Abstract
During premetamorphic stages, Xenopus laevis tadpoles expressing either a dominant-negative thyroid hormone (TH) receptor or a type-III iodothyronine deiodinase transgene in the nervous system have reduced TH-induced proliferation in the spinal cord and produce fewer hindlimb-innervating motorneurons. During prometamorphic stages, innervation of the hindlimbs is reduced, and few functional neuromuscular connections are formed. By metamorphic climax, limb movement is impaired, ranging from uncoordinated leg swimming to complete quadriplegia. This phenotype is due to transgene action in the tadpole spinal cord. The requirement of TH for neurogenesis during premetamorphosis is the earliest TH-regulated process reported to date in the sequence of metamorphic changes in anurans. The muscle formed during limb growth was previously shown to be a direct target of TH control. Here, we show that the same is true of the development of spinal cord cells that innervate the limbs.
During amphibian metamorphosis, thyroid hormone (TH) controls remodeling of the nervous system (1). TH removes neural circuits that are larval-specific, for example, the spinal cord Rohon–Beard cells (2). TH also promotes the development of cells that participate in frog-specific circuits such as within the olfactory (3), visual (4–7), and auditory (8) systems. In the retina TH acts, in part, by regulating the proliferation of neural progenitors. This action of TH appears to be cell-autonomous, because progenitor cells found in the dorsal retina, but not those found in the ventral retina, are protected from TH action by the precise expression of type-III iodothyronine deiodinase (D3), an enzyme that inactivates TH (7).
The spinal cord of anurans is remodeled during metamorphosis. The most dramatic change is the development of neural circuits within the brachial and lumbar spinal cord that control the movement of arms and legs, respectively. The spinal cord cell type that has been most studied during metamorphosis is the limb motorneuron, which is the only spinal cord cell that directly innervates limbs. Limb motorneuron number reach a peak as the limb bud emerges at the beginning of premetamorphosis and then decreases dramatically soon after the limb is innervated (9, 10). To date, it has been unclear whether any change observed in limb motorneurons is due to the action of TH within spinal cord cells. Alternatively, changes in limb motorneurons and other spinal cord cells might be a consequence of the growth and development of limbs, which is a known TH-dependent process.
In the current study, we show that inhibiting TH action in the spinal cord by means of neural-specific transgenes leads to a defect in TH-induced neurogenesis, and a decreased generation of lumbar motorneurons that innervate the hindlimb. This defect in neurogenesis is followed by a reduction in hindlimb innervation and a deficiency in functional connections between nerve and muscle within the hindlimb. In terms of hindlimb movement, the phenotypes in these transgenic animals range in severity from uncoordinated leg swimming to complete limb paralysis. These data demonstrate a direct role for TH in regulating spinal cord neurogenesis and function.
Materials and Methods
Plasmids, Animals, Transgenesis, and in Situ Hybridization. Two neural promoters were used in transgenes. The first, the Xenopus laevis neuro-β-tubulin (NβT) promoter drives neural expression (11) in a pattern indistinguishable from the NβT gene (12). The construction and activity of NβT-containing plasmids driving either the dominant-negative form of the TH receptor (TRDN) fused to GFP (NβT:GFP-TRDN) or D3 fused to GFP (NβT:GFP-D3) have been described (7). The second promoter controls expression of the rat tubulin α-1 gene (rTα1) (13). In X. laevis-transgenic animals, this promoter expresses throughout the nervous system, but also in the developing limbs (11). The constructs Tα1:GFP-TRDN and Tα1:GFP-D3 were made by replacing the NβT promoter with the rTα1 promoter, which was obtained from pCS2+[rTα1]GFPtag (11). The construct NβT:TRDN was made by cloning a PCR-amplified TRα cDNA lacking the last 12 amino acids behind the NβT promoter. Transgenesis was performed by restriction enzyme-mediated integration and nuclear transplantation (14). In the case of GFP-tagged transgenes, animals were killed with 1:1,000 (wt/vol) 3-aminobenzoic acid ethyl ester (MESAB) and screened for brain fluorescence between days 4 and 7 by using a Leica (Deerfield, IL) MZFLIII fluorescence stereomicroscope. Two-week-old animals that had been fed during the second week were induced with 10 nM 3,5,3′ triiodothyronine (T3) for 7 days. Animals used for in situ hybridization experiments were killed by MESAB overdose. Brains and spinal cords were dissected, fixed, and processed for in situ hybridization analyses by using probes and methods described (15).
Grafting. To produce animals that express transgenes in either the brain only or the spinal cord only, potentially transgenic animals were cut in half and grafted onto complimentary halves of control embryos. Two embryos at Nieuwkoop and Faber (NF) stages 20–23 were placed on top of Permoplast modeling clay (American Art Clay, Indianapolis) that was submerged in filtered 0.5× MMR (50 mM NaCl/1 mM KCl/0.5 mM MgCl2/1 mM CaCl2/2.5 mM Hepes, pH 7.6). Embryos were cut in half with a no. 11 surgical blade (Becton Dickinson). Transgenic and control halves were placed together in grooves in the clay and pressed onto each other by applying pressure through the modeling clay. Animals were allowed to heal for >1 h and were then transferred into 0.1× MMR with 50 μg/ml gentamycin that had been filtered through a 0.2-μm filter. The day after grafting, the transgenic status of chimeras was determined by GFP fluorescence in the brain. Animals that developed normally were raised to metamorphosis.
Labeling of Nerves and Neuromuscular Junctions. Animals were killed by MESAB overdose and their hindlimbs were dissected, skinned, fixed with 4% paraformaldehyde (PFA), and stained with 3A10 (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), a monoclonal antibody that recognizes a neurofilament-associated protein, and Alexa 488-labeled α-bungarotoxin (Molecular Probes), followed by a Alexa 568-labeled goat anti-mouse secondary antibody (Molecular Probes), by using conventional antibody protocols (16). NF stage 54 limbs were imaged from the dorsal side with a Leica MZFLIII fluorescence stereomicroscope equipped with a Roeper Scientific (Tucson, AZ) Coolsnap FX camera run by iplab spectrum 3.6 (Scanalytics, Fairfax, VA) imaging software. This software was used to obtain diameter measurements of tibial nerves by taking three measurements in three control and four transgenic right hindlimbs at NF stage 54 (shown in Fig. 5C). Older limbs were imaged with a Leica TCS NT confocal microscope. Stacks of 10 images, 5 μm apart, were used to image the limbs from ventral views.
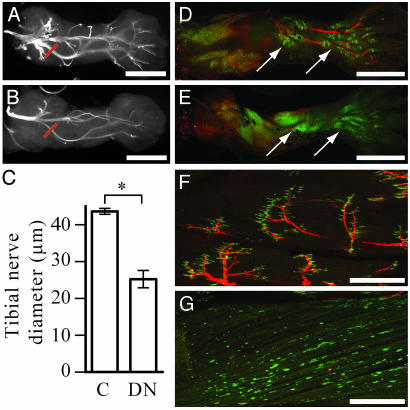
Fig. 5.
Transgenic animals develop defective neuromuscular junctions. Dorsal view of hindlimb nerves, labeled with 3A10 antibody, at NF stage 54 in control (A) and NβT:GFP-TRDN-transgenic (B) animals. (C) The diameter of tibial nerves, at positions marked in red in A and B, in control and NβT:GFP-TRDN animals at NF stage 54. Values are statistically different (*, P = 0.0014). Nerves and muscle synapses at NF stage 56 in control (D) and NβT:GFP-TRDN-transgenic (E) animals seen in a ventral view of the hindlimb. Arrows point to calf and foot muscles. Nerves and muscle synapses in the tibialis anterior muscle at NF Stage 60 in control (F) and NβT:GFP-TRDN-transgenic (G) animals. In D–G, the nerves are labeled with 3A10 antibody (red), and the AChRs are labeled with α-bungarotoxin (green). (Scale bars, 500 μmin A and B and D and E; 250 μm in F–G.)
Spinal Cord Antibody Labeling and Quantification. Spinal cords were dissected and fixed with 4% PFA. To not miss the lumbar region in young tadpoles that do not yet have a prominent lumbar bulge, a segment of spinal cord longer than the size of the brain was taken. For counts of retinaldehyde dehydrogenase-2 (RALDH-2)-positive cells between NF stages 54 and 66, dissected spinal cords were sectioned in a cryostat at 10-μm thickness along the entire length of the spinal cord. A rabbit polyclonal antibody (17) that recognizes RALDH-2 (gift from P. McCaffery, Eunice Kennedy Shriver Center, Waltham, MA) and an Alexa 568 goat anti-rabbit secondary antibody were used. Antibody stained sections were counterstained with 1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI; Sigma). Counts of RALDH-2 cells were obtained in every fifth section. The lumbar area was defined as the caudal peak of RALDH-2-positive cells flanked by at least two sections without any positive cells. The absolute number of motorneurons in the lumbar spinal cord was determined by plotting the number of RALDH-2-positive cells relative to section number and determining the area under the curve by using kaleidagraph software (Synergy Software, Reading, PA). In Fig. 4F, values in control animals represent the mean of three animals for NF stages 56, 58, 60, 63, and 66, respectively, and the values for control and NβT:GFP-TRDN-transgenic animals at stage 54 represent the mean of four animals in each group. The spinal cords of some 3-week-old animals used in the TH-induction study shown in Fig. 4 A–D were processed for RALDH-2 labeling as whole mounts. Different animals in the same TH-induction study were labeled as whole mounts with an antibody against phosphorylated histone H3 (PH-3) (Upstate Biotechnology, Lake Placid, NY). For PH-3 staining, the position of the lumbar spinal cord was only estimated, based on the position of the lumbar spinal cord in sibling animals stained with the RALDH-2 antibody. A third set of animals in the same TH-induction study were sectioned and processed for RALDH-2 staining in sections. Both whole-mounted and sectioned specimens were imaged with a Zeiss Axioplan compound fluorescence microscope and photographed with a Zeiss Axiocam digital camera run by Zeiss axiovision imaging software. All values were analyzed for statistical significance by using statview software (SAS Institute, Cary, NC), by performing factorial ANOVA analyses with Bonferroni–Dun posthoc set at 5% significance level.
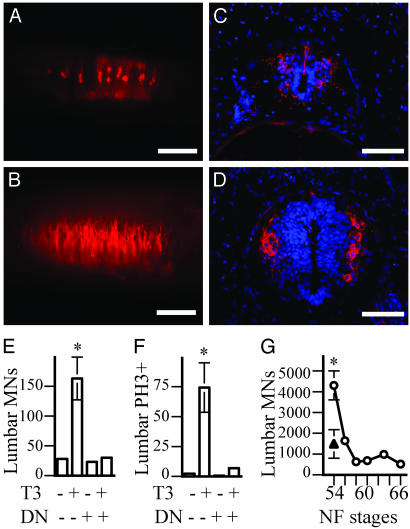
Fig. 4.
TH controls the early proliferation and production of limb motorneurons. (A–D) Lumbar spinal cords of 3-week-old control tadpoles. (A and B) Lateral view of whole mounts. (C and D) Transverse sections. (A and C) Controls. (B and D) Animals treated with 10 nM T3 during the third week. Limb motorneurons labeled with a RALDH-2 antibody are red and nuclei labeled by DAPI are blue. (Scale bars, 100 μmin A and B; 25 μmin C and D.) (E) The number of lumbar motorneurons (MNs), determined by counting RALDH-2-positive cells in whole-mount spinal cords. (F) The number of proliferating cells in the lumbar spinal cord, determined by counting PH3-positive cells in whole-mount spinal cords. The labeled groups (*) in E and F are significantly different from the other three groups (P < 0.001). (G) The number of lumbar motorneurons at various stages during metamorphosis in control animals (○), and at NF stage 54 in NβT:GFP-TRDN-transgenic animals (▴), determined by counting RALDH-2-positive cells in sections throughout the spinal cord. Values for control and NβT:GFP-TRDN-transgenic animals at stage 54 are significantly different (P = 0.005). Error bars in E–G represent the SEM.
Results
Control Tadpoles Change from Tail to Leg Swimming at the Climax of Metamorphosis. Locomotion in young X. laevis tadpoles is controlled by a spinal cord circuit that produces rhythmic tail movements (18). Before they lose their tails at the climax of metamorphosis, tadpoles acquire a second spinal cord circuit to control leg flexion and extension, their mode of locomotion as frogs. Here, we describe the onset and development of hindlimb movement and posture relative to NF stages (19). The hindlimbs, which develop during premetamorphosis, NF stages 52–56, and grow during prometamorphosis, NF stages 56–58, begin uncoordinated movements as early as NF stage 57. After this stage, tadpoles begin to hold their hindlimbs in a flexed position. The frequency of leg kicks, the strength and coordination of the kicks, and the development of a flexed leg position, become more pronounced in subsequent stages. At NF stage 59, the beginning of metamorphic climax, the tadpole still swims with its tail. However, in response to touch, NF stage 59 tadpoles extend their hindlimbs and attempt several uncoordinated kicks before assuming again a flexed hindlimb posture. One or two days later, when tadpoles reach NF stage 60, they begin using coordinated kicking of their hind legs as a primary means of locomotion. By NF stage 61, all control tadpoles have converted entirely to leg swimming. Typically, tail resorption will be complete at NF stage 66 approximately a week after this conversion from tail to leg swimming.
Interference with TH Action in the Nervous System Causes Limb Paralysis. TH action in X. laevis tadpoles can be inhibited by transgenes expressing either a TRDN, or D3, an enzyme that degrades TH (7, 16, 20–22). When the promoter from the X. laevis NβT gene, which drives widespread expression throughout the nervous system (11), is used to express these TH-interfering transgenes, tadpoles develop a range of neurological phenotypes. The most severely affected animals have a hindlimb posture defect detectable as early as NF stage 56. These animals will never flex their hindlimbs nor show any leg movement. By the time these transgenic tadpoles reach metamorphic climax, NF stage 59–66, many have their hindlimbs extended and crossed (Fig. 1B). The forelimbs of these animals point posteriorly and never move (Fig. 1B). Externally, only the limb posture and movement defects distinguish these transgenic tadpoles from control siblings (Fig. 1 A). However, dissection shows that in the most severely affected animals, the brain and spinal cord are smaller than in stage-matched controls (Fig. 1 C and D). The movement defect is specific to limbs, because even tadpoles with severe quadriplegic phenotypes continue to swim with their tails. (See Movies 1 and 2, which are published as supporting information on the PNAS web site.) Affected animals respond to sharp stimuli applied to their paralyzed toes in a tail-swimming escape response similar to wild-type animals, showing that hindlimbs have some degree of sensory functionality. Tadpoles with the severe transgene-induced phenotype arrest and die at the climax of metamorphosis, having resorbed gills but not tails (Fig. 1). Transgenic animals that are less severely affected have near normal brain size and leg posture but use their hindlimbs in an uncoordinated manner at a time when control animals have converted to leg swimming. Many of these tadpoles complete metamorphosis and ultimately leg swim normally. A continuum of phenotypic severities is observed in the transgenic animals; however, for the purpose of comparing different transgenes, phenotypes have been classified into severe and mild groups (Table 1). To a first approximation, the phenotype correlates with the expression level of the transgenes, as judged by the intensity of the GFP tag in the brains of transgenic animals (data not shown).
Fig. 1.
Transgenes that inhibit TH action in the nervous system produce quadriplegic tadpoles at the climax of metamorphosis. NF stage 62 control (A) and NβT:GFP-TRDN-transgenic (B) animals. Dissected brain and spinal cord of NF stage 60 control (C) and NβT:GFP-TRDN-transgenic (D) animals. (Scale bar for A–D, 1 cm.)
Table 1. Summary of phenotypes.
| Transgenes | Severe, % | Mild, % | Normal, % | n |
|---|---|---|---|---|
| NβT:GFP-TRDN | 56 | 32 | 12 | 123 |
| NβT:GFP-D3 | 35 | 53 | 12 | 57 |
| NβT:GFP-D3 (F1) | 0 | 60 | 40 | 10 |
| NβT:TRDN | 50 | 25 | 25 | 12 |
| Tα1:GFP-TRDN | 21 | 46 | 33 | 24 |
| Tα1:GFP-D3 | 0 | 22 | 78 | 18 |
Transgenes are described in Materials and Methods.
This limb movement phenotype has been observed only in tadpoles that express a transgene expected to interfere with TH function in the nervous system (Table 1). The construct used in most experiments is NβT:GFP-TRDN, in which the NβT promoter drives expression of a TRDN tagged with GFP. The GFP tag on this transgene does not affect its dominant-negative activity because untagged TRDN transgenes produce a similar range of phenotypes. Similar phenotypes are observed when the NβT promoter drives a GFP-tagged version of D3, an enzyme that degrades and inactivates TH. When the Tα1 gene promoter is used to drive either TRDN or D3 transgenes, a similar although generally weaker range of phenotypes is observed. This promoter also drives expression in the developing limbs (11), but the neural expression is generally weaker than that observed by using the NβT promoter (data not shown). Several animals with weaker phenotypes have been raised and bred, producing progeny that develop similar weak phenotypes. The progeny of one such animal is recorded in Table 1.
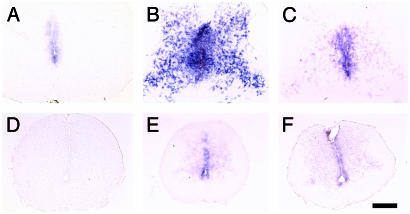
To test whether the transgenes inhibit TH-regulated gene expression in the nervous system, three TH-up-regulated genes were analyzed by in situ hybridization in the spinal cord and brain. These genes have reduced expression in the spinal cords (Fig. 2) and brains of NβT:GFP-TRDN- and NβT:GFP-D3-paralyzed animals, although the degree of inhibition varies for different genes in the same spinal cord. A gene regulated by TH in progenitors cells, TH/bZIP (Unigene Xl. 354; called gene 9 in ref. 23), is completely inhibited in some transgenic animals (Fig. 2 A and D). A second gene that is up-regulated by TH in both progenitor cells and differentiated cells, a gene with no ORF (Unigene Xl. 355; called gene 12 in ref. 23) is greatly inhibited in transgenic animals (Fig. 2 B and E). A third gene, the SP1-like gene xBTEB (Unigene Xl. 347; called gene 1 in ref. 23), which also is up-regulated by TH in both progenitors and differentiated cells, is only partially inhibited in transgenic animals (Fig. 2 C and F). The TH-dependent expression of these genes in the brain is similarly inhibited by the transgenes (data not shown).
Fig. 2.
Transgenes inhibit expression of TH-inducible genes in the spinal cord. In situ hybridization of cross-sections of the lumbar spinal cord at NF stage 62. TH/bZIP (A and D), gene 12 (B and E), xBTEB (C and F), control (A–C), and NβT:GFP-TRDN-transgenic (D–F) animals. (Scale bar, 200 μm.)
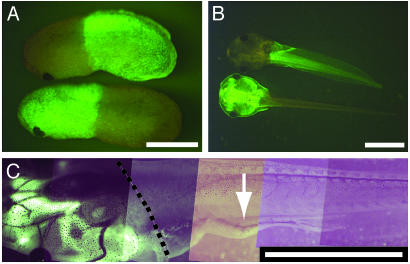
The Paralysis Phenotype Is Caused by Transgene Action in the Spinal Cord. To confirm that the paralysis is caused by transgene action within the spinal cord, grafting experiments between transgenic and nontransgenic animals were performed. X. laevis embryos can be cut in half and crossgrafted, and many of these animals develop normally (Fig. 3 A and B). Grafting experiments between NβT:GFP-TRDN-transgenic and either nontransgenic animals or animals expressing control transgenes, such as a cytomegalovirus (CMV) promoter driving GFP, demonstrate that TH acts directly on the spinal cord (Fig. 3 C and Table 2). Among the grafted animals that were raised to metamorphic climax, three of five animals with expression of NβT:GFP-TRDN in the spinal cord developed impaired hindlimb movement. None of five animals with expression of the same transgene in the brain and none of the eight chimeric animals with no transgene expression in either brain or spinal cord developed any hindlimb movement deficits.
Fig. 3.
Transgene expression only in the spinal cord affects hindlimb movement. (A) A pair of crossgrafted animals, half of which were derived from a CMV:GFP-expressing transgenic animal, at NF stage 25. (B) The same pair of crossgrafted animals at NF stage 46. (C) A limb-paralyzed animal with CMV:GFP expression in the head, and NβT:GFP-TRDN expression in the body, at NF stage 59. Arrow points to paralyzed hindlimb. The dashed line delimits the boundary of the graft as determined by the boundary of skin expression of the CMV:GFP transgene. (Scale bars, 100 μm in A and B; 1 mm in C.)
Table 2. Summary of grafting results.
| Head | Body | Severe, % | Mild, % | Normal, % | n |
|---|---|---|---|---|---|
| - | + | 20 | 40 | 40 | 5 |
| + | - | 0 | 0 | 100 | 5 |
| - | - | 0 | 0 | 100 | 8 |
+ and - represent whether the NβT:GFP-TRDN transgene was expressed in either the anterior (head) or posterior (body) half of the animal. See Fig. 3.
Transgenes Inhibit TH-Induced Proliferation and the Generation of Limb Motorneurons in the Lumbar Spinal Cord. Limb-innervating motorneurons in vertebrates express RALDH-2 (24), an enzyme that synthesizes retinoic acid (25). An antibody (17) that recognizes RALDH-2 in multiple species also labels X. laevis limb motorneurons (Fig. 4). In animals that have well differentiated functional limbs, the antibody labels large cells with characteristic motorneuron morphology in the ventral horn of the brachial and lumbar, but not the thoracic spinal cord (data not shown). Previous studies (9, 10) that identified limb motorneurons only by morphological criteria, large cells in the ventral horn with prominent nucleoli, were only able to detect these cells as early as NF stage 52. However, the RALDH-2 antibody identifies these cells much earlier in development. Starting at ≈3 days postfertilization, two small clusters of RALDH-2-positive cells with motorneuron morphology are found within the spinal cord at positions likely to be the brachial and lumbar spinal cord (data not shown). The number of RALDH-2 cells is small during the early larval period, before NF stage 50, but increases dramatically after treatment of tadpoles with T3, the more active form of TH (Fig. 4 A–E). This TH-induced increase in limb motorneurons can be blocked by TRDN transgenes (Fig. 4E). Sections through the lumbar spinal cord reveal that TH produces a large increase in the total number of nuclei in the spinal cord, as well as an increase in the number of RALDH-2-positive cells (Fig. 4 C and D). These effects of TH in the spinal cord are mediated mainly at the level of proliferation, because TH increases the number of mitotic cells (Fig. 4F and data not shown), but does not affect the number of cells that die (data not shown). Counts of RALDH-2-positive cells in sections throughout the spinal cord during NF stages 54–66 (Fig. 4G) are in good accordance with previous studies that identified limb motorneuron number by morphological criteria (9, 10). Such counts of RALDH-2-positive cells also show that NβT:GFP-TRDN-transgenic animals have significant reductions in the number of limb motorneurons at NF stage 54, a stage when the limb is actively becoming innervated (P = 0.005; Fig. 4G).
Transgenic Animals Have Aberrant Hindlimb Innervation. Labeling nerves in limbs with 3A10, an antibody that recognizes a neurofilament-associated protein, shows that transgenic animals have abnormal innervation of their hindlimbs (Fig. 5). At NF stage 54, the branching pattern of nerves within the limb appears normal (Fig. 5 A and B), but the thickness of nerves is reduced (P = 0.0014; Fig. 5C). The formation of functional synapses between motorneurons and muscle was assayed by the clustering of acetylcholine receptors (AChRs) on muscle cells as revealed by the use of fluorescently labeled α-bungarotoxin. Labeling by α-bungarotoxin is first seen in hindlimbs as punctate dispersed spots at NF stage 54 (data not shown). In control limbs, clustering of AChRs at the ends of nerves in the hindlimb begins at NF stage 56 (Fig. 5D). This clustering is most evident in the distal ventral muscles of the calf and foot. During this initial phase of synapse formation, clustering of AChRs in some NβT:GFP-TRDN-transgenic animals is reduced or delayed (Fig. 5E). By NF stage 56, these animals have very few nerves detectable by the 3A10 antibody. Their AChRs are expressed at high levels on muscle surfaces but fail to cluster. At the climax of metamorphosis, control tadpoles have large AChR clusters exclusively at the end of every nerve (Fig. 5F). In severely paralyzed transgenic animals at metamorphic climax, very few nerves innervate muscle, dispersed AChR staining is found on all muscle surfaces, and irregular clusters of AChRs are found, but not at the end of nerves (Fig. 5G). These results demonstrate that the NβT:GFP-TRDN transgenes interfere with the formation of limb-innervating nerves and the ability of those nerves to form functional connections with muscle.
Discussion
Inhibiting TH Function Perturbs the Development of the Spinal Cord. Interfering with TH function in the spinal cord produces phenotypes that range in severity from a delay in the onset of leg swimming to complete quadriplegia. This range of phenotypes correlates with the level of transgene expression. Yet, even in animals with the highest level of transgene expression and the most severe phenotypes, TH function is not inhibited completely, because some TH-regulated genes, whose mRNA is absent in the nervous systems of tadpoles raised in the goitrogen methimazole, are still expressed at low levels in these animals (Fig. 2). The fact that the same range of neural phenotypes is obtained by transgenic neural expression of D3 confirms that the TRDN transgene functions by interfering with TH signaling rather than indirectly by competing with another receptor that uses the same heterodimeric partner, the retinoic acid X receptor.
Because TH affects many aspects of neural development and the transgenes are expressed widely within the nervous system, experiments were performed to localize the paralytic effect of the transgenes to either brain or spinal cord. Whereas TH may regulate the development of circuits in the brain as well as the spinal cord, the limb paralysis phenotype is due to the action of TH within the spinal cord. Of the chimeras expressing TRDN transgenes in either the brain or the spinal cord, the only tadpoles that developed hindlimb movement deficits were those in which the transgenes were expressed in the spinal cord (Fig. 3 and Table 2).
Transgenic Animals Develop Defective Neuromuscular Junctions. The movement disorder seen in transgenic animals is most evident after NF stage 59, when control animals begin to use leg extension and flexion as their primary means of locomotion. At these late stages, affected animals have very few nerves that innervate hindlimb muscle. Muscle mass in these animals appears less than in control animals but all muscle groups are present. High levels of dispersed AChRs are distributed throughout the muscle cell surfaces, and irregularly shaped clusters develop at sites other than the contact point with nerve. Such irregular clustering of AChRs is known to occur in the absence of nerve contact in mammalian species as well (26, 27).
Even though the neuromuscular defects appear most severe near metamorphic climax, these defects likely result from an earlier inability to form functional contacts between nerve and muscle during premetamorphosis. At NF stage 56, when AChRs begin to cluster at nerve endings of control animals, muscle differentiation appears normal in transgenic animals, but severe defects in neuromuscular junction formation are evident. Even preceding the formation of neuromuscular synapses, at NF stage 54, the nerves that have grown into the hindlimbs are thinner than those in control tadpoles (Fig. 5 A–C). Why a reduced innervation of the limb should lead to such a complete failure of muscles to cluster AChRs is unexplained. One possibility is that the axons that enter the limb in the transgenic tadpoles are not only fewer in number but also compromised in their ability to form or keep functional synapses with muscle.
With the aid of an inducible promoter controlling the time of neural expression of the TRDN transgene (B. Das and D.D.B., unpublished work), impaired leg swimming results when the transgene is expressed during developmental windows before or after NF stage 52. Before NF stage 52 the effect of the transgenes is most likely confined to progenitor cells. After NF stage 52, the transgenes may act in the motorneurons themselves or in other differentiated cells of the spinal cord. Alternatively, the transgenes may still act in progenitor cells, because motorneurons produced after the peak of limb innervation contribute significantly to the innervation pattern observed in adult frogs (28).
TH Regulates the Generation of Limb Motorneurons. By use of a specific marker, RALDH-2, we counted limb motorneurons in young tadpoles (Fig. 4E) and during metamorphosis (Fig. 4F), and confirmed that the majority of limb motorneurons are generated between NF stages 46 and 52. Therefore, the majority of limb motorneuron generation occurs after the thyroid gland becomes functional, which is around NF stage 46, as determined by its ability to incorporate radioactive iodine (data not shown). A recent study (29) that used published counts of all spinal motorneurons in young tadpoles (30), published counts of limb motorneurons during metamorphosis (9, 10), and published measurements of TH levels (31–33), came to the same conclusion that TH likely regulates the generation of spinal cord cells in tadpoles. However, the fact that RALDH-2 cells are present in small numbers in brachial and lumbar spinal cord even during embryogenesis (data not shown) also suggests that TH may not be required for their generation. This conclusion is supported by the observation that hypophysectomized tadpoles develop large numbers of limb motorneurons (1). However, these same studies had already shown that TH produces an increase in the number of limb motorneurons (1). A recent study (29) showed that TH induces the reexpression in the tadpole spinal cord of markers first seen in the spinal cord during embryonic neurogenesis. We show here that TH can promote in young tadpoles robust proliferation and differentiation of spinal cord cells, including the lumbar limb motorneurons (Fig. 4 A–F). Moreover, we show that neural expression of TH-interfering transgenes prevents the proliferation and differentiation activities of TH (Fig. 4 E and F). This finding confirms that the action of TH in the spinal cord is mediated by the TR. More specifically, this action must be mediated by TRα, because this is the only TR isoform expressed in the spinal cord at relevant stages (data not shown). Most importantly, we show that in the absence of added TH, TH-interfering transgenes result in animals with a reduced number of lumbar limb motorneurons at the time that limb innervation begins, at NF stage 54. We suspect that they have reduced number of other spinal cord cells as well. All these data support the hypothesis that TH directly regulates the generation of spinal cord cells in tadpoles.
Growth and development of the limb bud itself does not come under the control of TH until NF stage 52, because tadpoles grown continuously in the presence of the potent goitrogen, methimazole, arrest development of their limb bud at that stage (data not shown). Thus, spinal cord progenitor cells are more sensitive to TH than even limb bud cells. A factor that enhances the sensitivity of spinal cord progenitors to TH is their high constitutive expression of a type-II iodothyronine deiodinase enzyme (34). This enzyme efficiently synthesizes the active hormone T3 locally from the circulating precursor thyroxine.
TH Regulates Multiple Aspects of Neural Development. We have concentrated in this study on the limb paralysis phenotype, but these transgenic animals have many other nervous system defects. They have reduced cell proliferation in the retina that likely results in a functional defect in binocular vision (11). It is likely that they have functional impairments in the brain as well. TH-induced proliferation occurs at the same time and at the same TH concentrations in brain and spinal cord, and NβT:GFP-TRDN transgenes inhibit TH-induced gene expression and proliferation equally in brain and spinal cord (ref. 16 and data not shown). We suspect that inhibition of one or more of these TH-dependent circuits in the brain underlie the developmental arrest and death at the climax of metamorphosis that is observed in many of the transgenic animals.
Building a Limb. The ability to direct expression of the TRDN transgene to specific cell types has enabled us to examine the role of different cells in the formation of the limb. Expressing the TRDN in muscle results in limbs that have only a deficiency in muscle (21). We concluded that limb muscle is a direct target of TH, and the loss of muscle has minimal if any effect on development of other limb cell types. In this study, we have extended these observations to the innervation of limbs. Expression of the TRDN transgene exclusively in the nervous system inhibits development of the circuitry that innervates the limb. The result is the development of paralyzed but otherwise normal limbs. Therefore, the innervation of limbs is also a direct and independent target of TH. How many additional yet independent programs under the control of TH are required to make an anuran limb, and how these separate programs are coordinated to form this single functional organ, represents an intriguing problem in organogenesis.
Supplementary Material
Acknowledgments
We thank Benjamin Remo and Rebecca Bruno for excellent technical support, and Shanthini Sockanathan, Catherine Thompson, and members of D.D.B.'s laboratory for helpful comments on the manuscript. This work was supported in part by grants from the National Institutes of Health and the G. Harold and Leila Y. Mathers Charitable Trust (to D.D.B.).
Abbreviations: TH, thyroid hormone; TR, TH receptor; TRDN, dominant-negative form of TR; D3, type-III iodothyronine deiodinase; NβT, neuro-β-tubulin; Tα1, tubulin α-1; RALDH-2, retinaldehyde dehydrogenase-2; AChR, acetylcholine receptor; NF, Nieuwkoop and Faber.
References
- 1.Kollros, J. J. (1981) in Metamorphosis: A Problem in Developmental Biology, eds. Gilbert, L. I. & Frieden, E. (Plenum, New York), pp. 445–459.
- 2.Coen, L., du Pasquier, D., Le Mevel, S., Brown, S., Tata, J., Mazabraud, A. & Demeneix, B. A. (2001) Proc. Natl. Acad. Sci. USA 98, 7869–7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fritz, A., Gorlick, D. L. & Burd, G. D. (1996) Int. J. Dev. Neurosci. 14, 931–943. [DOI] [PubMed] [Google Scholar]
- 4.Hollyfield, J. G. (1971) Dev. Biol. 24, 264–286. [DOI] [PubMed] [Google Scholar]
- 5.Beach, D. H. & Jacobson, M. (1979) J. Comp. Neurol. 183, 615–623. [DOI] [PubMed] [Google Scholar]
- 6.Beach, D. H. & Jacobson, M. (1979) J. Comp. Neurol. 183, 603–613. [DOI] [PubMed] [Google Scholar]
- 7.Marsh-Armstrong, N., Huang, H., Remo, B. F., Liu, T. T. & Brown, D. D. (1999) Neuron 24, 871–878. [DOI] [PubMed] [Google Scholar]
- 8.Boatright-Horowitz, S. S. & Simmons, A. M. (1997) Proc. Natl. Acad. Sci. USA 94, 14877–14882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes, A. (1961) J. Embryol. Exp. Morphol. 9, 269–284. [PubMed] [Google Scholar]
- 10.Prestige, M. C. (1967) J. Embryol. Exp. Morphol. 18, 359–387. [PubMed] [Google Scholar]
- 11.Marsh-Armstrong, N., Huang, H., Berry, D. L. & Brown, D. D. (1999) Proc. Natl. Acad. Sci. USA 96, 14389–14393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moody, S. A., Miller, V., Spanos, A. & Frankfurter, A. (1996) J. Comp. Neurol. 364, 219–230. [DOI] [PubMed] [Google Scholar]
- 13.Gloster, A., Wu, W., Speelman, A., Weiss, S., Causing, C., Pozniak, C., Reynolds, B., Chang, E., Toma, J. G. & Miller, F. D. (1994) J. Neurosci. 14, 7319–7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kroll, K. L. & Amaya, E. (1996) Development (Cambridge, U.K.) 122, 3173–3183. [DOI] [PubMed] [Google Scholar]
- 15.Berry, D. L., Schwartzman, R. A. & Brown, D. D. (1998) Dev. Biol. 203, 12–23. [DOI] [PubMed] [Google Scholar]
- 16.Schreiber, A. M., Das, B., Huang, H., Marsh-Armstrong, N. & Brown, D. D. (2001) Proc. Natl. Acad. Sci. USA 98, 10739–10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berggren, K., McCaffery, P., Dräger, U. & Forehand, C. J. (1999) Dev. Biol. 210, 288–304. [DOI] [PubMed] [Google Scholar]
- 18.Roberts, A., Soffe, S. R., Wolf, E. S., Yoshida, M. & Zhao, F. Y. (1998) Ann. N.Y. Acad. Sci. 860, 19–34. [DOI] [PubMed] [Google Scholar]
- 19.Nieuwkoop, P. D. & Faber, J. (1956) Normal Table of Xenopus laevis (Daudin): A Systematical and Chronological Survey of the Development from the Fertilized Egg Till the End of Metamorphosis (Elsevier/North-Holland, New York).
- 20.Huang, H., Marsh, A. N. & Brown, D. D. (1999) Proc. Natl. Acad. Sci. USA 96, 962–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das, B., Schreiber, A. M., Huang, H. & Brown, D. D. (2002) Proc. Natl. Acad. Sci. USA 99, 12230–12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreiber, A. M. & Brown, D. D. (2003) Proc. Natl. Acad. Sci. USA 100, 1769–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang, Z. & Brown, D. D. (1993) J. Biol. Chem. 268, 16270–16278. [PubMed] [Google Scholar]
- 24.Sockanathan, S. & Jessell, T. M. (1998) Cell 94, 503–514. [DOI] [PubMed] [Google Scholar]
- 25.Zhao, D., McCaffery, P., Ivins, K. J., Neve, R. L., Hogan, P., Chin, W. W. & Dräger, U. C. (1996) Eur. J. Biochem. 240, 15–22. [DOI] [PubMed] [Google Scholar]
- 26.Yang, X., Arber, S., William, C., Li, L., Tanabe, Y., Jessell, T. M., Birchmeier, C. & Burden, S. J. (2001) Neuron 30, 399–410. [DOI] [PubMed] [Google Scholar]
- 27.Lin, W., Burgess, R. W., Dominguez, B., Pfaff, S. L., Sanes, J. R. & Lee, K. F. (2001) Nature 410, 1057–1064. [DOI] [PubMed] [Google Scholar]
- 28.Clorfene, J. B. & Pollack, E. D. (1994) Brain Res. Dev. Brain Res. 79, 93–100. [DOI] [PubMed] [Google Scholar]
- 29.Schlosser, G., Koyano-Nakagawa, N. & Kintner, C. (2002) Dev. Dyn. 225, 485–498. [DOI] [PubMed] [Google Scholar]
- 30.Roberts, A., Walford, A., Soffe, S. R. & Yoshida, M. (1999) J. Comp. Neurol. 411, 472–486. [DOI] [PubMed] [Google Scholar]
- 31.Gancedo, B., Alonso-Gomez, A. L., de Pedro, N., Delgado, M. J. & Alonso-Bedate, M. (1997) Gen. Comp. Endocrinol. 107, 240–250. [DOI] [PubMed] [Google Scholar]
- 32.Leloup, J. & Buscaglia, M. (1977) C. R. Acad. Sci. Paris D. 284, 2261–2263. [Google Scholar]
- 33.Tata, J. R., Baker, B. S., Machuca, I., Rabelo, E. M. & Yamauchi, K. (1993) J. Steroid Biochem. Mol. Biol. 46, 105–119. [DOI] [PubMed] [Google Scholar]
- 34.Cai, L. & Brown, D. B. (2004) Dev. Biol., in press. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.