Abstract
Specific promoter recognition by bacterial RNA polymerase is mediated by σ subunits, which assemble with RNA polymerase core enzyme (E) during transcription initiation. However, σ70 (the housekeeping σ subunit) and σS (an alternative σ subunit mostly active during slow growth) recognize almost identical promoter sequences, thus raising the question of how promoter selectivity is achieved in the bacterial cell. To identify novel sequence determinants for selective promoter recognition, we performed run-off/microarray (ROMA) experiments with RNA polymerase saturated either with σ70 (Eσ70) or with σS (EσS) using the whole Escherichia coli genome as DNA template. We found that Eσ70, in the absence of any additional transcription factor, preferentially transcribes genes associated with fast growth (e.g. ribosomal operons). In contrast, EσS efficiently transcribes genes involved in stress responses, secondary metabolism as well as RNAs from intergenic regions with yet-unknown function. Promoter sequence comparison suggests that, in addition to different conservation of the −35 sequence and of the UP element, selective promoter recognition by either form of RNA polymerase can be affected by the A/T content in the −10/+1 region. Indeed, site-directed mutagenesis experiments confirmed that an A/T bias in the −10/+1 region could improve promoter recognition by EσS.
INTRODUCTION
Bacteria must cope with drastic changes in their environment, such as nutritional up- and downshifts, and variations in pH, osmolarity and temperature. Bacterial cells can quickly adapt to such environmental changes by modulating gene expression, at both transcriptional and post-transcriptional levels. At the transcription initiation level, gene expression can be regulated either through accessory transcription factors (activators and repressors), or via assembly of different forms of RNA polymerase. The latter mechanism of gene regulation involves the assembly of RNA polymerase core enzyme (indicated as E) with one of several σ factors that can direct RNA polymerase to specific promoter sequences (1). Typically, in the bacterial cell, one σ factor is devoted to transcription of a large part of the genome, including the essential cellular functions (housekeeping σ factor), while the so-called ‘alternative σ factors’ direct transcription of smaller sets of genes, often linked to specific functions (e.g. response to cellular stresses).
In Escherichia coli, seven σ factors have been identified: σ70 or σD (the housekeeping σ) and six alternativeσ factors: σE, σF, σH, σI, σN and σS (2). Most alternative σ factors recognize promoter sequences that strongly diverge from the consensus sequence for σ70; in contrast, genes under the control of σS are characterized by promoter sequences very similar to σ70-dependent genes (3,4). In line with this observation, in vitro selection of DNA sequences bound with high affinity by RNA polymerase associated with σS (EσS) led to the identification of a consensus sequence very similar to the one recognized by σ70 (5). Some level of overlapping in promoter recognition by σ70 and σS might be consistent with σS function: indeed, in conditions leading to slow metabolic activity, such as nutrient starvation or oxidative stress, σS might take over in transcription of genes important for cell survival that are under σ70 control during faster growth (6). However, in order to switch from the fully active to the slow metabolic state, specific gene expression, and thus specific recognition of σ70- versus σS-dependent promoters, must take place in the bacterial cell. Some promoter sequence determinants can favour recognition by either σ70 or σS (3): for instance, a C nucleotide upstream of the −10 promoter element (−13C) enhances transcription by EσS (7). However, some sequence features favouring promoter recognition by σS seem to be dependent on specific promoter contexts: for instance, at the EσS-dependent aidB promoter, EσS, but not Eσ70, can recognize with equal efficiency either C or T as the first nucleotide in the −10 promoter element (8). However, the percentage of σS-dependent promoters carrying a −12C element is not significantly higher than in σ70-dependent promoters (9), suggesting that the presence of a −12C might only contribute to specific promoter recognition by σS at selected promoters.
In addition to sequence determinants, it has been proposed that transcription factors such as CRP, IHF and Lrp can selectively block (or promote) promoter recognition by either Eσ70 or EσS (10). A transcription regulator important for the modulation of promoter accessibility to different RNA polymerase holoenzymes is the H-NS protein, which can repress transcription by Eσ70, but not by EσS, at various promoters (11), a phenomenon known as transcriptional silencing (12). Specific promoter recognition by EσS is also affected by the degree of DNA supercoiling (13). In addition, σS activity and intracellular concentrations are affected by various factors, such as the presence of an anti-sigma factor for σ70 (14), and by the accumulation of the signal molecules ppGpp (15) and polyphosphate (3).
Work aimed to the identification of σS-specific promoter elements has mostly been carried out in vivo, comparing relative gene expression in a wild type versus an rpoS mutant derivative unable to produce the σS protein (9,16–20). Although this approach has proven very useful for the identification of rpoS-dependent genes and in the characterization of σS-specific promoter elements such as the −13C, it cannot distinguish between promoters directly recognized by the EσS form of RNA polymerase and promoters under the indirect control of the rpoS gene. In contrast, dependence on EσS, as determined by biochemical experiments with purified RNA polymerase, has only been determined for a limited number of promoters [e.g. fic (21), csiD (22) and aidB (23)]. In this work, we have performed in vitro transcription experiments with either Eσ70 or EσS, using the whole E. coli genome as template, to identify promoter regions selectively recognized by two forms of RNA polymerase. Our results support previous observations that Eσ70- and EσS-dependent promoters differ in conservation of the UP element and of the −35 sequence, and in the sequence immediately upstream of the −10 promoter element. In addition, we show that differences in the A/T content in the −10/+1 promoter region can favour transcription by either form of RNA polymerase. Finally, our work has led to the identification of novel σS-dependent genes, thus providing further insight on the physiological role of σS.
MATERIALS AND METHODS
Protein purification and RNA polymerase reconstitution
Escherichia coli RNA polymerase core enzyme was purchased from Epicentre (Madison, WI, USA); histidine-tagged σ factors were produced and purified as described (8,24). The σS protein appeared totally pure from contaminants as determined by denaturing protein gel electrophoresis; in contrast, in the σ70 preparations the presence of faint additional bands, corresponding to the molecular weight of the core RNA polymerase subunits α, β and β′, could be detected (data not shown). Weak contamination of σ70 preparations by core RNA polymerase subunits is consistent with the high affinity of σ70 for the core enzyme. For reconstitution of RNA polymerase holoenzymes, the core enzyme was incubated for 10 min at 37°C with either σS or σ70 at a 1:10 ratio. For calculation of RNA polymerase concentrations in transcription assays, it was assumed that, after reconstitution, core enzyme would be 100% active and fully saturated by either σ factor.
In vitro transcription on supercoiled plasmids
Promoter regions of interest were amplified from the genome of E. coli MG1655 (25) and cloned into the pJCD01 plasmid (22) using the BamHI and EcoRI sites, with the exception of the ilvY promoter, which was cloned using the BamHI and SphI sites, due to the presence of an EcoRI site in the ilvY promoter region. Single-round in vitro transcription experiments were carried out on supercoiled templates (3 nM) in the presence of 10 nM reconstituted RNA polymerase holoenzyme. Plasmid DNA and reconstituted holoenzyme were incubated for 10 min at 37°C in 18 µl of transcription buffer (40 mM HEPES pH 8.0, 10 mM magnesium chloride, 150 mM potassium glutamate, 2 mM dithiothreitol, 100 µg/ml bovine serum albumin) prior to the addition of 2 µl of a mixture of ribonucleotide triphosphates and heparin to a final concentration of 500 µM each for ATP, CTP, GTP and UTP and 250 µg/ml heparin. Transcription reactions were allowed to proceed for 10 min at 37°C and were stopped by addition of NaCl to a final concentration of 0.5 M followed by incubation at 70°C for 5 min. Samples were extracted with a 1:1 phenol–chlorophorm mixture, precipitated with ethanol and resuspended in 12 µl TE buffer. A volume of 2.5 µl of resuspended samples were treated with DNaseI (1 U in 10 mM Tris–HCl pH 7.6, 2.5 mM MgCl2, 0.5 mM CaCl2, in a final volume of 20 µl) for 1 h at 37°C, and DNaseI was heat-inactivated at 65°C for 10 min. Transcript amounts were determined by quantitative real-time polymerase chain reaction (PCR): 1 µl of DNaseI-treated samples was retrotranscribed and the cDNAs were amplified in quantitative real-time PCR using the RNA-I transcript as reference gene. The sequences of the primers used in real-time PCR are available upon request. DNaseI-treated transcription reactions not incubated with reverse transcriptase were added as negative controls for the presence of undigested plasmid DNA. To verify that transcripts would indeed originate from the promoters of interest, and not from non-specific transcription initiation events, in vitro transcription start sites were determined by Rapid Amplification of cDNA Ends (RACE) analysis as described (26): start sites observed in the in vitro transcription experiments corresponded to those described in the literature (data not shown). Site-directed mutagenesis of the ssrS P1 promoter was performed using the three-step PCR method (27).
Run-off microarray experiments on E. coli genome
Genomic DNA from E. coli MG1655 was isolated with GenElute™ Bacterial Genomic DNA Kit (Sigma) and digested with EcoRI overnight. The size of digested genomic DNA ranged from 0.6 to 30 kb as judged from visualization on agarose gels. After purification with Wizard SV Gel and PCR Clean-Up System (Promega), 1 µg of genomic DNA was used in single transcription assay for run-off micro array (ROMA) experiments. RNA polymerase holoenzyme concentrations were 100 nM in 50-µl reaction mixture. Digested genomic DNA and reconstituted holoenzyme were incubated in transcription buffer (40 mM HEPES pH 8.0, 10 mM magnesium chloride, 150 mM potassium glutamate, 2 mM dithiothreitol, 100 µg/ml bovine serum albumin) for 15 min at 37°C to allow binding of RNA polymerase to DNA. Transcription reactions were started with the addition of 2.5 µl of ATP, CTP, GTP and UTP to a final concentration of 500 µM each, were allowed to proceed for 30 min at 37°C and were stopped by incubation at 65°C for 5 min. The reaction mixture was incubated with 10 U of DNaseI at 37°C for 30 min to degrade genomic DNA. RNA was purified from reaction with miRNeasy Mini Kit (Qiagen), which allows recovering of total RNA, including small RNA molecules (<200 nt). The quantity of RNA produced by in vitro transcription was determined using Nanodrop Spectrophotometer, while the average length of the transcripts, as well as of the corresponding cDNA from reverse transcription (see below), was evaluated by capillary electrophoresis on an Agilent Bioanalyzer 2100 using an RNA pico and nano assay, respectively. Capillary electrophoresis analysis of transcription reactions performed in the presence of EσS, Eσ70 or core RNA polymerase clearly showed that, while the average transcript size generated by any form of RNA polymerase was comparable, a significantly lower amount of RNA was produced in transcription reactions performed with core RNA polymerase alone compared to either form of RNA polymerase holoenzyme (Supplementary Figure S1). Indeed, in vitro transcription reactions performed using RNA polymerase core enzyme generated 4 ng/µl of transcripts, versus 31 ng/µl and 23 ng/µl total RNA produced in the presence of Eσ70 and EσS, respectively. Dependence on the presence of a σ factor for efficient in vitro transcription strongly suggests that, in our experimental conditions, transcription initiation does not originate from non-specific initiation sites such as 5′ DNA termini, but requires interaction of RNA polymerase holoenzyme with promoter sequences. Ten independent run-off transcription assays with either EσS or Eσ70 were performed, and transcripts were pooled together (∼1 µg total RNA). From the pool of the 10 independent multi-round transcription reactions two distinct hybridizations on microarrays were performed.
For hybridization onto microarrays, we used the Affymetrix Genechip E. coli Genome 2.0 array, which includes 10 144 probe sets covering all the predicted transcripts from four strains of E. coli: the laboratory strain MG1655, the uropathogenic CFT073 and the enteropathogenic strain OH157:N7, subtypes EDL933 and SAKAI. Due to the high degree of similarity between the E. coli strains, typically a single probe set is tiled to represent the equivalent orthologue in all four strains, and strain-specific probes are only used for genes displaying low levels of conservation, or present solely in one strain. Probe sets match every open reading frame (ORF) in E. coli; in addition, 1427 probe sets targeting 714 E. coli MG1655 intergenic regions, probes for various antibiotic resistance markers, and additional control and reporter genes from the previous generation E. coli arrays are also represented in the Affymetrix Genechip E. coli Genome 2.0 array.
The RNA samples were processed for microarray hybridization, following the instructions of the GeneChip® Expression Analysis Technical Manual (Chapter 5, 6 and 7, Prokaryotic target Preparation, Hybridization, Washing, Staining and Scanning), except that cDNA synthesis by reverse transcription was performed with 1 μg RNA. cDNA was fragmented with DNase I treatment and labelled with biotin using Terminal Deoxynucleotidyl Transferase. The fragmented and labelled cDNAs were then hybridized for 16 h at 45°C on individual E. coli Genome 2.0 arrays. After hybridization, GeneChips were washed and stained with streptavidin-conjugated phycoerythrin by using the Fluidic Station FS450 (Affymetrix) according to the FS450_0006 Protocol. Fluorescent images of the arrays were acquired using a GeneChip Scanner 3000 7G (Affymetrix). All Chip images and files have been deposited in the GEO (Gene Expression Omnibus) (http://www.ncbi.nlm.nih.gov/geo/) repository (accession number:GSE22207). After quality control of data distribution, the raw data (CEL files) were used to perform normalization and probe set summarization through Robust Multiarray Analysis (RMA) algorithm (by using the Affymetrix Gene Expression Console Software (www.affymetrix.com). Normalized data were also analyzed with OneChannelGUI Software (http://www.bioinformatica.unito.it/oneChannelGUI/) in order to perform an additional expression analysis based on different parameters. OneChannelGUI is an add-on Bioconductor package extending the capability of the affylmGUI package (28); it is a library providing a graphical interface (GUI) for Bioconductor libraries to be used for the complete single-channel microarray analysis.
To perform statistic analysis on the two hybridizations, we decided to apply a simple non-parametric statistical method based on ranks of fold changes to perform a two-class paired differential analysis. To select genes with significantly different transcription levels, we set the following parameters for the Rank Product analysis: 100 permutations and 0.1 cut-off percentage of false positives (pfp) which corresponds to a P-value <0.01. Fold differences higher than 1.5-fold were considered indicative of significantly different expression, similar to previous ROMA experiments (29): genes more efficiently transcribed by either EσS or Eσ70 are listed in Tables 1 and 2, respectively. Determination of start sites on transcripts generated in the in vitro transcription assays was carried out by RACE analysis (26).
Table 1.
Genes transcribed more efficiently in the presence of EσS
| Genes and promoter regionsa | Gene productb | b numberc | Transcription levels (EσS/Eσ70 ratio)d | Known regulatory factorse |
|---|---|---|---|---|
| Stress response | ||||
| yieF | Chromate reductase | b3713 | 1.50 | σE (59); σS (19) |
| IG1341353_1341620-r | Intergenic region including osmB promoter | N.A. | 1.51 | σS, RcsBA (60) |
| osmB | Osmolarity-inducible lipoprotein | b1283 | 1.51 | σS, RcsBA (60) |
| gshA | γ-glutamate–cysteine ligase (glutathione biosynthesis) | b2688 | 2.21 | |
| ahpF | Alkyl-peroxidase reductase | b0606 | 1.52 | OxyR (61) |
| pphA | Protein phosphatase | b1838 | 1.54 | σH (62,63) |
| macA | Macrolide resistance efflux pump | b0878 | 1.54 | |
| DNA and RNA metabolism and modification | ||||
| ppk | Polyphosphate kinase, part of RNA degradosome, | b2501 | 1.50 | Regulator of intracellular σS concentrations (3) |
| IG2522899_2523146-r | Intergenic region including xapA promoter | N.A. | 1.62 | XapR (64) |
| xapA | Xanthosine phosphorylase, nucleotide synthesis/degradation | b2407 | 1.56 | XapR (64) |
| gyrB | B subunit of DNA gyrase | b3699 | 1.65 | Fis (65) |
| recT | Rac prophage, recombinase, in recET-lar-ydaCQ operon | b1349 | 1.93 | σS-Dependent in biofilm-growing cells (18) |
| lar | Rac prophage,restriction alleviation and modification enhancement, in recET-lar-ydaCQ operon | b1348 | 1.93 | |
| ydaC | Rac prophage, in recET-lar-ydaCQ operon | b1347 | 1.74 | as recT |
| ydaQ | Rac prophage, possible recombinase, in recET-lar-ydaCQ operon | b1346 | 1.66 | as recT |
| Polyamine metabolism | ||||
| puuB | γ-glutamyl–putrescine oxidase | b1301 | 1.61 | σS (19) |
| gabD | Succinate semialdehyde dehydrogenase | b2661 | 1.65 | σS, Nac (9,66) |
| speB | Agmatinase (in arginine/putrescine degradation pathway) | b2937 | 1.66 | |
| Transcription regulation | ||||
| IG582284_582903-f | Intergenic region including appY promoter | N.A. | 1.69 | H-NS (67) |
| appY | Regulator of hyaABCDEF operon | b0564 | 1.51 | H-NS (67) |
| yciT | Putative deoR-type transcription regulator | b1284 | 1.56 | |
| ilvY | Regulator of ilvC operon | b3773 | 1.75 | |
| rhaS | Regulator of rhamnose transport | b3905 | 1.81 | CRP (29); RhaS (68) |
| Sugar metabolism | ||||
| treF | Trehalase | b3519 | 1.53 | σS (9,19) |
| ytfT | Putative galactose ABC transporter | b4230 | 1.55 | σS (19) |
| otsB | Trehalose-6-phosphate phosphatise | b1897 | 1.56 | σS (7) |
| araF | Arabinose transporter, component of an ABC transport system | b1901 | 1.57 | CRP, AraC (69); σS (19) |
| glgP | Glycogen phosphorylase, part of glgCAP operon | b3428 | 1.60 | CRP (70); σS controls glgCAP operon (19) |
| gpmM | Putative 2,3-bisphosphoglycerate mutase | b3612 | 1.61 | |
| glvC | Sugar transport phosphotransferase | b3683 | 1.79 | |
| Other metabolic functions | ||||
| IG1030936_1031361-f | Intergenic region including hyaABCDEF promoter | N.A. | 2.02 | |
| hyaA | Hydrogenase small subunit; in hyaABCDEF operon | b0972 | 1.68 | σS, AppY, anaerobic regulation by ArcA and NarP/NarL (20,71,72) |
| hyaB | Hydrogenase large subunit; in hyaABCDEF operon | b0973 | 1.66 | as hyaA |
| hyaF | Hydrogenase subunit (nickel-binding protein); in hyaABCDEF operon | b0977 | 1.50 | as hyaA |
| syd | SecY-interacting protein | b2793 | 1.59 | |
| murP | Acetyl-muramic acid permease | b2429 | 1.59 | CRP, MurR (73) |
| murR | Transcriptional repressor of murQP | b2427 | 1.62 | |
| tam | Trans-aconitate methylatransferase | b1519 | 1.60 | σS (74) |
| ydhY | Predicted oxidoreductase, Fe-S protein; in ydhYVWXUT operon | b1674 | 1.80 | FNR, NarL (75) |
| ydhV | Predicted oxidoreductase, Fe-S protein; in ydhYVWXUT operon | b1673 | 1.62 | FNR, NarL (75). σS-dependent in biofilm-growing cells (18) |
| Unknown and miscellaneous functions | ||||
| yccT | Unknown | b0964 | 1.51 | Induced in stationary phase (76) |
| yffP | Predicted protein, prophage | b2447 | 1.52 | |
| yidK | Putative membrane transporter | b3679 | 1.53 | |
| ynfD | Predicted protein | b1586 | 1.54 | |
| yfiL | Putative lipoprotein | b2602 | 1.55 | |
| yi91a | Unknown, in CP4-6 prophage sequence | b0255 | 1.56 | |
| yfjL | Unknown, possible prophage gene | b2625 | 1.57 | σS (9) |
| yagL | Unknown, prophage protein | b0278 | 1.59 | σS-Dependent in biofilm-growing cells (18) |
| eutA | Reactivating factor for ethanolamine ammonia lyase | b2451 | 1.59 | eutH, in eutHA operon, is σS-dependent (9) |
| yhjG | Predicted outer membrane protein | b3524 | 1.61 | σS (9) |
| ybeH | Hypothetical protein | b0625 | 1.66 | |
| yedS | Unknown | b1964 | 1.69 | |
| yfgJ | Unknown, mutant affecting swarming motility | b2510 | 1.74 | |
| G7353 | Phantom gene | b2596 | 1.79 | Upstream of a ribosome modulation factor induced in stationary phase (yfiA) |
| yqiG | Unknown, interrupted by IS element | b3046 | 1.81 | |
| IG1006824_1007066-r | Intergenic region including ycbX promoter | N.A. | 2.26 | |
| ycbX | Unknown | b0947 | 2.05 | |
| yfjH | Unknown | b2623 | 2.30 | |
| IG2755422_2755664-r | Intergenic region downstream of yfjH | N.A. | 2.01 | |
| ychS | Unknown | b1228 | 3.21 | |
| Non-coding RNAs | ||||
| ryeE (cyaR) | Small RNA, promotes degradation of ompX and nadE RNA | b4438 | 1.50 | CRP, σE (77,78) |
| sgrS/sgrT | Small RNA, inhibits ptsG translation/SgrT protein | b4577 | 1.51 | SgrR protein |
| ECs3934 | sibD/sibE non coding RNA- ibsD/ibsE toxic peptides | b4447 | 1.62 | Complex locus including two non coding RNAs overlapped by two small ORFs encoding a putative toxin/antitoxin system |
| b4664 | ||||
| micA | micA small RNA (downregulates ompA expression) | b4442 | 2.00 | |
| Intergenic regions, genes not annotated in MG1655 | ||||
| c3878 | Unknown, annotated in CFT073 | N.A. | 1.50 | |
| IG2922538_2922756-f | Intergenic region between syd (predicted protein) and csrB (ncRNA), antisense | N.A. | 1.50 | |
| c5008 | Unknown, annotated in CFT073, downstream of malM | N.A. | 1.50 | |
| IG2438141_2438404-f | Between fabB and ycfJ, antisense | N.A. | 1.50 | |
| ECs5537 | Unknown, annotated in O157:H7 SAKAI | N.A. | 1.51 | |
| IG2885243_2885600_r | Intergenic region, upstream of predicted helicase ygcB | N.A. | 1.52 | |
| c4656 | Unknown, annotated in CFT073 (antisense of atpC) | N.A. | 1.52 | |
| IG3665211_3665420-f | Intergenic region upstream of gadA, antisense | N.A. | 1.52 | |
| c1010 | Unknown, annotated in CFT073, upstream of aqpZ | N.A. | 1.52 | aqpZ (aquaporin) is σS-dependent (18,79) |
| c0723 | Unknown, annotated in CFT073, upstream of dacA, transcribed in opposite direction | N.A. | 1.54 | |
| IG2481360_2481774-r | Intergenic region upstream emrKY (TolC-multidrug efflux pump) | N.A. | 1.54 | |
| IG2228406_2228643-f | Intergenic region upstream of yohJK (inner membrane proteins) | N.A. | 1.54 | |
| IG2201932_2202549-r | Intergenic region between yehI-yehK, antisense | N.A. | 1.54 | |
| c2806 | Unknown, annotated in CFT073 (antisense of menB) | N.A. | 1.55 | |
| c3010 | Unknown, annotated in CFT073, upstream of perM gene, transcribed in opposite direction | N.A. | 1.55 | |
| IG3669525_3669971-r | Intergenic region, upstream of yhjB | N.A. | 1.58 | |
| IG2898371_2898613-f | Intergenic region upstream of yqcE | N.A. | 1.60 | |
| c5221 | Unknown, annotated in CFT073 (antisense of aspA) | N.A. | 1.63 | |
| IG223409_223770-r | Intergenic region upstream of rrsH ribosomal operon (transcribed in opposite direction) | N.A. | 1.64 | |
| IG3420831_3421058-r | Intergenic region, downstream of rrfF ribosomal operon | N.A. | 1.66 | |
| c4942 | Unknown, annotated in CFT073 (antisense of rplL) | N.A. | 1.67 | |
| IG2428784_2429041-f | Intergenic region, upstream of cvpA gene, transcribed in opposite direction | N.A. | 1.71 | |
| c1908 | Unknown, annotated in CFT073 (antisense of yddM) | N.A. | 1.71 | |
| c0703 | Unknown, annotated in CFT073 (antisense of citF) | N.A. | 1.74 | |
| ECs5165 | yjfO (biofilm-related protein) in O157:H7 SAKAI | N.A. | 1.86 | |
| IG1903284_1903567-r | Intergenic region downstream of yobD, transcribed in opposite direction | N.A. | 1.87 | |
| IG3358642_3358810-f | Intergenic region downstream of gltD | N.A. | 1.89 | |
| IG2190243_2190534-r | Intergenic region downstream of yehE | N.A. | 1.94 | |
| c3113 | Unknown, annotated in CFT073 (upstream of rrsG ribosomal operon, transcribed in opposite direction) | N.A. | 1.95 | |
| c2317 | Unknown, annotated in CFT073 (antisense of azuC) | N.A. | 1.99 | Complex locus including small RNA isrB |
| IG2755422_2755664-r | Intergenic region downstream yfjH | N.A. | 2.01 | |
| IG2519349_2519612-f | Intergenic region downstream of xapR | N.A. | 2.05 | |
| IG330721_331594-r | Intergenic region upstream of yahA, transcribed in opposite direction | N.A. | 2.07 | |
aFor known genes, we used the nomenclature reported in the NCBI database (http://www.ncbi.nlm.nih.gov/genomes/lproks.cgi); intergenic region are indicated using the nomenclature by Affymetrix, which indicates the start and the end of the intergenic region (IG) covered by the various probe sets; –f or –r indicate if the probe sets are in forward or reverse orientation relative to the (+) strand of the E. coli chromosome; genes already described as rpoS-dependent in vivo are underlined.
bGene product (or predicted product).
cRelative location of the various ORFs on the E. coli MG1655 chromosome.
dDetermined by microarray analysis as described in ‘Materials and Methods’ section.
eWhen not otherwise stated, the information is taken from http://ecocyc.org/.
Table 2.
Genes transcribed more efficiently in the presence of Eσ70
| Genes and promoter regionsa | Gene productb | b numberc | Transcription levels (Es70/Ess ratio)d | Known regulatory factorse |
|---|---|---|---|---|
| Ribosomal genes and protein synthesis | ||||
| IG1286552_1286760-r | Intergenic region including tyrT promoter | N.A. | 2.97 | FIS (80) |
| rttR | Non-coding RNA, part of tyrT transcript | b4425 | 1.97 | FIS (80) |
| tpr | Small protamine-like protein part of tyrT transcript | b1229 | 2.03 | |
| serT | Serine tRNAs gene | b0971 | 1.89 | FIS (79); upregulated in rpoS mutant of MG1655 (17) |
| rpmI | Ribosomal protein L35 | b1717 | 1.91 | |
| prfB | Release Factor RF2 | b2891 | 1.91 | Upregulated in a biofilm-growing rpoS mutant derivative of MG1655 (18) |
| rimP | Ribosomal maturation protein | b3170 | 2.01 | |
| rpsF | Ribosomal protein S6 | b4200 | 2.04 | |
| rpmG | Ribosomal protein L33 | b3636 | 2.10 | |
| relA | ppGpp alarmone biosynthetic enzyme | b2784 | 2.35 | |
| rplU | Ribosomal protein L21, in rplU-rpmA operon | b3186 | 2.50 | |
| rpmA | Ribosomal protein L27, in rplU-rpmA operon | b3185 | 2.58 | |
| rpsU | Ribosomal protein S21 | b3065 | 2.78 | Upregulated in a biofilm-growing rpoS mutant derivative of MG1655 (18) |
| IG3426400_3426656-r | Intergenic region including rrnH promoter | N.A. | 2.87 | |
| DNA repair | ||||
| sulA | SOS response inhibitor of cell division | b0958 | 2.04 | LexA (81) |
| dinI | AP endonuclease, SOS response | b1061 | 2.18 | LexA (82); upregulated in an rpoS mutant derivative of OH157:H7 EDL 933 (19) |
| Multifunctional operons | ||||
| cvpA | Colicin V production; in cvpA-purF-ubiX operon | b2313 | 1.85 | PurR (83); upregulated in an rpoS mutant derivative of OH157:H7 EDL 933 (19) |
| purF | Amidophosphoribosyl transferase (ribonucleotide metabolism); in cvpA-purF-ubiX operon | b2312 | 2.12 | as cvpA |
| ubiX | 3-octaprenyl-4-hydroxybenzoate decarboxylase (ubiquinone biosynthesis); in cvpA-purF-ubiX operon | b2311 | 1.90 | as cvpA |
| c2854 | Unknown, annotated in CFT073 as part of the cvpA-purF-ubiX operon | N.A. | 2.00 | |
| yjeF | Putative carbohydrate kinase; in yjeFE-amiB-mutL operon | b4167 | 1.88 | |
| yjeE | Essential protein with weak ATPase activity; in yjeEF-amiB-mutL operon | b4168 | 1.95 | |
| amiB | N-acetylmuramyl-l-alanine amidase needed for septum formation during cell division; in yjeEF-amiB-mutL operon. | b4169 | 2.02 | |
| mutL | Methyl-directed mismatch repair, subunit in MutHLS complex; in yjeEF-amiB-mutL operon. | b4170 | 2.06 | |
| yhbE | Inner membrane protein; in yhbE-obgE operon | b3184 | 2.36 | |
| obgE | GTP-binding protein, involved in ppGpp turnover; in yhbE-obgE operon | b3183 | 1.91 | |
| Transcription regulation | ||||
| IG3717398_3717677-f | Intergenic region including cspA promoter | N.A. | 1.85 | |
| cspA | Cold shock protein A | b3556 | 2.34 | Upregulated in an rpoS mutant derivative of OH157:H7 EDL 933 (19) |
| alpA | CP4-57 prophage gene, regulator of tmRNAs | b2624 | 2.31 | |
| Metabolic functions | ||||
| fhuF | Iron reductase | b4367 | 1.83 | Fur, OxyR (84) |
| ydhR | Putative monooxygenase | b1667 | 1.91 | |
| artJ | Arginine transporter | b0860 | 2.13 | Upregulated in a biofilm-growing rpoS mutant derivative of MG1655 (18) |
| Unknown and miscellaneous functions | ||||
| ycgY | Unknown | b1196 | 1.83 | |
| yefM | Antitoxin in yefM-yoeB toxin-antitoxin system | b2017 | 1.85 | |
| yehL | Unknown, possible component of ABC transport system | b2119 | 1.92 | |
| yebN | Unknown, putative membrane protein | b1821 | 2.06 | |
| ydiE | Putative lipoprotein | b1705 | 2.10 | |
| Non-coding RNAs | ||||
| isrB | Small RNA | b4434 | 2.25 | |
| spf | Small RNA, regulates DNA polymerase I activity | b3864 | 2.72 | |
| ssrS | 6S RNA, modulates σ70 activity | b2911 | 3.74 | Upregulated in a rpoS mutant derivative of MG1655 (20) |
| Intergenic regions, genes not annotated in MG1655 | ||||
| c1714 | Unknown, annotated in CFT073, upstream of cls, transcribed in opposite direction | N.A. | 1.83 | |
| IG330721_331594-f | Intergenic region including yahA promoter region | N.A. | 1.85 | |
| ECs1613 | Unknown, annotated in O157:H7 SAKAI, possible prophage gene (renD) | N.A. | 1.86 | |
| IG2424809_2425028-r | Intergenic region between argT and hisJ | N.A. | 1.92 | |
| IG1120179_1120464-r | Intergenic region upstream bssS (regulator of biofilm formation) | N.A. | 1.92 | |
| c2481 | Unknown, annotated in CFT073, upstream of cobU | N.A. | 1.94 | |
| IG127588_127911-f | Intergenic region upstream of lpd | N.A. | 1.96 | |
| IG2404662_2405580-r | Intergenic region upstream of lrhA | N.A. | 1.98 | |
| Z0043 | Annotated in O157:H7 OH157:H7 EDL 933, caiC | N.A. | 2.05 | |
| c2568 | Annotated in CFT073, wcaM | N.A. | 2.13 | |
| Z5055 | Annotated in O157:H7 OH157:H7 EDL 933, rfaG | N.A. | 2.16 | |
| c4052 | Unknown, annotated in CFT073 | N.A. | 2.27 | |
| IG2815526_2815805-r | Intergenic region upstream yqaBA | N.A. | 2.31 | |
| IG583654_583902-r | Intergenic region downstream of ompT | N.A. | 2.35 | |
| c2230 | Unknown, annotated in CFT073 (antisense of cspC) | N.A. | 2.42 | |
| IG1905616_1906284-f | Intergenic region, between yobF and yebO | N.A. | 2.44 | |
| Z3239 | Annotated in O157:H7 OH157:H7 EDL 933 (antisense of yegI) | N.A. | 2.49 | |
| c4719 | Annotated in CFT073 homologous to aslA | N.A. | 2.54 | |
| c4352 | Annotated in CFT073 as dppC | N.A. | 2.99 | |
| IG2428784_2429041-f | Intergenic region, between yobD and yebN | N.A. | 3.10 | |
| c1434 | Unknown, annotated in CFT073, ydfR | N.A. | 3.45 | |
| Z5945 | Annotated in O157:H7 OH157:H7 EDL 933 (toxic peptide) | N.A. | 4.02 | |
| Z5868 | Annotated in O157:H7 OH157:H7 EDL 933; yjgM, putative acetyltransferase | N.A. | 4.44 | |
aFor known genes, we used the nomenclature reported in the NCBI database (http://www.ncbi.nlm.nih.gov/genomes/lproks.cgi); intergenic region are indicated using the nomenclature by Affymetrix, which indicates the start and the end of the intergenic region (IG) covered by the various probe sets; –f or –r indicate if the probe sets are in forward or reverse orientation relative to the (+) strand of the E. coli chromosome; genes previously described as being upregulated in an rpoS mutant strain are double underlined.
bGene product (or predicted product).
cRelative location of the various ORFs on the E. coli MG1655 chromosome.
dDetermined by microarray analysis as described in ‘Materials and Methods’ section.
eWhen not otherwise stated, the information is taken from http://ecocyc.org/.
In order to manage and retrieve microarray data, a genome browser was set up (http://155.253.6.64/cgi-bin/gbrowse/provakappa/#search). The genome browser is based on the Generic Genome Browser (Gbrowse) which is a combination of database and interactive webpage for manipulating and displaying annotations on genomes. This bioinformatic tool allows users to view and navigate through the MG1655 genome (GenBank Accession Number:U00096) with information about the gene annotation, coming from the website http://www.genome.wisc.edu/tools/asap.htm (downloaded as GFF3 file), and all the probe sets contained in the Affymetrix E. coli Genome 2.0 Array manually remapped on the genome.
Determination of rpoS-dependent gene expression in bacterial cells
Bacterial strains used were MG1655 (wild type) and its rpoS mutant derivative EB1.3 (30). Strains were grown at 30°C in three different media: the complex Luria Bertani (LB) broth, the glucose-based M9Glu/sup medium (31) and LB medium diluted 1:4 (LB1/4). The LB1/4 medium was utilized since it was shown to stimulate expression of rpoS-dependent genes such as the csg operons (32). Thus, growth in LB1/4 medium might positively affect either σS concentration or σS activity. Samples for RNA extraction were taken both in late logarithmic (OD600 = 0.6–0.7) and in late stationary phase (overnight cultures, OD600≥1.5). RNA was extracted using the small RNA miRNeasy Mini Kit (Qiagen), and further reverse transcription and cDNA amplification in quantitative real-time-PCR were performed as described (33). Primer sequences are available upon request. All reactions were performed twice, each time in duplicate, and always showed very similar results. The relative amounts of the transcripts were determined using 16S rRNA as the reference gene ([CtGene of interest – Ct16S] = ΔCt).
RESULTS
ROMA analysis of σS- and σ70-dependent promoters
To identify sequence determinants that can direct selective promoter recognition by either the σ70- or the σS- associated form of RNA polymerase, we compared EσS and Eσ70 in ROMA experiments (29,34). Run-off transcription assays were performed using as DNA template the whole genome of E. coli MG1655 after digestion with EcoRI; cDNAs generated from the transcripts were hybridized on microarrays to determine their relative amounts. Unlike genome expression studies performed in living cells, which do not distinguish between direct and indirect effects, such as transcription factor-dependent promoter recognition, the ROMA analysis solely detects genes whose promoter are recognized by either EσS or Eσ70 (or both) in the absence of any additional factor. Out of ∼10 000 probe sets on the microarray, only 173 (∼1.7%) showed differences in transcription levels higher than 1.5-fold, considered to be significant (pfp<0.1, P-value <0.01; see ‘Materials and Methods’ section). For several operons, only a portion of the transcriptional unit (or even a single gene) showed significant difference in transcription levels by either EσS or Eσ70: in the case of reduced transcription of distal genes within a given operon, this effect might depend either on premature arrest of transcription by RNA polymerase or on the presence of an EcoRI restriction site within the operon, since the EcoRI restriction nuclease was used to digest chromosomal DNA used in ROMA experiments (see ‘Materials and Methods’ section). However, the effects of EcoRI digestion were not so severe as it could be expected, possibly due to the fact that chromosomal DNA was only partially digested by the enzyme in our conditions. For instance, despite the presence of an EcoRI site in the ydhY gene, transcription extending into the downstream genes was still detectable (Table 1 and Supplementary Table S1). In the case of distal genes of an operon showing more significant differences in EσS- versus Eσ70-dependent transcription than genes proximal to the promoter, this might be due to either lack of detection of proximal genes in the microarray experiment or the presence of unidentified promoters internal to the operons. We verified that genes within the same operon would not show dependence on different forms of RNA polymerase: out of the several hundred operons in the E. coli genome, only in the weakly Eσ70-dependent atpBEFHAGDC and flgBCDEFGHIJ operons could we observe the presence of a single gene showing dependence on EσS in our ROMA experiments (data not shown). This result might suggest the presence of EσS-dependent promoters, or promoter-like sequences, within these operons; however, we decided to focus our investigation on operons consistently showing preferential recognition by either form of RNA polymerase, and the atpBEFHAGDC and flgBCDEFGHIJ operons were not considered further in our study. In Supplementary Table S1, we show the ratios of EσS- versus Eσ70-dependent transcription for operons featuring genes preferentially transcribed in the presence of EσS.
Complete results of ROMA experiments are summarized in Tables 1 and 2, listing genes and intergenic regions transcribed more efficiently either by EσS (Table 1) or by Eσ70 (Table 2). Out of the 54 genes preferentially transcribed by EσS in ROMA experiments that are annotated in E. coli MG1655, 21 (39%) had already been described as rpoS-dependent genes, either from genetic characterization or from microarray experiments comparing wild-type and rpoS mutant strains (underlined in Table 1). In contrast, no known rpoS-dependent gene was found to be preferentially transcribed by Eσ70 (Table 2). However, several genes clearly identified by previous works as rpoS-dependent (e.g. katE, dps and gadA) only showed slight (<1.5-fold) preferential recognition by EσS in our experimental conditions (Supplementary Table S2). This would suggest that, for these rpoS-dependent genes, promoter recognition by EσS might be mediated by regulatory proteins, or facilitated by additional factors such as DNA supercoiling or effector molecules (e.g. ppGpp), missing in ROMA experiments. Alternatively, in ROMA experiments, Eσ70 might recognize promoters which might not be accessible to this form of RNA polymerase in vivo, due to selective negative recognition by regulatory proteins such as H-NS (11,12).
A significant fraction of genes more efficiently transcribed by EσS is involved in sugar and in polyamine metabolism, in response to cellular stresses and in nucleic acid synthesis, modification and turnover (Table 1), consistent with the role of σS as a starvation- and stress-related protein; no essential gene was found. Interestingly, several EσS-dependent transcripts correspond to non-coding RNAs, intergenic regions and ORFs of unknown function only annotated in pathogenic E. coli strains. This last result was surprising, since the DNA template used in the in vitro transcription experiments came from E. coli MG1655; however, probe set analysis and sequence comparison performed using BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) revealed that these ORFs are also present in MG1655, although they are not annotated in this strain. Several of these unlisted ORFs overlap known genes in an antisense direction, as indicated in Table 1. Interestingly, one of such ORFs (c3113, annotated in the uropathogenic strain CFT073), as well as one intergenic region (IG223409_223770-r), is located in the promoter regions of ribosomal operons rrsG, and rrsH in an antisense direction, possibly suggesting that they may play a role in EσS-dependent control of ribosomal operon transcription.
In contrast, Eσ70 appeared to transcribe with higher-efficiency genes encoding ribosomal proteins and other protein synthesis-related genes, such as rRNA- and tRNA-encoding genes and prfB, encoding for release factor 2 (Table 2). Several of the protein synthesis-related genes listed in Table 2 are essential, as are the yjeE and the amiB genes, part of a multifunctional operon also preferentially transcribed by Eσ70. Interestingly, regulatory genes directly affecting σ70 activity, such as relA, responsible for biosynthesis of the ppGpp alarmone in response to amino acid starvation (35), and ssrS, encoding a 6S RNA able to modulate Eσ70-dependent at several promoters (36), showed dependence on Eσ70 in ROMA experiments (Table 2). For ssrS, our data confirm literature data showing that the main ssrS promoter (ssrS P1) is strictly Eσ70-dependent in vitro (37). Interestingly, several genes preferentially transcribed by Eσ70 in ROMA experiments are upregulated in rpoS mutant strains (underlined in Table 2); it has been proposed that negative regulation by σS can depend on competition with σ70 for a limiting amount of RNA polymerase core enzyme, which results in lower intracellular Eσ70 concentrations and, in turn, in impaired transcription initiation at strictly σ70-dependent promoters (38).
In vivo validation of EσS-dependent genes identified in ROMA experiments
In order to validate the results of ROMA experiments, we tested in vivo expression of 18 genes preferentially transcribed by EσS, comparing their transcript levels in MG1655 and in its rpoS mutant derivative EB1.3. Transcript levels were determined by quantitative real-time PCR. Different genetic backgrounds (MG1655, MC4100 and OH157:H7 EDL933 strains) and different growth conditions (LB medium, glucose-based medium and biofilm growth) can strongly affect rpoS-dependent gene expression (9,17–20), suggesting that most genes belonging to the σS regulon are also subject to additional forms of gene expression regulation. Thus, in vivo gene expression was determined in three different growth media: the peptone-based LB medium, either full strength or diluted 1:4, and the glucose-based M9Glu/sup medium (Table 3). In addition, we took samples from cultures both in late exponential phase and in late stationary phase of growth; indeed, although σS-mediated gene expression is typically associated with stationary phase, several rpoS-dependent genes are maximally expressed at the transition between exponential and stationary phase or even in mid-exponential phase (19,39; Landini,P., unpublished data).
Table 3.
Gene expression in bacterial cells
| Gene | LB |
M9Glu/sup |
LB 1/4 |
|||
|---|---|---|---|---|---|---|
| Exp | Stat | Exp | Stat | Exp | Stat | |
| ychS | 2.4 | 0.1 | 1.1 | 6.7 | 1.7 | 2.0 |
| gshA | 1.0 | 1.5 | 1.0 | 1.1 | 1.9 | 3.3 |
| ycbX | 1.1 | 0.5 | 0.7 | 2.0 | 1.5 | 2.6 |
| recT | 0.9 | 0.4 | 0.7 | 2.5 | 1.8 | 2.0 |
| rhaS | 1.8 | 0.3 | 1.5 | 2.7 | 1.5 | 2.7 |
| cyaR | 1.0 | 1.7 | 1.5 | 10.0 | 0.8 | 0.6 |
| ilvY | 1.7 | 0.3 | 1.0 | 10.2 | 1.9 | 2.2 |
| hyaA | 1.2 | 5.6 | 2.1 | 2.9 | 2.3 | 17.5 |
| speB | 1.5 | 1.3 | 1.4 | 2.7 | 1.2 | 86.3 |
| gyrB | 1.4 | 0.8 | 0.9 | 2.8 | 1.0 | 3.1 |
| gabD | 4.8 | 0.7 | 12.1 | 1.2 | 5.1 | 69.0 |
| ydhY | 1.5 | 0.3 | 0.9 | 19.2 | 1.1 | 2.1 |
| appY | 1.2 | 0.7 | 0.9 | 3.4 | 2.0 | 11.4 |
| puuB | 1.7 | 0.2 | 0.6 | 3.5 | 3.0 | 1.8 |
| treF | 1.5 | 3.0 | 7.2 | 0.6 | 2.1 | 52.6 |
| bsmA | 1.1 | 1.0 | 2.3 | 0.9 | 1.0 | 2.1 |
| xapA | 2.4 | 0.2 | 0.8 | 4.3 | 1.1 | 2.7 |
| c3113 | 1.0 | 1.4 | 1.3 | 8.1 | 2.7 | 1.3 |
| dps | 0.9 | 10.7 | 10.7 | 0.3 | 14.2 | 30.3 |
| cspA | 1.3 | 0.2 | 1.1 | 0.8 | 0.7 | 0.2 |
Relative expression is indicated as WT/rpoS ratio.
Values higher than 2.5-fold were considered significant and are shown in boldface type.
Values are the average of two independent experiments performed in duplicate.
For five genes (recT, hyaA, gabD, puuB and treF), dependence on a functional rpoS gene has been described (Table 1); however, for the recT gene, part of the recET-lar-ydaCQ operon, both positive and negative control by rpoS was reported (17,18). As a positive control, we tested the expression of dps that, although showing only weak dependence on EσS in our ROMA experiments (Supplementary Table S2), has consistently been described as rpoS-dependent in vivo in several reports (9,16,17); in agreement with the literature data, dps expression showed strong rpoS-dependence in all conditions tested (Table 3). We also tested the expression of cspA, which was transcribed with higher efficiency by Eσ70 in ROMA experiments (Table 2); consistent with this result, in vivo gene expression studies show that a functional rpoS allele is not required for cspA expression, which is in fact upregulated in the rpoS mutant when grown in peptone-based media (Table 3), in agreement with previous observations (20). For in vivo gene expression experiments, we considered as significant a fold difference ≥2.5 in WT versus rpoS mutant relative expression ratio.
As shown in Table 3, growth conditions strongly affected gene expression: in LB medium, expression of only three genes (hyaA, gabD and treF) was dependent on a functional rpoS allele, while seven genes were in fact upregulated in the rpoS mutant, suggesting negative control by σS. In contrast, 12 genes were expressed in an rpoS-dependent fashion when bacteria were grown in 1:4 diluted LB, while 15 genes showed dependence on rpoS in M9Glu/sup medium (Table 3). Of all genes tested, only the bsmA gene did not show relative expression values ≥2.5 in any growth condition, while recT and ycbX only displayed weak dependence on a functional rpoS allele (2.5- and 2.6-fold increase in one growth condition). All other genes showed either strong dependence on the rpoS gene (up to 86.3-fold for speB in LB1/4 medium) or were affected by the rpoS mutation in more than one growth condition (e.g. rhaS, induced 2.7-fold in the wild-type strain both in M9Glu/sup and in LB1/4 growth media). Finally, in vivo gene expression experiments demonstrated that the c3113 gene, tested as a representative of ORFs only annotated in the uropathogenic E. coli CFT073, is indeed expressed in MG1655, and it shows dependence on the rpoS gene when bacteria are grown in M9Glu/sup (Table 3).
In vitro transcription assays on single promoters
To further confirm the results of ROMA experiments, we performed in vitro transcription assays comparing EσS and Eσ70 on single promoters. To this aim, promoter regions of the ilvY, speB, xapA, ydhY and ssrS genes were cloned into the pJCD01 vector (22) and the obtained plasmids were used as DNA template for in vitro transcription. The ilvY, speB, xapA and ydhY genes were selected since their promoter region has been experimentally identified and they show rpoS-dependence in vivo (Table 3). In contrast, ssrS shows the highest dependence on Eσ70 in ROMA experiments among genes whose promoter has been characterized (Table 2). Strong dependence on Eσ70 is in agreement with previous results showing that ssrS P1, the main ssrS promoter, is Eσ70-dependent in vitro (37), and consistent with upregulation of ssrS in an rpoS mutant of MG1655 (20). Transcription of the different genes of interest was normalized to the RNA-I transcript, as previously described (22), and transcript quantitation was performed by real-time PCR as described in ‘Materials and Methods’ section.
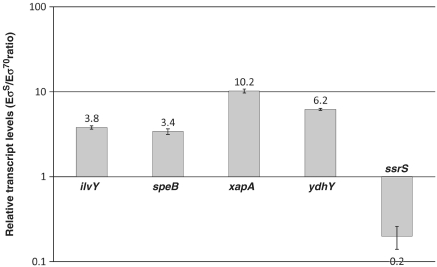
As shown in Figure 1, in vitro transcription experiments performed on plasmids showed that the ilvY, speB, xapA and ydhY are transcribed more efficiently in the presence of EσS. Extent of dependence on EσS was higher than in ROMA experiments, ranging from a 3.4-fold difference for speB to a 10.2-fold difference for xapA, thus suggesting that, at least at these promoters, DNA supercoiling does not negatively affect promoter recognition by EσS. In contrast, the ssrS P1 promoter showed clear dependence on Eσ70, in agreement with previous observations (37). Thus, results of in vitro transcription experiments performed on single promoters were fully consistent with ROMA experiments.
Figure 1.
In vitro transcription experiments on supercoiled plasmids. Transcription from the ilvY, speB, xapA, ydhY and ssrS P1 promoter regions cloned into pJCD01 plasmid was performed in the presence of either EσS or Eσ70; transcript amounts were determined by quantitative real-time PCR as described in ‘Materials and Methods’ section, using the RNA-I transcript as reference as previously described (22). Relative transcript levels are shown as EσS/Eσ70 ratio. Experiments were performed three times in duplicate, and standard errors are shown.
Sequence elements involved in specific EσS- versus Eσ70 recognition of promoter regions
The genes identified in ROMA experiments define, at least partially, what can be considered as the ‘core σS regulon’, i.e. a set of genes whose transcription is directly controlled by EσS. The core σS regulon can be opposed to the ‘expanded σS regulon’, i.e. genes dependent on a functional rpoS allele in vivo, whose promoters are, however, not necessarily recognized by EσS. In order to identify sequence features important for specific promoter recognition by EσS, it can be very informative to compare the sequences of EσS-dependent promoters; however, it is important to limit this comparison to the promoters that are exclusively, or at least preferentially, under EσS control. Thus, we performed a sequence alignment on the promoter sequences of genes preferentially transcribed by either EσS or Eσ70. Known promoter sequences were retrieved from the Ecocyc database (http://ecocyc.org/): only promoters whose transcription initiation start site had been experimentally determined were considered for sequence alignment. However, since many rpoS-dependent genes are controlled by multiple promoters in vivo, suggesting complex regulation that might involve different sigma factors, we verified that their transcription start sites in the in vitro transcription reactions did indeed correspond to the transcription sites reported in the literature. Thus, using RACE analysis, we determined transcription start sites on in vitro transcription assays carried out with EσS and performed as in the ROMA experiment (data not shown): we were able to identify precisely the in vitro transcription start sites at 31 promoter regions controlling 29 different genes, as listed in Supplementary File S1. For most genes with already known promoter regions, we could confirm the transcription start observed in vivo, although for several genes reported to be controlled by multiple promoters, only one promoter was found to be recognized by EσS in vitro (e.g. osmB, otsB, glgC and murQ). For the gabD gene, two of the three promoters described as functional in the bacterial cell were also recognized by EσS in the in vitro transcription assays. In contrast, for the araF gene, we identified a second promoter additional to the one already described in the literature. Finally, we determined the transcription start sites for eight genes (or operons) preferentially transcribed by EσS in ROMA experiments with yet-unknown transcription start sites, namely puuCBE, tam, treF, yciT, yffOP, yhjG, yi91a and yqiG: their transcription start site and putative promoter elements are listed in Supplementary File S1. For Eσ70-dependent promoters, we verified the transcription start sites for the ssrS transcript obtained in ROMA experiments performed with Eσ70: the transcription start site matched the known transcription site for the ssrS P1 promoter previously identified (37).
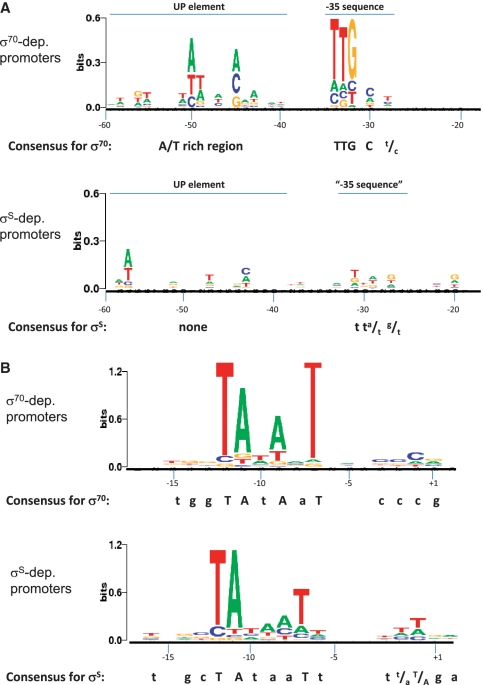
For promoter sequence comparison, we considered DNA sequences extending from −100 to +2 bp relative to the transcription start site, i.e. an area that includes all promoter elements described for Eσ70. For sequence analysis, we divided the −100 to +2 promoter sequence in three parts: the −17 to +2 region, carrying the −10 element and the transcription start site; the −60 to −18 region, containing the UP element and the −35 sequence; and the −100 to −61 region. Alignment of the −17 to +2 regions was centred on the first nucleotide of the −10 element (conventionally referred to as the −12 position, Figure 2B). For the −60 to −18 regions, the alignment was centred either on the first nucleotide of the −35 element (when present) or on the nucleotide located 22 bp upstream of the −10 element, which would correspond to the first nucleotide of a hypothetical −35 element placed at the optimal 17 bp distance from the −10 sequence (Figure 2A). In total, 31 promoters showing preferential recognition by EσS (listed in Supplementary File S1) and 30 promoters preferentially recognized by Eσ70 (Supplementary File S2) were selected for sequence analysis. Consensus sequence conservation within promoters preferentially recognized by either EσS or Eσ70 was displayed as sequence logos using the Weblogo 2.8.2 application (40) (http://weblogo.berkeley.edu/). Results of sequence analysis showed that, as expected, the most conserved sequences corresponded to the known promoter elements and were comprised between −60 and +2 (Figure 2). Comparison of the sequences located further upstream (−100 to −61) is shown in Supplementary Figure S2.
Figure 2.
Conserved sequence features in promoters of genes showing preferential transcription by either Eσ70 or EσS, shown as a sequence logo derived from multiple sequence alignments. (A) Comparison of alignments in the −60 to −18 promoter regions. (B) Comparison of alignments in the −17 to +2 promoter regions. Note that different y-axis scales were used in the two panels to account for the different levels of sequence conservation. Multiple alignment included 31 promoters controlling genes preferentially transcribed by EσS in ROMA experiments (listed in Supplementary File S1) and 29 promoters preferentially recognized by Eσ70 (listed in Supplementary File S2).
For promoters more efficiently recognized by Eσ70, the −35 and −10 sequences, i.e. the most important Eσ70-dependent promoter elements, are clearly recognizable in the sequence logos (Figure 2). In addition, the −60/−40 region is characterized by a high occurrence of A and T residues (Figure 2A), consistent with strong conservation of the UP element, i.e. a binding site for the α subunit of RNA polymerase (41). Other than the already known promoter features for σ70, only the transcription start site (−3/+1) showed a moderately conserved sequence (CCCG, Figure 2B).
Several differences in conserved regions were detectable in the promoter set for genes more efficiently transcribed by EσS in ROMA experiments. The −10 sequence is clearly the main conserved promoter element in this set (Figure 2B). No conserved sequences were detectable in the −35 region (Figure 2A), in agreement with previous works reporting that the −35 element does not play an important role in promoter recognition by EσS (42,43), while a weakly conserved sequence similar to a −35 element seems to be located at around −30 (Figure 2A). Another difference between the two promoter sets resides in the lack of an A/T rich region between −60 and −40 (Figure 2A). In contrast, however, promoters more efficiently transcribed by EσS seem to possess an increased occurrence of T residues in the −90 to −70 region (Supplementary Figure S2). Finally, the discriminator, i.e. the sequence located between the −10 element and the transcription start site, appears to be biased towards a high A/T content in promoters transcribed more efficiently by EσS. In particular, T residues appear to be conserved at positions −6, −2 and −1. Rather than being associated to specific nucleotide position, however, the bias towards an A/T-rich discriminator seems to be a common feature in promoters of genes preferentially transcribed by EσS. Indeed, while the average length of the discriminator is identical for both Eσ70- and EσS-dependent promoters (6.1 bp, Supplementary Table S3), the GC content is significantly higher in Eσ70-dependent promoters (0.58 versus 0.41, Supplementary Table S3).
Finally, an additional deviation between Eσ70- and EσS-specific promoter elements could be observed immediately upstream of the −10 hexamer: the Eσ70-dependent promoters showed some conservation of a TGG motif upstream of the −10 [the ‘extended −10′ (44)], which was replaced by a TNGC motif, which includes the characteristic −13C element, in EσS-dependent promoters.
Mutations in the discriminator region affect selective promoter recognition at the Eσ70-dependent promoter ssrS P1
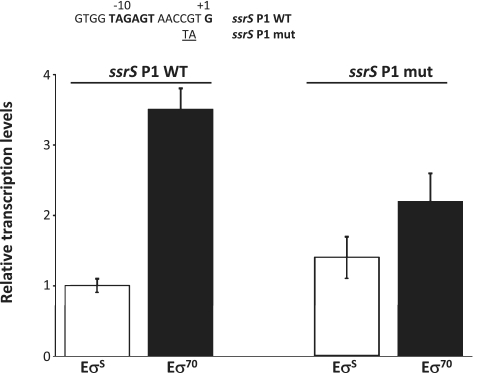
It might be inferred that differences in the specific elements of the promoter regions might account for different transcription efficiency by either EσS or Eσ70 in ROMA experiments. In particular, we investigated whether the presence of an A/T rich region immediately downstream of the −10 element, conserved among promoters preferentially recognized by EσS in ROMA experiment (Figure 2B), could indeed be a determinant for selective promoter recognition by EσS. To test this possibility, we targeted for mutagenesis the ssrS P1 promoter, which shows preferential recognition by Eσ70 in our in vitro assays (Table 1 and Figure 1) and whose dependence on Eσ70 had already been reported in the literature (37). We changed the CG nucleotides at positions −3/−2 of the ssrS P1 promoter to TA (Figure 3), thus making the −6/+1 region of the promoter A/T-rich. In vitro transcription experiments on supercoiled templates in the presence of either EσS or Eσ70 showed that CG to TA substitutions at positions −3/−2 of the ssrS P1 promoter resulted in loss of specific recognition by Eσ70 from 3.5- to 1.6-fold (Figure 3), suggesting that the A/T content in the −6/+1 region can indeed play a role in modulating transcription efficiency by either EσS or Eσ70.
Figure 3.
In vitro transcription experiments on pJCD01 plasmid derivatives in which either the ssrS P1 promoter (ssrS P1 WT) or a mutated derivative (ssrS P1 mut) had been cloned. The ssrS P1 mut carries a double substitution (CG to TA) at positions −3/−2 (shown in the figure). In vitro transcription was performed in the presence of either EσS or Eσ70; transcript amounts were determined by quantitative real-time PCR as described in ‘Materials and Methods’ section, using the RNA-I transcript as reference as previously described (22). Experiments were performed three times in duplicate, and standard errors are shown.
DISCUSSION
In this work, we have attempted to identify bona fide EσS-dependent promoters through in vitro transcription experiments using the whole E. coli genome as template, followed by identification of EσS-dependent transcripts by microarray analysis (ROMA experiments). The in vitro transcription experiments have been performed on linear DNA, in the absence of any additional factor or molecule able to affect transcription initiation by either EσS or Eσ70 (i.e. DNA supercoiling, transcription regulators, histone-like proteins, ppGpp), and thus they represent a direct measurement of sequence-specific interactions between promoters and RNA polymerase. Our results indicate that selective promoter utilization by Eσ70 and EσS is mediated by specific sequence features, and confirm the role of σS-specific promoter elements previously identified. Promoters more efficiently recognized by Eσ70 in ROMA experiments are characterized by a high occurrence of the UP-like element, i.e. an A/T-rich region located immediately upstream of the −35 sequence and acting as a binding site for the α subunit of RNA polymerase (41), suggesting that, at least in vitro, the UP element might favour promoter recognition by Eσ70. It is worth mentioning that, in Bacillus subtilis, UP elements are highly conserved among σA-dependent promoters (45), thus suggesting that UP elements might favour promoter recognition by the housekeeping σ factor in different bacteria. In contrast, UP-like elements flanking the −35 sequence are less conserved in promoters better recognized by EσS, where, however, A/T-rich elements seem to be scattered in the region spanning 70−90 nt upstream of the transcription start (Supplementary Figure S2). It might be speculated that, similar to the UP element for Eσ70-dependent promoters, AT-rich sequences located in the −70 to −90 region might also be involved in interaction with RNA polymerase α subunit at σS-dependent promoters. It is conceivable that the α subunit might contact alternative upstream promoter elements when assembled in different forms of RNA polymerase holoenzyme; indeed, differential ability to interact with UP elements has already been described for Eσ70 and EσS (46).
A sequence element showing strong differences between the two promoter sets is the discriminator, i.e. the region spanning between −10 and +1. This region shows a high T/A content for promoters more efficiently recognized by EσS; in contrast, Eσ70-dependent promoters identified in ROMA experiments are biased towards a CG-rich region in the −3/+1 residues (CCCG, Figure 2). Mutations in the discriminator region of the Eσ70-dependent ssrS P1 promoter increasing its A/T content result in partial loss of preferential recognition by Eσ70 (Figure 3), providing further confirmation for a role of the −10/+1 region in specific recognition by either form of RNA polymerase. GC versus AT content in the discriminator region can directly affect promoter melting, and GC-rich discriminators are a common feature among promoters subject to negative regulation by ppGpp (47,48), which, indeed, inhibits Eσ70-dependent transcription while promoting transcription by alternative forms of RNA polymerase (49).
The sequence features indicated by our promoter analysis are consistent with previous observations based on sequence analysis of rpoS-dependent promoters in the bacterial cell (4); this also includes the fairly strong conservation of a C nucleotide immediately upstream of the −10 hexamer (the −13 C element) among σS-dependent promoters. Indeed, the −13 C element occurs in almost half of the promoters transcribed more efficiently by EσS in ROMA experiments (14 out of 31 promoters; Supplementary File S1), opposed to a much lower frequency in Eσ70-dependent promoters (4 out of 29, Supplementary File S2). This result is not particularly surprising: indeed, a large amount of data has clearly shown that the presence of a C residue immediately upstream of the −10 nt favours promoter recognition by EσS (7,8,50). The −13C element might play a similar role as the TG motif at Eσ70-dependent promoters lacking a −35 region (44). It is noteworthy that an in vivo analysis suggests that the −13C element occurs in more than 70% of putative rpoS-dependent promoters (4): this observation would suggest that the −13C element might be needed to improve EσS–promoter interaction in the bacterial cell, possibly to overcome the negative effects of DNA-binding proteins, such as H-NS, that can modulate RNA polymerase–promoter interaction.
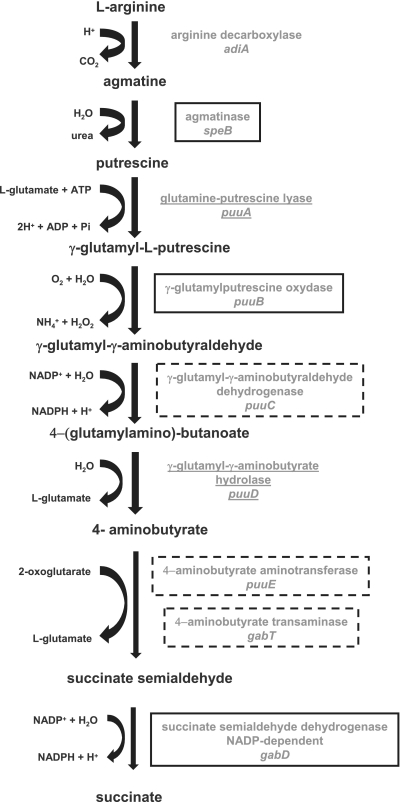
The results of the ROMA approach have also expanded our knowledge of the σS regulon: the σS protein is considered a central element of the so-called ‘general stress response’ (51). Intracellular σS concentration and expression of σS-dependent genes respond to reduction in growth rate (52); thus, any cellular stress affecting growth rate is likely to induce σS accumulation, which in turn plays a direct role in oxidative, acid and osmotic stress through activation of specific genes. In addition, σS activates metabolic genes associated to stationary-phase metabolism, in particular carbon storage genes involved in glycogen (19,20) and trehalose metabolism (9,20); consistent with these observations, stress response and carbon metabolism genes are highly represented among genes preferentially transcribed by EσS in ROMA experiments (Table 1). In addition, our results underline the importance of σS for the expression of genes involved in polyamine metabolism. Polyamines, in particular putrescine, play an important role in various cellular processes and can affect intracellular concentrations of the regulatory proteins σN, Cra and H-NS, thus impacting global gene expression (53). As shown in Figure 4, putrescine, the most abundant polyamine in bacterial cells (54), is a product of arginine degradation. Putrescine accumulates at the transition between exponential and stationary phase (55), and it can be subsequently converted into other polyamines or degraded to succinate, which can be shunted into the tricarboxylic acid (TCA) cycle, in a NADPH-generating process (Figure 4). Interestingly, mutants unable to synthesize polyamines are more sensitive to oxidative stress, since they cannot induce ahpC, katE and katG genes, encoding three different peroxidase, suggesting that polyamine accumulation might control expression of rpoS-dependent genes involved in the response to oxidative stress (54). In turn, our results indicate that genes encoding enzymes involved in both accumulation and degradation of putrescine, one of the main polyamines found in the bacterial cell, are rpoS-dependent. Indeed, the speB gene, encoding the putrescine-biosynthetic enzyme agmatinase, is preferentially transcribed by EσS in vitro (Table 1 and Figure 1) and is rpoS-dependent in vivo, in particular in LB1/4 medium (Table 3). Likewise, the genes involved in putrescine degradation, belonging to the puuCBE and gabDTP operons, are efficiently transcribed by EσS in ROMA experiments (Table 1 and Supplementary Table S1) and show dependence on a functional rpoS gene in vivo (Table 3). The gabDTP operon, as well as the puuA and puuD genes, also involved in putrescine degradation, have already been reported to be rpoS-dependent in vivo (20,55,56). Thus, σS could control every step in putrescine metabolism by regulating genes responsible for both its biosynthesis and its degradation, as shown in Figure 4. Activation of putrescine biosynthesis rather than degradation might respond to different growth conditions and environmental cues, as also suggested by the very different levels of rpoS-dependent regulation of putrescine-related genes (i.e. speB, gabD and puuB) in different growth media (Table 3).
Figure 4.
Schematic representation of a biochemical pathway under the control of the rpoS gene: arginine degradation to succinate via putrescine (adapted from EcoCyc; http://ecocyc.org/). Genes belonging to this pathway and found to be preferentially transcribed by EσS in ROMA experiments are boxed with solid lines (speB, puuB and gabD); genes co-transcribed with either puuB or gabD (i.e. belonging to the puuCBE and gabDTP operons; see also Supplementary Table 1) are boxed with dashed lines. Other genes of the pathway known to be dependent on the rpoS gene in vivo (puuA and puuD) (20,55) are underlined.
rpoS-dependent control of intracellular polyamines concentrations represents an important mechanism of indirect gene regulation by the σS protein, since polyamines act as signal molecules able to impact global transcription pattern in the bacterial cell (53). Indirect control of gene expression by σS can also occur through activation of regulatory proteins and of regulatory RNAs. Indeed, ROMA experiments would suggest that EσS directly controls at least four genes encoding regulatory proteins (appY, ilvY, rhaS and yciT) and four genetic loci encoding regulatory RNAs (cyaR, micA, sgrS/sgrT and ECs3934) (Table 1). Interestingly, the sgrS/sgrT locus, encoding both a non-coding RNA (SgrS) and a small regulatory protein (SgrT), negatively affects expression of the ptsG glucose uptake system (57), which is overexpressed in rpoS mutant strains of E. coli (20,58). However, the extent of σS-dependent indirect regulation of gene expression through non-coding RNA might not be limited to these four loci. Indeed, a significant number of intergenic regions, often located immediately upstream of known ORFs in antisense direction, was efficiently transcribed by EσS (Table 1). It is possible that at least a part of these intergenic regions might indeed be transcribed in the bacterial cell and function as cis-acting regulatory RNAs. Similarly, several ORFs only annotated in pathogenic E. coli strains were detectable as transcripts in the ROMA experiment, although MG1655 genomic DNA had been used in the experiments. Sequence comparison allowed us to determine that these ORFs are indeed present in MG1655, but they are not accounted for in the available databases. As observed for intergenic regions efficiently transcribed by EσS, several of these ORFs also overlap known genes in an antisense direction, suggesting that at least some of them might be involved in modulating gene expression. One ORF, c3113, partially overlaps the promoter region of the rrsG ribosomal operon, and quantitative real-time PCR experiments suggest that it is transcribed in a stationary-phase-dependent and rpoS-dependent manner in bacterial cells grown in glucose-based medium (Table 3). Similar to c3113, an intergenic region showing preferential transcription by EσS (IG223409_223770-r) also partially overlaps the ribosomal operon rrsH, but in an antisense direction. Thus, our observations would suggest that small proteins or non-coding RNAs might play an important role in rpoS-dependent negative regulation of genes associated with fast growth, such as ribosomal operons and the ptsG glucose uptake system.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
The Swiss National Fund for Scientific Research (Project 3100A0-109433 to T.E. and P.L.); Italian Ministry for University and Research (CHEM-PROFARMA-NET Research Program, Project RBPR05NWWC_004 to P.L., and FIRB-MIUR NG-LAB Research Program, Project RBLA03ER38_004 to G.D.B.). Funding for open access charge: Italian Ministry for University and Research (MIUR).
Conflict of interest statement. None declared.
Supplementary Material
REFERENCES
- 1.Lloyd G, Landini P, Busby S. Activation and repression of transcription initiation in bacteria. Essays Biochem. 2001;37:17–31. doi: 10.1042/bse0370017. [DOI] [PubMed] [Google Scholar]
- 2.Gourse RL, Ross W, Rutherford ST. General pathway for turning on promoters transcribed by RNA polymerases containing alternative sigma factors. J. Bacteriol. 2006;188:4589–4591. doi: 10.1128/JB.00499-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hengge-Aronis R. Stationary phase gene regulation: what makes an Escherichia coli promoter sigmaS-selective? Curr. Opin. Microbiol. 2002;5:591–595. doi: 10.1016/s1369-5274(02)00372-7. [DOI] [PubMed] [Google Scholar]
- 4.Typas A, Becker G, Hengge R. The molecular basis of selective promoter activation by the sigmaS subunit of RNA polymerase. Mol. Microbiol. 2007;63:1296–1306. doi: 10.1111/j.1365-2958.2007.05601.x. [DOI] [PubMed] [Google Scholar]
- 5.Gaal T, Ross W, Estrem ST, Nguyen LH, Burgess RR, Gourse RL. Promoter recognition and discrimination by EsigmaS RNA polymerase. Mol. Microbiol. 2001;42:939–954. doi: 10.1046/j.1365-2958.2001.02703.x. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka K, Takayanagi Y, Fujita N, Ishihama A, Takahashi H. Heterogeneity of the principal sigma factor in Escherichia coli: the rpoS gene product, sigma38, is a second principal sigma factor of RNA polymerase in stationary-phase Escherichia coli. Proc. Natl Acad. Sci. USA. 1993;90:8303. doi: 10.1073/pnas.90.17.8303a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker G, Hengge-Aronis R. What makes an Escherichia coli promoter sigma(S) dependent? Role of the -13/-14 nucleotide promoter positions and region 2.5 of sigma(S) Mol. Microbiol. 2001;39:1153–1165. doi: 10.1111/j.1365-2958.2001.02313.x. [DOI] [PubMed] [Google Scholar]
- 8.Lacour S, Kolb A, Landini P. Nucleotides from -16 to -12 determine specific promoter recognition by bacterial sigmaS-RNA polymerase. J. Biol. Chem. 2003;278:37160–37168. doi: 10.1074/jbc.M305281200. [DOI] [PubMed] [Google Scholar]
- 9.Weber H, Polen T, Heuveling J, Wendisch VF, Hengge R. Genome-wide analysis of the general stress response network in Escherichia coli: sigmaS-dependent genes, promoters, and sigma factor selectivity. J. Bacteriol. 2005;187:1591–1603. doi: 10.1128/JB.187.5.1591-1603.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colland F, Barth M, Hengge-Aronis R, Kolb A. sigma factor selectivity of Escherichia coli RNA polymerase: role for CRP, IHF and Lrp transcription factors. EMBO J. 2000;19:3028–3037. doi: 10.1093/emboj/19.12.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnqvist A, Olsen A, Normark S. Sigma S-dependent growth-phase induction of the csgBA promoter in Escherichia coli can be achieved in vivo by sigma 70 in the absence of the nucleoid-associated protein H-NS. Mol. Microbiol. 1994;13:1021–1032. doi: 10.1111/j.1365-2958.1994.tb00493.x. [DOI] [PubMed] [Google Scholar]
- 12.Mujacic M, Baneyx F. Regulation of Escherichia coli hchA, a stress-inducible gene encoding molecular chaperone Hsp31. Mol. Microbiol. 2006;60:1576–1589. doi: 10.1111/j.1365-2958.2006.05207.x. [DOI] [PubMed] [Google Scholar]
- 13.Kusano S, Ding Q, Fujita N, Ishihama A. Promoter selectivity of Escherichia coli RNA polymerase Esigma70 and Esigma38 holoenzymes. Effect of DNA supercoiling. J. Biol. Chem. 1996;271:1998–2004. doi: 10.1074/jbc.271.4.1998. [DOI] [PubMed] [Google Scholar]
- 14.Jishage M, Ishihama A. A stationary phase protein in Escherichia coli with binding activity to the major sigma subunit of RNA polymerase. Proc. Natl Acad. Sci. USA. 1998;95:4953–4958. doi: 10.1073/pnas.95.9.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gentry DR, Hernandez VJ, Nguyen LH, Jensen DB, Cashel M. Synthesis of the stationary-phase sigma factor sigmaS is positively regulated by ppGpp. J. Bacteriol. 1993;175:7982–7989. doi: 10.1128/jb.175.24.7982-7989.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lacour S, Landini P. SigmaS-dependent gene expression at the onset of stationary phase in Escherichia coli: function of sigmaS-dependent genes and identification of their promoter sequences. J. Bacteriol. 2004;186:7186–7195. doi: 10.1128/JB.186.21.7186-7195.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patten CL, Kirchhof MG, Schertzberg MR, Morton RA, Schellhorn HE. Microarray analysis of RpoS-mediated gene expression in Escherichia coli K-12. Mol. Genet. Genomics. 2004;272:580–591. doi: 10.1007/s00438-004-1089-2. [DOI] [PubMed] [Google Scholar]
- 18.Ito A, May T, Kawata K, Okabe S. Significance of rpoS during maturation of Escherichia coli biofilms. Biotechnol. Bioeng. 2008;99:1462–1471. doi: 10.1002/bit.21695. [DOI] [PubMed] [Google Scholar]
- 19.Dong T, Schellhorn HE. Control of RpoS in global gene expression of Escherichia coli in minimal media. Mol. Genet. Genomics. 2009;281:19–33. doi: 10.1007/s00438-008-0389-3. [DOI] [PubMed] [Google Scholar]
- 20.Dong T, Schellhorn HE. Global effect of RpoS on gene expression in pathogenic Escherichia coli O157:H7 strain EDL933. BMC Genomics. 2009;10:349. doi: 10.1186/1471-2164-10-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiratsu K, Shinagawa H, Makino K. Mode of promoter recognition by the Escherichia coli RNA polymerase holoenzyme containing the sigmaS subunit: identification of the recognition sequence of the fic promoter. Mol. Microbiol. 1995;18:841–850. doi: 10.1111/j.1365-2958.1995.18050841.x. [DOI] [PubMed] [Google Scholar]
- 22.Marschall C, Labrousse V, Kreimer M, Weichart D, Kolb A, Hengge-Aronis R. Molecular analysis of the regulation of csiD, a carbon starvation-inducible gene in Escherichia coli that is exclusively dependent on sigmaS and requires activation by cAMP-CRP. J. Mol. Biol. 1998;276:339–353. doi: 10.1006/jmbi.1997.1533. [DOI] [PubMed] [Google Scholar]
- 23.Landini P, Hajec LI, Nguyen LH, Burgess RR, Volkert MR. The leucine-responsive regulatory protein (Lrp) acts as a specific repressor for sigmaS-dependent transcription of the Escherichia coli aidB gene. Mol. Microbiol. 1996;20:947–955. doi: 10.1111/j.1365-2958.1996.tb02536.x. [DOI] [PubMed] [Google Scholar]
- 24.Checroun C, Bordes P, Leroy O, Kolb A, Gutierrez C. Interactions between the 2.4 and 4.2 regions of sigmaS, the stress-specific sigma factor of Escherichia coli, and the -10 and -35 promoter elements. Nucleic Acids Res. 2004;32:45–53. doi: 10.1093/nar/gkh155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blattner FR, Plunkett G, III, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, et al. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 26.Argaman L, Hershberg R, Vogel J, Bejerano G, Wagner EG, Margalit H, Altuvia S. Novel small RNA-encoding genes in the intergenic regions of Escherichia coli. Curr. Biol. 2001;11:941–950. doi: 10.1016/s0960-9822(01)00270-6. [DOI] [PubMed] [Google Scholar]
- 27.Li XM, Shapiro LJ. Three step PCR mutagenesis for “linker scanning”. Nucleic Acids Res. 1993;21:3745–3478. doi: 10.1093/nar/21.16.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wettenhall JM, Simpson KM, Satterley K, Smyth GK. affylmGUI: a graphical user interface for linear modeling of single channel microarray data. Bioinformatics. 2006;22:897–899. doi: 10.1093/bioinformatics/btl025. [DOI] [PubMed] [Google Scholar]
- 29.Zheng D, Constantinidou C, Hobman JL, Minchin SD. Identification of the CRP regulon using in vitro and in vivo transcriptional profiling. Nucleic Acids Res. 2004;32:5874–5893. doi: 10.1093/nar/gkh908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prigent-Combaret C, Brombacher E, Vidal O, Ambert A, Lejeune P, Landini P, Dorel C. Complex regulatory network controls initial adhesion and biofilm formation in Escherichia coli via regulation of the csgD gene. J. Bacteriol. 2001;183:7213–7223. doi: 10.1128/JB.183.24.7213-7223.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brombacher E, Dorel C, Zehnder AJ, Landini P. The curli biosynthesis regulator CsgD co-ordinates the expression of both positive and negative determinants for biofilm formation in Escherichia coli. Microbiology. 2003;149:2847–2857. doi: 10.1099/mic.0.26306-0. [DOI] [PubMed] [Google Scholar]
- 32.Perrin C, Briandet R, Jubelin G, Lejeune P, Mandrand-Berthelot MA, Rodrigue A, Dorel C. Nickel promotes biofilm formation by Escherichia coli K-12 strains that produce curli. Appl. Environ. Microbiol. 2009;75:1723–1733. doi: 10.1128/AEM.02171-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gualdi L, Tagliabue L, Landini P. Biofilm formation-gene expression relay system in Escherichia coli: modulation of sigmaS-dependent gene expression by the CsgD regulatory protein via sigmaS protein stabilization. J. Bacteriol. 2007;189:8034–8043. doi: 10.1128/JB.00900-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao M, Kobel PA, Morshedi MM, Wu MF, Paddon C, Helmann JD. Defining the Bacillus subtilis sigmaW regulon: a comparative analysis of promoter consensus search, run-off transcription/macroarray analysis (ROMA), and transcriptional profiling approaches. J. Mol. Biol. 2002;316:443–457. doi: 10.1006/jmbi.2001.5372. [DOI] [PubMed] [Google Scholar]
- 35.Cashel M, Gentry DR, Hernandez VJ, Vinella D. The Stringent Response. In Escherichia coli and Salmonella: Cellular and Molecular Biology. 2nd edn. Washington DC: American society for Microbiology; 1996. [Google Scholar]
- 36.Wassarman KM, Storz G. 6S RNA regulates E. coli. RNA polymerase activity. Cell. 2000;101:613–623. doi: 10.1016/s0092-8674(00)80873-9. [DOI] [PubMed] [Google Scholar]
- 37.Kim KS, Lee Y. Regulation of 6S RNA biogenesis by switching utilization of both sigma factors and endoribonucleases. Nucleic Acids Res. 2004;32:6057–6068. doi: 10.1093/nar/gkh939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farewell A, Kvint K, Nystrom T. Negative regulation by RpoS: a case of sigma factor competition. Mol. Microbiol. 1998;29:1039–1051. doi: 10.1046/j.1365-2958.1998.00990.x. [DOI] [PubMed] [Google Scholar]
- 39.Dong T, Kirchhof MG, Schellhorn HE. RpoS regulation of gene expression during exponential growth of Escherichia coli K12. Mol. Genet. Genomics. 2008;279:267–277. doi: 10.1007/s00438-007-0311-4. [DOI] [PubMed] [Google Scholar]
- 40.Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ross W, Gosink KK, Salomon J, Igarashi K, Zou C, Ishihama A, Severinov K, Gourse RL. A third recognition element in bacterial promoters: DNA binding by the alpha subunit of RNA polymerase. Science. 1993;262:1407–1413. doi: 10.1126/science.8248780. [DOI] [PubMed] [Google Scholar]
- 42.Espinosa-Urgel M, Chamizo C, Tormo A. A consensus structure for sigma S-dependent promoters. Mol. Microbiol. 1996;21:657–659. doi: 10.1111/j.1365-2958.1996.tb02573.x. [DOI] [PubMed] [Google Scholar]
- 43.Lee SJ, Gralla JD. Sigma38 (rpoS) RNA polymerase promoter engagement via -10 region nucleotides. J. Biol. Chem. 2001;276:30064–30071. doi: 10.1074/jbc.M102886200. [DOI] [PubMed] [Google Scholar]
- 44.Barne KA, Bown JA, Busby SJ, Minchin SD. Region 2.5 of the Escherichia coli RNA polymerase sigma70 subunit is responsible for the recognition of the 'extended-10' motif at promoters. EMBO J. 1997;16:4034–4040. doi: 10.1093/emboj/16.13.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Helmann JD. Compilation and analysis of Bacillus subtilis sigmaA-dependent promoter sequences: evidence for extended contact between RNA polymerase and upstream promoter DNA. Nucleic Acids Res. 1995;23:2351–2360. doi: 10.1093/nar/23.13.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Typas A, Hengge R. Differential ability of sigmaS and sigma70 of Escherichia coli to utilize promoters containing half or full UP-element sites. Mol. Microbiol. 2005;55:250–260. doi: 10.1111/j.1365-2958.2004.04382.x. [DOI] [PubMed] [Google Scholar]
- 47.Travers AA. Promoter sequence for stringent control of bacterial ribonucleic acid synthesis. J. Bacteriol. 1980;141:973–976. doi: 10.1128/jb.141.2.973-976.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Travers AA. A tRNATyr promoter with an altered in vitro response to ppGpp. J. Mol. Biol. 1980;141:91–97. doi: 10.1016/s0022-2836(80)80030-1. [DOI] [PubMed] [Google Scholar]
- 49.Condon C, Squires C, Squires CL. Control of rRNA transcription in Escherichia coli. Microbiol. Rev. 1995;59:623–645. doi: 10.1128/mr.59.4.623-645.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bordes P, Repoila F, Kolb A, Gutierrez C. Involvement of differential efficiency of transcription by EsigmaS and Esigma70 RNA polymerase holoenzymes in growth phase regulation of the Escherichia coli osmE promoter. Mol. Microbiol. 2000;35:845–853. doi: 10.1046/j.1365-2958.2000.01758.x. [DOI] [PubMed] [Google Scholar]
- 51.Hengge-Aronis R. A role for the sigmaS subunit of RNA polymerase in the regulation of bacterial virulence. Adv. Exp. Med. Biol. 2000;485:85–93. doi: 10.1007/0-306-46840-9_11. [DOI] [PubMed] [Google Scholar]
- 52.Ihssen J, Egli T. Specific growth rate and not cell density controls the general stress response in Escherichia coli. Microbiology. 2004;150:1637–1648. doi: 10.1099/mic.0.26849-0. [DOI] [PubMed] [Google Scholar]
- 53.Terui Y, Higashi K, Taniguchi S, Shigemasa A, Nishimura K, Yamamoto K, Kashiwagi K, Ishihama A, Igarashi K. Enhancement of the synthesis of RpoN, Cra, and H-NS by polyamines at the level of translation in Escherichia coli cultured with glucose and glutamate. J .Bacteriol. 2007;189:2359–2368. doi: 10.1128/JB.01562-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jung IL, Kim IG. Transcription of ahpC, katG, and katE genes in Escherichia coli is regulated by polyamines: polyamine-deficient mutant sensitive to H2O2-induced oxidative damage. Biochem. Biophys. Res. Commun. 2003;301:915–922. doi: 10.1016/s0006-291x(03)00064-0. [DOI] [PubMed] [Google Scholar]
- 55.Kurihara S, Oda S, Kumagai H, Suzuki H. Gamma-glutamyl-gamma-aminobutyrate hydrolase in the putrescine utilization pathway of Escherichia coli K-12. FEMS Microbiol. Lett. 2006;256:318–323. doi: 10.1111/j.1574-6968.2006.00137.x. [DOI] [PubMed] [Google Scholar]
- 56.Mendoza-Vargas A, Olvera L, Olvera M, Grande R, Vega-Alvarado L, Taboada B, Jimenez-Jacinto V, Salgado H, Juarez K, Contreras-Moreira B, et al. Genome-wide identification of transcription start sites, promoters and transcription factor binding sites in E. coli. PLoS ONE. 2009;4:e7526. doi: 10.1371/journal.pone.0007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vanderpool CK, Gottesman S. Involvement of a novel transcriptional activator and small RNA in post-transcriptional regulation of the glucose phosphoenolpyruvate phosphotransferase system. Mol. Microbiol. 2004;54:1076–1089. doi: 10.1111/j.1365-2958.2004.04348.x. [DOI] [PubMed] [Google Scholar]
- 58.Seeto S, Notley-McRobb L, Ferenci T. The multifactorial influences of RpoS, Mlc and cAMP on ptsG expression under glucose-limited and anaerobic conditions. Res. Microbiol. 2004;155:211–215. doi: 10.1016/j.resmic.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 59.Rhodius VA, Suh WC, Nonaka G, West J, Gross CA. Conserved and variable functions of the sigmaE stress response in related genomes. PLoS Biol. 2006;4:e2. doi: 10.1371/journal.pbio.0040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boulanger A, Francez-Charlot A, Conter A, Castanie-Cornet MP, Cam K, Gutierrez C. Multistress regulation in Escherichia coli: expression of osmB involves two independent promoters responding either to sigmaS or to the RcsCDB His-Asp phosphorelay. J. Bacteriol. 2005;187:3282–3286. doi: 10.1128/JB.187.9.3282-3286.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Storz G, Tartaglia LA, Ames BN. Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science. 1990;248:189–194. doi: 10.1126/science.2183352. [DOI] [PubMed] [Google Scholar]
- 62.Missiakas D, Raina S. Signal transduction pathways in response to protein misfolding in the extracytoplasmic compartments of E. coli: role of two new phosphoprotein phosphatases PrpA and PrpB. EMBO J. 1997;16:1670–1685. doi: 10.1093/emboj/16.7.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Missiakas D, Raina S. Protein misfolding in the cell envelope of Escherichia coli: new signaling pathways. Trends Biochem. Sci. 1997;22:59–63. doi: 10.1016/s0968-0004(96)10072-4. [DOI] [PubMed] [Google Scholar]
- 64.Seeger C, Poulsen C, Dandanell G. Identification and characterization of genes (xapA, xapB, and xapR) involved in xanthosine catabolism in Escherichia coli. J. Bacteriol. 1995;177:5506–5516. doi: 10.1128/jb.177.19.5506-5516.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schneider R, Travers A, Kutateladze T, Muskhelishvili G. A DNA architectural protein couples cellular physiology and DNA topology in Escherichia coli. Mol. Microbiol. 1999;34:953–964. doi: 10.1046/j.1365-2958.1999.01656.x. [DOI] [PubMed] [Google Scholar]
- 66.Metzner M, Germer J, Hengge R. Multiple stress signal integration in the regulation of the complex sigmaS-dependent csiD-ygaF-gabDTP operon in Escherichia coli. Mol. Microbiol. 2004;51:799–811. doi: 10.1046/j.1365-2958.2003.03867.x. [DOI] [PubMed] [Google Scholar]
- 67.Atlung T, Sund S, Olesen K, Brondsted L. The histone-like protein H-NS acts as a transcriptional repressor for expression of the anaerobic and growth phase activator AppY of Escherichia coli. J. Bacteriol. 1996;178:3418–3425. doi: 10.1128/jb.178.12.3418-3425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wickstrum JR, Skredenske JM, Kolin A, Jin DJ, Fang J, Egan SM. Transcription activation by the DNA-binding domain of the AraC family protein RhaS in the absence of its effector-binding domain. J. Bacteriol. 2007;189:4984–4993. doi: 10.1128/JB.00530-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hendrickson W, Stoner C, Schleif R. Characterization of the Escherichia coli araFGH and araJ promoters. J. Mol. Biol. 1990;215:497–510. doi: 10.1016/S0022-2836(05)80163-9. [DOI] [PubMed] [Google Scholar]
- 70.Romeo T, Preiss J. Genetic regulation of glycogen biosynthesis in Escherichia coli: in vitro effects of cyclic AMP and guanosine 5'-diphosphate 3'-diphosphate and analysis of in vivo transcripts. J. Bacteriol. 1989;171:2773–2782. doi: 10.1128/jb.171.5.2773-2782.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Atlung T, Knudsen K, Heerfordt L, Brondsted L. Effects of sigmaS and the transcriptional activator AppY on induction of the Escherichia coli hya and cbdAB-appA operons in response to carbon and phosphate starvation. J. Bacteriol. 1997;179:2141–2146. doi: 10.1128/jb.179.7.2141-2146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Richard DJ, Sawers G, Sargent F, McWalter L, Boxer DH. Transcriptional regulation in response to oxygen and nitrate of the operons encoding the [NiFe] hydrogenases 1 and 2 of Escherichia coli. Microbiology. 1999;145:2903–2912. doi: 10.1099/00221287-145-10-2903. [DOI] [PubMed] [Google Scholar]
- 73.Jaeger T, Mayer C. The transcriptional factors MurR and catabolite activator protein regulate N-acetylmuramic acid catabolism in Escherichia coli. J. Bacteriol. 2008;190:6598–6608. doi: 10.1128/JB.00642-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cai H, Clarke S. A novel methyltransferase catalyzes the methyl esterification of trans-aconitate in Escherichia coli. J. Biol. Chem. 1999;274:13470–13479. doi: 10.1074/jbc.274.19.13470. [DOI] [PubMed] [Google Scholar]
- 75.Partridge JD, Browning DF, Xu M, Newnham LJ, Scott C, Roberts RE, Poole RK, Green J. Characterization of the Escherichia coli K-12 ydhYVWXUT operon: regulation by FNR, NarL and NarP. Microbiology. 2008;154:608–618. doi: 10.1099/mic.0.2007/012146-0. [DOI] [PubMed] [Google Scholar]
- 76.Bergholz TM, Wick LM, Qi W, Riordan JT, Ouellette LM, Whittam TS. Global transcriptional response of Escherichia coli O157:H7 to growth transitions in glucose minimal medium. BMC Microbiol. 2007;7:97. doi: 10.1186/1471-2180-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johansen J, Eriksen M, Kallipolitis B, Valentin-Hansen P. Down-regulation of outer membrane proteins by noncoding RNAs: unraveling the cAMP-CRP- and sigmaE-dependent CyaR-ompX regulatory case. J. Mol. Biol. 2008;383:1–9. doi: 10.1016/j.jmb.2008.06.058. [DOI] [PubMed] [Google Scholar]
- 78.De Lay N, Gottesman S. The Crp-activated small noncoding regulatory RNA CyaR (RyeE) links nutritional status to group behavior. J. Bacteriol. 2009;191:461–476. doi: 10.1128/JB.01157-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Soupene E, King N, Lee H, Kustu S. Aquaporin Z of Escherichia coli: reassessment of its regulation and physiological role. J. Bacteriol. 2002;184:4304–4307. doi: 10.1128/JB.184.15.4304-4307.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Neidhardt FC, Curtiss R, III, Ingraham JL, Lin ECC, Low KB, Jr, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE. Escherichia coli and Salmonella: Cellular and Molecular Biology. 2nd edn. Washington DC: American Society for Microbiology; 1996. [Google Scholar]
- 81.Mizusawa S, Court D, Gottesman S. Transcription of the sulA gene and repression by LexA. J. Mol. Biol. 1983;171:337–343. doi: 10.1016/0022-2836(83)90097-9. [DOI] [PubMed] [Google Scholar]
- 82.Yasuda T, Nagata T, Ohmori H. Multicopy suppressors of the cold-sensitive phenotype of the pcsA68 (dinD68) mutation in Escherichia coli. J. Bacteriol. 1996;178:3854–3859. doi: 10.1128/jb.178.13.3854-3859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rolfes RJ, Zalkin H. Escherichia coli gene purR encoding a repressor protein for purine nucleotide synthesis. Cloning, nucleotide sequence, and interaction with the purF operator. J. Biol. Chem. 1988;263:19653–19661. [PubMed] [Google Scholar]
- 84.Zheng M, Wang X, Doan B, Lewis KA, Schneider TD, Storz G. Computation-directed identification of OxyR DNA binding sites in Escherichia coli. J. Bacteriol. 2001;183:4571–4579. doi: 10.1128/JB.183.15.4571-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.