Abstract
OBJECTIVE
Cluster analysis was performed on the results of self-monitoring of blood glucose (SMBG) to discriminate islet graft function after islet cell transplantation (ICT) in patients with type 1 diabetes.
RESEARCH DESIGN AND METHODS
Eleven islet recipients were included in this study. The patients visited our clinic monthly after ICT and provided blood samples for fasting C-peptide (n = 270), which were used to evaluate islet graft function. They also provided their SMBG data through an automatic data collection system. The SMBG data for 3 days immediately before each clinic visit were evaluated using the following assessments: M value, mean amplitude of glycemic excursions, J index, index of glycemic control, average daily risk range, and glycemic risk assessment diabetes equation. The cluster analysis was performed for both SMBG assessments and samples. Multivariate logistic regression analysis was used to evaluate the clusters of SMBG for assessing islet graft function.
RESULTS
Analysis for SMBG assessments revealed five types of clusters, which showed similar patterns according to functional or dysfunctional islet graft phase. Two clusters, the euglycemia cluster (P < 0.001) and the hypoglycemia cluster (P = 0.001), were significant factors in the logistic model for islet graft function. The SMBG clusters had significant correlations with clinical graft indexes (P < 0.001).
CONCLUSIONS
Cluster analysis of SMBG data as part of an automated data quality system could allow discrimination of islet graft dysfunction after ICT. This approach should be considered for islet recipients.
Intensive glycemic control in patients with type 1 diabetes can reduce the risk of microvascular complications (1), but those with brittle type 1 diabetes have difficulty with glycemic control (2). β-Cell replacement therapies, including whole pancreas transplantation and islet cell transplantation (ICT), improve glycemic control without exogenous insulin (3). Although ICT is a promising treatment and a minimally invasive procedure, one of the major difficulties is maintaining long-term graft function and insulin independence as a result of the loss of transplanted islets by inflammatory reactions, allogeneic rejection, autoimmune recurrence, and senescence (3). Therefore, one of the greatest concerns in ICT is to clarify clinical features of islet graft dysfunction.
On the other hand, self-monitoring of blood glucose (SMBG) is still widely used for glycemic control. The raw data of SMBG in themselves are helpful for diabetologists in determining exogenous insulin amounts for intensive therapy, whereas clinical indexes of SMBG, such as the M value and the mean amplitude of glycemic excursion (MAGE), can serve as landmarks for the quality of glycemic management (4). Recently, newly developed SMBG assessment methods have been proposed and their usefulness has been demonstrated; the average daily risk range (ADRR) is associated with both low and high blood glucose excursions (5), and the glycemic risk assessment diabetes equation (GRADE) considers the risk of diabetes complications (6). However, information on the interpretation of SMBG assessments in ICT, which is accompanied with a dramatic change in glycemic control, is limited.
Cluster analyses are frequently used in the field of genomics or proteomics together with heatmapping (7) to handle and interpret large amounts of data by classifying them on the basis of their similarity. The heatmap is also helpful in recognizing patterns in the data. Therefore, we hypothesized that these analytic methods would also be useful for the evaluation of SMBG in ICT. The aim of this study was to perform a cluster analysis of SMBG for assessing islet graft function in ICT, which would contribute to the clinical management of patients who receive β-cell replacement therapies.
RESEARCH DESIGN AND METHODS
Patients
This study included 11 patients with type 1 diabetes who underwent ICTs under the Food and Drug Administration–approved investigational new drug application at our hospitals (Baylor University Medical Center at Dallas, Dallas, TX, and Baylor All Saints Medical Center at Fort Worth, Fort Worth, TX) from March 2005 until February 2010. Inclusion criteria were based on the Edmonton protocol (8). This study was approved by the institutional review board of Baylor Research Institute, and all patients provided written informed consent.
Islet preparation and transplantation
All pancreata were procured from brain-dead donors, and pancreatic islets were isolated by the modified Ricordi method (9). Total islet yield averaged 13,998 ± 1,676 islet equivalent (IE)/kg. Immediately after the islet isolation, prepared islets were transplanted into the portal vein by percutaneous transhepatic cannulation. The first seven patients received an Edmonton-like immunosuppressive regimen (8), and the later four patients received immunosuppressive induction with antithymocyte globulin and maintenance with tacrolimus and mycophenolate mofetil (10).
SMBG assessments
For measurement of SMBG, an automatic, wireless blood glucose monitoring and transmittal system (GlucoMON-ADMS; Diabetech, Dallas, TX) was used with the OneTouch Ultra glucose meter (LifeScan, Milpitas, CA). SMBG measurements for 3 consecutive days immediately before each clinic visit were included; data with inadequate frequency of monitoring (<3 readings per day) were excluded. Percentage in target range, percentage below target range, percentage above target range, mean glucose levels, SD of glucose levels, percent coefficient of variation, and differences between maximum and minimum glucose levels were calculated. M value, MAGE, J index, index of glycemic control, lability index, ADRR, and GRADE were calculated according to definitions (4–6,11,12). The ideal blood glucose level for the M value was set at 100 mg/dL (5.5 mmol/L). Settings to calculate the index of glycemic control were 80 mg/dL for the lower limit of target range, 2.0 for parameter b, 30 for parameter d, 140 mg/dL for the upper limit of target range, 1.1 for parameter a, and 30 for parameter c (4). The lability index was calculated as an accumulation for 3 days of daily glycemic lability, as defined in the original article (12).
Evaluation of graft function and glycemic control
During monthly clinic visits after ICTs, patients’ fasting blood samples were collected and used to measure glucose level, plasma C-peptide, and HbA1c. Samples within 1 month of ICT were excluded from this study, since intensive insulin therapy was provided during this period based on our protocol irrespective of islet function. After 1 month, exogenous insulin therapy was discontinued or resumed to maintain favorable glycemic control, with the goal of a fasting blood glucose <7 mmol/L or HbA1c <6.5%. Four graft indexes for ICT were calculated: the secretory unit of islet transplant objects index, i.e., fasting C-peptide (ng/mL) × 1,500/(fasting blood glucose [mg/dL] − 63) (13); the β-score (14); the C-(peptide-to-glucose ratio), i.e., C-peptide (ng/mL) × 100/blood glucose (mg/dL) (15); and the corrected homeostasis model assessment (16).
Statistical analysis
Data analyses were performed using R 2.9.0 (R Foundation for Statistical Computing, Vienna, Austria) and PASW Statistics 18 (SPSS Inc., Chicago, IL). All SMBG assessments were rescaled to range from 0 to 1 (17). The cluster analysis with complete linkage methods was used to discover the pattern similarities of SMBG assessments (18). The optimal number of clusters and their stabilities were determined with 1,000 bootstrap resamplings (19). The average of elements in every cluster was calculated and used as a representative value of each cluster in further analysis. Hierarchical clustering for all time points including all patients was also used with a single linkage method (nearest neighbor), where the dendrogram was coupled with the heatmap (20). Similar to the β-score (14), graft function was classified into three categories: nonfunction, nondetectable level of fasting C-peptide; partial function, <0.3 mmol/L of detectable fasting C-peptide; and full function, >0.3 mmol/L of detectable fasting C-peptide.
To determine the contribution of the SMBG clusters to islet graft function, backward stepwise multivariate logistic regression analysis was used. In the analysis, the outcome variable was defined as functional (detectable fasting C-peptide levels) or nonfunctional (undetectable fasting C-peptide levels). The variables on individual patients and time after the first transplantation were also included in the logistic model. Using the final logistic model, the probability of graft function could be expressed (21). To determine the discrimination of the probability of graft function, receiver operating characteristic (ROC) analysis was performed and the area under the curve (AUC) on ROC analysis was evaluated.
We also compared the SMBG clusters with the islet graft indexes of the secretory unit of islet transplant objects index, C-peptide-to-glucose ratio, β-score, and corrected homeostasis model assessment using the Spearman correlation coefficient. In addition, the SMBG clusters were compared with the frequency of SMBG measurements per day. A two-sided P value of < 0.05 was considered statistically significant. Values were expressed as mean ± SE.
RESULTS
Patient characteristics and clinical results
The 11 patients (9 women and 2 men) had an average age of 43.8 ± 3.3 years; body weight, 67.0 ± 3.6 kg; BMI, 24.3 ± 1.1 kg/m2; duration of diabetes, 33.4 ± 2.6 years; daily insulin use/body weight, 0.48 ± 0.03 unit/kg; fasting C-peptide, 0.0 ± 0.0 ng/dL; and HbA1c, 7.9 ± 0.3%. The follow-up period was 23.6 ± 4.1 months from first ICT, including a total of 270 clinical visits. Eight patients achieved insulin independence after ICT; their average total islet yield/body weight was 15,696 IE/kg. Four patients received one dose of islets, five patients received two, and two patients received three. Four patients remain insulin independent at the time of this analysis. A total of 4,861 SMBG measurements were included in this study.
Hierarchical cluster analysis
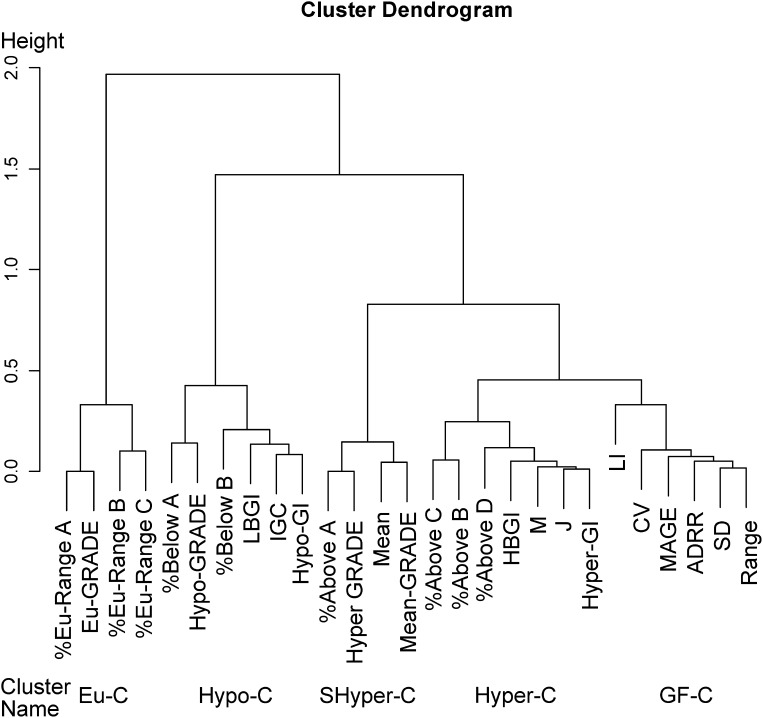
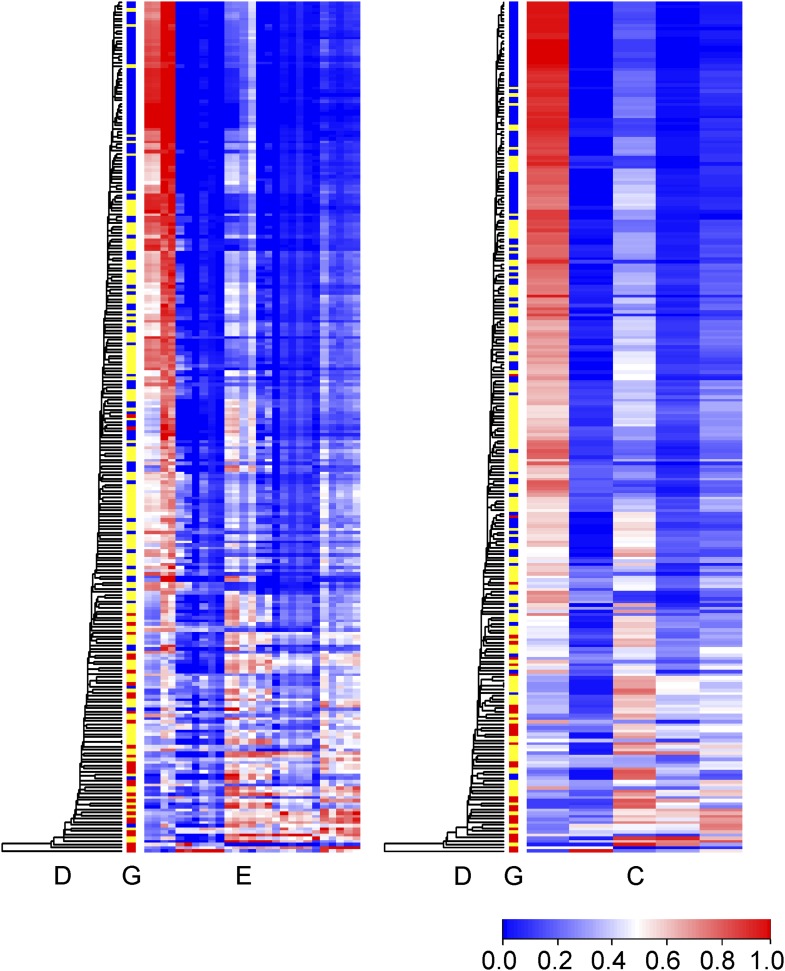
SMBG assessment elements were classified into five clusters with 100% stability under 1,000 resamplings. The five clusters were named euglycemia cluster, hypoglycemia cluster, semihyperglycemia cluster, hyperglycemia cluster, and glucose fluctuation cluster, based on the elements in each cluster (Fig. 1). Hierarchical cluster analysis with the single linkage method was also used across all time points including all patients. The dendrogram for samples was coupled with the heatmaps of graft function, the SMBG assessment elements, and the SMBG clusters (Fig. 2). The phase of full graft function was observed in the upper rows and nonfunction in the bottom rows.
Figure 1.
Dendrogram of hierarchical cluster analysis for SMBG evaluations. Height indicates the distance of correlation method between substructures. %Eu-Range A, B, and C, percentage of blood glucose between 70 and 140, 80 and 200, and 70 and 180 mg/dL; %above A, B, C, and D, percentage of blood glucose above 140, 180, 200, and 250 mg/dL; %below A and B, percentage of blood glucose below 80 and 50 mg/dL. LBGI and HBGI, low and high blood glucose index; mean-, Hypo-, Eu-, and Hyper-GRADE, the average of GRADE values and the percentage of hypoglycemia, euglycemia, and hyperglycemia in all GRADE values; IGC, the index of glycemic control; Hyper-GI and Hypo-GI, hyper- and hypoglycemia index; LI, liability index; M, M value; J, J index; CV, coefficient of variation; Eu-C, euglycemia cluster; Hypo-C, hypoglycemia cluster; SHyper-C, semihyperglycemia cluster; Hyper-C, hyperglycemia cluster; GF-C, glucose fluctuation cluster.
Figure 2.
Heatmap of SMBG assessments and SMBG clusters. Time points (rows) were reordered according to hierarchical cluster analysis with the single linkage method, and the dendrograms (Column D) are shown. Column E shows nonclustered SMBG assessment elements ordered the same as in Fig 1. Column C shows the SMBG clusters ordered the same as in Fig 1. Column G shows graft function as full function (blue), partial function (yellow), and nonfunction (red).
Multivariate logistic analysis for islet graft function
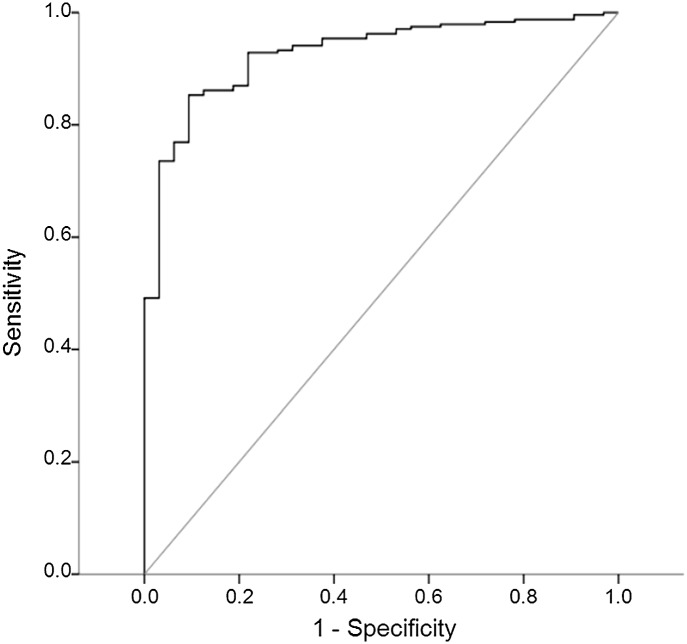
The final multiple logistic regression model included the euglycemia (P < 0.001) and hypoglycemia (P = 0.001) clusters as significant variables. Conversely, patient variables and the time period after transplantation were not independent variables in the model. The probability of graft function was obtained using the final logistic model and shown as a heatmap in the clinical courses of all patients as well as SMBG assessment elements and SMBG clusters (Supplementary Fig. 1). The AUC of ROC curve of this probability model displayed excellent discrimination for graft function (AUC = 0.927, P < 0.001, Fig. 3). More than 0.85 of the probability had 90.6, 85.3, 98.1, and 43.5% specificity, sensitivity, positive predictive value, and negative predictive value, respectively, for a functional islet graft.
Figure 3.
ROC curve of probability based on the SMBG clusters for islet graft function. The AUC was 0.927 (95% CI 0.887–0.967, P < 0.001), indicating that SMBG clusters were able to achieve excellent discrimination of islet graft function.
Correlation of SMGB clusters with islet graft indexes and frequency of SMBG measurements
SMBG clusters were compared with islet graft assessments as well as frequency of SMBG measurements per day by Spearman correlation coefficients. All clusters significantly correlated with islet graft indexes (P < 0.001), whereas no significant coefficients were found between the SMBG clusters and the frequency of SMBG measurements (Table 1).
Table 1.
Correlations between clusters and graft assessments
| Cluster | SUITO index | CP/G | β-Score | HOMA2β | Frequency of daily SMBG measurements |
|---|---|---|---|---|---|
| Euglycemia | 0.702* | 0.681* | 0.774* | 0.631* | 0.114 |
| Hypoglycemia | −0.627* | −0.693* | −0.585* | −0.576* | 0.048 |
| Semihyperglycemia | −0.462* | −0.384* | −0.570* | −0.425* | −0.117 |
| Hyperglycemia | −0.669* | −0.649* | −0.751* | −0.638* | −0.087 |
| Glucose fluctuation | −0.735* | −0.765* | −0.760* | −0.718* | 0.054 |
Spearman correlation coefficients are shown. SUITO, secretory unit of islet transplant objects; CP/G, C-peptide-to-glucose ratio; HOMA2β, corrected homeostasis model assessment.
*P < 0.001.
CONCLUSIONS
This report demonstrated that cluster analysis of SMBG can provide helpful information on glycemic profiles and discriminate graft function from nonfunction in islet recipients. Although recent studies have proposed several tools to evaluate SMBG (4–6,11,12), this study discovered that the SMBG assessment tools had similar patterns in the same clusters, along with similar islet graft function. In this study, SMBG results were clustered by euglycemia, hyperglycemia, hypoglycemia, and glycemic fluctuation, which supports the view that glycemic variability and fluctuation should be accounted for in the evaluation of SMBG. Theoretically, ideal glycemic control subjects provide low scores (blue) in all clusters except for the euglycemia cluster, and the ideal pattern was observed in the patients with full islet graft function. Thus, the SMBG clusters and heatmap were also useful in detecting islet graft functionality.
From the patient’s perspective, it is beneficial to calculate the SMBG clusters using 3 consecutive days of SMBG results. Even though the patients regained glucose stability after ICT, they did not always have ideal blood glucose levels. Usually, the patients with enough function to achieve insulin independence reduce the number of SMBG assessments, and clinicians cannot follow their blood glucose profiles in detail. However, durations of SMBG as short as 3 days provided enough data for evaluation of glycemic control in this study. In addition, we used an automated data system for collecting SMBG data. This system is important for analyzing SMBG, because it was shown that patient compliance regarding SMBG might be limited (22).
There are some limitations to our study. We used a relatively small number of participants, and our analysis was entirely retrospective, albeit on prospectively collected data. Additionally, this study used advanced analysis methods for research purposes, which requires a simplified methodology for application to clinical practice. A prospective large-scale study is also needed to show the usefulness of the SMGB clusters.
We analyzed the contribution of the SMBG clusters, as well as patient factors and time points of clinical follow-up, on islet graft function. A multivariate logistic regression model revealed that the euglycemia and hypoglycemia clusters were significant independent variables. The probability of successful graft function obtained from the final logistic model was supported by ROC analysis, which yielded excellent discrimination in predicting islet graft function. The negative predictive value was low, however, implying a high rate of false-negative results. Both prevalence and the ability of the index affect predictive values (23), so that the uneven prevalence of islet graft function in this study (88.1% functional islet grafts) would be associated with a poor negative predictive value. The probability obtained from the scores of the SMBG clusters might be able to predict islet graft dysfunction; however, further validation is needed because of the small number of subjects in this study.
Interestingly, the SMBG clusters had significant correlations with clinical indexes of islet graft function. This finding suggests that better islet graft function could lead to better glycemic control. We also evaluated the association between the SMBG clusters and the frequency of the measurements, since it was reported that higher frequency was significantly correlated with better metabolic control (24). In this study, no significant correlations between the SMBG clusters and frequency were observed, which suggests that fully functional islet transplants eliminate the necessity of frequent measurement of blood glucose to maintain excellent glycemic control.
Single-donor ICT does successfully make patients with type 1 diabetes insulin independent (10). One of the advantages of ICT is the relative ease of repeated transplantation. The SMBG cluster analysis can be used to estimate the timing of additional ICT, providing uninterrupted periods of long-term insulin independence by using retransplantation. The possible causes of graft loss were marginal yield of transplanted islets for the initial three patients (Patients A–C in Supplementary Figs.), which resulted in occasional hyperglycemia and glucotoxicity to transplanted islets (9), and the toxicity of immunosuppressive drugs such as diabetogenic tacrolimus and antiproliferative sirolimus. We have implemented an improved islet preparation method for higher islet yield and a sirolimus-free immunosuppression protocol to overcome those issues and have had promising results (10).
Continuous glucose monitoring (CGM) has recently been developed as an advanced technological version of SMBG. In clinical studies of ICT, it has been reported that CGM could detect graft dysfunction (25). However, there are major limitations for the clinical use of CGM, particularly decreased accuracy of blood glucose measurements and limited recording time.
In summary, the SMBG cluster analysis provided excellent discrimination of islet graft function and helpful information on glycemic profiles in ICT. A large-scale prospective study will reveal the value of cluster analysis of SMGB for assessing islet graft function and predicting the timing for retransplantation of islets.
Supplementary Material
Acknowledgments
This work was supported in part by the All Saints Health Foundation.
No potential conflicts of interest relevant to this article were reported.
M.T. and S.M. researched data and wrote and edited the manuscript. H.N., M.S., D.C., T.I., K.S., J.A.S., and N.O. researched data and contributed to discussion. B.N. and M.F.L. researched data and reviewed and edited the manuscript.
The authors thank Ms. Yoshiko Tamura (Baylor Regional Transplant Institute [BRTI]) and Ms. Ana M. Rahman (BRTI) for their technical support, Ms. Kerri Purcell (BRTI) for her patient care, and Ms. Cynthia Orticio (scientific publications office of Baylor University Medical Center) for editorial assistance.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-1938/-/DC1.
References
- 1.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.Zgibor JC, Songer TJ, Kelsey SF, et al. The association of diabetes specialist care with health care practices and glycemic control in patients with type 1 diabetes: a cross-sectional analysis from the Pittsburgh epidemiology of diabetes complications study. Diabetes Care 2000;23:472–476 [DOI] [PubMed] [Google Scholar]
- 3.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes 2005;54:2060–2069 [DOI] [PubMed] [Google Scholar]
- 4.Rodbard D. Interpretation of continuous glucose monitoring data: glycemic variability and quality of glycemic control. Diabetes Technol Ther 2009;11(Suppl. 1):S55–S67 [DOI] [PubMed] [Google Scholar]
- 5.Kovatchev BP, Otto E, Cox D, Gonder-Frederick L, Clarke W. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care 2006;29:2433–2438 [DOI] [PubMed] [Google Scholar]
- 6.Hill NR, Hindmarsh PC, Stevens RJ, Stratton IM, Levy JC, Matthews DR. A method for assessing quality of control from glucose profiles. Diabet Med 2007;24:753–758 [DOI] [PubMed] [Google Scholar]
- 7.Zhao Y, Karypis G. Data clustering in life sciences. Mol Biotechnol 2005;31:55–80 [DOI] [PubMed] [Google Scholar]
- 8.Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006;355:1318–1330 [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto S, Noguichi H, Shimoda M, et al. Seven consecutive successful clinical islet isolations with pancreatic ductal injection. Cell Transplant 2010;19:291–297 [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto S, Takita M, Chaussabel D, et al. Improving efficacy of clinical islet transplantation with iodixanol based islet purification, thymoglobulin induction and blockage of IL-1 beta and TNF-alpha. Cell Transplant. 8 March 2011 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Wójcicki JM. “J”-index. A new proposition of the assessment of current glucose control in diabetic patients. Horm Metab Res 1995;27:41–42 [DOI] [PubMed] [Google Scholar]
- 12.Ryan EA, Shandro T, Green K, et al. Assessment of the severity of hypoglycemia and glycemic lability in type 1 diabetic subjects undergoing islet transplantation. Diabetes 2004;53:955–962 [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto S, Noguchi H, Hatanaka N, et al. SUITO index for evaluation of efficacy of single donor islet transplantation. Cell Transplant 2009;18:557–562 [DOI] [PubMed] [Google Scholar]
- 14.Ryan EA, Paty BW, Senior PA, Lakey JR, Bigam D, Shapiro AM. β-Score: an assessment of β-cell function after islet transplantation. Diabetes Care 2005;28:343–347 [DOI] [PubMed] [Google Scholar]
- 15.Faradji RN, Monroy K, Messinger S, et al. Simple measures to monitor beta-cell mass and assess islet graft dysfunction. Am J Transplant 2007;7:303–308 [DOI] [PubMed] [Google Scholar]
- 16.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998;21:2191–2192 [DOI] [PubMed] [Google Scholar]
- 17.Gentleman R, Carey V, Huber W, Hahne F. Genefilter [reference manual], 2010. Seattle, WA, Bioconductor. Available from http://bioconductor.org/packages/2.6/bioc/manuals/genefilter/man/genefilter.pdf. Accessed 9 February 2011
- 18.Webb AR. Clustering. In Statistical Pattern Recognition. 2nd ed. Webb AR, Ed. Hoboken, NJ, John Wiley & Sons, 2003, p. 361–407 [Google Scholar]
- 19.Smolkin M, Ghosh D. Cluster stability scores for microarray data in cancer studies. BMC Bioinformatics 2003;4:36–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warnes GR. Package ‘gplots' [reference manual], 2010. Vienna, Austria, The R Project for Statistical Computing. Available from http://cran.r-project.org/web/packages/gplots/gplots.pdf. Accessed 9 February 2011
- 21.Hosmer DW, Lemeshow S. Multiple logistic regression. In Applied Logistic Regression. 2nd ed. Hosmer DW, Lemeshow S, Eds. Hoboken, NJ, John Wiley & Sons, 2000, p. 31–46 [Google Scholar]
- 22.Hansen MV, Pedersen-Bjergaard U, Heller SR, et al. Frequency and motives of blood glucose self-monitoring in type 1 diabetes. Diabetes Res Clin Pract 2009;85:183–188 [DOI] [PubMed] [Google Scholar]
- 23.Holmes EW. The interpretation of laboratory tests. In Clinical Laboratory Medicine. McClatchey KD, Ed. Philadelphia, PA, Lippincott Williams & Wilkins, 2002, p. 97–121 [Google Scholar]
- 24.Schütt M, Kern W, Krause U, et al. ; DPV Initiative. Is the frequency of self-monitoring of blood glucose related to long-term metabolic control? Multicenter analysis including 24,500 patients from 191 centers in Germany and Austria. Exp Clin Endocrinol Diabetes 2006;114:384–388 [DOI] [PubMed] [Google Scholar]
- 25.Faradji RN, Monroy K, Riefkohl A, et al. Continuous glucose monitoring system for early detection of graft dysfunction in allogenic islet transplant recipients. Transplant Proc 2006;38:3274–3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.