Abstract
Genetic cases of congenital pituitary hormone deficiency are common and many are caused by transcription factor defects. Mouse models with orthologous mutations are invaluable for uncovering the molecular mechanisms that lead to problems in organ development and typical patient characteristics. We are using mutant mice defective in the transcription factors PROP1 and POU1F1 for gene expression profiling to identify target genes for these critical transcription factors and candidates for cases of pituitary hormone deficiency of unknown etiology. These studies reveal critical roles for Wnt signalling pathways including the TCF/LEF transcription factors and interacting proteins of the groucho family, bone morphogenetic proteins antagonists, and targets of notch signalling. Current studies are investigating roles of novel homeobox genes and pathways that regulate the transition from proliferation to differentiation, cell adhesion and cell migration.
Pituitary adenomas are a common human health problem, yet most cases are sporadic, necessitating alternative approaches to traditional Mendelian genetic studies. Mouse models of adenoma formation offer the opportunity for gene expression profiling during progressive stages of hyperplasia, adenoma and tumorigenesis. This approach holds promise for identification of relevant pathways and candidate genes as risk factors for adenoma formation, understanding mechanisms of progression, and identifying drug targets and clinically relevant biomarkers.
Keywords: cell proliferation, apoptosis, transcription factors, Prop1, Emx2
Introduction
The study of single gene defects that lead to congenital pituitary hormone deficiency in mouse and man has uncovered a cadre of transcription factors that are involved in development of the pituitary gland and transcription of hormone genes (Table 1) [1, 2]. Several of the genes identified in this manner belong to multigene families and some exhibit overlapping functions with related genes within the family, such as Pitx1 and Pitx2, Lhx3 and Lhx4, and Sox2 and Sox3 [3–9]. This supports the hypothesis that additional related genes could contribute to a genetic predisposition for pituitary hormone deficiencies. PROP1 is an intriguing member of this group of critical pituitary transcription factors. It is a member of the paired class of homeobox transcription factors, but phylogenetic analysis places it in an orphan class [9]. Thus, there are no immediately obvious genes that are predicted to exhibit overlapping functions.
Table 1.
Transcription factors defects that cause congenital pituitary hormone deficiency
| Gene name | Type of protein | Disease * |
|---|---|---|
| PITX2 | Bicoid-paired homeo | Rieger syndrome (eyes, teeth), GHD |
| LHX4 | LIM homeo | MPHD, cerebellum and skull defects |
| LHX3 | LIM homeo | MPHD, rigid cervical spine |
| SOX2 | HMG box | Brain and eye defects, growth insufficiency |
| SOX3 | HMG box | Mental retardation, hypopituitarism, pituitary dysmorphology |
| HESX1 | Paired homeo | Septo-optic dysplasia, MPHD, GHD |
| PROP1 | Paired homeo | MPHD: lack LH, FSH, GH, TSH, PRL |
| POU1F1 | POU homeo | MPHD: lack GH, TSH, PRL |
| TBX19 | T box | Isolated ACTH deficiency |
| NR5A1 | Nuclear receptor | LH, FSH deficient, hypogonadism, adrenal failure |
| OTX1 | Bicoid-paired | Mice: transient dwarfism and hypogonadism |
| OTX2 | Bicoid-paired | Dysgnathia Complex |
Abbreviations: GHD = growth hormone deficiency, MPHD = multiple pituitary hormone deficiency
Please consult OMIM (http://www.ncbi.nlm.nih.gov/sites/entrez?db=OMIM) for complete disease annotations
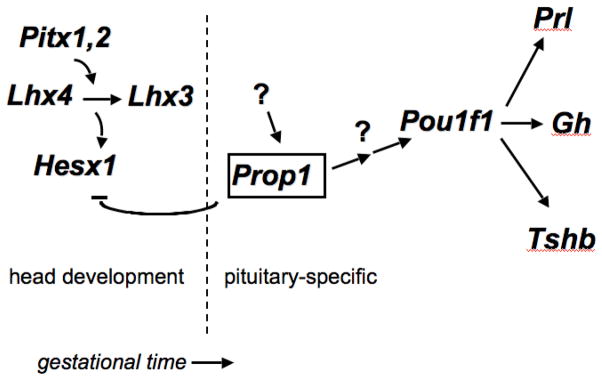
Prop1 is a pivotal regulatory gene within the hierarchy of pituitary transcription factors. It is the first known pituitary-specific gene in the pathway, and it is required for repression of the paired homeobox gene Hesx1 and for activation of Pou1f1 (a founding member of the POU homeodomain family, formerly known as Pit1 or Ghf-1) [10] (Fig. 1). Hesx1 is expressed in embryonic stem cells and in Rathke’s pouch until approximately e12.5 [11, 12]. Prop1 expression is detectable in the developing pituitary gland, with onset around e10.5 in mice, peaking at e12.5 and waning to barely detectable levels by e14.5, while Pou1f1 transcription is detectable at e14.5 and persists through adulthood [7, 13]. The distinctive lag time between peak Prop1 expression and detection of Pou1f1 transcripts suggests that there are additional steps in the hierarchy of transcriptional control. Such gene(s) are candidates to investigate causes of panhypopituitarism in patients with features typical of PROP1 and POU1F1 mutations, but for which the molecular lesions remain elusive.
Fig. 1.
Genetic hierarchy of transcription factor regulation of pituitary development. Studies with individual mutants and double mutants have suggested that Pitx1 and Pitx2 have overlapping functions required for activation of the LIM homeobox genes Lhx3 and Lhx4. These genes also have overlapping functions and are required for the maintenance of Hesx1 expression. Prop1 is critical for timely suppression of Hesx1 expression, but the genes required for initial activation of Prop1 transcription are not known. Prop1 is necessary for activation of Pou1f1, but the lag of several days between detection of Prop1 transcripts and Pou1f1 transcripts suggests that additional genes are required to activate Pou1f1. Pou1f1 is necessary for expansion of precursor cells that give rise to cells producing Tshb, Gh and Prl. THE Correct figure is shown below. IT SHOULD HAVE A REPRESSOR ARROW BETWEEN HESX1 and PROP1, not an activator.
Lesions in Prop1 and Pou1f1 produce essentially indistinguishable phenotypes in adult mice, namely anterior pituitary hypoplasia, undetectable circulating levels of thyroid-stimulating hormone (TSH), growth hormone (GH) and prolactin (PRL) owing to the failure to produce the cells specialized in synthesis and secretion of these hormones, and dramatically reduced gonadotropin production, which can be corrected by thyroid hormone and GH replacement therapy [14–17]. We selected these genes for the focus of our studies because they are more commonly mutated in patients with panhypopituitarism than any of the other genes discovered to date, and they are the first known genes in the genetic hierarchy that are pituitary specific in their expression [7, 13, 18–20].
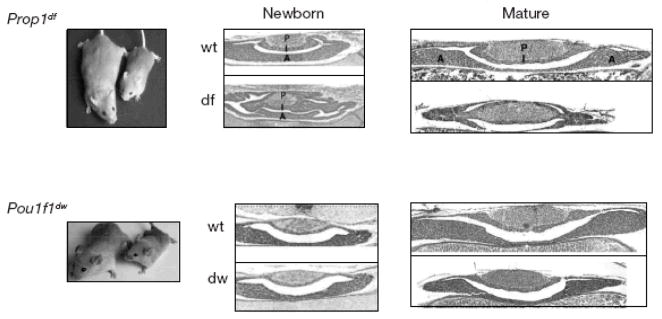
Despite the similar phenotypes in adult mutant mice, there are striking differences in the appearance of the pituitary glands of newborn mutants (Fig. 2). At birth the Prop1 mutants have a highly dysmorphic organ that likely arises from failure of progenitor cells to undergo the transition from proliferation to differentiation. These cells maintain the appearance of flattened, tightly adhered cells instead of adopting a more rounded shape and loosely packed appearance typical of glandular cells, suggesting failure of the epithelial to mesenchymal transition. The changes in migration, cellular conformation and function in the presence of Prop1 suggests pituitary cells may be regulated by analogous, though opposite, transformations typical of the planar cell polarity pathway [21]. In the absence of Prop1, progenitor cells fail to migrate and colonize the anterior lobe, which results in fewer proliferation competent cells in the anterior lobe by birth than is characteristic of normal mice [22, 23]. In addition, the Prop1 mutant pituitary gland shows signs of poor vascularization and enhanced cell death. In contrast, the Pou1f1 mutant pituitary glands are indistinguishable from normal glands at birth and exhibit evidence of anterior lobe hypoplasia during the first weeks of life. These striking phenotypic differences in the pituitary glands of Prop1 and Pou1f1 mutants suggest that these genes have fundamentally different roles in organ development. Furthermore, these observations suggest that identification of Prop1 target genes could reveal promising candidate genes for understanding additional cases of hypopituitarism that remain unexplained.
Fig. 2.
Striking differences in pituitary morphology at birth suggest major differences in Prop1 and Pou1f1 function. Prop1 mutants carrying the spontaneous Ser180Pro mutation, or Ames dwarfs (df), are shown next to normal mice (wt) of the same strain, a non-inbred DF/B stock carrying the pink-eyed dilute mutation p [17]. A mouse homozygous for the spontaneous G261T mutation in Pou1f1 is shown next to a normal mouse on the inbred DW/J stock. Both mutations produce a similar degree of adult pituitary hypoplasia, dwarfism, hypothyroidism and infertility. The lobes of the pituitary are posterior (P), intermediate (I), and anterior (A). Newborn = day of birth, “Mature” = 11 days old. The image of the Prop1df/df mouse and wild type littermate is from Buckwalter et al., Genomics, 10:515–526, 1991, the Pou1f1dw/dw mouse and wild type littermate is from Karolyi et al., Mammalian Genome, 18:596–608, 2007, and the pituitary gland sections are all from Ward, et al., Mol Endocrinol. 20:1378–90, 2006.
We have taken several approaches to identifying potential Prop1 target genes and genes expressed at the critical time of e12.5–e14.5 in mouse pituitary development. Our first forays utilized differential display and subtractive hybridization techniques [24, 25]. Limitations of these approaches are their relatively inefficient nature and the fact that they generate short clones, which are not useful for validation of expression by in situ hybridization or for functional studies. Nevertheless, they were fruitful in uncovering some critical signalling pathways. We noticed an enrichment of clones in the canonical and non-canonical Wnt signalling pathways and pursued validation and functional studies with individual knockout mice to determine functional roles for these genes. This work revealed the important roles of Tcf7l2 (Tcf4), Wnt5a, and amino-terminal enhancer of split (Aes) [26–29]. Although none of these genes are Prop1 targets, at least one candidate for growth insufficiency in humans arose from these studies, namely AES, also known as groucho-related gene 5 (GRG5), which produces a protein that can bind to engrailed repressor domains and act as a co-repressor or de-repressor in a context-dependent manner [30, 31]. The role of the Tcf7l2 in suppression of pituitary growth suggests that the role of the human orthologue in pituitary adenomas should be explored.
We undertook the generation of full-length cDNAs from developing pituitary gland and deep sequencing to provide a more complete picture of the pituitary transcriptome and to provide cDNA clones suitable for validation and functional studies [32]. In addition, we carried out subtractive hybridization using these full-length clones and methods that were technically advanced compared to our initial efforts (Brinkmeier et al., in press). Preliminary analysis has revealed that this approach was successful in uncovering potential Prop1 targets and candidate genes for pituitary hypoplasia. We report here the evaluation of the functional role of the empty spiracles homolog 2 gene (Emx2) in pituitary gland development and provide the results of a bioinformatic analysis that suggest candidate genes for involvement in the biological processes of cell proliferation, cell adhesion, cell migration and apoptosis.
Materials and Methods
We prepared RNA and cDNA from pituitary glands dissected from wild-type mice at e12.5 and e14.5 and from Prop1 mutants at e14.5 using techniques designed to maximize full-length cDNA clones [32]. In addition two sets of subtractive hybridizations were carried out in order to enrich for potential Prop1 target genes. Details of this research will be published elsewhere (Brinkmeier et al., in press). One subtracted library was optimized for genes transcriptionally activated between e12.5 and e14.5 (e14.5 wild type minus e12.5 wild type) and the other enriched for genes dependent upon Prop1 for expression at e14.5 (wild type e14.5 minus Prop1 mutant littermates at e14.5). Single-pass DNA sequencing was carried out from either the 5′ or the 3′ end on over 50,000 clones representing all five libraries approximately equally. We organized the sequences into a database searchable by gene name, RefSeq and Unigene ID, gene ontology terms (GO), and DNA sequence. Preliminary studies suggest that these gene identifications based on single-pass sequence analysis yield a 1–3% false identification rate. (Footnote: To obtain database access contact Drs. Camper or Lyons at scamper@umich.edu or boblyons@umich.edu.)
Emx2 null mutants were kindly provided by A. Mansouri and P Gruss from the Max-Planck-Institute, Germany, on a part 129/Sv, part C57BL/6J genetic background [33]. They were generated by replacing the second and part of the third helix of the homeobox. For genotyping, a single forward primer, 5′ CAC AAG TCC CGA GAG TTT CCT TTT GCA CAA CG 3′ was used for both alleles, and the reverse primers 5′ ACC TGA GTT TCC GTA AGA CTG AGA CTG TGA GC 3′ was used for the wild type and 5′ ACT TCC TGA CTA GGG GAG GAG TAG AAG GTG G 3′ was used for the mutant sequence [34] Mice for this paper were maintained at the Wellcome Trust Sanger Institute, Hinxton, UK in compliance with UK Home Office requirements. Wild type, heterozygous, and homozygous null animals were collected from three separate litters at e16.5, fixed and sectioned for immunohistochemical analysis. Four embryos were examined for each genotype, and 10–12 sections were examined for each embryo. Immunohistochemistry with antibodies specific for various pituitary hormones and transcription factors was carried out as described previously [35].
Results and Discussion
cDNA Libraries from Developing Pituitary Provide Transcriptome Information
We have created a searchable database of cDNA libraries representing a partial transcriptome of the developing pituitary that includes cDNA from e12.5 and e14.5 wild-type pituitaries and e14.5 pituitaries deficient for Prop1 [32]. This database has proven extremely valuable in aiding gene discovery efforts as we detail below.
Transcription Factors are Highly Represented in the Pituitary Transcriptome
Transcription factors comprised the largest class of genes we identified. Over 700 transcription factor genes were detected. Intriguingly, more than 40 of these transcription factors contained homeodomains. At least 12 of these homeobox genes have previously known roles in pituitary development, approximately 6 are known to be expressed in the pituitary gland but their functional roles in intact animals have not been studied directly, and over 20 were completely unexpected. Many of the novel homeobox genes detected in this analysis have only been studied in invertebrates. We present some examples of genes in each of these groups.
Zinc Finger Homeobox Genes
We found expression of two genes in developing pituitary glands, Zfhx3 and Zeb2, which contain numerous zinc fingers in addition to homeodomain(s) (unpublished). Zfhx3 was originally named Atbf1 because it was identified as a factor binding to the alpha-fetoprotein enhancer, and it was recently discovered to bind the Pou1f1 early enhancer [36, 37]. Analysis of a hypomorphic gene trap allele demonstrated that this unusual protein, which contains 4 homeodomains and 23 zinc fingers, is necessary for robust expression of Pou1f1, Gh, and Tshb genes [37]. Zeb2, formerly named Zfhx1b, is predicted to encode a multifunctional protein with a single homeodomain and eight zinc fingers that interacts with Smads and nucleosome remodelling complexes, acts upstream of Wnt signalling, and effects expression of Sox2 and E-cadherin [38, 39]. ZEB2 was also detected in a screen for genes expressed in human pituitary adenomas [40]. Individuals with mutations in ZEB2 have Mowat–Wilson Syndrome, a dominant disorder characterized by a distinctive facial phenotype in association with mental retardation, microcephaly and short stature [41]. Neural crest-specific disruption in mice causes neurocristopathies that mimic Mowat-Wilson syndrome, but the etiology of the short stature and the role of Zeb2 in pituitary development have not been defined [42]. Future studies may uncover a role for ZEB2 in pituitary function and growth regulation.
LIM Homeobox Genes
Several LIM homeodomain genes play important roles in pituitary development, including Lhx3, Lhx4, and Isl1 [43–45]. We found a related gene, Lhx2, represented by cDNAs in multiple developing pituitary cDNA libraries (unpublished). This gene was cloned based on its ability to bind an essential promoter element in the Cga gene, which encodes the alpha subunit of the glycoprotein hormones TSH, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) [46]. The role of this gene in pituitary development has not been explored, although mice homozygous for a disruption of Lhx2 revealed important requirements for Lhx2 in multiple organs [47]. It will be intriguing to determine whether its role in the pituitary gland involves cell identity or maintenance of growth and undifferentiated character of progenitors, as has been shown in other organs, and whether there is any link to pituitary adenoma risk, given the role in myeloproliferative disorders [48–50].
ADNP, a POU-Like Homeodomain Protein
Some of the homeobox genes we found in one or more of the five libraries were completely unexpected. An example is the activity-dependent neuroprotective protein gene (Adnp), which encodes a POU-like homeodomain protein. Adnp-deficient animals die by e8.5–9.5, making it unlikely that ADNP loss of function accounts for panhypopituitarism, but it is intriguing that some individuals with Okihiro syndrome are haploinsufficient for ADNP [51, 52]. Recent studies have shown that ADNP is bound to several members of the SWI/SNF chromatin-remodelling complex and it is necessary for upregulation of Neurod1, a gene important for corticotrope development [51, 53, 54]. Thus, future studies may uncover a basic role for ADNP in pituitary cell differentiation or maintenance [55].
EMX2, a Potential Tumour Suppressor and Regulator of Progenitor Migration and Differentiation
The presence of Emx2 cDNAs amongst the pituitary libraries is unexpected and intriguing. Emx2 encodes a homeodomain protein that is required for viability of mouse embyros and development of multiple organs including the central nervous and urogenital systems [56], yet its expression in the pituitary gland has not been previously described. In humans, EMX2 is required for normal cortical development and brain function, and it has been implicated as a tumour suppressor due to the presence of EMX2 loss-of-function mutations in endometrial cancers [57]. In the brain, EMX2 regulates the transition from early neural progenitors to more mature cells that migrate out of the proliferative zone and differentiate into a variety of neural cell types [58]. This feature is interesting in light of the requirement for pituitary progenitors to migrate away from the lumen of Rathke’s pouch and seed the anterior lobe with precursors for several different cell lineages [22, 23]. Another captivating potential parallel is the importance of several signalling pathways in pituitary development, including sonic hedgehog (SHH), beta-catenin, bone morphogenetic protein (BMP) and fibroblast growth factor (FGF), and the involvement of the same signalling pathways in regulation of Emx2 expression ([27, 59–63]. Taken together these findings suggest the possibility that Emx2 functions in pituitary ontogeny.
To explore the role of Emx2 in pituitary development, we compared pituitary morphology and expression of pituitary hormones and lineage-specific transcription factors in normal and Emx2 mutant mice (Fig. 3). We found no difference in the intensity or pattern of immunohistochemical staining for pro-opiomelanocortin (POMC) in the intermediate or anterior lobes of the pituitary gland, suggesting that differentiation of melanotropes and corticotropes has proceeded normally in the absence of Emx2. Somatotropes and thyrotropes appear unaffected also, as immunostaining revealed no differences in expression of Pou1f1 or its dependent targets, GH and TSH beta in normal and mutant mice. Finally, steroidogenic factor 1 (SF1 or NR5A1) is expressed equivalently in mutant and normal pituitary glands, suggesting that commitment to the gonadotrope lineage has occurred normally. While these results suggest that there is no essential role for Emx2 in pituitary ontogeny, its role in postnatal pituitary function or in pituitary adenoma risk still remains to be explored.
Fig. 3.
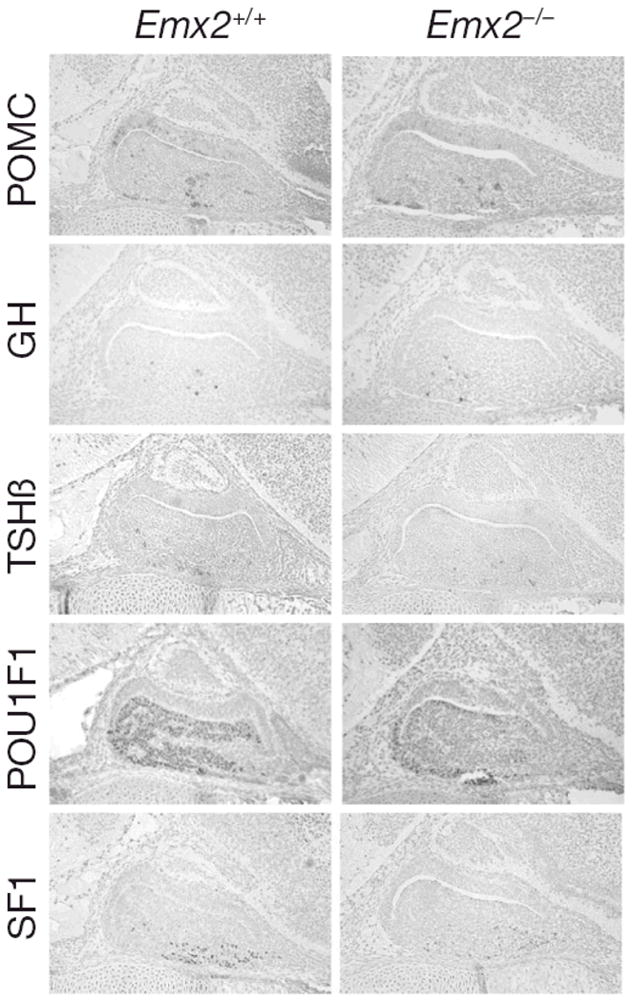
Emx2 is not required for normal anterior pituitary morphology or cell specification during development. Sagittal sections from heads of wild type and mutant Emx2 litter mates were immunostained with antibodies specific for POMC, GH, TSH, POU1F1 and SF1, developed with diaminobenzidine to produce a brown precipitate and counterstained with hematoxylin and eosin. The intensity of staining and the number and distribution of stained cells was similar in mutants and wild types. Typical examples are shown.
Signalling Pathways
Many signalling pathways have been implicated in early pituitary gland ontogeny. Most reviews of early pituitary development posit the hypothesis that opposing dorsal FGF signalling and ventral BMP signalling gradients establish domains of transcription factor expression that produce cell-specific differentiation of unique cell types. However, BMP4 is known to be required for induction of Rathke’s pouch [45], and it is produced in the ventral diencephalon, which is located dorsal to the developing pituitary primordium [60, 61]. FGFs are also produced in the ventral diencephalon and are required in a dosage-sensitive manner for stimulation of Rathke’s pouch development [60, 61]. In the absence of these FGFs or their receptors, Rathke’s pouch undergoes massive cell death resulting in extremely hypoplastic pituitaries [45, 64, 65]. We recently showed that the BMP antagonist, NOGGIN, plays an essential role in attenuating BMP activity in the ventral diencephalon during pouch induction, and that it is necessary to maintain a balance between BMP, Wnt, FGF, and SHH signalling pathways [59]. Noggin is also expressed in the adult pituitary where it may regulate the balance of BMP signalling that is known to promote prolactinoma formation, but that is also protective against corticotropinoma formation [59, 66, 67].
We found over 100 genes involved in signalling pathways or in the interaction of signalling pathways in the developing pituitary libraries (Brinkmeier, et al., in press). We find several BMP antagonists in addition to Noggin that are expressed in the developing pituitary gland [59]. These antagonists could be important in attenuating BMP activity in a temporally and spatially critical manner, as well as contributing to establishing or maintaining the appropriate balance between signalling pathways. We also detect expression of several members of the Notch pathway (unpublished). This pathway appears to be regulated directly by Prop1 because Notch2 is not expressed in Prop1 mutants [68]. Moreover, some putative downstream targets of this pathway are important for normal pituitary development [69, 70]. Additional studies are necessary to explore the roles of these newly identified signalling genes.
Genes Involved in Proliferation, Apoptosis, Cell Migration and Cell Adhesion
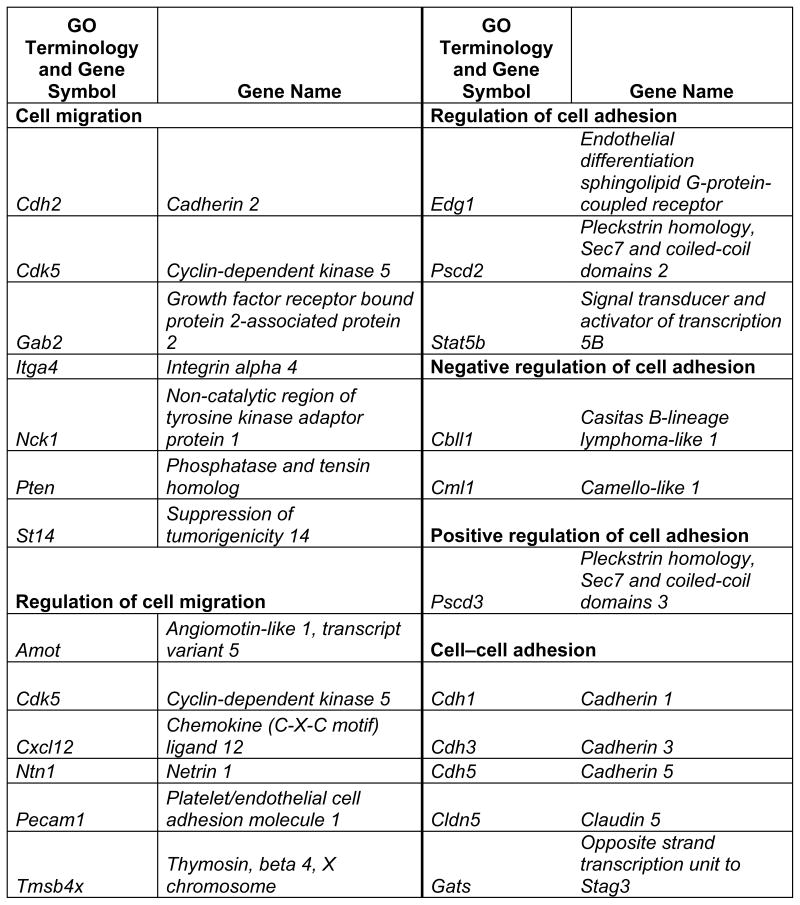
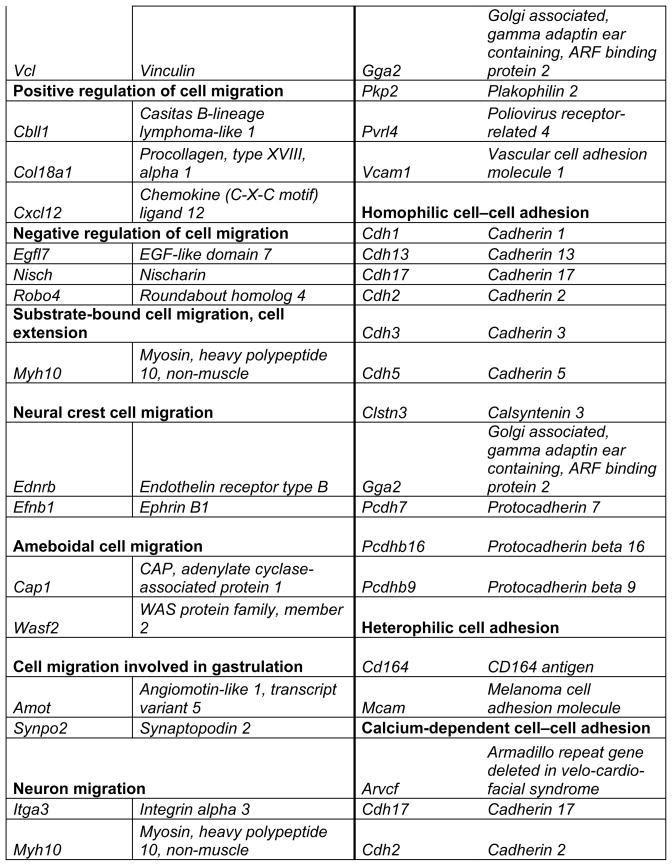
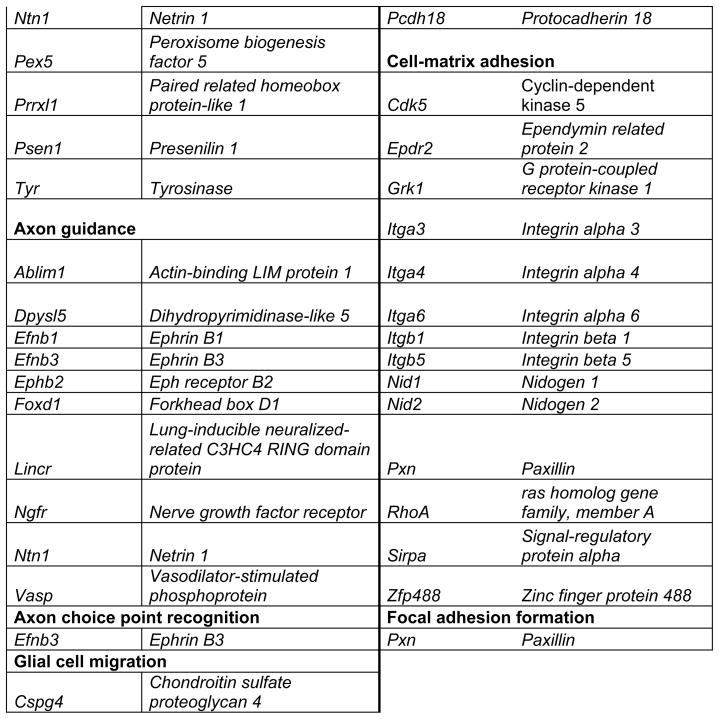
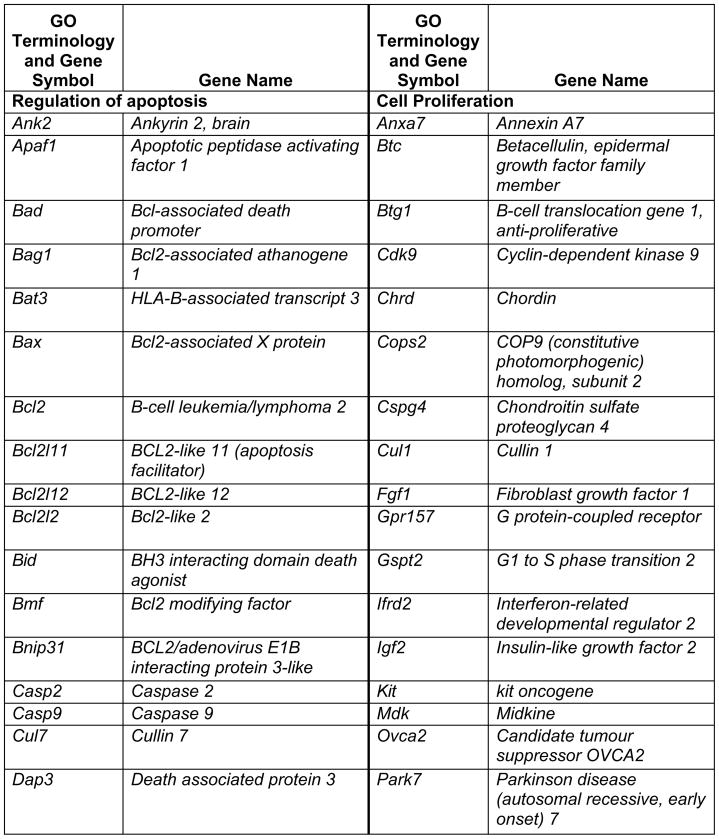
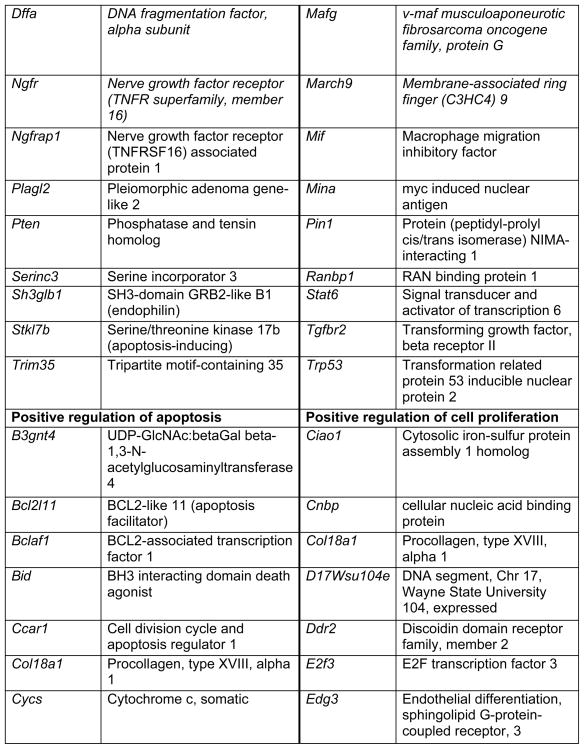
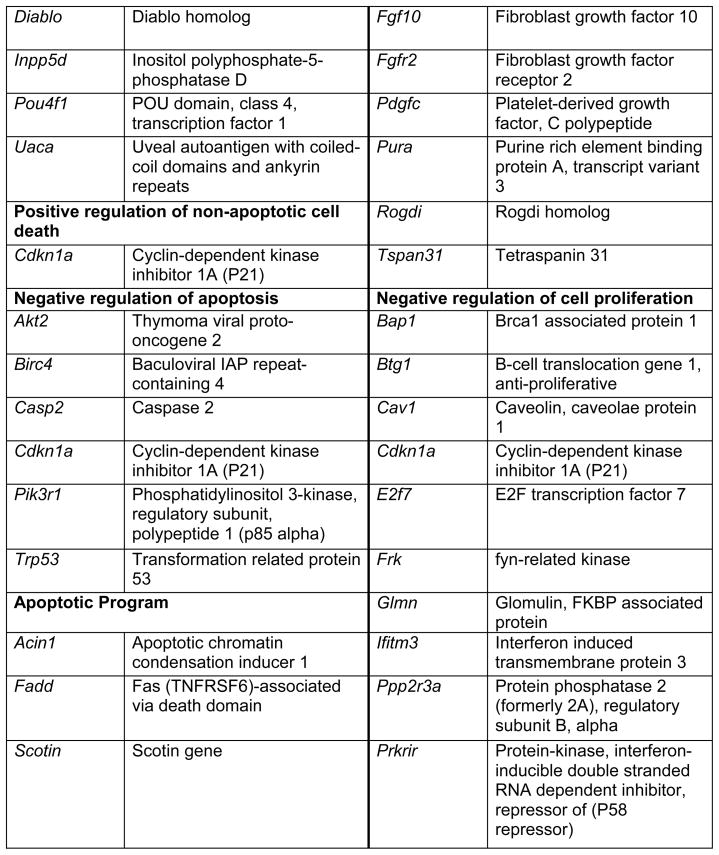
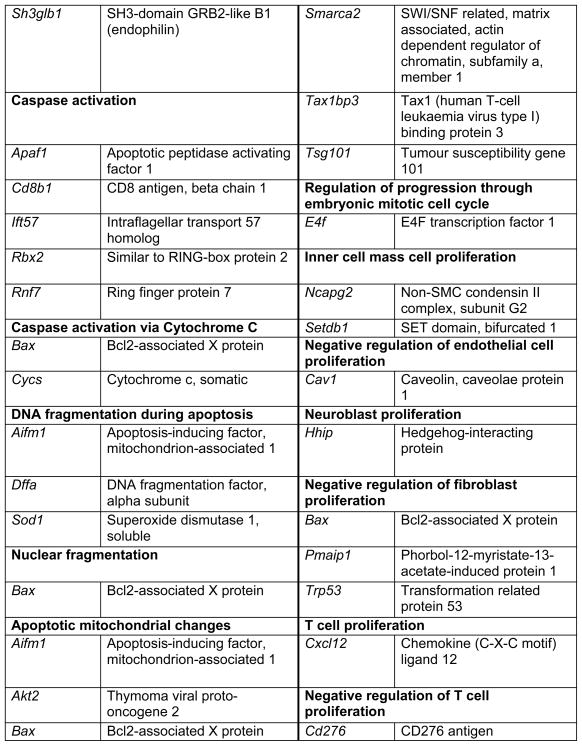
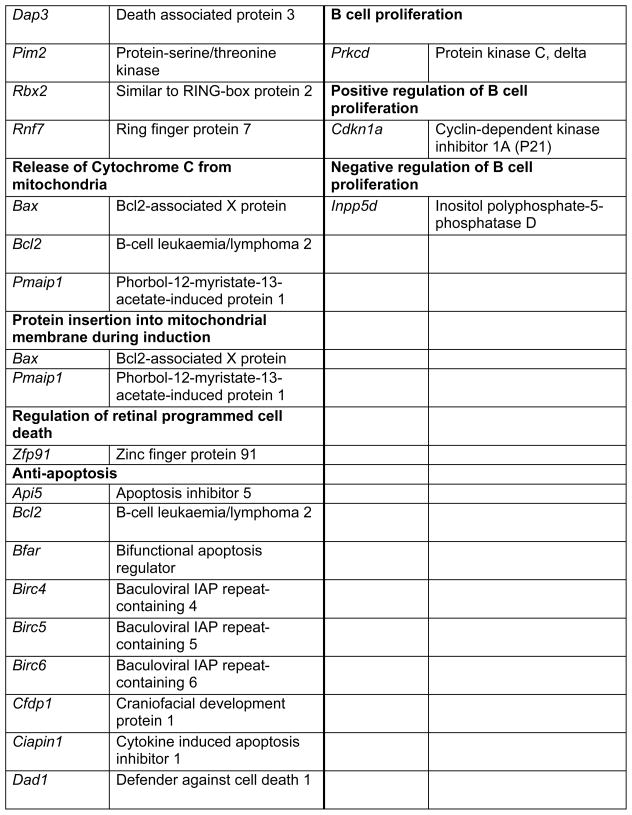
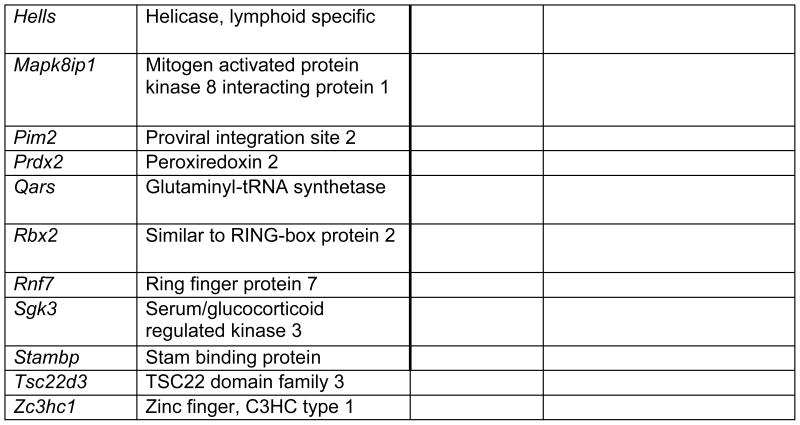
Prop1 is required for migration of precursors away from the lumen of Rathke’s pouch, for the production of proliferating cells in the developing anterior lobe, and for invasion of the vasculature [22, 23]. The movement of precursor cells occurs concomitant with changes in cell adhesion and cell shape. The failure of these Prop1 dependent processes is associated with enhanced cell death. We expected to find cDNAs in the developing pituitary libraries with GO terms relevant to the affected processes in Prop1-mutant mice. In fact we found approximately 80 genes with the GO terms proliferation and regulation proliferation, including some known oncogenes and tumour suppressors (unpublished). There were approximately 100 genes with apoptosis as a GO term, 37 with ‘cell migration’, and 96 with ‘cell adhesion’. These are catalogued in Table 2a and b. These cDNAs require DNA sequence verification and confirmation of expression by reverse transcription polymerase chain reaction, in situ hybridization, or immunohistochemistry as a basis for future functional studies.
Table 2a.
Genes identified with relationships to cell migration and cell adhesion pathways*
|
|
|
This table lists mouse genes. For human orthologues refer to http://www.informatics.jax.org/
Table 2b.
Genes identified with relationships to cell apoptosis and proliferation*
|

|
|
|
|
|
|
This table lists mouse genes. For human orthologues refer to http://www.informatics.jax.org/
Pituitary Adenomas
Pituitary adenomas are common, comprising 15% of intracranial tumours [71–73]. They tend to be slow growing, benign neoplasms. About one-third of pituitary adenomas are silent, coming to the patient or physician’s attention because of compressive effects such as headaches and visual problems. The remaining two thirds of these adenomas secrete hormones.
A few Mendelian disorders predispose to pituitary adenoma formation: multiple endocrine neoplasia (MEN), Carney complex, and McCune–Albright syndrome [73]. MENIN is mutated in MEN1 [74]. It encodes a tumour suppressor; loss of heterozygosity causes parathyroid, endocrine pancreas, and anterior pituitary neoplasias. Carney complex is a genetically heterogeneous, chromosome instability syndrome. Lesions in protein kinase, cAMP-dependent regulatory, type I, alpha (PRKAR1A) cause adrenal and anterior pituitary neoplasms that are multifocal, commonly involving GH hypersecretion. McCune–Albright syndrome causes precocious puberty and pituitary hyperplasia due to activating mutations in GNAS1 leading to excess cAMP production. Each of these Mendelian disorders has provided insight into the pathways that regulate pituitary growth. Intriguingly, all three of these genes are represented in the developmental database, implying an early role in pituitary development.
Most pituitary adenomas are sporadic and monoclonal. They often originate as hyperplasia that progresses to adenoma and have evidence of chromosome instability and aneuploidy [71–73]. Mutations that cause these sporadic adenomas are largely unknown. Although approximately 40% of acromegaly cases are caused by GNAS mutations that lead to excess GH production, the aetiology of most cases remains elusive. Mutations in the gene for aryl hydrocarbon receptor interacting protein (AIP) have been reported to cause GH-producing adenomas in Finnish populations, and many laboratories are working to replicate this result in other ethnic groups and discover the mechanism whereby lesions in this gene could enhance the risk for acromegaly [75]. In addition, the aryl hydrocarbon receptor gene (Ahr) and Aip are present in our developmental database. Pituitary tumour transforming gene (PTTG) expression correlates with tumour size and invasiveness [76, 77]. It was discovered by differential gene expression and led to understanding the role of securin proteins in mitosis, which is relevant to many types of cancers [78, 79]. This suggests that expanded application of the differential expression approach could provide further insight into pituitary adenoma risk factors, biomarkers and treatments. As with Ahr and Aip, Pttg is represented in the developmental database, lending support to the idea that early developmental roles are recapitulated during tumorigenesis.
Gene expression studies have been carried out using human pituitary adenoma tissue, mouse pituitary tumour tissue, and immortalized murine cell lines thought to represent different stages of differentiation [40, 80–83]. An advantage of animal models is that the pituitary tissue is readily accessible at various stages during the progression from hyperplasia to adenoma formation, and following treatment with hormones or drugs that suppress adenoma growth.
We developed two animal models that could be useful in identifying candidate genes involved in progression to adenoma and growth suppression. Constitutive expression of Prop1 in transgenic mice produced a model with a low penetrance of adenomas. In this model Prop1 expression was driven by the Cga promoter and enhancer sequences, plus an intronic element of the Prop1 gene that confers expression in the pituitary progenitor cells that line the lumen of Rathke’s pouch [84, 85]. One transgenic line exhibits delayed gonadotropin production and delayed puberty, but eventually achieves sexual maturation and fertility [86]. The genetic background and transgene integration site both appear to influence the penetrance of adenoma development in this model. On a mixed background one line developed large nodules that did not express Prop1, but fewer adenomas were observed after repeated backcrossing to C57BL6/J mice (unpublished). Recent studies show that female mice with the constitutive Prop1 transgene can develop GH, PRL, TSH, and/or gonadotropin-producing adenomas [87]. In contrast the Cga (α-Gsu) knockout mouse develops thyrotrope adenomas that are multifocal, very highly penetrant, and responsive to thyroid hormone mediated growth suppression [88–90]. We are in the process of using this model to uncover genes that are differentially expressed between normal and mutant mice at a variety of ages (unpublished). We expect that this approach could shed light on growth dysregulation in thyrotrope adenomas, which are rare, but very tenacious, recurrent and difficult to treat.
Conclusion and future directions
The deep sequencing approach we are taking is fruitful for identification of individual genes and pathways that regulate pituitary development. We have proven that several genes discovered with this approach play important roles in pituitary development and function, and at least some of these are candidates for screening patients with panhypopituitarism. This approach can now be done in a less laborious manner using the new technologies that facilitate massively parallel DNA sequencing and bioinformatic analysis of the data using the complete genome sequence and dense annotation of genes. Pyrosequencing using the 454 genome analyzer by Roche can produce 400,000 sequences of up to 250 nucleotides each in a single run [91], and the Illumina (formerly Solexa) Genome Analyzer generates 100 million sequences, each approximately 25–35 base pairs, in a single run [92]. These and other new DNA sequencing technologies promise to provide information with ease and more depth than the traditional approach we report here. These new technologies should also enhance the identification of additional pituitary adenoma risk factors, biomarkers and drug targets.
Table 3.
CLINICAL AND THERAPEUTIC IMPLICATIONS
*Mouse mutants can model human-pituitary disease
|
*Tumor suppressors and oncogenes mutated in familial pituitary adenoma syndromes are expressed in early mouse pituitary development, suggesting roles in normal pituitary growth regulation.
|
*Gene discovery with mouse mutants reveals new candidates for functional tests and human screening
|
Acknowledgments
We thank the University of Michigan DNA Sequencing Core Facility, Comprehensive Cancer Center; and other members of the Camper laboratory, past and present, for their contributions to this work; Ken Morohashi and Simon Rhodes for providing critical reagents; and financial support from the National Institutes of Health (NICHD R3730428 and R01 HD34283 to SAC), a Pilot Feasibility Study from the University of Michigan Center for Computational Medicine and Biology (to SAC), and the Wellcome Trust (to KPS).
Footnotes
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Kelberman D, Dattani MT. Hypothalamic and pituitary development: Novel insights into the aetiology. Eur J Endocrinol. 2007;157 (Suppl 1):S3–14. doi: 10.1530/EJE-07-0156. [DOI] [PubMed] [Google Scholar]
- 2.Cushman LJ, Showalter AD, Rhodes SJ. Genetic defects in the development and function of the anterior pituitary gland. Ann Med. 2002;34:179–191. [PubMed] [Google Scholar]
- 3.Collignon J, Sockanathan S, Hacker A, Cohen-Tannoudji M, Norris D, Rastan S, Stevanovic M, Goodfellow PN, Lovell-Badge R. A comparison of the properties of sox-3 with sry and two related genes, sox-1 and sox-2. Development. 1996;122:509–520. doi: 10.1242/dev.122.2.509. [DOI] [PubMed] [Google Scholar]
- 4.Kelberman D, Rizzoti K, Avilion A, Bitner-Glindzicz M, Cianfarani S, Collins J, Chong WK, Kirk JM, Achermann JC, Ross R, Carmignac D, Lovell-Badge R, Robinson IC, Dattani MT. Mutations within sox2/sox2 are associated with abnormalities in the hypothalamo-pituitary-gonadal axis in mice and humans. J Clin Invest. 2006;116:2442–2455. doi: 10.1172/JCI28658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meijlink F, Beverdam A, Brouwer A, Oosterveen TC, Berge DT. Vertebrate aristaless-related genes. Int J Dev Biol. 1999;43:651–663. [PubMed] [Google Scholar]
- 6.Rizzoti K, Brunelli S, Carmignac D, Thomas PQ, Robinson IC, Lovell-Badge R. Sox3 is required during the formation of the hypothalamo-pituitary axis. Nat Genet. 2004;36:247–255. doi: 10.1038/ng1309. [DOI] [PubMed] [Google Scholar]
- 7.Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O’Connell SM, Gukovsky I, Carrière C, Ryan AK, Miller AP, Zuo L, Gleiberman AS, Andersen B, Beamer WG, Rosenfeld MG. Pituitary lineage determination by the prophet of pit-1 homeodomain factor defective in ames dwarfism. Nature. 1996;384:327–333. doi: 10.1038/384327a0. [DOI] [PubMed] [Google Scholar]
- 8.Suh H, Gage PJ, Drouin J, Camper SA. Pitx2 is required at multiple stages of pituitary organogenesis: Pituitary primordium formation and cell specification. Development. 2002;129:329–337. doi: 10.1242/dev.129.2.329. [DOI] [PubMed] [Google Scholar]
- 9.Galliot B, de Vargas C, Miller D. Evolution of homeobox genes: Q50 paired-like genes founded the paired class. Dev Genes Evol. 1999;209:186–197. doi: 10.1007/s004270050243. [DOI] [PubMed] [Google Scholar]
- 10.Gage PJ, Brinkmeier ML, Scarlett LM, Knapp LT, Camper SA, Mahon KA. The ames dwarf gene, df, is required early in pituitary ontogeny for the extinction of rpx transcription and initiation of lineage specific cell proliferation. Mol Endocrinol. 1996;10:1570–1581. doi: 10.1210/mend.10.12.8961267. [DOI] [PubMed] [Google Scholar]
- 11.Hermesz E, Mackem S, Mahon KA. Rpx: A novel anterior-restricted homeobox gene progressively activated in the prechordal plate, anterior neural plate, and rathke’s pouch of the mouse embryo. Development. 1996;122:41–52. doi: 10.1242/dev.122.1.41. [DOI] [PubMed] [Google Scholar]
- 12.Thomas PQ, Johnson BV, Rathjen J, Rathjen PD. Sequence, genomic organization, and expression of the novel homeobox gene hesx1. J Biol Chem. 1995;270:3869–3875. doi: 10.1074/jbc.270.8.3869. [DOI] [PubMed] [Google Scholar]
- 13.Li S, Crenshaw EB, 3rd, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG. Dwarf locus mutants lacking three pituitary cell types result from mutations in the pou-domain gene pit-1. Nature. 1990;347:528–533. doi: 10.1038/347528a0. [DOI] [PubMed] [Google Scholar]
- 14.Nasonkin IO, Ward RD, Raetzman LT, Seasholtz AF, Saunders TL, Gillespie PJ, Camper SA. Pituitary hypoplasia and respiratory distress syndrome in prop1 knockout mice. Hum Mol Genet. 2004;13:2727–2735. doi: 10.1093/hmg/ddh311. [DOI] [PubMed] [Google Scholar]
- 15.Tang K, Bartke A, Gardiner CS, Wagner TE, Yun JS. Gonadotropin secretion, synthesis, and gene expression in human growth hormone transgenic mice and in ames dwarf mice. Endocrinology. 1993;132:2518–2524. doi: 10.1210/endo.132.6.8504754. [DOI] [PubMed] [Google Scholar]
- 16.O’Hara BF, Bendotti C, Reeves RH, Oster-Granite Ml, Coyle JT, Gearhart JD. Genetic mapping and analysis of somatostatin expression in snell dwarf mice. Mol Brain Res. 1988;4:283–292. doi: 10.1016/0169-328x(88)90037-x. [DOI] [PubMed] [Google Scholar]
- 17.Buckwalter MS, Katz RW, Camper SA. Localization of the panhypopituitary dwarf mutation (df) on mouse chromosome 11 in an intersubspecific backcross. Genomics. 1991;10:515–526. doi: 10.1016/0888-7543(91)90430-m. [DOI] [PubMed] [Google Scholar]
- 18.Cogan J, Wu W, Phillips JI, Arnhold I, Agapito A, Fofanova O, Osorio M, Bircan I, Moreno A, Mendonca B. The prop1 2-base pair deletion is a common cause of combined pituitary hormone deficiency. Journal of Clinical Endocrinology & Metabolism. 1998;83:3346–3349. doi: 10.1210/jcem.83.9.5142. [DOI] [PubMed] [Google Scholar]
- 19.Cohen LE, Radovick S. Molecular basis of combined pituitary hormone deficiencies. Endocr Rev. 2002;23:431–442. doi: 10.1210/er.2001-0030. [DOI] [PubMed] [Google Scholar]
- 20.Deladoey J, Fluck C, Buyukgebiz A, Kuhlmann BV, Eble A, Hindmarsh PC, Wu W, Mullis PE. “Hot spot” In the prop1 gene responsible for combined pituitary hormone deficiency. J Clin Endocrinol Metab. 1999;84:1645–1650. doi: 10.1210/jcem.84.5.5681. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: New insights and new questions. Development. 2007;134:647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- 22.Ward RD, Raetzman LT, Suh H, Stone BM, Nasonkin IO, Camper SA. Role of prop1 in pituitary gland growth. Mol Endocrinol. 2005;19:698–710. doi: 10.1210/me.2004-0341. [DOI] [PubMed] [Google Scholar]
- 23.Ward RD, Stone BM, Raetzman LT, Camper SA. Cell proliferation and vascularization in mouse models of pituitary hormone deficiency. Mol Endocrinol. 2006;20:1378–1390. doi: 10.1210/me.2005-0409. [DOI] [PubMed] [Google Scholar]
- 24.Douglas KR, Camper SA. Partial transcriptome of the developing pituitary gland. Genomics. 2000;70:335–346. doi: 10.1006/geno.2000.6400. [DOI] [PubMed] [Google Scholar]
- 25.Douglas KR, Kennell JA, Eswara P, Harrison TA, Patrianakos AI, Sprecher BS, Potok MA, Lyons RH, MacDougald OA, Camper SA. Identification of members of the wnt signaling pathway in the embryonic pitutiary gland. Mammalian Genome. 2001 doi: 10.1007/s00335-001-2076-0. [DOI] [PubMed] [Google Scholar]
- 26.Brinkmeier ML, Potok MA, Cha KB, Gridley T, Stifani S, Meeldijk J, Clevers H, Camper SA. Tcf and groucho-related genes influence pituitary growth and development. Mol Endocrinol. 2003;17:2152–2161. doi: 10.1210/me.2003-0225. [DOI] [PubMed] [Google Scholar]
- 27.Brinkmeier ML, Potok MA, Davis SW, Camper SA. Tcf4 deficiency expands ventral diencephalon signaling and increases induction of pituitary progenitors. Dev Biol. 2007;311:396–407. doi: 10.1016/j.ydbio.2007.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cha KB, Douglas KR, Potok MA, Liang H, Jones SN, Camper SA. Wnt5a signaling affects pituitary gland shape. Mech Dev. 2004;121:183–194. doi: 10.1016/j.mod.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Potok MA, Cha KB, Hunt A, Brinkmeier ML, Leitges M, Kispert A, Camper SA. Wnt signaling affects gene expression in the ventral diencephalon and pituitary gland growth. Dev Dyn. 2008;237:1006–1020. doi: 10.1002/dvdy.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brantjes H, Roose J, Wetering Mvd, Clevers H. All tcf hmg box transcription factors interact with groucho-related co-repressors. Nucleic Acids Res. 2001;29:1410–1419. doi: 10.1093/nar/29.7.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu C, Dyer M, Uchikawa M, Kondoh H, Lagutin O. Six3-mediated auto repression and eye development requires its interaction with members of the groucho-related family of co-repressors. Development. 2002;129:2835–2849. doi: 10.1242/dev.129.12.2835. [DOI] [PubMed] [Google Scholar]
- 32.Carninci P, Waki K, Shiraki T, Konno H, Shibata K, Itoh M, Aizawa K, Arakawa T, Ishii Y, Sasaki D, Bono H, Kondo S, Sugahara Y, Saito R, Osato N, Fukuda S, Sato K, Watahiki A, Hirozane-Kishikawa T, Nakamura M, Shibata Y, Yasunishi A, Kikuchi N, Yoshiki A, Kusakabe M, Gustincich S, Beisel K, Pavan W, Aidinis V, Nakagawara A, Held WA, Iwata H, Kono T, Nakauchi H, Lyons P, Wells C, Hume DA, Fagiolini M, Hensch TK, Brinkmeier M, Camper S, Hirota J, Mombaerts P, Muramatsu M, Okazaki Y, Kawai J, Hayashizaki Y. Targeting a complex transcriptome: The construction of the mouse full-length cdna encyclopedia. Genome Res. 2003;13:1273–1289. doi: 10.1101/gr.1119703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pellegrini M, Mansouri A, Simeone A, Boncinelli E, Gruss P. Dentate gyrus formation requires emx2. Development. 1996;122:3893–3898. doi: 10.1242/dev.122.12.3893. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Bendito G, Chan CH, Mallamaci A, Parnavelas J, Molnar Z. Role of emx2 in the development of the reciprocal connectivity between cortex and thalamus. J Comp Neurol. 2002;451:153–169. doi: 10.1002/cne.10345. [DOI] [PubMed] [Google Scholar]
- 35.Kendall SK, Gordon DF, Birkmeier TS, Petrey D, Sarapura VD, O’Shea KS, Wood WM, Lloyd RV, Ridgway EC, Camper SA. Enhancer-mediated high level expression of mouse pituitary glycoprotein hormone α-subunit transgene in thyrotropes, gonadotropes, and developing pituitary gland. Mol Endocrinol. 1994;8:1420–1433. doi: 10.1210/mend.8.10.7531821. [DOI] [PubMed] [Google Scholar]
- 36.Morinaga T, Yasuda H, Hashimoto T, Higashio K, Tamaoki T. A human alpha-fetoprotein enhancer-binding protein, atbf1, contains four homeodomains and seventeen zinc fingers. Mol Cell Biol. 1991;11:6041–6049. doi: 10.1128/mcb.11.12.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qi Y, Ranish JA, Zhu X, Krones A, Zhang J, Aebersold R, Rose DW, Rosenfeld MG, Carriere C. Atbf1 is required for the pit1 gene early activation. Proc Natl Acad Sci U S A. 2008;105:2481–2486. doi: 10.1073/pnas.0712196105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miquelajauregui A, Van de Putte T, Polyakov A, Nityanandam A, Boppana S, Seuntjens E, Karabinos A, Higashi Y, Huylebroeck D, Tarabykin V. Smad-interacting protein-1 (zfhx1b) acts upstream of wnt signaling in the mouse hippocampus and controls its formation. Proc Natl Acad Sci U S A. 2007;104:12919–12924. doi: 10.1073/pnas.0609863104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verstappen G, van Grunsven LA, Michiels C, Van de Putte T, Souopgui J, Van Damme J, Bellefroid E, Vandekerckhove J, Huylebroeck D. Atypical mowat-wilson patient confirms the importance of the novel association between zfhx1b/sip1 and nurd corepressor complex. Hum Mol Genet. 2008;17:1175–1183. doi: 10.1093/hmg/ddn007. [DOI] [PubMed] [Google Scholar]
- 40.Moreno CS, Evans CO, Zhan X, Okor M, Desiderio DM, Oyesiku NM. Novel molecular signaling and classification of human clinically nonfunctional pituitary adenomas identified by gene expression profiling and proteomic analyses. Cancer Res. 2005;65:10214–10222. doi: 10.1158/0008-5472.CAN-05-0884. [DOI] [PubMed] [Google Scholar]
- 41.Mowat DR, Croaker GD, Cass DT, Kerr BA, Chaitow J, Ades LC, Chia NL, Wilson MJ. Hirschsprung disease, microcephaly, mental retardation, and characteristic facial features: Delineation of a new syndrome and identification of a locus at chromosome 2q22–q23. J Med Genet. 1998;35:617–623. doi: 10.1136/jmg.35.8.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van de Putte T, Francis A, Nelles L, van Grunsven LA, Huylebroeck D. Neural crest-specific removal of zfhx1b in mouse leads to a wide range of neurocristopathies reminiscent of mowat-wilson syndrome. Hum Mol Genet. 2007;16:1423–1436. doi: 10.1093/hmg/ddm093. [DOI] [PubMed] [Google Scholar]
- 43.Raetzman LT, Ward R, Camper SA. Lhx4 and prop1 are required for cell survival and expansion of the pituitary primordia. Development. 2002;129:4229–4239. doi: 10.1242/dev.129.18.4229. [DOI] [PubMed] [Google Scholar]
- 44.Sheng HZ, Zhadanov AB, Mosinger BJ, Fujii T, Bertuzzi S, Grinberg A, Lee EJ, Huang SP, Mahon KA, Westphal H. Specification of pituitary cell lineages by the lim homeobox gene lhx3. Science. 1996;272:1004–1007. doi: 10.1126/science.272.5264.1004. [DOI] [PubMed] [Google Scholar]
- 45.Takuma N, Sheng HZ, Furuta Y, Ward JM, Sharma K, Hogan BLM, Pfaff SL, Westphal H, Kimura S, Mahon KA. Formation of rathke’s pouch requires dual induction from the diencephalon. Development. 1998;125:4835–4840. doi: 10.1242/dev.125.23.4835. [DOI] [PubMed] [Google Scholar]
- 46.Roberson MS, Schoderbek WE, Tremml G, Maurer RA. Activation of the glycoprotein hormone alpha-subunit promoter by a lim-homeodomain transcription factor. Mol Cell Biol. 1994;14:2985–2993. doi: 10.1128/mcb.14.5.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porter FD, Drago J, Xu Y, Cheema SS, Wassif C, Huang SP, Lee E, Grinberg A, Massalas JS, Bodine D, Alt F, Westphal H. Lhx2, a lim homeobox gene, is required for eye, forebrain, and definitive erythrocyte development. Development. 1997;124:2935–2944. doi: 10.1242/dev.124.15.2935. [DOI] [PubMed] [Google Scholar]
- 48.Mangale VS, Hirokawa KE, Satyaki PR, Gokulchandran N, Chikbire S, Subramanian L, Shetty AS, Martynoga B, Paul J, Mai MV, Li Y, Flanagan LA, Tole S, Monuki ES. Lhx2 selector activity specifies cortical identity and suppresses hippocampal organizer fate. Science. 2008;319:304–309. doi: 10.1126/science.1151695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rhee H, Polak L, Fuchs E. Lhx2 maintains stem cell character in hair follicles. Science. 2006;312:1946–1949. doi: 10.1126/science.1128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richter K, Pinto do OP, Hagglund AC, Wahlin A, Carlsson L. Lhx2 expression in hematopoietic progenitor/stem cells in vivo causes a chronic myeloproliferative disorder and altered globin expression. Haematologica. 2003;88:1336–1347. [PubMed] [Google Scholar]
- 51.Mandel S, Rechavi G, Gozes I. Activity-dependent neuroprotective protein (adnp) differentially interacts with chromatin to regulate genes essential for embryogenesis. Dev Biol. 2007;303:814–824. doi: 10.1016/j.ydbio.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 52.Borozdin W, Graham JM, Jr, Bohm D, Bamshad MJ, Spranger S, Burke L, Leipoldt M, Kohlhase J. Multigene deletions on chromosome 20q13.13-q13. 2 including sall4 result in an expanded phenotype of okihiro syndrome plus developmental delay. Hum Mutat. 2007;28:830. doi: 10.1002/humu.9502. [DOI] [PubMed] [Google Scholar]
- 53.Mandel S, Gozes I. Activity-dependent neuroprotective protein constitutes a novel element in the swi/snf chromatin remodeling complex. J Biol Chem. 2007;282:34448–34456. doi: 10.1074/jbc.M704756200. [DOI] [PubMed] [Google Scholar]
- 54.Poulin G, Turgeon B, Drouin J. Neurod1/β2 contributes to cell-specific transcription of the proopiomelanocortin gene. Mol Cell Biol. 1997;17:6673–6682. doi: 10.1128/mcb.17.11.6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mandel S, Spivak-Pohis I, Gozes I. Adnp differential nucleus/cytoplasm localization in neurons suggests multiple roles in neuronal differentiation and maintenance. J Mol Neurosci. 2008 doi: 10.1007/s12031-007-9013-y. [DOI] [PubMed] [Google Scholar]
- 56.Miyamoto N, Yoshida M, Kuratani S, Matsuo I, Aizawa S. Defects of urogenital development in mice lacking emx2. Development. 1997;124:1653–1664. doi: 10.1242/dev.124.9.1653. [DOI] [PubMed] [Google Scholar]
- 57.Noonan FC, Mutch DG, Ann Mallon M, Goodfellow PJ. Characterization of the homeodomain gene emx2: Sequence conservation, expression analysis, and a search for mutations in endometrial cancers. Genomics. 2001;76:37–44. doi: 10.1006/geno.2001.6590. [DOI] [PubMed] [Google Scholar]
- 58.Gangemi RM, Daga A, Muzio L, Marubbi D, Cocozza S, Perera M, Verardo S, Bordo D, Griffero F, Capra MC, Mallamaci A, Corte G. Effects of emx2 inactivation on the gene expression profile of neural precursors. Eur J Neurosci. 2006;23:325–334. doi: 10.1111/j.1460-9568.2005.04559.x. [DOI] [PubMed] [Google Scholar]
- 59.Davis S, Camper S. Noggin regulates bmp4 activity during pituitary induction. Dev Biol. 2007;305:145–160. doi: 10.1016/j.ydbio.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ericson J, Norlin S, Jessell TM, Edlund T. Integrated fgf and bmp signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development. 1998;125:1005–1015. doi: 10.1242/dev.125.6.1005. [DOI] [PubMed] [Google Scholar]
- 61.Treier M, Gleiberman AS, O’Connell SM, Szeto DP, McMahon JA, McMahon AP, Rosenfeld MG. Multistep signaling requirements for pituitary organogenesis in vivo. Genes & Development. 1998;12:1691–1704. doi: 10.1101/gad.12.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Treier M, O’Connell S, Gleiberman A, Price J, Szeto DP, Burgess R, Chuang P-T, McMahon AP, Rosenfeld MG. Hedgehog signaling is required for pituitary gland development. Development. 2001;128:377–386. doi: 10.1242/dev.128.3.377. [DOI] [PubMed] [Google Scholar]
- 63.Olson LE, Tollkuhn J, Scafoglio C, Krones A, Zhang J, Ohgi KA, Wu W, Taketo MM, Kemler R, Grosschedl R, Rose D, Li X, Rosenfeld MG. Homeodomain-mediated beta-catenin-dependent switching events dictate cell-lineage determination. Cell. 2006;125:593–605. doi: 10.1016/j.cell.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 64.De Moerlooze L, Spencer-Dene B, Revest J, Hajihosseini M, Rosewell I, Dickson C. An important role for the iiib isoform of fibroblast growth factor receptor 2 (fgfr2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000;127:483–492. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- 65.Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N. Fgf10 acts as a major ligand for fgf receptor 2 iiib in mouse multi-organ development. Biochem Biophys Res Commun. 2000;277:643–649. doi: 10.1006/bbrc.2000.3721. [DOI] [PubMed] [Google Scholar]
- 66.Paez-Pereda M, Giacomini D, Refojo D, Nagashima AC, Hopfner U, Grubler Y, Chervin A, Goldberg V, Goya R, Hentges ST, Low MJ, Holsboer F, Stalla GK, Arzt E. Involvement of bone morphogenetic protein 4 (bmp-4) in pituitary prolactinoma pathogenesis through a smad/estrogen receptor crosstalk. Proc Natl Acad Sci U S A. 2003;100:1034–1039. doi: 10.1073/pnas.0237312100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giacomini D, Paez-Pereda M, Theodoropoulou M, Labeur M, Refojo D, Gerez J, Chervin A, Berner S, Losa M, Buchfelder M, Renner U, Stalla GK, Arzt E. Bone morphogenetic protein-4 inhibits corticotroph tumor cells: Involvement in the retinoic acid inhibitory action. Endocrinology. 2006;147:247–256. doi: 10.1210/en.2005-0958. [DOI] [PubMed] [Google Scholar]
- 68.Raetzman LT, Ross SA, Cook S, Dunwoodie SL, Camper SA, Thomas PQ. Developmental regulation of notch signaling genes in the embryonic pituitary: Prop1 deficiency affects notch2 expression. Dev Biol. 2004;265:329–340. doi: 10.1016/j.ydbio.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 69.Raetzman LT, Cai JX, Camper SA. Hes1 is required for pituitary growth and melanotrope specification. Dev Biol. 2007;304:455–466. doi: 10.1016/j.ydbio.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raetzman LT, Wheeler BS, Ross SA, Thomas PQ, Camper SA. Persistent expression of notch2 delays gonadotrope differentiation. Mol Endocrinol. 2006;20:2898–2908. doi: 10.1210/me.2005-0394. [DOI] [PubMed] [Google Scholar]
- 71.Asa SL, Ezzat S. Genetics and proteomics of pituitary tumors. Endocrine. 2005;28:43–47. doi: 10.1385/ENDO:28:1:043. [DOI] [PubMed] [Google Scholar]
- 72.Farrell WE. Pituitary tumours: Findings from whole genome analyses. Endocr Relat Cancer. 2006;13:707–716. doi: 10.1677/erc.1.01131. [DOI] [PubMed] [Google Scholar]
- 73.Heaney AP, Melmed S. Molecular targets in pituitary tumours. Nat Rev Cancer. 2004;4:285–295. doi: 10.1038/nrc1320. [DOI] [PubMed] [Google Scholar]
- 74.Chandrasekharappa SC, Guru SC, Manickam P, Olufeni S-E, Collins FS, Emmert-Buck MR, Debelenko LV, Zhuang Z, Lubensky IA, Liotta LA, Crabtree JS, Wang Y, Agarwal SK, Kester MB, Kim YS, Heppner C, Dong Q, Spiegel AM, Burns AL, Marx SJ. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276:404–406. doi: 10.1126/science.276.5311.404. [DOI] [PubMed] [Google Scholar]
- 75.Vierimaa O, Georgitsi M, Lehtonen R, Vahteristo P, Kokko A, Raitila A, Tuppurainen K, Ebeling TM, Salmela PI, Paschke R, Gundogdu S, De Menis E, Makinen MJ, Launonen V, Karhu A, Aaltonen LA. Pituitary adenoma predisposition caused by germline mutations in the aip gene. Science. 2006;312:1228–1230. doi: 10.1126/science.1126100. [DOI] [PubMed] [Google Scholar]
- 76.Heaney AP, Horwitz GA, Wang Z, Singson RSM. Early involvement of estrogen-induced pituitary tumor transforming gene and fibroblast growth factor expression in prolactinoma pathogenesis. Nat Med. 1999;5:1317–1321. doi: 10.1038/15275. [DOI] [PubMed] [Google Scholar]
- 77.Zhang X, Horwitz GA, Heaney AP, Nakashima M, Bronstein M, Melmed S. Pituitary tumor transforming gene expression in human pituitary adenomas. J Clin Endocrinol Metab. 1999;84:761–767. doi: 10.1210/jcem.84.2.5432. [DOI] [PubMed] [Google Scholar]
- 78.Zou H, McGarry TJ, Bernal T, Kirschner MW. Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science. 1999;285:418–422. doi: 10.1126/science.285.5426.418. [DOI] [PubMed] [Google Scholar]
- 79.Yu R, Ren SG, Horwitz GA, Wang Z, Melmed S. Pituitary tumor transforming gene (pttg) regulates placental jeg-3 cell division and survival: Evidence from live cell imaging. Mol Endocrinol. 2000;14:1137–1146. doi: 10.1210/mend.14.8.0501. [DOI] [PubMed] [Google Scholar]
- 80.Qian X, Scheithauer BW, Kovacs K, Lloyd RV. DNA microarrays: Recent developments and applications to the study of pituitary tissues. Endocrine. 2005;28:49–56. doi: 10.1385/ENDO:28:1:049. [DOI] [PubMed] [Google Scholar]
- 81.Abbud RA, Kelleher R, Melmed S. Cell-specific pituitary gene expression profiles after treatment with leukemia inhibitory factor reveal novel modulators for proopiomelanocortin expression. Endocrinology. 2004;145:867–880. doi: 10.1210/en.2003-0897. [DOI] [PubMed] [Google Scholar]
- 82.Ruebel KH, Leontovich AA, Jin L, Stilling GA, Zhang H, Qian X, Nakamura N, Scheithauer BW, Kovacs K, Lloyd RV. Patterns of gene expression in pituitary carcinomas and adenomas analyzed by high-density oligonucleotide arrays, reverse transcriptase-quantitative pcr, and protein expression. Endocrine. 2006;29:435–444. doi: 10.1385/ENDO:29:3:435. [DOI] [PubMed] [Google Scholar]
- 83.Mould AW, Duncan R, Serewko-Auret M, Loffler KA, Biondi C, Gartside M, Kay GF, Hayward NK. Global expression profiling of murine men1-associated tumors reveals a regulatory role for menin in transcription, cell cycle and chromatin remodelling. Int J Cancer. 2007;121:776–783. doi: 10.1002/ijc.22734. [DOI] [PubMed] [Google Scholar]
- 84.Cushman LJ, Watkins-Chow DE, Brinkmeier ML, Raetzman LT, Radak AL, Lloyd RV, Camper SA. Persistent prop1 expression delays gonadotrope differentiation and enhances pituitary tumor susceptibility. Hum Mol Genet. 2001;10:1141–1153. doi: 10.1093/hmg/10.11.1141. [DOI] [PubMed] [Google Scholar]
- 85.Ward RD, Davis SW, Cho M, Esposito C, Lyons RH, Cheng JF, Rubin EM, Rhodes SJ, Raetzman LT, Smith TP, Camper SA. Comparative genomics reveals functional transcriptional control sequences in the prop1 gene. Mamm Genome. 2007;18:521–537. doi: 10.1007/s00335-007-9008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vesper AH, Raetzman LT, Camper SA. Role of prophet of pit1 (prop1) in gonadotrope differentiation and puberty. Endocrinology. 2006;147:1654–1663. doi: 10.1210/en.2005-1080. [DOI] [PubMed] [Google Scholar]
- 87.Egashira N, Minematsu T, Miyai S, Takekoshi S, Camper S, Osamura R. Pituitary changes in prop1 transgenic mice: Hormone producing tumors and signet-ring type gonadotropes. Acta Histochem Cytochem. 2008 doi: 10.1267/ahc.08007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brinkmeier ML, Stahl JH, Gordon D, Ross BD, Sarapura VD, Dowding JM, Kendall SK, Lloyd RV, Ridgway EC, Camper SA. Thyroid hormone responsive pituitary hyperplasia independent of somatostatin receptor 2. Mol Endocrinol. 2001 doi: 10.1210/mend.15.12.0744. in press. [DOI] [PubMed] [Google Scholar]
- 89.Kendall SK, Samuelson LC, Saunders TL, Wood RI, Camper SA. Targeted disruption of the pituitary glycoprotein hormone alpha-subunit produces hypogonadal and hypothyroid mice. Genes Dev. 1995;9:2007–2019. doi: 10.1101/gad.9.16.2007. [DOI] [PubMed] [Google Scholar]
- 90.Stahl J, Kendall S, Brinkmeier M, Greco T, Watkins-Chow D, Canpos-Barros A, Lloyd R, Camper S. Thyroid hormone is essential for pituitary somatotropes and lactotropes. Endocrinol. 1999;140:1884–1892. doi: 10.1210/endo.140.4.6627. [DOI] [PubMed] [Google Scholar]
- 91.Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, Braverman MS, Chen YJ, Chen Z, Dewell SB, Du L, Fierro JM, Gomes XV, Godwin BC, He W, Helgesen S, Ho CH, Irzyk GP, Jando SC, Alenquer ML, Jarvie TP, Jirage KB, Kim JB, Knight JR, Lanza JR, Leamon JH, Lefkowitz SM, Lei M, Li J, Lohman KL, Lu H, Makhijani VB, McDade KE, McKenna MP, Myers EW, Nickerson E, Nobile JR, Plant R, Puc BP, Ronan MT, Roth GT, Sarkis GJ, Simons JF, Simpson JW, Srinivasan M, Tartaro KR, Tomasz A, Vogt KA, Volkmer GA, Wang SH, Wang Y, Weiner MP, Yu P, Begley RF, Rothberg JM. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376–380. doi: 10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hillier LW, Marth GT, Quinlan AR, Dooling D, Fewell G, Barnett D, Fox P, Glasscock JI, Hickenbotham M, Huang W, Magrini VJ, Richt RJ, Sander SN, Stewart DA, Stromberg M, Tsung EF, Wylie T, Schedl T, Wilson RK, Mardis ER. Whole-genome sequencing and variant discovery in c. Elegans Nat Methods. 2008;5:183–188. doi: 10.1038/nmeth.1179. [DOI] [PubMed] [Google Scholar]