Abstract
The Wnt/β-catenin signaling pathway is one of several key conserved intercellular signaling pathways in animals, and plays fundamental roles in the proliferation, regeneration, differentiation, and function of many cell and tissue types. This pathway is activated in a dynamic manner during the morphogenesis of oral organs, including teeth, taste papillae, and taste buds, and is essential for these processes to occur normally. Conversely, forced activation of Wnt/β-catenin signaling promotes the formation of ectopic teeth and taste papillae. In this review, we discuss our current understanding of the roles of Wnt/β-catenin signaling in oral tissue development and in related human diseases, and the potential of manipulating this pathway for therapeutic purposes.
Keywords: Wnt, β-catenin, development, tooth, taste, lip, oral tissue, cancer
Introduction
Oral diseases present major and prevalent public health problems, and oral cancers can be life-threatening. Elucidation of the molecular mechanisms regulating the development and regeneration of oral tissues will provide insight into the etiology underlying these disorders, and has the potential to identify novel therapeutic targets, as well as to contribute to regenerative medicine. Recent advances in oral tissue research have revealed essential roles for the Wnt/β-catenin signaling pathway in the development of many oral tissues.
The Wnt/β-Catenin Pathway
WNT paracrine signaling molecules form a family of evolutionarily conserved secreted glycoproteins with at least 19 members in humans and mice (Mikels and Nusse, 2006). WNT proteins signal across cell membranes by interacting with serpentine receptors of the Frizzled (Fzd) family. WNT ligands activate several known pathways: the Wnt/β-catenin or canonical pathway; the Wnt/Ca+2 pathway involving Protein Kinase A; the planar cell polarity pathway; and a pathway involving Protein Kinase C that functions in muscle myogenesis (Gordon and Nusse, 2006). The Wnt/β-catenin pathway is the best-studied of these. Membrane β-catenin is a component of intercellular adhesion junctions, where it connects cadherins to α-catenin and forms a dynamic link to the cytoskeleton (Drees et al., 2005; Yamada et al., 2005). In the absence of canonical Wnt signaling, cytoplasmic β-catenin is associated with adenomatous polyposis coli (APC) and Axin proteins, and is phosphorylated by glycogen synthase kinase 3β (GSK3β) and casein kinase I (CKI) in its N-terminal degradation box, resulting in polyubiquitination by βTRCP1 or βTRCP2 complexes and targeting for protease-mediated degradation (Liu et al., 1999). Binding of WNT ligand to a Frizzled (FZD) receptor and a low-density lipoprotein-related-receptor protein (LRP) 5 or 6 co-receptor, and interaction of FZD with the cytoplasmic protein Disheveled (DSH), result in phosphorylation of cytoplasmic DSH by CKI and its binding to GSK3β with the help of Frequently Rearranged in Advanced T-cell lymphomas (FRAT) protein. These interactions cause inactivation of the Axin/APC/GSK3β/CKI complex and stabilization of cytoplasmic β-catenin. A novel member of the FRL1/Crypto class of receptors can also mediate Wnt/β-catenin signaling triggered by Wnt11, together with Frizzled in Xenopus embryos (Tao et al., 2005).
Nuclear β-catenin interacts with members of the Lymphoid Enhancer Factor/T-cell factor (LEF/TCF) family of transcription factors and also with Legless family docking proteins BCL9/BCL9L, which associate with the transcription co-activator pygopus (PYGO) 1/2 (Kramps et al., 2002). The LEF/TCF/β-catenin complex recruits transcription co-activator CREB binding protein (CBP) and the closely related protein p300. This complex activates the transcription of target genes. β-catenin also binds to the TATA box-binding protein (TBP), either directly or through another factor, TIP49 (also known as Pontin52) (Bauer et al., 2000), thus connecting to the basal transcription machinery.
Traditionally, WNT proteins have been divided into two groups based on their ability to activate Wnt/β-catenin signaling: Members of the Wnt1 class (WNT1, WNT3, WNT3A, WNT7A, WNT7B, WNT8A) have been considered effective activators of the canonical pathway, whereas members of the Wnt5a class (WNT4, WNT5A, and WNT11) have been considered poor activators or even inhibitors of the canonical pathway (Maye et al., 2004). However, analysis of recent data indicates that the outcome of signaling is regulated by specific Wnt-receptor pairings rather than being intrinsic to a particular Wnt. For instance, WNT11 and WNT5A are able to signal via β-catenin in a Frizzled-FRL- or Frizzled4- and LRP5-dependent manner, respectively (Tao et al., 2005; Mikels and Nusse, 2006).
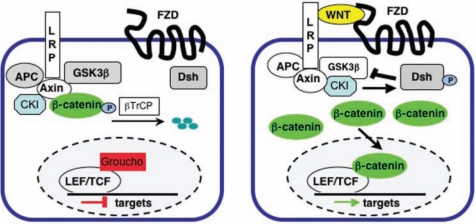
Multiple secreted and intracellular inhibitors control activity of the Wnt/β-catenin signaling pathway. Among the secreted Wnt inhibitors, Dickkopf (Dkk) family members interact with LRP5/LRP6 co-receptors and cause endocytosis of the WNT/LRP5/6 complex, while SFRP (secreted Frizzled related protein) family members and WIF1 (Wnt inhibitory factor 1) inhibit binding of WNT ligands to FZD family receptors (Kawano and Kypta, 2003). In addition to components of the β-catenin destruction complex, intracellular inhibitors include the Dishevelled-binding protein NKD (naked cuticle) (Wharton et al., 2001), and nemo-like kinase (NLK), which phosphorylates LEF/TCF proteins and inhibits the DNA-binding ability of β-catenin/TCF complexes (Ishitani et al., 1999). A simplified version of the canonical Wnt signaling pathway is shown in Fig. 1; for a more comprehensive survey of canonical Wnt signaling components, see www.stanford.edu/~rnusse/pathways/cell2.html.
Figure 1.
Simplified schematic of the Wnt/β-catenin signaling pathway. (Left) In the absence of a WNT ligand, cytoplasmic β-catenin is associated with APC and Axin, phosphorylated by GSK3β and CKI, and polyubiquitinated by the βTRCP complex, targeting it for proteosomal degradation; under these conditions, LEF/TCF transcription factors in the nucleus associate with transcriptional co-repressors, such as Groucho, and the transcription of Wnt target genes is repressed. (Right) In the presence of a WNT ligand, phosphorylation and degradation of β-catenin are inhibited, allowing it to accumulate in the cytoplasm and translocate into the nucleus. Nuclear β-catenin interacts with LEF/TCF family transcription factors and several other transcriptional co-activators to initiate transcription of target genes. Abbreviations: FZD, Frizzled; LRP, low-density lipoprotein-receptor-related protein; APC, adenomatous polyposis coli; GSK, glycogen synthase kinase; CKI, casein kinase I; DSH, Disheveled; LEF/TCF, Lymphoid enhancer factor/T-cell factor.
Wnt/β-Catenin Signaling in the Development of the CraniofacialSkeleton and Muscles
Wnt/β-catenin signaling is activated in a dynamic fashion in cranial neural crest cells (NCCs) that contribute extensively to forming craniofacial structures (Mani et al., 2010). NCCs migrate into the first branchial arch from the midbrain and anterior hindbrain at around the four-somite stage (Serbedzija et al., 1992). These cells will form the skeleton of the upper and lower jaws, and contribute to the trigeminal ganglion (Tan and Morriss-Kay, 1985). In neural-crest-specific β-catenin knockout mice, these cranial NCC-derived structures are absent, and apoptosis is increased in cells migrating to the cranial ganglia and in areas of prechondrogenic condensations, indicating the pivotal role of Wnt/β-catenin signaling in neural crest cell survival and/or differentiation (Brault et al., 2001).
Wnt/β-catenin signaling also plays essential roles in general skeletal development. Skeletons develop by two processes: intramembranous and endochondral ossification. The majority of skeletons are formed by endochondral ossification, whereby mesenchymal cells condense and cells within the condensations differentiate into chondrocytes, forming a cartilaginous template that prefigures the future skeletal element, while the surrounding cells form the perichondrium. In micromass cultures in the mouse and chicken, Wnt/β-catenin signaling represses chondrogenesis in limb buds and in the craniofacial region by repressing Sox9 transcription (Cheung et al., 2005; Hill et al., 2005; Später et al., 2006). Wnt signaling also controls chondrocyte maturation. Overexpression of Wnt4, Wnt8, or a stabilized form of β-catenin in chicken embryos results in acceleration of chondrocyte maturation (Hartmann and Tabin, 2000; Enomoto-Iwamoto et al., 2002). Conversely, overexpression of a Wnt-antagonist, SFRP3 (FRZB1), results in delayed chondrocyte differentiation, by antagonizing β-catenin-mediated maturation of hypertrophic chondrocytes (Enomoto-Iwamoto et al., 2002), while loss of SFRP1 results in acceleration of chondrocyte hypertrophy and mineralization (Gaur et al., 2006).
About 40 skeletal muscles are present in the vertebrate head, and most of them (the “genuine” head muscles) develop from the paraxial and prechordal mesoderm located in the preotic levels of the head. Although the genuine head muscles eventually exhibit the same tissue architecture as muscles in the trunk, their development is remarkably distinct and is regulated by different molecular mechanisms (Carvajal et al., 2001). The cranial muscle anlagen in the chick are surrounded by NCCs and other tissues secreting the Wnt ligands WNT1, WNT3A, and WNT13, and the Wnt inhibitor FRZB. Interestingly, although myogenesis in trunk paraxial mesoderm is induced by Wnt/β-catenin signaling from the dorsal neural tube, myogenesis in cranial paraxial mesoderm is blocked by the same signal; conversely, skeletal myogenesis in the head is induced by the Wnt inhibitor FRZB in combination with the Bone Morphogenetic Protein (BMP) inhibitors Noggin and Gremlin (Tzahor et al., 2003).
The Wnt/β-Catenin Pathway in Tooth Development
Tooth development is regulated by reciprocal interactions between the dental epithelium and mesenchyme through a complex signaling network (Thesleff, 2006). Tooth development is initiated with thickening of the ectodermally derived oral epithelium. The epithelium invaginates into mesenchyme that originates from the neural crest of the first branchial arch, and forms a tooth bud. Morphogenesis from the bud to the cap stage involves the formation of a transient signaling center at the base of the epithelial bud, termed an “enamel knot”, which expresses multiple signaling molecules, and the development of a mesenchymal papilla. The single primary enamel knot disappears at the bell stage. At this stage in developing molar teeth, several secondary enamel knots are formed, which determine the locations and shapes of the molar cusps. Subsequent differentiation gives rise to dentin-secreting odontoblasts of mesenchymal origin, and enamel-secreting ameloblasts derived from the inner dental epithelium (Jernvall and Thesleff, 2000). Wnt/β-catenin signaling is dynamically active in tooth-forming regions at all of these stages of tooth development, and plays multiple roles in these events (Jarvinen et al., 2006; Liu et al., 2008).
(1) Initiation Stage
Multiple Wnt genes are expressed at the initiation of mouse tooth development. These include Wnt10b, which is expressed specifically in the molar and incisor dental epithelial thickenings, and Wnt5a and the Wnt agonist/antagonists MFrzb1 and Mfrp2 that are expressed in a graded proximo-distal (P-D) manner in mesenchymal cells, with no overlying expression in the oral or dental epithelium (Sarkar and Sharpe, 1999). At this same stage, expression of Wnt4, Wnt6, and the Wnt receptor gene Fz6 is observed in facial, oral, and dental epithelium. Tissue recombination experiments revealed that epithelial Wnt4 induces expression of Sema3a, a secreted repulsive axon guidance molecule, in the presumptive dental mesenchyme, which in turn guides the arrival of the first dental nerve fibers (Kettunen et al., 2005). Wnt3 and Wnt7b are expressed in the oral epithelium, but are absent from the presumptive dental epithelium. Ectopic expression of Wnt7b in presumptive dental ectoderm in mandibular arch explants down-regulates Sonic Hedgehog (Shh) expression in the presumptive dental ectoderm and the Shh effector and target gene Patched in the underlying ectomesenchyme, and tooth development is subsequently arrested, suggesting that the expression of Wnt7b in presumptive oral ectoderm and Shh in presumptive dental ectoderm positions the sites of tooth formation (Sarkar et al., 2000). However, the signaling pathway activated by ectopic Wnt7b in these experiments was not identified. Activity of the canonical Wnt/β-catenin pathway in developing molar teeth in vivo has been monitored by immunostaining for nuclear β-catenin, by the use of Wnt reporter transgenes that contain multimerized LEF/TCF binding sites upstream of a minimal promoter and a lacZ reporter (TOPGAL, BAT-gal, and Lef/Tcf-lacZ), and by means of a knockin of lacZ into the endogenous Axin2 locus, a direct Wnt target gene (Liu et al., 2008; Lohiet al., 2010). While expression patterns vary slightly for different reporters, these studies, taken together, indicate that Wnt/β-catenin signaling localizes specifically to the epithelium of the dental lamina and forming dental placodes at the initiation stage of tooth development (Fig. 2), suggesting that it plays a positive, rather than inhibitory, role in dental initiation. This hypothesis is supported by observations of continuous ectopic dental development in embryos bearing an activating, stabilizing mutation in epithelial β-catenin, or homozygous for a null mutation in the intracellular Wnt antagonist APC (Jarvinen et al., 2006; Kuraguchi et al., 2006; Liu et al., 2008).
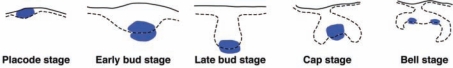
Figure 2.
The Wnt/β-catenin signaling pathway is activated in a dynamic fashion in molar tooth development. Summary of data from analyses of Wnt/β-catenin pathway activity in developing molar teeth in vivo with immunostaining for nuclear β-catenin; X-gal staining of embryos carrying Wnt reporter transgenes that contain multimerized LEF/TCF binding sites upstream of a minimal promoter and a lacZ reporter (TOPGAL, BAT-gal, and Lef/Tcf-lacZ); and X-gal staining of embryos in which lacZ has been inserted into the endogenous Axin2 locus, a direct Wnt target gene (Liu et al., 2008; Lohi et al., 2010). While expression patterns vary slightly for different reporters, these studies, taken together, indicate that Wnt/β-catenin signaling localizes specifically to the epithelium of the dental lamina at the placode stage; to the primary epithelial enamel knot and underlying mesenchymal cells at the bud and cap stages; and to the secondary enamel knots and underlying mesenchymal cells at the bell stage. Wnt/β-catenin pathway activity is indicated by grey or blue shading in the print and online versions, respectively. Dashed black lines indicate epithelial-mesenchymal borders. Diagrams are not to scale.
(2) Bud Stage
At the bud stage, Wnt/β-catenin reporter gene expression reveals that Wnt/β-catenin signaling is active in dental epithelial cells and immediately underlying mesenchymal cells (Liu et al., 2008; Lohi et al., 2010) (Fig. 2). When dental epithelial and mesenchymal Wnt/β-catenin signaling is inhibited soon after tooth initiation by forced epithelial expression of the secreted Wnt inhibitor DKK1, tooth development is arrested at the early bud stage (Liu et al., 2008). Depletion of epithelial β-catenin produces a similar, albeit less severe, phenotype, demonstrating a requirement for epithelial Wnt/β-catenin signaling at early stages of tooth development. Arrested dental development in Dkk1-expressing embryos appears to result from loss of expression of Bmp4 and its target genes Msx1 and Msx2, consistent with the similar tooth phenotypes observed in Dkk1-expressing and Msx1−/−; Msx2−/− embryos (Bei and Maas, 1998; Liu et al., 2008). Deletion of β-catenin in oral mesenchyme results in failure of progression from the bud to the cap stage of tooth development. Mesenchymal β-catenin is required for expression of Lef1 and Fgf3 in the dental mesenchyme and for induction of the primary enamel knot in the epithelium (J Chen et al., 2009). These data indicate essential roles for β-catenin signaling within both epithelial and mesenchymal compartments of the developing tooth. Interestingly, the Wnt-activated populations in developing dental epithelium and mesenchyme directly abut each other, suggesting intimate crosstalk between these compartments that likely involves Wnt10a, Wnt10b, Wnt6, and Wnt3, expressed in the primary enamel knot, and Wnt5a, expressed in adjacent mesenchyme.
β-catenin and Lef1 expression in the dental epithelium of developing tooth buds overlaps with expression of PITX2, an essential transcription factor for tooth development (Hjalt et al., 2000). Expression of Lef1 is regulated through direct physical interactions of PITX2, LEF1, and ß-catenin (Vadlamudi et al., 2005), and PITX2 enhances endogenous expression of the full-length β-catenin-dependent Lef1 isoform while decreasing expression of the N-terminally truncated β-catenin-independent isoform (Amen et al., 2007). Targeted inactivation of the Lef1 gene in mice results in arrested tooth development at the bud stage (van Genderen et al., 1994), slightly later than the block to tooth development seen in Dkk1-expressing embryos. The difference in the stage of arrest is likely due to partially redundant functions of LEF1 with other LEF/TCF family members in tooth development. Recombinations of epithelial and mesenchymal tissues from developing teeth of wild-type and Lef1-/- embryos showed that LEF1 is required only transiently in the epithelium in a tissue-non-autonomous manner (Kratochwil et al., 1996). The Fgf4 gene is a direct transcriptional target of LEF1, and in turn induces expression of Fgf3 in dental mesenchyme followed by Shh in the epithelium, indicating sequential and reciprocal communication between the epithelium and mesenchyme (Kratochwil et al., 2002). Loss of Lef1 also causes failure of survival of dental epithelial cells (Sasaki et al., 2005). Conversely, overexpression of Lef1 in the epithelium can trigger the epithelial cells in the lip furrow of developing transgenic animals to invaginate, sometimes leading to inappropriate adoption of hair follicle and tooth cell fates (Zhou et al., 1995).
(3) Cap Stage
At the early cap stage, Lef1, Wnt3, Wnt6, Wnt10b, and MFz6 are detected in the primary enamel knot, a transient signaling center in the developing tooth, while Wnt5a and MFrzb1 show strong expression in the dental papilla mesenchyme (Sarkar and Sharpe, 1999). Conversely, expression of Wnt inhibitors of the Dkk family is up-regulated in developing teeth and surrounding oral mesenchyme, but is excluded from enamel knots (Fjeld et al., 2005). Consistent with these data, expression of Wnt/β-catenin reporter genes and immunostaining for nuclear β-catenin indicate that Wnt/β-catenin signaling is active in the enamel knot (Liu et al., 2008; Lohi et al., 2010) (Fig. 2), and that β-catenin mRNA is also prominently up-regulated in this structure (Obara et al., 2006; Liu et al., 2008). In mutant embryos carrying an activating, stabilizing mutation in epithelial β-catenin, supernumerary teeth develop by a process in which new enamel knots bud off from the existing dental epithelium, suggesting β-catenin signaling as an upstream activator of enamel knot formation (Jarvinen et al., 2006).
(4) Bell Stage
The secondary enamel knots provide the earliest morphologic indication of species-specific cusp patterns. Cusp patterning is thought to begin in molar teeth of mice and voles at the late cap stage, prior to morphological evidence of cusp development (Keranen et al., 1998). Thus, the molecular signals regulating the relative positions of cusps are predicted to be active immediately after apoptosis of the primary enamel knot. In line with this expected pattern, Wnt/β-catenin signaling is active in developing secondary enamel knots (Liu et al., 2008; Lohi et al., 2010) (Fig. 2). Treatment of molar tooth germ explants with the Wnt inhibitor MFRZB1, followed by transplantation to renal capsules for further development, causes teeth to be reduced in size and with blunter cusps than controls, suggesting that Wnt signaling regulates molar tooth size and cusp development (Sarkar and Sharpe, 2000). Consistent with this, forced inducible in vivo expression of Dkk1 in oral and dental epithelia from the cap stage results in formation of blunted molar cusps, down-regulation of the enamel knot marker p21, and expanded expression of the Sclerostin-domain-containing-1 gene (Sostdc1), also known as Uterine Sensitization Associated Gene-1 (Usag-1), Ectodin, or Wise, whose expression is normally excluded from the enamel knot. Sostdc1 modulates the Wnt and BMP pathways in a context-dependent fashion (Itasaki et al., 2003). Consistent with roles for Wnt and BMP signaling in regulating cusp patterning, molar teeth in mouse mutants lacking Sostdc1 have enlarged enamel knots and extra cusps (Kassai et al., 2005). The results of these experiments reveal requirements for Wnt activity in maintaining secondary enamel knots and indicate that tight regulation of this pathway is essential for controlling the shapes of individual teeth (Liu et al., 2008).
(5) Secretory Stage
Wnt10a is expressed in secretory odontoblasts and co-localizes with dentin sialophosphoprotein (Dspp), a tooth-specific non-collagenous matrix protein regulating dentin mineralization. Analysis of in vitro data indicates that Wnt10a is an upstream regulatory molecule for Dspp expression, and that cell-matrix interaction is essential for the induction of Dspp expression (Yamashiro et al., 2007). Overexpression of Wnt3 in dental epithelium results in the loss of ameloblasts and a reduction of enamel in post-natal incisors, suggesting that inhibition of the Wnt pathway might play a role in enamel formation (Millar et al., 2003). In line with this, Dkk1 is prominently up-regulated in pre-odontoblasts and secretory odontoblasts, while Dkk3 is transiently expressed in ameloblasts before enamel matrix secretion (Fjeld et al., 2005).
(6) Post-natal Stages
It is generally accepted that Hertwig’s epithelial root sheath (HERS) is important for tooth root formation. Syndecan-1, a cell-surface heparan sulfate proteoglycan that enhances Wnt/β-catenin signaling (Liu et al., 2003), is expressed in the HERS (Yamamoto et al., 2004). The formation of cementum is sensitive to intra- and extracellular phosphate/pyrophosphate distribution, and treatment of immortalized mouse cementoblasts with inorganic phosphate alters the expression of several Wnt pathway genes: Expression of the secreted canonical Wnt signaling Sfrp4 inhibitor is enhanced, while expression of the Wif1 and Dkk3 inhibitors and the Wnt10b and Wnt4 ligands is diminished (Rutherford et al., 2006). Sfrp4 has been identified as a potential phosphatonin (Berndt et al., 2003), a putative circulating regulator of phosphate concentration. Analysis of these data suggests that Wnt signaling may be involved in cementum formation.
Unlike molar teeth, rodent incisor teeth erupt continuously, a process thought to depend on a pool of stem cells in the cervical loop. Wnt/β-catenin pathway activity is detected in mesenchymal and epithelial cells of post-natal incisor teeth (Lohi et al., 2010; Rooker et al., 2010; Suomalainen and Thesleff, 2010). Interestingly, however, pathway activity is excluded from the cervical loop, suggesting that, in contrast to its roles in controlling stem cell function in several other regenerating epithelial tissues, such as the hair follicle and intestine, Wnt/β-catenin signaling does not directly regulate the proliferation and self-renewal of incisor tooth stem cells (Suomalainen and Thesleff, 2010).
(7) β-catenin Signaling and Patterning of the Dentition
Rodents have a toothless region, known as the diastema, between their incisor and molar regions. However, in many rodents, including mice, rudimentary supernumerary tooth germs are present in the diastema and incisor regions early in development, and regress through apoptosis (Keranen et al., 1999; Murashima-Suginami et al., 2007). In mice lacking Sostdc1, in addition to abnormal molar cusp patterning, rudimentary incisors survive and erupt as supernumerary teeth, and ectopic molars develop (Kassai et al., 2005; Murashima-Suginami et al., 2007; Munne et al., 2009). Similar phenotypes are observed in mice hypomorphic for LRP4, a SOSTDC1 receptor (Ohazama et al., 2008). Nuclear β-catenin and BMP signaling pathways are up-regulated in dental mesenchymal cells within the rudimentary incisors of Sostdc1 mutant mice, and supernumerary tooth formation can be rescued by application of either the BMP inhibitor Noggin or the Wnt inhibitor DKK1 in organ culture, suggesting that Sostdc1 suppresses tooth development by inhibiting Wnt and BMP signaling in the dental mesenchyme (Murashima-Suginami et al., 2008; Munne et al., 2009). Consistent with these data, forced activation of β-catenin signaling by mutation of the Ctnnb1 gene to a constitutively active form in oral epithelial cells, or by deletion of epithelial Apc, results in continuous tooth development in embryogenesis (Jarvinen et al., 2006; Kuraguchi et al., 2006; Liu et al., 2008). Strikingly, inducible deletion of epithelial Apc or activation of β-catenin can result in new tooth development, even in adult mice. This effect is seen only in the continuously erupting incisor, likely reflecting the continued presence of a dental lamina in these specialized teeth (Wang et al., 2009) (F.L and S.E.M., unpublished observations). Analysis of these data, taken together, identifies Wnt/β-catenin as a pro-odontogenic signal at tooth initiation and in cusp development, and suggests manipulation of this pathway as an important component in strategies for dental tissue engineering.
Wnt/β-Catenin Signaling in the Development of Taste Papillae And Taste Buds
Taste buds (TBs) are found on the dorsal surface of the tongue, the posterior soft palate, and the epiglottis. In the tongue, they are located within the tips of the fungiform papillae and in clusters in the epithelium of vallate and foliate papillae. TBs are derived from surface oral epithelial cells (Barlow and Northcutt, 1995; Stone et al., 1995), and, like other epithelial cells, are regenerated at regular intervals during adult life. Unlike other epithelia, however, taste buds have several characteristics usually associated with neurons, including their expression of chemoreceptors, and their ability to synapse with nerve fibers. Each TB consists of ~50 to 100 cells, and is flask-like in shape, with its broad base resting on the corium, and its neck opening into an orifice, the gustatory pore, between the cells of the epithelium. TBs are composed of basal cells and 3 kinds of elongated cells derived from basal cells, denoted types I, II, and III. The type I cells, also called dark cells, are considered supporting cells. Type II cells, also called light cells, are taste sensory cells that detect sweet, umami, or bitter tastes and express the corresponding G-protein-coupled taste receptors. Most type II cells appear to respond to only one type of taste stimulus (Tomchik et al., 2007). Type III cells, also called intermediate cells, are generally considered to be the only taste cell type that makes conventional synaptic contacts with nerve fibers (Yang et al., 2000), and most of these pre-synaptic cells respond to two or more different taste qualities, including sour and salty stimuli (Tomchik et al., 2007).
The fungiform papillae form in a stereotypical array on the anterior two-thirds of the dorsal lingual surface. In mice, these papillae appear first at embryonic day (E) 12.5 as localized epithelial thickenings, termed ‘placodes’, that show specifically elevated expression of the signaling molecules Shh and Bmp4 (Hall et al., 1999; Zhou et al., 2006). By E14.5, fungiform placodes begin to evaginate, forming raised papillae with mesenchymal cores. Taste buds are first morphologically evident at approximately E18 as onion-shaped aggregates of elongated cells within apical papillary epithelium (Stone et al., 1995). However, early in papillary morphogenesis, a cluster of cells at the center of the papillary apex can be distinguished from surrounding cells by virtue of their mitotic quiescence and expression of the taste cell marker cytokeratin 8 (Farbman and Mbiene, 1991; Mbiene and Roberts, 2003; Zhou et al., 2006). Recent evidence suggests that Shh-expressing cells in the fungiform placode are determined precursor cells of taste buds, and do not differentiate into surface epithelial cells (Thirumangalathu et al., 2009). These results indicate that taste bud progenitor cells are established early in papillary development.
Several Wnt pathway genes, including Wnt6, Wnt10a, Wnt10b, Fzd6, β-catenin (Ctnnb1), and Lef1, are expressed in developing fungiform placodes, and Wnt reporter transgenes detect Wnt/β-catenin signaling pathway activity in developing taste papillae and taste buds. Forced epithelial expression of the secreted Wnt inhibitor DKK1, or genetic deletion of epithelial β-catenin, down-regulates expression of Shh and Bmp4, two early markers of taste organ development, and blocks initiation of the development of fungiform papillae and taste buds (Liu et al., 2007). These data indicate that Wnt signaling is required within epithelial cells for fungiform placode formation. Similar, though less severe, phenotypes are detected in embryos lacking either Lef1 or Wnt10b (Iwatsuki et al., 2007), indicating that these Wnt pathway components play key roles in taste development, but may overlap in their functions with other LEF/TCF family members and Wnt ligands, respectively. Conversely, forced activation of epithelial Wnt signaling in vivo up-regulates expression of Shh, and produces numerous massively enlarged ectopic fungiform papillae and taste buds in the tongue, while filiform papilla morphogenesis is almost absent, indicating that early up-regulation of Wnt signaling promotes development of fungiform taste papilla and taste bud development at the expense of filiform papillae (Liu et al., 2007). Up-regulation of Shh by Wnt signaling has been confirmed by in vitro culture of embryonic mouse tongues, while blocking Shh signaling in vitro enhances papilla formation and is accompanied by an up-regulation of Wnt/β-catenin signaling, indicating that Shh limits Wnt/β-catenin pathway activity in this system (Iwatsuki et al., 2007). Another downstream target of Wnt/β-catenin in papillary development is the SoxB1-HMG-box transcription factor gene Sox2. Sox2 is expressed in basal epithelial cells of the tongue, with high levels in taste bud placodes, fungiform papillae, and mature taste cells, and low levels in filiform papillae. In hypomorphic Sox2 embryos whose Sox2 expression is 20% of the normal level, fungiform placodes form, but no mature taste buds develop. Conversely, transgenic overexpression of Sox2 in basal cells inhibits differentiation of filiform keratinocytes, suggesting that Sox2 functions in a dose-dependent manner to promote the differentiation of tongue progenitor cells into taste bud sensory cells rather than keratinocytes (Okubo et al., 2006). Deletion of the TGFβ antagonist follistatin, which is expressed in tongue mesenchyme, causes increased expression of mesenchymal BMP7 and altered patterning of epithelial Wnt/β-catenin activation and papilla formation, indicating important roles for mesenchymal-epithelial signaling in controlling Wnt pathway activity and papillary development (Beites et al., 2009).
In vitro experiments have shown that BMP and SHH signaling pathways regulate the formation of taste papillae (Hall et al., 2003; Mistretta et al., 2003; Zhou et al., 2006). However, the in vivo roles of these pathways in taste organ development, and in vivo interactions among Wnt, BMP, and SHH signaling pathways, have not yet been fully elucidated. Another key question for future research is how activation of the Wnt pathway at similar embryonic stages in different oral epithelia leads to such different outcomes, namely, formation of teeth in the jaws, and taste papillae in the tongue. In each case, activation of β-catenin signaling provides the instruction “make an appendage”, but the nature of the appendage formed seems to rely on pre-existing regional signals. Possible mechanisms underlying these regional differences in response to Wnt activation are discussed in more detail below. Another critical question for oral-regenerative medicine is whether Wnt, BMP, and SHH pathways are involved in the cyclical regeneration of taste buds that occurs in adult life and is essential for homeostatic maintenance of taste function, and for recovery from taste cell injury such as can occur following chemotherapy. Periodic growth of hair follicles, another type of ectodermal appendage, is known to be regulated by Wnt, SHH, and BMP signaling (Millar, 2002; Fuchs, 2008). It will be fascinating to determine whether similar principles apply to the taste bud.
Wnt/β-Catenin Signaling in Development of the Lip and Palate
Cleft lip with or without cleft palate (CLP) has an occurrence of 1 in 500 to 1 in 2500 live births worldwide, making it the most common craniofacial birth defect in humans (Vanderas, 1987; Schutte and Murray, 1999). In embryogenesis, the vertebrate upper lip forms from initially freely projecting maxillary, medial nasal, and lateral nasal prominences at the rostral and lateral boundaries of the primitive oral cavity, that subsequently undergo fusion to form a mature lip (Jiang et al., 2006). Mutations in the WNT3 and WNT9B genes are associated with CLP in both humans and mice, indicating that Wnt signaling plays a critical role in this process.
In a mouse model of human CLP, the A/WySn strain, an essential causal recessive mutation, clf1, was mapped to a small region of mouse chromosome 11 containing the closely linked Wnt3 and Wnt9b genes (Juriloff et al., 2001). Sequence analysis showed that clf1 is associated with a retrotransposon insertion at 6.6 kb downstream of the Wnt9b gene, and non-complementation of clf1 and a Wnt9b null mutation in a standard genetic test of allelism confirmed that clf1 affects the Wnt9b gene (Juriloff et al., 2006). In line with this, a targeted mutation in the mouse Wnt9b gene caused an incomplete penetrance of CLP in homozygous mutants (Carroll et al., 2005).
The clf1 region of mouse chromosome 11 is syntenic to human Chromosome 17q21, a chromosomal region strongly associated with non-syndromic CLP in some human populations (Chenevix-Trench et al., 1992; Shaw et al., 1993; Mitchell, 1994; Peanchitlertkajorn et al., 2003; Marazita et al., 2004; Moreno et al., 2004). In humans, a nonsense mutation in the WNT3 gene is associated with tetra-amelia, a rare recessive genetic disorder characterized by complete absence of all 4 limbs and other anomalies, including CLP (Niemann et al., 2004). In addition, non-syndromic cleft lip with or without cleft palate (NSCLP) is associated with chromosome 3p21.2, which contains the WNT5A gene (Blanton et al., 2004).
Both Wnt3 and Wnt9b are expressed in the facial ectoderm at critical stages of midfacial morphogenesis during mouse embryogenesis (Lan et al., 2006), with Wnt3 expression in the maxillary and medial nasal ectoderm, and Wnt9b expression in the maxillary, medial nasal, and lateral nasal ectoderm. During mouse lip fusion, Wnt9b, but not Wnt3, is expressed in the epithelial seam between the fusing medial and lateral nasal processes, and the Wnt/β-catenin reporter transgene TOPGAL is specifically activated in distal regions of the medial nasal, lateral nasal, and maxillary processes prior to lip fusion. During lip fusion, the epithelial seam between the medial and lateral nasal processes, and facial mesenchyme directly beneath the fusing epithelia, express TOPGAL. Together with Wnt3 and Wnt9b mutant phenotypes, analysis of these data suggests that Wnt3 and Wnt9b signal through the Wnt/β-catenin pathway to regulate lip fusion (Lan et al., 2006). Interestingly, deletion of R-spondin2, another secreted protein capable of activating the Wnt pathway, also results in craniofacial malformations in mice (Yamada et al., 2009). Combined mutation in mouse embryos of the downstream Wnt pathway components Lef1 and Tcf4 results in a more “human-like” appearance of the fetal head, indicating a critical role for Wnt signaling in the growth of the maxillary and frontonasal prominences. This finding has led to the suggestion that subtle differences in the timing or duration of Wnt signaling may underpin species-specific differences in facial growth (Liu et al., 2009).
Tissue-Specific Roles of Wnt/β-Catenin Signaling in the Development of Oral Tissues
As discussed above, initiation of the development of oral ectodermal appendage organs, such as taste buds and teeth, is dependent on activity of the Wnt/β-catenin pathway, and can be promoted by forced activation of β-catenin signaling (Jarvinen et al., 2006; Liu et al., 2007, 2008; Wang et al., 2009). How does activation of the same signaling pathway have such diverse effects on the oral ectoderm, promoting tooth development in some locations, and taste bud formation in others, as well as being required in a host of other critical functions, including skeletal development and lip and palate fusion? The answer to this question is likely to lie, at least in part, in a thorough understanding of the biological contexts in which various craniofacial cells find themselves at different developmental time-points and in different spatial locations. In other words, the precise response to the activation of Wnt signaling must depend on combinations of interacting signaling pathways, transcription factors and co-factors, and other intracellular and extracellular modulators of pathway activity that are expressed in, or received by, the cell at the time of Wnt ligand reception. The site-specific differentiation of oral tissues is likely determined by signals from the mesenchyme, as indicated by the ability of dental mesenchyme to reprogram non-dental epithelia to a dental fate in tissue-recombination experiments (Mucchielli et al., 1997). The mesenchymal signals may be determined by the expression patterns of HOX genes, a family of homeodomain transcription factors that act to specify positional identity during development, similar to a mechanism that has been described to control regional development of the epidermis (Rinn et al., 2006). TCF/LEF transcription factors exist as multiple isoforms that display distinct expression patterns and co-factor binding properties (Hoppler and Kavanagh, 2007). TCF/LEF isoform diversity likely contributes significantly to context-specific Wnt activity. For example, in Xenopus development, Tcf1 and Tcf3 mediate Wnt signaling in mesoderm induction, while Wnt-mediated regulation of ventrolateral patterning, which occurs slightly later in development, requires Tcf1 and Lef1 (Liu et al., 2005). In mouse embryos, both Lef1 and Tcf1 are expressed in tooth buds, while Tcf11 is exclusively expressed in the palate and Lef1 is exclusively expressed in the tongue and lips (Oosterwegel et al., 1993; van Genderen et al., 1994). These observations suggest that different utilization of Tcf/Lef factors may contribute to the specificity of Wnt signaling in the development of these distinct oral tissues. Analysis of emerging data suggests that cell-type-specific chromatin remodeling complexes also play key roles in mediating tissue-specific transcriptional responses to activation of generally utilized signaling pathways (Lange et al., 2008). While we are beginning to understand some of these interactions, gaining a full picture of the signaling, transcriptional, and epigenetic regulators of these complex processes will likely require computer simulation and mathematical modeling approaches in addition to genetic, genomic, developmental, molecular, and bioinformatics studies.
The Wnt/β-Catenin Pathway in Oral Disease
(1) Inherited Diseases
In addition to the association of WNT3 mutations with cleft lip and palate, as described above, several other congenital human syndromes are associated with mutations in Wnt pathway genes (Table 1). Nonsense, missense, and translation termination mutations in the WNT10A gene are associated with ectodermal dysplasias, including odonto-onycho-dermal dysplasia, a rare autosomal-recessive syndrome in which patients display dry hair, severe hypodontia, smooth tongue with marked reduction of fungiform and filiform papillae, onychodysplasia, keratoderma and hyperhidrosis of palms and soles, and hyperkeratosis of the skin (Adaimy et al., 2007; Bohring et al., 2009; Nawaz et al., 2009). These characteristics are reminiscent of phenotypes caused by inhibiting Wnt/β-catenin signaling in mouse embryonic ectoderm (Kratochwil et al., 1996; Jarvinen et al., 2006; Iwatsuki et al., 2007; Liu et al., 2007, 2008), suggesting that WNT10A activates the β-catenin pathway in the development of these epithelial organs.
Table 1.
Human Oral Diseases with Aberrant Wnt/β-catenin Signaling Activity
| Disease | Abnormality of Oral Tissue | Mutated Gene | Effect on Canonical Wnt Signaling | Reference |
|---|---|---|---|---|
| Tetra-amelia | Cleft lip | WNT3 | Down | Niemann et al., 2004 |
| Odonto-onycho-dermal dysplasia | Severe hypodontia, smooth tongue | WNT10A | Down | Adaimy et al., 2007; Bohring et al., 2009; Nawaz et al., 2009 |
| Gardener’s syndrome | Jaw osteomas, odontomas, and supernumerary or unerupted teeth | APC | Up | Foulkes, 1995 |
| Oligodontia | Severe permanent tooth agenesis | AXIN2 | Up | Lammi et al., 2004 |
| COC | Calcifying cystic odontogenic tumor | CTNNB1 | Up | Sekine et al., 2003 |
| Adenoid cystic carcinoma | Salivary gland tumor | CTNNB1, APC, AXIN1 | Up | Daa et al., 2004 |
| Pleomorphic adenomas | Salivary gland tumor | WIF | Up | Queimado et al., 2007 |
Loss-of-function mutations in the APC gene, the activity of which normally limits Wnt pathway activity, cause oral and maxillofacial symptoms of familial adenomatous polyposis (FAP), including an increased risk of jaw osteomas, odontomas, and supernumerary or unerupted teeth (Gardner’s syndrome), in addition to colorectal cancer (Foulkes, 1995). By contrast, nonsense or frameshift loss-of-function mutations in the intracellular Wnt pathway inhibitor AXIN2 are associated with dominant inherited severe permanent tooth agenesis (oligodontia) and colorectal neoplasia (Lammi et al., 2004). In addition, AXIN2 polymorphisms are reported as a risk factor for selective tooth agenesis, the most common developmental abnormality of the human dentition (Mostowska et al., 2006). Tooth agenesis in these conditions is likely the result of continuous Wnt pathway stimulation in the absence of AXIN2, resulting in disrupted dental morphogenesis and development of ectopic, abnormal teeth that do not erupt. A distinct form of inherited agenesis of permanent teeth, He-Zhao deficiency, was discovered in a Chinese kindred, in which the affected locus was mapped to chromosome 10q11.2 (Liu et al., 2001), close to the gene encoding the secreted Wnt inhibitor DKK1 (Roessler et al., 2000). Consistent with results from mouse models, analysis of these data indicates that inappropriate activation of Wnt signaling can lead to either supernumerary teeth or tooth agenesis, and indicates that tight control of Wnt pathway activity is necessary for normal tooth development. Importantly, a diagnosis of supernumerary teeth or congenital tooth agenesis may be considered a possible indicator of colorectal cancer susceptibility.
Other craniofacial structures, in addition to the lip, palate, teeth, hair follicles, and taste papillae, can also be affected by Wnt pathway mutations. For instance, gain-of-function mutations in the gene encoding LRP5 causes high bone mass. A 59-year-old woman carrying a novel LRP5 missense mutation, Arg154Met, manifested extensive oropharyngeal exostoses as well as dense bones, showing that exuberant LRP5 effects are not always benign (Rickels et al., 2005).
(2) Tumors
Ectopic activation of the Wnt signaling pathway is associated with many human tumors, including those derived from oral tissues (Table 1). Nuclear localized β-catenin is observed in follicular and plexiform-type ameloblastomas, and these tumors are occasionally associated with gain-of-function mutation of β-catenin or loss-of-function mutation of APC (Kawabata et al., 2005; Miyake et al., 2006; Siriwardena et al., 2009). Craniopharyngioma is a rare neoplasm that occurs in the sellar region. No β-catenin mutation is present in any papillary craniopharyngiomas analyzed. By contrast, gain-of-function β-catenin gene mutations are present in all adamantinomatous craniopharyngiomas examined; these lesions have a histological appearance similar to that of ameloblastoma (Sekine et al., 2002). Calcifying odontogenic cyst (COC), a rare odontogenic lesion that displays histologic features such as ghost cell formation, a predominantly cystic morphology, and calcification, is also frequently associated with gain-of-function mutation of β-catenin (Sekine et al., 2003). These data indicate that Wnt/β-catenin signaling is important in the formation of certain oral tissue tumors, as well as in embryonic development of the various structures of the oral cavity.
Although little is known about the role of Wnt signaling in development of the salivary gland, the proto-oncogenic effects of Wnt activation in mouse salivary gland tissue have been well-established by studying mutant Apc allele Multiple intestinal neoplasia (Min/+);Tcf1-/- mice (Roose et al., 1999), MMTV-Wnt1 transgenic mice (Tsukamoto et al., 1988; Li et al., 2001), and mice with MMTV-Cre-mediated forced activation of Wnt/β-catenin signaling (Bierie et al., 2003). Among human pleomorphic adenomas, the most common tumors of the salivary gland, chromosome 12q13-15 re-arrangements involving the oncogene HMGA2 gene are frequent. In some cases, these re-arrangements cause production of a fusion protein containing HMGA2 and WNT inhibitory factor 1 (WIF1), resulting in increased HMGA2 and decreased WIF1 expression in the salivary tissue, and tumors possibly arising due to synergistic effects of increased Wnt and HMGA2 activity (Queimado et al., 2007). Wnt pathway components are overexpressed in adenoid cystic carcinoma (ACC), another common salivary gland tumor subtype, and nuclear localized β-catenin and mutations in Wnt pathway genes, including β-catenin, AXIN1, and APC, are detected in some cases (Frierson et al., 2002; Daa et al., 2004, 2005). However, in an immunohistochemical study of biopsy specimens including 12 pleomorphic adenoma, 17 ACC, 10 epithelial-myoepithelial carcinomas, and 4 polymorphous low-grade adenocarcinomas, nuclear staining of β-catenin was observed only in the epithelial-myoepithelial carcinomas, and not in other salivary tumors (Furuse et al., 2006), indicating the divergence in tumorigenesis of salivary gland tumors, and lack of consensus on the probable role of β-catenin signaling in various subtypes.
Squamous cell carcinoma of the head and neck (HNSCC) represents a large health burden worldwide, with approximately 500,000 cases diagnosed annually (Kim et al., 2001). The oral cavity is a major site of origin for HNSCC; for instance, in the year 2000, 30,200 cases of oral cavity and pharynx cancer were diagnosed and were responsible for an estimated 7800 deaths in the United States (Greenlee et al., 2000). Expression analyses of HNSCC and HNSCC-derived cell lines have found that most tumors and cell lines examined overexpress Wnt signaling pathway components (Leethanakul et al., 2000; Uraguchi et al., 2004), and that nuclear β-catenin localizes at the invasive front in carcinomas (Uraguchi et al., 2004). Treatment of HNSCC cells with anti-WNT1 antibody inhibits proliferation and induces apoptosis (Rhee et al., 2002), while enhancing Wnt/β-catenin signaling inhibits apoptosis, induces cell scattering and invasive growth in matrigel, and promotes HNSCC tumor growth in nude mice (Yang et al., 2006).
In oral squamous cell carcinoma (OSCC), cytoplasmic accumulation of β-catenin is a common characteristic, but is not closely associated with mutational alterations in the APC, β-catenin, and AXIN1 genes (Iwai et al., 2005; Odajima et al., 2005). Analysis of these data suggests that Wnt/β-catenin signaling may play a role in oral squamous cell carcinoma, but that this aberrant signaling may be secondary or due to epigenetic changes. Interestingly, epigenetic inactivation of SFRP genes, that encode secreted WNT inhibitors, is frequently detected in OSCC (Sogabe et al., 2008).
In oral leukoplakia, a precancerous lesion that sometimes develops into squamous cell carcinoma, WNT3 expression, and nuclear β-catenin are detected in individuals with dysplasia, but not in normal oral epithelium, suggesting potential involvement of this pathway in dysplasia (Ishida et al., 2007). This oral pre-malignant lesion is the subject of study in many primary prevention trials, and small-molecule Wnt signaling antagonists may be promising candidates for such purposes.
Future Directions
The advances outlined here underscore the broad utilization of Wnt/β-catenin signaling in the development of a diverse array of oral tissues. While research over the past several decades has revealed many of the functions of this pathway in the oral cavity, some areas have received little attention to date. The role of Wnt/β-catenin signaling in salivary gland morphogenesis and regeneration is one such area. Delineating the role of Wnt signaling in salivary gland biology is likely to be of value in improving our understanding of the molecular mechanisms at work in salivary cancers, as well as in normal salivary development.
Current findings raise several questions and opportunities related to clinical applications. A major question is whether we can utilize our knowledge of the molecular controls of taste, tooth, and other oral tissue development in bioengineering approaches to generate new organs such as teeth, in promoting taste bud regeneration following chemotherapy, or in therapeutic contexts—for instance, in repairing cleft lip and palate. Based on the known effects of forced activation of Wnt/β-catenin signaling in oral tissues, small-molecule agonists of this pathway could potentially be useful in such applications. For instance, one such agonist, IQ-1, has been shown to be capable of maintaining long-term expansion of mouse embryonic stem cells and preventing spontaneous differentiation (Miyabayashi et al., 2007). As discussed above, numerous lines of evidence suggest that inappropriate activation of Wnt/β-catenin signaling underlies the etiology of at least some oral cancers. Therefore, development of any such therapeutic applications must include careful regulation of dose, and methods for temporal and spatial control of pathway activation. In addition, a better understanding of the biological context required for specific organ development and/or regeneration in response to activation of Wnt signaling, as discussed above, will facilitate the development of novel therapeutic approaches.
A second avenue of interest lies, conversely, in the development of methods for dampening Wnt/β-catenin signaling activity as a contributing therapy in oral cancer treatment. A promising approach in this area is the use of small-molecule Wnt pathway inhibitors. For instance, nitric-oxide-donating non-steroidal anti-inflammatory drugs (NO-NSAIDs), which have pleiotropic effects, including the inhibition of Wnt/β-catenin signaling, are emerging as a promising class of compounds for the chemoprevention of colon cancer (Rigas, 2007). Progress is also being made in the development of specific Wnt/β-catenin small-molecule antagonists. Some examples include FJ9, a small molecule that targets interaction of Fzd and Dsh, induces apoptosis in human lung cancer and melanoma cells, and suppresses tumor cell growth in a mouse xenograft model (Fujii et al., 2007). Another small-molecule antagonist, PKF115-584, which disrupts transcriptionally active β-catenin/TCF protein complexes, blocks expression of Wnt target genes and induces cytotoxicity in multiple myeloma cells from both patients and cell lines without a significant effect in normal plasma cells (Sukhdeo et al., 2007). Some more recently discovered examples include small molecules that abrogate destruction of Axin proteins or inhibit the activity of Porcupine, a membrane-bound acyltransferase essential for Wnt protein production (B Chen et al., 2009; Huang et al., 2009). A more thorough understanding of the precise contributions of Wnt pathway activation to the development of oral cancers will clearly be a prerequisite for the development of successful therapies for these classes of disease. If aberrant Wnt/β-catenin signaling is confirmed as an underlying mechanism in certain oral cancers, small-molecule Wnt pathway antagonists may hold promise in the prevention and treatment of oral tumors.
Acknowledgments
We thank Dr. Linda Barlow for critical reading of the manuscript.
Footnotes
Research in S.E.M.’s laboratory is funded by NIH grants RO1AR47709, RO1DE015342, R01HD053829, RO1AR055241, and RC1DE020337. Research in F.L.’s laboratory is funded by RC1DE020595 and by Scott & White Research Advancement Award (SWRGP) #90183.
References
- Adaimy L, Chouery E, Megarbane H, Mroueh S, Delague V, Nicolas E, et al. (2007). Mutation in WNT10A is associated with an autosomal recessive ectodermal dysplasia: the odonto-onycho-dermal dysplasia. Am J Hum Genet 81:821-828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amen M, Liu X, Vadlamudi U, Elizondo G, Diamond E, Engelhardt JF, et al. (2007). PITX2 and beta-catenin interactions regulate Lef-1 isoform expression. Mol Cell Biol 27:7560-7573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow LA, Northcutt RG. (1995). Embryonic origin of amphibian taste buds. Dev Biol 169:273-285 [DOI] [PubMed] [Google Scholar]
- Bauer A, Chauvet S, Huber O, Usseglio F, Rothbacher U, Aragnol D, et al. (2000). Pontin52 and reptin52 function as antagonistic regulators of beta-catenin signalling activity. EMBO J 19:6121-6130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei M, Maas R. (1998). FGFs and BMP4 induce both Msx1-independent and Msx1-dependent signaling pathways in early tooth development. Development 125:4325-4333 [DOI] [PubMed] [Google Scholar]
- Beites CL, Hollenbeck PL, Kim J, Lovell-Badge R, Lander AD, Calof AL. (2009). Follistatin modulates a BMP autoregulatory loop to control the size and patterning of sensory domains in the developing tongue. Development 136:2187-2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt T, Craig TA, Bowe AE, Vassiliadis J, Reczek D, Finnegan R, et al. (2003). Secreted frizzled-related protein 4 is a potent tumor-derived phosphaturic agent. J Clin Invest 112:785-794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierie B, Nozawa M, Renou JP, Shillingford JM, Morgan F, Oka T, et al. (2003). Activation of beta-catenin in prostate epithelium induces hyperplasias and squamous transdifferentiation. Oncogene 22:3875-3887 [DOI] [PubMed] [Google Scholar]
- Blanton SH, Bertin T, Serna ME, Stal S, Mulliken JB, Hecht JT. (2004). Association of chromosomal regions 3p21.2, 10p13, and 16p13.3 with nonsyndromic cleft lip and palate. Am J Med Genet A 125:23-27 [DOI] [PubMed] [Google Scholar]
- Bohring A, Stamm T, Spaich C, Haase C, Spree K, Hehr U, et al. (2009). WNT10A mutations are a frequent cause of a broad spectrum of ectodermal dysplasias with sex-biased manifestation pattern in heterozygotes. Am J Hum Genet 85:97-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, et al. (2001). Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128:1253-1264 [DOI] [PubMed] [Google Scholar]
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. (2005). Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell 9:283-292 [DOI] [PubMed] [Google Scholar]
- Carvajal JJ, Cox D, Summerbell D, Rigby PW. (2001). A BAC transgenic analysis of the Mrf4/Myf5 locus reveals interdigitated elements that control activation and maintenance of gene expression during muscle development. Development 128:1857-1868 [DOI] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, et al. (2009). Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol 5:100-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lan Y, Baek JA, Gao Y, Jiang R. (2009). Wnt/beta-catenin signaling plays an essential role in activation of odontogenic mesenchyme during early tooth development. Dev Biol 334:174-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenevix-Trench G, Jones K, Green AC, Duffy DL, Martin NG. (1992). Cleft lip with or without cleft palate: associations with transforming growth factor alpha and retinoic acid receptor loci. Am J Hum Genet 51:1377-1385 [PMC free article] [PubMed] [Google Scholar]
- Cheung M, Chaboissier MC, Mynett A, Hirst E, Schedl A, Briscoe J. (2005). The transcriptional control of trunk neural crest induction, survival, and delamination. Dev Cell 8:179-192 [DOI] [PubMed] [Google Scholar]
- Daa T, Kashima K, Kaku N, Suzuki M, Yokoyama S. (2004). Mutations in components of the Wnt signaling pathway in adenoid cystic carcinoma. Mod Pathol 17:1475-1482 [DOI] [PubMed] [Google Scholar]
- Daa T, Kaku N, Kashima K, Nakayama I, Yokoyama S. (2005). Expression of beta-catenin, E-cadherin and cyclin D1 in adenoid cystic carcinoma of the salivary gland. J Exp Clin Cancer Res 24:83-87 [PubMed] [Google Scholar]
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. (2005). Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell 123:903-915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto-Iwamoto M, Kitagaki J, Koyama E, Tamamura Y, Wu C, Kanatani N, et al. (2002). The Wnt antagonist Frzb-1 regulates chondrocyte maturation and long bone development during limb skeletogenesis. Dev Biol 251:142-156 [DOI] [PubMed] [Google Scholar]
- Farbman AI, Mbiene JP. (1991). Early development and innervation of taste bud-bearing papillae on the rat tongue. J Comp Neurol 304:172-186 [DOI] [PubMed] [Google Scholar]
- Fjeld K, Kettunen P, Furmanek T, Kvinnsland IH, Luukko K. (2005). Dynamic expression of Wnt signaling-related Dickkopf1, -2, and -3 mRNAs in the developing mouse tooth. Dev Dyn 233:161-166 [DOI] [PubMed] [Google Scholar]
- Foulkes WD. (1995). A tale of four syndromes: familial adenomatous polyposis, Gardner syndrome, attenuated APC and Turcot syndrome. QJM 88:853-863 [PubMed] [Google Scholar]
- Frierson HF, Jr, El-Naggar AK, Welsh JB, Sapinoso LM, Su AI, Cheng J, et al. (2002). Large scale molecular analysis identifies genes with altered expression in salivary adenoid cystic carcinoma. Am J Pathol 161:1315-1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. (2008). Skin stem cells: rising to the surface. J Cell Biol 180:273-284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii N, You L, Xu Z, Uematsu K, Shan J, He B, et al. (2007). An antagonist of dishevelled protein-protein interaction suppresses beta-catenin-dependent tumor cell growth. Cancer Res 67:573-579 [DOI] [PubMed] [Google Scholar]
- Furuse C, Cury PR, Altemani A, dos Santos Pinto DJ, Araújo NS, de Araújo VC. (2006). Beta-catenin and E-cadherin expression in salivary gland tumors. Int J Surg Pathol 14:212-217 [DOI] [PubMed] [Google Scholar]
- Gaur T, Rich L, Lengner CJ, Hussain S, Trevant B, Ayers D, et al. (2006). Secreted frizzled related protein 1 regulates Wnt signaling for BMP2 induced chondrocyte differentiation. J Cell Physiol 208:87-96 [DOI] [PubMed] [Google Scholar]
- Gordon MD, Nusse R. (2006). Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem 281:22429-22433 [DOI] [PubMed] [Google Scholar]
- Greenlee RT, Murray T, Bolden S, Wingo PA. (2000). Cancer statistics, 2000. CA Cancer J Clin 50:7-33 [DOI] [PubMed] [Google Scholar]
- Hall JM, Hooper JE, Finger TE. (1999). Expression of sonic hedgehog, patched, and Gli1 in developing taste papillae of the mouse. J Comp Neurol 406:143-155 [DOI] [PubMed] [Google Scholar]
- Hall JM, Bell ML, Finger TE. (2003). Disruption of sonic hedgehog signaling alters growth and patterning of lingual taste papillae. Dev Biol 255:263-277 [DOI] [PubMed] [Google Scholar]
- Hartmann C, Tabin CJ. (2000). Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development 127:3141-3159 [DOI] [PubMed] [Google Scholar]
- Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. (2005). Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell 8:727-738 [DOI] [PubMed] [Google Scholar]
- Hjalt TA, Semina EV, Amendt BA, Murray JC. (2000). The Pitx2 protein in mouse development. Dev Dyn 218:195-200 [DOI] [PubMed] [Google Scholar]
- Hoppler S, Kavanagh CL. (2007). Wnt signalling: variety at the core. J Cell Sci 120(Pt 3):385-393 [DOI] [PubMed] [Google Scholar]
- Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, et al. (2009). Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461:614-620 [DOI] [PubMed] [Google Scholar]
- Ishida K, Ito S, Wada N, Deguchi H, Hata T, Hosoda M, et al. (2007). Nuclear localization of beta-catenin involved in precancerous change in oral leukoplakia. Mol Cancer 6:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani T, Ninomiya-Tsuji J, Nagai S, Nishita M, Meneghini M, Barker N, et al. (1999). The TAK1-NLK-MAPK-related pathway antagonizes signalling between beta-catenin and transcription factor TCF. Nature 399:798-802 [DOI] [PubMed] [Google Scholar]
- Itasaki N, Jones CM, Mercurio S, Rowe A, Domingos PM, Smith JC, et al. (2003). Wise, a context-dependent activator and inhibitor of Wnt signalling. Development 130:4295-4305 [DOI] [PubMed] [Google Scholar]
- Iwai S, Katagiri W, Kong C, Amekawa S, Nakazawa M, Yura Y. (2005). Mutations of the APC, beta-catenin, and axin 1 genes and cytoplasmic accumulation of beta-catenin in oral squamous cell carcinoma. J Cancer Res Clin Oncol 131:773-782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki K, Liu HX, Gronder A, Singer MA, Lane TF, Grosschedl R, et al. (2007). Wnt signaling interacts with Shh to regulate taste papilla development. Proc Natl Acad Sci USA 104:2253-2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvinen E, Salazar-Ciudad I, Birchmeier W, Taketo MM, Jernvall J, Thesleff I. (2006). Continuous tooth generation in mouse is induced by activated epithelial Wnt/beta-catenin signaling. Proc Natl Acad Sci USA 103:18627-18632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernvall J, Thesleff I. (2000). Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev 92:19-29 [DOI] [PubMed] [Google Scholar]
- Jiang R, Bush JO, Lidral AC. (2006). Development of the upper lip: morphogenetic and molecular mechanisms. Dev Dyn 235:1152-1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juriloff DM, Harris MJ, Brown CJ. (2001). Unravelling the complex genetics of cleft lip in the mouse model. Mamm Genome 12:426-435 [DOI] [PubMed] [Google Scholar]
- Juriloff DM, Harris MJ, McMahon AP, Carroll TJ, Lidral AC. (2006). Wnt9b is the mutated gene involved in multifactorial nonsyndromic cleft lip with or without cleft palate in A/WySn mice, as confirmed by a genetic complementation test. Birth Defects Res A Clin Mol Teratol 76:574-579 [DOI] [PubMed] [Google Scholar]
- Kassai Y, Munne P, Hotta Y, Penttila E, Kavanagh K, Ohbayashi N, et al. (2005). Regulation of mammalian tooth cusp patterning by ectodin. Science 309:2067-2070 [DOI] [PubMed] [Google Scholar]
- Kawabata T, Takahashi K, Sugai M, Murashima-Suginami A, Ando S, Shimizu A, et al. (2005). Polymorphisms in PTCH1 affect the risk of ameloblastoma. J Dent Res 84:812-816 [DOI] [PubMed] [Google Scholar]
- Kawano Y, Kypta R. (2003). Secreted antagonists of the Wnt signalling pathway. J Cell Sci 116(Pt 13):2627-2634 [DOI] [PubMed] [Google Scholar]
- Keranen SV, Aberg T, Kettunen P, Thesleff I, Jernvall J. (1998). Association of developmental regulatory genes with the development of different molar tooth shapes in two species of rodents. Dev Genes Evol 208:477-486 [DOI] [PubMed] [Google Scholar]
- Keranen SV, Kettunen P, Aberg T, Thesleff I, Jernvall J. (1999). Gene expression patterns associated with suppression of odontogenesis in mouse and vole diastema regions. Dev Genes Evol 209:495-506 [DOI] [PubMed] [Google Scholar]
- Kettunen P, Loes S, Furmanek T, Fjeld K, Kvinnsland IH, Behar O, et al. (2005). Coordination of trigeminal axon navigation and patterning with tooth organ formation: epithelial-mesenchymal interactions, and epithelial Wnt4 and Tgfbeta1 regulate semaphorin 3a expression in the dental mesenchyme. Development 132:323-334 [DOI] [PubMed] [Google Scholar]
- Kim KB, Khuri FR, Shin DM. (2001). Recent advances in the management of squamous cell carcinoma of the head and neck. Expert Rev Anticancer Ther 1:99-110 [DOI] [PubMed] [Google Scholar]
- Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, et al. (2002). Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell 109:47-60 [DOI] [PubMed] [Google Scholar]
- Kratochwil K, Dull M, Farinas I, Galceran J, Grosschedl R. (1996). Lef1 expression is activated by BMP-4 and regulates inductive tissue interactions in tooth and hair development. Genes Dev 10:1382-1394 [DOI] [PubMed] [Google Scholar]
- Kratochwil K, Galceran J, Tontsch S, Roth W, Grosschedl R. (2002). FGF4, a direct target of LEF1 and Wnt signaling, can rescue the arrest of tooth organogenesis in Lef1(-/-) mice. Genes Dev 16:3173-3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraguchi M, Wang XP, Bronson RT, Rothenberg R, Ohene-Baah NY, Lund JJ, et al. (2006). Adenomatous polyposis coli (APC) is required for normal development of skin and thymus. PLoS Genet 2:e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammi L, Arte S, Somer M, Jarvinen H, Lahermo P, Thesleff I, et al. (2004). Mutations in AXIN2 cause familial tooth agenesis and predispose to colorectal cancer. Am J Hum Genet 74:1043-1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y, Ryan RC, Zhang Z, Bullard SA, Bush JO, Maltby KM, et al. (2006). Expression of Wnt9b and activation of canonical Wnt signaling during midfacial morphogenesis in mice. Dev Dyn 235:1448-1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange M, Kaynak B, Forster UB, Tonjes M, Fischer JJ, Grimm C, et al. (2008). Regulation of muscle development by DPF3, a novel histone acetylation and methylation reader of the BAF chromatin remodeling complex. Genes Dev 22:2370-2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leethanakul C, Patel V, Gillespie J, Pallente M, Ensley JF, Koontongkaew S, et al. (2000). Distinct pattern of expression of differentiation and growth-related genes in squamous cell carcinomas of the head and neck revealed by the use of laser capture microdissection and cDNA arrays. Oncogene 19:3220-3224 [DOI] [PubMed] [Google Scholar]
- Li Y, Podsypanina K, Liu X, Crane A, Tan LK, Parsons R, et al. (2001). Deficiency of Pten accelerates mammary oncogenesis in MMTV-Wnt-1 transgenic mice. BMC Mol Biol 2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Rooker SM, Helms JA. (2009). Molecular control of facial morphology. Semin Cell Dev Biol. [Epub ahead of print; Sept 10, 2009] (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BY, Kim YC, Leatherberry V, Cowin P, Alexander CM. (2003). Mammary gland development requires syndecan-1 to create a beta-catenin/TCF-responsive mammary epithelial subpopulation. Oncogene 22:9243-9253 [DOI] [PubMed] [Google Scholar]
- Liu C, Kato Y, Zhang Z, Do VM, Yankner BA, He X. (1999). beta-Trcp couples beta-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc Natl Acad Sci USA 96:6273-6278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, van den Broek O, Destrée O, Hoppler S. (2005). Distinct roles for Xenopus Tcf/Lef genes in mediating specific responses to Wnt/beta-catenin signalling in mesoderm development. Development 132:5375-5385 [DOI] [PubMed] [Google Scholar]
- Liu F, Thirumangalathu S, Gallant NM, Yang SH, Stoick-Cooper CL, Reddy ST, et al. (2007). Wnt-beta-catenin signaling initiates taste papilla development. Nat Genet 39:106-112 [DOI] [PubMed] [Google Scholar]
- Liu F, Chu EY, Watt B, Zhang Y, Gallant NM, Andl T, et al. (2008). Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Dev Biol 313:210-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Wang H, Zhao S, Zhao W, Bai S, Zhao Y, et al. (2001). The novel gene locus for agenesis of permanent teeth (He-Zhao deficiency) maps to chromosome 10q11.2. J Dent Res 80:1716-1720 [DOI] [PubMed] [Google Scholar]
- Lohi M, Tucker AS, Sharpe PT. (2010). Expression of Axin2 indicates a role for canonical Wnt signaling in development of the crown and root during pre- and postnatal tooth development. Dev Dyn 239:160-167 [DOI] [PubMed] [Google Scholar]
- Mani P, Jarrell A, Myers J, Atit R. (2010). Visualizing canonical Wnt signaling during mouse craniofacial development. Dev Dyn 239:354-363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazita ML, Murray JC, Lidral AC, Arcos-Burgos M, Cooper ME, Goldstein T, et al. (2004). Meta-analysis of 13 genome scans reveals multiple cleft lip/palate genes with novel loci on 9q21 and 2q32-35. Am J Hum Genet 75:161-173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maye P, Zheng J, Li L, Wu D. (2004). Multiple mechanisms for Wnt11-mediated repression of canonical Wnt signaling pathway. J Biol Chem 279:24659-24665 [DOI] [PubMed] [Google Scholar]
- Mbiene JP, Roberts JD. (2003). Distribution of keratin 8-containing cell clusters in mouse embryonic tongue: evidence for a prepattern for taste bud development. J Comp Neurol 457:111-122 [DOI] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. (2006). Wnts as ligands: processing, secretion and reception. Oncogene 25:7461-7468 [DOI] [PubMed] [Google Scholar]
- Millar SE. (2002). Molecular mechanisms regulating hair follicle development. J Invest Dermatol 118:216-225 [DOI] [PubMed] [Google Scholar]
- Millar SE, Koyama E, Reddy ST, Andl T, Gaddapara T, Piddington R, et al. (2003). Over- and ectopic expression of Wnt3 causes progressive loss of ameloblasts in postnatal mouse incisor teeth. Connective Tissue Res 44(Suppl 1):124-129 [PubMed] [Google Scholar]
- Mistretta CM, Liu HX, Gaffield W, MacCallum DK. (2003). Cyclopamine and jervine in embryonic rat tongue cultures demonstrate a role for Shh signaling in taste papilla development and patterning: fungiform papillae double in number and form in novel locations in dorsal lingual epithelium. Dev Biol 254:1-18 [DOI] [PubMed] [Google Scholar]
- Mitchell LE. (1994). Interpreting the evidence for an association between the retinoic acid receptor locus and non-syndromic cleft lip with or without cleft palate. J Med Genet 31:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyabayashi T, Teo JL, Yamamoto M, McMillan M, Nguyen C, Kahn M. (2007). Wnt/beta-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency. Proc Natl Acad Sci USA 104:5668-5673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake T, Tanaka Y, Kato K, Tanaka M, Sato Y, Ijiri R, et al. (2006). Gene mutation analysis and immunohistochemical study of beta-catenin in odontogenic tumors. Pathol Int 56:732-727 [DOI] [PubMed] [Google Scholar]
- Moreno LM, Arcos-Burgos M, Marazita ML, Krahn K, Maher BS, Cooper ME, et al. (2004). Genetic analysis of candidate loci in non-syndromic cleft lip families from Antioquia-Colombia and Ohio. Am J Med Genet A 125:135-144 [DOI] [PubMed] [Google Scholar]
- Mostowska A, Biedziak B, Jagodzinski PP. (2006). Axis inhibition protein 2 (AXIN2) polymorphisms may be a risk factor for selective tooth agenesis. J Hum Genet 51:262-266 [DOI] [PubMed] [Google Scholar]
- Mucchielli ML, Mitsiadis TA, Raffo S, Brunet JF, Proust JP, Goridis C. (1997). Mouse Otlx2/RIEG expression in the odontogenic epithelium precedes tooth initiation and requires mesenchyme-derived signals for its maintenance. Dev Biol 189:275-284 [DOI] [PubMed] [Google Scholar]
- Munne PM, Tummers M, Jarvinen E, Thesleff I, Jernvall J. (2009). Tinkering with the inductive mesenchyme: Sostdc1 uncovers the role of dental mesenchyme in limiting tooth induction. Development 136:393-402 [DOI] [PubMed] [Google Scholar]
- Murashima-Suginami A, Takahashi K, Kawabata T, Sakata T, Tsukamoto H, Sugai M, et al. (2007). Rudiment incisors survive and erupt as supernumerary teeth as a result of USAG-1 abrogation. Biochem Biophys Res Commun 359:549-555 [DOI] [PubMed] [Google Scholar]
- Murashima-Suginami A, Takahashi K, Sakata T, Tsukamoto H, Sugai M, Yanagita M, et al. (2008). Enhanced BMP signaling results in supernumerary tooth formation in USAG-1 deficient mouse. Biochem Biophys Res Commun 369:1012-1016 [DOI] [PubMed] [Google Scholar]
- Nawaz S, Klar J, Wajid M, Aslam M, Tariq M, Schuster J, et al. (2009). WNT10A missense mutation associated with a complete Odonto-Onycho-Dermal Dysplasia syndrome. Eur J Hum Genet 17:1600-1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann S, Zhao C, Pascu F, Stahl U, Aulepp U, Niswander L, et al. (2004). Homozygous WNT3 mutation causes tetra-amelia in a large consanguineous family. Am J Hum Genet 74:558-563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara N, Suzuki Y, Takeda M. (2006). Gene expression of beta-catenin is up-regulated in inner dental epithelium and enamel knots during molar tooth morphogenesis in the mouse. Cell Tissue Res 325:197-201 [DOI] [PubMed] [Google Scholar]
- Odajima T, Sasaki Y, Tanaka N, Kato-Mori Y, Asanuma H, Ikeda T, et al. (2005). Abnormal beta-catenin expression in oral cancer with no gene mutation: correlation with expression of cyclin D1 and epidermal growth factor receptor, Ki-67 labeling index, and clinicopathological features. Hum Pathol 36:234-241 [DOI] [PubMed] [Google Scholar]
- Ohazama A, Johnson EB, Ota MS, Choi HY, Porntaveetus T, Oommen S, et al. (2008). Lrp4 modulates extracellular integration of cell signaling pathways in development. PLoS One 3:e4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo T, Pevny LH, Hogan BL. (2006). Sox2 is required for development of taste bud sensory cells. Genes Dev 20:2654-2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterwegel M, van de Wetering M, Timmerman J, Kruisbeek A, Destree O, Meijlink F, et al. (1993). Differential expression of the HMG box factors TCF-1 and LEF-1 during murine embryogenesis. Development 118:439-448 [DOI] [PubMed] [Google Scholar]
- Peanchitlertkajorn S, Cooper ME, Liu YE, Field LL, Marazita ML. (2003). Chromosome 17: gene mapping studies of cleft lip with or without cleft palate in Chinese families. Cleft Palate Craniofac J 40:71-79 [DOI] [PubMed] [Google Scholar]
- Queimado L, Lopes CS, Reis AM. (2007). WIF1, an inhibitor of the Wnt pathway, is rearranged in salivary gland tumors. Genes Chromosomes Cancer 46:215-225 [DOI] [PubMed] [Google Scholar]
- Rhee CS, Sen M, Lu D, Wu C, Leoni L, Rubin J, et al. (2002). Wnt and frizzled receptors as potential targets for immunotherapy in head and neck squamous cell carcinomas. Oncogene 21:6598-6605 [DOI] [PubMed] [Google Scholar]
- Rickels MR, Zhang X, Mumm S, Whyte MP. (2005). Oropharyngeal skeletal disease accompanying high bone mass and novel LRP5 mutation. J Bone Miner Res 20:878-885 [DOI] [PubMed] [Google Scholar]
- Rigas B. (2007). The use of nitric oxide-donating nonsteroidal anti-inflammatory drugs in the chemoprevention of colorectal neoplasia. Curr Opin Gastroenterol 23:55-59 [DOI] [PubMed] [Google Scholar]
- Rinn JL, Bondre C, Gladstone HB, Brown PO, Chang HY. (2006). Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet 2:e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler E, Du Y, Glinka A, Dutra A, Niehrs C, Muenke M. (2000). The genomic structure, chromosome location, and analysis of the human DKK1 head inducer gene as a candidate for holoprosencephaly. Cytogenet Cell Genet 89:220-224 [DOI] [PubMed] [Google Scholar]
- Rooker SM, Liu B, Helms JA. (2010). Role of Wnt signaling in the biology of the periodontium. Dev Dyn 239:140-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roose J, Huls G, van Beest M, Moerer P, van der Horn K, Goldschmeding R, et al. (1999). Synergy between tumor suppressor APC and the beta-catenin-Tcf4 target Tcf1. Science 285:1923-1926 [DOI] [PubMed] [Google Scholar]
- Rutherford RB, Foster BL, Bammler T, Beyer RP, Sato S, Somerman MJ. (2006). Extracellular phosphate alters cementoblast gene expression. J Dent Res 85:505-509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar L, Sharpe PT. (1999). Expression of Wnt signalling pathway genes during tooth development. Mech Dev 85:197-200 [DOI] [PubMed] [Google Scholar]
- Sarkar L, Sharpe PT. (2000). Inhibition of Wnt signaling by exogenous Mfrzb1 protein affects molar tooth size. J Dent Res 79:920-925 [DOI] [PubMed] [Google Scholar]
- Sarkar L, Cobourne M, Naylor S, Smalley M, Dale T, Sharpe PT. (2000). Wnt/Shh interactions regulate ectodermal boundary formation during mammalian tooth development. Proc Natl Acad Sci USA 97:4520-4524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Ito Y, Xu X, Han J, Bringas P, Jr, Maeda T, et al. (2005). LEF1 is a critical epithelial survival factor during tooth morphogenesis. Dev Biol 278:130-143 [DOI] [PubMed] [Google Scholar]
- Schutte BC, Murray JC. (1999). The many faces and factors of orofacial clefts. Hum Mol Genet 8:1853-1859 [DOI] [PubMed] [Google Scholar]
- Sekine S, Shibata T, Kokubu A, Morishita Y, Noguchi M, Nakanishi Y, et al. (2002). Craniopharyngiomas of adamantinomatous type harbor beta-catenin gene mutations. Am J Pathol 161:1997-2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine S, Sato S, Takata T, Fukuda Y, Ishida T, Kishino M, et al. (2003). Beta-catenin mutations are frequent in calcifying odontogenic cysts, but rare in ameloblastomas. Am J Pathol 163:1707-1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbedzija GN, Bronner-Fraser M, Fraser SE. (1992). Vital dye analysis of cranial neural crest cell migration in the mouse embryo. Development 116:297-307 [DOI] [PubMed] [Google Scholar]
- Shaw D, Ray A, Marazita M, Field L. (1993). Further evidence of a relationship between the retinoic acid receptor alpha locus and nonsyndromic cleft lip with or without cleft palate (CL +/- P). Am J Hum Genet 53:1156-1157 [PMC free article] [PubMed] [Google Scholar]
- Siriwardena BS, Kudo Y, Ogawa I, Tilakaratne WM, Takata T. (2009). Aberrant beta-catenin expression and adenomatous polyposis coli gene mutation in ameloblastoma and odontogenic carcinoma. Oral Oncol 45:103-108 [DOI] [PubMed] [Google Scholar]
- Sogabe Y, Suzuki H, Toyota M, Ogi K, Imai T, Nojima M, et al. (2008). Epigenetic inactivation of SFRP genes in oral squamous cell carcinoma. Int J Oncol 32:1253-1261 [DOI] [PubMed] [Google Scholar]
- Später D, Hill TP, Gruber M, Hartmann C. (2006). Role of canonical Wnt-signalling in joint formation. Eur Cell Mater 12:71-80 [DOI] [PubMed] [Google Scholar]
- Stone LM, Finger TE, Tam PP, Tan SS. (1995). Taste receptor cells arise from local epithelium, not neurogenic ectoderm. Proc Natl Acad Sci USA 92:1916-1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhdeo K, Mani M, Zhang Y, Dutta J, Yasui H, Rooney MD, et al. (2007). Targeting the beta-catenin/TCF transcriptional complex in the treatment of multiple myeloma. Proc Natl Acad Sci USA 104:7516-7521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen M, Thesleff I. (2010). Patterns of Wnt pathway activity in the mouse incisor indicate absence of Wnt/beta-catenin signaling in the epithelial stem cells. Dev Dyn 239:364-372 [DOI] [PubMed] [Google Scholar]
- Tan SS, Morriss-Kay G. (1985). The development and distribution of the cranial neural crest in the rat embryo. Cell Tissue Res 240:403-416 [DOI] [PubMed] [Google Scholar]
- Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, et al. (2005). Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell 120:857-871 [DOI] [PubMed] [Google Scholar]
- Thesleff I. (2006). The genetic basis of tooth development and dental defects. Am J Med Genet A 140:2530-2535 [DOI] [PubMed] [Google Scholar]
- Thirumangalathu S, Harlow DE, Driskell AL, Krimm RF, Barlow LA. (2009). Fate mapping of mammalian embryonic taste bud progenitors. Development 136:1519-1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. (2007). Breadth of tuning and taste coding in mammalian taste buds. J Neurosci 27:10840-10848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE. (1988). Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell 55:619-625 [DOI] [PubMed] [Google Scholar]
- Tzahor E, Kempf H, Mootoosamy RC, Poon AC, Abzhanov A, Tabin CJ, et al. (2003). Antagonists of Wnt and BMP signaling promote the formation of vertebrate head muscle. Genes Dev 17:3087-3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uraguchi M, Morikawa M, Shirakawa M, Sanada K, Imai K. (2004). Activation of WNT family expression and signaling in squamous cell carcinomas of the oral cavity. J Dent Res 83:327-332 [DOI] [PubMed] [Google Scholar]
- Vadlamudi U, Espinoza HM, Ganga M, Martin DM, Liu X, Engelhardt JF, et al. (2005). PITX2, beta-catenin and LEF-1 interact to synergistically regulate the LEF-1 promoter. J Cell Sci 118(Pt 6):1129-1137 [DOI] [PubMed] [Google Scholar]
- van Genderen C, Okamura RM, Farinas I, Quo RG, Parslow TG, Bruhn L, et al. (1994). Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev 8:2691-2703 [DOI] [PubMed] [Google Scholar]
- Vanderas AP. (1987). Incidence of cleft lip, cleft palate, and cleft lip and palate among races: a review. Cleft Palate J 24:216-225 [PubMed] [Google Scholar]
- Wang XP, O’Connell DJ, Lund JJ, Saadi I, Kuraguchi M, Turbe-Doan A, et al. (2009). Apc inhibition of Wnt signaling regulates supernumerary tooth formation during embryogenesis and throughout adulthood. Development 136:1939-1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton KA, Jr, Zimmermann G, Rousset R, Scott MP. (2001). Vertebrate proteins related to Drosophila Naked Cuticle bind Dishevelled and antagonize Wnt signaling. Dev Biol 234:93-106 [DOI] [PubMed] [Google Scholar]
- Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. (2005). Deconstructing the cadherin-catenin-actin complex. Cell 123:889-901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada W, Nagao K, Horikoshi K, Fujikura A, Ikeda E, Inagaki Y, et al. (2009). Craniofacial malformation in R-spondin2 knockout mice. Biochem Biophys Res Commun 381:453-458 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Cho SW, Kim EJ, Kim JY, Fujiwara N, Jung HS. (2004). Developmental properties of the Hertwig’s epithelial root sheath in mice. J Dent Res 83:688-692 [DOI] [PubMed] [Google Scholar]
- Yamashiro T, Zheng L, Shitaku Y, Saito M, Tsubakimoto T, Takada K, et al. (2007). Wnt10a regulates dentin sialophosphoprotein mRNA expression and possibly links odontoblast differentiation and tooth morphogenesis. Differentiation 75:452-462 [DOI] [PubMed] [Google Scholar]
- Yang F, Zeng Q, Yu G, Li S, Wang CY. (2006). Wnt/beta-catenin signaling inhibits death receptor-mediated apoptosis and promotes invasive growth of HNSCC. Cell Signal 18:679-687 [DOI] [PubMed] [Google Scholar]
- Yang R, Crowley HH, Rock ME, Kinnamon JC. (2000). Taste cells with synapses in rat circumvallate papillae display SNAP-25-like immunoreactivity. J Comp Neurol 424:205-215 [DOI] [PubMed] [Google Scholar]
- Zhou P, Byrne C, Jacobs J, Fuchs E. (1995). Lymphoid enhancer factor 1 directs hair follicle patterning and epithelial cell fate. Genes Dev 9:700-713 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liu HX, Mistretta CM. (2006). Bone morphogenetic proteins and noggin: inhibiting and inducing fungiform taste papilla development. Dev Biol 297:198-213 [DOI] [PubMed] [Google Scholar]