms of the beneficial effect from this type of cell transfer are not clear. It is more likely that transplanted stem cells can provide trophic support to promote survival of the spared host cells in the lesion and/or to prevent degeneration of projection neurons that lose synaptic innervation from neurons residing at the lesion site[20, 21].
Endogenous neural stem cell
Adult neurogenesis is not exceptionally limited to SVZ and SGZ[22]. NSCs in the SVZ migrate along the rostral migratory stream (RMS) to the olfactory bulb and differentiate into granular inter neurons[22]. Stroke may activate neurogenesis in the SVZ and SGZ. The newborn neural cells are capable to migrate and reach the ischemic boundary and differentiate into neurons[23]. Unlike other brain regions of mammals, the spinal cord does not seem to support the stem cell niche, a neurogenic region providing the microenvironment for neurogenesis[24]. It is becoming evident that endogenous neurogenesis is not enough to repair brain function. To repair the damaged neural networks, the NSCs are required to differentiate and integrate into the circuitry. A variety of endogenous and exogenous factors have been shown to be able to promote neurogenesis[25]. Development of novel interventions to stimulate this process is crucial for the repair of CNS.
Challenges in cell replacement
Given the fact that exogenous stem cells provide a potentially useful resource to replace neural cell loss, accumulating data indicate that transplanted cells may not function mainly by reconstructing the neural circuitry. Instead, the observed beneficial activity is actually a “bystander activity”, that modulates immune responses and produces neurotrophic factors rather than that the graft differentiates to replace the damaged neural cells. NSCs transplanted intravenously formed ectopic niches that release neuroprotective neurotrophins and immunomodulatory factors[20, 21]. Thus the efficacy of the transplanted stem cells is still far in its infancy. There are also special concerns regarding the stem cell strategy. First, there is a potential tumorigenesis concern as well as ethical problems. Second, the transplanted cells may be unable to migrate to the lesions far from the injection site, especially when there is widespread progressive neural degenerations in patients with AD, ALS and MS. Third, the microenvironment in mature CNS tissue of the diseased patient, that is different from the developmental stage, may not facilitate differentiation and survival of the newly formed neural cells[26]. Programmed neural migration and guidance are required for functional synaptic connection. Finally, there are difficulties for recovery of neuronal activities, such as learning and memory, that are developed on a basis of synaptic circuitry formed during tens of years of experience.
Promoting neural growth
The neurological deficits of CNS injury are largely the result of breaking neural circuitry. In combination with restoring the lost neural cells, another strategy for repairing the CNS is to promote neural growth, especially axonal regeneration and extension to reconstruct the neural circuitry.
Neurodegeneration In CNS Repair
Neurodegeneration is traditionally referred to as the progressive loss of neurons/axons[27]. However, chronic damage to oligodendroctyes and astrocytes leads to MS and neuromyelitis optica (NMO)[28] respectively. Together with the finding that astrocytes and microglia are also involved in the classic degenerative neurologic diseases such as AD, PD and ALS[29], these results indicate that insults to glial cells also participate in degenerative CNS disorders. Thus, we utilize neurodegeneration to describe chronic deterioration of both neurons and glial cells in CNS disease. CNS repair must therefore be viewed in a broad way.
Neural degeneration: the hallmark of CNS injury
Insults to the CNS frequently lead to neurodegeneration, which includes progressive neuron (axon) atrophy/loss, astrocyte/microglia activation and damage, demyelination or any of these combinations. Insults to one neural cell type may have a subsequent effect on another. Although a specific neurologic disease often presents with a predominant pathological characteristic accompanied by some secondary changes, the interwoven deterioration of all the neural cells occurs as the disease worsens.
Alzheimer’s disease (AD) is the most common degenerative disorder of the brain. When there is gross cortical atrophy, the major pathological changes in the brain are extracellular amyloid plaques and cytosolic neurofibrillary tangles. Amyloid plaques are mainly composed of β-amyloid protein (Aβ); that is, the peptide cleavage of amyloid precursor protein (APP). APP is synthesized in neurons and transported to neuron membranes and subsequently cleaved. Two enzymes involved in severing APP pathologically are first β-secretase, and second γ-secretase. The peptide Aβ fragments form oligomers called Aβ42 that interfere with neuronal communication. Aβ42 also produces fibrils that stick together to form amyloid plaques. Some plaques insert into neuron membranes to cause further damage. This neuronal damage and buildup of Aβ42 lead to neuron dysfunction and death[4]. The second major pathological change in AD is the insoluble neurofibrillary tangles formed by hyperphosphorylated tau protein, the so-called “tauopathy”. It is still controversial whether the tau tangle is the primary causative factor for cell degeneration[30]. Both the formation and precise role of amyloid plaques and neurofibrillary tangles on brain function are not fully understood. Compared to AD, neurodegenerations in PD and ALS mainly involve dopaminergic neurons in the brain stem and motor neurons of the spinal cord respectively. The pathological hallmark of PD is the selective loss of dopaminergic neurons from the pars compacta of the substantia nigra accompanied by intraneuronal accumulations of the Lewy body, the pale body and the Lewy neurite. The post-translationally modified α-synuclein, which is a normal presynaptic protein abnormally aggregated intracellularly in PD patients, is the main component of Lewy bodies. The abnormally accumulated α-synuclein may affect protein transport between the endoplasmic reticulum (ER) and the Golgi apparatus, but the precise mechanism of how aggregated α-synuclein damages neurons is not known[31]. The general mechanism underlying ALS is the accumulation of mutated proteins, such as superoxide dismutase 1 (SOD1) that are mis-folded to induce toxicity in spinal motor neurons. Although no consensus has been reached as to the primary toxicity of SOD1, a number of toxic mechanisms have been proposed including excitatory effect of glutamate, ER stress, dysfunction of proteasome and mitochondria, production of superoxide, defect in axonal transport and formation of micro-hemorrhages in the spinal cord[32].
Another important finding in degenerative CNS diseases is the presence of activated microglia and astrocyte[29]. Astrocyte and microglia dysfunction may exacerbate neurodegeneration or may even be the cause of the disease[33], though astrocytes are capable to ingest and degrade the pathogenic Aβ peptides in AD[34]. In support of the concept that astrocytes are the primary site of injury in some disorders, anti-aquaporin-4 (AQP4) IgG deposition in astrocyte endfeet was found to induce complement-dependent necrosis of astrocytes that underlies the pathogenesis of NMO[28]. Similar, but distinct from NMO, MS is another demyelinating CNS disease in which astrocytes were shown to present antigens to CNS-infiltrating T cells[35, 36]. The irreversible neurological decline in MS patients, accompanied by multifocal lymphocyte infiltration, demyelination, activation of astrocyte and microglia and axon transection[37], supports MS also as a neurodegenerative disorder. Reactive microglia and astrocytes, and activated complement components also suggest that some aspects of chronic inflammation occur in most degenerative CNS disorders[38, 39]. However, further characterization is needed about the roles of microglia and astrocytes in chronic inflammation and their contribution to neurodegeneration.
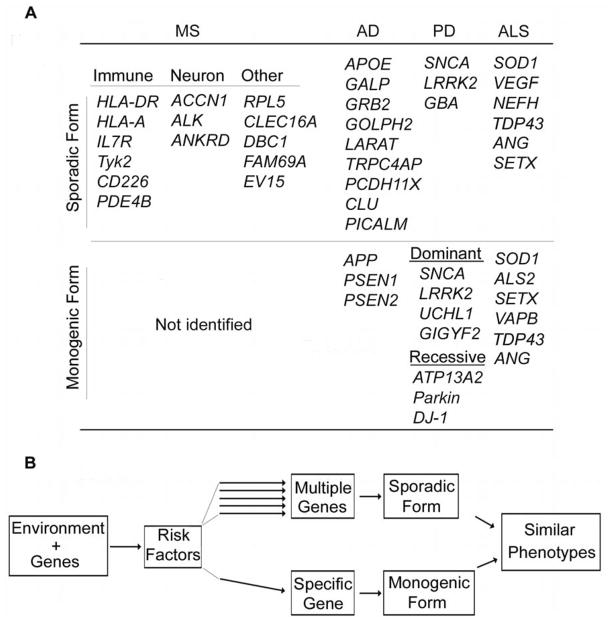
The major challenge of neurodegeneration is that the etiology is elusive, which becomes the major obstacle towards complete repair. Epidemiological studies indicate that both environmental and genetic components contribute to the etiology of neurodegenerative diseases such as AD, PD or ALS and MS (Fig. 1). Although a specific gene mutation has not been identified so far in MS, the association of MS with both multiple genetic loci and environmental factors indicate that gene-environment interactions involving the immune system, CNS and vasculature determine MS onset[40]. This concept is further strengthened by the findings in classic neurodegenerative CNS disorders. For example, early-onset familial AD (EOFAD), determined by APP (chr21)[41, 42], PSEN1 (chr14)[43] and PSEN2 (chr1)[44, 45] mutations, accounts for less than 1% of all AD patients. The majority, late-onset Alzheimer disease (LOAD), is linked with multiple genes[46], some of which affect APP function[47–50]. Similarly, less than 10% of the PD or ALS cases are identified as monogenic forms; the majority of sporadic cases are determined by multiple genetic loci in distinct environment[51–53] (Fig. 1A). Thus, the unique gene-environment interaction concerning multiple organs/systems may confer the risk and determine the onset of the disease cumulatively (such as MS and sporadic forms of AD, PD and ALS).
Figure 1. The genes associated with neurodegenerative CNS diseases indicate that gene-environment interactions produce major risk factors for injury.
A. The upper row shows some of the genes associated with sporadic CNS degenerative diseases including MS, AD, PD and ALS. The lower row indicates the genes involved in monogenic diseases (for details see reviews[27, 40, 51, 53, 122]). B. Gene-environment interactions produce risk factors that contribute to neurodegenerative disease at multiple levels. In sporadic forms of the disease, each individual risk factor functions in parallel, and their cummulative effects cause the disease. In contrast, risk factors may converge to induce monogenic mutation(s) that lead to the monogenic or familial form of the same degenerative CNS disorder. Of interest the phenotype of the sporadic and monogenic diseases are extremely similar.
Alternatively different risk factors produced by the interactions converge on monogene(s) that determine the initiation of the disorder (such as monogenic forms of AD, PD and ALS) (Fig. 1B). How multiple genetic variants confer the risk to the disease is undergoing extensive study in both patients and animal models. Nevertheless, the deposition of amyloid plaques and neurofibrillary tangles, aggregation of α-synuclein and accumulation of mutated proteins such as SOD1, all indicate that homeostasis in the CNS parenchyma is broken. Developing interventions to restore this homeostasis may be a resolution for repair.
Blood-brain barrier in neural degeneration
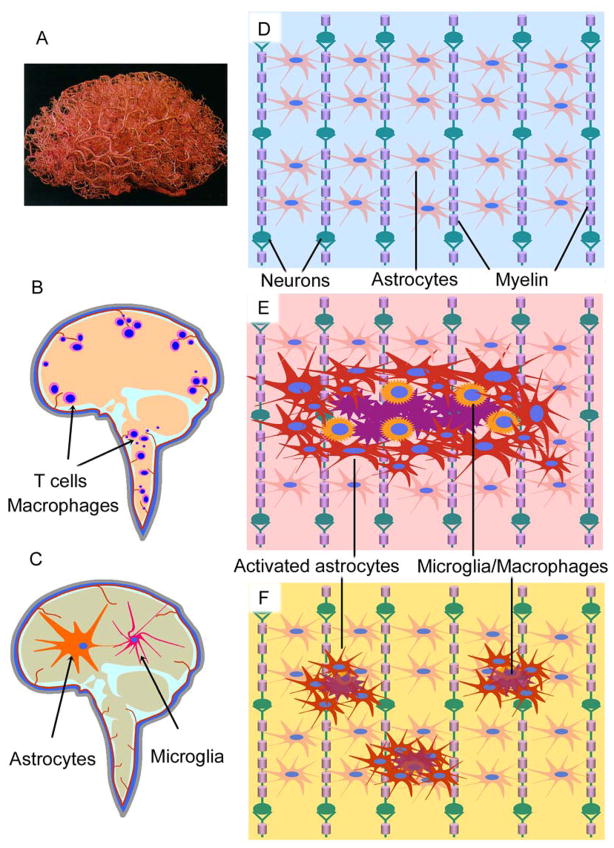
The CNS is an organ relatively isolated from the blood circulation by an interface barrier that includes the neurovascular unit (NVU) and the meninges (the dura mater, arachnoid, and pia mater). This interface affects almost every aspect of CNS function. During development, neuron-derived guidance molecules guide angiogenesis and blood-brain barrier (BBB) formation[54]. Accordingly, the micro-capillaries form an ~400 mile-length vascular web in the human brain[55] (Fig. 2A) that encloses the neural networks. As a result, neurons are located within ~20 μm from a brain capillary[56]. More than 20% of the cardiac output goes through this vasculature that brings nutrients and takes away the metabolic wastes. The NVU is composed of endothelial cells, astrocytes, neurons and pericytes/smooth muscle cells. The orchestrated intercellular interactions and communications within NVU allow the CNS to regulate the cerebral blood supply and maintain the homeostasis of the CNS microenvironment[57]. Astrocyte-endothelial cell interactions, the proposed gliovascular unit, regulate the vascular blood flow by conveying signals from neurons to the micro-capillary[58]. Astrocytes, which are connected by intercellular gap-junctions, receive and respond to neural (both neurons and glia) signals synergistically[59, 60]. Some astrocytes contact endothelial cells of a segment of micro-capillary with their endfeet, whereas others surround a population of neuronal synapses with their processes. The adjacent gliovascular units form contiguous but non-overlapping ensheathment along the capillary web[61]. Also the BBB, which controls the permeability of the CNS capillary, is formed by the vascular endothelial cells and constitutes the central structure of NVU. The tight junctions (TJ) localized on the apical side of the adjacent endothelial cells force molecules other than gaseous or lipophilic to traffic across BBB transcelluarly. The localization of specific transporters and expression of metabolic enzymes on the luminal and/or abluminal membranes of endothelial cells allows selected transport of certain molecules. The nutrients are transported to the neural tissue by GLUT1 (glucose carrier), LAT1 (large neutral amino acids, amino acids carrier) and nucleotide transporters, whereas many excitatory toxic molecules such as glutamate are removed by efflux transporters like EAAT1-3 (excitatory amino acid transporters 1-3) and inactivated intracellularly by enzymes such as monoamine oxidase and cytochrome P450[58]. These barriers maintain a stable environment for CNS function characterized by electrical transductions. However, when the CNS becomes inflamed or injured in this relatively isolated space, the pathological process may progress to chronic neurodegeneration.
Figure 2. CNS vasculature and inflammation in neurological diseases.
A. Vasculature in the brain. To reveal the vascular web, the brain was injected with a plastic emulsion and the parenchymal tissue was dissolved subsequently. The brain capillaries form a web that encloses the neural networks, supplying CNS nutrients and taking away unneeded wastes (Permission obtained from Wolters Kluwer Health C. Zlokovic, B. V. & Apuzzo, M. L. J. Strategies to circumvent vascular barriers of the central nervous system. Neurosurgery 43(4), 877–878, 1998.). B-F. Schematic drawing shows the CNS in normal or different disease conditions. Two types of inflammation are proposed to contribute to CNS diseases. One is characterized by infiltrating T cells and macrophages (B), which are mainly observed in classical inflammatory disorders such as MS, whereas the other is featured by activation of astrocytes and microglia (C) that is the typical pathological change of the degenerative CNS diseases such as AD, PD and ALS. (D) Neural cells in the CNS form network structures to support each other, in which oligodendrocytes ensheath the axon fibers and astrocytes form nonoverlapping domains. In response to CNS injury, astrocytes get activated with hypertrophy and seal the lesions by forming glial scar (E) or by inducing mild astrogliosis (F). Note that normal astroglial domains are interrupted by astrogliosis, which effects neuron functions.
How do the barriers facilitate CNS degeneration? Inflammation in the CNS may attenuate efflux activity of BBB, which promotes accumulation of pro-inflammatory cytokines and cell lytic products. T cells activated by a retroviral infection have been shown to reduce the transporter-mediated cerebrospinal fluid (CSF)-to-blood efflux of an organic anion, prostaglandin PGE2. Among the pro-inflammatory cytokines secreted by these T cells, tumor necrosis factor and interleukin 1 are directly involved in the mechanisms underlying decreased anion efflux[62]. Another mechanism that may affect progressive degeneration is saturation of CNS-to-blood efflux. The accumulation of adaptive immune cells and cytokines in both the CSF and CNS parenchyma[63] (Fig. 2B) suggest that the CNS is not effectively drained when inflammation develops. Inflammation enhances the “permeability” of the BBB, which may create a gradient of chemokines to recruit leukocytes to the CNS parenchyma. Finally, inflammation and/or neural injury may pose obstacles to the BBB, that supplies the CNS nutrients. This may partially explain CNS atrophy observed in degenerative CNS disorders such as AD and MS. Thus the anatomical structure of the BBB prevents efficient drainage of the CNS. It is not clear whether the inflammation-specific transporters and/or enzymes, that may not be expressed in normal individuals, are present in the inflamed BBB. The accumulation of inflammatory products in an inefficiently drained CNS environment exacerbates the original CNS damage, which develops into a chronic progressive disease process.
Contribution of the CNS networks to degeneration
Because the CNS is enclosed in the skeletal structures and secluded from the circulation environment by the BBB, maintaining the homeostasis relies much more on the network functions of the CNS itself. The CNS is constituted by interwoven multiple cell types including neurons, astrocytes, oligodendrocytes and microglia (Fig. 2D). The vasculature and immune cells, which survey the CNS and infiltrate to clear the pathogens and the damaged CNS tissues, must also receive special consideration. These cells form interacting networks to support each other in fulfilling their functions[64], that include processing information and controlling movements. During development, neuroectoderm-derived neural cells differentiate to form the neurotube first, and then undergo several steps of differentiation to develop into the layered architecture of the brain and spinal cord. Neurons generate first. The new-born neurons migrate to their destination and form a wide variety of groups or subgroups of functional populations with distinct localizations in the CNS. Neurons send out neurites, which sequentially differentiate into both axons and dendrites and extend locally and/or for long distances to innervate their target cells or organs[65]. Signals are generated in neurons and propagated along the neuron fibers. The neuroglia, which include astrocytes and oligodendrocytes, are later produced. Some astrocytes, which are connected by gap junctions, localize between neurons to form astroglial network domains that closely surround neuronal synaptic connections, whereas others extend their processes to contact blood vessels and the pia matter. Thus astrocytes are actively involved in neuron transmission and orchestrate energy supply for neural activities[66]. Oligodendrocytes are derived from oligodendrocyte precursor or progenitor cells (OPCs). The OPCs migrate to the vicinity of axons and differentiate into mature OLs that interact with and wrap axons to form the myelin sheath. Myelination provides efficient saltatory conduction of action potentials as well as important mutual dependencies between axons and OLs[67]. The processes of some astrocytes also contact OLs at the nodes of Ranvier to support OL function[68]. Microglia, the resident macrophages of the brain and spinal cord, are derived from bone marrow hematopoietic stem cells. During development, bone marrow stem cells migrate into the CNS and further differentiate into microglia. Microglia function together with astrocytes to maintain the homeostasis in the CNS[69]. The characteristic network organizations of the CNS make it vulnerable to many varieties of insults. The interdependence of neural cells makes it possible that primary injury to one component causes later degeneration in other members. Alternatively, repair of one component may beneficially impact on the other interacting cells.
Challenges of neurodegeneration in CNS repair
In addition to the unknown etiology for most CNS degenerative disorders, neurodegeneration often proceeds as a chronically progressive process. Even in SCI, the spared neurons may degenerate chronically as a result of lost synaptic innervation to and/or from neurons or muscles[1]. Grafted neural cells may degenerate chronically in the PD brain[70]. The challenge is that there is yet no effective treatment to stop or even slow down neurodegeneration.
Rewiring The CNS
The characteristic function of CNS is built on its wiring which connects different local circuits. Injury to the neural tissue, either by loss of neurons or damage to axonal fibers, results into a broken network. Beside cell-replacement, another strategy to repair the damaged CNS requires rewiring the CNS circuitry, a process for the regenerating axons to grow and innervate the postsynaptic targets and/or the differentiation of newly formed neurons to incorporate into the preexisting network. This process concerns communication between neurons and the CNS environment in a diseased condition, in which the regenerating axon interacts with reactive astrocytes, microglia, oligodendrocytes and ECM and non-ECM molecules in the extracellular space.
Astrogliosis and axon growth
Astrocytes, the essential component of the neural network, play crucial roles in both physiology and pathology. Astrocytes can respond to any CNS insult, including damage induced by infection or ischemia and all forms of traumatic or degenerative injury, by a process of astrogliosis or reactive astrocytosis[66]. Astrogliosis can present with distinct levels of change from mild to moderate to severe depending on different pathological conditions[66, 71]. A typical change in astrocyte morphology is the increased expression of glial fibrillary acid protein (GFAP), which is enriched in the cell body and branched processes undergoing hypertrophy. In the meanwhile, both gain and/or loss of function can occur to reactive astrocytes, which may be beneficial or detrimental to neural tissues in a context-dependent manner.
Detrimental effect
Reactive astroglial scars have been traditionally regarded to exert a detrimental effect in the disease process. This is largely because the scar formation in CNS trauma inhibits axonal regeneration. Severe traumatic lesions such as in SCI induce astrocyte proliferation and hypertrophy[2]. Astrocytic processes extend beyond the territorial domain of the individual cell and intermingle with neighboring astrocytic processes. In some conditions, the dense and compact glial scars develop and further disrupt the domain structures. It is likely that the reactive glial scars and disrupted astrocyte domains form a physical barrier to prevent regenerating axon from traversing the lesion[66]. In addition to the morphological change, there are also intracellular molecular, cellular and even functional modifications in response to CNS injury. Astrocytes secrete proteoglycans, the ECM molecules with a protein core linked by four sugar moieties to a sulphated glycosaminoglycan (GAG) chain that contains repeating disaccharide units. One of the proteoglycans, chondroitin sulphate proteoglycan (CSPG), is the major inhibitor to the regenerating axons. The CSPGs contain a large family of molecules including aggrecan, brevican, neurocan, NG2, phosphacan and versican. All have chondroitin sulphate side chains, but differ in the protein core and on the number, length and pattern of sulphation in the side chains[72]. The negatively charged chondroitin sulphate deposition interacts with different proteins in the ECM to regulate cellular activities from pathfinding to plasticity. The GAG sugar chain, the major inhibitory component of CSPGs, can be released by the bacterial enzyme chondroitinase ABC (ChABC) from the CSPG core protein[73]. Treatment with ChABC both in vitro and in vivo has been shown to benefit axon regeneration and functional recovery in the SCI model[74]. In chronic disease conditions, astrocytes can also be stimulated to produce deleterious molecules such as pro-inflammatory cytokines to exacerbate inflammation[75], and to release reactive oxygen species[76] and glutamate[77] to exert neurotoxicity. As the result to distinct varieties of insult, astrogliosis can be detrimental to axon regeneration.
Beneficial effect
In addition to regulating energy provision and maintaining synaptic plasticity and neural network integrity physiologically[66], astrocytes can be activated to confine lesions or pathogenic invasions within a smaller territory. Reactive astrogliosis, with/without compact scar formation, are often observed to seal the lesion[78]. After ischemic stroke, astrocytes proliferate and migrate to the borders of the infarct and form scar structures[79]. Reactive astrocytes are usually scattered among and surround the demyelinated lesions of patients with multiple sclerosis[80]. In experimental autoimmune encephalomyelitis (EAE), glial scars were observed to form around inflammatory cells infiltrating the perivascular space[81]. Reactive astrocyte processes were shown to surround amyloid plaques in the Alzheimer’s brain[82] and can degrade Aβ protein in vitro[34]. It is not known how reactive astrocytes fight these distinct varieties of insult. An emerging consensus is that astrocytes react to and regulate inflammation. Indeed, experimental disruption of the astroglial scar formation in transgenic mice resulted in spread of inflammation and increased lesion sizes in SCI[83], stroke[84] and EAE[81].
With both the detrimental and beneficial effects in mind, it is becoming evident that astrocytes respond to insults and maintain homeostasis in the CNS. Even though glial scar does inhibit axon regeneration, astrocytes may exacerbate neurologic diseases when they are chronically stimulated or when genetic mutations develop[85, 86](Fig. 2E & F). The molecular events of reactive astrocytes are just beginning to be discovered[71]. The fact is that astrocytes react not only to insults, but also to growing axons. When astrocytes are targeted for repair, the major challenge is how to regulate the homeostasis established by reactive astrocytes to facilitate neural growth, rather than to simply inhibiting reactive astrogliosis. Developing reagents to overcome inhibition and promote growth, for example by regulating both neurons and astrocytes, is a likely solution.
Myelin-derived axonal growth inhibitors
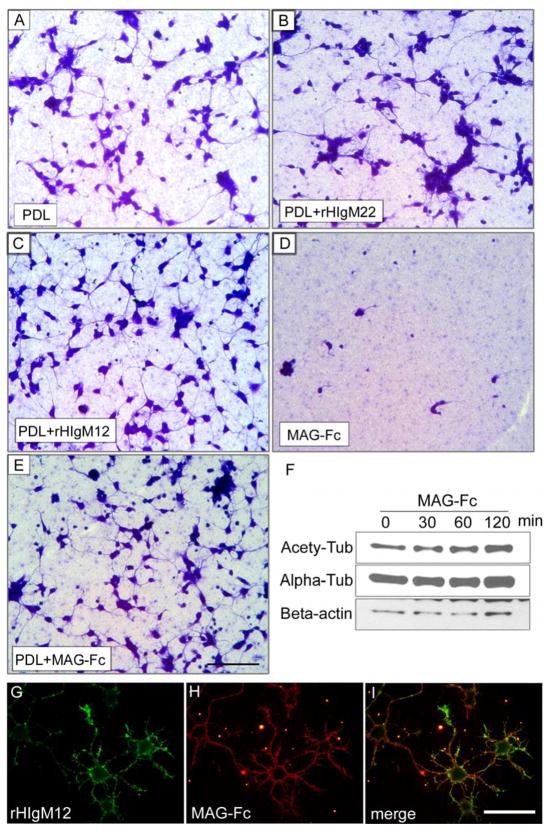
Myelin components have been identified to inhibit axon growth since the early 1990s. The major myelin inhibitors include myelin-associated glycoprotein (MAG)[87, 88], Nogo[89–91] and oligodendrocyte myelin glycoprotein (OMGp)[92]. Intriguing, these inhibitors all function through a receptor complex composed of NgR-p75/TROY-Lingo-1[93–96]. Downstream to the receptor complex, the myelin inhibitor-induced signaling converges on Rho GTPases and effects RhoA-activated kinase (ROCK), which regulates cytoskeleton dynamics[93, 94]. In comparison to other myelin inhibitors, MAG has also been shown to bind gangliosides[97, 98], paired immunoglobulin-like receptor B (PirB)[99] and β1-integrin[100]. Thus MAG binds and interacts with multiple distinct molecules on axon membranes. MAG, also known as Siglec-4, contains five Ig-like extracellular domains. Thirty percent of MAG is carbohydrate consisting of heterogeneous N-linked oligosaccharides at eight extracellular sites. MAG is negatively charged because of the sialic acid on the side chains. MAG binds specifically to α2,3-linked sialic acid (2,3-SA) found in O-linked oligosaccharides on glycoproteins and some gangliosides, such as GD1a and GT1b. It also binds N-linked oligosaccharides with 2,3-SA on glycoproteins[93, 94, 101, 102]. These observations suggest that interactions between myelin and axolemma are likely accomplished by a pattern composed of multiple distinct molecules rather than through traditional ligand-receptor binding. Nevertheless myelin components are not natural axon growth inhibitors and RhoA is part of the common regulators to cytoskeleton dynamics. Mice with experimental MAG knockout showed no significant axonal regeneration after injury[103]. However with time axons do degenerate eventually in MAG-deficient mice. Even the Nogo-MAG-OMGp triple mutant mice failed to exhibit enhanced axonal regeneration in the SCI model[104]. More importantly, MAG, which localizes at the inner surface of the myelin sheath and closely interacts with the periaxonal membranes, is an axonal stabilizer[105, 106]. MAG regulates cytoskeleton stability and may not necessarily inhibit neurite outgrowth in some conditions (Fig. 3). Thus myelin-induced signals, that inhibit axon outgrowth in vitro, are actually required for axonal function in vivo. Elucidating the signaling pathways at the myelin-axon interface directly influences how to design reagents for repair.
Figure 3. MAG supports neuron stability.
A–E. Primary hippocampal neurons were seeded on nitrocellulose-attached 24-well plates pre-coated with different substrates. The cultures were fixed and stained with Coomassie blue to show the attached neurons 16 hours after plating. Note that neurons grew on myelin-associated glycoprotein fused with human immunoglobulin Fc fragment (MAG-Fc) substrates (D) did not attach well and showed shorter neurites, the floating cells were washed away after fixation. When poly-D-lysine (PDL) plus MAG-Fc were used as substrates (E), MAG-Fc did not inhibit neurite extension as compared with PDL alone (A) or PDL+rHIgM22 (B). HIgM12, which was shown to promote neurite outgrowth[114], was used as positive control (C). F. DIV3 hippocampal neurons were treated with 2.5 μg/ml of MAG-Fc at different times. The level of acetylated tubulin increased after 60 min of treatment, indicating a change in microtubule stability. G–I. DIV1 live hippocampal neurons were incubated with 100 μg/ml of MAG-Fc for 30 min on ice and then stained with rHIgM12. MAG-Fc did not block rHIgM12 binding, and neither did rHIgM12 block MAG-Fc binding (data now shown). Scale bar 50 μm.
Distinct strategies were designed to reverse the inhibition of myelin debris. Including antibodies against Nogo-A to neutralize the effect of Nogo ligands[107, 108], other interventions tested thus far have been to block the receptor complex of myelin by injecting synthetic segments of the extracellular domains of NgR1, p75, Lingo-1, or PirB[95, 96]. The regenerating axon after injury does show features distinct from that of mature ones. Lingo-1 expression, which peaks after birth and decreases thereafter, is up-regulated following injury[95]. Thus regulating the specific pattern of signaling on the regenerating axonal membranes with either less or no effect to myelin signaling is a potential repair strategy. Therefore, molecules, which can bind the axolemma and promote axonal outgrowth but do not interfere with myelin signaling, are attractive future targets for inducing CNS repair (Fig. 3C&G, Fig. 4 & Fig. 5B).
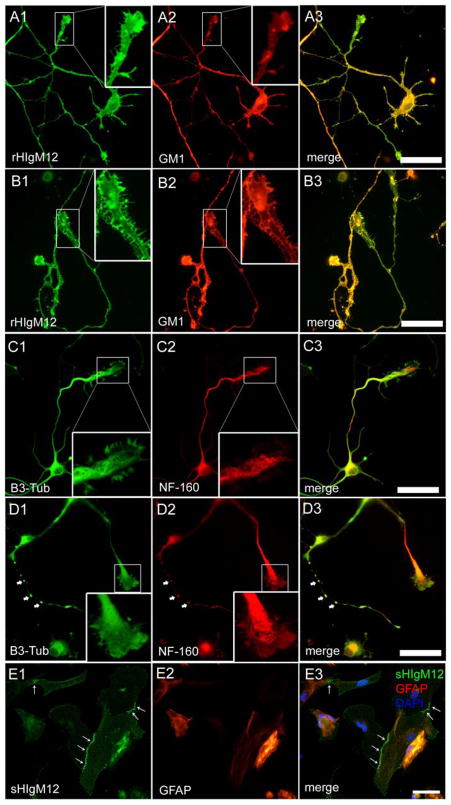
Figure 4. rHIgM12 binds neural cells in different patterns.
A–D. DIV3 hippocampal neurons treated with (B & D) or without (A & C) 100 nM H2O2 were stained with rHIgM12 or anti-cytoskeletal protein antibodies after fixation. Note that rHIgM12 bound both healthy and injured neuronal membranes (not permeabilized) in similar but distinct patterns. However, H2O2 treatment changed cytoskeletal organization substantially. In H2O2-treated neurons, varicosity structures containing both beta-3-tubulin (B3-Tub) and neurofilament 160 (NF-160) were formed along some neurites (arrow head), and cytoskeletons were depolymerized (see high power growth cone regions). E. Compared to the even distribution on neurons, rHIgM12 staining (E1) was enriched along the cell-cell contact in some astrocytes (E2) indicating a distinct binding pattern. Scale bar 50 μm.
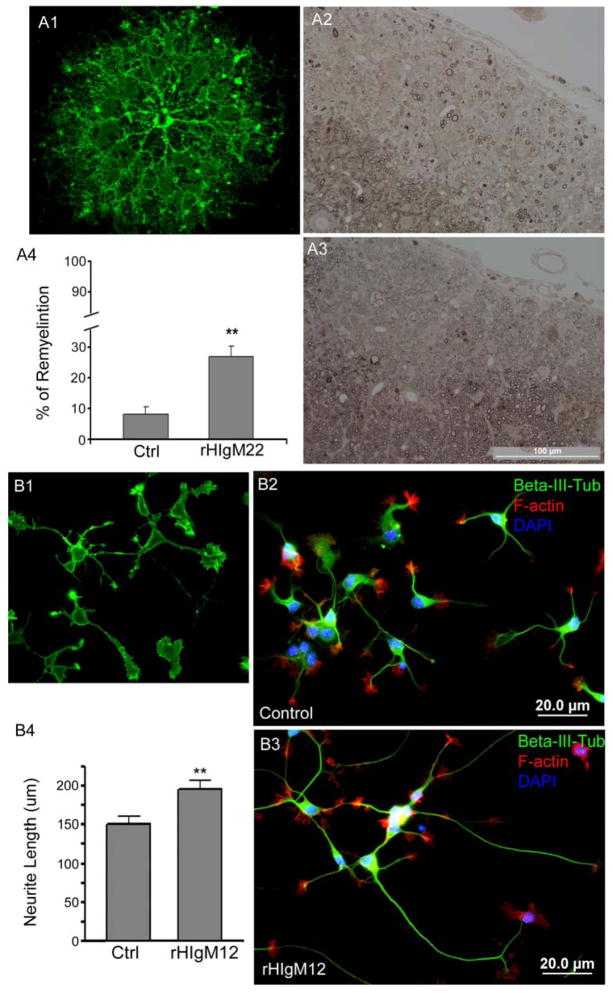
Figure 5. Human IgMs promote neural growth.
A. A single dose of 500 μg of rHIgM22 promotes remyelination of the demyelinated lesions in the TMEV MS model. A1. rHIgM22 binds to primary rat oligodendrocyte. A2. Demyelinated lesions as seen in the control IgM-treated mice. A3. Enhanced remyelination was observed following rHIgM22 treatment. A4. Quantitation of remyelination following treatment with control IgM versus rHIgM22 (p<0.05, unpaired t-test). B. rHIgM12 bound to neuronal surface (B1). E15 hippocampal neurons from FVB mice were grown on nitrocellulose-attached glass coverslips coated with PDL (B2) or rHIgM12 (B3) substrates. Neurite length was measured 12 hr after plating of cells. Total neurite length was quantified and neurons grown on rHIgM12 substrate showed increased neurite length compared to the control (B4) (p<0.05, unpaired t-test). PDL was used as the control substrate because the non-specific IgMs did not support neuron attachment.
Developing reagents to promote rewiring
Rewiring the CNS requires stimulation that can reverse the inhibitors in a nonpermissive environment to promote axonal outgrowth or enhance remyelination. Earlier studies in our laboratory demonstrated that immunization with CNS homogenates or transfer of the anti-serum to CNS homogenates promoted repair of demyelinated CNS lesions in miouse MS models[109]. This observation indicated that it might be possible to promote CNS repair by regulating the immune system. In support of this hypothesis, antibodies in the sera of immunized mice were identified to bind myelin, protect oligodendrocytes from death[110] and promote remyelination[111]. Similar remyelination-promoting antibodies have also been characterized in human sera and cloned[112] (Fig. 5A). The recombinant form of one of the human IgMs, rHIgM22, has been synthesized and is nearing Phase I clinical trials[113]. In addition to IgMs that interact with myelin, human IgMs that bound neurons and supported CNS neurite outgrowth were identified in a subsequent screening of a sera bank of patients with monoclonal gammopathies[114](Fig. 5B). One of the IgMs, sHIgM12, promoted neurologic function in the TMEV MS model[113]. These monoclonal antibodies belong to the IgM repertoire of natural antibodies. Thus the concept of natural IgMs that bind neural cells and promote CNS repair is firmly established.
Mechanism of action of neural-binding IgMs
Natural antibodies or autoantibodies are produced in healthy individuals without obvious antigenic stimuli which can react with self-molecules[115]. Natural antibodies, that are mostly IgMs, usually display low to moderate affinity but high avidity for self-antigens, and are encoded by germ line sequences without or with few somatic mutations[116]. They are generally regarded as first line defenders against infections or even serving as housekeeping molecules to maintain homeostasis of the host[117, 118]. First, natural IgMs may maintain homeostasis by pattern recognition[119]. This function relies on the pentameric structure and poly-reactivity to bind a wide variety of molecules comprising of proteins, nucleic acid, carbohydrates, lipids or even their various combinations. Natural IgMs recognize conserved patterns independent of somatic mutation, which may be shared by healthy cells, injured tissue, transformed cells and pathogens such as bacteria and viruses, though some patterns may be found on the surface of a specific cell type of an organ (Fig. 4A, B & E). Natural IgMs may be involved in house-keeping function to remove cell debris, apoptotic or even precancerous and cancerous cells[118, 120]. How natural IgMs recognize distinct patterns remains not fully understood. Also natural IgMs may induce signaling in a context-dependent manner. Natural IgMs have been identified in patients, though they do exist in healthy individuals. Why do natural IgMs remain “silent” normally, but are “active” in pathological conditions? One reason is that signals induced by natural IgMs in vivo have been difficult to clarify. Nevertheless they do mediate reparative signaling on primary cells. HIgM22 induced calcium influx[110] and promoted Lyn expression[121] in cell cultures. HIgM22 protected cells from apoptosis in vitro[110] and promoted remyelination of the demyelinated lesions in vivo[112]. HIgM12, which binds primarily to CNS neurons, modulated microtubule dynamics (unpublished observation). Both HIgM22 and HIgM12 bind lipid raft microdomains on cell membranes, in the case of HIgM12 that depends on carbohydrates[114]. HIgM22 was found to be polyreactive to multiple known and novel antigens[121]. HIgM12 bound both live and injured neuronal surface (Fig. 4A & B). The reorganized cytoskeletons suggest that HIgM12 may mediate a distinct downstream signaling in injured neurons (Fig. 4C & D). Thus IgMs may induce different signals by binding to the same or modified patterns in a context dependent manner.
Conclusion
Repairing the CNS will require combinatorial therapies. Preventing neural degeneration to stop progressive neurologic deterioration is crucial for repair to take place. However, effective treatments to stop and/or reverse the neurodegeneration are yet not available. Potential therapies include ion channel blockers, which inhibit neurotransmitter-induced excitatory toxicity; neurotrophic factors, which potentially protect neural cells; agents that target the immune system to eliminate immune response-induced injury; and neutralizers to reactive oxygen species. The risk-factors that cause these diseases are not fully understood. Nevertheless the adult CNS has the intrinsic ability to regenerate and produce neural cells, though this capacity following neural injury is limited and the regions able to produce neural cells are restricted. Emerging reagents such as human monoclonal IgMs that regulate homeostasis to produce a permissive environment for neuroregeneration may shed new light on CNS repair.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01 NS 24180, R01 NS 32129, R01 CA104996, R01 CA096859), the National Multiple Sclerosis Society (CA 1011 A8-3), the Applebaum Foundation, the Hilton Foundation, and the Peterson Foundation. Patents for antibodies that promote remyelination and central nervous system repair are issued and are owned by Mayo Foundation.
Footnotes
Therefore, the authors have a potential future financial conflict of interest.
References
- 1.Hagg T, Oudega M. Degenerative and spontaneous regenerative processes after spinal cord injury. J Neurotrauma. 2006 Mar–Apr;23(3–4):264–80. doi: 10.1089/neu.2006.23.263. [DOI] [PubMed] [Google Scholar]
- 2.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004 Feb;5(2):146–56. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 3.Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008 May;14(5):497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 4.LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci. 2007 Jul;8(7):499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 5.Daniela F, Vescovi AL, Bottai D. The stem cells as a potential treatment for neurodegeneration. Methods Mol Biol. 2007;399:199–213. doi: 10.1007/978-1-59745-504-6_14. [DOI] [PubMed] [Google Scholar]
- 6.Leker RR, Lasri V, Chernoguz D. Growth factors improve neurogenesis and outcome after focal cerebral ischemia. J Neural Transm. 2009 Nov;116(11):1397–402. doi: 10.1007/s00702-009-0329-3. [DOI] [PubMed] [Google Scholar]
- 7.Sahni V, Kessler JA. Stem cell therapies for spinal cord injury. Nat Rev Neurol. Jul;6(7):363–72. doi: 10.1038/nrneurol.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.0McDonald JW, Liu XZ, Qu Y, et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999 Dec;5(12):1410–2. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- 9.Erdo F, Buhrle C, Blunk J, et al. Host-dependent tumorigenesis of embryonic stem cell transplantation in experimental stroke. J Cereb Blood Flow Metab. 2003 Jul;23(7):780–5. doi: 10.1097/01.WCB.0000071886.63724.FB. [DOI] [PubMed] [Google Scholar]
- 10.Bjorklund LM, Sanchez-Pernaute R, Chung S, et al. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci U S A. 2002 Feb 19;99(4):2344–9. doi: 10.1073/pnas.022438099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 2000 Jun;18(6):675–9. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]
- 12.Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998 Nov;4(11):1313–7. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 13.Toda H, Takahashi J, Iwakami N, et al. Grafting neural stem cells improved the impaired spatial recognition in ischemic rats. Neurosci Lett. 2001 Dec 4;316(1):9–12. doi: 10.1016/s0304-3940(01)02331-x. [DOI] [PubMed] [Google Scholar]
- 14.Shim JW, Park CH, Bae YC, et al. Generation of functional dopamine neurons from neural precursor cells isolated from the subventricular zone and white matter of the adult rat brain using Nurr1 overexpression. Stem Cells. 2007 May;25(5):1252–62. doi: 10.1634/stemcells.2006-0274. [DOI] [PubMed] [Google Scholar]
- 15.Bonner JF, Blesch A, Neuhuber B, Fischer I. Promoting directional axon growth from neural progenitors grafted into the injured spinal cord. J Neurosci Res. May 1;88(6):1182–92. doi: 10.1002/jnr.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001 Apr;32(4):1005–11. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 17.Newman MB, Davis CD, Kuzmin-Nichols N, Sanberg PR. Human umbilical cord blood (HUCB) cells for central nervous system repair. Neurotox Res. 2003;5(5):355–68. doi: 10.1007/BF03033155. [DOI] [PubMed] [Google Scholar]
- 18.Sykova E, Homola A, Mazanec R, et al. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant. 2006;15(8–9):675–87. doi: 10.3727/000000006783464381. [DOI] [PubMed] [Google Scholar]
- 19.Munoz-Elias G, Marcus AJ, Coyne TM, Woodbury D, Black IB. Adult bone marrow stromal cells in the embryonic brain: engraftment, migration, differentiation, and long-term survival. J Neurosci. 2004 May 12;24(19):4585–95. doi: 10.1523/JNEUROSCI.5060-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pluchino S, Zanotti L, Rossi B, et al. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005 Jul 14;436(7048):266–71. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- 21.Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006 May;7(5):395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- 22.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–50. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 23.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002 Sep;8(9):963–70. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton LK, Truong MK, Bednarczyk MR, Aumont A, Fernandes KJ. Cellular organization of the central canal ependymal zone, a niche of latent neural stem cells in the adult mammalian spinal cord. Neuroscience. 2009 Dec 15;164(3):1044–56. doi: 10.1016/j.neuroscience.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Bath KG, Lee FS. Neurotrophic factor control of adult SVZ neurogenesis. Dev Neurobiol. Apr;70(5):339–49. doi: 10.1002/dneu.20781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Case LC, Tessier-Lavigne M. Regeneration of the adult central nervous system. Curr Biol. 2005 Sep 20;15(18):R749–53. doi: 10.1016/j.cub.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Bredesen DE, Rao RV, Mehlen P. Cell death in the nervous system. Nature. 2006 Oct 19;443(7113):796–802. doi: 10.1038/nature05293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005 Aug 15;202(4):473–7. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener. 2009;4:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007 Sep;8(9):663–72. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 31.Auluck PK, Caraveo G, Lindquist S. alpha-Synuclein: Membrane Interactions and Toxicity in Parkinson’s Disease. Annu Rev Cell Dev Biol. May 25; doi: 10.1146/annurev.cellbio.042308.113313. [DOI] [PubMed] [Google Scholar]
- 32.Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009 Dec 14;187(6):761–72. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. Mar 19;140(6):918–34. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wyss-Coray T, Loike JD, Brionne TC, et al. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med. 2003 Apr;9(4):453–7. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- 35.Tan L, Gordon KB, Mueller JP, Matis LA, Miller SD. Presentation of proteolipid protein epitopes and B7–1-dependent activation of encephalitogenic T cells by IFN-gamma-activated SJL/J astrocytes. J Immunol. 1998 May 1;160(9):4271–9. [PubMed] [Google Scholar]
- 36.Constantinescu CS, Tani M, Ransohoff RM, et al. Astrocytes as antigen-presenting cells: expression of IL-12/IL-23. J Neurochem. 2005 Oct;95(2):331–40. doi: 10.1111/j.1471-4159.2005.03368.x. [DOI] [PubMed] [Google Scholar]
- 37.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998 Jan 29;338(5):278–85. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez M, Scheithauer B. Ultrastructure of multiple sclerosis. Ultrastruct Pathol. 1994 Jan-Apr;18(1–2):3–13. doi: 10.3109/01913129409016267. [DOI] [PubMed] [Google Scholar]
- 39.Minghetti L. Role of inflammation in neurodegenerative diseases. Curr Opin Neurol. 2005 Jun;18(3):315–21. doi: 10.1097/01.wco.0000169752.54191.97. [DOI] [PubMed] [Google Scholar]
- 40.Fugger L, Friese MA, Bell JI. From genes to function: the next challenge to understanding multiple sclerosis. Nat Rev Immunol. 2009 Jun;9(6):408–17. doi: 10.1038/nri2554. [DOI] [PubMed] [Google Scholar]
- 41.Goate AM, Haynes AR, Owen MJ, et al. Predisposing locus for Alzheimer’s disease on chromosome 21. Lancet. 1989 Feb 18;1(8634):352–5. doi: 10.1016/s0140-6736(89)91725-x. [DOI] [PubMed] [Google Scholar]
- 42.Goate A, Chartier-Harlin MC, Mullan M, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991 Feb 21;349(6311):704–6. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 43.Sherrington R, Rogaev EI, Liang Y, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995 Jun 29;375(6534):754–60. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 44.Levy-Lahad E, Wijsman EM, Nemens E, et al. A familial Alzheimer’s disease locus on chromosome 1. Science. 1995 Aug 18;269(5226):970–3. doi: 10.1126/science.7638621. [DOI] [PubMed] [Google Scholar]
- 45.Rogaev EI, Sherrington R, Rogaeva EA, et al. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature. 1995 Aug 31;376(6543):775–8. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- 46.Bertram L, Tanzi RE. Genome-wide association studies in Alzheimer’s disease. Hum Mol Genet. 2009 Oct 15;18(R2):R137–45. doi: 10.1093/hmg/ddp406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borchelt DR, Thinakaran G, Eckman CB, et al. Familial Alzheimer’s disease-linked presenilin 1 variants elevate Abeta1–42/1–40 ratio in vitro and in vivo. Neuron. 1996 Nov;17(5):1005–13. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 48.Xia W, Zhang J, Kholodenko D, et al. Enhanced production and oligomerization of the 42-residue amyloid beta-protein by Chinese hamster ovary cells stably expressing mutant presenilins. J Biol Chem. 1997 Mar 21;272(12):7977–82. doi: 10.1074/jbc.272.12.7977. [DOI] [PubMed] [Google Scholar]
- 49.Holtzman DM, Bales KR, Tenkova T, et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2000 Mar 14;97(6):2892–7. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baulac S, LaVoie MJ, Kimberly WT, et al. Functional gamma-secretase complex assembly in Golgi/trans-Golgi network: interactions among presenilin, nicastrin, Aph1, Pen-2, and gamma-secretase substrates. Neurobiol Dis. 2003 Nov;14(2):194–204. doi: 10.1016/s0969-9961(03)00123-2. [DOI] [PubMed] [Google Scholar]
- 51.Beleza-Meireles A, Al-Chalabi A. Genetic studies of amyotrophic lateral sclerosis: controversies and perspectives. Amyotroph Lateral Scler. 2009 Feb;10(1):1–14. doi: 10.1080/17482960802585469. [DOI] [PubMed] [Google Scholar]
- 52.Dion PA, Daoud H, Rouleau GA. Genetics of motor neuron disorders: new insights into pathogenic mechanisms. Nat Rev Genet. 2009 Nov;10(11):769–82. doi: 10.1038/nrg2680. [DOI] [PubMed] [Google Scholar]
- 53.Lesage S, Brice A. Parkinson’s disease: from monogenic forms to genetic susceptibility factors. Hum Mol Genet. 2009 Apr 15;18(R1):R48–59. doi: 10.1093/hmg/ddp012. [DOI] [PubMed] [Google Scholar]
- 54.Tam SJ, Watts RJ. Connecting vascular and nervous system development: angiogenesis and the blood-brain barrier. Annu Rev Neurosci. 33:379–408. doi: 10.1146/annurev-neuro-060909-152829. [DOI] [PubMed] [Google Scholar]
- 55.Begley DJ, Brightman MW. Structural and functional aspects of the blood-brain barrier. Prog Drug Res. 2003;61:39–78. doi: 10.1007/978-3-0348-8049-7_2. [DOI] [PubMed] [Google Scholar]
- 56.Schlageter KE, Molnar P, Lapin GD, Groothuis DR. Microvessel organization and structure in experimental brain tumors: microvessel populations with distinctive structural and functional properties. Microvasc Res. 1999 Nov;58(3):312–28. doi: 10.1006/mvre.1999.2188. [DOI] [PubMed] [Google Scholar]
- 57.Banerjee S, Bhat MA. Neuron-glial interactions in blood-brain barrier formation. Annu Rev Neurosci. 2007;30:235–58. doi: 10.1146/annurev.neuro.30.051606.094345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006 Jan;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 59.Rouach N, Glowinski J, Giaume C. Activity-dependent neuronal control of gap-junctional communication in astrocytes. J Cell Biol. 2000 Jun 26;149(7):1513–26. doi: 10.1083/jcb.149.7.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008 Dec 5;322(5907):1551–5. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- 61.Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002 Jan 1;22(1):183–92. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khuth ST, Strazielle N, Giraudon P, Belin MF, Ghersi-Egea JF. Impairment of blood-cerebrospinal fluid barrier properties by retrovirus-activated T lymphocytes: reduction in cerebrospinal fluid-to-blood efflux of prostaglandin E2. J Neurochem. 2005 Sep;94(6):1580–93. doi: 10.1111/j.1471-4159.2005.03309.x. [DOI] [PubMed] [Google Scholar]
- 63.Navikas V, Link H. Review: cytokines and the pathogenesis of multiple sclerosis. J Neurosci Res. 1996 Aug 15;45(4):322–33. doi: 10.1002/(SICI)1097-4547(19960815)45:4<322::AID-JNR1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 64.Nave KA, Trapp BD. Axon-glial signaling and the glial support of axon function. Annu Rev Neurosci. 2008;31:535–61. doi: 10.1146/annurev.neuro.30.051606.094309. [DOI] [PubMed] [Google Scholar]
- 65.Rakic P, Ayoub AE, Breunig JJ, Dominguez MH. Decision by division: making cortical maps. Trends Neurosci. 2009 May;32(5):291–301. doi: 10.1016/j.tins.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. Jan;119(1):7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001 Apr;81(2):871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 68.Black JA, Waxman SG. The perinodal astrocyte. Glia. 1988;1(3):169–83. doi: 10.1002/glia.440010302. [DOI] [PubMed] [Google Scholar]
- 69.Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–45. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 70.Lindvall O, Kokaia Z. Prospects of stem cell therapy for replacing dopamine neurons in Parkinson’s disease. Trends Pharmacol Sci. 2009 May;30(5):260–7. doi: 10.1016/j.tips.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 71.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009 Dec;32(12):638–47. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Margolis RK, Margolis RU. Nervous tissue proteoglycans. Exs. 1994;70:145–77. doi: 10.1007/978-3-0348-7545-5_9. [DOI] [PubMed] [Google Scholar]
- 73.Yamagata T, Saito H, Habuchi O, Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–35. [PubMed] [Google Scholar]
- 74.Bradbury EJ, Carter LM. Manipulating the glial scar: Chondroitinase ABC as a therapy for spinal cord injury. Brain Res Bull. Jul 8; doi: 10.1016/j.brainresbull.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 75.Brambilla R, Persaud T, Hu X, et al. Transgenic inhibition of astroglial NF-kappa B improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation. J Immunol. 2009 Mar 1;182(5):2628–40. doi: 10.4049/jimmunol.0802954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hamby ME, Hewett JA, Hewett SJ. TGF-beta1 potentiates astrocytic nitric oxide production by expanding the population of astrocytes that express NOS-2. Glia. 2006 Nov 1;54(6):566–77. doi: 10.1002/glia.20411. [DOI] [PubMed] [Google Scholar]
- 77.Takano T, Kang J, Jaiswal JK, et al. Receptor-mediated glutamate release from volume sensitive channels in astrocytes. Proc Natl Acad Sci U S A. 2005 Nov 8;102(45):16466–71. doi: 10.1073/pnas.0506382102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rolls A, Shechter R, Schwartz M. The bright side of the glial scar in CNS repair. Nat Rev Neurosci. 2009 Mar;10(3):235–41. doi: 10.1038/nrn2591. [DOI] [PubMed] [Google Scholar]
- 79.Trendelenburg G, Dirnagl U. Neuroprotective role of astrocytes in cerebral ischemia: focus on ischemic preconditioning. Glia. 2005 Jun;50(4):307–20. doi: 10.1002/glia.20204. [DOI] [PubMed] [Google Scholar]
- 80.Kuhlmann T, Lassmann H, Bruck W. Diagnosis of inflammatory demyelination in biopsy specimens: a practical approach. Acta Neuropathol. 2008 Mar;115(3):275–87. doi: 10.1007/s00401-007-0320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Voskuhl RR, Peterson RS, Song B, et al. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci. 2009 Sep 16;29(37):11511–22. doi: 10.1523/JNEUROSCI.1514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thal DR, Schultz C, Dehghani F, Yamaguchi H, Braak H, Braak E. Amyloid beta-protein (Abeta)-containing astrocytes are located preferentially near N-terminal-truncated Abeta deposits in the human entorhinal cortex. Acta Neuropathol. 2000 Dec;100(6):608–17. doi: 10.1007/s004010000242. [DOI] [PubMed] [Google Scholar]
- 83.Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004 Mar 3;24(9):2143–55. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li L, Lundkvist A, Andersson D, et al. Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab. 2008 Mar;28(3):468–81. doi: 10.1038/sj.jcbfm.9600546. [DOI] [PubMed] [Google Scholar]
- 85.Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007 May;10(5):608–14. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nagai M, Re DB, Nagata T, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007 May;10(5):615–22. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994 Oct;13(4):805–11. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- 88.Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994 Sep;13(3):757–67. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 89.Chen MS, Huber AB, van der Haar ME, et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000 Jan 27;403(6768):434–9. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- 90.GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000 Jan 27;403(6768):439–44. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- 91.Prinjha R, Moore SE, Vinson M, et al. Inhibitor of neurite outgrowth in humans. Nature. 2000 Jan 27;403(6768):383–4. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- 92.Wang KC, Koprivica V, Kim JA, et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002 Jun 27;417(6892):941–4. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- 93.Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003 Sep;4(9):703–13. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- 94.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006 Aug;7(8):617–27. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mi S, Sandrock A, Miller RH. LINGO-1 and its role in CNS repair. Int J Biochem Cell Biol. 2008;40(10):1971–8. doi: 10.1016/j.biocel.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 96.Cao Z, Gao Y, Deng K, Williams G, Doherty P, Walsh FS. Receptors for myelin inhibitors: Structures and therapeutic opportunities. Mol Cell Neurosci. Jan;43(1):1–14. doi: 10.1016/j.mcn.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 97.Yang LJ, Zeller CB, Shaper NL, et al. Gangliosides are neuronal ligands for myelin-associated glycoprotein. Proc Natl Acad Sci U S A. 1996 Jan 23;93(2):814–8. doi: 10.1073/pnas.93.2.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vyas AA, Patel HV, Fromholt SE, et al. Gangliosides are functional nerve cell ligands for myelin-associated glycoprotein (MAG), an inhibitor of nerve regeneration. Proc Natl Acad Sci U S A. 2002 Jun 11;99(12):8412–7. doi: 10.1073/pnas.072211699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Atwal JK, Pinkston-Gosse J, Syken J, et al. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008 Nov 7;322(5903):967–70. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- 100.Goh EL, Young JK, Kuwako K, et al. beta1-integrin mediates myelin-associated glycoprotein signaling in neuronal growth cones. Mol Brain. 2008;1(1):10. doi: 10.1186/1756-6606-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Quarles RH. Myelin-associated glycoprotein (MAG): past, present and beyond. J Neurochem. 2007 Mar;100(6):1431–48. doi: 10.1111/j.1471-4159.2006.04319.x. [DOI] [PubMed] [Google Scholar]
- 102.Schnaar RL, Lopez PH. Myelin-associated glycoprotein and its axonal receptors. J Neurosci Res. 2009 Nov 15;87(15):3267–76. doi: 10.1002/jnr.21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bartsch U, Bandtlow CE, Schnell L, et al. Lack of evidence that myelin-associated glycoprotein is a major inhibitor of axonal regeneration in the CNS. Neuron. 1995 Dec;15(6):1375–81. doi: 10.1016/0896-6273(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 104.Lee JK, Geoffroy CG, Chan AF, et al. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron. Jun 10;66(5):663–70. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yin X, Crawford TO, Griffin JW, et al. Myelin-associated glycoprotein is a myelin signal that modulates the caliber of myelinated axons. J Neurosci. 1998 Mar 15;18(6):1953–62. doi: 10.1523/JNEUROSCI.18-06-01953.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nguyen T, Mehta NR, Conant K, et al. Axonal protective effects of the myelin-associated glycoprotein. J Neurosci. 2009 Jan 21;29(3):630–7. doi: 10.1523/JNEUROSCI.5204-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990 Jan 18;343(6255):269–72. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- 108.Brosamle C, Huber AB, Fiedler M, Skerra A, Schwab ME. Regeneration of lesioned corticospinal tract fibers in the adult rat induced by a recombinant, humanized IN-1 antibody fragment. J Neurosci. 2000 Nov 1;20(21):8061–8. doi: 10.1523/JNEUROSCI.20-21-08061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rodriguez M, Lennon VA, Benveniste EN, Merrill JE. Remyelination by oligodendrocytes stimulated by antiserum to spinal cord. J Neuropathol Exp Neurol. 1987 Jan;46(1):84–95. doi: 10.1097/00005072-198701000-00008. [DOI] [PubMed] [Google Scholar]
- 110.Howe CL, Bieber AJ, Warrington AE, Pease LR, Rodriguez M. Antiapoptotic signaling by a remyelination-promoting human antimyelin antibody. Neurobiol Dis. 2004 Feb;15(1):120–31. doi: 10.1016/j.nbd.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 111.Miller DJ, Sanborn KS, Katzmann JA, Rodriguez M. Monoclonal autoantibodies promote central nervous system repair in an animal model of multiple sclerosis. J Neurosci. 1994 Oct;14(10):6230–8. doi: 10.1523/JNEUROSCI.14-10-06230.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Warrington AE, Asakura K, Bieber AJ, et al. Human monoclonal antibodies reactive to oligodendrocytes promote remyelination in a model of multiple sclerosis. Proc Natl Acad Sci U S A. 2000 Jun 6;97(12):6820–5. doi: 10.1073/pnas.97.12.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rodriguez M, Warrington AE, Pease LR. Invited Article: Human natural autoantibodies in the treatment of neurologic disease. Neurology. 2009 Apr 7;72(14):1269–76. doi: 10.1212/01.wnl.0000345662.05861.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Warrington AE, Bieber AJ, Van Keulen V, Ciric B, Pease LR, Rodriguez M. Neuron-binding human monoclonal antibodies support central nervous system neurite extension. J Neuropathol Exp Neurol. 2004 May;63(5):461–73. doi: 10.1093/jnen/63.5.461. [DOI] [PubMed] [Google Scholar]
- 115.Casali P, Schettino EW. Structure and function of natural antibodies. Curr Top Microbiol Immunol. 1996;210:167–79. doi: 10.1007/978-3-642-85226-8_17. [DOI] [PubMed] [Google Scholar]
- 116.Boes M. Role of natural and immune IgM antibodies in immune responses. Mol Immunol. 2000 Dec;37(18):1141–9. doi: 10.1016/s0161-5890(01)00025-6. [DOI] [PubMed] [Google Scholar]
- 117.Elkon K, Casali P. Nature and functions of autoantibodies. Nat Clin Pract Rheumatol. 2008 Sep;4(9):491–8. doi: 10.1038/ncprheum0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Binder CJ. Natural IgM antibodies against oxidation-specific epitopes. J Clin Immunol. May;30(Suppl 1):S56–60. doi: 10.1007/s10875-010-9396-3. [DOI] [PubMed] [Google Scholar]
- 119.Jeannin P, Jaillon S, Delneste Y. Pattern recognition receptors in the immune response against dying cells. Curr Opin Immunol. 2008 Oct;20(5):530–7. doi: 10.1016/j.coi.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 120.Vollmers HP, Brandlein S. Natural antibodies and cancer. J Autoimmun. 2007 Dec;29(4):295–302. doi: 10.1016/j.jaut.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 121.Watzlawik J, Holicky E, Edberg DD, et al. Human remyelination promoting antibody inhibits apoptotic signaling and differentiation through Lyn kinase in primary rat oligodendrocytes. Glia. Nov 15;58(15):1782–93. doi: 10.1002/glia.21048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ertekin-Taner N. Genetics of Alzheimer disease in the pre- and post-GWAS era. Alzheimers Res Ther. 2(1):3. doi: 10.1186/alzrt26. [DOI] [PMC free article] [PubMed] [Google Scholar]