Abstract
Endothelin-1 is the most potent vasoconstrictor agent currently identified, and it was originally isolated and characterized from the culture media of aortic endothelial cells. Two other isoforms, termed endothelin-2 and endothelin-3, were subsequently identified, along with structural homologues isolated from the venom of Actractapis engaddensis known as the sarafotoxins. In this review, we will discuss the basic science of endothelins, endothelin-converting enzymes, and endothelin receptors. Only concise background information pertinent to clinical physician is provided. Next we will describe the pathophysiological roles of endothelin-1 in pulmonary arterial hypertension, heart failure, systemic hypertension, and female malignancies, with emphasis on ovarian cancer. The potential intervention with pharmacological therapeutics will be succinctly summarized to highlight the exciting pre-clinical and clinical studies within the endothelin field. Of note is the rapid development of selective endothelin receptor antagonists, which has led to an explosion of research in the field.
Keywords: Endothelin, Endothelin-converting enzymes, Endothelin receptor, Endothelin signal, Pathophysiology
Introduction
The main purpose of this review is to provide a broad overview on the basic science of the endothelin system and its clinical relevance. In the basic science portions of the review, we will begin our discussion on the synthesis of endothelins, endothelin-converting enzymes, and endothelin receptors. For our clinical discussion, we will describe the pathophysiological intervention of pulmonary arterial hypertension with regard to the endothelin system. We will also visit much-discussed topics of endothelin in heart failure, systemic hypertension, and ovarian cancer. Other clinical interventions and diseases within the context of endothelin have also been suggested, and we will conclude our discussion with future possibilities for endothelin antagonist therapy.
Endothelins
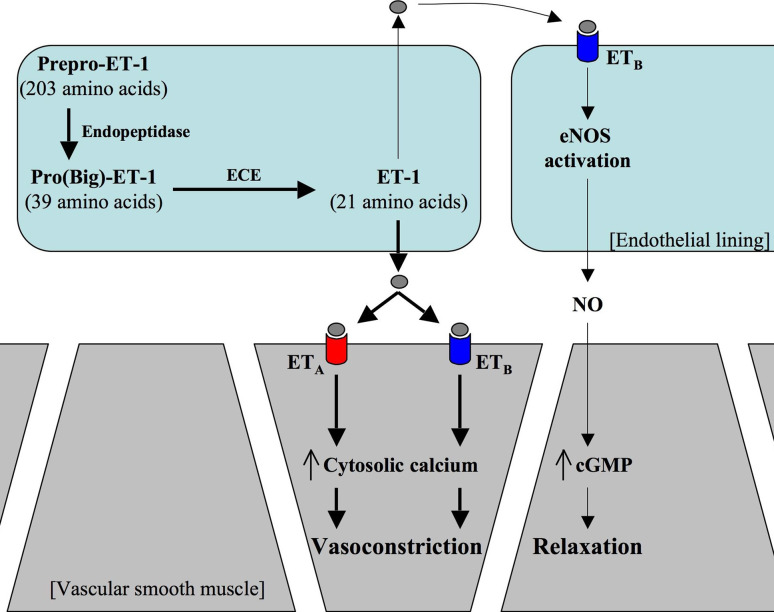
The human genes of endothelin-1 (ET-1), endothelin-2 (ET-2), and endothelin-3 (ET-3) are located on chromosomes 6, 1, and 20, respectively. Endothelin-1 expression is determined primarily at the level of gene transcription regulated by a promoter region located upstream (5′) of the preproendothelin-1 gene. A binding site of GATA mediates basal levels of gene transcription of preproendothelin-1 gene. Ap-1 nuclear factor and a hexonucleotide sequence that control gene transcription are thought to be regulated by angiotensin II, transforming growth factor beta, and/or acute phase reactants. Further post-transcriptional modulation occurs via selective destabilization of preproendothelin-1 mRNA via ‘suicide motifs’ present in the non-translated 3′ region. This may account for a short, 15-min half-life of preproendothelin-1 mRNA and thereby prevent excessive endothelin-1 production. Factors known to promote endothelin-1 production include thrombin, insulin, cyclosporine, epinephrine, angiotensin II, cortisol, inflammatory mediators, hypoxia, and vascular shear stress. Endothelin production is inhibited by nitric oxide, nitric oxide donor drugs, and dilator prostanoids via an increase in cellular cGMP, and natriuretic peptides via an increase in cAMP levels [1]. The mature endothelin-1 peptide is generated by enzymatic cleavage of the initial preproendothelin-1 gene product (Fig. 1). A short hydrophobic secretory sequence is first removed to produce proendothelin-1, which is further cleaved at dibasic amino acid pairs by the endopeptidase furin generating the 39-amino acid peptide big endothelin-1 [2]. Subsequent production of mature endothelin-1 by a proteolytic cleavage between Trp21 and Val22 is catalyzed by the membrane bound metalloprotease endothelin-converting enzyme-1 (ECE-1) [3]. Although additional ECE isoforms have been identified in animals, a human ECE-2 and ECE-3 have yet to be identified [4]. ECE gene knockout studies suggest that ECE-1 is the major functional ECE for all three endothelin isoforms in vivo [5]. Endothelin-1 was initially considered to be produced de novo in response to the factors described earlier. However, secretory vesicles containing both mature endothelin-1 and ECE have been identified in endothelial cells [6]. Recently, a new endothelin peptide with 31 amino acids has been identified in humans. This endothelin is formed through the cleavage of the big endothelin-1 between the Tyr31 and Gly32 amino acids by a human chymase enzyme expressed in mast cells. This product has been termed endothelin-11–31 [7]. Endothelin-11–31 triggered pressor responses that were reduced by endothelin receptor antagonists. These pressor responses to endothelin-11–31 were abolished by the neutral endopeptidase inhibitor thiorphan, but were unaffected by the endothelin-converting enzyme inhibitor CGS35066 [8]. Each of the three endothelin peptides is expressed in various tissues and cells. ET-1 is produced by vascular endothelial and smooth muscle cells, airway epithelial cells, macrophages, fibroblasts, cardiac myocytes, brain neurons, and pancreatic islets [3, 9]. ET-2 is expressed in the ovary and intestinal epithelial cells [3]. ET-3 is found in endothelial cells and intestinal epithelial cells. ET-3 mediates release of vasodilators, including NO and prostacyclin [3].
Fig. 1.
Endothelin-1 (ET-1) is transcripted and translated as a prepro-ET-1. Dibasic-pair-specific endopeptidase cleaves prepro-ET-1 to form pro-ET-1 or big ET-1. The precursor big ET-1 is further cleaved by endothelin-converting enzyme (ECE) to the vaso-active peptide ET-1. ET-1 can activate endothelin receptors type-A (ETA) and type-B (ETB). While ETA is localized in vascular smooth muscle cells, ETB resides in vascular smooth muscle and endothelial cells. Activation of ETA or ETB in smooth muscle cells results in vasoconstriction. Activation of ETB in endothelial cells will induce nitric oxide (NO) production through endothelial nitric oxide synthase (eNOS). NO is a potent vasodilator in smooth muscle cells. Activation of ET receptors in non-vascular cells has also been implicated with other cellular functions (see text for more details)
Endothelin-converting enzyme
ECE-1 was first isolated and purified from aortic endothelial cells [10]. It is inhibited by the combined ECE and neutral endopeptidase (NEP) inhibitor phosphoramidon or selective ECE inhibitor CGS35066, but not by selective NEP inhibitors such as thiorphan or kelatorphan [11]. Structurally, ECE-1 exists as a transmembrane 758-amino acid dimer, linked by a single disulphide bridge. A short (1–56) N-terminal intracellular region is connected by a 21-amino acid transmembrane portion. ECE-1 belongs to a family of neutral metalloprotease enzymes, which includes NEP and the human Kell blood group proteins [12]. However, ECE is unique amongst this group in that it recognizes a relatively long C-terminal portion of big endothelin-1 (residues His27 to Gly34) in addition to the cleavage site between residues 21 and 22 [13]. The ECE-1 gene is located on chromosome 1 at the p36 band [14]. cDNA cloning studies have demonstrated that differential gene splicing leads to the production of four isoforms of ECE-1, termed ECE-1a, ECE-1b, ECE-1c, and ECE-1d, which differ in structure only at the N-terminus. ECE-1a is responsible for generating the majority of functional endothelin-1 from big endothelin-1. ECE-1a is expressed by endothelial cells and is located intracellularly. The enzymatically active C-terminal segment faces the intra-luminal region of the Golgi apparatus. A generator role for ECE-1a is further suggested by the presence of characteristic promoter regions for this gene, indicating that it is a constitutively expressed ‘housekeeping’ gene. In contrast, ECE-1b spans the plasma membrane of effector cells, such as vascular smooth muscle cells, converting extracellular big endothelin-1 to endothelin-1. A ‘responder/regulator’ role for ECE-1b to extracellular big endothelin-1 is suggested from its promoter region containing potential receptor sites for transcription factors, allowing modulation activity. ECE-1c contributes to the elevation of ET-1 peptide levels in diabetes. In particular, expression of ECE-1c seems to respond to high glucose levels in endothelial cells [15]. It is also speculated that different ECE-1 isoforms may be responsible for different cellular functions in cancers. For example, transient ECE-1c overexpression increased cancer invasiveness through Matrigel™, whereas transient ECE-1a expression suppressed invasion. In addition, transient ECE-1a expression in stromal cells strongly counteracts the effect of transient ECE-1c expression in cancer cells. Thus, it is concluded that ECE-1a and ECE-1c are significant, but with reciprocal effects on cell invasion [16].
Transfection of preproendothelin-1 and ECE-1b genes into cultured cells demonstrates that ECE-1b expressed at the cell surface is relatively inefficient at proteolysis of exogenous big endothelin-1, with only around 10% converted to endothelin-1. On the other hand, between 50 and 90% of the endothelin peptides secreted are in the mature endothelin-1 form [12], which suggests that endogenously generated endothelin-1 secreted luminally is the most functionally important source and confirms a predominantly autocrine/paracrine function of endothelin-1. Such a theory is supported by the low concentration of endothelin-1 in the plasma, which is probably insufficient to activate endothelin receptors. Concentrations of angiotensin II and atrial natriuretic peptide in plasma are normally up to ten times greater than those of circulating endothelin-1. In addition, endothelin-1 has a half-life of less than 5 min in plasma, with the main clearance in the lungs and kidneys [17, 18]. It is likely that much higher concentrations of endothelin-1 occur at the junctions between endothelial and vascular smooth muscle cells and that at least some of the plasma endothelin-1 represents overspill from this site. One might conclude, therefore, that plasma levels of endothelin-1 in pathological states represent an unreliable index of vascular endothelin activity [19]. Conversely, urinary concentrations of endothelin-1 may reflect local renal endothelin activity, but not systemic endothelin function.
Endothelin receptors
Another important discovery is the identification of two seven-transmembrane G protein-coupled endothelin receptors, endothelinA and endothelinB receptor (ETA and ETB, respectively) [20, 21]. The isoforms of endothelin exert their physiological effects in a receptor-mediated fashion. The two subtypes of endothelin receptors can be distinguished pharmacologically by the order of their affinity for the three endothelin isopeptides; ETA receptor is ET-1-selective, with an affinity order of ET-1 ≥ ET-2 > ET-3, whereas ETB receptor exhibits similar affinities for all three isopeptides [20, 21]. These receptors are both distributed in various tissues and cells, but with different levels of expression, suggesting the presence of a multifunctional ET system. ETA receptors are located on vascular smooth muscle cells [20], and when activated, produce a sustained vasoconstriction that has a slow onset. In contrast, ETB receptors are located on both endothelial [22] and vascular smooth muscle cells [23]. Activation of ETB receptors on endothelial cells causes vasodilation through the release of vasodilators acting on smooth muscle cells [24]. Moreover, ETB receptor inhibits cell growth and vasoconstriction in the vascular system and functions as a clearance receptor, based on the evidence that selective ETB receptor blockade inhibits the accumulation of intravenously administered radiolabeled ET-1 in tissue [3, 25]. The ETB receptor-mediated clearance mechanism is particularly important in the lung, which clears about 80% of circulating ET-1 [26]. The ETA receptor couples to the pertussis toxin insensitive Gαq/11 to cause activation of phospholipase C, leading to an increase in inositol phosphate production and activation of protein kinase C in vascular smooth muscle cells [27, 28]. Judging from the data that activation of the ETA receptor increases in cAMP production and protein kinase A activity [29–31], the ETA receptor couples to Gαs, the G protein that activates adenylate cyclase. Moreover, the ETA receptor couples to Gα12 and induces the stress fiber formation [31, 32]. On the other hand, the ETB receptor couples to Gαi/o to inhibit cAMP formation [33–35], Gαq/11 to stimulate phosphoinositide hydrolysis [36, 37], and Gα13 to induce stress fiber formation [32, 38].
Pathophysiology of ET in pulmonary arterial hypertension
Pulmonary arterial hypertension (PAH) is a progressive and fatal condition characterized by a sustained increase in pulmonary vascular resistance, leading to right ventricular failure and premature death. ET-1 has been implicated as a mediator of increased vascular tone and vascular remodeling in pulmonary hypertension [39]. There is increasing evidence that pulmonary vascular smooth muscle cells, as well as endothelial cells, synthesize and release ET-1, particularly when stimulated by cytokines [40]. ET-1 is also produced in the lung in response to increased pressure. Expression of ET-1 mRNA increases in pulmonary vascular endothelial cells of patients with pulmonary hypertension [39]. In patients with pulmonary hypertension, a significant correlation between serum levels of ET and pulmonary vascular resistance, right atrial pressure, and oxygen saturation has been reported [41, 42]. In thromboembolic pulmonary hypertension, it was shown that there is upregulation of the ETB receptor in the pulmonary artery [43]. Overexpression of ET-1 in experimental PAH models is localized to the medial layer of the pulmonary arterial tree [44]. ETA and ETB receptors are also upregulated [44]. Importantly, both nonselective (bosentan) and selective (sitaxentan, atrasentan, and TBC-3711) ETA receptor antagonists are effective in PAH, reducing pulmonary artery pressure and inhibiting vascular remodeling in animal model studies. Bosentan was granted FDA approval for treatment of patients with NYHA/WHO functional class III or IV symptoms based on the results of two trials [45, 46]. Open-label continuation studies in each trial population demonstrated that the effects of bosentan were maintained beyond the initial 12-week study period. Survival at 1 and 2 years in the patients initially treated with bosentan was 96 and 89%, respectively, compared to predicted rates in historical controls of 69 and 57%. Complementary outcomes were obtained with sitaxentan [47]. Sitaxentan was granted a license for use in PAH in 2006. ET receptor antagonists are now established in American and European guidelines for the treatment of NYHA class III or IV patients with idiopathic PAH who either do not respond to acute vasodilators or remain class III despite vasodilator responsiveness.
Pathophysiology of ET in heart failure
Circulating ET-1 levels have been correlated with the severity of hemodynamics and with symptoms in patients with congestive heart failure [48, 49]. ET-1 contributes to acute and chronic increases in vascular resistance, ventricular and vascular remodeling, inflammation and arrhythmogenesis in models of heart failure. Tissue endothelin levels are increased in the failing human heart. Studies have also shown that big endothelin can be used as an independent predictor of survival [50]. It is likely that there is interplay between the ET system and neurohormonal system, because the activation of one system appears to increase levels of the other. ET-1 appears to exert differential effects on the normal and failing myocardium. Patients with reduced left ventricular function have increased contractility in response to ETA receptor blockade, whereas patients with normal left ventricular function manifest a reduction in contractility. ETA receptors are upregulated in heart failure, whereas the ETB receptor appears to be downregulated [51, 52]. Preliminary clinical trials with bosentan, darusentan, and BQ-123 show short-term hemodynamic benefits [53–55]. In addition, studies of intravenous tezosentan for patients with acutely decompensated heart failure were reported to improve cardiac index and pulmonary capillary wedge pressures [56]. However, five Randomized Intravenous TeZosentan (RITZ) trials for the treatment of congestive cardiac heart failure have been disappointing [57–63]. In stable patients with chronic heart failure, clinical trials of endothelin receptor antagonism also failed to demonstrate any benefit in clinically relevant end points (clinical status, mortality or hospitalization for heart failure) and were beset with toxicity problems [64, 65].
Pathophysiology of ET in systemic hypertension
The identification of endothelin as a vasoconstrictor [2] and the discovery of its release from vascular endothelial cells suggested that ET was involved in the pathogenesis of hypertension and vascular disease [66]. Further support for this hypothesis came from case reports of hemangioendothelioma patients who presented with markedly elevated levels of plasma ET-1 and hypertension, but who showed normalization of elevated ET-1 and blood pressure levels after tumor surgery [67]. In contrast, ET-1 plasma levels are generally normal in patients with essential hypertension; however, local ET-1 levels increase in the vascular wall with hypertension [68, 69]. Some studies demonstrate that the potent antihypertensive effects and end-organ protection by endothelin receptor antagonists in experimental hypertension are more effective in patients with high salt intake or angiotensin II level [70]. In addition, acute blockade of ETA receptors ameliorates myocardial ischemia and biochemical changes caused by infarction in mice with coronary atherosclerosis [71]. Indeed, ET has strong growth-promoting activity in the vascular wall, and both endothelin and its receptors are widely expressed in macrophages, vascular smooth muscle cells, and fibroblasts [72]. Although plasma levels of ET are not consistently elevated in patients with systemic hypertension, there is often an exaggerated vasodilator response to ET receptor blockade in these patients [73]. This could contribute to a change in the sensitivity of the vasculature to endogenous ET-1 being altered as part of the disease. Other studies suggest that certain polymorphisms of the genes coding for ET-1 and endothelin receptors could be associated with chronic elevations in blood pressure [74]. In experimental animals with induced hypertension, ETA receptor blockade prevents vascular hypertrophy and attenuates left ventricular hypertrophy [75]. Hypertension develops in ETB knockout mice and blood pressure rises after ETB blockade in humans [76, 77]. In patients with essential hypertension, the nonselective ET receptor antagonist TAK-044 caused greater forearm vasodilatation compared with normotensive controls, and the nonselective antagonist bosentan resulted in greater forearm vasodilatation than the selective ETA receptor blocker BQ-123 [78, 79]. A 4-week treatment trial with bosentan, at a fairly high dose of 1,000 mg twice per day, produced a fall in ambulatory diastolic blood pressure of approximately 10 mmHg, an effect similar to treatment with 20 mg of enalapril [80]. These data suggest that ET antagonists may represent a new class of drug in the treatment of patients with uncontrolled hypertension. Moreover, ETA receptor antagonists can reduce blood pressure substantially in hypertensive patients with chronic kidney disease [81]. This effect is synergistic to angiotensin-converting enzyme inhibitors and abolished by significant concurrent ETB receptor blockade [82]. Furthermore, in diabetic and nondiabetic renal disease patients, ET receptor antagonists may produce favorable renal hemodynamic changes that reduce proteinuria [83, 84]. Thus, the ET antagonist may offer benefits to patients with chronic kidney disease that extend beyond blood pressure lowering.
Pathophysiology of ET in ovarian cancer
In addition to its role as a vasoconstrictor, ET-1 is known to be a potent mitogen that stimulates proto-oncogene expression in vascular and non-vascular cells. Elevated expression of ET-1 has been reported in many tumors, and it is believed to be a vital “hormone” in the growth and progression of prostate, ovarian, colorectal, bladder, breast, and lung carcinomas. Currently, the endothelin system in cancer biology has been an intense focus in both basic and clinical science settings. In particular, ET receptor activation plays a huge role in cancer cells or cancer-related cells, including proliferation, resistance to apoptosis, angiogenesis, migration, neovascularization, and subsequent invasion [85].
The ET-1 and ETA receptor mRNA levels are detected in almost all primary and metastatic ovarian carcinomas. Their mRNA levels are especially higher in tumors than in normal ovarian tissues. A high level of ET-1 is found in ascitic fluids of ovarian cancer patients. In addition, ETA expression is higher in grade 3 and 4 cancers than early grade ovarian cancers correlated. The high expression of ET-1 and its receptors in human cancer cells and human tumors further suggests a potential role for ET-1 in tumor growth promotion or maintenance through a possible autocrine or paracrine mechanism [86–90]. More importantly, ETA is one of the genes more highly expressed in post-chemotherapy samples than in samples of untreated primary ovarian tumors [91].
As a growth-regulatory peptide, ET-1 also acts synergistically with other growth factors that have been implicated in cancer progression [92]. In particular, high ET-1 level is correlated with an increased vascular endothelial growth factor (VEGF), which is associated with neovascularization [90]. Transactivation of the epidermal growth factor (EGF) receptor in ET-1-induced mitogenic signaling in human ovarian carcinoma cells has been reported [93]. Furthermore, ET-1-induced cyclooxygenase-2 and prostaglandin E2 release and estrogen signaling have been shown to be important in the overall cellular proliferation processes [94, 95].
Many potential targets for the endothelin system have been suggested in ovarian carcinoma cells. One of the remedies includes the anti-tumor effect of green tea polyphenol epigallocatechin-3-gallate [96]. Two most actively studied drugs, however, are atrasentan (ABT-627, Abbott Laboratories, Abbott Park, IL) and zibotentan (ZD4054, AstraZeneca, Macclesfield, UK). These two pharmacological agents have oral bioavailability and bind to ET-1 receptor blocking signal transduction pathways implicated in cell proliferation and processes involving cancer growth [97–99].
Both atrasentan and zibotentan prevent ET-1-mediated survival signaling pathways and decreased proliferation in ovarian OVCA 433 and HEY cells. These agents significantly reduce tumor growth in animals bearing ovarian tumor xenografts in vivo. Furthermore, many clinical studies have shown that blocking ET-1 receptor inhibits tumor growth and/or reduces metastatic potential of ovarian cancer [100–104]. Treatment with either agent provides no detectable signs of acute or delayed toxicity. Both atrasentan and zibotentan give comparable results to those of paclitaxel. However, a combinational therapy of ET-receptor blocker and paclitaxel provide a better recovery or prolonged tumor growth inhibition. It is believed that atrasentan or zibotentan can actually enhance paclitaxel activity in human ovarian carcinoma in vitro and in vivo [89, 102, 104].
Conclusions and perspective
Since its discovery over 20 years ago, endothelin has been recognized to function not only as a vasoconstrictor but also as a multifunctional peptide with cytokine- or hormone-like activity. From the basic science perspective, ET has been shown to regulate a wide spectrum of physiological cellular activities, including mitogenesis, cell survival, angiogenesis, bone remodeling, stimulation of nociceptors, tumor-infiltrating immune cells, epithelial-to-mesenchymal transition, invasion, and metastatic dissemination. From the clinical perspective, the therapeutic efficacy of ET antagonists has become the first clinical indication for pulmonary arterial hypertension. Other cardiovascular-related diseases have shown promising clinical indications. Furthermore, exciting new results from basic science studies have associated endothelin antagonist therapy to many other cancer-related diseases, including targets for pharmacotherapy of female malignancies [105]. Without doubt, a detailed understanding of the molecular mechanisms of endothelin is a crucial step in identifying new effective therapies for other diseases.
Acknowledgments
Due to restricted space, we apologize to those whose work is not described in this review. Work from our laboratory that is cited in this review has been supported by grants from the Merck Company Foundation, Banyu Fellowship in Cardiovascular Medicine, the National Institutes of Health (NIH: DK080640), and the Japan Society for the Promotion of Science (JSPS: S-10707). We thank Charisse Montgomery for her editorial support. Y. Kawanabe is the recipient of the Merck Company Foundation and Banyu fellowship awards in cardiovascular medicine.
References
- 1.Gray GA, Webb DJ. The endothelin system and its potential as a therapeutic target in cardiovascular disease. Pharmacol Ther. 1996;72(2):109–148. doi: 10.1016/S0163-7258(96)00101-5. [DOI] [PubMed] [Google Scholar]
- 2.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 3.Kedzierski RM, Yanagisawa M. Endothelin system: the double-edged sword in health and disease. Annu Rev Pharmacol Toxicol. 2001;41:851–876. doi: 10.1146/annurev.pharmtox.41.1.851. [DOI] [PubMed] [Google Scholar]
- 4.Emoto N, Yanagisawa M. Endothelin-converting enzyme-2 is a membrane-bound, phosphoramidon-sensitive metalloprotease with acidic pH optimum. J Biol Chem. 1995;270(25):15262–15268. doi: 10.1074/jbc.270.25.15262. [DOI] [PubMed] [Google Scholar]
- 5.Yanagisawa H, Hammer RE, Richardson JA, Emoto N, Williams SC, Takeda S, Clouthier DE, Yanagisawa M. Disruption of ECE-1 and ECE-2 reveals a role for endothelin-converting enzyme-2 in murine cardiac development. J Clin Invest. 2000;105(10):1373–1382. doi: 10.1172/JCI7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner AJ, Murphy LJ. Molecular pharmacology of endothelin-converting enzymes. Biochem Pharmacol. 1996;51(2):91–102. doi: 10.1016/0006-2952(95)02036-5. [DOI] [PubMed] [Google Scholar]
- 7.Nakano A, Kishi F, Minami K, Wakabayashi H, Nakaya Y, Kido H. Selective conversion of big endothelins to tracheal smooth muscle-constricting 31-amino acid-length endothelins by chymase from human mast cells. J Immunol. 1997;159(4):1987–1992. [PubMed] [Google Scholar]
- 8.Simard E, Jin D, Takai S, Miyazaki M, Brochu I, D’Orleans-Juste P. Chymase-dependent conversion of big endothelin-1 in the mouse in vivo. J Pharmacol Exp Ther. 2009;328(2):540–548. doi: 10.1124/jpet.108.142992. [DOI] [PubMed] [Google Scholar]
- 9.Ortmann J, Nett PC, Celeiro J, Traupe T, Tornillo L, Hofmann-Lehmann R, Haas E, Frank B, Terraciano LM, Barton M. Endothelin inhibition delays onset of hyperglycemia and associated vascular injury in type I diabetes: evidence for endothelin release by pancreatic islet beta-cells. Biochem Biophys Res Commun. 2005;334(2):689–695. doi: 10.1016/j.bbrc.2005.06.140. [DOI] [PubMed] [Google Scholar]
- 10.Ohnaka K, Takayanagi R, Yamauchi T, Okazaki H, Ohashi M, Umeda F, Nawata H. Identification and characterization of endothelin converting activity in cultured bovine endothelial cells. Biochem Biophys Res Commun. 1990;168(3):1128–1136. doi: 10.1016/0006-291X(90)91146-J. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto T, Ozawa Y, Taguchi K, Kobayashi T, Kamata K. Diabetes-associated changes and role of N epsilon-(carboxymethyl)lysine in big ET-1-induced coronary vasoconstriction. Peptides. 2010;31(2):346–353. doi: 10.1016/j.peptides.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 12.Xu D, Emoto N, Giaid A, Slaughter C, Kaw S, de Wit D, Yanagisawa M. ECE-1: a membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell. 1994;78(3):473–485. doi: 10.1016/0092-8674(94)90425-1. [DOI] [PubMed] [Google Scholar]
- 13.Takayanagi R, Liu W, Ito T, Ohnaka K, Nawata H. Big endothelin analogues with inhibitory activity on endothelin-converting enzyme-1. J Cardiovasc Pharmacol. 1998;31(Suppl 1):S62–S63. doi: 10.1097/00005344-199800001-00020. [DOI] [PubMed] [Google Scholar]
- 14.Valdenaire O, Rohrbacher E, Mattei MG. Organization of the gene encoding the human endothelin-converting enzyme (ECE-1) J Biol Chem. 1995;270(50):29794–29798. doi: 10.1074/jbc.270.50.29794. [DOI] [PubMed] [Google Scholar]
- 15.Keynan S, Khamaisi M, Dahan R, Barnes K, Jackson CD, Turner AJ, Raz I. Increased expression of endothelin-converting enzyme-1c isoform in response to high glucose levels in endothelial cells. J Vasc Res. 2004;41(2):131–140. doi: 10.1159/000077132. [DOI] [PubMed] [Google Scholar]
- 16.Lambert LA, Whyteside AR, Turner AJ, Usmani BA. Isoforms of endothelin-converting enzyme-1 (ECE-1) have opposing effects on prostate cancer cell invasion. Br J Cancer. 2008;99(7):1114–1120. doi: 10.1038/sj.bjc.6604631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dupuis J, Goresky CA, Fournier A. Pulmonary clearance of circulating endothelin-1 in dogs in vivo: exclusive role of ETB receptors. J Appl Physiol. 1996;81(4):1510–1515. doi: 10.1152/jappl.1996.81.4.1510. [DOI] [PubMed] [Google Scholar]
- 18.Dupuis J, Stewart DJ, Cernacek P, Gosselin G. Human pulmonary circulation is an important site for both clearance and production of endothelin-1. Circulation. 1996;94(7):1578–1584. doi: 10.1161/01.cir.94.7.1578. [DOI] [PubMed] [Google Scholar]
- 19.Goddard J, Webb DJ. Endothelin receptor antagonists. Promising new agents in the management of cardiovascular disorders. Drugs R D. 1999;2(1):1–12. doi: 10.2165/00126839-199902010-00001. [DOI] [PubMed] [Google Scholar]
- 20.Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348(6303):730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- 21.Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348(6303):732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- 22.Hosoda K, Nakao K, Hiroshi A, Suga S, Ogawa Y, Mukoyama M, Shirakami G, Saito Y, Nakanishi S, Imura H. Cloning and expression of human endothelin-1 receptor cDNA. FEBS Lett. 1991;287(1–2):23–26. doi: 10.1016/0014-5793(91)80007-P. [DOI] [PubMed] [Google Scholar]
- 23.Davenport AP, O’Reilly G, Molenaar P, Maguire JJ, Kuc RE, Sharkey A, Bacon CR, Ferro A. Human endothelin receptors characterized using reverse transcriptase-polymerase chain reaction, in situ hybridization, and subtype-selective ligands BQ123 and BQ3020: evidence for expression of ETB receptors in human vascular smooth muscle. J Cardiovasc Pharmacol. 1993;22(Suppl 8):S22–S25. doi: 10.1097/00005344-199322008-00008. [DOI] [PubMed] [Google Scholar]
- 24.Takayanagi R, Kitazumi K, Takasaki C, Ohnaka K, Aimoto S, Tasaka K, Ohashi M, Nawata H. Presence of non-selective type of endothelin receptor on vascular endothelium and its linkage to vasodilation. FEBS Lett. 1991;282(1):103–106. doi: 10.1016/0014-5793(91)80454-B. [DOI] [PubMed] [Google Scholar]
- 25.Luscher TF, Barton M. Endothelins and endothelin receptor antagonists: therapeutic considerations for a novel class of cardiovascular drugs. Circulation. 2000;102(19):2434–2440. doi: 10.1161/01.cir.102.19.2434. [DOI] [PubMed] [Google Scholar]
- 26.Luscher TF. Endothelium-derived vasoactive factors and regulation of vascular tone in human blood vessels. Lung. 1990;168(Suppl):27–34. doi: 10.1007/BF02718110. [DOI] [PubMed] [Google Scholar]
- 27.Griendling KK, Tsuda T, Alexander RW. Endothelin stimulates diacylglycerol accumulation and activates protein kinase C in cultured vascular smooth muscle cells. J Biol Chem. 1989;264(14):8237–8240. [PubMed] [Google Scholar]
- 28.Takuwa Y, Kasuya Y, Takuwa N, Kudo M, Yanagisawa M, Goto K, Masaki T, Yamashita K. Endothelin receptor is coupled to phospholipase C via a pertussis toxin-insensitive guanine nucleotide-binding regulatory protein in vascular smooth muscle cells. J Clin Invest. 1990;85(3):653–658. doi: 10.1172/JCI114488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eguchi S, Hirata Y, Imai T, Marumo F. Endothelin receptor subtypes are coupled to adenylate cyclase via different guanyl nucleotide-binding proteins in vasculature. Endocrinology. 1993;132(2):524–529. doi: 10.1210/en.132.2.524. [DOI] [PubMed] [Google Scholar]
- 30.Eguchi S, Hirata Y, Marumo F. Endothelin subtype B receptors are coupled to adenylate cyclase via inhibitory G protein in cultured bovine endothelial cells. J Cardiovasc Pharmacol. 1993;22:S161–3. doi: 10.1097/00005344-199322008-00043. [DOI] [PubMed] [Google Scholar]
- 31.Kawanabe Y, Okamoto Y, Hashimoto N, Masaki T. Molecular mechanisms for activation of voltage-independent Ca2 + channels by endothelin-1/endothelin-A receptors. J Cardiovasc Pharmacol. 2004;44(Suppl 1):S219–S223. doi: 10.1097/01.fjc.0000166252.66486.06. [DOI] [PubMed] [Google Scholar]
- 32.Gohla A, Offermanns S, Wilkie TM, Schultz G. Differential involvement of Galpha12 and Galpha13 in receptor-mediated stress fiber formation. J Biol Chem. 1999;274(25):17901–17907. doi: 10.1074/jbc.274.25.17901. [DOI] [PubMed] [Google Scholar]
- 33.Aramori I, Nakanishi S. Coupling of two endothelin receptor subtypes to differing signal transduction in transfected Chinese hamster ovary cells. J Biol Chem. 1992;267(18):12468–12474. [PubMed] [Google Scholar]
- 34.Aramori I, Nirei H, Shoubo M, Sogabe K, Nakamura K, Kojo H, Notsu Y, Ono T, Nakanishi S. Subtype selectivity of a novel endothelin antagonist, FR139317, for the two endothelin receptors in transfected Chinese hamster ovary cells. Mol Pharmacol. 1993;43(2):127–131. [PubMed] [Google Scholar]
- 35.Kawanabe Y, Okamoto Y, Miwa S, Hashimoto N, Masaki T. Molecular mechanisms for the activation of voltage-independent Ca2 + channels by endothelin-1 in Chinese hamster ovary cells stably expressing human endothelin(A) receptors. Mol Pharmacol. 2002;62(1):75–80. doi: 10.1124/mol.62.1.75. [DOI] [PubMed] [Google Scholar]
- 36.Masaki T, Miwa S, Sawamura T, Ninomiya H, Okamoto Y. Subcellular mechanisms of endothelin action in vascular system. Eur J Pharmacol. 1999;375(1–3):133–138. doi: 10.1016/S0014-2999(99)00252-6. [DOI] [PubMed] [Google Scholar]
- 37.Takigawa M, Sakurai T, Kasuya Y, Abe Y, Masaki T, Goto K. Molecular identification of guanine-nucleotide-binding regulatory proteins which couple to endothelin receptors. Eur J Biochem. 1995;228(1):102–108. doi: 10.1111/j.1432-1033.1995.tb20236.x. [DOI] [PubMed] [Google Scholar]
- 38.Kawanabe Y, Okamoto Y, Nozaki K, Hashimoto N, Miwa S, Masaki T. Molecular mechanism for endothelin-1-induced stress-fiber formation: analysis of G proteins using a mutant endothelin(A) receptor. Mol Pharmacol. 2002;61(2):277–284. doi: 10.1124/mol.61.2.277. [DOI] [PubMed] [Google Scholar]
- 39.Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, Kimura S, Masaki T, Duguid WP, Stewart DJ. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328(24):1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 40.Corder R, Carrier M, Khan N, Klemm P, Vane JR. Cytokine regulation of endothelin-1 release from bovine aortic endothelial cells. J Cardiovasc Pharmacol. 1995;26(Suppl 3):S56–S58. [PubMed] [Google Scholar]
- 41.Nootens M, Kaufmann E, Rector T, Toher C, Judd D, Francis GS, Rich S. Neurohormonal activation in patients with right ventricular failure from pulmonary hypertension: relation to hemodynamic variables and endothelin levels. J Am Coll Cardiol. 1995;26(7):1581–1585. doi: 10.1016/0735-1097(95)00399-1. [DOI] [PubMed] [Google Scholar]
- 42.Stewart DJ, Levy RD, Cernacek P, Langleben D. Increased plasma endothelin-1 in pulmonary hypertension: marker or mediator of disease? Ann Intern Med. 1991;114(6):464–469. doi: 10.7326/0003-4819-114-6-464. [DOI] [PubMed] [Google Scholar]
- 43.Bauer M, Wilkens H, Langer F, Schneider SO, Lausberg H, Schafers HJ. Selective upregulation of endothelin B receptor gene expression in severe pulmonary hypertension. Circulation. 2002;105(9):1034–1036. doi: 10.1161/hc0902.105719. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi H, Soma S, Muramatsu M, Oka M, Ienaga H, Fukuchi Y. Discrepant distribution of big endothelin (ET)-1 and ET receptors in the pulmonary artery. Eur Respir J. 2001;18(1):5–14. doi: 10.1183/09031936.01.00075501. [DOI] [PubMed] [Google Scholar]
- 45.Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, Badesch DB, Roux S, Rainisio M, Bodin F, Rubin LJ. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358(9288):1119–1123. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- 46.Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, Landzberg M, Simonneau G. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346(12):896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 47.Barst RJ, Langleben D, Frost A, Horn EM, Oudiz R, Shapiro S, McLaughlin V, Hill N, Tapson VF, Robbins IM, Zwicke D, Duncan B, Dixon RA, Frumkin LR. Sitaxentan therapy for pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;169(4):441–447. doi: 10.1164/rccm.200307-957OC. [DOI] [PubMed] [Google Scholar]
- 48.Cody RJ, Haas GJ, Binkley PF, Capers Q, Kelley R. Plasma endothelin correlates with the extent of pulmonary hypertension in patients with chronic congestive heart failure. Circulation. 1992;85(2):504–509. doi: 10.1161/01.cir.85.2.504. [DOI] [PubMed] [Google Scholar]
- 49.Pacher R, Stanek B, Hulsmann M, Koller-Strametz J, Berger R, Schuller M, Hartter E, Ogris E, Frey B, Heinz G, Maurer G. Prognostic impact of big endothelin-1 plasma concentrations compared with invasive hemodynamic evaluation in severe heart failure. J Am Coll Cardiol. 1996;27(3):633–641. doi: 10.1016/0735-1097(95)00520-X. [DOI] [PubMed] [Google Scholar]
- 50.Hulsmann M, Stanek B, Frey B, Sturm B, Putz D, Kos T, Berger R, Woloszczuk W, Putz D, Kos T, Berger R, Woloszczuk W, Maurer G, Pacher R. Value of cardiopulmonary exercise testing and big endothelin plasma levels to predict short-term prognosis of patients with chronic heart failure. J Am Coll Cardiol. 1998;32(6):1695–1700. doi: 10.1016/S0735-1097(98)00437-9. [DOI] [PubMed] [Google Scholar]
- 51.Ponicke K, Vogelsang M, Heinroth M, Becker K, Zolk O, Bohm M, Zerkowski HR, Brodde OE. Endothelin receptors in the failing and nonfailing human heart. Circulation. 1998;97(8):744–751. doi: 10.1161/01.cir.97.8.744. [DOI] [PubMed] [Google Scholar]
- 52.Zolk O, Quattek J, Sitzler G, Schrader T, Nickenig G, Schnabel P, Shimada K, Takahashi M, Bohm M. Expression of endothelin-1, endothelin-converting enzyme, and endothelin receptors in chronic heart failure. Circulation. 1999;99(16):2118–2123. doi: 10.1161/01.cir.99.16.2118. [DOI] [PubMed] [Google Scholar]
- 53.Cowburn PJ, Cleland JG, McArthur JD, MacLean MR, McMurray JJ, Dargie HJ. Short-term haemodynamic effects of BQ-123, a selective endothelin ET(A)-receptor antagonist, in chronic heart failure. Lancet. 1998;352(9123):201–202. doi: 10.1016/S0140-6736(05)77807-7. [DOI] [PubMed] [Google Scholar]
- 54.Kiowski W, Sutsch G, Hunziker P, Muller P, Kim J, Oechslin E, Schmitt R, Jones R, Bertel O. Evidence for endothelin-1-mediated vasoconstriction in severe chronic heart failure. Lancet. 1995;346(8977):732–736. doi: 10.1016/S0140-6736(95)91504-4. [DOI] [PubMed] [Google Scholar]
- 55.Spieker LE, Mitrovic V, Noll G, Pacher R, Schulze MR, Muntwyler J, Schalcher C, Kiowski W, Luscher TF. Acute hemodynamic and neurohumoral effects of selective ET(A) receptor blockade in patients with congestive heart failure. ET 003 Investigators. J Am Coll Cardiol. 2000;35(7):1745–1752. doi: 10.1016/S0735-1097(00)00649-5. [DOI] [PubMed] [Google Scholar]
- 56.Torre-Amione G, Young JB, Colucci WS, Lewis BS, Pratt C, Cotter G, Stangl K, Elkayam U, Teerlink JR, Frey A, Rainisio M, Kobrin I. Hemodynamic and clinical effects of tezosentan, an intravenous dual endothelin receptor antagonist, in patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2003;42(1):140–147. doi: 10.1016/S0735-1097(03)00556-4. [DOI] [PubMed] [Google Scholar]
- 57.Cotter G, Kiowski W, Kaluski E, Kobrin I, Milovanov O, Marmor A, Jafari J, Reisin L, Krakover R, Vered Z, Caspi A. Tezosentan (an intravenous endothelin receptor A/B antagonist) reduces peripheral resistance and increases cardiac power therefore preventing a steep decrease in blood pressure in patients with congestive heart failure. Eur J Heart Fail. 2001;3(4):457–461. doi: 10.1016/S1388-9842(01)00168-4. [DOI] [PubMed] [Google Scholar]
- 58.Kaluski E, Kobrin I, Zimlichman R, Marmor A, Krakov O, Milo O, Frey A, Kaplan S, Krakover R, Caspi A, Vered Z, Cotter G. RITZ-5: randomized intravenous TeZosentan (an endothelin-A/B antagonist) for the treatment of pulmonary edema: a prospective, multicenter, double-blind, placebo-controlled study. J Am Coll Cardiol. 2003;41(2):204–210. doi: 10.1016/S0735-1097(02)02708-0. [DOI] [PubMed] [Google Scholar]
- 59.O’Connor CM, Gattis WA, Adams KF, Jr, Hasselblad V, Chandler B, Frey A, Kobrin I, Rainisio M, Shah MR, Teerlink J, Gheorghiade M. Tezosentan in patients with acute heart failure and acute coronary syndromes: results of the Randomized Intravenous TeZosentan Study (RITZ-4) J Am Coll Cardiol. 2003;41(9):1452–1457. doi: 10.1016/S0735-1097(03)00194-3. [DOI] [PubMed] [Google Scholar]
- 60.O’Connor CM, Gattis WA, Adams KF, Jr, Shah MR, Kobrin I, Frey A, Gheorghiade M. Tezosentan in patients with acute heart failure and acute coronary syndromes: design of the Randomized Intravenous Tezosentan study (RITZ-4) Am Heart J. 2002;144(4):583–588. doi: 10.1016/s0002-8703(02)00127-8. [DOI] [PubMed] [Google Scholar]
- 61.Schalcher C, Cotter G, Reisin L, Bertel O, Kobrin I, Guyene TT, Kiowski W. The dual endothelin receptor antagonist tezosentan acutely improves hemodynamic parameters in patients with advanced heart failure. Am Heart J. 2001;142(2):340–349. doi: 10.1067/mhj.2001.116760. [DOI] [PubMed] [Google Scholar]
- 62.Torre-Amione G, Durand JB, Nagueh S, Vooletich MT, Kobrin I, Pratt C. A pilot safety trial of prolonged (48 h) infusion of the dual endothelin-receptor antagonist tezosentan in patients with advanced heart failure. Chest. 2001;120(2):460–466. doi: 10.1378/chest.120.2.460. [DOI] [PubMed] [Google Scholar]
- 63.Torre-Amione G, Young JB, Durand J, Bozkurt B, Mann DL, Kobrin I, Pratt CM. Hemodynamic effects of tezosentan, an intravenous dual endothelin receptor antagonist, in patients with class III to IV congestive heart failure. Circulation. 2001;103(7):973–980. doi: 10.1161/01.cir.103.7.973. [DOI] [PubMed] [Google Scholar]
- 64.Kalra PR, Moon JC, Coats AJ. Do results of the ENABLE (Endothelin Antagonist Bosentan for Lowering Cardiac Events in Heart Failure) study spell the end for non-selective endothelin antagonism in heart failure? Int J Cardiol. 2002;85(2–3):195–197. doi: 10.1016/S0167-5273(02)00182-1. [DOI] [PubMed] [Google Scholar]
- 65.Mylona P, Cleland JG. Update of REACH-1 and MERIT-HF clinical trials in heart failure Cardio.net Editorial Team. Eur J Heart Fail. 1999;1(2):197–200. doi: 10.1016/S1388-9842(99)00022-7. [DOI] [PubMed] [Google Scholar]
- 66.Iwasa S, Fan J, Miyauchi T, Watanabe T. Blockade of endothelin receptors reduces diet-induced hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice. Pathobiology. 2001;69(1):1–10. doi: 10.1159/000048751. [DOI] [PubMed] [Google Scholar]
- 67.Yokokawa K, Tahara H, Kohno M, Murakawa K, Yasunari K, Nakagawa K, Hamada T, Otani S, Yanagisawa M, Takeda T. Hypertension associated with endothelin-secreting malignant hemangioendothelioma. Ann Intern Med. 1991;114(3):213–215. doi: 10.7326/0003-4819-114-3-213. [DOI] [PubMed] [Google Scholar]
- 68.Barton M, Shaw S, d’Uscio LV, Moreau P, Luscher TF. Angiotensin II increases vascular and renal endothelin-1 and functional endothelin converting enzyme activity in vivo: role of ETA receptors for endothelin regulation. Biochem Biophys Res Commun. 1997;238(3):861–865. doi: 10.1006/bbrc.1997.7394. [DOI] [PubMed] [Google Scholar]
- 69.Schiffrin EL. Role of endothelin-1 in hypertension and vascular disease. Am J Hypertens. 2001;14(6 Pt 2):83S–89S. doi: 10.1016/s0895-7061(01)02074-x. [DOI] [PubMed] [Google Scholar]
- 70.Barton M. Reversal of proteinuric renal disease and the emerging role of endothelin. Nat Clin Pract Nephrol. 2008;4(9):490–501. doi: 10.1038/ncpneph0891. [DOI] [PubMed] [Google Scholar]
- 71.Caligiuri G, Levy B, Pernow J, Thoren P, Hansson GK. Myocardial infarction mediated by endothelin receptor signaling in hypercholesterolemic mice. Proc Natl Acad Sci USA. 1999;96(12):6920–6924. doi: 10.1073/pnas.96.12.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barton M. Endothelial dysfunction and atherosclerosis: endothelin receptor antagonists as novel therapeutics. Curr Hypertens Rep. 2000;2(1):84–91. doi: 10.1007/s11906-000-0064-5. [DOI] [PubMed] [Google Scholar]
- 73.Taddei S, Virdis A, Ghiadoni L, Sudano I, Notari M, Salvetti A. Vasoconstriction to endogenous endothelin-1 is increased in the peripheral circulation of patients with essential hypertension. Circulation. 1999;100(16):1680–1683. doi: 10.1161/01.cir.100.16.1680. [DOI] [PubMed] [Google Scholar]
- 74.Agapitov AV, Haynes WG. Role of endothelin in cardiovascular disease. J Renin Angiotensin Aldosterone Sys. 2002;3(1):1–15. doi: 10.3317/jraas.2002.001. [DOI] [PubMed] [Google Scholar]
- 75.Moreau P, d’Uscio LV, Shaw S, Takase H, Barton M, Luscher TF. Angiotensin II increases tissue endothelin and induces vascular hypertrophy: reversal by ET(A)-receptor antagonist. Circulation. 1997;96(5):1593–1597. doi: 10.1161/01.cir.96.5.1593. [DOI] [PubMed] [Google Scholar]
- 76.Ohuchi T, Kuwaki T, Ling GY, Dewit D, Ju KH, Onodera M, Cao WH, Yanagisawa M, Kumada M. Elevation of blood pressure by genetic and pharmacological disruption of the ETB receptor in mice. Am J Physiol. 1999;276(4 Pt 2):R1071–7. doi: 10.1152/ajpregu.1999.276.4.R1071. [DOI] [PubMed] [Google Scholar]
- 77.Strachan FE, Spratt JC, Wilkinson IB, Johnston NR, Gray GA, Webb DJ. Systemic blockade of the endothelin-B receptor increases peripheral vascular resistance in healthy men. Hypertension. 1999;33(1 Pt 2):581–585. doi: 10.1161/01.hyp.33.1.581. [DOI] [PubMed] [Google Scholar]
- 78.Haynes WG, Ferro CJ, O’Kane KP, Somerville D, Lomax CC, Webb DJ. Systemic endothelin receptor blockade decreases peripheral vascular resistance and blood pressure in humans. Circulation. 1996;93(10):1860–1870. doi: 10.1161/01.cir.93.10.1860. [DOI] [PubMed] [Google Scholar]
- 79.Haynes WG, Webb DJ. Contribution of endogenous generation of endothelin-1 to basal vascular tone. Lancet. 1994;344(8926):852–854. doi: 10.1016/S0140-6736(94)92827-4. [DOI] [PubMed] [Google Scholar]
- 80.Krum H, Viskoper RJ, Lacourciere Y, Budde M, Charlon V. The effect of an endothelin-receptor antagonist, bosentan, on blood pressure in patients with essential hypertension. Bosentan hypertension Investigators. N Engl J Med. 1998;338(12):784–790. doi: 10.1056/NEJM199803193381202. [DOI] [PubMed] [Google Scholar]
- 81.Goddard J, Johnston NR, Hand MF, Cumming AD, Rabelink TJ, Rankin AJ, Webb DJ. Endothelin-A receptor antagonism reduces blood pressure and increases renal blood flow in hypertensive patients with chronic renal failure: a comparison of selective and combined endothelin receptor blockade. Circulation. 2004;109(9):1186–1193. doi: 10.1161/01.CIR.0000118499.69469.51. [DOI] [PubMed] [Google Scholar]
- 82.Goddard J, Eckhart C, Johnston NR, Cumming AD, Rankin AJ, Webb DJ. Endothelin A receptor antagonism and angiotensin-converting enzyme inhibition are synergistic via an endothelin B receptor-mediated and nitric oxide-dependent mechanism. J Am Soc Nephrol. 2004;15(10):2601–2610. doi: 10.1097/01.ASN.0000141313.84470.4B. [DOI] [PubMed] [Google Scholar]
- 83.Dhaun N, Goddard J, Webb DJ. The endothelin system and its antagonism in chronic kidney disease. J Am Soc Nephrol. 2006;17(4):943–955. doi: 10.1681/ASN.2005121256. [DOI] [PubMed] [Google Scholar]
- 84.Dhaun N, Macintyre IM, Melville V, Lilitkarntakul P, Johnston NR, Goddard J, Webb DJ. Blood pressure-independent reduction in proteinuria and arterial stiffness after acute endothelin-a receptor antagonism in chronic kidney disease. Hypertension. 2009;54(1):113–119. doi: 10.1161/HYPERTENSIONAHA.109.132670. [DOI] [PubMed] [Google Scholar]
- 85.Bhalla A, Haque S, Taylor I, Winslet M, Loizidou M. Endothelin receptor antagonism and cancer. Eur J Clin Invest. 2009;39(Suppl 2):74–77. doi: 10.1111/j.1365-2362.2009.02123.x. [DOI] [PubMed] [Google Scholar]
- 86.Bagnato A, Catt KJ. Endothelins as autocrine regulators of tumor cell growth. Trends Endocrinol Metab. 1998;9(9):378–383. doi: 10.1016/S1043-2760(98)00094-0. [DOI] [PubMed] [Google Scholar]
- 87.Bagnato A, Salani D, Di Castro V, Wu-Wong JR, Tecce R, Nicotra MR, Venuti A, Natali PG. Expression of endothelin 1 and endothelin A receptor in ovarian carcinoma: evidence for an autocrine role in tumor growth. Cancer Res. 1999;59(3):720–727. [PubMed] [Google Scholar]
- 88.Bagnato A, Tecce R, Moretti C, Di Castro V, Spergel D, Catt KJ. Autocrine actions of endothelin-1 as a growth factor in human ovarian carcinoma cells. Clin Cancer Res. 1995;1(9):1059–1066. [PubMed] [Google Scholar]
- 89.Rosano L, Spinella F, Di Castro V, Nicotra MR, Dedhar S, de Herreros AG, Natali PG, Bagnato A. Endothelin-1 promotes epithelial-to-mesenchymal transition in human ovarian cancer cells. Cancer Res. 2005;65(24):11649–11657. doi: 10.1158/0008-5472.CAN-05-2123. [DOI] [PubMed] [Google Scholar]
- 90.Salani D, Di Castro V, Nicotra MR, Rosano L, Tecce R, Venuti A, Natali PG, Bagnato A. Role of endothelin-1 in neovascularization of ovarian carcinoma. Am J Pathol. 2000;157(5):1537–1547. doi: 10.1016/S0002-9440(10)64791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jazaeri AA, Awtrey CS, Chandramouli GV, Chuang YE, Khan J, Sotiriou C, Aprelikova O, Yee CJ, Zorn KK, Birrer MJ, Barrett JC, Boyd J. Gene expression profiles associated with response to chemotherapy in epithelial ovarian cancers. Clin Cancer Res. 2005;11(17):6300–6310. doi: 10.1158/1078-0432.CCR-04-2682. [DOI] [PubMed] [Google Scholar]
- 92.Komuro I, Kurihara H, Sugiyama T, Yoshizumi M, Takaku F, Yazaki Y. Endothelin stimulates c-fos and c-myc expression and proliferation of vascular smooth muscle cells. FEBS Lett. 1988;238(2):249–252. doi: 10.1016/0014-5793(88)80489-7. [DOI] [PubMed] [Google Scholar]
- 93.Vacca F, Bagnato A, Catt KJ, Tecce R. Transactivation of the epidermal growth factor receptor in endothelin-1-induced mitogenic signaling in human ovarian carcinoma cells. Cancer Res. 2000;60(18):5310–5317. [PubMed] [Google Scholar]
- 94.Pedram A, Razandi M, Evinger AJ, Lee E, Levin ER. Estrogen inhibits ATR signaling to cell cycle checkpoints and DNA repair. Mol Biol Cell. 2009;20(14):3374–3389. doi: 10.1091/mbc.E09-01-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Spinella F, Rosano L, Elia G, Di Castro V, Natali PG, Bagnato A. Endothelin-1 stimulates cyclooxygenase-2 expression in ovarian cancer cells through multiple signaling pathways: evidence for involvement of transactivation of the epidermal growth factor receptor. J Cardiovasc Pharmacol. 2004;44(Suppl 1):S140–S143. doi: 10.1097/01.fjc.0000166255.12229.0d. [DOI] [PubMed] [Google Scholar]
- 96.Spinella F, Rosano L, Decandia S, Di Castro V, Albini A, Elia G, Natali PG, Bagnato A. Antitumor effect of green tea polyphenol epigallocatechin-3-gallate in ovarian carcinoma cells: evidence for the endothelin-1 as a potential target. Exp Biol Med (Maywood) 2006;231(6):1123–1127. [PubMed] [Google Scholar]
- 97.Jimeno A, Carducci M. Atrasentan: a novel and rationally designed therapeutic alternative in the management of cancer. Expert Rev Anticancer Ther. 2005;5(3):419–427. doi: 10.1586/14737140.5.3.419. [DOI] [PubMed] [Google Scholar]
- 98.Rosano L, Di Castro V, Spinella F, Decandia S, Natali PG, Bagnato A. ZD4054, a potent endothelin receptor A antagonist, inhibits ovarian carcinoma cell proliferation. Exp Biol Med (Maywood) 2006;231(6):1132–1135. [PubMed] [Google Scholar]
- 99.Warren R, Liu G. ZD4054: a specific endothelin A receptor antagonist with promising activity in metastatic castration-resistant prostate cancer. Expert Opin Investig Drugs. 2008;17(8):1237–1245. doi: 10.1517/13543784.17.8.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Growcott JW. Preclinical anticancer activity of the specific endothelin A receptor antagonist ZD4054. Anticancer Drugs. 2009;20(2):83–88. doi: 10.1097/CAD.0b013e328320791c. [DOI] [PubMed] [Google Scholar]
- 101.Rosano L, Cianfrocca R, Masi S, Spinella F, Di Castro V, Biroccio A, Salvati E, Nicotra MR, Natali PG, Bagnato A. Beta-arrestin links endothelin A receptor to beta-catenin signaling to induce ovarian cancer cell invasion and metastasis. Proc Natl Acad Sci USA. 2009;106(8):2806–2811. doi: 10.1073/pnas.0807158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rosano L, Di Castro V, Spinella F, Nicotra MR, Natali PG, Bagnato A. ZD4054, a specific antagonist of the endothelin A receptor, inhibits tumor growth and enhances paclitaxel activity in human ovarian carcinoma in vitro and in vivo. Mol Cancer Ther. 2007;6(7):2003–2011. doi: 10.1158/1535-7163.MCT-07-0151. [DOI] [PubMed] [Google Scholar]
- 103.Rosano L, Spinella F, Di Castro V, Natali PG, Bagnato A. Therapeutic targeting of the endothelin-A receptor in human ovarian carcinoma: efficacy of cytotoxic agents is markedly enhanced by co-administration with atrasentan. J Cardiovasc Pharmacol. 2004;44(Suppl 1):S132–S135. doi: 10.1097/01.fjc.0000166259.96980.6a. [DOI] [PubMed] [Google Scholar]
- 104.Rosano L, Spinella F, Salani D, Di Castro V, Venuti A, Nicotra MR, Natali PG, Bagnato A. Therapeutic targeting of the endothelin a receptor in human ovarian carcinoma. Cancer Res. 2003;63(10):2447–2453. [PubMed] [Google Scholar]
- 105.Smollich M, Wulfing P. The endothelin axis: a novel target for pharmacotherapy of female malignancies. Curr Vasc Pharmacol. 2007;5(3):239–248. doi: 10.2174/157016107781024082. [DOI] [PubMed] [Google Scholar]