Abstract
Background
Despite their high risk for adverse cardiac outcomes, persons on chronic dialysis have been shown to have lower use of antihypertensive medications with cardioprotective properties, such as angiotensin converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARBs), β-blockers, and calcium channel blockers (CCBs), than might be expected. We constructed a novel database that permits detailed exploration into the demographic, clinical and geographic factors associated with the use these agents of among hypertensive chronic dialysis patients.
Study Design
National cross-sectional retrospective analysis linking Medicaid prescription drug claims with United States Renal Data System core data.
Setting & Participants
48,882 hypertensive chronic dialysis patients who were dually-eligible for Medicaid and Medicare services in 2005.
Factors
Demographics, comorbidities, functional status, and state of residence.
Outcomes
Prevalence of cardioprotective antihypertensive agents in Medicaid pharmacy claims and state-specific observed:expected odds ratios of medication exposure.
Measurements
Factors associated with medication use were modeled using multi-level logistic regression models.
Results
In multivariable analyses, cardioprotective antihypertensive medication exposure was significantly associated with younger age, female sex, non-Caucasian race, intact functional status, and use of in-center hemodialysis. Diabetes was associated with a statistically-significant 28% higher odds of ACE inhibitor/ARB use, but congestive heart failure (CHF) was associated with only a 9% increase in the odds of β-blockers and no increase in ACE inhibitor/ARB use. There was substantial state-by-state variation in use of all classes of agents, with a greater than 2.9-fold difference in adjusted rate odds ratios between the highest- and lowest-prescribing states for ACE inhibitors/ARBs and a 3.6-fold difference for β-blockers.
Limitations
Limited generalizability beyond study population.
Conclusions
Among publicly insured chronic dialysis patients with hypertension, there were marked differences in use rates by state, in part potentially due to differences in Medicaid benefits. However, geographic characteristics were also associated with exposure suggesting clinical uncertainty about the utility of these medications.
Keywords: antihypertensive drugs, cardioprotection, dialysis, end stage renal disease, Medicare, Medicaid, Insurance
Hypertension and cardiovascular disease (CVD) are common in end stage renal disease (ESRD) patients on dialysis. Data from the Dialysis Outcomes and Practice Patterns Study (DOPPS) revealed that in US chronic dialysis patients, 78% have hypertension, 46% coronary artery disease, 29% congestive heart failure, and 17% cerebrovascular disease.1 A strong clinical rationale therefore exists to preferentially prescribe antihypertensive agents with cardioprotective properties, even though the CVD benefits of these agents have only been shown in the non-dialysis population 2-14 or in animal models.15, 16 Currently, antihypertensive agents with the most well-established cardioprotective properties are renin-angiotensin-aldosterone system inhibitors (i.e., angiotensin converting enzyme [ACE] inhibitors and angiotensin receptor blockers [ARBs]), β-adrenergic blockers (β-blockers), and calcium channel blockers (CCBs).1
While previous studies have reported use rates of these agents in the dialysis population, the estimates have varied considerably. In Waves 3 and 4 of the Dialysis Morbidity and Mortality Study (DMMS), which recorded medication use among dialysis patients in 1993, ACE inhibitors were used by 13.9% of incident dialysis patients, β-blockers in 8.5%, and CCBs in 35.0%.17 Generally higher use rates were reported in both incident and prevalent patients of the DMMS Wave 2 study, which spanned 1996-97: ACE inhibitors were prescribed for 23.6% – 31.6% of individuals 18,19, 20, β-blockers in 19.2% – 25.4% 18-20 and CCBs in 50.7% – 52.5%.18, 20,21
While these and other 22 studies have examined use of cardioprotective medications to treat hypertension in chronic dialysis patients, no study has specifically examined whether there is substantial geographic variation in care. If there is substantial geographic variability in the use of these agents that cannot be accounted for by differences in underlying comorbidity burden, it could prompt future efforts to uncover the reasons for these differences and might also provide an opportunity to provide more consistent care throughout the U.S. A particularly important subset of patients are Medicaid-eligible individuals, who comprise nearly a third of prevalent dialysis patients23 and who have not been systematically explored. Since Medicaid-enrolled dialysis patients are more financially disadvantaged and medically-needy, relative to non-Medicaid-enrolled dialysis patients,24 understanding practice patterns in this subgroup has important policy implications for vulnerable publicly-assisted patients’ access to medications. While Medicaid is a joint federal-state program, each state offers prescription benefits with varied degrees of restrictions which may translate into different exposure rates.
To address these gaps in knowledge, we examined Medicaid-enrolled patients with hypertension undergoing long-term dialysis to determine how the use of ACE inhibitors/ARBs, β-blockers, and CCBs was associated with demographic, risk behavior, functional status, comorbidity, dialysis modality, and laboratory factors, and how practice patterns varied by state across the US.
Methods
Data Sources for Analysis
We conducted a cross-sectional retrospective analysis of antihypertensive prescription drug claims for prevalent dually-eligible (Medicare-Medicaid) chronic dialysis patients during a four-month period, September through December 2005. Data were obtained from the United States Renal Data System (USRDS) and the Centers for Medicare & Medicaid Services (CMS) Medicaid files. From CMS, we obtained Medicaid Analytic eXtract 25 Personal Summary Files as well as the final action prescription drug claims files. The USRDS performed a deterministic match of these Medicaid beneficiaries against the core files to identify dually-eligible individuals on chronic dialysis.24 The MAX final action prescription drug claims were used to determine medication exposure.
Study Cohort and Rationale for Analytic Approach
We identified unique individuals over the age of 18 years who were on chronic dialysis, diagnosed with hypertension on the CMS-2728 dialysis intake form, were enrolled in Medicaid and Medicare programs simultaneously during the 4-month period of September through December 2002, and who filled at least one Medicaid prescription during this time. Patients with Medicaid managed care plans were excluded since medication data were not available. We excluded Arizona and Tennessee because all Medicaid patients in these states are enrolled in managed care plans, and Delaware and Kentucky because there were < 25 eligible dialysis patients. Of note, persons on chronic dialysis were not generally eligible for Medicare managed care plans prior to 2006.
Descriptive Variables
Descriptive variables were drawn from the CMS-2728 dialysis intake form, completed at the time of dialysis initiation. Demographic variables included age, sex, and race by ethnicity (four mutually-exclusive groups comprising non-Hispanic Caucasians, non-Hispanic African-Americans, Hispanics, and Others), and employment. Risk behavior factors were smoking and substance abuse (alcohol or illicit drugs), and functional status markers were inability to ambulate and inability to transfer. Cause of ESRD was categorized as diabetes, hypertension, glomerulonephritis, or other. Major comorbidities were diabetes (types I and II combined), hypertension, congestive heart failure, coronary artery disease, cerebrovascular disease, peripheral vascular disease, and cardiac dysrhythmia. Since the CMS-2728 form is structured such that diseases like diabetes or hypertension may be considered as both a cause of ESRD and/or a comorbidity, diabetes and hypertension were considered present in an individual if they were listed as either the cause of ESRD or as a comorbidity. The sole laboratory value analyzed was hemoglobin, which was dichotomized level at 11g/dL. Dialysis modality was categorized as in-center hemodialysis (HD) or self-care dialysis (home HD or peritoneal dialysis (PD)).
Medication Exposure
We matched drug name and therapeutic class information in the Medicaid drug claims at the national drug code (NDC) level using Multum Lexicon (Cerner Corporation, www.cerner.com). β-blockers were divided into cardioselective, non-selective, and α1 agents. Renin-angiotensin-aldosterone system agents were subclassified as ACE inhibitors or ARBs. Calcium channel blockers were initially grouped into four classes: class I (short-acting nifedipine), class II (long-acting dihydropyridines), class III (diltiazem), and class IV (verapamil). As there is evidence of adverse cardiac events associated with short-acting nifedipine,26 we eliminated it from the list or cardioprotective anti-hypertensive agents. We looked across a 4-month period of exposure since some state Medicaid programs allow for 100-day supplies of maintenance medications. We limited the analysis to the first prescription for each person from each class of medications.
Statistical Analyses
Person-Level Analyses
We generated descriptive statistics (means and standard deviations for continuous variables and frequencies and percentages for categorical variables) to illustrate how users of each class of agents differed from each other. In addition, bivariate analyses comparing each of the explanatory variables by use versus non-use were performed using Pearson’s chi-squared test or Student’s t-test, as appropriate. To specifically determine the factors associated with medication status, we generated multi-level logistic regression models using generalized linear mixed models27 (GLMM) with medication status being regressed simultaneously on all of these a priori selected explanatory variables. These models included a random effect for state. To assess the fit, we also generated unconditional logistic regression models that treated state as a fixed effect, and the Hosmer-Lemeshow goodness-of-fit test was conducted.28
Due to the large sample size, statistical significance was inferred only when P <0.01. All statistical analyses were done with SAS 9.2 (SAS Institute, Inc., www.sas.com).
State-by-State Medication Exposure
In addition to the person-level analysis, we conducted a state-by-state comparison of medication treatment. For each state, we determined whether the observed proportion treated was above or below what was expected based on the cross-state averages from the multi-level models or GLMMs. We utilized the random coefficients for state from our GLMMs to facilitate these state-level observed versus expected comparisons. Specifically, we derived the estimates of the random coefficients for each state as these parameters modify each state’s log-odds of medication treatment -- and hence its proportion treated -- from the overall cross-state (fixed) model effects. Taking the anti-log of these estimates generated state-specific observed vs expected odds ratios. Using the estimated standard errors of the predictions we estimated confidence intervals for these state-specific observed vs expected odds ratios.29
Compliance and Research Participant Protection
The research protocol was approved by the institutional review board at the University of Kansas Medical Center (KUMC), and the project was undertaken according to the principles of the Declarations of Helsinki. Data Use Agreements (DUA) between KUMC and the USRDS and CMS permitted the data linking across the USRDS, Medicare and Medicaid files.
Results
Study Population and Demographics
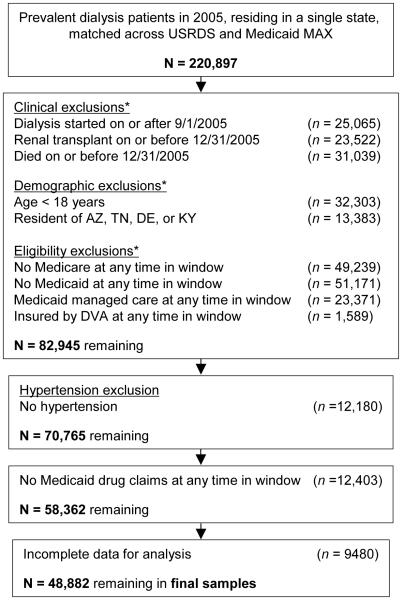
There were over 220,000 dually eligible ESRD patients in 2005 who matched across the USRDS and Medicaid MAX files (Figure 1). The initial exclusions for clinical, demographic and eligibility criteria led to 82,945 persons. (Note that individuals could be excluded for more than one reason.) After limiting the cohort to persons with hypertension who had at least one prescription and who had complete data (CMS 2728 data fields), there were 48,882 prevalent chronic dialysis patients. The sample included more females (54.7%) than males and more African-Americans (46.4%) than Caucasians (27.8%), Hispanics (18.3%), or individuals of other races/ethnicities (7.5%) (Table 1). The mean age was 60.8 years. Only 6.0% were employed, consistent with Medicaid’s means-tested eligibility criteria. Nearly half (47.4%) of the cohort had diabetes as the primary cause for ESRD followed by hypertension (32.2%). Congestive heart failure (CHF) was present in 29.0%, coronary artery disease (CAD) in 20.2%, and 8.6% had a history of a cerebrovascular accident (CVA). Nearly 95% were using in-center HD.
Figure 1.
Construction of the analytic sample. *Exclusions are not mutual, so individual totals do not sum to N. “Window” refers to the observation window of 9/1/2005 – 12/31/2005.[nd2]
Table 1.
Descriptive characteristics of the total eligible cohort and of the users of selected antihypertensive agents.
| Users of Selected Antihypertensive Agents | |||||
|---|---|---|---|---|---|
| Characteristics | Total eligible cohort1 | =>1 Class2 | ACEi/ARBs2 | β-Blockers2 | CCBs2 |
| Number of cases | 48,882 (100) | 39,411 (80.6) | 23,791(48.7) | 26,661 (54.5) | 23,740 (48.6) |
| Age, yr3 | 60.4 ± 15.1 | ||||
| Drug | 60.2 ± 14.9 | 59.6 ± 14.8 | 59.8 ± 15.0 | 59.7 ± 14.9 | |
| No drug[nd1] | 60.8 ± 15.6 | 61.1 ± 15.3 | 61.1 ± 15.2 | 61.0 ± 15.2 | |
| Sex, n (%) | |||||
| Female | 26,725 (54.7) | 21,584 (80.8) | 13,005 (48.7) | 14,326 (53.6)* | 13,231 (49.5)* |
| Male | 22,157 (45.3) | 17,827 (80.5) | 10,786 (48.7) | 12,335 (55.7)* | 10,509 (47.4)* |
| Race/Ethnicity, n (%) | |||||
| African-American | 22,667 (46.4) | 18,539 (81.8)* | 11,242 (49.6)* | 12,689 (56.0%)* | 11,644 (51.4)* |
| Caucasian | 13,595 (27.8) | 10,636 (78.2)* | 6116 (45.0)* | 7543 (55.5%)* | 5660 (41.6)* |
| Hispanic | 8937 (18.3) | 7199 (80.6)* | 4464 (50.0)* | 4442 (49.7%)* | 4457 (51.0)* |
| Other | 3683 (7.5) | 3037 (82.5)* | 1969 (53.5)* | 1987 (54.0%)* | 1879 (51.0)* |
| BMI category, n (%) | |||||
| < 20 kg/m2 | 4094 (8.4) | 3291 (80.4)* | 2,014 (49.2)* | 2167 (52.9)* | 2055 (50.2)* |
| 20-24.9 kg/m2 | 13,830 (28.3) | 11,460 (82.9)* | 7145 (51.7)* | 7853 (56.8)* | 7179 (51.9)* |
| 25-29.9 kg/m2 | 13,357 (27.3) | 10,922 (81.8)* | 6583 (49.3)* | 7468 (55.9)* | 6664 (49.9)* |
| 30+ kg/m2 | 17,601 (36.0) | 13,738 (78.1)* | 8049 (45.7)* | 9173 (52.1)* | 7842 (44.6)* |
| Smoker, n (%) | |||||
| Yes | 3242 (6.6) | 2645 (81.6) | 1700 (52.4)* | 1855 (57.2)† | 1638 (50.5) |
| No | 45,640 (93.4) | 36,766 (80.6) | 22,091 (48.4)* | 24,806 (54.4)† | 22,102 (48.4) |
| Substance abuser, n (%) | |||||
| Yes | 1595 (3.3) | 1319 (82.7) | 818 (51.3) | 928 (58.2)† | 861 (54.0)* |
| No | 47,287 (96.7) | 38,092 (80.6) | 22,973 (48.6) | 25,733 (54.4)† | 22,879 (48.4)* |
| Unemployed, n (%) | |||||
| Yes | 45936 (94.0) | 37,007 (80.6) | 22,293 (48.5) | 24,986 (54.4)† | 22,230 (48.4)† |
| No | 2946 (6.0) | 2404 (81.6) | 1498 (50.9) | 1675 (56.9)† | 1510 (51.3)† |
| Unable to ambulate, n (%) | |||||
| Yes | 1670 (3.4) | 1243 (74.4)* | 700 (41.9)* | 853 (51.1)† | 648 (38.8)* |
| No | 47,212 (96.6) | 38,168 (80.4)* | 23,091 (48.9)* | 25,808 (54.7)† | 23,092 (48.9)* |
| Unable to transfer, n (%) | |||||
| Yes | 517 (1.1) | 379 (73.3)* | 197 (38.1)* | 261 (50.5) | 187 (36.2)* |
| No | 48,365 (98.9) | 39,032 (80.7)* | 23,594 (48.8)* | 26,400 (54.6) | 23,553 (48.7)* |
| Cause of ESRD, n (%) | |||||
| Diabetes | 23,192 (47.4) | 19,041 (82.1)* | 12,006 (51.8)* | 12,729 (54.9)* | 1,1447 (49.4)* |
| Hypertension | 15,736 (32.2) | 12,847 (81.6)* | 7483(47.6)* | 8652 (55.0)* | 7967 (50.6)* |
| GN | 4783 (9.8) | 3703 (77.4)* | 2230 (46.6)* | 2623 (54.8)* | 2167 (45.5)* |
| Other | 5171 (10.6) | 3820 (73.9)* | 2072 (40.1)* | 2657 (51.4)* | 2150 (41.6)* |
| Comorbidities | |||||
| Diabetes, n (%) | |||||
| Yes | 27,930 (57.1) | 22,897 (82.0)* | 14,318 (51.3)* | 15,350 (55.0) | 13,686 (49.0) |
| No | 20,952 (42.9) | 16,514 (78.8)* | 9473 (45.2)* | 11,311 (54.0) | 10,054 (48.0) |
| CHF, n (%) | |||||
| Yes | 14,158 (29.0) | 11,488 (81.1) | 6928 (48.9) | 8029 (56.7)* | 6437 (45.5)* |
| No | 34,724 (71.0) | 27,923 (80.4) | 16,863 (48.6) | 18,632 (53.7)* | 17,303 (49.8)* |
| CAD, n (%) | |||||
| Yes | 9867 (20.2) | 8097 (82.0)* | 4767 (48.3) | 6059 (61.4)* | 4173 (42.3)* |
| No | 39,006 (79.8) | 31,314 (80.3)* | 19024 (48.8) | 20,602 (52.8)* | 19,567 (50.2)* |
| PVD, n (%) | |||||
| Yes | 5417 (11.1) | 4330 (79.9) | 2587 (47.8) | 3038 (56.1) | 2367 (43.7)* |
| No | 43465 (88.9) | 35,081 (80.7) | 21,204 (48.8) | 23,623 (54.4) | 21,373 (49.2)* |
| CVA, n (%) | |||||
| Yes | 4202 (8.6) | 3450 (82.1) | 2036 (48.5) | 2389 (56.9)† | 1941 (46.2)† |
| No | 44,680 (91.4) | 35,961 (80.5) | 21755 (48.7) | 24,272 (54.3)† | 21799 (48.8)† |
| Dysrhythmia, n (%) | |||||
| Yes | 1719 (3.5) | 1311 (76.3)* | 731 (42.5)* | 929 (54.0) | 645 (37.5)* |
| No | 47,163 (96.5) | 38,100 (80.8)* | 23,060 (48.9)* | 25,732 (54.6) | 23,095 (49.0)* |
| Dialysis type, n (%) | |||||
| In-center HD | 46,305 (94.7) | 37,408 (80.8)* | 22,533 (48.7) | 25,298 (54.6) | 22,591 (48.8)* |
| Self-care | 2577 (5.3) | 2003 (77.7)* | 1258 (48.8) | 1363 (52.9) | 1149 (44.6)* |
| Hemoglobin, n (%) | |||||
| < 11.0 g/dL | 37,471 (76.7) | 30,242 (80.7) | 18,258 (48.7) | 20,475 (54.6) | 18,429 (49.2)* |
| ≥ 11.0 g/dL | 11,411 (23.3) | 9169 (80.4) | 5533 (48.5) | 6186 (54.2) | 5311 (46.5)* |
Percentages in this column show the distribution of characteristics across the entire cohort of users and nonusers of selected agents.
Percentages in these columns are the percent of participants with the given characteristic who used the specific agent or agents.
values shown as mean +/− sd
p -values for bivariate tests of differences between users and non-users within each group denoted as: p < 0.001,
p < 0.01
ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; BMI, body mass index; ESRD, end stage renal disease; CHF, congestive heart failure; CAD, coronary artery disease; PVD; peripheral vascular disease; CVA, cerebrovascular accident; HD, hemodialysis;; GN, Glomerulonephritis
Patient characteristics associated with use of any cardioprotective antihypertensive medication
A total of 39,411 (80.6%) of the individuals received at least cardioprotective antihypertensive medication. In bivariate analyses (Table 1), males and females were equally likely to be treated. Overall, Caucasians were treated at lower rates than non-Caucasians. Persons within both the lowest and highest BMI categories were treated less frequently, and persons with functional limitations were treated at significantly lower rates. Persons with diabetes and CAD had higher rates of treatment. Participation in self-care dialysis was associated with significantly lower rates of treatment with cardioprotective agents. For many of the observed characteristics, absolute differences in treatment rates were quite small.
Patient characteristics associated with specific cardioprotective antihypertensive medications
With respect to individual classes of agents, β-blockers (54.5%) surpassed ACE inhibitor/ARBS (48.7%) and CCBs (48.6) in rates of use. Broadly speaking, the proportion of individuals within each demographic (sex and race/ethnicity) category treated with an individual class was relatively homogeneous, at 41.6 – 56.0% (Table 1). Males were prescribed significantly more β-blockers, and females significantly more CCBs. African-Americans and other non-Caucasians were notable for appearing to have substantially higher rates of treatment with CCBs than Caucasians (51.4% vs 41.6%). For persons with diabetes, rates of ACE inhibitor/ARBs, but not β-blockers or CCBs, were higher than in non-diabetic patients, although only about half of diabetics received ACE inhibitor/ARBs. Importantly, in persons with CHF, β-blockers, but not ACE inhibitor/ARBs, were prescribed more often than in persons without CHF. Among those with CAD, β-blockers were used more often than in those without CAD, but the difference was fairly modest (< 9%) and only about 60% of those with CAD were receiving the drug.
Among the ACE inhibitor/ARBs (Table 2), ACE inhibitors were prescribed much more frequently than ARBs (62.6% vs 37.4%, respectively), and lisinopril was the most-frequently prescribed ACE inhibitor (at 32.8% of all ACE inhibitor/ARBs). In the case of the β-blockers, cardioselective agents were the most frequently used subclass (69.8%), while 29.2% received α1 β-blockers; carvedilol was used only 17.9% of the time. For the CCBs, class II agents predominated (87.6%), with amlodipine being most common. We also examined the proportion of the cohort with concurrent use of these three classes: 32.8% had prescriptions for a drug from any of the two classes, and 19.2% had prescriptions from each of the three classes during the four-month study period (data not shown in the table).
Table 2.
Distribution of the antihypertensive agents and their subclasses used by dually-eligible ESRD patients with hypertension
| Class/Subclass/Specific Drug | Number | % of Class | ||
|---|---|---|---|---|
| Renin angiotensin system antagonists | 23,791 | 100.0% | ||
| Angiotensin converting enzyme inhibitors | 14,883 | 62.6% | ||
| Lisinopril | 7,797 | 32.8% | ||
| Enalapril | 2,501 | 10.5% | ||
| Benazepril | 2,064 | 8.7% | ||
| Ramipril | 1,017 | 4.3% | ||
| Fosinopril | 509 | 2.1% | ||
| Captopril | 390 | 1.6% | ||
| Quinapril | 383 | 1.6% | ||
| Trandolopril/Moexipril/Perindopril | 222 | 0.9% | ||
| Angiotensin receptor blockers | 8,908 | 37.4% | ||
| Valsartan | 3,657 | 15.4% | ||
| Losartan | 2,980 | 12.5% | ||
| Irbesartan | 1,188 | 5.0% | ||
| Olmesartan | 517 | 2.2% | ||
| Telmisartan/ Candesartan /Eprosartan | 566 | 2.4% | ||
| Beta-adrenergic blockers | 26,661 | 100.0% | ||
| Cardioselective | 18,619 | 69.8% | ||
| Metoprolol | 14,385 | 54.0% | ||
| Atenolol | 4,155 | 15.6% | ||
| Bisoprolol/Acebutolol/Betaxolol | 79 | 0.3% | ||
| Alpha-1 | 7,782 | 29.2 | ||
| Carvedilol | 4,780 | 17.9% | ||
| Labetalol | 3,002 | 11.3% | ||
| Non-selective | ||||
| Propranolol/Nadolol/Pindolol/Timolol/Penbutolol | 260 | 1.0% | ||
| Calcium Channel Blockers | 23,740 | 100.0% | ||
| Class II | 20,793 | 87.6% | ||
| Amlodipine | 14,838 | 62.5% | ||
| Nifedipine long-acting | 4,940 | 20.8% | ||
| Felodipine | 669 | 2.8% | ||
| Nisoldipine/Isradipine/Nicardipine/Nimodipine | 346 | 1.5% | ||
| Class III | ||||
| Diltiazem | 2,381 | 10.0% | ||
| Class IV | ||||
| Verapamil | 566 | 2.4% | ||
Abbreviation: ESRD, end-stage renal disease
Patient characteristics associated with cardioprotective antihypertensive use
Multivariable analysis (Table 3) demonstrates that each one-year increase in age was significantly associated with approximately a 1% decline in the odds of use of any agent as well as agents within each class. Males had significantly lower odds to receive at least one of the classes (adjusted odds ratio (AOR), 0.90; 95% confidence interval (CI), 0.86-0.94), to receive an ACE inhibitor/ARB (AOR, 0.93; 95% CI, 0.89-0.96), or to receive a CCB (AOR, 0.84; CI, 0.81-0.87) but no difference when it came to β-blocker use (AOR, 0.99; CI, 0.95-1.03). Non-Caucasians were significantly more likely to use any agent as well as ACE inhibitors/ARBs and CCBs; the most pronounced differences were in CCB exposure (AORs of 1.28-1.36 across non-Caucasians). Persons in the highest category of BMI had 32% lower odds (AOR, 0.68; 95% CI, 0.64-0.72) of any of the three classes of agents compared with persons in the referent BMI category; this lower odds was seen for each of the three individual classes. Interestingly, while individuals with inability to ambulate had significantly less use of these agents, this was not the case for individuals unable to transfer. Diabetes was associated with a 28% higher odds of ACE inhibitor/ARB use (AOR, 1.28; 95% CI, 1.20-1.37), but use of all classes of medications was higher in diabetics. CHF was associated with only a 9% increase in the odds of β-blocker use (AOR, 1.09; 95% CI, 1.05-1.14) but no increase in ACE inhibitor/ARB use, and CAD with more β-blocker use (AOR, 1.47; 95% CI, 1.40-1.55) but less CCB use. Self-care dialysis was associated with significantly less use of β-blockers and CCBs, and a trend towards less use of ACE inhibitors/ARBs.
Table 3.
Factors associated with use for any of the selected antihypertensive agents.
| >=1 Class | ACEi/ARBs | β-Blockers | CCBs | |
|---|---|---|---|---|
| Age, per year | 0.993 (0.992-0.995) | 0.991 (0.990-0.992) | 0.99 (0.989-0.992) | 0.994 (0.993-0.995) |
| Male sex | 0.90 ( 0.86-0.94) | 0.93 (0.89-0.96) | 0.99 (0.95-1.03) | 0.84 (0.81-0.87) |
| Race/Ethnicity | ||||
| Caucasian | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| African-American | 1.20 (1.13-1.27) | 1.15 (1.10-1.21) | 1.09 (1.04-1.14) | 1.28 (1.22-1.35) |
| Hispanic | 1.23 (1.14-1.33) | 1.23 (1.15-1.31) | 0.99 (0.93-1.05) | 1.36 (1.28-1.45) |
| Other | 1.26 (1.13-1.39) | 1.30 (1.20-1.41) | 1.04 (0.96-1.13) | 1.33 (1.23-1.44) |
| BMI category | ||||
| < 20 kg/m2 | 0.90 (0.82-0.98) | 0.96 (0.89-1.03) | 0.89 (0.82-0.95) | 0.95 (0.89-0.1.02) |
| 20-24.9 kg/m2 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 25-29.9 kg/.m2 | 0.90 (0.84-0.96) | 0.88 (0.84-0.93) | 0.94 (0.89-0.99) | 0.92 (0.87-0.96) |
| 30+ kg/m2 | 0.68 (0.64-0.72) | 0.73 (0.70-0.76) | 0.76 (0.72-0.80) | 0.72 (0.68-0.75) |
| Smoker | 1.07 (0.98-1.18) | 1.17 (1.09-1.26) | 1.01 (0.94-1.09) | 1.12 (1.04-1.21) |
| Substance abuser | 1.08 (0.94-1.23) | 1.03 (0.93-1.15) | 1.02 (0.92-1.14) | 1.13 (1.02-1.26) |
| Employed | 1.08 (0.98-1.19) | 1.07 (0.99-1.16) | 1.01 (0.94-1.10) | 1.07 ( 0.99-1.16) |
| Inability to ambulate | 0.70 (0.62-0.81) | 0.82 (0.73-0.92) | 0.84 (0.75-0.94) | 0.79 (0.70-0.89) |
| Inability to transfer | 0.91 (0.72-1.15) | 0.81 (0.65-0.99) | 0.96 (0.78-1.18) | 0.85 (0.69-1.05) |
| Cause of ESRD | ||||
| Diabetes | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Hypertension | 1.07 (0.98-1.16) | 0.94 (0.88-1.01) | 1.07 (1.00-1.14) | 0.99 (0.93-1.06) |
| Glomerulonephritis | 0.80 (0.72-0.89) | 0.85 (0.78-0.93) | 0.96 (0.88-1.05) | 0.76 (0.71-0.83) |
| Other | 0.69 (0.62-0.76) | 0.72 (0.66-0.78) | 0.85 (0.78-0.92) | 0.73 (0.67-0.79) |
| Comorbidities | ||||
| Diabetes | 1.26 (1.16-1.37) | 1.28 (1.20-1.37) | 1.12 (1.05-1.20) | 1.08 (1.02-1.16) |
| CHF | 1.04 (0.98-1.10) | 1.04 (0.99-1.08) | 1.09 (1.05-1.14) | 0.93 (0.89-0.97) |
| CAD | 1.17 (1.10-1.25) | 1.04 (0.99-1.09) | 1.47 (1.40-1.55) | 0.82 (0.78-0.87) |
| PVD | 0.95 (0.88-1.02) | 0.98 (0.92-1.04) | 0.99 (0.93-1.05) | 0.96 (0.90-1.02) |
| CVA | 1.12 (1.02-1.22) | 1.02 (0.95-1.09) | 1.05 (0.98-1.12) | 1.00 (0.93-1.07) |
| Dysrhythmia | 0.78 (0.70-0.88) | 0.84 (0.76-0.93) | 0.88 (0.80-0.97) | 0.78 (0.70-0.86) |
| Self-care dialysis | 0.83 (0.75-0.91) | 0.98 (0.90-1.06) | 0.91 (0.84-0.99) | 0.83 (0.76-0.90) |
| Hemoglobin < 11.0 | 1.00 (0.94-1.05) | 0.99 (0.94-1.03) | 1.01 (0.96-1.05) | 1.06 (1.02-1.11) |
Note: values shown are AORs and 95% CIs for each Selected Antihypertensive Agent.
AOR, adjusted odds ratio; CI, confidence interval; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; M, male; F, female; BMI, body mass index; Emp, employed; Unemp, unemployed; ESRD, end stage renal disease; CHF, congestive heart failure; CAD, coronary artery disease; PVD; peripheral vascular disease; CVA, cerebrovascular accident; HD, hemodialysis;
Geographic variation in cardioprotective antihypertensive use
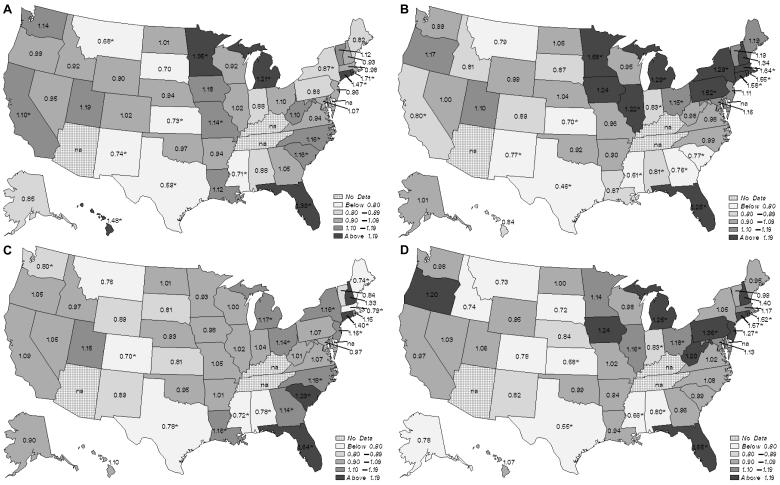
In the state-by-state geographic comparisons (Figure 2), we categorized states according to their adjusted rates of any antihypertensive use and of each of the individual classes. The shadings demonstrate wide state-by-state variation. For the analysis of any of the agents, the observed vs expected odds ratios ranged from 0.55 (0.48-0.62) up to 1.65 (1.40-1.94), a 3.0-fold difference. For ACE inhibitors/ARBs, the observed vs expected odds ratios ranged from 0.59 (95% confidence intervals [CIs], 0.53-0.66) up to 1.71 (1.28-2.30), a 2.9-fold difference. β-blocker observed vs expected odds ratios ranged from 0.46 (0.40-0.51) to 1.64 (1.38-1.95), a 3.6-fold difference, and CCB odds ratios from 0.70 (0.56-0.87) to 1.64 (1.46-1.84), a 2.3-fold difference.
Figure 2.
Observed vs expected odds ratios for use of (A) angiotensin converting enzyme inhibitors/angiotensin receptor blockers, (B) β-blockers, (C) calcium channel blockers, and (D) any of the three classes, by state.
Discussion
In examining the use of antihypertensive agents with cardioprotective properties in a large prevalent cohort of dually-eligible hypertensive chronic dialysis patients, we found that several patient characteristics, namely age, gender, race/ethnicity, ESRD etiology, diabetes, and use of self-care dialysis were independently associated with the use such medications. However, the most striking association is attributable to state of residence, where a greater than 2.3-fold (indeed, up to 3.6-fold) variation in use was found. To improve confidence in our results, we instituted a variety of analytical safeguards, such as utilizing a modeling approach that takes into account uncertainty in the expected state ratios (thereby accounting for higher uncertainty in states with smaller numbers of patients), excluding patients with any form of managed care (so as to study only “truly observable” patients), examining only medications which are widely covered on state formularies30 and which have only nominal copayments, using a 4-month day prescription window (so as to encompass states which permit more than 100-day supplies), and, finally, studying only individuals who filled a prescription (thereby demonstrating actual utilization of Medicaid services).
Although residual state-specific programmatic differences probably partially contribute to observed differences in medication use, the unintuitively large variations in care we report may well reflect a lack of consensus regarding optimal hypertension management. Our findings may reflect a therapeutic nihilism about the benefits of these particular agents, given that patients with kidney disease have been systematically excluded from large randomized controlled trials (RCTs) of these agents.31 Since few RCTs exist in dialysis patients 32, 33, practitioners must rely in large measure on observational trials, in which the evidence is mixed. For example, for ACE inhibitors, the evidence of a benefit in undifferentiated diaysis patients is far from unanimous31, with some studies demonstrating a mortality benefits 34-37 but several others not.17, 38-41 For β-blockers, the one small RCT demonstrating a beneficial effect of these agents studied only in individuals with New York Heart Association Class II or III heart failure.32 Although one large observation study demonstrated a substantial beneficial effect of β-blockers on mortality17, numerous other studies did not.18, 19, 21, 34, 35, 37, 39, 40, 42 Studies of CCBs are also have considerable uncertainty as to their benefits; this class of medicines, which is traditionally considered “less cardioprotective” than ACE inhibitors or β-blockers in the general population, appears to have considerable evidence in support of their benefits for dialysis patients 18, 21, 42 but even here, the evidence is far from unequivocal 17, 19, 34, 36, 37, 39.
Our results extend an emerging literature identifying regional differences in the care of chronic dialysis patients. For example, hemodialysis catheter use43, 44, access to kidney transplantation45, arteriovenous fistula creation46 , and even access to pre-ESRD care47 have all been recently found to vary geographically. Our study extends this realm of inquiry into prescriptions for potentially-beneficial antihypertensive medications, and suggest that further research is needed into how to guide use of more consistent, effective therapy in this high-risk population.
Given the lack of evidence demonstrating the putative cardioprotective properties of these agents in dialysis patients, no definitive conclusions about the appropriateness of prescribing patterns can be drawn; only relative, rather than absolute, conclusions can be drawn. However, specific examination of the use of such agents in patients with comorbidities associated with, or those having manifestations of, CVD may reveal insights into additional opportunities to improve care. While diabetics were more likely to be prescribed ACE inhibitors/ARBs than non-diabetics, it was nonetheless the case that only half of such patients were prescribed them. Additionally, diabetics had only slightly more use of β-blockers, which is troubling given the risk of CVD events in such patients. In the case of CHF, it is of interest that ACE inhibitor/ARBs were not prescribed at significantly higher rates, despite what might be as would be expected from other reports,1, 48, suggesting that the additional benefits of ACE inhibitors/ARBs (e.g., ventricular remodeling49-51) are not being delivered to CHF patients. Even β-blockers, which decrease sympathetic tone52 and which are a Class IA recommendation in CHF patients,53 were only slightly more likely to be prescribed in patients with heart failure than without, suggesting that there may be opportunities for improvement of care. Additionally, while individuals with CAD are indeed more likely to receive β-blockers, as reported by other authors1, 48, disease in other vascular beds, as indicated by PVD or a history of a CVA and which may be a marker for undiagnosed CAD54-56, was not associated with greater use of β-blockers.
Although we limited our analysis to patients with hypertension, the high prevalence of hypertension in the dialysis population means that we found comparable overall use rates of these drugs compared to other studies of chronic dialysis patients. ACE inhibitors/ARBs were prescribed in 48.7% of these hypertensive individuals, similar to the 39-49% use of ACE inhibitors reported in other studies. This is more than the 23.1% – 25.5% of undifferentiated individuals reported by others to be on ACE inhibitors alone during the 1990’s18, 20, 21 but identical to privately insured USRDS patients(48.7%) in 2002.22 These rates were also generally comparable to analyses based on Dialysis Clinics, Inc.(DCI) patients (43.8%)57 as well as DOPPS II data from 2002-04 (38.9%).1 Use of β-blockers, at 54.5%, was consistently higher than in older reports (17.8-19.3%18, 20, 21) as well as in more contemporary findings from USRDS (39.0%)22 and DOPPS II (26.4%) data.1 CCBs were prescribed to 48.6% of individuals, a rate in the middle of the range (40-55%) from other studies.1, 18, 20, 21, 57, 58 Slight discrepancies in estimates across reports are likely to be the result of differing use rates of other classes of agents and sample inclusion criteria, the most important of which is the definition and prevalence of hypertension.
One of the restrictions we did not explicitly model, which is employed by many states’ Medicaid programs and which could have affected our results, are the state-specific limitations on the numbers of prescriptions per month. There were thirteen states that reportedly had monthly caps 30; however, in our analyses of raw claims, we have found that these caps were not strictly enforced. In general, the purported limits range from three to eight prescriptions per month, though some apply only to brand-name drugs and some states allow for overrides of the policy. For instance, our own state, Kansas, purportedly has a limit of five brand- name medications per month, but pharmacists can easily electronically override the limit at the point of sale. Details of which states actually enforced such policies are not readily available, but clearly persons on dialysis who resided in states with caps were routinely able to exceed them to some degree.
It is important to consider several limitations in this study. First, we studied only dually-eligible prevalent chronic dialysis patients. By virtue of having Medicaid, the patients we studied probably represented the neediest patients, and compared to the general chronic dialysis population, are more likely to be female and non-Caucasian, have functional limitations and engage in risk behaviors, and be on in-center hemodialysis.24 Although this somewhat decreases our ability to generalize findings to the US dialysis population as a whole, dually-eligible patients are in many ways reflective of growing trends in dialysis patients, such as the increase in females, Hispanics, individuals with functional limitations, and in those on in-center HD. Second, we relied on the CMS-2728 dialysis intake form to determine comorbidities, including hypertension. While this source is suboptimal compared to more rigorous approaches59, it has been shown to have good sensitivity and specificity for most major comorbidities60 and it seems it seems unlikely that use of this form would substantially undermine our primary finding, namely that of substantial geographic variability, which is an issue worthy of future detailed study. An important limitation is that, like most observational studies, we do not have actual data on blood pressure readings, so we are unable to determine if patients were indeed hypertensive over time. However, while patients may have acquired hypertension after the initiation of dialysis, it is unlikely that it would have resolved after starting it. As such, our findings may be a conservative estimate of the population who may potentially benefit from selecting medications with putative cardioprotective properties. Additionally, we do not examine use of all classes of antihypertensives. However, our goal was not study antihypertensive treatment as a whole, but rather whether and how medications with ostensible cardioprotective properties vary geographically in their prescription patterns, and, by extension, whether geographic variation exists in whether prescribers appear to believe such medications provide benefits for CVD outcomes. While a strength of our study is our use of prescription medication records, rather than chart abstraction,61, 62 to determine that patients had been actively filled at least one prescription medication during the observation window, the presence of a claim for medication dispensation is of course not equivalent to consumption, which is difficult and costly to quantify.
In conclusion, we used a novel linked database which included both clinical and medication data for a national cohort of chronic dialysis patients in order to provide a detailed description of the prevalence of antihypertensive agents with cardioprotective properties in dually-eligible chronic dialysis patients with hypertension. We noted wide variations across US states, suggesting that some regions were preferentially using antihypertensive agents with cardioprotective properties and others were not. Use of antihypertensive agents with cardioprotective properties in the general population was somewhat lower than expected, particularly when specific indications or risk profiles were considered, given the overall burden of CVD in this population. More work is needed to determine whether use of these medications is “appropriate” or whether opportunities exist to improve medication prescription in dialysis patients. Such efforts will require randomized controlled trials, coupled with comparative effectiveness research focusing on clinically-important outcomes and employing rigorous analytic techniques to account for the variability in socio-demographic, clinical and geographic characteristics associated with medication use.
Supplementary Material
Acknowledgements
The authors thank Connie Wang, MD, for technical assistance with manuscript preparation.
The data reported here have been supplied by the United States Renal Data System (DUA#2007-10 & 2009-19) and the Centers for Medicare & Medicaid Services (DUA#19707). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. government.
Support: Funding for this study was provided by NIH (NIDDK) grants R01 DK080111-02 (to Dr Shireman) and K23 DK085378-01 (to Dr Wetmore), by a National Kidney Foundation Young Investigator Award (to Dr Wetmore), and by a Sandra A. Daugherty Foundation Grant (to Dr Wetmore).
Footnotes
Support: The authors declare that they have no relevant financial interests.
Supplementary Material Table S1: List of observed vs expected ORs and accompanying 95% CIs for use of any medication, by state
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org.
Descriptive Text for Online Delivery Hyperlink: Supplementary Table S1 (PDF)
About: List of observed vs expected ORs and accompanying 95% CIs for use of any medication, by state
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lopes AA, Bragg-Gresham JL, Ramirez SP, et al. Prescription of antihypertensive agents to haemodialysis patients: time trends and associations with patient characteristics, country and survival in the DOPPS. Nephrol Dial Transplant. 2009;24(9):2809–2816. doi: 10.1093/ndt/gfp212. [DOI] [PubMed] [Google Scholar]
- 2.Estacio RO, Jeffers BW, Hiatt WR, Biggerstaff SL, Gifford N, Schrier RW. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non-insulin-dependent diabetes and hypertension. N Engl J Med. 1998;338(10):645–652. doi: 10.1056/NEJM199803053381003. [DOI] [PubMed] [Google Scholar]
- 3.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353(9169):2001–2007. [PubMed] [Google Scholar]
- 4.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342(3):145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 5.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357(9266):1385–1390. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 6.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 7.Kjeldsen SE, Dahlof B, Devereux RB, et al. Effects of losartan on cardiovascular morbidity and mortality in patients with isolated systolic hypertension and left ventricular hypertrophy: a Losartan Intervention for Endpoint Reduction (LIFE) substudy. Jama. 2002;288(12):1491–1498. doi: 10.1001/jama.288.12.1491. [DOI] [PubMed] [Google Scholar]
- 8.Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362(9377):7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 9.McMurray JJ, Ostergren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362(9386):767–771. doi: 10.1016/S0140-6736(03)14283-3. [DOI] [PubMed] [Google Scholar]
- 10.Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349(20):1893–1906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 11.Wassertheil-Smoller S, Psaty B, Greenland P, et al. Association between cardiovascular outcomes and antihypertensive drug treatment in older women. Jama. 2004;292(23):2849–2859. doi: 10.1001/jama.292.23.2849. [DOI] [PubMed] [Google Scholar]
- 12.Demers C, McMurray JJ, Swedberg K, et al. Impact of candesartan on nonfatal myocardial infarction and cardiovascular death in patients with heart failure. Jama. 2005;294(14):1794–1798. doi: 10.1001/jama.294.14.1794. [DOI] [PubMed] [Google Scholar]
- 13.Dahlof B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366(9489):895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 14.Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359(23):2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 15.Mohan IK, Khan M, Wisel S, et al. Cardioprotection by HO-4038, a novel verapamil derivative, targeted against ischemia and reperfusion-mediated acute myocardial infarction. Am J Physiol Heart Circ Physiol. 2009;296(1):H140–151. doi: 10.1152/ajpheart.00687.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed LA, Salem HA, Attia AS, El-Sayed ME. Enhancement of amlodipine cardioprotection by quercetin in ischaemia/reperfusion injury in rats. J Pharm Pharmacol. 2009;61(9):1233–1241. doi: 10.1211/jpp/61.09.0014. [DOI] [PubMed] [Google Scholar]
- 17.Foley RN, Herzog CA, Collins AJ. Blood pressure and long-term mortality in United States hemodialysis patients: USRDS Waves 3 and 4 Study. Kidney Int. 2002;62(5):1784–1790. doi: 10.1046/j.1523-1755.2002.00636.x. [DOI] [PubMed] [Google Scholar]
- 18.Ishani A, Herzog CA, Collins AJ, Foley RN. Cardiac medications and their association with cardiovascular events in incident dialysis patients: cause or effect? Kidney Int. 2004;65(3):1017–1025. doi: 10.1111/j.1523-1755.2004.00473.x. [DOI] [PubMed] [Google Scholar]
- 19.Griffith TF, Chua BS, Allen AS, Klassen PS, Reddan DN, Szczech LA. Characteristics of treated hypertension in incident hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. 2003;42(6):1260–1269. doi: 10.1053/j.ajkd.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 20.Abbott KC, Trespalacios FC, Agodoa LY, Taylor AJ, Bakris GL. beta-Blocker use in long-term dialysis patients: association with hospitalized heart failure and mortality. Arch Intern Med. 2004;164(22):2465–2471. doi: 10.1001/archinte.164.22.2465. [DOI] [PubMed] [Google Scholar]
- 21.Kestenbaum B, Gillen DL, Sherrard DJ, Seliger S, Ball A, Stehman-Breen C. Calcium channel blocker use and mortality among patients with end-stage renal disease. Kidney Int. 2002;61(6):2157–2164. doi: 10.1046/j.1523-1755.2002.00355.x. [DOI] [PubMed] [Google Scholar]
- 22.United States Renal Data System. USRDS . Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2003. 2003. [Google Scholar]
- 23.United States Renal Data System. USRDS . Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2006. 2006. [Google Scholar]
- 24.Wetmore JB, Rigler SK, Mahnken JD, Mukhopadhyay P, Shireman TI. Considering health insurance: how do dialysis initiates with Medicaid coverage differ from persons without Medicaid coverage? Nephrol Dial Transplant. 25(1):198–205. doi: 10.1093/ndt/gfp396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Douglas JG, Bakris GL, Epstein M, et al. Management of high blood pressure in African Americans: consensus statement of the Hypertension in African Americans Working Group of the International Society on Hypertension in Blacks. Arch Intern Med. 2003;163(5):525–541. doi: 10.1001/archinte.163.5.525. [DOI] [PubMed] [Google Scholar]
- 26.Furberg CD, Psaty BM, Meyer JV. Nifedipine. Dose-related increase in mortality in patients with coronary heart disease. Circulation. 1995;92(5):1326–1331. doi: 10.1161/01.cir.92.5.1326. [DOI] [PubMed] [Google Scholar]
- 27.McCulloch C, Searle S. Generalized, Linear, and Mixed Models. John Wiley & Sons, Inc; New York, NY.: 2001. Ch. 8. [Google Scholar]
- 28.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed John Wiley & Sons, Inc.; New York: 2000. [Google Scholar]
- 29.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS® for Mixed Models. 2nd ed SAS Institute Inc; Cary, NC: 2006. p. 745. [Google Scholar]
- 30. http://www.npcnow.org/Public/Research___Publications/Publications/pub_rel_research/pub_medicaid/Pharmaceutical_Benefits_Under_State_Medical_Assistance_Programs_2003.aspx.
- 31.Wetmore JB, Shireman TI. The ABCs of cardioprotection in dialysis patients: a systematic review. Am J Kidney Dis. 2009;53(3):457–466. doi: 10.1053/j.ajkd.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 32.Cice G, Ferrara L, D’Andrea A, et al. Carvedilol increases two-year survivalin dialysis patients with dilated cardiomyopathy: a prospective, placebo-controlled trial. J Am Coll Cardiol. 2003;41(9):1438–1444. doi: 10.1016/s0735-1097(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 33.Zannad F, Kessler M, Lehert P, et al. Prevention of cardiovascular events in end-stage renal disease: results of a randomized trial of fosinopril and implications for future studies. Kidney Int. 2006;70(7):1318–1324. doi: 10.1038/sj.ki.5001657. [DOI] [PubMed] [Google Scholar]
- 34.Efrati S, Zaidenstein R, Dishy V, et al. ACE inhibitors and survival of hemodialysis patients. Am J Kidney Dis. 2002;40(5):1023–1029. doi: 10.1053/ajkd.2002.36340. [DOI] [PubMed] [Google Scholar]
- 35.McCullough PA, Sandberg KR, Borzak S, Hudson MP, Garg M, Manley HJ. Benefits of aspirin and beta-blockade after myocardial infarction in patients with chronic kidney disease. Am Heart J. 2002;144(2):226–232. doi: 10.1067/mhj.2002.125513. [DOI] [PubMed] [Google Scholar]
- 36.Berger AK, Duval S, Krumholz HM. Aspirin, beta-blocker, and angiotensin-converting enzyme inhibitor therapy in patients with end-stage renal disease and an acute myocardial infarction. J Am Coll Cardiol. 2003;42(2):201–208. doi: 10.1016/s0735-1097(03)00572-2. [DOI] [PubMed] [Google Scholar]
- 37.Winkelmayer WC, Charytan DM, Levin R, Avorn J. Poor short-term survival and low use of cardiovascular medications in elderly dialysis patients after acute myocardial infarction. Am J Kidney Dis. 2006;47(2):301–308. doi: 10.1053/j.ajkd.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 38.de Boer IH, Ioannou GN, Kestenbaum B, Brunzell JD, Weiss NS. 25-Hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2007;50(1):69–77. doi: 10.1053/j.ajkd.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 39.Trespalacios FC, Taylor AJ, Agodoa LY, Abbott KC. Incident acute coronary syndromes in chronic dialysis patients in the United States. Kidney Int. 2002;62(5):1799–1805. doi: 10.1046/j.1523-1755.2002.00638.x. [DOI] [PubMed] [Google Scholar]
- 40.Chow FY, Polkinghorne KR, Chadban SJ, Atkins RC, Kerr PG. Cardiovascular risk in dialysis patients: a comparison of risk factors and cardioprotective therapy between 1996 and 2001. Nephrology (Carlton) 2003;8(4):177–183. doi: 10.1046/j.1440-1797.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- 41.Boger CA, Gotz AK, Kruger B, et al. Effect of genetic variation on therapy with angiotensin converting enzyme inhibitors or angiotensin receptor blockers in dialysis patients. Eur J Med Res. 2005;10(4):161–168. [PubMed] [Google Scholar]
- 42.Tepel M, Giet MV, Park A, Zidek W. Association of calcium channel blockers and mortality in haemodialysis patients. Clin Sci (Lond) 2002;103(5):511–515. doi: 10.1042/cs1030511. [DOI] [PubMed] [Google Scholar]
- 43.Hopson S, Frankenfield D, Rocco M, McClellan W. Variability in reasons for hemodialysis catheter use by race, sex, and geography: findings from the ESRD Clinical Performance Measures Project. Am J Kidney Dis. 2008;52(4):753–760. doi: 10.1053/j.ajkd.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 44.Foley RN, Chen SC, Collins AJ. Hemodialysis access at initiation in the United States, 2005 to 2007: still “catheter first”. Hemodial Int. 2009;13(4):533–542. doi: 10.1111/j.1542-4758.2009.00396.x. [DOI] [PubMed] [Google Scholar]
- 45.Ashby VB, Kalbfleisch JD, Wolfe RA, Lin MJ, Port FK, Leichtman AB. Geographic variability in access to primary kidney transplantation in the United States, 1996-2005. Am J Transplant. 2007;7(5 Pt 2):1412–1423. doi: 10.1111/j.1600-6143.2007.01785.x. [DOI] [PubMed] [Google Scholar]
- 46.O’Hare AM, Dudley RA, Hynes DM, et al. Impact of surgeon and surgical center characteristics on choice of permanent vascular access. Kidney Int. 2003;64(2):681–689. doi: 10.1046/j.1523-1755.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- 47.McClellan WM, Wasse H, McClellan AC, Kipp A, Waller LA, Rocco MV. Treatment center and geographic variability in pre-ESRD care associate with increased mortality. J Am Soc Nephrol. 2009;20(5):1078–1085. doi: 10.1681/ASN.2008060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sood MM, Battistella M, Lok CE. Patterns of cardioprotective medication prescription in incident hemodialysis patients. Int Urol Nephrol. 2009;41(4):1021–1027. doi: 10.1007/s11255-009-9606-1. [DOI] [PubMed] [Google Scholar]
- 49.Wong M, Staszewsky L, Latini R, et al. Valsartan benefits left ventricular structure and function in heart failure: Val-HeFT echocardiographic study. J Am Coll Cardiol. 2002;40(5):970–975. doi: 10.1016/s0735-1097(02)02063-6. [DOI] [PubMed] [Google Scholar]
- 50.Greenberg B, Quinones MA, Koilpillai C, et al. Effects of long-term enalapril therapy on cardiac structure and function in patients with left ventricular dysfunction. Results of the SOLVD echocardiography substudy. Circulation. 1995;91(10):2573–2581. doi: 10.1161/01.cir.91.10.2573. [DOI] [PubMed] [Google Scholar]
- 51.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348(20):2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 52.Malfatto G, Facchini M, Branzi G, Riva B, Sala L, Perego GB. Long-term treatment with the beta-blocker carvedilol restores autonomic tone and responsiveness in patients with moderate heart failure. J Cardiovasc Pharmacol. 2003;42(1):125–131. doi: 10.1097/00005344-200307000-00019. [DOI] [PubMed] [Google Scholar]
- 53.Smith SC, Jr., Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update endorsed by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol. 2006;47(10):2130–2139. doi: 10.1016/j.jacc.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 54.Mohler ER., 3rd Peripheral arterial disease: identification and implications. Arch Intern Med. 2003;163(19):2306–2314. doi: 10.1001/archinte.163.19.2306. [DOI] [PubMed] [Google Scholar]
- 55.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113(11):e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 56.DeLoach SS, Mohler ER., 3rd Peripheral arterial disease: a guide for nephrologists. Clin J Am Soc Nephrol. 2007;2(4):839–846. doi: 10.2215/CJN.04101206. [DOI] [PubMed] [Google Scholar]
- 57.Manley HJ, Garvin CG, Drayer DK, et al. Medication prescribing patterns in ambulatory haemodialysis patients: comparisons of USRDS to a large not-for-profit dialysis provider. Nephrol Dial Transplant. 2004;19(7):1842–1848. doi: 10.1093/ndt/gfh280. [DOI] [PubMed] [Google Scholar]
- 58.United States Renal Data System. USRDS . Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2005. 2005. [Google Scholar]
- 59.Eggers PW. CMS 2728: what good is it? Clin J Am Soc Nephrol. 5(11):1908–1909. doi: 10.2215/CJN.08170910. [DOI] [PubMed] [Google Scholar]
- 60.How good are the data? USRDS data validation special study. Am J Kidney Dis. 1992;20(5 Suppl 2):68–83. [PubMed] [Google Scholar]
- 61.Glintborg B, Poulsen HE, Dalhoff KP. The use of nationwide on-line prescription records improves the drug history in hospitalized patients. Br J Clin Pharmacol. 2008;65(2):265–269. doi: 10.1111/j.1365-2125.2007.03017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Warholak TL, McCulloch M, Baumgart A, Smith M, Fink W, Fritz W. An exploratory comparison of medication lists at hospital admission with administrative database records. J Manag Care Pharm. 2009;15(9):751–758. doi: 10.18553/jmcp.2009.15.9.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.