Abstract
OBJECTIVE
Aging increases the risk of developing impaired glucose tolerance (IGT) and type 2 diabetes. It has been proposed that increased reactive oxygen species (ROS) generation by dysfunctional mitochondria could play a role in the pathogenesis of these metabolic abnormalities. We examined whether aging per se (in subjects with normal glucose tolerance [NGT]) impairs mitochondrial function and how this relates to ROS generation, whether older subjects with IGT have a further worsening of mitochondrial function (lower ATP production and elevated ROS generation), and whether exercise reverses age-related changes in mitochondrial function.
RESEARCH DESIGN AND METHODS
Mitochondrial ATP and ROS production were measured in muscle from younger individuals with NGT, older individuals with NGT, and older individuals with IGT. Measurements were performed before and after 16 weeks of aerobic exercise.
RESULTS
ATP synthesis was lower in older subjects with NGT and older subjects with IGT versus younger subjects. Notably, mitochondria from older subjects (with NGT and IGT) displayed reduced ROS production versus the younger group. ATP and ROS production were similar between older groups. Exercise increased ATP synthesis in the three groups. Mitochondrial ROS production also increased after training. Proteomic analysis revealed downregulation of several electron transport chain proteins with aging, and this was reversed by exercise.
CONCLUSIONS
Old mitochondria from subjects with NGT and IGT display mitochondrial dysfunction as manifested by reduced ATP production but not with respect to increased ROS production. When adjusted to age, the development of IGT in elderly individuals does not involve changes in mitochondrial ATP and ROS production. Lastly, exercise reverses the mitochondrial phenotype (proteome and function) of old mitochondria.
Several mitochondrial alterations have been described with aging, including reduced synthesis of mitochondrial proteins, reduced activity of oxidative enzymes, and lower mitochondrial mass (1–5). Collectively, these alterations lead to a decrease in mitochondrial ATP synthesis (6,7). These changes in mitochondrial structure and function are thought to result, in part, from an increased prevalence of mitochondrial DNA (mtDNA) mutations, decreased mtDNA abundance, and a lower content of mRNA transcripts encoding mitochondrial proteins (4,8–10). Despite the evidence demonstrating that these molecular alterations play an important role in the mitochondrial dysfunction of aging, the underlying cause of these age-induced changes is unclear. Apart from producing energy, mitochondria are a major source of reactive oxygen species (ROS) (11). According to the free radical and mitochondrial theories of aging (12,13), ROS emanating from mitochondrial respiration damage macromolecules (mtDNA, proteins, and lipids), and over time, the abnormal function of these cellular constituents induces the changes associated with aging.
Although several studies have documented a decline in mitochondrial function with aging (3,6,7,14,15), the physiologic relevance of this decline is not clear. An alteration in glucose homeostasis is thought to be one of the most important consequences of the aging-related decrease in mitochondrial function. Substantial evidence has demonstrated that increasing age leads to decreased glucose tolerance (16–19). For example, the Baltimore Longitudinal Study of Aging showed a progressive decline in glucose tolerance from the 3rd through the 9th decade of life (18). The fasting plasma glucose increased on average 1 mg/dL per decade, and the 2-h glucose during an oral glucose tolerance test (OGTT) increased by 5.3 mg/dL per decade. This decline in glucose tolerance is also evident in the National Health and Nutrition Examination Survey III (20). The cause for the higher prevalence of impaired glucose tolerance (IGT) and type 2 diabetes in elderly individuals is unknown. However, decreased insulin sensitivity at the level of the skeletal muscle is thought to play an important role in the deterioration in glucose homeostasis seen in older subjects (6,21,22).
It has been proposed that alterations in mitochondrial function, as seen in older subjects, could be responsible for the decrease in insulin action that occurs with aging (6). Aging-related decreases in mitochondrial oxidative capacity could lead to insulin resistance by promoting the accumulation of intramyocellular lipids, which interfere with the insulin signaling cascade (6). Another important mechanism by which aging-related mitochondrial dysfunction could affect insulin action is by increasing oxidative stress. A fundamental concept within the free radical and mitochondrial theories of aging is that the mitochondrial dysfunction resulting from the presence of damaged/oxidized mitochondrial components (mtDNA, lipids, and proteins) leads to the leakage of electrons from the electron transport chain, inducing the generation of ROS, which can further damage the mitochondria and impair their oxidative function. In addition, data from cell culture and animal studies suggest that ROS play a direct role in the pathogenesis of insulin resistance. For example, exposing adipocytes to H2O2 impairs activation of the insulin signaling cascade (23), and antioxidants such as tetrakis (4-benzoic acid)-porphyrin improves insulin sensitivity in obese mice (24).
Despite the evidence that mitochondrial function declines with age (6,7,14) and that oxidative stress can impair insulin action (24), the role that ROS play in the insulin resistance of aging in humans is unclear. The goal of this study was to determine whether aging-induced changes in mitochondrial function lead to excessive ROS production in muscle from older subjects with normal glucose tolerance (NGT) and IGT. Also, we evaluated the effect of physical activity on mitochondrial function (ATP synthesis and ROS production) in older subjects. We predicted that by improving mitochondrial efficiency, exercise would lead to a decrease in mitochondrial ROS production, an effect that would result in improved insulin sensitivity.
RESEARCH DESIGN AND METHODS
Twenty-two young (age 18–30 years) and 35 older (age ≥65 years) healthy, nondiabetic, community-dwelling subjects were studied. Each subject underwent a physical examination, screening laboratory tests, and a 75-g OGTT. All subjects were sedentary (≤1 exercise session per week). Activity levels were confirmed with a leisure-time activity questionnaire (25). Subjects had a BMI of 23–26 kg/m2 and did not have a family history (first-degree relative) of diabetes. Body weight was stable (± 1 kg) for ≥3 months before enrollment. Subjects were not taking medications known to affect glucose metabolism. Older subjects were subdivided into NGT or IGT groups based on the OGTT (26). The young group was not subdivided because only 7% of the screened subjects had IGT. The study was approved by the University of Texas Health Science Center at San Antonio Institutional Review Board, and all subjects gave written consent.
OGTT.
Plasma glucose and insulin were measured before and every 15 min for 2 h after the ingestion of 75 g of glucose. The Matsuda index of insulin sensitivity was calculated as described (27).
Dual X-ray absorptiometry.
Dual X-ray absorptiometry (Hologic, Bedford, MA) was used to measure fat and fat-free mass (FFM).
Exercise testing.
Vo2max was determined using a cycle ergometer and a Metabolic Measurement System (Sensormedics, Savi Park, CA) (21).
Muscle biopsy and insulin clamp.
After a 10- to 12-h overnight fast, subjects underwent a vastus lateralis muscle biopsy (28). Subjects refrained from exercise, other than habitual walking, for 48 h. A portion of fresh tissue was used for immediate measurement of mitochondrial ATP and ROS production, whereas another portion was rapidly frozen in liquid nitrogen. The biopsy was followed by a 120-min euglycemic, hyperinsulinemic (40 mU/m2/min) clamp study. Insulin-stimulated glucose metabolism (M) was determined based on the average glucose infusion rate during the last 30 min of the clamp (29) and adjusted to the plasma insulin concentration (M/I).
Exercise program.
At ∼1 week after the biopsy, 12 older subjects with NGT, 5 older subjects with IGT, and 10 young subjects initiated a 16-week aerobic exercise program (30) on a stationary bicycle under supervision. For the first 4 weeks, subjects underwent three sessions per week at 65% Vo2max for 20 min per session. During weeks 5–8, the intensity, duration, and number of sessions were gradually increased so that during weeks 9–16, subjects underwent four sessions per week for 45 min per session at 80% Vo2max. Vo2peak was tested every 4 weeks to adjust exercise intensity. Compliance with the target intensity and number of sessions was recorded. Subjects maintained their usual dietary intake throughout the training protocol and were asked to increase their caloric intake to avoid weight loss, if needed. Vo2peak and body composition were measured after completion of the program. A posttraining muscle biopsy and insulin clamp were performed 48–72 h after the last exercise bout.
Laboratory analyses.
Plasma insulin was measured by radioimmunoassay (Diagnostic Products, Los Angeles, CA), glucose was measured using a Beckman analyzer (Fullerton, CA), and hemoglobin A1c was measured using a DCA2000 analyzer (Bayer, Tarrytown, NY). Free fatty acid (FFA) concentrations were determined by an enzymatic method (Wako, Neuss, Germany), and plasma interleukin (IL)-6 and tumor necrosis factor-α (TNF-α) concentrations were measured using an ELISA (R&D Systems, Minneapolis, MN).
Mitochondrial isolation.
Muscle tissue was minced in medium A (100 mmol/L KCl, 50 mmol/L MOPS, 5 mmol/L MgSO4 ⋅ 7 H2O, 1 mmol/L ATP, pH 7.4), and trypsin (10 mg/g) and neutral protease (1 mg/g) were added for 20 min and homogenized (31). An equal volume of medium B (100 mmol/L KCl, 50 mmol/L MOPS, 5 mmol/L MgSO4 ⋅ 7 H2O, 1 mmol/L ATP, 1 mmol/L EDTA, pH 7.4) was added and centrifuged at 12,000g for 10 min. The pellet was suspended in medium B, homogenized, and centrifuged at 300g for 10 min. The supernatant was centrifuged at 7,000g for 20 min. The pellet was washed once with medium B and once with KME buffer (100 mmol/L KCl, 50 mmol/L MOPS, 0.5 mmol/L EGTA) and centrifuged at 7,000g for 20 min.
ATP production.
Mitochondrial ATP production was determined using a luminescent assay (CLSII; Roche, Indianapolis, IN) (32). Mitochondria were suspended in assay buffer (125 mmol/L KCl, 10 mmol/L HEPES, 5 mmol/L MgCl2, 2 mmol/L K2HPO4, pH 7.4). For each subject, equal amounts of mitochondrial protein were added to the wells of a 96-well plate in the presence of complex I (2.5 mmol/L glutamate plus 2.5 mmol/L malate; 2.5 mmol/L pyruvate plus 2.5 mmol/L malate) and complex II (5 mmol/L succinate with 0.5 μmol/L rotenone to inhibit reverse electron transport through complex I) substrates. The reaction was initiated by adding Luciferase (Roche) and 0.3 mmol/L ADP. The luminescence was followed at 560 nm for 5 min. The slope in luminescence was converted to the ATP production rate using a standard curve.
ROS production.
Mitochondrial H2O2 production was measured as described (31). Amplex Red reagent (80 μmol/L, Molecular Probes, Eugene, OR), horseradish peroxidase (1 unit/mL, Sigma, St. Louis, MO), and superoxide dismutase (SOD; 30 units/mL, Sigma) were added to mitochondrial protein. SOD was included to convert all superoxide into H2O2. Glutamate plus malate or pyruvate plus malate (all at 2.5 mmol/L) were added to stimulate respiration through complex I, III, and IV. Succinate (5 mmol/L) was added to drive respiration through complex II, III, and IV. Rotenone and antimycin A (both at 0.5 µmol/L), inhibitors of complex I and III, respectively, were added to determine maximal rates of H2O2 production. Fluorescence was observed at 530-nm excitation and 590-nm emission for 5 min. The slope in fluorescence was converted to the H2O2 production rate using a standard curve.
Western blotting.
Muscle was homogenized in lysis buffer (20 mmol/L Tris, pH 7.5, 5 mmol/L EDTA, 10 mmol/L Na3PO4, 100 mmol/L NaF, 2 mmol/L Na3VO4, 1% Nonidet P-40, 10 μmol/L leupeptin, 3 mmol/L benzamidine, 10 μg/mL aprotinin, 1 mmol/L phenylmethylsulfonyl fluoride), and immunoblotting was performed using antibodies against mitochondrial transcription factor A (TFAM; Thermo Scientific, Rockford, IL), nuclear respiratory factor 1 (NRF1; Santa Cruz Biotechnology, Santa Cruz, CA), 4 hydroxynonenal (4HNE; α-Diagnostics, San Antonio, TX), MnSOD (Stressgen, Ann Arbor, MI), and Cu-ZnSOD (R&D Systems). Bands were quantitated using ImageQuant.
Citrate synthase, MnSOD, and Cu-ZnSOD activity.
Citrate synthase, MnSOD, and Cu-ZnSOD activities were measured in whole cell lysates (33,34).
Quantitative PCR.
Total RNA was isolated using TRIZOL reagent (Sigma). One-step real-time RT-PCR was performed on an ABI-Prism-7900HT System (Applied Biosystems, Foster City, CA). mRNA levels were normalized to 18S. The primers/probe used can be found in the Supplementary Data.
Proteomic analysis.
Mitochondria-enriched fraction were prepared as described (35). Forty micrograms of mitochondria-enriched fraction from older before and after exercise (n = 8) and younger (n = 7) subjects were resolved on a 10% SDS-PAGE. Each lane was divided into 20 slices, prepared for mass spectrometry, and subjected to tandem mass spectrometry as described (36). Normalized spectral abundance factors were calculated for every protein identified, and the mean log2 fold changes between young versus older before exercise and between older after exercise versus older before exercise were calculated.
Statistical analysis.
Data are expressed as means ± SE. Differences between groups were analyzed using unpaired t test or ANOVA, as appropriate. The effects of exercise within each group were analyzed using paired t test. Differences with P < 0.05 were considered statistically significant. For proteomic analysis, a paired t test was performed on the older group before and after exercise. Protein changes with a P ≤ 0.05 were selected. The log2 fold changes of these proteins in older after exercise versus older before exercise and in young versus older were compared.
RESULTS
Clinical characteristics.
BMI and fasting plasma glucose concentrations were similar in older subjects with NGT and older subjects with IGT (Table 1). Older subjects with NGT and IGT had elevated glucose levels at the 2-h time point during the OGTT compared with younger subjects with NGT. Older subjects with IGT also had elevated fasting insulin, FFA, and TNF-α concentrations in plasma. Vo2max was lower in the older NGT and IGT groups. Peripheral insulin sensitivity (M/I) was significantly (P < 0.05) and marginally (P = 0.09) decreased in the older IGT and older NGT groups, respectively, versus the young subjects. The Matsuda index of insulin sensitivity (27) also was significantly reduced in older subjects with NGT (P < 0.05) and IGT (P < 0.05) compared with younger subjects. These indices of insulin sensitivity (M/I and Matsuda index) were not significantly different between the older NGT and IGT groups.
TABLE 1.
Baseline subject characteristics
| Younger (n = 22) | Older with NGT and IGT (n = 35) | Older with NGT (n = 24) | Older with IGT (n = 11) | |
|---|---|---|---|---|
| Age (years) | 26 ± 0.717 | 74 ± 1.277* | 72.62 ± 1.49* | 80 ± 1.72* |
| Sex (men/women) | 8/14 | 17/18 | 12/12 | 5/6 |
| BMI (kg/m2) | 23.9 ± 0.49 | 24.4 ± 0.52 | 24.9 ± 0.61 | 23.1 ± 0.92 |
| OGTT fasting glucose (mg/dL) | 93 ± 2 | 96 ± 2 | 95 ± 2 | 97 ± 2 |
| OGTT 2-h glucose (mg/dL) | 95 ± 4 | 125 ± 5** | 109 ± 4* | 161 ± 4**† |
| OGTT fasting insulin (mU/mL) | 4.1 ± 0.64 | 6.4 ± 0.67* | 5.6 ± 0.55 | 8.1 ± 1.74* |
| OGTT fasting FFA (mmol/L) | 0.51 ± 0.055 | 0.59 ± 0.03 | 0.52 ± 0.04 | 0.71 ± 0.05*† |
| HbA1c (%) | 5.1 ± 0.1 | 5.5 ± 0.1** | 5.5 ± 0.1** | 5.5 ± 0.1** |
| IL-6 (pg/mL) | 1.8 ± 0.4 | 2.8 ± 0.3 | 2.8 ± 0.4 | 3.1 ± 0.8 |
| TNF-α (pg/mL) | 1.1 ± 0.2 | 1.7 ± 0.2 | 1.7 ± 0.3 | 1.8 ± 0.3* |
| Vo2max (mL/kg/min) | 25.2 ± 1.8 | 15.0 ± 0.7** | 14.9 ± 0.9** | 15.1 ± 1.2** |
| Daily activity index | 8.0 ± 0.4 | 8.2 ± 0.3 | 7.9 ± 0.3 | 8.9 ± 0.4 |
| Matsuda index | 11.8 ± 1.98 | 6.5 ± 0.52** | 6.9 ± 0.78* | 5.6 ± 0.52** |
| M/I (mg/kg FFM.min/μU/mL × 100) | 17.6 ± 1.7 | 13.0 ± 1.1* | 13.6 ± 1.6 | 11.9 ± 1.1* |
| FFM (%) | 69 ± 2 | 72 ± 2.7 | 75 ± 6 | 68 ± 3 |
| Fat mass (%) | 27 ± 2 | 31 ± 1 | 32 ± 1 | 28 ± 3 |
Data are mean ± SE.
*P < 0.05 vs. younger, **P < 0.005 vs. younger, †P < 0.05 vs. older with NGT.
Clinical effects of exercise.
Ten younger subjects with NGT and 17 older subjects (12 with NGT, 5 with IGT) underwent the exercise training program. In the older group, training caused a 15% increase in Vo2max (P < 0.05). In young subjects, training increased Vo2max by 18%, although this change did not reach statistical significance (P = 0.2). In a similar manner, the training program led to a significant increase in insulin sensitivity (M/I) in the older subjects by 1.38-fold (P < 0.05) and tended (P = 0.21) to increase insulin sensitivity in the younger group by 1.25-fold (Supplementary Fig. 1). In addition, training reduced baseline plasma insulin concentrations in younger and older subjects by 47 and 32%, respectively (P < 0.05 vs. preexercise in both groups). BMI, FFM, fat mass, hemoglobin A1c, and plasma concentrations of glucose, FFA, TNF-α, and IL-6 were unaffected by exercise.
Effect of aging and exercise on ATP production.
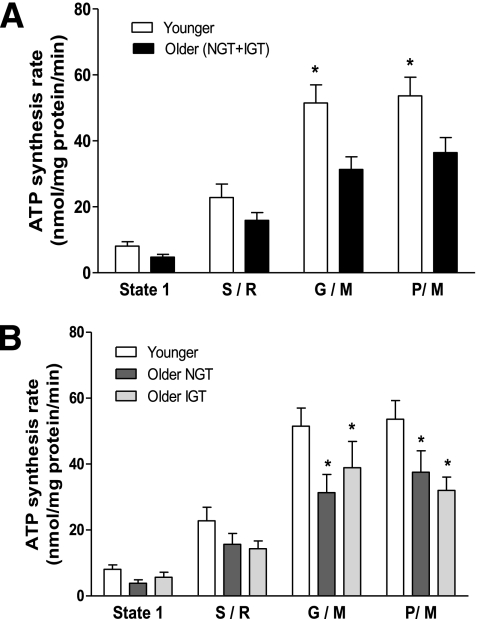
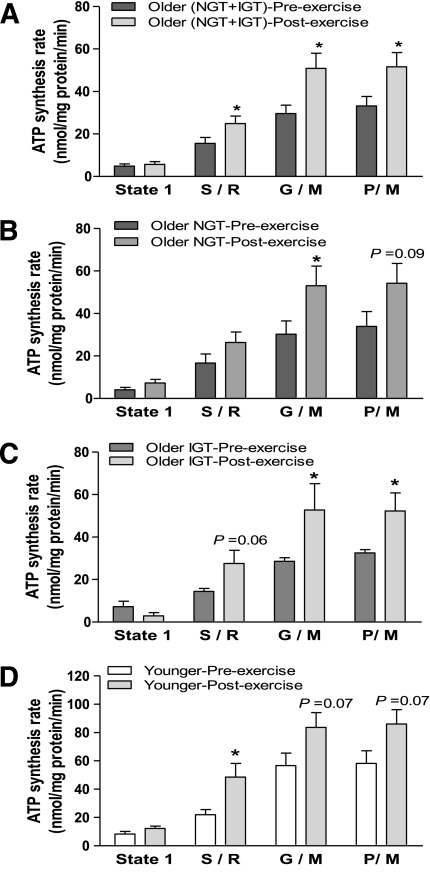
Figure 1 shows mitochondrial ATP synthesis rates in older and younger subjects. When accounting all older subjects (with NGT and IGT), ATP synthesis was significantly (P < 0.05) decreased in older versus younger subjects (Fig. 1A). After subdividing the older group into subjects with NGT and subjects with IGT, both had a lower ATP production rate compared with the younger subjects (P < 0.05 in both groups) (Fig. 1B). There was no difference in ATP synthesis between older subjects with IGT and older subjects with NGT (Fig. 1B). The exercise intervention caused a significant (P < 0.05) increase in ATP production in the older (NGT plus IGT) group (Fig. 2A), reaching levels similar to those in the young group at baseline. The increase in ATP production was of similar magnitude in older individuals with NGT and IGT (P < 0.05 in both groups) (Fig. 2B and C). Exercise also tended (P = 0.07) to increase ATP production in the younger individuals; the study was likely underpowered to detect a statistically significant effect of exercise in this population (Fig. 2D).
FIG. 1.
Production of ATP by isolated human skeletal muscle mitochondria. ATP production was measured in isolated skeletal muscle mitochondria during state 1 (no added substrates) and by using substrates succinate plus rotenone (S/R), glutamate plus malate (G/M), and pyruvate plus malate (P/M) in (A) younger and older (NGT + IGT) subjects and (B) younger, older with NGT, and older with IGT subjects. Data are means ± SE. *P < 0.05 vs. younger group.
FIG. 2.
Effect of exercise training on the production of ATP by isolated human skeletal muscle mitochondria. Mitochondrial ATP production was measured during state 1 (no added substrates) and in the presence of respiratory substrates/inhibitors succinate plus rotenone (S/R), glutamate plus malate (G/M), and pyruvate plus malate (P/M) in (A) older (NGT + IGT) subjects before and after exercise, (B) older subjects with NGT before and after exercise, (C) older subjects with IGT before and after exercise, and (D) younger subjects before and after exercise. Data are means ± SE. *P < 0.05 vs. before exercise of respective group.
Effect of aging and exercise on mitochondrial ROS production.
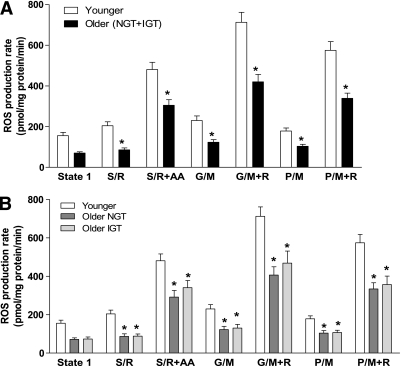
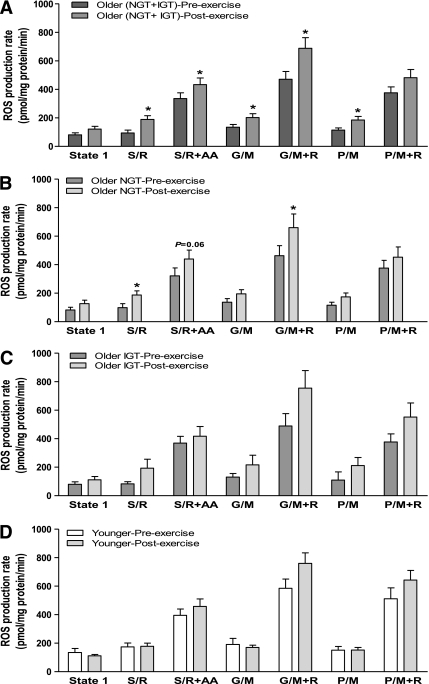
Figure 3A shows the mitochondrial ROS production rates in muscle from older and younger subjects. Surprisingly, H2O2 production was significantly (P < 0.05) decreased in older (NGT plus IGT) subjects (Fig. 3A), despite the finding that aging caused mitochondrial dysfunction (lower ATP synthesis). The decrease in ROS production was similar in older individuals with NGT and older individuals with IGT, and both were significantly (P < 0.05) reduced compared with the younger subjects (Fig. 3B). We had predicted that improving mitochondrial efficiency through exercise would decrease ROS production in aging mitochondria. To the contrary, training caused a significant (P < 0.05) increase in mitochondrial H2O2 production in the older (NGT plus IGT) subjects (Fig. 4A), and this effect occurred in both older individuals with NGT and older individuals with IGT (Fig. 4B and C). Exercise also tended (P = 0.1) to increase mitochondrial ROS production in younger subjects (Fig. 4D).
FIG. 3.
Production of hydrogen peroxide by isolated muscle mitochondria. Mitochondrial hydrogen peroxide production was measured during state 1 (no added substrates) and in the presence of respiratory substrates/inhibitors succinate plus rotenone (S/R), S/R plus antimycin A (S/R+AA), glutamate plus malate (G/M), G/M plus rotenone (G/M+R), pyruvate plus malate (P/M), and P/M plus rotenone (P/M+R) in (A) younger and older (NGT + IGT) subjects and (B) younger, older with NGT, and older with IGT subjects. Data are means ± SE. *P < 0.05 vs. younger group.
FIG. 4.
Effect of exercise training on the production of hydrogen peroxide by isolated muscle mitochondria. Mitochondrial hydrogen peroxide production was measured in isolated skeletal muscle during state 1 (no added substrates) and in the presence of respiratory substrates/inhibitors succinate plus rotenone (S/R), S/R plus antimycin A (S/R+AA), glutamate plus malate (G/M), G/M plus rotenone (G/M+R), pyruvate plus malate (P/M), and P/M plus rotenone (P/M+R) in (A) older (NGT + IGT) subjects before and after exercise, (B) older subjects with NGT before and after exercise, (C) older subjects with IGT before and after exercise, and (D) younger subjects before and after exercise. Data are means ± SE. *P < 0.05 vs. before exercise of respective group.
We also calculated the ROS/ATP production ratio, which reflects the number of ROS molecules generated during the production of one ATP molecule. ROS/ATP ratio was similar between the younger and the older group (Supplementary Fig. 2).
Overall, exercise increased ATP and ROS production in younger and older subjects. Nonetheless, the effect was more pronounced in the older group. This difference could be the result of elevated rates of ATP and ROS production at baseline in the younger group, leaving less room for improvement after training.
Citrate synthase activity.
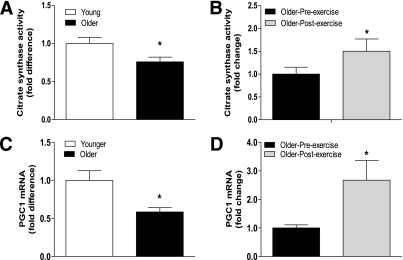
Citrate synthase activity, an indirect measurement of mitochondrial mass, was higher in the younger versus the older group by 23% (P < 0.05) (Fig. 5A). Exercise increased citrate synthase activity in the older group by 33% (P < 0.05) (Fig. 5B).
FIG. 5.
Citrate synthase activity and gene expression of transcription factor PGC-1α. Citrate synthase activity was measured in whole cell lysates from (A) younger and older subjects and (B) older subjects before and after exercise. PGC-1α mRNA levels were measured at baseline in (C) younger vs. older subjects and (D) older subjects before and after exercise. Data are means ± SE; n = 11–13 per group. *P < 0.05.
Peroxisome proliferator–activated receptor γ coactivator-1α, NRF1, and TFAM protein content and mRNA levels.
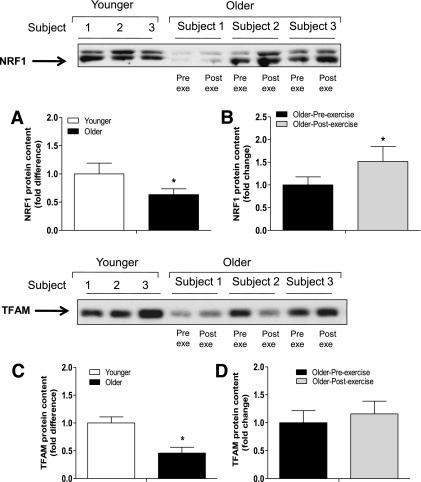
The gene expression of numerous proteins involved in the Krebs cycle, oxidative phosphorylation, and mtDNA replication/transcription is regulated by peroxisome proliferator–activated receptor γ coactivator (PGC)-1α through the activation of transcription factors such as NRF1 and TFAM (37). In this study, basal PGC-1α mRNA levels were reduced in older subjects by 42% (P < 0.05) (Fig. 5C). Older subjects also had significant (P < 0.05) decreases in baseline NRF1 (by 36%) and TFAM (by 65%) protein content (Fig. 6A and C). Exercise increased PGC-1α mRNA (Fig. 5D) and NRF1 protein content (Fig. 6B) levels in older subjects by 2.6- and 1.5-fold (P < 0.05), respectively. Training also increased NRF1 mRNA levels by 1.5-fold (P < 0.05) (Supplementary Fig. 3B) in older subjects, whereas TFAM protein (Fig. 6D) and mRNA levels (Supplementary Fig. 3D) were not affected by exercise.
FIG. 6.
Protein expression of mitochondrial transcription factors. NRF1 (A and B) and TFAM (C and D) protein content was measured at baseline in (A and C) younger vs. older subjects and (B and D) older subjects before and after exercise. Data are means ± SE; n = 11–13 per group. Pre exe, preexercise; Post exe, postexercise. *P < 0.05.
Lipid peroxidation.
As an indication of lipid peroxidation, we measured levels of 4HNE-modified proteins. There were no differences in 4HNE-modified proteins between younger and older subjects (Supplementary Fig. 4A), and these proteins did not change after exercise (Supplementary Fig. 4B).
Antioxidant enzymes.
MnSOD protein was similar between younger and older subjects (Supplementary Fig. 5A and B), whereas MnSOD activity was significantly (P < 0.05) lower in older individuals by 45% (Supplementary Fig. 5C). The protein content and activity of Cu-ZnSOD was similar between the groups (Supplementary Fig. 5E and F). The activity of MnSOD and Cu-ZnSOD did not change after exercise (Supplementary Fig. 5D and H). GPX1, MnSOD, and Cu-ZnSOD gene expression was not different between younger and older individuals, and exercise did not change the mRNA levels of these antioxidant enzymes (Supplementary Fig. 6).
Proteomic analysis of mitochondria-enriched fraction.
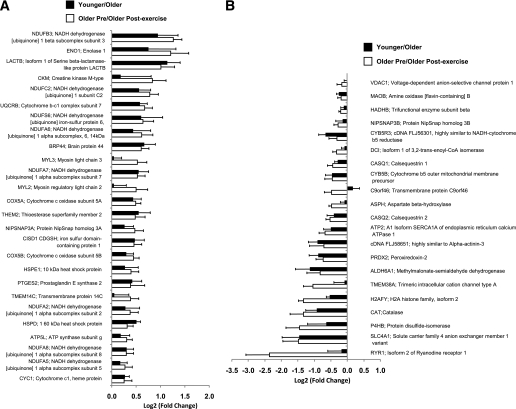
We detected 385 annotated (Gene Ontology term: 5739) mitochondrial proteins. Significant differences (P ≤ 0.05) in the abundance of 47 proteins were observed between younger and older subjects (Fig. 7A and B). The protein abundance of 26 proteins was increased in the young mitochondria (Fig. 7A). Most notable, the abundance of eight subunits of complex I (NDUFB3, NDUFC2, NDUFS6, NDUFA6, NDUFA7, NDUFA2, NDUFA8, NDUFA5) as well as five other electron transport chain proteins (UQCRB, COX5A, COX5B, ATP5 L, and CYC1) were reduced in mitochondria from older subjects. The content of four proteins, including the antioxidant enzymes peroxiredoxin 2 (PRDX2) and catalase (CAT), was increased in the old mitochondria (Fig. 7B). It is interesting to note that when the fold differences of the 47 proteins that were different between younger and older subjects were compared with the fold changes of these same proteins in the older preexercise versus older postexercise subjects, the direction of change (increased or decreased protein abundance) for these proteins was identical (Fig. 7A and B). The list of all proteins (International Protein Index number, sequence coverage, and number of unique peptides assigned) detected in the mitochondrial fractions is shown in Supplementary Table 1. The number of spectra and normalized spectral abundance factors for each protein used for determining differences between groups can also be found in Supplementary Table 1.
FIG. 7.
Relative protein abundance in the mitochondria-enriched fraction. A: Twenty-six proteins that were significantly increased in younger vs. older subjects at baseline (black bars) and the same proteins that were significantly increased in older subjects before vs. after exercise (white bars). B: Twenty-one proteins that were significantly increased in younger vs. older subjects at baseline (black bars) and the same proteins that were significantly reduced in older subjects before vs. after exercise (white bars). See Supplementary Table 1 for raw data and detailed listing of mitochondrial proteins with change in abundance from older subjects before exercise.
DISCUSSION
In this study, ATP synthesis was significantly decreased in older subjects, supporting the concept that aging is associated with a decrease in mitochondrial function. This finding is consistent with previous investigations that also have reported decreased ATP production by mitochondria isolated from aged human muscle (7). According to the free radical and mitochondrial theories of aging, this decrease in mitochondrial function is the result of damaged/oxidized mitochondrial components (mtDNA, proteins, and lipids). These theories also propose that dysfunctional mitochondria perpetuate oxidative damage by promoting further leakage of electrons, which generate more ROS. In line with this model, mice with defective oxidative phosphorylation as a result of genetic ablation of adenine nucleotide translocator (Ant1), an enzyme that exchanges ADP to ATP through the inner mitochondrial membrane, display marked increases in mitochondrial ROS production in muscle, heart, and brain (38). Nonetheless, in the current study, we found the opposite; ROS production was actually lower in mitochondria from older subjects. These results indicate that old mitochondria are dysfunctional with respect to substrate oxidation and ATP synthesis, but they are not dysfunctional with regard to leaking more electrons during respiration. In fact, the ROS/ATP production ratio, which reflects the number of ROS molecules generated during the production of one ATP molecule, were similar in younger and older individuals, indicating that differences in ROS production between groups are a function of the decreased ATP synthesis rate.
Older and younger subjects had similar levels of 4HNE, a frequently used marker of lipid peroxidation of proteins. This finding, together with the lower ROS production seen in older subjects, does not support the hypothesis that aging-related mitochondrial dysfunction increases oxidative stress. It is important to note, however, that differences in oxidative stress between younger and older subjects cannot be completely ruled out, considering that other important markers of oxidative stress, such as 8-oxo-deoxyguanosine (7), F2-isoprostanes (31), and gluthatione redox state, were not measured because there was insufficient tissue to carry out all these experiments. Furthermore, there are other nonmitochondrial sources of ROS that were not evaluated in this study, including peroxisomes, nicotinamide adenine dinucleotide phosphate oxidases, xanthine oxidases, and nitric oxide synthases. It would be interesting to evaluate in future studies whether these sources of ROS compensate for the diminished ROS production observed in old mitochondria.
Most of the evidence suggesting that oxidative stress increases with age comes from studies that have documented alterations in antioxidant enzyme levels (39–41) and in oxidative damage to mtDNA, lipids, and proteins (7–9). To the best of our knowledge, this is the first study that has directly tested whether aging is associated with increased mitochondrial ROS production in human muscle using Amplex Red, a highly sensitive and stable fluorescent reagent. This finding is in contrast to results of a prior study done by our group in which we found that decreased ATP production in muscle from old mice was accompanied by higher ROS production (32). The discrepant results with the current study could be due to species differences.
Another goal of this study was to establish the relationship between age-related changes in mitochondrial function (ATP synthesis) and insulin sensitivity. To avoid the confounding effects of obesity and inactivity, we enrolled younger individuals with NGT and older individuals with NGT who were lean and had similar activity levels. We also enrolled older subjects with IGT. If mitochondrial dysfunction plays a role in the pathogenesis of type 2 diabetes, one might expect that it would be present in the earlier stages of the disease (i.e., IGT). Older diabetic subjects were not studied because of the negative effect that high glucose per se has on mitochondrial function (42–44) and insulin sensitivity (45,46). ATP synthesis was reduced in both older subjects with NGT and older subjects with IGT, compared with younger individuals. In line with the reduction in mitochondrial ATP synthesis, insulin sensitivity also tended to be modestly reduced in older subjects with NGT. ATP production also was lower in older subjects with IGT compared with the younger group. Nonetheless, there were no differences in ATP (and ROS) production levels between older subjects with NGT and older subjects with IGT, suggesting that changes in mitochondrial function do not contribute to the transition from NGT to IGT in elderly individuals. Considering that IGT is a prediabetic state, these results also do not support the hypothesis that mitochondrial dysfunction is involved in the pathogenesis of type 2 diabetes, although it would be important to confirm this by performing measurements of mitochondrial function in older subjects with type 2 diabetes.
An analysis of the mitochondrial proteome was performed to determine whether the decreases in mitochondrial function observed in old mitochondria were associated with changes in the content of mitochondrial proteins. The most striking finding was that the content of several electron transport proteins was reduced in the older subjects, which likely contributed to the lower capacity to synthesize ATP. In addition, the expression/content of PGC-1α, NRF1, and TFAM also was reduced in older subjects, which helps explain the reductions in mitochondrial function and mass.
Physical activity is an effective modality to improve insulin sensitivity. Exercise is particularly effective in elderly individuals in reducing the progression from IGT to type 2 diabetes (47). We hypothesized that training would improve mitochondrial function in older subjects and that improving mitochondrial efficiency would result in decreased oxidative stress. As predicted, exercise caused a significant increase in mitochondrial ATP synthesis in older subjects. However, contrary to our hypothesis, the improvement in mitochondrial function was accompanied by an increase in mitochondrial ROS production. The increase in mitochondrial ROS production caused by exercise seems counterintuitive, considering that oxidative stress is thought to impair insulin action (24). On the other hand, recent studies suggest that ROS might have a beneficial effect on insulin sensitivity. For example, GPX1 null mice are partially protected from high-fat diet-induced insulin resistance (48). Furthermore, muscle contraction/exercise increases ROS production (49), and antioxidants such as n-acetylcysteine blunt contraction-stimulated muscle glucose uptake (50). Collectively, the results from these studies, along with the present data, suggest that ROS do not impair insulin action and actually might be involved in the mechanism by which exercise improves insulin sensitivity.
The training program led to major changes in the mitochondrial proteome in older subjects. As mentioned above, numerous proteins, many of them involved in the electron transport chain, were downregulated at baseline in older subjects. Training resulted in increased expression of most downregulated proteins to levels similar to those observed in young mitochondria prior to exercise. In addition, training lowered the expression of several proteins that were upregulated at baseline in the older subjects. Overall, the mitochondrial proteome of the older subjects after training was remarkably similar to the mitochondrial proteome of the younger individuals at baseline. A prior cross-sectional study found that highly trained (≥1 h of exercise for 6 days per week for ≥4 years) older subjects have increased content of proteins involved in ATP synthesis versus untrained older individuals (1). The present prospective, interventional study highlights the ability of exercise to rapidly (within a few months) counteract aging at the molecular level.
In conclusion, aging is associated with reductions in mitochondrial function, mitochondrial protein content, and expression of transcriptional regulators that control mitochondrial function/biogenesis. This global downregulation in mitochondrial function and mass in older subjects was accompanied with a modest reduction in insulin sensitivity. However, the severity of mitochondrial dysfunction was similar between older subjects with NGT and older subjects with IGT, indicating that declines in mitochondrial function do not contribute to the transition from NGT to IGT in elderly individuals. Furthermore, age-related declines in mitochondrial function were not associated with increased production of ROS in human muscle, suggesting that mitochondria-derived ROS do not promote insulin resistance in aging muscle. This study also demonstrates that training reverses the phenotype of mitochondria from older, sedentary subjects with respect to ATP synthesis, the mitochondrial proteome, and ROS production. Lastly, the increase in ROS generation could have a beneficial effect on insulin action.
Supplementary Material
ACKNOWLEDGMENTS
S.G. has received an Institutional National Research Service Award (T32-HL-04776). A.R. has received a grant from the National Institutes of Health (NIH; AG-019316). R.A.D. has received grants from the American Diabetes Association, the NIH (DK-24092), and the U.S. Department of Veterans Affairs. H.V.R. has received a grant from the NIH (PO1-AG-020591). N.M. has received grants from the American Diabetes Association, the NIH (DK-80157), the San Antonio Nathan Shock Center, and the South Texas Health Research Center and was the recipient of a Paul B. Beeson Career Development Award (AG-030979) from the American Federation for Aging Research and the National Institute on Aging. No potential conflicts of interest relevant to this article were reported.
S.G. researched data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. R.L. and N.L. researched data and reviewed and edited the manuscript. M.M.-C., J.J.-G., B.P.B., J.J.G.-G., and M.A.-G. researched data. A.R., R.A.D., L.M., and H.V.R. researched data and reviewed and edited the manuscript. N.M. researched data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript.
Parts of this study were presented in abstract form at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009, and the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
The authors thank all the volunteers that participated in the study.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0121/-/DC1.
REFERENCES
- 1.Lanza IR, Short DK, Short KR, et al. Endurance exercise as a countermeasure for aging. Diabetes 2008;57:2933–2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Short KR, Vittone JL, Bigelow ML, et al. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes 2003;52:1888–1896 [DOI] [PubMed] [Google Scholar]
- 3.Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci USA 1996;93:15364–15369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barazzoni R, Short KR, Nair KS. Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart. J Biol Chem 2000;275:3343–3347 [DOI] [PubMed] [Google Scholar]
- 5.Welle S, Bhatt K, Thornton CA. High-abundance mRNAs in human muscle: comparison between young and old. J Appl Physiol 2000;89:297–304 [DOI] [PubMed] [Google Scholar]
- 6.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 2003;300:1140–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA 2005;102:5618–5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melov S, Shoffner JM, Kaufman A, Wallace DC. Marked increase in the number and variety of mitochondrial DNA rearrangements in aging human skeletal muscle. Nucleic Acids Res 1995;23:4122–4126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee CM, Weindruch R, Aiken JM. Age-associated alterations of the mitochondrial genome. Free Radic Biol Med 1997;22:1259–1269 [DOI] [PubMed] [Google Scholar]
- 10.Welle S, Bhatt K, Shah B, Needler N, Delehanty JM, Thornton CA. Reduced amount of mitochondrial DNA in aged human muscle. J Appl Physiol 2003;94:1479–1484 [DOI] [PubMed] [Google Scholar]
- 11.Qu B, Li QT, Wong KP, Ong CN, Halliwell B. Mitochondrial damage by the “pro-oxidant” peroxisomal proliferator clofibrate. Free Radic Biol Med 1999;27:1095–1102 [DOI] [PubMed] [Google Scholar]
- 12.Harman D. Free radical theory of aging: dietary implications. Am J Clin Nutr 1972;25:839–843 [DOI] [PubMed] [Google Scholar]
- 13.Miquel J, Economos AC, Fleming J, Johnson JE., Jr Mitochondrial role in cell aging. Exp Gerontol 1980;15:575–591 [DOI] [PubMed] [Google Scholar]
- 14.Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci 2006;61:534–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coggan AR, Spina RJ, King DS, et al. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol 1992;47:B71–B76 [DOI] [PubMed] [Google Scholar]
- 16.Andres R. Aging and diabetes. Med Clin North Am 1971;55:835–846 [DOI] [PubMed] [Google Scholar]
- 17.Broughton DL, Taylor R. Review: deterioration of glucose tolerance with age: the role of insulin resistance. Age Ageing 1991;20:221–225 [DOI] [PubMed] [Google Scholar]
- 18.Davidson MB. The effect of aging on carbohydrate metabolism: a review of the English literature and a practical approach to the diagnosis of diabetes mellitus in the elderly. Metabolism 1979;28:688–705 [DOI] [PubMed] [Google Scholar]
- 19.Reaven GM, Chen N, Hollenbeck C, Chen YD. Effect of age on glucose tolerance and glucose uptake in healthy individuals. J Am Geriatr Soc 1989;37:735–740 [DOI] [PubMed] [Google Scholar]
- 20.Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971-1993. Diabetes Care 1998;21:1138–1145 [DOI] [PubMed] [Google Scholar]
- 21.DeFronzo RA. Glucose intolerance and aging: evidence for tissue insensitivity to insulin. Diabetes 1979;28:1095–1101 [DOI] [PubMed] [Google Scholar]
- 22.Fink RI, Kolterman OG, Griffin J, Olefsky JM. Mechanisms of insulin resistance in aging. J Clin Invest 1983;71:1523–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudich A, Tirosh A, Potashnik R, Hemi R, Kanety H, Bashan N. Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. Diabetes 1998;47:1562–1569 [DOI] [PubMed] [Google Scholar]
- 24.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006;440:944–948 [DOI] [PubMed] [Google Scholar]
- 25.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 1982;36:936–942 [DOI] [PubMed] [Google Scholar]
- 26.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 28.Cusi K, Maezono K, Osman A, et al. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest 2000;105:311–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 1981;30:1000–1007 [DOI] [PubMed] [Google Scholar]
- 30.Coker RH, Hays NP, Williams RH, et al. Exercise-induced changes in insulin action and glycogen metabolism in elderly adults. Med Sci Sports Exerc 2006;38:433–438 [DOI] [PubMed] [Google Scholar]
- 31.Lustgarten MS, Jang YC, Liu Y, et al. Conditional knockout of Mn-SOD targeted to type IIB skeletal muscle fibers increases oxidative stress and is sufficient to alter aerobic exercise capacity. Am J Physiol Cell Physiol 2009;297:C1520–C1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mansouri A, Muller FL, Liu Y, et al. Alterations in mitochondrial function, hydrogen peroxide release and oxidative damage in mouse hind-limb skeletal muscle during aging. Mech Ageing Dev 2006;127:298–306 [DOI] [PubMed] [Google Scholar]
- 33.Jang YC, Pérez VI, Song W, et al. Overexpression of Mn superoxide dismutase does not increase life span in mice. J Gerontol A Biol Sci Med Sci 2009;64:1114–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Röckl KS, Hirshman MF, Brandauer J, Fujii N, Witters LA, Goodyear LJ. Skeletal muscle adaptation to exercise training: AMP-activated protein kinase mediates muscle fiber type shift. Diabetes 2007;56:2062–2069 [DOI] [PubMed] [Google Scholar]
- 35.Højlund K, Yi Z, Hwang H, et al. Characterization of the human skeletal muscle proteome by one-dimensional gel electrophoresis and HPLC-ESI-MS/MS. Mol Cell Proteomics 2008;7:257–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hwang H, Bowen BP, Lefort N, et al. Proteomics analysis of human skeletal muscle reveals novel abnormalities in obesity and type 2 diabetes. Diabetes 2010;59:33–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 2006;27:728–735 [DOI] [PubMed] [Google Scholar]
- 38.Esposito LA, Melov S, Panov A, Cottrell BA, Wallace DC. Mitochondrial disease in mouse results in increased oxidative stress. Proc Natl Acad Sci USA 1999;96:4820–4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hollander J, Bejma J, Ookawara T, Ohno H, Ji LL. Superoxide dismutase gene expression in skeletal muscle: fiber-specific effect of age. Mech Ageing Dev 2000;116:33–45 [DOI] [PubMed] [Google Scholar]
- 40.Pansarasa O, Castagna L, Colombi B, Vecchiet J, Felzani G, Marzatico F. Age and sex differences in human skeletal muscle: role of reactive oxygen species. Free Radic Res 2000;33:287–293 [DOI] [PubMed] [Google Scholar]
- 41.Tiidus PM, Pushkarenko J, Houston ME. Lack of antioxidant adaptation to short-term aerobic training in human muscle. Am J Physiol 1996;271:R832–R836 [DOI] [PubMed] [Google Scholar]
- 42.Toledo FG, Menshikova EV, Azuma K, et al. Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes 2008;57:987–994 [DOI] [PubMed] [Google Scholar]
- 43.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002;51:2944–2950 [DOI] [PubMed] [Google Scholar]
- 44.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 2005;54:8–14 [DOI] [PubMed] [Google Scholar]
- 45.Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest 1987;79:1510–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kahn BB, Shulman GI, DeFronzo RA, Cushman SW, Rossetti L. Normalization of blood glucose in diabetic rats with phlorizin treatment reverses insulin-resistant glucose transport in adipose cells without restoring glucose transporter gene expression. J Clin Invest 1991;87:561–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loh K, Deng H, Fukushima A, et al. Reactive oxygen species enhance insulin sensitivity. Cell Metab 2009;10:260–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reid MB, Haack KE, Franchek KM, Valberg PA, Kobzik L, West MS. Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. J Appl Physiol 1992;73:1797–1804 [DOI] [PubMed] [Google Scholar]
- 50.Sandström ME, Zhang SJ, Bruton J, et al. Role of reactive oxygen species in contraction-mediated glucose transport in mouse skeletal muscle. J Physiol 2006;575:251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.