Abstract
Our previous studies have shown that neonatal exposure to lipopolysaccharide (LPS) resulted in long-lasting dopaminergic injury and enhanced methamphetamine (METH)-induced increase of locomotion in the adult male rat. To further investigate the effect of neonatal LPS exposure-induced dopaminergic injury, we used our neonatal rat model of LPS exposure (1 mg/kg, intracerebral injection in postnatal day 5, P5, rats) to examine the METH sensitization as an indicator of drug addiction in the adult rats. On P70, animals began a treatment schedule of 5 daily subcutaneous (s.c.) administration of METH (0.5 mg/kg) or saline (P70-P74) to induce behavioral sensitization. Ninety-six hours after the 5th treatment with METH or saline (P78), animals received a single dose of 0.5 mg/kg METH (s.c.) or saline. Neonatal LPS exposure enhanced the level of development of behavioral sensitization including distance traveled, rearing events and stereotypy to METH administration in both male and female rats. Neonatal LPS exposure also enhanced the reinstated behavioral sensitization in both male and female rats after the administration had ceased for ninety-six hours. However, neonatal LPS exposure induced alteration in the reinstated behaviors sensitization of distance traveled and rearing events to METH administration appears to be greater in male than in female rats. These results indicate that neonatal brain LPS exposure produces a persistent lesion in the dopaminergic system, as indicated by enhanced METH-induced locomotor and stereotyped behavioral sensitization in later life. These findings show that early-life brain inflammation may enhance susceptibility to the development of drug addiction in later life.
Keywords: Lipopolysaccharide, Methamphetamine, Locomotion, Stereotypy, Behavioral sensitization
1. Introduction
Perinatal or early life exposure to an acute immune activation may induce long-lasting dopaminergic damage and is associated with the subsequent development of motor disturbances in adults [1–4]. In our previous studies, we developed a neonatal rat model with central exposure to lipopolysaccharide (LPS), an endotoxin which is a component of the cell wall of Gram-negative bacteria and responsible for most of the inflammatory effects of infection from Gram-negative bacteria, in order to study the infection/inflammation-associated brain injury during the early developmental period (postnatal day 5, P5, 1 mg/kg, intracerebral injection) [5–7]. In this neonatal rat model we have found that central inflammation induced by LPS produces lesions in the dopaminergic system such as loss of dendrites and reduced tyrosine hydroxylase immunoreactivity in the substantia nigra and ventral tegmental area which is persistent into adult life (P70) [2].
Our previous study also indicates that neonatal LPS-exposure causes an increased locomotor behavioral response after a single dose challenge of methamphetamine (METH) in adult male rats [2]. Lesions of the dopaminergic system, such as dysregulation of presynaptic striatal dopamine, alteration of central dopamine turnover, or dopaminergic receptor sensitization, were evidenced by enhanced behavioral reaction after METH administration to the animals [8–10]. To further investigate the effect of neonatal LPS exposure on the dopaminergic system, the long-lasting changes in the brain reward system were examined by METH behavioral sensitization. Animals began a treatment schedule of 5 daily subcutaneous (s.c.) injections of METH (0.5 mg/kg) to induce behavioral sensitization. Ninety-six hours after the 5th treatment with METH, animals received a single dose of 0.5 mg/kg METH (s.c.) to reintroduce behavioral sensitization. Behavioral sensitization is the progressive increase in behavioral responses following repeated treatments of a drug, and is mediated by long-lasting changes in the neural substrates mediating reward and motivation; namely, the mesolimbic dopamine system, consisting of reciprocal projections between the ventral tegmental area and the nucleus accumbens [4, 11–13]. Thus behavioral sensitization resulted from neuroadaptations within the mesolimbic dopamine system caused by repeated drug exposure can be used as an indicator of drug addiction [4, 11–13].
The aim of this study was to determine whether the brain inflammation early in life leads to a long-lasting change in the brain reward system which can enhance adult susceptibility to the development of addictive-like behavior in later life. The craving for addictive METH has shown gender differences in humans [14]. The second objective of this study was to determine whether there are any gender differences in early life LPS exposure-enhanced reinstated behavioral sensitization to methamphetamine.[5–7, 15]
2. Materials and methods
2.1. Chemicals
Unless otherwise stated, all chemicals used in this study were purchased from Sigma (St. Louis, MO., USA).
2.2. Animals
Timed pregnant Sprague-Dawley rats arrived in the laboratory on day 19 of gestation. Animals were maintained in a room with a 12-h light/dark cycle and at constant temperature (22 ± 2°C). The day of birth was defined as postnatal day 0. After birth, the litter size was adjusted to twelve pups per litter to minimize the effect of litter size on body weight and brain size. All procedures for animal care were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center or Fu Jen Catholic University. Every effort was made to minimize the number of animals used and their suffering.
2.3. Surgery procedures and animal treatment
Intracerebral injection of LPS to 5-day old Sprague-Dawley rat pups of both sexes was performed as previously described [5–7, 15]. Under light anesthesia with isoflurane (1.5%), LPS (1 mg/kg, from Escherichia coli, serotype 055: B5) in sterile saline (total volume of 2 μl) was administered to the rat brain at the location of 1.0 mm posterior and 1.0 mm left to the bregma, and 2.0 mm deep to the scalp at the left hemisphere in a stereotaxic apparatus with a neonatal rat adapter. The injection was completed in 5 min and the needle was kept in this position for an additional 2 min and then retrieved slowly out of the brain. The wound was sutured and the pups were placed on a thermal blanket (34°C-35°C) for recovery before being returned to their dams. The dose of LPS was chosen based on the previously reported results which produced reproducible brain injury [5–7]. The injection site was located at the area just above the left cingulum (white matter). LPS distributed from injection site (left cingulum) to the right cingulum through the corpus callosum track within 30 minutes. The control rats were injected with the same volume of sterile saline. All animals survived the intracerebral injection. Each dam had the same litter size (12 pups) and equal numbers of LPS-treated and saline-treated rat pups were included in a litter. The pups were weaned at P21 and four rats (2 LPS-treated and 2 saline-treated) per cage were housed after weaning. Ten rats from each group and each gender were used in the present study.
Ten rats from the LPS- or saline-injected group were further divided into two groups: one received 5 doses of the subcutaneous (s.c.) injection of methamphetamine (METH) (0.5 mg/kg) (5 male and 5 female rats) and the other with saline (5 male and 5 female rats) on P70. Animals began a treatment schedule of 5 daily injections of METH (0.5 mg/kg, s.c.) to induce behavioral sensitization from P70 to P74. Ninety-six hours after the 5th treatment with METH, animals received a single dose of 0.5 mg/kg METH (s.c.) to reintroduce behavioral sensitization on P78.
2.4. Behavioral testing: methamphetamine-induced locomotor and stereotyped behavioral sensitization
METH-induced locomotor and stereotyped behaviors were performed as previously described [2, 16] with modifications. All animals were tested and video taped in the same order once a week from P70 to P78. Male and female rats were tested on different occasions in chambers with fresh litter to avoid behavioral bias due to the odors or contact with the opposite sex. Rats received their drug treatments at 9:00 AM on experimental days. Locomotor activity was measured using the ANY-maze Video Tracking System (Stoelting Co., Wood Dale, IL, USA). On the experimental day, rats were placed in the activity chambers (42 × 25 × 40 cm3) in a quiet room with dimmed light 1 hour before the administration of saline or METH to acclimatize them to their surroundings. Baseline levels of locomotion were determined during the 10 min prior to METH or saline administration (−10 to 0 min). After rats received saline or METH (0.5 mg/kg, s.c.), their activities were recorded for 150 min. The total distance traveled by the animal was determined during every 10-min testing period for 150 min. Rating of rearing events including: exposure rearing responses (body inclined vertically with hindpaws on the floor of the activity cage and forepaws on the wall of the chamber) and sniffing-air responses (rearing in the open area of the active chamber) were determined at the first minute of every 10-min intervals during 150 min after METH or saline administration. The summation of exposure rearing and sniffing-air responses reflects vertical activity which has been used apart from locomotion [17].
The stereotyped behaviors including: standing (on all four feet, essentially motionless and not actively sniffing), grooming (washing the face or any other part of its body with the forepaws, with the mouth generally in contact with the body), scratching (raising of hindpaw to touch any part of its body), head-swinging (standing on all four feet, moving its head from side to side), sniffing (not moving, but sniffing parts of the walls or floor of the apparatus) and freezing (standing on all four feet, freezing position, completely inactive, i.e. head oriented forwards and eyes fixed at a point of the upper side of the cage) were determined at the first minute of every 10-min intervals during 150 min after METH or saline administration. Quantification of stereotyped behaviors was achieved by counting the frequency of discrete episodes and the summation of all stereotyped responses during the testing period was scored for each rat [17–19].
2.5. Data analysis and statistics
The behavioral data were presented as the mean±SEM and analyzed by two-way repeated measures ANOVA for data from tests conducted continuously at different experimental times or days or by two-way ANOVA for the behavioral response in specific time intervals on experimental day followed by Student-Newman-Keuls test.
The increment of distance traveled, rearing responses, or stereotyped behaviors in LPS-exposure enhanced METH-increased behavioral responses in male or female rats were calculated as follows, ΔMale (LPS+METH – Saline+METH) = LPS+METH Male group – saline+METH Male group; ΔFemale (LPS+METH – Saline+METH) = LPS+METH Female group – saline+METH Female group. The data for the alteration in the behavioral sensitization (P74) and reinstated behavioral sensitization (P78) to METH following LPS exposure were presented as the mean±SEM and analyzed by Student’s t-test. Results with a p<0.05 were considered statistically significant.
3. Results
3.1. Neonatal LPS exposure enhanced methamphetamine-induced locomotor behavioral response, behavioral sensitization, and reinstated behavioral sensitization in adult rats
The upper panel of Fig. 1(A & B) shows the total distance traveled by the rats after a single dose injection of METH which was determined during every 10-min testing period for 150 min. After a single dose injection of METH in the male rats (P70), both groups including neonatal exposure to saline or LPS showed increased total distance traveled from 10 min to 50 min, and from 10 min to 80 min, respectively [F(3, 319) = 53.848, p<0.001] (P<0.05) (Fig. 1A). Two-way ANOVA on behavioral data (summation for 120 min) following the METH challenge showed that adult (P70) METH treatment and the interaction of neonatal (P5) LPS treatment x adult (P70) METH treatment have significant effects on the total distance traveled [F(1, 19) = 178.342, p<0.001; F(1, 19) = 13.158, p=0.02, respectively, Fig. 1C]. Consistent with our previous study (Fan et al., 2011), neonatal LPS exposure enhanced METH (0.5 mg/kg, s.c.)-induced locomotor behavioral response in P70 male rats (p<0.05) (Fig. 1C). In the female rats, both groups including neonatal exposure to saline or LPS showed increased total distance traveled from 10 min to 70 min [F(3, 319) = 8.12, p=0.002] (P<0.05) after a single dose injection of METH (Fig. 1B). However, no difference was found between subjects with neonatal exposure to LPS and their control counterparts after increased response to a single METH injection [F(1, 19) = 0.0844, p=0.775] (Fig. 1C).
Fig. 1.
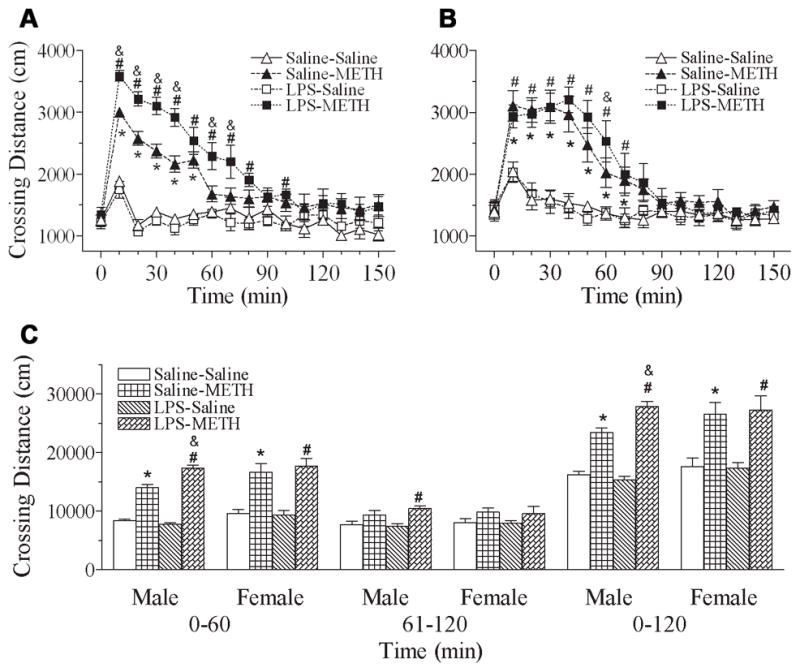
Effect of neonatal LPS-exposure on METH-induced locomotor activity, as determined by the total distance traveled in the open field test in the P70 male (A) and female (B) rats. A & B, average values for the distance traveled by the P70 rats after saline or METH (0.5 mg/kg) treatment. Each point represents the cumulative distance (cm) traveled in 10-min intervals for a total duration of 150 min. C, bar graphs represent the average values for the distance traveled by P70 rats in specific time intervals from 0 to 60 min, 61 to 120 min, and 0 to 120 min, after saline or METH (0.5 mg/kg) treatment. The results are expressed as the mean±SEM of five animals in each group and analyzed by two-way repeated measures ANOVA for data from tests conducted continuously at different experimental times or by two-way ANOVA for the behavioral response in the specific time intervals followed by Student-Newman-Keuls test. * P<0.05 represents significant difference for the saline+METH group as compared with that of the saline+saline group. # P<0.05 represents significant difference for the LPS+METH group as compared with that of the LPS+saline group. & P<0.05 represents significant difference for the LPS+METH group as compared with that of the saline+METH group.
Repeated administration of METH produced progressively increased behavioral response in rats (Fig. 2). The METH-induced behavioral sensitization occurred in the day showed significant greater than that of the results on day 1 (P70). Male subjects exposed as neonates to LPS showed that the METH induced behavioral sensitization in the total distance traveled occurred one day earlier (day 4, P73) than that of the control group (day 5, P74) [F(15, 119) = 43.346, p<0.001] (p<0.05) (Fig. 2B). The present results also demonstrated that there is a gender difference in the rate of development of locomotor sensitization to METH. For females, METH-induced behavioral sensitization was determined on day 2 (P71) [F(15, 119) = 36.950, p<0.001] (p<0.05) of both neonatal LPS exposure and the control groups (Fig. 2C).
Fig. 2.
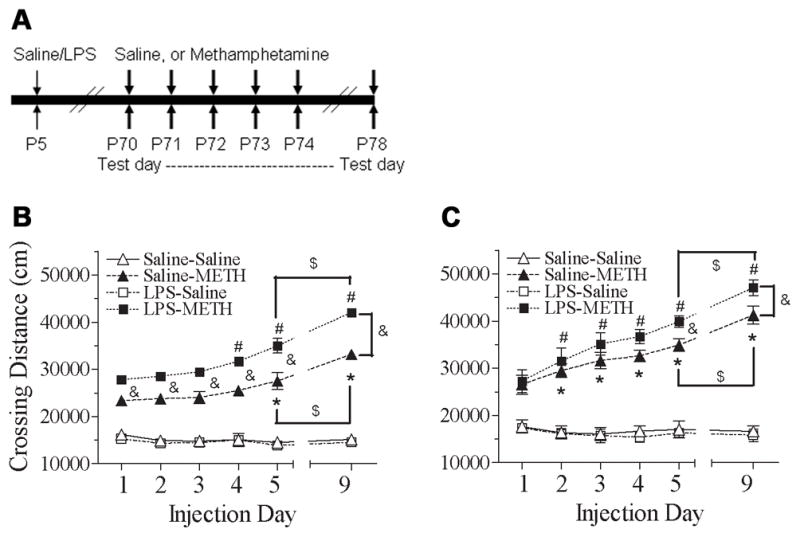
Effect of neonatal LPS-exposure on METH-induced behaviors sensitization (from P70 to P74) and reinstated behavioral sensitization (P78), as determined by the total distance traveled in the open field test in the male (B) and female (C) rats. A, treatment procedure. Repeated administration of METH in rats developed behavioral sensitization as indicated by the progressively increased behavioral response of the total distance traveled as compared with the data of day 1 (P70). After 5 injections from P70 to P74 (day 1 to day 5), animals received a single injection of saline or METH after ninety-six hour drug abstinence from the 5th injection of METH to assess the reinstated behavioral sensitization (P78, day 9). Each point represents the cumulative distance (cm) traveled for a total duration of 120 min on the different experimental day. The results are expressed as the mean±SEM of five animals in each group and analyzed by two-way repeated measures ANOVA for data from tests conducted continuously at different experimental day followed by Student-Newman-Keuls test. * P<0.05 represents significant difference for the experimental day as compared with the data of day 1 in the saline+METH group. # P<0.05 represents significant difference for the experimental day as compared with the data of day 1 in the LPS+METH group. & P<0.05 represents significant difference for the LPS+METH group as compared with that of the saline+METH group in the same experimental day. $ P<0.05 represents significant difference for the day 9 as compared with the data of day 5 in the saline+METH or LPS+METH group.
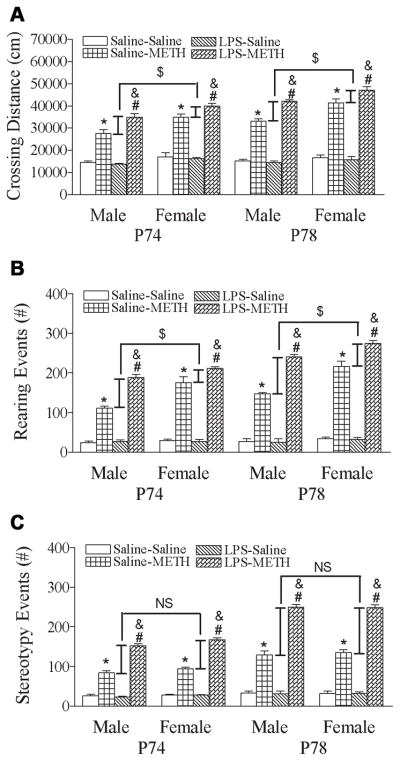
To test whether repeated METH administration produced a long-lasting locomotor sensitization, animals received a single dose of METH (0.5 mg/kg, s.c.) or saline on P78 (day 9) ninety-six hours after the 5th treatment with METH or saline (day 5, P74). Both neonatal saline and LPS-treated groups showed increased response to METH challenge on day 9 (P78) as compared with the response to METH on day 5 (P74) (p<0.05) (Figs. 2B & 2C). Neonatal LPS exposure also enhanced the reinstated behavioral sensitization in both male and female rats after administration had ceased for ninety-six hours (p<0.05) (Figs. 2B & 2C). However, neonatal LPS exposure induced alteration in the behavioral sensitization (P74) and reinstated behavioral sensitization (P78) of total distance to METH administration that appears to be greater in male rats as compared with that of female rats [P74: Δ Male (LPS+METH – Saline+METH) = 7467.7±862.3 cm, Δ Female (LPS+METH – Saline+METH) = 4971.2±490.6 cm; t(8) = 2.516, p<0.05] [P78: Δ Male (LPS+METH – Saline+METH) = 8881.4±562.5 cm, ΔFemale (LPS+METH – Saline+METH) = 5843.9±940.6 cm; t(8) = 2.771, p<0.05] (Fig. 7A).
Fig. 7.
Effect of neonatal LPS-exposure induced alteration in METH-induced behavioral sensitization (P74) and reinstated behavioral sensitization (P78), as determined by the total distance traveled (A), rearing responses (B), and stereotyped behaviors (C) in the male and female rats. The results are expressed as the mean±SEM of five animals in each group and analyzed by two-way ANOVA for the behavioral response followed by Student-Newman-Keuls test. * P<0.05 represents significant difference for the saline+METH group as compared with that of the saline+saline group. # P<0.05 represents significant difference for the LPS+METH group as compared with that of the LPS+saline group. & P<0.05 represents significant difference for the LPS+METH group as compared with the saline+METH group. The narrow bars represent the increment in LPS-exposure enhanced METH-induced behaviors responses in male or female rats. $ P<0.05 represents significant difference between the increment of distance traveled or rearing responses in LPS-exposure enhanced METH-increased behavioral responses in male rats as compared with that of the female rats. NS represents no significant difference between the increment of stereotyped behaviors in LPS-exposure enhanced METH-increased behavioral responses in male rats as compared with that of the female rats.
3.2. Neonatal LPS exposure enhanced methamphetamine-induced rearing behavioral response, behavioral sensitization, and reinstated behavioral sensitization in adult rats
The summation of exposure rearing and sniffing-air responses reflects vertical activity which has been used apart from locomotion (Antoniou et al., 2004). The upper panel of Fig. 3 shows the rearing events determined at the first minute of every 10-min intervals during 150 min after administration of METH or saline. In male rats, both groups including neonatal exposure to saline or LPS showed the increased rearing events from 20 min to 40 min, and from 10 min to 70 min, respectively [F(3, 319) = 33.238, p<0.001] (P<0.05) after a single dose injection of METH (P70) (Fig. 3A). Two-way ANOVA on behavioral data (summation for 120 min) following the METH challenge showed that adult (P70) METH treatment and the interaction of neonatal (P5) LPS treatment x adult (P70) METH treatment have significant effects on the rearing events [F(1, 19) = 86.056, p<0.001; F(1, 19) = 19.506, p<0.001, respectively, Fig. 3C]. The neonatal LPS exposure enhanced the METH-induced rearing events in P70 male rats (p<0.05) (Fig. 3C). In the female rats, both groups including neonatal exposure to saline or LPS showed increased rearing events from 20 min to 80 min [F(3, 319) = 10.890, p<0.001] (P<0.05) after a single dose injection of METH (Fig. 3B). However, no difference was found between subjects with neonatal exposure to LPS and their control counterparts after increased response to a single METH injection [F(1, 19) = 0.0468, p=0.831] (Fig. 3C).
Fig. 3.
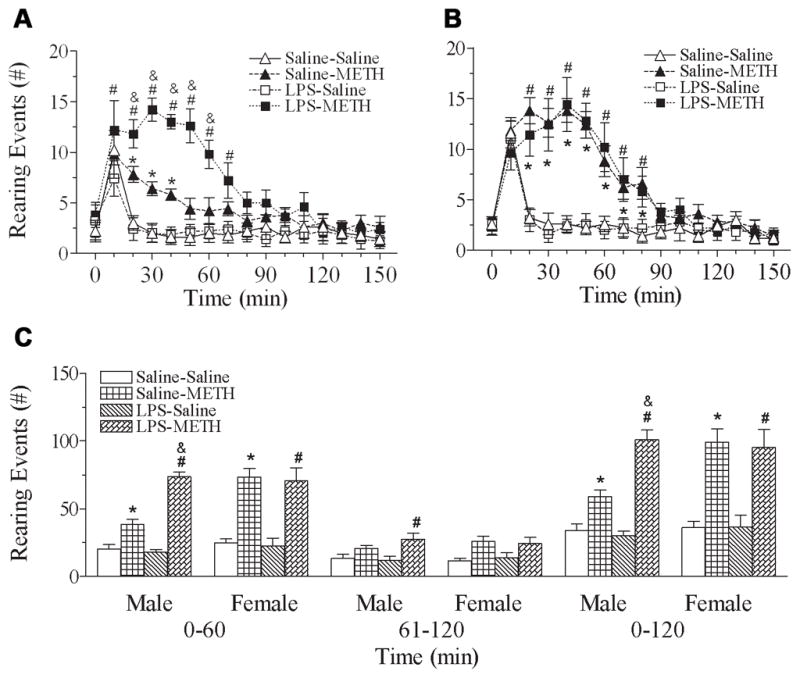
Effect of neonatal LPS-exposure on METH-induced rearing responses in the P70 male (A) and female (B) rats. A & B, average values for rearing responses by the P70 rats after saline or METH (0.5 mg/kg) treatment. Each point represents the cumulative rearing events determined at the first minute of every 10-min intervals during 150 min. C, bar graphs represent the average values for rearing events by P70 rats in specific time intervals from 0 to 60 min, 61 to 120 min, and 0 to 120 min, after saline or METH (0.5 mg/kg) treatment. The results are expressed as the mean±SEM of five animals in each group and analyzed by two-way repeated measures ANOVA for data from tests conducted continuously at different experimental times or by two-way ANOVA for the behavioral response in the specific time intervals followed by Student-Newman-Keuls test. * P<0.05 represents significant difference for the saline+METH group as compared with that of the saline+saline group. # P<0.05 represents significant difference for the LPS+METH group as compared with the LPS+saline group. & P<0.05 represents significant difference for the LPS+METH group as compared with the saline+METH group.
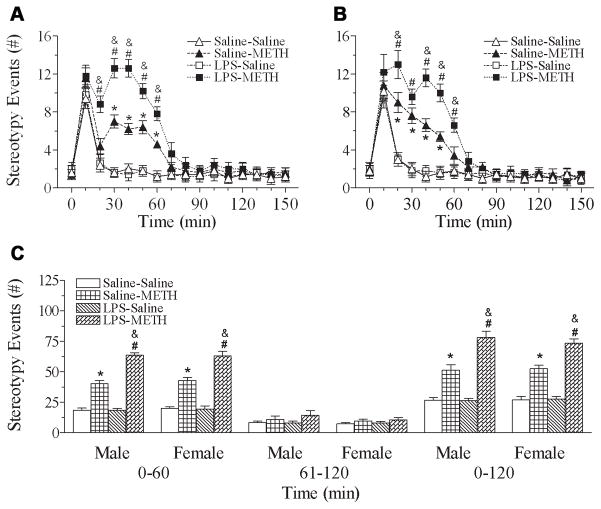
Fig. 4 shows that the repeated administration of METH in rats produced progressively increased rearing events. In both male and female rats, neonatal exposed to LPS showed that the METH induced behavioral sensitization in rearing events occurred on day 2 (P71) and the level was greater than that in the control group [male, F(5, 119) = 47.120, p<0.001; female, F(5, 119) = 57.347, p<0.001] (p<0.05) (Figs. 4A & 4B).
Fig. 4.
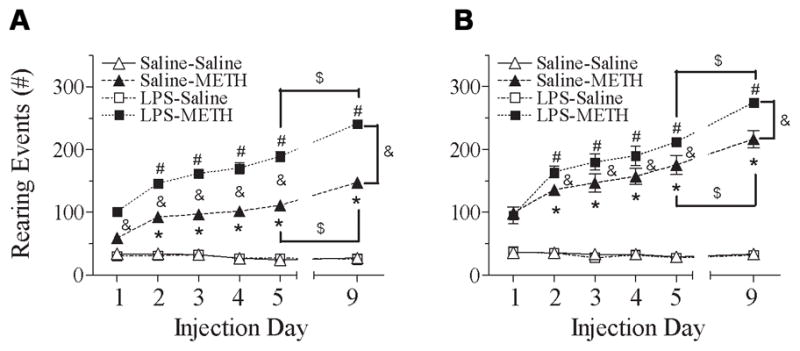
Effect of neonatal LPS-exposure on METH-induced behaviors sensitization (from P70 to P74) and reinstated behaviors sensitization (P78), as determined by rearing events in the male (A) and female (B) rats. Repeated administration of METH in rats developed behavioral sensitization as indicated by the progressively increased behavioral response of the rearing events as compared with the data of day 1 (P70). After 5 injections from P70 to P74 (day 1 to day 5), animals received a single injection of saline or METH after ninety-six hour drug withdrawal from the 5th injection of METH to assess the reinstated behaviors sensitization (P78, day 9). Each point represents the cumulative rearing events of 120 min (first minute of every 10-min intervals during 120 min) on the different experimental day. The results are expressed as the mean±SEM of five animals in each group and analyzed by two-way repeated measures ANOVA for data from tests conducted continuously at different experimental day followed by Student-Newman-Keuls test. * P<0.05 represents significant difference for the experimental day as compared with the data of day 1 in the saline+METH group. # P<0.05 represents significant difference for the experimental day as compared with the data of day 1 in the LPS+METH group. & P<0.05 represents significant difference for the LPS+METH group as compared with that of the saline+METH group in the same experimental day. $ P<0.05 represents significant difference for the day 9 as compared with the data of day 5 in the saline+METH or LPS+METH group.
The reinstated behavioral sensitization was induced by a single dose challenge of METH or saline after ninety-six hour drug abstinence following the 5th treatment with METH or saline (day 5, P74). Both neonatal saline and LPS-treated groups showed increased response to METH challenge on day 9 (P78) as compared with the response to METH on day 5 (P74) (p<0.05) (Fig. 4). Neonatal LPS exposure enhanced the reinstated behavioral sensitization in both male and female rats after administration had ceased for ninety-six hours (Figs. 4A & 4B) (p<0.05). However, neonatal LPS exposure induced alteration in the behavioral sensitization (P74) and reinstated behavioral sensitization (P78) of rearing events to METH administration that appears to be greater in male rats as compared with that of female rats [P74: ΔMale (LPS+METH – Saline+METH) = 77.80±4.47 events, ΔFemale (LPS+METH – Saline+METH) = 36.40±12.57 events; t(8) = 3.103, p<0.05] [P78: ΔMale (LPS+METH – Saline+METH) = 93.40±3.11 events, ΔFemale (LPS+METH – Saline+METH) = 58.40±8.08 events; t(8) = 4.044, p<0.05] (Fig. 7B).
3.3. Neonatal LPS exposure enhanced methamphetamine-induced stereotyped behavioral response, behavioral sensitization, and reinstated behavioral sensitization in adult rats
The rating of stereotyped behaviors including: standing, grooming, scratching, head-swinging, sniffing and freezing were determined at the first minute of every 10-min intervals during 150 min after METH or saline administration. In both male and female rats, both groups including neonatal exposure to saline or LPS showed increased stereotyped behaviors [male, F(3, 319) = 26.447, p<0.001; female, F(3, 319) = 58.616, p<0.001] (P<0.05) after a single dose injection of METH (P70) (Figs. 5A & 5B). Two-way ANOVA on behavioral data (summation for 120 min) following the METH challenge showed that adult (P70) METH treatment and the interaction of neonatal (P5) LPS treatment x adult (P70) METH treatment have significant effects on stereotyped behaviors [male, F(1, 19) = 104.207, p<0.001; F(1, 19) = 13.474, p=0.002, respectively, Fig. 5C] [female, F(1, 19) = 166.016, p<0.001; F(1, 19) = 14.010, p=0.002, respectively, Fig. 5C]. The neonatal LPS exposure enhanced the METH-induced stereotyped behaviors in P70 male and female rats (p<0.05) (Fig. 5C).
Fig. 5.
Effect of neonatal LPS-exposure on METH-induced stereotyped behaviors in the P70 male (A) and female (B) rats. A & B, average values for stereotyped behaviors by the P70 rats after saline or METH (0.5 mg/kg) treatment. Each point represents the cumulative stereotyped behaviors determined at the first minute of every 10-min intervals during 150 min. C, bar graphs represent the average values for stereotyped behaviors by P70 rats in specific time intervals from 0 to 60 min, 61 to 120 min, and 0 to 120 min, after saline or METH (0.5 mg/kg) treatment. The results are expressed as the mean±SEM of five animals in each group and analyzed by two-way repeated measures ANOVA for data from tests conducted continuously at different experimental times or by two-way ANOVA for the behavioral response in the specific time intervals followed by Student-Newman-Keuls test. * P<0.05 represents significant difference for the saline+METH group as compared with that of the saline+saline group. # P<0.05 represents significant difference for the LPS+METH group as compared with that of the LPS+saline group. & P<0.05 represents significant difference for the LPS+METH group as compared with that of the saline+METH group.
Fig. 6 shows that the repeated administration of METH in rats produced progressively increased stereotyped behaviors. In both male and female rats, neonatal rats exposed to LPS showed that the METH-induced behavioral sensitization in stereotyped behaviors occurred one day earlier (day 2, P71) than that of the control group (day 3, P72) [male, F(5, 119) = 112.327, p<0.001; female, F(5, 119) = 190.355, p<0.001] (p<0.05) (Figs. 6A & 6B).
Fig. 6.
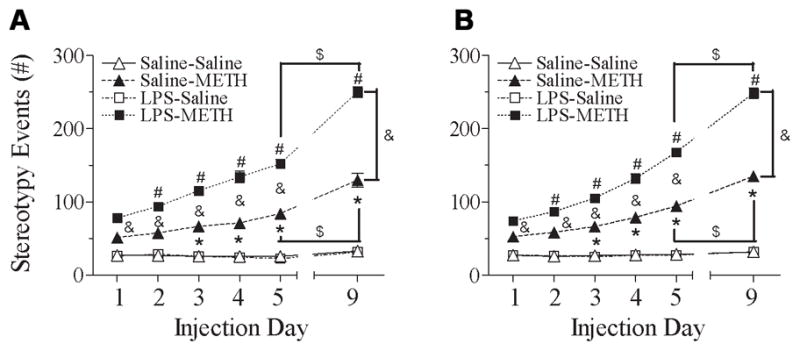
Effect of neonatal LPS-exposure on METH-induced behaviors sensitization (from P70 to P74) and reinstated behaviors sensitization (P78), as determined by stereotyped behaviors in the male (A) and female (B) rats. Repeated administration of METH in rats developed behavioral sensitization as indicated by the progressively increased behavioral response of the stereotyped behaviors as compared with the data of day 1 (P70). After 5 injections from P70 to P74 (day 1 to day 5), animals received a single injection of saline or METH after ninety-six hour drug withdrawal from the 5th injection of METH to assess the reinstated behavioral sensitization (P78, day 9). Each point represents the cumulative stereotyped behaviors of 120 min (first minute of every 10-min intervals during 120 min) on the different experimental day. The results are expressed as the mean±SEM of five animals in each group and analyzed by two-way repeated measures ANOVA for data from tests conducted continuously at different experimental day followed by Student-Newman-Keuls test. * P<0.05 represents significant difference for the experimental day as compared with the data of day 1 in the saline+METH group. # P<0.05 represents significant difference for the experimental day as compared with the data of day 1 in the LPS+METH group. & P<0.05 represents significant difference for the LPS+METH group as compared with that of the saline+METH group in the same experimental day. $ P<0.05 represents significant difference for the day 9 as compared with the data of day 5 in the saline+METH or LPS+METH group.
Both neonatal saline and LPS-treated groups showed increased stereotyped behaviors to METH challenge on day 9 (P78) as compared with the response to METH on day 5 (P74) (p<0.05) (Fig. 6). Neonatal LPS exposure enhanced the reinstated behavioral sensitization of stereotyped behaviors in both male and female rats after administration had ceased for ninety-six hours (Figs. 6A & 6B) (p<0.05). The neonatal LPS exposure induced alteration in the behavioral sensitization (P74) and reinstated behavioral sensitization (P78) of stereotyped behaviors to METH and showed no difference between male and female rats [P74: ΔMale (LPS+METH – Saline+METH) = 68.00±6.37 events, ΔFemale (LPS+METH – Saline+METH) = 73.60±2.58 events; t(8) = −0.815, p=0.439] [P78: ΔMale (LPS+METH – Saline+METH) = 120.20±4.33 events, ΔFemale (LPS+METH – Saline+METH) = 113.40±4.76 events; t(8) = 1.057, p=0.321] (Fig. 7C).
4. Discussion
Perinatal or early life infection/inflammation has been shown to increase the risk for central nervous system disorders in human (as indicated by epidemiological studies) and animal models of Parkinson’s disease, schizophrenia, autism and cerebral palsy [1, 20–22]. We have recently reported that exposure to LPS (P5, 1 mg/kg, intracerebral injection) in white matter during early development produced not only white matter injury around the ventricle areas [6, 24], but also dopaminergic neuronal injury and chronic inflammation in the adult rat brain [2]. We also found that there was a functional disability in juvenile rats, suggesting possible involvement of dopaminergic impairment in this neonatal rat model of central inflammation-induced neurological dysfunction [15, 23–24]. METH-induced locomotion is used to assess the dopaminergic function as a behavioral indicator of activity in the dopamine pathway [8–9, 25]. Consistent with our previous studies [2]; the P70 male rats with neonatal LPS exposure enhanced the METH (0.5 mg/kg, s.c.)-induced locomotor behavioral responses, including distances traveled and rearing events, and stereotyped behaviors as compared with those without LPS exposure (Figs. 1A, 3A & 5A). Similarly, the neonatal LPS exposure enhanced the METH (0.5 mg/kg, s.c.)-induced stereotyped behaviors in P70 female rats (Fig. 5B). Another study also reported that maternal exposure to LPS during pregnancy enhances amphetamine (a METH analog)-induced locomotion in adult rat offspring [3]. These results suggest that the dopaminergic system in the LPS-exposed rat brain is more sensitive to METH stimulation. The enhanced METH-induced reaction in the LPS-exposed animal observed in these studies suggests the existence of functional alterations in the dopaminergic system of the LPS-exposed rat brain.
To further investigate the effect of neonatal LPS exposure on the dopaminergic system, the long-lasting changes in the brain reward system were examined by METH behavioral sensitization. Behavioral sensitization is the progressive increase in the behavioral responses following repeated treatments of a drug, such as METH, and is mediated by long-lasting changes in the neural substrates mediating reward and motivation (Anderson and Pierce, 2005; Robinson and Berridge, 1993, 2008; Tenk et al., 2007). METH behavioral sensitization resulting from neuroadaptations within the mesolimbic dopamine system caused by repeated METH exposure can be used as an indicator of drug addiction [3–4, 11–13]. The present data indicate that neonatal LPS exposure enhanced the level of development of behavioral sensitization including distances traveled, rearing events and stereotypy to 5 treatments of METH administration (from P70 to P74) in both adult male and female rats. Neonatal LPS exposure enhanced reinstated behavioral sensitization in both adult male and female rats after METH administration had ceased for ninety-six hours (P78). These results show that neonatal brain LPS exposure produces a persistent lesion in the dopaminergic system and enhances METH-induced locomotor and stereotyped behavioral sensitization in later life. Therefore, these findings indicate that brain inflammation early in life leads to a long-lasting change in the brain reward system which may enhance susceptibility to the development of addictive-like behavior in later life.
In normal Sprague-Dawley rats, the METH and amphetamine induced behavioral responses are greater in adult female than male rats [26]. Female rats are more vulnerable to the acquisition of METH self-administration, and they are more motivated to self-administer METH as compared with that of male rats [27]. The current study shows that P70 female rats without LPS treatment display greater distance traveled and rearing events to a single dose injection of METH (0.5 mg/kg, s.c.) than that of male rats. P70 female rats without LPS treatment performed a greater behavioral sensitization and reinstated behavioral sensitization of distance traveled and rearing events to METH than that of male rats (Figs. 7A & 7B). However, the neonatal LPS exposure (P5) induced alteration in the behavioral sensitization and reinstated behavioral sensitization of distance traveled and rearing events to METH that appears to be greater in male than in female Sprague-Dawley rats (Figs. 7A & 7B). This result indicates that there are gender differences in early life LPS exposure-enhanced behavioral sensitization and reinstated behavioral sensitization to METH. The present finding also shows that female rats were more sensitive to METH sensitization in the absence of LPS pretreatment and neonatal exposure to LPS diminished this difference between male and female. The male METH users showed a longer craving period for METH than females in abstinent METH [14]. Craving for addictive drugs may predict relapse in abstinent addicts [14]. Therefore, the present rat model may be useful to investigate the addiction and relapse in abstinent addicts with the gender differences. The male METH users showed greater amphetamine-stimulated dopamine release than female, but the female METH users showed a decreased degree of toxicity [28]. The present rat model may be also useful to investigate the pattern of male versus female differences in the METH-induced toxicity in the doparminergic system. In contrast, another study indicated that systemical neonatal LPS (50 mg/kg) treatment (P3 and P5) potentiated both the level and the rate of development of locomotor sensitization to quinpirole (0.5 mg/kg, s.c., a dopamine D2/D3 agonist) administration in adult female Long-Evans rats, but not in males [4]. These conflicting finding may be because of rat strain differences (Sprague-Dawley rats or Long-Evans rats), route differences (central LPS exposure or systemically LPS exposure), treatment differences (METH, a psychostimulant; or quinpirole, a dopamine D2/D3 agonist), or other factors between this and other studies. Both the results from our current study and Tenk’s study may further implicate that neonatal LPS exposure causes dopaminergic injury including the long-lasting changes in the brain reward system with sex differences. However, further studies are necessary.
We have reported that neonatal LPS exposure produces dopaminergic injury and chronic inflammation in the adult rat brain [2]. Lesions of the dopaminergic system, such as dysregulation of presynaptic striatal dopamine, alteration in central dopamine turnover, or dopaminergic receptor sensitization, were evidenced by enhanced behavioral reaction after METH administration in the animals [3, 8–10, 25]. Therefore, the present results of enhanced behavioral sensitization and reinstated behavioral sensitization to METH indicate that exposure to LPS in the early developing brain may induce long-lasting dopaminergic injury in later life. Besides the dopaminergic injury, perinatal LPS exposure also causes a long-lasting increase in inflammatory cytokines including interleukin-1β (IL-1β) and IL-6 in the adult rat brain [2, 29]. Systemic treatment with IL-6 produces long-lasting sensitization to the locomotor-stimulating effects of amphetamine [30]. The prenatal inflammation alters the dopaminergic function to produce greater response to a single injection of amphetamine and enhanced behavioral sensitization following repeated exposure to amphetamine in the adult offspring rat [31]. Therefore, the above findings suggest that perinatal inflammation may have direct effects on the dopaminergic system mediating behavioral sensitization and the detailed mechanisms involved need to be further investigated.
Our study is the first investigation to examine the effect of neonatal LPS exposure on the persistent lesion in the dopaminergic system as determined by the METH induced behavioral sensitization in the adult rats. These results indicate that neonatal brain LPS exposure enhanced METH-induced locomotor and stereotyped behavioral sensitization in the adult rats. These findings show that early-life brain inflammation may enhance susceptibility to the development of drug addiction such as METH in later life. In addition, this study also implicates that there are gender differences in early life LPS exposure-enhanced behavioral sensitization to METH.
Research Highlights.
Neonatal LPS exposure enhanced the behavioral sensitization to METH in both male and female rats.
Neonatal LPS exposure enhanced the reinstated behavioral sensitization to METH in both male and female rats.
Neonatal LPS exposure induced alteration in the reinstated behaviors sensitization to METH is greater in male than in female rats.
Acknowledgments
This work was supported by funds from the NIH (NS 54278), Newborn Medicine Funds, a research grant from the Department of Pediatrics, UMC, Jackson, MS, USA, and NSC 99-2320-B-030-003-MY3 from National Science Council of Taiwan.
Footnotes
Conflict of Interest Statement: All authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun. 2010;24:881–897. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Fan LW, Tien LT, Zheng B, Pang Y, Lin RC, Simpson KL, Ma T, Rhodes PG, Cai Z. Dopaminergic neuronal injury in the adult rat brain following neonatal exposure to lipopolysaccharide and the silent neurotoxicity. Brain Behav Immun. 2011;25:286–297. doi: 10.1016/j.bbi.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fortier ME, Joober R, Luheshi GN, Boksa P. Maternal exposure to bacterial endotoxin during pregnancy enhances amphetamine-induced locomotion and startle responses in adult rat offspring. J Psychiatr Res. 2004;38:335–345. doi: 10.1016/j.jpsychires.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Tenk CM, Foley KA, Kavaliers M, Ossenkopp KP. Neonatal immune system activation with lipopolysaccharide enhances behavioural sensitization to the dopamine agonist, quinpirole, in adult female but not male rats. Brain Behav Immun. 2007;21:935–945. doi: 10.1016/j.bbi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Cai Z, Pang Y, Lin S, Rhodes PG. Differential roles of tumor necrosis factor-alpha and interleukin-1 beta in lipopolysaccharide-induced brain injury in the neonatal rat. Brain Res. 2003;975:37–47. doi: 10.1016/s0006-8993(03)02545-9. [DOI] [PubMed] [Google Scholar]
- 6.Fan LW, Pang Y, Lin S, Rhodes PG, Cai Z. Minocycline attenuates lipopolysaccharide-induced white matter injury in the neonatal rat brain. Neuroscience. 2005;133:159–168. doi: 10.1016/j.neuroscience.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Pang Y, Cai Z, Rhodes PG. Disturbance of oligodendrocyte development, hypomyelination and white matter injury in the neonatal rat brain after intracerebral injection of lipopolysaccharide. Brain Res Dev Brain Res. 2003;140:205–214. doi: 10.1016/s0165-3806(02)00606-5. [DOI] [PubMed] [Google Scholar]
- 8.Wakuda T, Matsuzaki H, Suzuki K, Iwata Y, Shinmura C, Suda S, Iwata K, Yamamoto S, Sugihara G, Tsuchiya KJ, et al. Perinatal asphyxia reduces dentate granule cells and exacerbates methamphetamine-induced hyperlocomotion in adulthood. PLoS One. 2008;3:e3648. doi: 10.1371/journal.pone.0003648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace TL, Gudelsky GA, Vorhees CV. Methamphetamine-induced neurotoxicity alters locomotor activity, stereotypic behavior, and stimulated dopamine release in the rat. J Neurosci. 1999;19:9141–9148. doi: 10.1523/JNEUROSCI.19-20-09141.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallace TL, Gudelsky GA, Vorhees CV. Neurotoxic regimen of methamphetamine produces evidence of behavioral sensitization in the rat. Synapse. 2001;39:1–7. doi: 10.1002/1098-2396(20010101)39:1<1::AID-SYN1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Anderson SM, Pierce RC. Cocaine-induced alterations in dopamine receptor signaling: implications for reinforcement and reinstatement. Pharmacol Ther. 2005;106:389–403. doi: 10.1016/j.pharmthera.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 13.Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galloway GP, Singleton EG, Buscemi R, Baggott MJ, Dickerhoof RM, Mendelson JE. An examination of drug craving over time in abstinent methamphetamine users. Am J Addict. 2010;19:510–514. doi: 10.1111/j.1521-0391.2010.00082.x. [DOI] [PubMed] [Google Scholar]
- 15.Fan LW, Pang Y, Lin S, Tien LT, Ma T, Rhodes PG, Cai Z. Minocycline reduces lipopolysaccharide-induced neurological dysfunction and brain injury in the neonatal rat. J Neurosci Res. 2005;82:71–82. doi: 10.1002/jnr.20623. [DOI] [PubMed] [Google Scholar]
- 16.Tien LT, Park Y, Fan LW, Ma T, Loh HH, Ho IK. Increased dopamine D2 receptor binding and enhanced apomorphine-induced locomotor activity in mu-opioid receptor knockout mice. Brain Res Bull. 2003;61:109–115. doi: 10.1016/s0361-9230(03)00077-7. [DOI] [PubMed] [Google Scholar]
- 17.Antoniou K, Papathanasiou G, Panagis G, Nomikos GG, Hyphantis T, Papadopoulou-Daifoti Z. Individual responses to novelty predict qualitative differences in d-amphetamine-induced open field but not reward-related behaviors in rats. Neuroscience. 2004;123:613–623. doi: 10.1016/j.neuroscience.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Antoniou K, Kafetzopoulos E, Papadopoulou-Daifoti Z, Hyphantis T, Marselos M. D-amphetamine, cocaine and caffeine: a comparative study of acute effects on locomotor activity and behavioural patterns in rats. Neurosci Biobehav Rev. 1998;23:189–196. doi: 10.1016/s0149-7634(98)00020-7. [DOI] [PubMed] [Google Scholar]
- 19.Gentry WB, Ghafoor AU, Wessinger WD, Laurenzana EM, Hendrickson HP, Owens SM. (+)-Methamphetamine-induced spontaneous behavior in rats depends on route of (+)METH administration. Pharmacol Biochem Behav. 2004;79:751–760. doi: 10.1016/j.pbb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Bakos J, Duncko R, Makatsori A, Pirnik Z, Kiss A, Jezova D. Prenatal immune challenge affects growth, behavior, and brain dopamine in offspring. Ann N Y Acad Sci. 2004;1018:281–287. doi: 10.1196/annals.1296.033. [DOI] [PubMed] [Google Scholar]
- 21.Breivik T, Stephan M, Brabant GE, Straub RH, Pabst R, von Horsten S. Postnatal lipopolysaccharide-induced illness predisposes to periodontal disease in adulthood. Brain Behav Immun. 2002;16:421–438. doi: 10.1006/brbi.2001.0642. [DOI] [PubMed] [Google Scholar]
- 22.Feleder C, Tseng KY, Calhoon GG, O’Donnell P. Neonatal intrahippocampal immune challenge alters dopamine modulation of prefrontal cortical interneurons in adult rats. Biol Psychiatry. 2010;67:386–392. doi: 10.1016/j.biopsych.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan LW, Mitchell HJ, Rhodes PG, Cai Z. Alpha-Phenyl-n-tert-butyl-nitrone attenuates lipopolysaccharide-induced neuronal injury in the neonatal rat brain. Neuroscience. 2008;151:737–744. doi: 10.1016/j.neuroscience.2007.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan LW, Mitchell HJ, Tien LT, Zheng B, Pang Y, Rhodes PG, Cai Z. alpha-Phenyl-n-tert-butyl-nitrone reduces lipopolysaccharide-induced white matter injury in the neonatal rat brain. Dev Neurobiol. 2008;68:365–378. doi: 10.1002/dneu.20591. [DOI] [PubMed] [Google Scholar]
- 25.Karila L, Weinstein A, Aubin HJ, Benyamina A, Reynaud M, Batki SL. Pharmacological approaches to methamphetamine dependence: a focused review. Br J Clin Pharmacol. 2010;69:578–592. doi: 10.1111/j.1365-2125.2010.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milesi-Halle A, McMillan DE, Laurenzana EM, Byrnes-Blake KA, Owens SM. Sex differences in (+)-amphetamine- and (+)-methamphetamine-induced behavioral response in male and female Sprague-Dawley rats. Pharmacol Biochem Behav. 2007;86:140–149. doi: 10.1016/j.pbb.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roth ME, Carroll ME. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology (Berl) 2004;172:443–449. doi: 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- 28.Dluzen DE, Liu B. Gender differences in methamphetamine use and responses: a review. Gend Med. 2008;5:24–35. doi: 10.1016/s1550-8579(08)80005-8. [DOI] [PubMed] [Google Scholar]
- 29.Borrell J, Vela JM, Arevalo-Martin A, Molina-Holgado E, Guaza C. Prenatal immune challenge disrupts sensorimotor gating in adult rats. Implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology. 2002;26:204–215. doi: 10.1016/S0893-133X(01)00360-8. [DOI] [PubMed] [Google Scholar]
- 30.Zalcman S, Savina I, Wise RA. Interleukin-6 increases sensitivity to the locomotor-stimulating effects of amphetamine in rats. Brain Res. 1999;847:276–283. doi: 10.1016/s0006-8993(99)02063-6. [DOI] [PubMed] [Google Scholar]
- 31.Aguilar-Valles A, Flores C, Luheshi GN. Prenatal inflammation-induced hypoferremia alters dopamine function in the adult offspring in rat: relevance for schizophrenia. PLoS One. 2010;5:e10967. doi: 10.1371/journal.pone.0010967. [DOI] [PMC free article] [PubMed] [Google Scholar]