Abstract
Background: Acne vulgaris affects individuals of all races and ethnicities. Understanding the safety and efficacy of topical agents benefits the practicing clinician when treating patients with skin of color. Purpose: To report observations in acne patients representing all six Fitzpatrick skin types based on a Phase 3 study that evaluated the efficacy and safety of a clindamycin phosphate 1.2% tretinoin 0.025% gel versus a clindamycin phosphate 1.2% gel alone. Methods: The two treatments were compared in a randomized, double-blind, multicenter, parallel, 12-week study employing a total of 2,010 patients with moderate-to-severe acne. Primary efficacy endpoints were 1) treatment success defined as percentage of patients who were clear or almost clear or achieved at least a 2-grade improvement in Evaluators Global Severity Scores at Week 12 and 2) percent change from baseline versus 12-week scores for noninflamed, inflamed, and total lesions. Results: The 12-week, 37.8-percent Evaluators Global Severity Scores treatment success for clindamycin phosphate 1.2% tretinoin 0.025% gel was greater than the 31.7 percent observed for clindamycin phosphate 1.2% gel alone (P = 0.002). Percent changes from baseline versus 12-week scores for noninflamed, inflamed, and total lesions obtained with clindamycin phosphate 1.2% tretinoin 0.025% gel (49.8, 60.9, and 54.5%, respectively) were significantly greater than those observed for clindamycin phosphate 1.2% gel alone (41.3, 54.8, and 46.9%, respectively); all comparisons P<0.001. Conclusion: Use of clindamycin phosphate 1.2% tretinoin 0.025% gel resulted in greater percent reductions of Evaluators Global Severity Scores treatment success scores and acne lesions in patients with all six Fitzpatrick skin types combined than clindamycin phosphate 1.2% gel alone. Both products were well tolerated, with no hypo- or hyperpigmentation noted. Side effects observed were similar to those previously reported for the individual ingredients.
Acne vulgaris is a common disorder of the skin that affects individuals of all races and ethnicities.1,2 The prevalence of acne in patients with light skin (Fitzpatrick skin types I–III) and darker skin (Fitzpatrick skin types IV–VI) appears to be similar.3,4 Treating patients with differing skin colors presents a challenge to clinicians, as patients may respond differently to topical therapy and experience distinct adverse events, such as the development of postinflammatory hypo- or hyper-pigmentation in patients with darker skin types.5,6
Treatment of acne vulgaris with clindamycin and tretinoin inhibits the growth of Propionibacterium acnes, normalizes desquamation, prevents follicular plugging, and may provide anti-inflammatory effects.7–10 A number of clinical trials support the collective therapeutic properties and safety of clindamycin and tretinoin in treating the various stages of acne.11–17
The aim of this paper is to report observations in acne patients with skin types that ranged from very light to very dark pigmented skin representing all six Fitzpatrick3 skin types from a Phase 3 study conducted in 2,010 patients that evaluated the efficacy and safety of a clindamycin phosphate 1.2% tretinoin 0.025% gel versus a clindamycin phosphate 1.2 % gel alone.
Methods
In this Phase 3, randomized, controlled, double-blind, multicenter study, clindamycin phosphate 1.2% tretinoin 0.025% gel (CLIN/RA gel [ZIANA® Gel, Medicis Pharmaceutical Company, Scottsdale, Arizona]) was compared to clindamycin phosphate 1.2% gel alone (hereafter referred to as clindamycin gel) for a period of 12 weeks. Following baseline measurements, patients were instructed to apply their assigned product to the face once a day at bedtime for the duration of the study. All lesion counts and safety exams were conducted by board-eligible or board-certified dermatologists.
Inclusion/exclusion criteria. Primary inclusion criteria included patients 1) who were male and female over 12 years of age and exhibited facial acne vulgaris; 2) with 20 to 50 inflammatory lesions (papules and pustules), 20 to 100 noninflammatory lesions (open and closed comedones), and no more than two nodules; 3) who exhibited an Evaluators Global Severity Score (EGSS) of moderate (3) or severe (4);18 4) who were willing to undergo the specified washout periods for topical antibiotics and other topical antibacterial drugs (2 weeks), facial anti-inflammatory agents and corticosteroids (4 weeks), retinoids, including retinol (4 weeks); and 5) who had undergone the specified washout periods of systemic medications that included corticosteroids and intramuscular injections (4 weeks), antibiotics (4 weeks), other systemic acne treatments (4 weeks), and systemic retinoids (6 months).
Exclusion criteria included those individuals who 1) had participated in a similar study within 30 days of enrollment or were participating in another research study; 2) exhibited any facial dermatological conditions that could hinder or obstruct clinical assessments; 3) needed to use another non-acne topical medication that could interfere with study treatment; 4) were pregnant, nursing, planning a pregnancy, or who became pregnant during the trial; and 5) did not conform to the topical or systemic washout criteria.
Patient screening took place 30 days prior to the baseline exam during which time demographic information (age, sex, race), medical history, previous acne medication, numbers of inflammatory and noninflammatory lesions, EGSS (Table 1), and Fitzpatrick skin type (Table 2) were determined.
Table 1.
The Evaluator's Global Severity Scale18
| SCORE | GRADE | DESCRIPTION |
|---|---|---|
| 0 | Clear | Normal, clear skin with no evidence of acne vulgaris |
| 1 | Almost clear | Rare noninflammatory lesions present, with rare noninflamed papules (papules must be resolving and may be hyperpigmented, though not pink-red) |
| 2 | Mild | Some noninflammatory lesions are present, with few inflammatory lesions (papules/pustules only; no nodulocystic lesions) |
| 3 | Moderate | Noninflammatory lesions predominate, with multiple inflammatory lesions evident: Several to many comedones and papules/pustules, and there may or may not be one small nodulocystic lesion |
| 4 | Severe | Inflammatory lesions are more apparent, many comedones and papules/pustules, there may or may not be a few nodulo-cystic lesions |
| 5 | Very Severe | Highly inflammatory lesions predominate, variable number of comedones, many papules/pustules and many nodulocystic lesions |
Table 2.
Fitzpatrick Skin Type Classification Scale3
| PHOTOTYPE | SUNBURN & TANNING HISTORY |
CONSTITUTIVE COLOR (UNEXPOSED BUTTOCK SKIN) |
|---|---|---|
| I | Burns easily, never tans | Ivory white |
| II | Burns easily, tans minimally, with difficulty | White |
| III | Burns moderately, tans moderately, and uniformly | White |
| IV | Burns minimally, tans moderately, and easily | Beige-olive, lightly tanned |
| V | Rarely burns, tans profusely | Moderate brown or tanned |
| VI | Never burns, tans profusely | Dark brown or black |
Efficacy. Treatment success was defined as either clear or almost clear (EGSS score = 0 or 1) or at least a 2-grade reduction from the baseline score. Primary efficacy analyses were conducted on the intent-to-treat (ITT) population as follows: The percentage of patients who were clear to almost clear at Week 12 or achieved at least 2 grades of improvement in the EGSS (treatment success) from baseline to Week 12 and percent change from baseline to Week 12 in noninflammatory, inflammatory, and total lesion counts. Secondary efficacy analyses of efficacy were conducted using the Per Protocol (PP) population in the same manner as in the primary analyses.
The ITT population included all randomized study participants who used the study drug. Data from these individuals was used for efficacy analyses regardless of whether they provided any post-baseline data. EGSS treatment success was considered a failure in the event that a patient did not meet the criteria for success or did not record a value at the visit. Lesion counts were carried forward from the last observation. A subset of the ITT population consisted of PP patients who completed the Week-12 evaluation and had no noteworthy protocol violations.
Efficacy assessments. The study protocol stipulated that, in a blinded fashion, CLIN/RA gel was to be considered superior in efficacy to clindamycin gel alone if statistical significance in the ITT population was achieved in 1) the analysis of 12-week versus baseline EGSS treatment success findings and 2) any two of the three analyses of percent changes from baseline at Week 12 for noninflammatory lesions, inflammatory lesions, or total lesions. Differences between treatments were tested using the Cochran-Mantel-Haenszel Row Mean Score Statistic, which was stratified by investigational center. Data and analyses from the PP population were used for secondary support. Time points, efficacy measurements, and comparisons between treatments for this population were the same as the primary analyses for the ITT population.
Safety assessments. Safety analyses were conducted with all randomized patients in both test groups at least once throughout the duration of the study. This included all ITT study participants except those who returned the study medication unopened and then discontinued study participation.
Cutaneous safety and tolerability evaluations, as well as adverse event (AE) monitoring, were conducted at each visit by the site dermatologist. Measurements of cutaneous safety were done by the investigator who used a graded (0–3) scale to assess facial scaling and erythema. The dermatologist also obtained measures of itching, burning, and stinging tolerability measurements from the patients using a 0 to 3 scale.
At each visit, beginning with the dosing visit, the investigator questioned each subject about AEs using an open question. Any AE, whether or not it was related to the study products, was recorded. Patients experiencing AEs were referred to the appropriate physician for diagnosis and treatment.
Statistical analyses. Prior to the start of the study, estimates of per-treatment group sample size were based on 1) approximations of dichotomized (both test group) EGSS treatment success and 2) percent changes in lesion counts in order to achieve a two-sided five-percent level of significance, at 90-percent statistical power for specified detectable clinical differences between CLIN/RA gel and clindamycin gel. The selection of sample size for the study was based on estimates of the effect sizes from previous Phase 3 studies of the investigational studies, using standard statistical methods for t tests and chi-square tests.19
The method of random permuted blocks (of patients) within strata (skin types) was used to ensure that for each stratum equal numbers of patients entered each treatment group as required. The randomization and stratification process was not intended to be used as factors in the inferential statistical evaluation of treatment efficacy.
Subgroup analyses of primary efficacy endpoints were conducted in the ITT populations by Fitzpatrick skin type, baseline EGSS value of 3 (moderate) or 4 (severe), gender, age, and ethnicity. Patients were divided into two groups by age: less than the median age of patients in the ITT population and greater than or equal to the median age of patients in the ITT population. Subset analyses were conducted on the variables, such as treatment success evaluation at Week 12 as well as by percent change from baseline in noninflammatory, inflammatory, and total lesions at Week 12. As stipulated in the study protocol, these analyses contained only descriptive statistics at Week 12 for the ITT population.
Ethics and internal review board. The study was carried out in compliance with the protocol, International Conference on Harmonisation, Good Clinical Practice, and applicable regulatory requirements. The protocol, all appropriate amendments to the protocol, and subject information that included a written informed consent, description of study drug, study procedures, and protocol adherence guidelines were reviewed and approved by an Institutional Review Board/Independent Ethics Committee. Written informed consent, in accordance with local clinical investigation regulations, was obtained prior to participation in the study.
Results
Patient disposition. The ITT population comprised 1,008 patients randomly assigned to the CLIN/RA gel group and 1,002 study participants assigned to the clindamycin gel arm. At the completion of the study, 859 patients (85.2%) remained in the CLIN/RA gel group and 838 (83.6%) remained in the clindamycin gel treatment group. Ninety-two patients (9.1%) were lost to follow up in the CLIN/RA gel group, and 108 (10.8%) were lost to follow up in the clindamycin gel arm. A patient disposition flow chart is provided in Table 3.
Table 3.
Patient disposition, number (%) of patients
| CLIN/RA GEL | CLINDAMYCIN GEL | TOTAL | |
|---|---|---|---|
| Safety evaluable patients | 1008 | 1002 | 2010 |
| ITT patients | 1008 | 1002 | 2010 |
| PP patients | 727 (72.1) | 718 (71.7) | 1445 (71.9) |
| NUMBER OF PATIENTS ATTENDING VISIT-N (%) | |||
| Screening (Visit 1) | 1008 (100) | 1002 (100) | 2010 (100) |
| Baseline (Week 0, Visit 2) | 1008 (100) | 1002 (100) | 2010 (100) |
| Week 2 (Visit 3) | 886 (87.9) | 866 (86.4) | 1752 (87.2) |
| Week 4 (Visit 4) | 835 (82.8) | 823 (82.1) | 1658 (82.5) |
| Week 8 (Visit 5) | 743 (73.7) | 732 (73.1) | 1475 (73.4) |
| Week 12 (Final Visit) | 790 (78.4) | 781 (77.9) | 1571 (78.2) |
| Patients completing the study | 859 (85.2) | 838 (83.6) | 1697 (84.4) |
| Patients withdrawn from the study | 149 (14.8) | 164 (16.4) | 313 (15.6) |
| REASON PATIENT DID NOT COMPLETE TREATMENT PHASE | |||
| Patient request | 17 (1.7) | 28 (2.8) | 45 (2.2) |
| Adverse event | 6 (0.6) | 2 (0.2) | 8 (0.4) |
| Protocol violation | 1 (0.1) | 0 (0.0) | 1 (0.05) |
| Withdrawal of consent | 27 (2.7) | 20 (2.0) | 47 (2.3) |
| Lost to follow up | 92 (9.1) | 108 (10.8) | 210 (10.0) |
| Noncompliance | 1 (0.1) | 3 (0.3) | 4 (0.2) |
| Other | 5 (0.5) | 3 (0.3) | 8 (0.4) |
- ITT
- intent-to-treat
- PP
- per protocol
Demographic information and baseline characteristics for study participants are summarized in Table 4. Mean ages for the CLIN/RA gel- and clindamycin gel-treated groups were 19.1 and 19.0 years, and the gender ratio of males and females was 51.1 to 45.4 percent and 48.9 to 54.6 percent, respectively.
Table 4.
Demographics and baseline characteristics: intent-to-treat population
| PARAMETER |
CLIN/RA GEL n=1008 |
CLINDAMYCIN GEL n=1002 |
|---|---|---|
| AGE (YEARS) | ||
| Mean Age ±SD | 19.1 ±7.5 | 19.0 ±7.0 |
| GENDER —n (%) | ||
| Male | 515 (51.1) | 455 (45.4) |
| Female–n (%) | 493 (48.9) | 547 (54.6) |
| RACE –n (%) | ||
| Caucasian | 765 (75.9) | 758 (75.6) |
| African American | 102 (10.1) | 97 (9.7) |
| Hispanic/Latino | 100 (9.9) | 103 (10.3) |
| American Indian/Alaskan | 1 (0.1) | 5 (0.5) |
| Asian/Pacific Islander | 25 (2.5) | 28 (2.8) |
| Other | 15 (1.5) | 11 (1.1) |
| FITZPATRICK SKIN TYPE —n (%) | ||
| Type I | 47 (4.7) | 47 (4.7) |
| Type II | 214 (21.2) | 214 (21.4) |
| Type III | 341 (33.8) | 339 (33.8) |
| Type IV | 231 (22.9) | 230 (23.0) |
| Type V | 106 (10.5) | 103 (10.3) |
| Type VI | 69 (6.8) | 69 (6.9) |
| BASELINE GLOBAL SEVERITY SCORE —n (%) | ||
| 3 (moderate) | 753 (74.7) | 747 (74.6) |
| 4 (severe) | 255 (25.3) | 255 (25.4) |
| BASELINE ABSOLUTE MEAN LESION COUNTS ± SD | ||
| Noninflammatory lesions | 49.0 ±20.7 | 48.9 ± 21.1 |
| Inflammatory lesions | 30.6 ±8.3 | 30.9 ±8.7 |
| Total lesions | 79.6 ± 24.1 | 79.8 ± 25.1 |
The distribution of ethnicity in both treatment groups was similar in the two groups. Caucasian patients totaled 765 (75.9%) and non-Caucasian patients 243 (24.1%) in the CLIN/RA gel population, whereas the distribution of ethnicity in the clindamycin gel arm comprised 758 (75.6%) Caucasian and 244 (24.4%) non-Caucasian patients.
Both treatment groups used similar amounts of once-daily test product, with a total of 79 mean applications for CLIN/RA gel and 80 mean uses for clindamycin gel recorded.
Efficacy. Analyses of the ITT population's 12-week EGSS treatment success scores, as well as the mean percent reductions in noninflammatory, inflammatory, and total lesion counts, are summarized in Table 5 and Figure 1. The 12-week EGSS treatment success findings demonstrated that the percentage of patients who achieved a clear or almost clear score, or at least a 2-grade improvement in EGSS, was significantly greater in the CLIN/RA gel-treated group than in the clindamycin gel-treated group (37.8 versus 31.7%; P = 0.002).
Table 5.
Primary efficacy endpoints: Intent-to-treat population —CLIN/RA gel versus clindamycin gel alone
| EFFICACY PARAMETER |
CLIN/RA GEL n=1008 |
CLINDAMYCIN GEL n=1002 |
PVALUE* |
|---|---|---|---|
| Dichotomized EGSS† percent success | 37.8 | 31.7 | 0.002 |
| PERCENT CHANGE FROM BASELINE TO WEEK 12 | |||
| Noninflammatory lesion count mean ± SD | 49.8 ±37.1 | 41.3 ±38.6 | <0.001 |
| Inflammatory lesion count mean ± SD | 60.9 ± 35.8 | 54.8 ± 38.0 | <0.001 |
| Total lesions mean ± SD | 54.5 ±31.6 | 46.9 ±32.9 | <0.001 |
P values are based on a two-sided 5% test of the Cochran-Mantel-Haenszel Row Mean Score Statistic, adjusting for investigational center.
EGSS success was defined as clear or almost clear, or a 2-grade improvement from baseline.
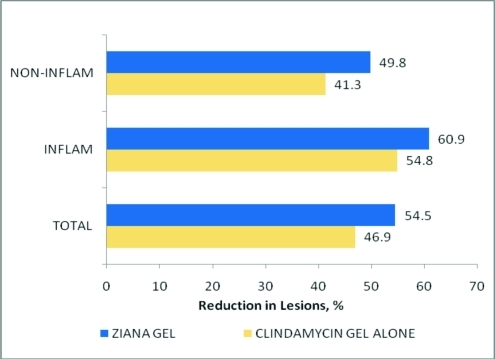
Figure 1.
Mean percent acne lesion reductions following treatment with clindamycin phosphate 1.2% tretinoin 0.025% gel or clindamycin phosphate 1.2 % gel alone: Intent-to-treat population, all six Fitzpatrick groups combined. All clindamycin phosphate 1.2% tretinoin 0.025% gel percent reductions were greater than clindamycin gel alone; P<0.001
After 12 weeks of treatment, the mean percent reductions in lesion counts in noninflammatory, inflammatory, and total lesions for the ITT population were 49.8, 60.9, and 54.5 percent, respectively, in the CLIN/RA gel-treated group and 41.3, 54.8, and 46.9 percent, respectively, in the clindamycin gel-treated group. In all instances, differences between the two treatments favored CLIN/RA gel with all comparisons being statistically significant (P<0.001).
When these same comparisons were made in the PP population, the EGSS treatment success scores, and the mean 12-week percent reductions from baseline in noninflammatory, inflammatory, and total lesion counts observed for CLIN/RA gel, were similar to those seen for the ITT population; all were statistically significantly in favor of the combination treatment when compared to clindamycin gel alone (P<0.001).
Subset analyses by Fitzpatrick skin type. The EGSS treatment score comparisons for skin types III and VI favored CLIN/RA (38.1 vs. 30.7% and 44.9 vs. 31.9%, respectively; P≤0.045). In patient subgroups with Fitzpatrick skin types I, II, IV, and V, there were no statistical differences in EGSSs for CLIN/RA (range: 30.8–41.6%) versus EGSSs obtained for clindamycin gel alone (range: 29.9–34.0%). These results are summarized in Table 6.
Table 6.
Analysis of primary efficacy endpoints by Fitzpatrick skin type in the intent-to-treat population: Twelve-week-percent improvement from Baseline in EGSS and mean lesion counts
|
FITZPATRICK SKIN TYPE |
TREATMENT |
NUMBER OF PATIENTS |
EGGS SUCCESSa |
NONINFLAMMATORY LESIONS |
INFLAMMATORY LESIONS |
TOTAL LESIONS |
|---|---|---|---|---|---|---|
| I | CLIN/RA | N=47 | 16 (34.0)b | 50.4 ± 44.9c | 67.1 ± 24.5 | 58.4 ± 31.0 |
| Clin | N=47 | 16 (34.0) | 47.8 ± 32.7 | 54.2 ± 35.7 | 51.1 ± 28.9 | |
| P valued | 0.771 | 0.938 | 0.079 | 0.436 | ||
| II | CLIN/RA | N=214 | 66 (30.8) | 49.1 ± 36.6 | 56.7 ± 37.0 | 52.3 ±32.1 |
| Clin | N=214 | 64 (29.9) | 36.9 ± 44.9 | 49.1 ± 42.1 | 42.2 ± 36.4 | |
| P value | 0.994 | 0.010 | 0.170 | 0.015 | ||
| III | CLIN/RA | N=341 | 130 (38.1) | 48.8 ± 37.2 | 59.0 ± 38.8 | 53.2 ± 32.2 |
| Clin | N=339 | 104 (30.7) | 44.9 ± 36.4 | 56.1 ± 35.4 | 49.8 ± 30.6 | |
| P value | 0.031 | 0.118 | 0.173 | 0.087 | ||
| IV | CLIN/RA | N=231 | 96 (41.6) | 52.5 ± 34.6 | 63.0 ± 34.2 | 57.1 ± 30.8 |
| Clin | N=230 | 78 (33.9) | 40.6 ± 39.3 | 55.9 ± 39.3 | 46.8 ± 34.5 | |
| P value | 0.145 | 0.001 | 0.101 | 0.003 | ||
| V | CLIN/RA | N=106 | 42 (39.6) | 50.7 ± 41.9 | 65.6 ± 30.3 | 56.8 ± 31.4 |
| Clin | N=103 | 34 (33.0) | 36.8 ± 34.4 | 59.1 ± 30.5 | 45.6 ± 29.5 | |
| P value | 0.370 | 0.004 | 0.076 | 0.004 | ||
| VI | CLIN/RA | N=69 | 31 (44.9) | 46.9 ± 33.7 | 64.7 ± 34.6 | 53.0 ± 30.5 |
| Clin | N=69 | 22 (31.9) | 41.3 ±34.3 | 56.2 ± 43.5 | 46.5 ± 34.0 | |
| P value | 0.045 | 0.174 | 0.154 | 0.147 |
- EGSS
- evaluator's global severity score
- CLIN/RA gel
- clindamycin phosphate 1.2% tretinoin 0.025%
- Clin
- clindamycin gel
EGSS success was defined as clear or almost clear, or a 2-grade improvement from baseline.
Number of subjects (percent success)
Percent ± standard deviation
P values are based on a 2-sided 5% test of the Cochran-Mantel-Haenszel Row Mean Score Statistic, adjusting for investigational center.
The mean percent decrease in lesion counts was greater in the CLIN/RA group (range: 46.9–67.1%) than in the clindamycin gel treatment group (range: 36.8–59.1%) for all Fitzpatrick skin types. Individually, 1) all comparisons by skin type for inflammatory lesions were not statistically significant, 2) for noninflammatory lesions, comparisons for skin types II, IV, and V favored CLIN/RA (P≤0.010), and 3) for total lesions, treatment differences were statistically significant in favor of CLIN/RA for skin types II, IV, and V (P≤0.015). These results are summarized in Table 6.
Subset analyses by baseline EGSS, gender, age, and ethnicity. Differences in EGSS demonstrated statistical significance in favor of CLIN/RA gel in populations with a baseline EGSS value of 3 (moderate) or 4 (severe), female patients, patients equal to or older than the median age (16.4 years), and non-Caucasian patients (P<0.041 to P = 0.001).
Statistically significant differences were observed for CLIN/RA gel in the mean percent reduction in noninflammatory, inflammatory, and total lesion counts in both baseline severity subpopulations, males and females, older and younger patients, and Caucasian and non-Caucasian patients (P =0.055 to P<0.001).
Safety. The CLIN/RA gel and the clindamycin gel only treatment were well tolerated. Patients applied product for the first time at the baseline visit. Baseline investigator assessments of scaling and erythema were 15.7 and 40.6 percent, respectively, for CLIN/RA gel-treated patients; corresponding values for the clindamycin gel arm were similar at 15.4 and 38.2 percent, respectively. Patient evaluations for itching, burning, and stinging at baseline were 13.7, 3.2, and 2.4 percent, respectively, for patients using the combination drug. Corresponding scores for patients in the clindamycin gel group were similar and were 14.1, 2.7, and 2.5 percent, respectively.
At Week 12, investigator evaluation of scaling and erythema observed in the CLIN/RA gel-treated group were 20.5 and 28.9 percent, respectively. Corresponding scores noted for the clindamycin gel treatment group were 11.3 percent for scaling and 29.0 percent for erythema.
Patient evaluations of itching, stinging, and burning at 12 weeks following CLIN/RA gel use were 5.3, 3.9, and 1.6 percent. Patient reports in the clindamycin gel group were 4.5, 0.7, and 0.7 percent.
During the course of the study, 270 (26.8%) patients in the CLIN/RA gel group and 236 (23.6%) patients in the clindamycin gel arm reported one or more AEs. Based upon investigator assessments, 42 (4.2%) patients in the CLIN/RA gel group and 17 (1.7%) patients in the clindamycin gel group experienced AEs that were related to treatment. Of these treatment-associated AEs related to skin and subcutaneous tissue reactions, with dry skin being the most frequent, 34 events were reported in patients in the CLIN/RA gel group and 14 AEs were reported in patients in the clindamycin gel arm. By study's end, 7 (0.7%) and 4 (0.4%) patients discontinued after using CLIN/RA gel and clindamycin gel, respectively. Ninety-nine percent of the AEs reported for each of the two treatment groups were mild to moderate. There were no reports of hypo- or hyperpigmentation for any of the patients treated with either test product. AEs are summarized in Tables 7 and 8.
Table 7.
Summary of adverse event incidence after 12 weeks of treatment: Number (%) of safety evaluable patients
| PARAMETER |
CLIN/RA GEL n=1008 |
CLINDAMYCIN GEL n=1002 |
|---|---|---|
| Patients with at least one AE | 270 (26.8) | 236 (23.6) |
| Total number of AEs | 409 | 387 |
| Patients with at least one serious AE | 2 (0.2) | 3 (0.3) |
| Patients with at least one severe AE | 4 (0.4) | 9 (0.9) |
| Patients with at least one treatment-related AE | 42 (4.2) | 17 (1.7) |
| Patients discontinued due to an AE | 7 (0.7) | 4 (0.4) |
- AE
- adverse event
Table 8.
Number (%) of patients with treatment-related, treatment-emergent adverse events during the treatment phase by system organ class and preferred term*
| PARAMETER |
CLIN/RA GEL n=1008 |
CLINDAMYCIN GEL n=1002 |
|---|---|---|
| At least one treatment-related-emergent adverse event | 42 (4.2) | 12 (1.7) |
| Skin and subcutaneous tissue disorders | 34 (3.4) | 14 (1.4) |
| • Dry skin | 23 (2.3) | 6 (0.6) |
| • Rash scaly | 7 (0.7) | 1 (0.1) |
| • Skin burning sensation | 4 (0.4) | 1 (0.1) |
| • Erythema | 4 (0.4) | 0 |
| • Pruritus | 3 (0.3) | 1 (0.1) |
| • Skin exfoliation | 3 (0.3) | 0 |
| • Rash | 2 (0.2) | 0 |
| • Skin tightness | 0 | 2 (0.2) |
| General disorders and administration site conditions | 7 (0.7) | 0 |
| • Application site reaction | 3 (0.3) | 0 |
| • Pain | 2 (0.2) | 0 |
At least two patients in either treatment group
Discussion
This controlled, Phase 3, randomized, double-blind, parallel, multicenter, 12-week study in patients with EGSSs of moderate-to-severe and multiple noninflammatory and inflammatory lesions at baseline, compared the once-daily use of a topical gel containing the combination drug clindamycin phosphate 1.2% tretinoin 0.025% to the once-daily use of a clindamycin phosphate 1.2% gel for the treatment of acne.
After 12 weeks of treatment, both products were found to be effective in reducing noninflammatory and inflammatory lesion counts in patients representing all six Fitzpatrick skin types combined. Analysis of 12-week EGSSs in this population demonstrated that the percentage of patients with clear (0) or almost clear (1) ratings, or those showing at least a 2-grade improvement in EGSS, were statistically greater for the CLIN/RA gel group compared to the clindamycin gel alone arm (P = 0.002). Percent reduction scores for noninflammatory, inflammatory, and total lesions were statistically greater for the CLIN/RA gel product versus clindamycin gel alone in an analysis where all Fitzpatrick skin types were combined (all comparisons P<0.001).
This report also provides an analysis of the study population based on subgroups by Fitzpatrick skin type, baseline EGSS of 3 (moderate) or 4 (severe), gender, age, and race (Caucasian and non-Caucasian). Regarding individual Fitzpatrick skin types, EGSS treatment score comparisons for types III and VI favored CLIN/RA gel over clindamycin gel alone (P≤0.045); all other comparisons were not statistically significant. When each of the six Fitzpatrick skin type groups were compared by treatment for mean percent reductions in lesion counts, the analyses demonstrated the following: 1) no statistically significant differences were noted for inflammatory lesions, 2) three comparisons for skin types II, IV, and V were P≤0.010 for noninflammatory lesions in favor of CLIN/RA gel, and 3) for total lesions, three comparisons for skin types II, IV, and V were P≤0.015, again in favor of the combination product. Overall, out of a total of 24 possible Fitzpatrick skin type comparisons between products, eight were P<0.001 to P<0.045 and favored CLIN/RA gel. The remaining comparisons were not statistically significant.
Statistically significant treatment differences also emerged in subpopulations with a baseline EGSS value of 3 (moderate) or 4 (severe), female patients, patients older than the median age (16.4 years), and non-Caucasian patients. Differences in the mean percent reduction in lesion count were noted in both EGGS severity groups, males and females, older and younger patients, and Caucasian and non-Caucasian patients. All other comparisons were not statistically significant.
Tolerability of a single, daily application of both test products was favorable over the 12-week test period. Investigator-based evaluations showed that patients in the CLIN/RA gel group did exhibit more scaling and dryness than participants in the clindamycin gel only arm, likely reflecting the pharmacological activity of tretinoin. Interestingly, the erythema scores for both groups were similar. When study participants evaluated itching, stinging, and burning 12 weeks following treatment, those applying CLIN/RA gel recorded similar scores to corresponding patients using clindamycin gel. There were no reports of hypo- or hyper-pigmentation for the combination gel. Overall, the side effects noted for both products were similar with those previously reported for each of the individual ingredients.
Topical clindamycin has been used over the years due to its ability to reduce levels of P. acnes. 7 Additionally, tretinoin has been widely prescribed for its well-know comedolytic effects.9 Recent review papers suggest that these two drugs also possess a broad range of potential anti-inflammatory properties.8,10 A topical drug that contains both clindamycin and tretinoin appears to possess additive features that can be of valuable therapeutic benefit when treating various stages of acne vulgaris.11–17 The current large-scale clinical trial described herein supports this body of evidence by demonstrating effective reduction in both noninflammatory and inflammatory acne lesions for CLIN/RA gel in a large group of individuals containing all six Fitzpatrick skin types combined. It has also been reported that 1) the combination of clindamycin and tretinoin in the same topical acne treatment may be more therapeutically effective than either drug used alone, 2) clindamycin appears to enhance the comedolytic activity of tretinoin through its ability to loosen and prevent follicular impactions, and 3) the comedolytic property of tretinoin may provide greater accessibility and penetration of clindamycin into the follicular environment, possibly leading to less bacterial resistance.9,20–32
Acne affects individuals of all races and ethnicities. The overall goal of acne management in patients is to select an effective treatment with minimal side effects. This is also important in darker patients who may develop post-inflammatory hypo- or hyperpigmentation, as a result of acne itself or because of treatment. This Phase 3 study included a substantial number of acne patients in each of the six pigment groups representing a diverse population, from very light to very dark pigmented skin, and demonstrated that the use of a combination gel containing clindamycin and tretinoin provided effective anti-acne treatment with minimal side effects.
Conclusion
This Phase 3 study demonstrated that the use of a combination gel containing clindamycin phosphate 1.2% tretinoin 0.025% resulted in greater percent reductions of EGSS treatment success scores and acne lesions in patients with all six Fitzpatrick skin types combined than a clindamycin phosphate 1.2% gel alone. Both products were well tolerated.
References
- 1.White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. 1998;39:S34–S37. doi: 10.1016/s0190-9622(98)70442-6. [DOI] [PubMed] [Google Scholar]
- 2.Davis EC, Callender VD. A review of acne in ethnic skin Pathogenesis, clinical manifestations and management strategies. J Clin Aesthetic Dermatol. 2010;3(4):24–38. [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzpatrick TB. Soleil et peau. J Med Esthet. 1975;2:33–34. [Google Scholar]
- 4.Halder RM, Grimes PE, McLaurin CI, et al. Incidence of common dermatoses in a predominantly black dermatologic practice. Cutis. 1983;32:388–390. [PubMed] [Google Scholar]
- 5.Bulengo-Ransby SM, Griffiths CEM, Kimbrough-Green CK, et al. Topical tretinoin (retinoic acid) therapy for hyperpigmented lesions caused by inflammation of the skin in black patients. N Engl J Med. 1993;328:1438–1443. doi: 10.1056/NEJM199305203282002. [DOI] [PubMed] [Google Scholar]
- 6.Kenny JA., Jr. Pigmentary disorders in black skin. Clin Dermatol. 1989;7(2):1–10. doi: 10.1016/0738-081x(89)90052-7. [DOI] [PubMed] [Google Scholar]
- 7.Guay DR. Topical clindamycin in the management of acne vulgaris. Expert Opin Pharacother. 2007;8(18):2625–2664. doi: 10.1517/14656566.8.15.2625. [DOI] [PubMed] [Google Scholar]
- 8.Del Rosso JQ, Schmidt NF. A review of the anti-inflammatory properties of clindamycin in the treatment of acne vulgaris. Cutis. 2010;85:15–24. [PubMed] [Google Scholar]
- 9.Lavker RM, Leyden JJ. An ultrastructural study of the effects of topical tretinoin on microcomedomes. Clinical Therapeutics. 1992;14(60):773–780. [PubMed] [Google Scholar]
- 10.Schmidt N, Gans EH. Tretinoin: a review of its anti-inflammatory properties in the treatment of acne. J Clin Aesthet Dermatol. In press. [PMC free article] [PubMed] [Google Scholar]
- 11.Richter JR, Forstrom LR, Kiistala UO, et al. Efficacy of the fixed 1.2% clindamycin phosphate, 0.025% tretinoin gel formulation (Velac) and a proprietary 0.025% tretinoin gel formulation (Aberela) in the topical control of facial acne. J Eur Acad Venereol. 1998;11:227–233. [PubMed] [Google Scholar]
- 12.Zouboulis CC, Derumeaux L, Decroix J, et al. A multicentre, single blind, randomized comparison of a fixed clindamycin phosphate/tretinoin gel formation (Velac) applied once daily and a clindamycin lotion formulation (Dalacin T) applied twice daily in the topical treatment of acne vulgaris. Br J Dermatol. 2000;143:498–505. doi: 10.1111/j.1365-2133.2000.03701.x. [DOI] [PubMed] [Google Scholar]
- 13.Schlessinger J, Menter A, Gold M, et al. Clinical safety and efficacy studies of a novel formulation combining 1.2% clindamycin phosphate and 0.025% tretinoin for the treatment of acne vulgaris. J Drugs Dermatol. 2007;6(6):607–615. [PubMed] [Google Scholar]
- 14.Rietschel R, Duncan BS. Clindamycin phosphate used in combination with tretinoin in the treatment of acne. Int J Dermatol. 1983;22:42–43. doi: 10.1111/j.1365-4362.1983.tb02113.x. [DOI] [PubMed] [Google Scholar]
- 15.Richter JRA, Bonsema MT, De Boulle KLVM, et al. Efficacy of a fixed clindamycin phosphate 1.2% tretinoin 0.025% gel formulation (Velac) in the topical control of facial acne lesions. J Dermatol Treat. 1998;9:81–90. [Google Scholar]
- 16.Leyden JJ, Krochmal L, Yaroshinsky A. Two randomized, double-blind, controlled trials of 2219 subjects to compare the combination clindamycin/tretinoin hydrogel with each agent alone and vehicle for the treatment of acne vulgaris. J Amer Acad Dermatol. 2006;54:73–81. doi: 10.1016/j.jaad.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 17.Yaroshinsky A, Leyden JJ. The safety and efficacy of clindamycin (1%), as clindamycin phosphate and tretinoin (0.025%) for the treatment of acne vulgaris: a combined analysis of results from six controlled safety and efficacy trials conducted in Europe [abstract] J Amer Acad Dermatol. 2004;50:P23. [Google Scholar]
- 18.The Evaluator's Global Severity Score (proposed at the Division of Dermatology Advisory Committee [DODAC] meeting of November 4 and 5, 2002).. [Google Scholar]
- 19.Scottsdale, Arizona: Medicis Pharmaceutical Corporation; 2006. Data on File. [Google Scholar]
- 20.Richter JR, Forstrom LR, Kiistala UO, et al. Efficacy of the fixed 1.2% clindamycin phosphate, 0.025% tretinoin gel formulation (Velac) and a proprietary 0.025% tretinoin gel formulation (Aberela) in the topical control of facial acne. J Eur Acad Venereol. 1998;11:227–233. [PubMed] [Google Scholar]
- 21.Zouboulis CC, Derumeaux L, Decroix J, et al. A multicentre, single blind, randomized comparison of a fixed clindamycin phosphate/tretinoin gel formulation (Velac) applied once daily and a clindamycin lotion formulation (Dalacin T) applied twice daily in the topical treatment of acne vulgaris. Br J Dermatol. 2000;143:498–505. doi: 10.1111/j.1365-2133.2000.03701.x. [DOI] [PubMed] [Google Scholar]
- 22.Leyden JJ. Open-label evaluation of topical antimicrobial and anti-acne preparations for effectiveness versus Propionibacterium acnes in vivo. Cutis. 1992;49:8–11. [Google Scholar]
- 23.Berson DS, Shalita AR. The treatment of acne: the role of combination therapies. J Am Acad Dermatol. 1995;32:S31–S41. doi: 10.1016/0190-9622(95)90418-2. [DOI] [PubMed] [Google Scholar]
- 24.Rietschel R, Duncan BS. Clindamycin phosphate used in combination with tretinoin in the treatment of acne. Int J Dermatol. 1983;22:42–43. doi: 10.1111/j.1365-4362.1983.tb02113.x. [DOI] [PubMed] [Google Scholar]
- 25.Abdel-Nasser MB, Zouboulis CC. Clindamycin phosphate/ tretinoin gel formulation in the treatment of acne vulgaris. Expert Opinion Pharmacother. 2008;9(16):2931–2937. doi: 10.1517/14656566.9.16.2931. [DOI] [PubMed] [Google Scholar]
- 26.Dreno B. Topical antibacterial therapy for acne vulgaris. Drugs. 2004;64(21):2389–2397. doi: 10.2165/00003495-200464210-00002. [DOI] [PubMed] [Google Scholar]
- 27.Glass D, Boorman GC, Stables GI, et al. A placebo-controlled clinical trial to compare a gel containing a combination of isotretinoin (0.05%) and erythromycin (2%) with gels containing isotretinoin (0.05%) or erythromycin (2%) alone in the topical treatment of acne vulgaris. Dermatology. 1999;199:242–247. doi: 10.1159/000018255. [DOI] [PubMed] [Google Scholar]
- 28.Griffiths CE, Voorhees JJ, Nickoloff BJ. Characterization of intracellular adhesion molecule-1 and HLA-DR expression in normal and inflamed skin: modulation by recombinant gamma interferon and tumor necrosis factor. J Am Acad Dermatol. 1989;20:617–629. doi: 10.1016/s0190-9622(89)70073-6. [DOI] [PubMed] [Google Scholar]
- 29.Gollnick H, Schramm M. Topical treatment of acne. J Eur Acad Dermatol Venereol. 1998;11(Suppl 1):S8–S12. discussion S28–S29. [PubMed] [Google Scholar]
- 30.Orfanos CE, Zouboulis CC, Almond-Roesler B, Geilen CC. Current use and future potential role of retinoids in dermatology. Drugs. 1997;53(3):358–388. doi: 10.2165/00003495-199753030-00003. [DOI] [PubMed] [Google Scholar]
- 31.Bergfeld WF. Topical retinoids in the management of acne vulgaris. J Drug Dev Clin Pract. 1996;8:151–160. [Google Scholar]
- 32.Weiss JS. Current options for the topical treatment of acne vulgaris. Pediatr Dermatol. 1997;14(6):480–488. doi: 10.1111/j.1525-1470.1997.tb00696.x. [DOI] [PubMed] [Google Scholar]