Abstract
The simplest null hypothesis for evolutionary time series is that the observed data follow a random walk. We examined whether aspects of Sepkoski's compilation of marine generic diversity depart from a random walk by using statistical tests from econometrics. Throughout most of the Phanerozoic, the random-walk null hypothesis is not rejected for marine diversity, accumulated origination or accumulated extinction, suggesting that either these variables were correlated with environmental variables that follow a random walk or so many mechanisms were affecting these variables, in different ways, that the resultant trends appear random. The only deviation from this pattern involves rejection of the null hypothesis for roughly the last 75 million years for the diversity and accumulated origination time series.
It is impossible to reject the null hypothesis that throughout much of the Phanerozoic [the last 540 million years (Myr)], marine diversity, accumulated origination, and accumulated extinction (Fig. 1) conform to a random-walk model. The only departure from this general pattern involves the diversity and accumulated origination time series, which do not conform to a random walk for roughly the last 50–75 Myr (Figs. 2 A and B). These deviations are probably not related to sampling issues or the pull of the recent. Instead, they may reflect real changes in the biota that occurred in the late Mesozoic and Cenozoic.
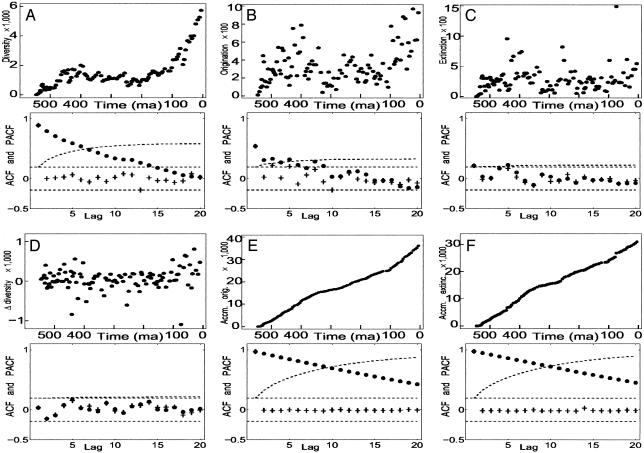
Fig. 1.
Graphs of marine data and their autocorrelation and partial autocorrelation functions. The plots shown in A, B, and C are of 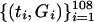 ,
,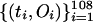 , and
, and 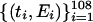 , respectively. A graph of the first difference of
, respectively. A graph of the first difference of 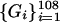 ,
, 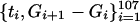 , is shown in D. The graphs of accumulated origination, (
, is shown in D. The graphs of accumulated origination, (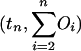 ), and accumulated extinction, (
), and accumulated extinction, (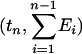 ), are shown in E and F, respectively. Autocorrelations (•) and partial autocorrelations (+) of the time series are shown below the data. Each unit of lag is one (sub)stage and averages 5.1 Myr. The total range of the lags is ≈100 Myr. In A, E, and F, none of the partial autocorrelations for lag greater than one are significantly different from zero, suggesting that first-order autoregressive models are appropriate for the corresponding time series. The autocorrelations for D, the first difference of diversity, are not significantly different from zero for any positive lag; thus, the first difference of diversity may be white noise.
), are shown in E and F, respectively. Autocorrelations (•) and partial autocorrelations (+) of the time series are shown below the data. Each unit of lag is one (sub)stage and averages 5.1 Myr. The total range of the lags is ≈100 Myr. In A, E, and F, none of the partial autocorrelations for lag greater than one are significantly different from zero, suggesting that first-order autoregressive models are appropriate for the corresponding time series. The autocorrelations for D, the first difference of diversity, are not significantly different from zero for any positive lag; thus, the first difference of diversity may be white noise.
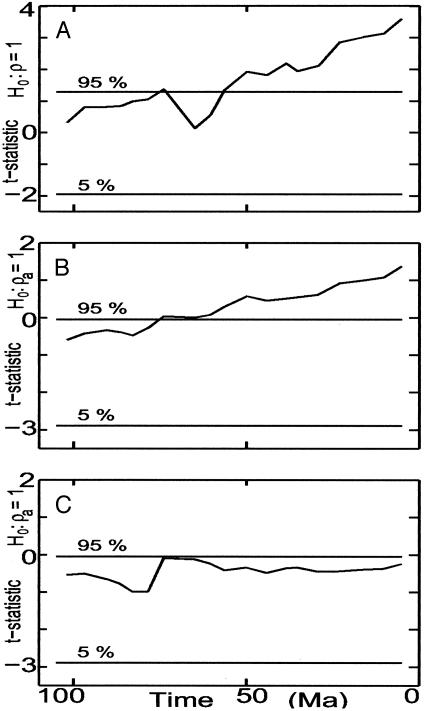
Fig. 2.
(A) Graphs of 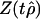 for fit of Eq. 3, yt = ρ yt–1 + εt, to windows of width 90 within total marine diversity (Fig. 1 A), and of
for fit of Eq. 3, yt = ρ yt–1 + εt, to windows of width 90 within total marine diversity (Fig. 1 A), and of 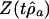 for fit of Eq. 4, yt = a0 + ρayt–1 + εt (B), to windows of width 90 within total accumulated origination (Fig. 1E) and (C) within total accumulated extinction (Fig. 1F). The time associated with each data point is the time of the 90th (sub)stage included in the window. The Lower and Upper horizontal lines mark the 5th percentile and 95th percentile thresholds for the t statistics. In A and B the t statistic exceeds the 95% threshold and the random-walk hypothesis is rejected only for windows ending in approximately the last 75 Myr. In C the random-walk hypothesis is not rejected for any window.
for fit of Eq. 4, yt = a0 + ρayt–1 + εt (B), to windows of width 90 within total accumulated origination (Fig. 1E) and (C) within total accumulated extinction (Fig. 1F). The time associated with each data point is the time of the 90th (sub)stage included in the window. The Lower and Upper horizontal lines mark the 5th percentile and 95th percentile thresholds for the t statistics. In A and B the t statistic exceeds the 95% threshold and the random-walk hypothesis is rejected only for windows ending in approximately the last 75 Myr. In C the random-walk hypothesis is not rejected for any window.
The overall result is perhaps surprising, because the analysis of Phanerozoic diversity, origination, and extinction curves has revealed a variety of interesting events, patterns, and trends. Examples include the Cambrian and Ordovician radiations, the big five mass extinctions, and a diversity plateau in the mid-Paleozoic (1–6). Moreover, coincident with recognition of these patterns, paleontologists have ascribed a series of processes to explain individual events and trends (5, 7–15). It is instructive to compare the reality of these patterns with some underlying null model of random patterns in the history of life, because apparent trends through time or even sudden shifts in time series can result just from random fluctuations (16–21). For time series data like the Phanerozoic diversity, accumulated origination and extinction curves for marine fossil taxa the random walk is the simplest possible null hypothesis (17).
Although our results may not obviate the need to invoke complex mechanisms to explain these apparent trends, caution needs to be exercised when looking for trends in these time series. Furthermore, the randomness that we see may not be intrinsic biotic randomness but might be driven by extrinsic environmental randomness. There is, for example, a significant correlation between marine fractional origination rates and estimates (22) of Phanerozoic atmospheric carbon dioxide levels (23). The CO2 time series also exhibits random walk character during 545–570 million years ago (mya) and nonrandom walk character when data from the last 70 Myr is included (see Supporting Text and Figs. 3–5, which are published as supporting information on the PNAS web site), but, with only 58 data points, this result requires further investigation.
Sepkoski and coworkers' (6, 24) compilation of marine generic diversity forms the template for the data and statistical tests to determine whether the time series depart from a random walk derive from econometrics (25–30). It has been argued that the nature of the biased fossil record can make it hard to quantify paleontological diversity at any one point in time and also track changes in diversity through time (11, 31–36). It is becoming increasingly apparent, however, that the magnitude and shape of the Phanerozoic biodiversity curve compiled by Sepkoski and coworkers (6, 10, 37) represents a reasonable approximation of animal diversity through time (38–48).
Primary Data and Analytic Methods
Graphs of the marine genera database assembled by Sepkoski and coworkers (6, 24) including generic diversity, origination, and extinction are shown in Fig. 1. The Phanerozoic is partitioned into 108 stages and substages, and recorded for each (sub)stage, [ti, ti+1] (Myr) are Gi, the total number of genera that appeared at some time during [ti, ti+1]; Oi, the number of genera that first appeared in [ti, ti+1]; and Ei, the number of genera that last appeared in [ti, ti+1]. The sequences {Gi}, {Oi), and {Ei} are related by
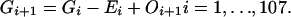 |
[1] |
Summation of this equation leads to
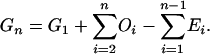 |
Autoregressive models of time series of order p are of the form
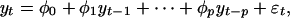 |
where φ0,..., φp are constants and 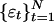 is a sequence of random variables. Given N observations,
is a sequence of random variables. Given N observations, 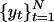 , an autoregressive model may be fit to the data by estimating the coefficients φ0,..., φp by using least-squares regression. When {εt} are independent and identically distributed random variables of mean zero and finite variance a model of the form yt = εt is said to be white noise and a model of the form Yt = Yt–1 + εt is said to be a random walk. The accumulation,
, an autoregressive model may be fit to the data by estimating the coefficients φ0,..., φp by using least-squares regression. When {εt} are independent and identically distributed random variables of mean zero and finite variance a model of the form yt = εt is said to be white noise and a model of the form Yt = Yt–1 + εt is said to be a random walk. The accumulation, 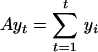 , of white noise
, of white noise 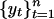 is a random walk, and the first difference, Yt – Yt–1, of a random walk
is a random walk, and the first difference, Yt – Yt–1, of a random walk 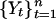 is white noise. A good test of whether a series
is white noise. A good test of whether a series 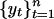 is white noise is to test whether the accumulated series
is white noise is to test whether the accumulated series 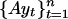 is a random walk. A model of the form
is a random walk. A model of the form
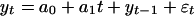 |
[2] |
is a random walk with linear drift, a0 + a1t, if a1 ≠ 0, and a random walk with constant drift if a1 = 0 and a0 ≠ 0. We model some of the macroevolutionary series as a random walk with constant drift, yt = a0 + yt–1 + εt, where a0 ≠ 0. In this case, the first difference is yt – yt–1 = a0 + εt and we call this white noise with nonzero mean (a0).
Initial investigation of time series data includes examination of plots of the data, plots of first differences of the data, and plots of the autocorrelation and the partial autocorrelation functions. Formulas for these functions and their 5% significance levels appear in Supporting Text.
Autocorrelations, 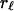 , of the macroevolutionary data shown in Fig. 1 are given in panels below the data graphs. The (sub)stages of the data average 5.1 Myr, so that lags 1–20 cover ≈5–100 Myr. The basic question about autocorrelations for a given time series is whether for some lag,
, of the macroevolutionary data shown in Fig. 1 are given in panels below the data graphs. The (sub)stages of the data average 5.1 Myr, so that lags 1–20 cover ≈5–100 Myr. The basic question about autocorrelations for a given time series is whether for some lag, 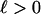 ,
, 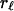 is significantly different from zero. The 5% significance levels are shown as dashed lines in Fig. 1. In Fig. 1D, none of the autocorrelations of the first difference of the diversity data are significantly different from zero; autocorrelation functions of white noise and white noise with positive mean are zero at every lag. This finding suggests that the first difference of diversity may be white noise or white noise with positive mean and that diversity may be a random walk. The autocorrelation function of accumulated origination (Fig. 1B) is significantly different from zero at lags 1, 2, 3, and 5, suggesting some structure to this data and that accumulated origination, at least for the entire Phanerozoic, may not be a random walk. The lag-5 autocorrelation of extinction (Fig. 1C) is 0.2271 and exceeds the 5% significance level of 0.2065, pointing to a previously described 26 Myr extinction periodicity (3, 24). However, the 103 summands in the autocorrelation are dominated by the single-end Cretaceous 0.0814 summand [for (sub)stages 91 and 96]; without this term the lag-5 autocorrelation would not be significantly different from zero. The summands for (sub)stages 86 and 91 and for 96 and 101 are both negative and do not support the strong five-step correlation for 91 and 96. Stage 96 is the end-Cretaceous Maastrichtian in which extinction was 5.8 SD above average extinction; this singular event combined with a 1.5 SD above average extinction in (sub)stage 91 accounts for the 0.0814 summand.
is significantly different from zero. The 5% significance levels are shown as dashed lines in Fig. 1. In Fig. 1D, none of the autocorrelations of the first difference of the diversity data are significantly different from zero; autocorrelation functions of white noise and white noise with positive mean are zero at every lag. This finding suggests that the first difference of diversity may be white noise or white noise with positive mean and that diversity may be a random walk. The autocorrelation function of accumulated origination (Fig. 1B) is significantly different from zero at lags 1, 2, 3, and 5, suggesting some structure to this data and that accumulated origination, at least for the entire Phanerozoic, may not be a random walk. The lag-5 autocorrelation of extinction (Fig. 1C) is 0.2271 and exceeds the 5% significance level of 0.2065, pointing to a previously described 26 Myr extinction periodicity (3, 24). However, the 103 summands in the autocorrelation are dominated by the single-end Cretaceous 0.0814 summand [for (sub)stages 91 and 96]; without this term the lag-5 autocorrelation would not be significantly different from zero. The summands for (sub)stages 86 and 91 and for 96 and 101 are both negative and do not support the strong five-step correlation for 91 and 96. Stage 96 is the end-Cretaceous Maastrichtian in which extinction was 5.8 SD above average extinction; this singular event combined with a 1.5 SD above average extinction in (sub)stage 91 accounts for the 0.0814 summand.
The partial autocorrelation functions for the macroevolutionary data are shown with the autocorrelation functions in Fig. 1. None of the partial autocorrelations of diversity, accumulated origination, or accumulated extinction beyond the lag-1 partial autocorrelations are significantly different from zero. This result suggests that we may model these data with first-order autoregressive models of the form in Eq. 2.
We have modeled the marine macroevolution data with equations of the form
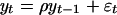 |
[3] |
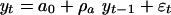 |
[4] |
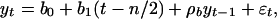 |
[5] |
where ρ, a0, ρa, b0, b1, and ρb are constants and εt is a random variable with mean zero and variance 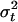 . These models were analyzed by Fuller (25) and Dickey and Fuller (26, 27) by using the assumption that {εt} is a sequence of independent normal random variables with mean zero and constant variance σ2. Phillips (28) and Phillips and Perron (29) extended that analysis using a greatly relaxed assumption about {εt} that did not require normality and allowed a weak dependence between the members of {εt} and slowly changing variance σ2t. D. A. Dickey (personal communication) has suggested that we use a model in deviation form, yt – ft = ρ(yt–1 – ft–1) + εt, where ft is either 0, a0, or b0 + b1(t – n/2) and that in this form the case ρ = 1 would lead to use of ft = b0 + b1(t – n/2). We draw the same conclusions with both forms and because the original development and most subsequent work is based on Eqs. 3–5, we report here results using Eqs. 3–5. We report results using the deviation form in Supporting Text and Fig. 6, which is published as supporting information on the PNAS web site.
. These models were analyzed by Fuller (25) and Dickey and Fuller (26, 27) by using the assumption that {εt} is a sequence of independent normal random variables with mean zero and constant variance σ2. Phillips (28) and Phillips and Perron (29) extended that analysis using a greatly relaxed assumption about {εt} that did not require normality and allowed a weak dependence between the members of {εt} and slowly changing variance σ2t. D. A. Dickey (personal communication) has suggested that we use a model in deviation form, yt – ft = ρ(yt–1 – ft–1) + εt, where ft is either 0, a0, or b0 + b1(t – n/2) and that in this form the case ρ = 1 would lead to use of ft = b0 + b1(t – n/2). We draw the same conclusions with both forms and because the original development and most subsequent work is based on Eqs. 3–5, we report here results using Eqs. 3–5. We report results using the deviation form in Supporting Text and Fig. 6, which is published as supporting information on the PNAS web site.
Parameter Estimation and Significance of Parameter Estimates
For a given set of macroevolutionary data, y1, y2,..., yn, the parameters of Eqs. 3–5 are estimated by linear regression. Equations defining these estimates are included in Supporting Text. Which of the models Eqs. 3–5 are to use revolves first around the question of whether b̂1 is significantly different from zero. If so, use Eq. 5. Otherwise, move to Eq. 4 and test whether â0 is significantly different from zero. If so, use Eq. 4. If â0 is not significantly different from zero, use Eq. 3. Regression t statistics for the conditions â0 ≠ 0, b̂0 ≠ 0, and b̂1 ≠ 0 are
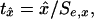 |
[6] |
where x is a0, b0, or b1 and Se,x is the regression SE of x.
Whether the original time series is a random walk revolves around the question of whether ρ = 1, ρa = 1, or ρb = 1, depending on which of Eqs. 3–5 has been selected. Regression t statistics for these hypotheses are
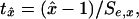 |
[7] |
where x is ρ, ρa, or ρb and Se,x is the regression SE of x.
Dickey and Fuller (26, 27) examined the t statistics in Eqs. 6 and 7 for testing the significance of the parameters estimated by the previous regressions. Assuming that the innovations {εt} are normal, independent, and of constant variance σ2, Dickey and Fuller (26) analytically found limiting distributions of the tx̂ for increasing sequence length, and they found, by Monte Carlo simulation, distributions of the tx̂ for sequences of finite length. The empirical distributions of the statistics in each case of Eq. 6 is larger than that of Student's t distribution. For time series of length 100, they found the 10% confidence thresholds of |tâ0|, |tb̂0|, and |tb̂1| to be 2.17, 2.73, and 2.38, respectively. In contrast, the Student's t distribution threshold is 1.66. Our time series are of length 90 or 108, and we use the thresholds for series of length 100 just noted to select among Eqs. 3–5. Fuller (Table 8.5.2 in ref. 25) reported distributions of the statistics given in Eq. 7, also determined by Monte Carlo modeling. We have used the fifth and ninety-fifth percentiles for sequences of length 100 from that table for testing the t statistics; the thresholds appear in Tables 1, 2, 3.
Table 1. Results of fitting Eq. 5 to marine diversity, accumulated originations, and accumulated extinctions in the linear-drift model.
| b̂0 | b̂1 | 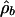 |
Z(tb̂0) | Z(tb̂1) | |
|---|---|---|---|---|---|
| Diversity | 34.48 | 1.16 | 1.0131 | 1.38 | 0.62 |
| Accumulated originations | -371.0 | -11.78 | 1.0451 | -1.83 | 0.06 |
| Accumulated extinctions | 912.4 | 13.19 | 0.9572 | 0.69 | 2.15 |
| 90% thresholds | 2.73 | 2.38 |
Table 2. Results of fitting Eq. 4 to marine diversity, accumulated originations, and accumulated extinctions in the constant-drift model.
| â0 | 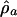 |
Z(tâ0) | 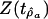 |
|
|---|---|---|---|---|
| Diversity | -2.49 | 1.0387 | -0.65 | |
| Accumulated originations | 216.92 | 1.0077 | 3.62 | 2.18 |
| Accumulated extinctions | 252.46 | 1.0025 | 5.10 | 0.80 |
| 5%, 95% thresholds | -2.89, -0.05 | |||
| 90% threshold | 2.17 |
Table 3. Results of fitting Eq. 3 to marine diversity in the no-drift model.
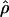 |
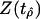 |
|
|---|---|---|
| Diversity | 1.0375 | 3.80 |
| 5%, 95% thresholds | -1.95, 1.29 |
Modifications by Phillips and Perron
Phillips (28) and Phillips and Perron (29) greatly relaxed this assumption about {εt} – normality was not required and they allowed a weak dependence between the members of {εt} and slowly changing variance σ2t. They defined a modified t statistic, Z(tx̂), which has the same limiting distribution as tx̂. For the macroevolutionary data the Z(tx̂) differ somewhat from tx̂, and because the Phillips and Peron (29) analysis allows more general innovations {εt}, we have chosen to use the modified t statistics, Z(tx̂). Because the limiting distributions are the same, the distributional thresholds found by Dickey and Fuller (26) tx̂ have been used with Z(tx̂) to evaluate the significance of x̂. The equations for computing Z(tx̂) appear in Phillips (28) and Phillips and Perron (29) and are summarized in Supporting Text.
Results
We first analyze the time series over the complete Phanerozoic. Because diversity sharply increases beginning ≈100 mya (Fig. 1A), we also analyze 90 (sub)stage windows of the series. Each window spans ≈450 Myr. Window 1 consists of (sub)stages 1–90 and precedes the sharp increase in diversity, window 2 consists of (sub)stages 2–91, and window 19 consists of (sub)stages 19–108.
The modified t statistics, Z(tx̂), for estimates of the parameters of Eqs. 3–5 fit to diversity, accumulated origination and accumulated extinction time series are shown in Tables 1, 2, 3. In Table 1, Z(tb̂1) is 0.62, 0.06, and 2.15 for diversity, accumulated origination, and accumulated extinction, respectively. Because the 90% threshold is 2.38, the null hypothesis, H0: b1 = 0, is not rejected for either of the three series, and a linear-drift model is not appropriate.
In Table 2, the values of Z(tâ0) for accumulated origination (3.62) and accumulated extinction (5.10) exceed the 90% threshold (2.17) for this t statistic, implying that the constant-drift model may be accepted for these series. The values of 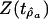 in Table 2 for accumulated origination (2.18) and accumulated extinction (0.80) series are greater than the 95% threshold of –0.05, and for both series the null hypothesis that the series is a random walk is rejected. The value of Z(tâ0) for diversity (–0.65) is in magnitude less than the 90% threshold (2.17) for this t statistic, and a linear-drift model may not be appropriate for diversity.
in Table 2 for accumulated origination (2.18) and accumulated extinction (0.80) series are greater than the 95% threshold of –0.05, and for both series the null hypothesis that the series is a random walk is rejected. The value of Z(tâ0) for diversity (–0.65) is in magnitude less than the 90% threshold (2.17) for this t statistic, and a linear-drift model may not be appropriate for diversity.
Turning to the no-drift model for diversity (Table 3) we observe that 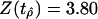 exceeds the 95% threshold of 1.29. The null hypothesis that generic diversity is a random walk over the whole Phanerozoic is rejected.
exceeds the 95% threshold of 1.29. The null hypothesis that generic diversity is a random walk over the whole Phanerozoic is rejected.
The models in Eqs. 3–5 assume a uniform time interval. The Sepkoski and coworkers (6, 24) (sub)stages average 5.1 Myr, but range from 2.5 to 12.5 Myr in length. There are substantial statistical problems associated with estimating model parameters of time series with irregularly spaced data (48). To examine this problem, we selected values for series at 108 uniformly distributed times over the Phanerozic by using both liner interpolation and cubic spline interpolation on the original series; the random-walk character of the series distributed uniformly in time is the same as that just reported for the original series. Furthermore, although there is variation in the lengths of the time intervals of the (sub)stages, the (sub)stage boundaries are marked by discrete events in the fossil record. The time lapse between events is less important than the fact that the events occurred, and we think of the macroevolutionary series as progressing from event to event.
Analysis of Windows of the Series. It may not be a surprise at first glance that the macroevolutionary data examined do not form random walks. As noted above, however, the autocorrelation function of the first difference of diversity suggests that it may be white noise so that diversity may be a random walk. Thus, the data were examined in more detail.
We applied the analysis represented in Tables 1, 2, 3 to 19 time windows of length 90 (sub)stages, where each window represents ≈450 Myr of the Phanerozoic. Windows of length 90 are long enough to retain some of the statistical significance of the total data set but allow some adjustments of coefficients to probe more deeply into the series. Other window sizes are discussed below. All statistics computed for a window were associated with the time of the ninetieth entry in the window (the most recent time of the window).
The no-drift model 3, yt = ρyt–1 + εt, accurately describes all of the windows of the diversity series. (The constant-drift model is actually specified for the first window, and shows a random walk, the same as shown by the no-drift model; for all remaining windows, the constant-drift model is rejected).
Fig. 2 A summarizes the results of fitting the no-drift model to windows of the total diversity data of Fig. 1 A. With one exception (at ≈75 mya), the random-walk hypothesis is not rejected for windows ending before 60 mya, and is rejected for all of the subsequent windows.
The linear-drift model was fit to windows of the accumulated origination series. For no window did Z(tb̂1) exceed the ninety percentile threshold (data not shown) so we turned to the constant-drift model. For all windows, Z(tâ0) exceeded its 90% threshold value (data not shown) and the constant-drift model is accepted. The graph of 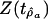 shown in Fig. 2B exhibits roughly the same pattern as found for the diversity data. For windows terminating before 75 mya, the random-walk hypothesis is not rejected; for windows terminating after 75 mya, the random-walk hypothesis is rejected.
shown in Fig. 2B exhibits roughly the same pattern as found for the diversity data. For windows terminating before 75 mya, the random-walk hypothesis is not rejected; for windows terminating after 75 mya, the random-walk hypothesis is rejected.
As with accumulated origination, for every window of accumulated extinction, the linear-drift model is not accepted and the constant-drift model is accepted (data not shown). In a departure from the previous pattern, accumulated extinction is a random walk for all windows (Fig. 2C). Because accumulated extinction is a random walk, extinction is white noise with positive mean within each of those windows that progressively cover the whole Phanerozoic.
Windows of widths other than 90 were also considered. Windows of length 100 all lap into the Cenozoic and retain the random walk character of the total series. For windows of length 90 and 100 the model (Eqs. 3, 4, or 5) used for a window was the same as the model used for the total series that contains the window with only one exception, the first window of length 90 of the diversity series. With windows of length 80, basically the same conclusion is drawn as that illustrated in Fig. 2 A–C but with a more complex development. For the diversity time series, for example, the constant-drift model was appropriate for windows 1–11, 13, 18, and 19, and for each of these windows the random-walk hypothesis is not rejected; the no-drift model was appropriate for the remaining windows and the random-walk hypothesis is rejected only for windows 17 and 20–29. The random-walk hypothesis is not rejected for any window ending before 75 mya and is rejected for all windows ending after 60 mya, which is similar to Fig. 2 A. The complexity increases and the statistical significance of the analysis decreases with series length, and thus we have not used windows of length 70 or less.
Cointegration. In the early windows, both accumulated origination and accumulated extinction are random walks: the null hypothesis that ρ = 1 is not rejected and they are called “unit root” processes. Origination and extinction are significantly correlated (correlation = 0.61), and the question arises as to whether accumulated origination and accumulated extinction are cointegrated. Time series xn and yn are cointegrated if each is a unit root process and there is a linear combination a xn + b yn for which all roots are less than one in magnitude (30). We have concluded that 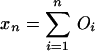 and
and 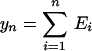 are not cointegrated. Only in the first six windows are both xn and yn unit root processes. For each of those windows we analyzed xn + a yn and a xn + yn for –1 ≤ a ≤1 in 0.01 increments of a and using all three models (Eqs. 3–5). In no case was the estimate of ρ significantly <1. We furthermore examined the autocorrelation and partial autocorrelation functions for a sample of these linear combinations to ensure that a first-order autoregressive model was appropriate.
are not cointegrated. Only in the first six windows are both xn and yn unit root processes. For each of those windows we analyzed xn + a yn and a xn + yn for –1 ≤ a ≤1 in 0.01 increments of a and using all three models (Eqs. 3–5). In no case was the estimate of ρ significantly <1. We furthermore examined the autocorrelation and partial autocorrelation functions for a sample of these linear combinations to ensure that a first-order autoregressive model was appropriate.
Disscussion and Conclusions
These analyses provide evidence that for much of its history the diversity of animal life and also the total amount of macroevolutionary origination and extinction follow a random walk. This conclusion does not necessarily entail that the trends previously identified in these series are actually random fluctuations in drifting systems. Instead, what may be occurring is that so many mechanisms are affecting these series in different ways and at different times that they effectively behave randomly. Another possibility is that these series are themselves being influenced by one or more environmental variables that in turn are after a random walk. The primary deviation from the overall pattern of a random walk involves the latest Mesozoic and Cenozoic parts of the diversity and origination series, although, interestingly, not the extinction series. Diversity and rates of origination actually seem to climb higher than random expectations in roughly the last 75 Myr. New findings by Jablonski et al. (50) suggest this result is likely not attributable to the pull of the recent. Some have argued (31, 36) that the apparent increase in accumulated origination and diversity in the fossil record could be due to sampling effects related to the improving quality of the fossil record through time, rather than true biological changes. Although this is possible, a variety of geological and paleontological studies (38–40, 42, 48, 51) suggest that this is not the case and instead speak to real biotic changes in the last ≈75 mya.
It is interesting that the extinction data do not conform to this pattern and still appear to match a random walk pattern even for the last 75 Myr. The different results for the accumulated origination and diversity time series relative to the accumulated extinction series may be related to the patterns Gilinsky and Bambach (52) documented: origination rates vary systematically through the life spans of most taxa, whereas extinction does not trend up or down. Related patterns identified by Raup and Sepkoski (53) and Gilinsky (54) may also be responsible. Kirchner and Weil (55) also found that the autocorrelations of extinction were no greater than would be found in a random series, which supports the null hypothesis that fluctuations in extinction rates are largely random or more abrupt than the time scale of the data; in addition, Kirchner (56) found that origination and extinction seem to operate on different characteristic time scales. The results about the random nature of accumulated origination and extinction through time suggest that origination and extinction may behave as white noise, although we have some evidence for periodicity in the record of extinction, matching the results from Raup and Sepkoski (3) and Plotnick and Sepkoski (24). However, the evidence for periodicity is primarily governed by the end-Cretaceous event, because it is so large. If this extinction event is not included, the evidence for cyclicity is no longer statistically significant, although it still bears mentioning.
Supplementary Material
Acknowledgments
We thank the innumerable scientists whose work contributed to the data shown, and specifically J. John Sepkoski, Jr., for his work on the marine fossil record, and Richard K. Bambach, David A. Dickey, Mike Foote, Wayne A. Fuller, Roger Kaesler, Jim Kirchner, Peter C. B. Phillips, and an anonymous reviewer for helpful discussions about the work or comments on an earlier version of this paper. This work was supported by the Department of Geology and the Self Fellowship of the University of Kansas and by the National Science Foundation.
Abbreviations: mya, million years ago; Myr, million years.
References
- 1.Raup, D. M. (1972) Science 177, 1065–1071. [DOI] [PubMed] [Google Scholar]
- 2.Smith, A. B. (1988) Paleontology 31, 799–828. [Google Scholar]
- 3.Raup, D. M. & Sepkoski, J. J., Jr. (1984) Proc. Natl. Acad. Sci. USA 81, 801–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vermeij, G. J. (1987) Evolution and Escalation (Princeton Univ. Press, Princeton).
- 5.Bambach, R. K., Knoll, A. H. & Sepkoski, J. J., Jr. (2002) Proc. Natl. Acad. Sci. USA 99, 6854–6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sepkoski, J. J., Jr. (1998) Philos. Trans. R. Soc. London B 353, 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sepkoski, J. J., Jr. (1979) Paleobiology 4, 222–251. [Google Scholar]
- 8.Sepkoski, J. J., Jr., Bambach, R. K. Raup, D. M. & Valentine, J. W. (1981) Nature 293, 435–437. [Google Scholar]
- 9.Sepkoski, J. J., Jr. (1987) Science 235, 64–66. [DOI] [PubMed] [Google Scholar]
- 10.Sepkoski, J. J., Jr. (1997) J. Paleontol. 71, 533–539. [DOI] [PubMed] [Google Scholar]
- 11.Miller, A. I. & Foote, M. (1996) Paleobiology 22, 304–309. [DOI] [PubMed] [Google Scholar]
- 12.Jablonski, D. & Bottjer, D. J. (1991) Science 252, 1831–1833. [DOI] [PubMed] [Google Scholar]
- 13.Valentine, J. W. (1980) Paleobiology 6, 444–450. [Google Scholar]
- 14.Foote, M. (2000) Paleobiology 26, 578–605. [Google Scholar]
- 15.Miller, A. I. & Mao, S. (1995) Geology 23, 305–308. [DOI] [PubMed] [Google Scholar]
- 16.Raup, D. M., Gould, S. J., Schopf, T. J. M. & Simberloff, D. S. (1973) J. Geol. 81, 525–542. [Google Scholar]
- 17.Raup, D. M. (1977) Am. Sci. 65, 50–57. [PubMed] [Google Scholar]
- 18.Raup, D. M. & Crick, R. E. (1981) Palebiology 7, 200–215. [Google Scholar]
- 19.Stanley, S. M., Signor, P. W., III, Lidgard, S. & Karr, A. F. (1981) Paleobiology 7, 115–127. [Google Scholar]
- 20.Bookstein, F. L. (1987) Paleobiology 13, 446–464. [Google Scholar]
- 21.Roopnarine, P. D. (2001) Paleobiology 27, 446–465. [Google Scholar]
- 22.Berner, R. A. & Kothavala, Z. (2001) Am. J. Sci. 301, 182–204. [Google Scholar]
- 23.Cornette, J. L., Lieberman, B. S. & Goldstein, R. H. (2002) Proc. Natl. Acad. Sci. USA 99, 7832–7835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plotnick, R. E. & Sepkoski, J. J, Jr. (2001) Paleobiology 27, 126–139. [Google Scholar]
- 25.Fuller, W. A. (1976) Introduction to Statistical Time Series (Wiley, New York).
- 26.Dickey, D. A. & Fuller, W. A. (1979) J. Amer. Stat. Assoc. 74, 427–431. [Google Scholar]
- 27.Dickey, D. A. & Fuller, W. A. (1981) Econometrica 49, 1057–1072. [Google Scholar]
- 28.Phillips, P. C. B. (1987) Econometrica 55, 277–301. [Google Scholar]
- 29.Phillips, P. C. B. & Perron, P. (1988) Biometrika 75, 335–346. [Google Scholar]
- 30.Tsay, R. S. (2002) Analysis of Financial Time Series (Wiley, New York).
- 31.Raup, D. M. (1976) Paleobiology 2, 289–297. [Google Scholar]
- 32.Adrain, J. M., Fortey, R. A. & Westrop, S. R. (1998) Science 280, 1922–1925. [DOI] [PubMed] [Google Scholar]
- 33.Alroy, J. (2000) Geology 28, 1023–1026. [Google Scholar]
- 34.Smith, A. B. (2000) Philos. Trans. R. Soc. London, B 356, 351–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alroy, J., Marshall, C. R., Bambach, R. K., Bezusko, K., Foote, M., Fursich, F. T., Hansen, T. A., Holland, S. M., Ivany, L. C., Jablonski, D., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 6261–6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters, S. & Foote, M. (2001) Paleobiology 27, 583–601. [Google Scholar]
- 37.Sepkoski, J. J., Jr. (2002) Bull. Am. Paleontol. 363, 5–56. [Google Scholar]
- 38.Bambach, R. K. (1977) Paleobiology 3, 152–167. [Google Scholar]
- 39.Bambach, R. K. (1993) Paleobiology 19, 372–397. [Google Scholar]
- 40.Gregor, C. B. (1985) in The Chronology of the Geological Record, ed. Snelling, N. J. (Blackwell Scientific, Boston) Geological Society, London, Memoir 10, pp. 284–289.
- 41.Signor, P. W. (1990) Annu. Rev. Ecol. Syst. 21, 509–539. [Google Scholar]
- 42.Sepkoski, J. J., Jr., Bambach, R. K. & Droser, M. L. (1991) in Cycles and Events in Stratigraphy, eds. Einselle, G., Ricken, W. & Seilacher, A. (Springer, Berlin), pp. 298–312.
- 43.Miller, A. I. (1998) Science 281, 1157–1160. [DOI] [PubMed] [Google Scholar]
- 44.Miller, A. I. (2000) Paleobiology Suppl. 26, 53–73. [Google Scholar]
- 45.Benton, M. J., Wills, M. A. & Hitchin, R. (2000) Nature 403, 534–537. [DOI] [PubMed] [Google Scholar]
- 46.Owen, A. W. & Crame, J. A. (2002) in Palaeobiogeography and Biodiversity Change: The Ordovician and Mesozoic-Cenozoic Radiations, eds. Crame, J. A. & Owen, A. W. (Geological Society, London), Special Publications 194, pp. 1–12.
- 47.Crame, J. A. & Rosen, B. R. (2002) in Palaeobiogeography and Biodiversity Change: The Ordovician and Mesozoic-Cenozoic Radiations, eds. Crame, J. A. & Owen, A. W. (Geological Society, London), Special Publications 194, pp. 153–168.
- 48.Powell, M. G. & Kowalewski, M. (2002) Geology 30, 331–334. [Google Scholar]
- 49.Conner, E. F. (1986) in Patterns and Processes in the History of Life, eds. Raup, D. M. & Jablonski, D. (Springer, Berlin), pp. 119–147.
- 50.Jablonski, D., Roy, K., Valentine, J. W., Price, R. M. & Anderson, P. S. (2003) Science 300, 1133–1135. [DOI] [PubMed] [Google Scholar]
- 51.Thayer, C. W. (1983) in Biotic Interactions in Recent and Fossil Benthic Communities, eds. Tavesz, M. & McCall, P. (Plenum, New York), pp. 479–625.
- 52.Gilinsky, N. L. & Bambach, R. D. (1987) Paleobiology 13, 427–445. [Google Scholar]
- 53.Raup, D. M. & Sepkoski, J. J., Jr. (1982) Science 215, 1501–1503. [DOI] [PubMed] [Google Scholar]
- 54.Gilinsky, N. L. (1994) Paleobiology 20, 445–458. [Google Scholar]
- 55.Kirchner, J. W. & Weil, A. (2000) Proc. R. Soc. London Ser. B 267, 1301–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kirchner, J. W. (2002) Nature 415, 65–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.