Abstract
RNA editing involves a variety of genetic systems and occurs by different mechanisms. In higher plant chloroplasts, specific sites of some transcripts are subject to C-to-U conversion. We have previously shown that site-specific trans-acting factors for psbE and petB mRNA editing bind corresponding cis-elements, which are located 5 nucleotides upstream from the editing site. Here we report that, by using mRNAs labeled either at the center of the upstream cis-element or at the editing site, the site-specific factors can be cross-linked with nucleotides at both positions. Mutations of nucleotides in the proximal region of the editing site revealed a correlation between editing activity and cross-linking efficiency of factors with the editing site, even though cross-linking with the upstream cis-element was unaffected. These observations suggest that the site-specific factor binds stably to the upstream ciselement, whereas it interacts weakly with the editing site. This finding raises the intriguing possibility that the site-specific factor is involved in both site-determination and C-to-U conversion in chloroplast RNA editing.
RNA editing is one of the posttranscriptional processes involved in transcript maturation in a variety of organisms, including viruses, fungi, plants, and mammals (1). This process can be subdivided into insertion/deletion of nucleotides and base modification. RNA editing in plant organelles belongs to the latter case; specific sites of some transcripts are subject to C-to-U (and rarely U-to-C) conversions (2–7). Most editing events occur in protein-coding regions and restore codons to conserved amino acids. In chloroplasts, editing of psbF, petB, and accD mRNAs has been reported to be necessary for the function of their products (8–10), indicating that at least some RNA editing events are essential posttranscriptional steps. However, one RNA editing event was observed in the third position of a codon and did not lead to amino acid substitution (11). Hence, at least one case of editing seems to have no biological significance.
At present, 34 C-to-U editing sites have been found in tobacco chloroplast transcripts (12). Cis-analysis has been pursued for several sites by transplastomic approaches, and all defined cis-acting elements are located within ≈30 nucleotides of editing sites (13–15). Sequences surrounding editing sites exhibit no common characteristics, except most of the 5′ neighboring residues of editing sites are pyrimidines and the 3′ neighbors are A residues, both in chloroplast and mitochondrial transcripts (16, 17). The importance of 5′ neighbors for editing efficiency has been reported in psbL and ndhB mRNA (site V) editing (13, 14).
We have recently studied the mechanism of psbE and petB mRNA editing in tobacco and pea chloroplasts (18). Both chloroplast genes possess single editing sites that depend on plant species. The 72nd codon of psbE is encoded as Ser (UCU) at the DNA level in spinach, pea, and maize, whereas tobacco, Arabidopsis, and black pine restore this codon from Pro (CCU) by RNA editing. The 204th codon of petB is Leu (CUA) in spinach, Arabidopsis and black pine, whereas tobacco, pea, and maize restore it from Pro (CCA). By using an improved in vitro RNA editing system from tobacco chloroplasts, we unambiguously showed the involvement of distinct proteins in psbE and petB mRNA editing (18). Competition analysis revealed that they specifically bind ≈10-nt sequences located ≈5 nt upstream of the editing site (upstream cis-element). However, editing assays with mutated mRNA substrates demonstrated that specific sequences in the 5′ proximal region of editing sites are also required for editing. This observation raises the problem of how the 5-nt region is important for RNA editing.
To explore the function of the proximal region for editing, we performed detailed mutation analysis monitored by editing activity and UV cross-linking. Here we report that the proper sequence in the proximal region is required for the site-specific trans-acting factor to interact with the editing site. A model of the mechanism of chloroplast RNA editing is discussed.
Materials and Methods
Preparation of RNA Substrates and Tobacco Chloroplast Extracts. RNA substrates were prepared essentially as described (18). In brief, 20 pmol of 5′-32P-labeled downstream RNAs (21–28 nucleotides) was ligated to 60 pmol of the corresponding upstream RNAs (106–128 nucleotides) with the aid of 40 pmol of a bridging DNA oligonucleotide and T4 DNA ligase in 20-μl reaction mixtures at 25°C overnight. The ligated mRNAs were purified by 5% PAGE containing 7 M urea. Tobacco (Nicotiana tabacum var. Bright Yellow 4) was grown in a growth chamber at 25°C in a 16-h light/8-h dark cycle for 6 weeks. Tobacco chloroplast extracts were prepared as described (18).
In Vitro RNA Editing and UV Cross-Linking Assays. RNA editing and UV cross-linking assays were carried out essentially as described (18) with slight modifications. Both reaction mixtures contained 5 μl of chloroplast extract (≈50 μg of protein) and a similar amount (1–3 fmol) of mRNA substrate. For RNA editing assays, an mRNA substrate was incubated at 28°C for 2 h. RNA was isolated and digested into 5′ mononucleotides with 1 μg of nuclease P1 (Wako Pure Chemical, Osaka) in the presence of 50 mM ammonium acetate (pH 4.8) at 37°C for 3 h. Mononucleotides were separated on cellulose TLC plates (20 × 20 cm, Funakoshi, Tokyo) by using isopropyl alcohol/HCl/water (70:15:15). For UV cross-linking assays, an mRNA substrate was incubated at 28°C for 1 h in the editing mixture. Reaction mixtures were irradiated with UV light (254 nm, 1.8 J/cm2) by using a Funacrosslinker (Funakoshi). This step was followed by digestion of the RNA with a mixture of 2 μg of RNase A, 0.25 units of nuclease P1 (Wako Pure Chemical), and 0.05 unit of Crotalus adamanteus venom phosphodiesterase I (Pharmacia) at 37°C for 15 min. Protein samples were separated by 12.5% PAGE containing 0.1% SDS. 32P-labeled mononucleotides on TLC and 32P-cross-linked proteins on PAGE were visualized by a Bioimaging Analyzer BAS2000 (Fuji).
Results
Cis-Analysis in the Proximal Region of Editing Sites. Previously, we showed that site-specific trans-acting factors bind to the upstream cis-elements, a 10-nt sequence from positions –15 to –6 (editing site at position +1) of psbE mRNA and a 15-nt sequence from –20 to –6 of petB mRNA in tobacco chloroplasts (18). Sequences between the –5/–1 regions of psbE and petB mRNAs are not required for binding of the factors. However, mutations of these five nucleotides of both mRNA substrates completely abolished editing. These results suggest that sequences between upstream cis-elements and editing sites (proximal regions) are also involved in editing reactions.
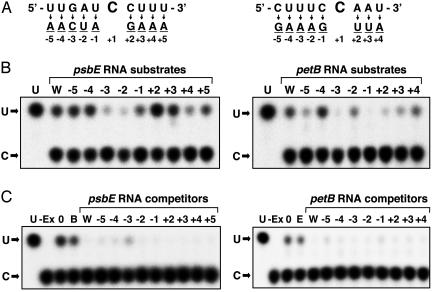
To examine sequence specificity in the proximal region, we constructed a series of mutated mRNA substrates with individual nucleotides converted to their complementary nucleotides (psbE mRNA, –5 to + 5 and petB mRNA, –5 to + 4) and used these substrates for in vitro editing assays. As shown in Fig. 1B Left, editing of psbE mRNAs was severely impaired by mutations at –3 and –2, whereas mutations at –1 and +4 resulted in slight inhibition. Mutation at +2 gave the opposite effect, a slight increase in editing. On the other hand, the editing of petB mRNAs seems to require a higher sequence specificity than that of psbE mRNAs. Mutations at –3 and –1 abolished editing, and those at –5, –2, +2, and +3 also exerted an inhibitory effect (Fig. 1B Right). Based on these results, it is difficult to define specific residues in common that are critical for editing. In the proximal region, the sequence specificity seems to be different for these editing sites.
Fig. 1.
Cis-analysis in the proximal region of psbE and petB mRNAs. (A) Schematic representation of altered regions in mRNA substrates (Left, psbE mRNA; Right, petB mRNA). The top sequence represents a portion of the wild-type mRNAs (19), and mutated residues are shown below. (B) Editing activity of mutated mRNA substrates. One femtomole of each mutated mRNA was incubated with a chloroplast extract as described in Materials and Methods, and resulting 32P-mononucleotides were separated by TLC. (C) Competition analysis using the mutated mRNAs as competitors. One femtomole of each 32P-labeled wild-type mRNA substrate was incubated with 1 pmol of each unlabeled mutated mRNA as competitor. U, marker pU; W, wild-type mRNA; E, psbE wild-type mRNA as competitor; B, petB wild-type mRNA as competitor; –Ex, without chloroplast extract; 0, without competitor.
To confirm that the mutations above do not affect binding of the site-specific factors, we examined the ability of the mutated RNAs to compete for binding. The editing of wild-type psbE mRNAs (labeled at C to be edited) was inhibited with an excess amount of the same psbE mRNA (unlabeled) but not with that of the heterologous mRNA (unlabeled) (Fig. 1C Left, lanes W vs. B), indicating that the site-specific factor for psbE mRNA editing was specifically depleted and was unavailable for the labeled mRNA substrate. As a result, most of these mutated psbE mRNA competitors trapped the site-specific factor and hence arrested the editing of wild-type psbE mRNAs (lanes –5 to +5). Therefore, the observed difference in editing efficiencies of mutated psbE mRNA substrates was not due to the extent of factor binding. A mutation at –3 in psbE mRNA was somewhat of an exception, and slight editing was observed when it was used as a competitor (Fig. 1C Left, lane –3), although it was a poor substrate (Fig. 1B Left, lane –3). The G residue at –3 may have some role in binding of the site-specific factor. In petB mRNA, all these mutated mRNAs were effective as competitors (Fig. 1C Right).
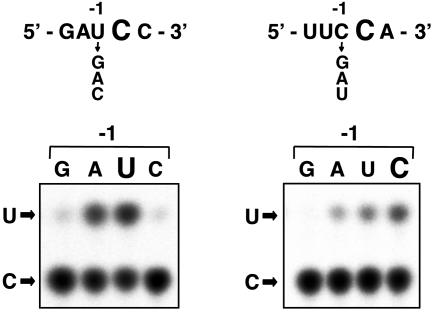
Preference for 5′ Neighboring Residues. The 5′ neighboring nucleotides of editing sites were reported to be important for psbL and ndhB (site V) mRNA editing (13, 14). If sequences in the proximal region are recognized site-specifically, it is possible that the nucleotide preference for that position (–1) differs for psbE and petB mRNA editing. We examined the effect of mutations at the –1 position on editing activity (Fig. 2). In psbE mRNA, the intrinsic U residue was the most favorable for editing, and conversion to the A residue exhibited slight inhibition. However, conversion to a G or C residue essentially abolished editing. In petB mRNA, the intrinsic C residue was also the most favorable. All the other residues were inhibitory, and conversion to G abolished editing activity completely. These results clearly show that the preference for 5′ neighboring residues is different for the editing of psbE and petB mRNAs. Taken together, these observations support the idea that the requirement for sequences in the proximal region is site-specific.
Fig. 2.
Preference for the 5′ neighboring residue for psbE mRNA (Left) and petB mRNA (Right) editing. The –1 residue of psbE and petB mRNAs was changed to three other nucleotides. Editing activities of mutated RNA substrates were assayed as in Fig. 1.
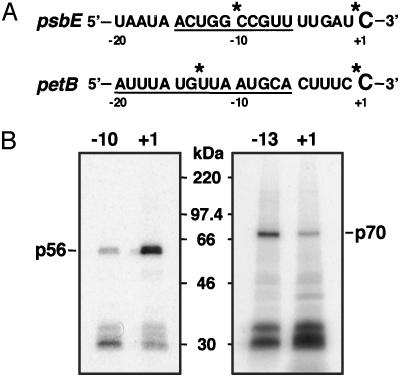
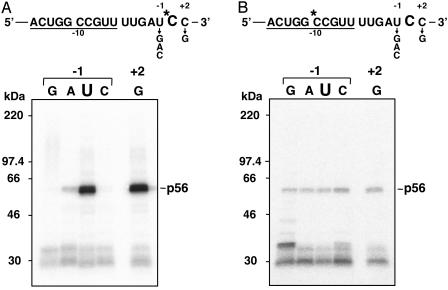
Site-Specific Factors Are Cross-Linked with the Editing Site. By using UV cross-linking approaches with mRNAs labeled at the upstream cis-elements, we detected two site-specific factors, p56 for psbE mRNA editing and p70 for petB mRNA editing (18). If the editing sites are also recognized by a protein factor(s), the protein may be cross-linked with the editing site. To improve the site specificity of cross-linking, nuclease P1 and C. adamanteus venom phosphodiesterase I (3′ exonuclease) were used in addition to RNase A for extensive RNA digestion after UV irradiation. By using the psbE mRNA substrate labeled at the center of the upstream cis-element (–10), p56 and abundant chloroplast RNA-binding proteins (20) were detected (Fig. 3B Left, lane –10). Surprisingly, by using the same substrate labeled at the editing site (+1), p56 was again cross-linked (Fig. 3B Left, lane +1). In petB mRNA, we found that p70 was also cross-linked with both positions, the center of the upstream cis-element (–13) and the editing site (+1) (Fig. 3B Right, lanes –13 and +1). These results imply that the site-specific factors directly interact with the editing sites. Note that the cross-linking intensities of the site-specific factors were different for the upstream cis-elements and the editing sites. This finding may reflect nonuniform distributions of UV-reactive residues, such as aromatic and/or sulfur-containing amino acids, in the factors.
Fig. 3.
UV cross-linking with the upstream cis-element or the editing site of psbE and petB mRNAs. (A) Schematic representation of mRNA substrates with 32P-labels (marked by asterisks) at the center of the upstream cis-element (underlined) or at the editing site (C). (B) Gel patterns of proteins bound to the upstream cis-element and the editing site. (Left) psbE mRNA. (Right) perB mRNA. Each mRNA was incubated for 1 h at 28°C in the editing reaction mixture and then UV-irradiated (254 nm). The mixture was treated with a mixture of RNase A, nuclease P1, and C. adamanteus venom phosphodiesterase I followed by SDS/PAGE. Protein size markers (Rainbow, Amersham Pharmacia) are shown between the two gel patterns.
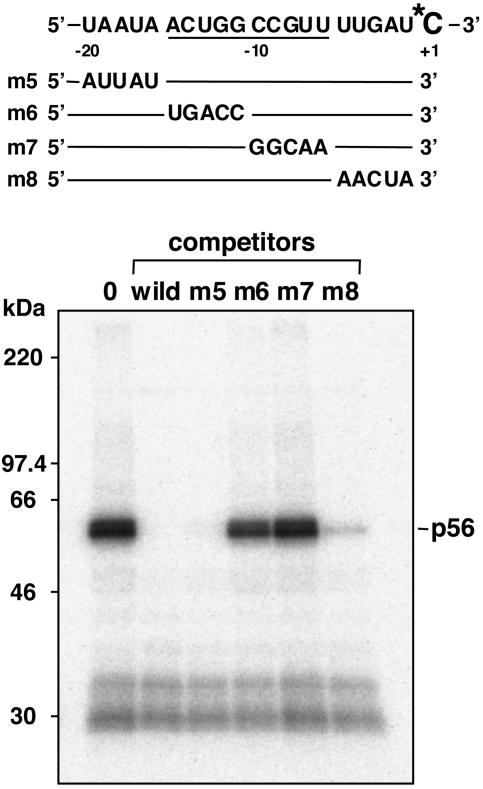
The Cross-Linking of p56 with the Editing Site Is Correlated with the Editing Activity. Our previous study showed that binding regions of site-specific factors are apart from editing sites (18). Thus, the sequence in proximal regions must be dispensable for binding of the factor to mRNAs. To confirm this idea, we defined sequences necessary for p56 binding to psbE mRNA by scanning mutated competitors. Binding was assayed by UV cross-linking with psbE mRNA substrate labeled at the C to be edited. Addition of excess amounts of competitors m5 and m8 (mutations in the –20 to –16 and in the –5 to –1 regions, respectively) specifically prevented p56 from cross-linking with the editing site (Fig. 4). Conversely, addition of competitors m6 and m7 (mutations in the –15 to –11 and in the –10 to –6 regions, respectively) showed no inhibition. These results confirmed that binding of p56 to psbE mRNA depends solely on the –15 to –6 region, and that the proximal 5-nt sequence is dispensable.
Fig. 4.
Determination of the sequence involved in p56 binding to psbE mRNA. Schematic representation of altered regions of psbE mRNAs used as competitors are shown above. One femtomole of the wild-type psbE RNA labeled at the +1 residue was used as a substrate, and 1 pmol of each competitor was added. Cross-linking assays were as described in Fig. 3. 0, without competitor.
The observation that the site-specific factors are cross-linked with the editing sites raises the possibility that these factors recognize not only the upstream cis-elements but also the editing sites and proximal regions. If so, mutations in the proximal region may affect the cross-linking of the site-specific factor with the editing site. To examine this hypothesis, we performed UV cross-linking experiments using psbE RNA substrates with 32P-label at the editing site and with a mutation of the 5′ neighboring residue (–1). The extent of p56 cross-linking with the substrate possessing A at –1 was strongly reduced relative to the intrinsic U (Fig. 5A). Moreover, mutations to G and C abolished this cross-linking. Conversely, mutation to G at the 3′ neighboring residue (+2) exerted no effect or slightly enhanced cross-linking. The chloroplast RNA-binding proteins (28–33 kDa) were cross-linked to a similar extent with all substrates, suggesting that these mutations specifically affect the cross-linking of p56. Thus, we conclude that the 5′ neighboring residue (–1) is involved in the interaction of p56 with the editing site (+1).
Fig. 5.
Effect of mutations in neighboring residues of the editing site in psbE mRNAs on the cross-linking of p56 with the editing site or the upstream cis-element. UV cross-linking experiments were performed by using psbE mRNAs with mutations in 5′ and 3′ neighboring residues (–1 and +2). (A) Assays with mRNAs labeled at the editing site (+1). (B) Assays with mRNAs labeled at the center of the upstream cis-element (–10).
Most importantly, the extent of p56 cross-linking correlated roughly with the editing activity of each RNA substrate (Fig. 5A vs. Figs. 1 Left and 2 Left). Mutation to G or C at –1 abolished both editing activity and cross-linking of p56 (Fig. 2 Left, lanes G and C vs. Fig. 5A, lanes G and C). Mutation to A at –1 did not reduce editing so much despite severe reduction in cross-linking (Fig. 2 Left, lane A vs. Fig. 5A, lane A). This is probably due to different thresholds of interaction for UV cross-linking and editing reaction. Conversely, mutation to G at +2 slightly enhanced both (Fig. 1B Left, lane +2 vs. Fig. 5A, lane +2). Taken together, these observations suggest that direct interaction of p56 with the editing site is a prerequisite for psbE mRNA editing.
Because the binding of p56 to psbE mRNA depends on the upstream cis-element (–15 to –6) but not on the proximal region (–5 to –1), it is expected that p56 binds to the upstream cis-element despite mutation in the proximal region. To examine this possibility, we carried out UV cross-linking experiments with the same series of mutated mRNAs, except they contained 32P-label at the –10 position. As shown in Fig. 5B, p56 was cross-linked with the –10 residue of all the mutated mRNAs. Even with the mRNAs that possess G or C at –1, cross-linking of p56 was observed at a level similar to that of wild-type mRNAs (U at –1), although they were neither edited nor cross-linked with the editing site. This result confirms that the binding of p56 to the upstream cis-element is unrelated to its downstream sequence.
Discussion
The results presented here provide evidence that p56, the site-specific trans-acting factor for psbE mRNA editing, interacts directly with the editing site in a sequence-specific manner. We reported that the binding regions of site-specific factors are distinct from the editing site (18). Consistent with that observation, here we have demonstrated that p56 can bind the upstream cis-element without interacting with the editing site. However, mutation of the nucleotides flanking the psbE editing site demonstrates a correlation between the extent of interaction with the editing site and the editing activity. Our results therefore suggest that direct interaction of p56 with the editing site is a prerequisite for psbE mRNA editing.
Nearest neighboring nucleotides were reported to be critical for efficient editing of psbL and ndhB (site V) mRNA (13, 14). Consistent with this observation, we showed that some mutations at these positions abolished the editing of psbE and petB mRNAs. However, scanning mutation analysis revealed that critical residues are not confined to particular positions but are scattered around the editing sites. It is likely that all nucleotides required for the editing reaction in the proximal region are recognized by the site-specific factor, because the sequence requirement in the region is site-specific.
In apoB mRNA editing, a well characterized example of C-to-U conversion in mammals, an essential cis-element (mooring sequence) is also distinct from the editing site, located 4 nucleotides downstream (21). The sequence specificity in the proximal region is relatively relaxed (22). Instead, distal ciselements in addition to the mooring sequence may contribute to the site specificity (23).
Editing sites possessing G at the 5′ neighboring position (–1) are not found in tobacco chloroplast transcripts (12, 17), and only a limited number are found in Arabidopsis mitochondrial mRNAs (16). Consistent with these observations, conversion to G abolished both psbE and petB mRNA editing in vitro. Similarly, the conversion of 5′ neighboring residues to G in chloroplast ndhB (site V) mRNA and mitochondrial cox II (C259) mRNA were reported to inhibit editing in vivo (13, 24). A guanosine at the 5′ neighboring position is inhibitory for most editing sites studied; therefore, the strong bias against G may be due to mechanical constraints.
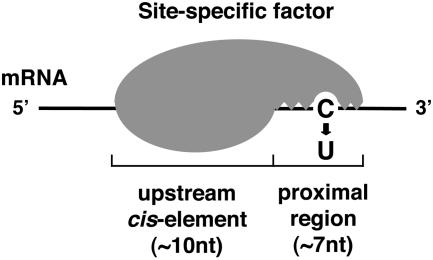
Our results suggest that p56 recognizes the psbE editing site by two separable steps (Fig. 6). In the first step, p56 binds to the upstream cis-element, which brings its part to contact with the proximal region. If the sequence in this region is compatible enough to establish a close interaction with the C residue to be edited (enough for UV cross-linking), the editing reaction proceeds. It is interesting that p56 binds psbE mRNA in different strengths. Binding to the upstream cis-element is strong, enough for p56 to hold onto RNA, whereas binding to the editing site/proximal region is so weak that it has almost no contribution to overall binding (see Fig. 4). Our model suggests that binding of the site-specific factor to the upstream cis-element acts as a scaffold for the interaction with its downstream region.
Fig. 6.
Model of chloroplast RNA editing. First, a site-specific trans-acting factor binds an upstream cis-element in a sequence-specific manner. Based on this binding, the factor then interacts with an editing site also in a sequence-specific manner. This interaction is most likely necessary for C-to-U conversion, possibly by allowing contact of the putative catalytic domain of the site-specific factor with the target C residue.
Why a direct interaction of p56 with the editing site is required for the editing reaction is unknown. The simplest hypothesis would be that p56 itself carries out C-to-U conversion (deamination of C residues); therefore, the interaction represents a contact of its deamination domain with the target C residue to be edited. This model is supported by the observation that no additional protein was specifically cross-linked with the editing site. Although the possibility that the deaminase is distinct from p56 cannot be ruled out, the RNA-binding features of p56 are reminiscent of the holoenzyme involved in apoB mRNA editing, in which a cytidine deaminase [apoB mRNA editing enzyme catalytic polypeptide 1 (APOBEC-1)] and a sequence-specific RNA-binding protein [APOBEC-1 complementation factor (ACF)] are the minimal complex for editing in vitro (25). Recognition of the apoB editing site is directed by ACF through binding to the mooring sequence (25, 26). APOBEC-1 also possesses a weak RNA-binding activity, which is a prerequisite for editing (27). The binding of p56 to the upstream cis-element seems to correspond to the binding of ACF to the mooring sequence, and the weak binding of p56 to the editing site/proximal region to that of APOBEC-1. In this respect, p56 would be expected to possess both RNA-binding forms characteristic of the holoenzyme of apoB mRNA editing. We therefore favor the hypothesis that both the site recognition and the cytidine deamination of psbE mRNA editing are carried out by p56 alone.
The most prominent feature of chloroplast RNA editing is that distinct proteins recognize each site (18, 28). However, it cannot be ruled out that the same trans-factors recognize several editing sites and that binding depends on cis-elements (29). Here we provide evidence of direct interaction of the site-specific factor with the editing site only for psbE mRNA editing. The site-specific factor of petB mRNA editing is also cross-linked with both the upstream cis-element and the editing site (see Fig. 3). Previous cis-analysis of other editing sites has shown that locations of cis-elements are invariable, occurring at similar upstream positions (13–15). Thus, our model is likely to be applicable to most, if not all, site-specific trans-acting factors in higher plant chloroplasts.
Acknowledgments
We thank T. Hirose, T. Wakasugi, M. Matsuo, and K. Masuyama for continuous discussions and suggestions. This work was supported in part by a Grant-in-Aid for Scientific Research in Priority Areas C (no. 13201001) from the Ministry of Education, Science, Sports and Culture of Japan.
References
- 1.Smith, H. C., Gott, J. M. & Hanson, M. R. (1997) RNA 3, 1105–1123. [PMC free article] [PubMed] [Google Scholar]
- 2.Maier, R. M., Zeltz, P., Kössel, H., Bonnard, G., Gualberto, J. M. & Grienenberger, J. M. (1996) Plant Mol. Biol. 32, 343–365. [DOI] [PubMed] [Google Scholar]
- 3.Marchfelder, A., Binder, S., Brennicke, A. & Knoop, V. (1998) in Modification and Editing of RNA, eds. Grosjean, H. & Benne, R. (Am. Soc. Microbiol., Washington, DC), pp. 307–323.
- 4.Mulligan, R. M. & Maliga, P. (1998) in A Look Beyond Transcription, eds. Bailey-Serres, J. & Gallie, D. R. (Am. Soc. Plant Physiol., Rockville, MD), pp. 153–161.
- 5.Sugiura, M., Hirose, T. & Sugita, M. (1998) Annu. Rev. Genet. 32, 437–459. [DOI] [PubMed] [Google Scholar]
- 6.Bock, R. (2001) in RNA Editing, ed. Bass, B. L. (Oxford Univ. Press, Oxford), pp. 38–60.
- 7.Tsudzuki, T., Wakasugi, T. & Sugiura, M. (2001) J. Mol. Evol. 53, 327–332. [DOI] [PubMed] [Google Scholar]
- 8.Bock, R., Kössel, H. & Maliga, P. (1994) EMBO J. 13, 4623–4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zito, F., Kuras, R., Choquet, Y., Kössel, H. & Wollman, F.-A. (1997) Plant Mol. Biol. 33, 79–86. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki, Y., Kozaki, A., Ohmori, A., Iguchi, H. and Nagano, Y. (2001) J. Biol. Chem. 276, 3937–3940. [DOI] [PubMed] [Google Scholar]
- 11.Hirose, T., Fan, H., Suzuki, J. Y., Wakasugi, T., Tsudzuki, T., Kössel, H. & Sugiura, M. (1996) Plant Mol. Biol. 30, 667–672. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki, T., Yukawa, Y., Miyamoto, T., Obokata, J. & Sugiura, M. (2003) Mol. Biol. Evol. 20, 1028–1035. [DOI] [PubMed] [Google Scholar]
- 13.Bock, R., Hermann, M. & Kössel, H. (1996) EMBO J. 15, 5052–5059. [PMC free article] [PubMed] [Google Scholar]
- 14.Chaudhuri, S. & Maliga, P. (1996) EMBO J. 15, 5958–5964. [PMC free article] [PubMed] [Google Scholar]
- 15.Reed, M. L., Peeters, N. M. & Hanson, M. R. (2001) Nucleic Acids Res. 29, 1507–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giege, P. & Brennicke, A. (1999) Proc. Natl. Acad. Sci. USA 96, 15324–15329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirose, T., Kusumegi, T., Tsudzuki, T. & Sugiura, M. (1999) Mol. Gen. Genet. 262, 462–467. [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto, T., Obokata, J. & Sugiura, M. (2002) Mol. Cell. Biol. 22, 6726–6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shinozaki, K., Ohme, M., Tanaka, M., Wakasugi, T., Hayashida, N., Matsubayashi, T., Zaita, N., Chunwongse, J., Obokata, J., Yamaguchi-Shinozaki, K., et al. (1986) EMBO J. 5, 2043–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, Y. & Sugiura, M. (1990) EMBO J. 9, 3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shar, R. R., Knott, T. J., Legros, J. E., Navaratnam, N., Greeve, J. C. & Scott, J. (1991) J. Biol. Chem. 266, 16301–16304. [PubMed] [Google Scholar]
- 22.Chen, S.-H., Li, X., Liao, W. S. L., Wu, J. H. & Chan, L. (1990) J. Biol. Chem. 265, 6811–6816. [PubMed] [Google Scholar]
- 23.Driscoll, D. M. & Innerarity, T. L. (2001) in RNA Editing, ed. Bass, B. L. (Oxford Univ. Press, Oxford), pp. 61–76.
- 24.Farre, J.-C., Leon, G., Jordana, X. & Araya, A. (2001) Mol. Cell. Biol. 21, 6731–6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta, A., Kinter, M. T., Sherman, N. E. & Driscoll, D. M. (2000) Mol. Cell. Biol. 20, 1846–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta, A. & Driscoll, D. M. (1998) Mol. Cell. Biol. 18, 4426–4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navaratnam, N., Bhattacharya, S., Fujino, T., Patel, D., Jarmuz, A. L. & Scott, J. (1995) Cell 81, 187–195. [DOI] [PubMed] [Google Scholar]
- 28.Hirose, T. & Sugiura, M. (2001) EMBO J. 20, 1144–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chateigner-Boutin, A.-L. & Hanson, M. R. (2002) Mol. Cell. Biol. 22, 8448–8456. [DOI] [PMC free article] [PubMed] [Google Scholar]