Abstract
OBJECTIVE
We have previously shown that overexpression of the Na-Ca exchanger (NCX1), a protein responsible for Ca2+ extrusion from cells, increases β-cell programmed cell death (apoptosis) and reduces β-cell proliferation. To further characterize the role of NCX1 in β-cells under in vivo conditions, we developed and characterized mice deficient for NCX1.
RESEARCH DESIGN AND METHODS
Biologic and morphologic methods (Ca2+ imaging, Ca2+ uptake, glucose metabolism, insulin release, and point counting morphometry) were used to assess β-cell function in vitro. Blood glucose and insulin levels were measured to assess glucose metabolism and insulin sensitivity in vivo. Islets were transplanted under the kidney capsule to assess their performance to revert diabetes in alloxan-diabetic mice.
RESULTS
Heterozygous inactivation of Ncx1 in mice induced an increase in glucose-induced insulin release, with a major enhancement of its first and second phase. This was paralleled by an increase in β-cell proliferation and mass. The mutation also increased β-cell insulin content, proinsulin immunostaining, glucose-induced Ca2+ uptake, and β-cell resistance to hypoxia. In addition, Ncx1+/− islets showed a two- to four-times higher rate of diabetes cure than Ncx1+/+ islets when transplanted into diabetic animals.
CONCLUSIONS
Downregulation of the Na/Ca exchanger leads to an increase in β-cell function, proliferation, mass, and resistance to physiologic stress, namely to various changes in β-cell function that are opposite to the major abnormalities seen in type 2 diabetes. This provides a unique model for the prevention and treatment of β-cell dysfunction in type 2 diabetes and after islet transplantation.
The prevalence of type 2 diabetes is progressing in an alarming way in most regions of the world (1,2). Type 2 diabetes is a complex disease characterized by insulin resistance and β-cell dysfunction. One of the earliest abnormalities occurring in this disease is the alteration in pulsatile insulin release with the suppression of the first phase of insulin response to glucose (3). The second phase of insulin release is also diminished and a number of abnormalities of continuous insulin release have been observed (4,5). In addition to a defect in β-cell function, a reduction in islet and β-cell mass has been observed (6,7). This reduction could be related to increased programmed cell death (apoptosis), to decreased β-cell replication, or both (8).
In a previous work, we observed that overexpression of the Na/Ca exchanger (isoform 1: Na-Ca exchanger [NCX1]), a protein responsible for Ca2+ extrusion from cells (9,10), increased β-cell apoptosis and reduced β-cell proliferation (11). The increase in apoptosis resulted from endoplasmic reticulum (ER) Ca2+ depletion with resulting ER stress (11).
If it is possible to increase apoptosis and to decrease β-cell proliferation by increasing the activity of NCX1, it may be possible to obtain the opposite effects by downregulating such a mechanism. To test this hypothesis, we generated Ncx1 heterozygous deficient mice (Ncx1+/−). Our data show that Ncx1+/− heterozygous inactivation induces several β-cell modifications, including an increase in glucose-induced insulin release and in β-cell proliferation and mass. Ncx1+/− islets also displayed an increased resistance to hypoxia, and when transplanted in diabetic animals, showed a two- to four-times higher rate of diabetes cure than Ncx1+/+ islets.
RESEARCH DESIGN AND METHODS
Generation of Ncx1−/− mice.
Exon 11 of the murine Ncx1 gene (GenBank, accession number AF409089) was cloned from a 129/Sv genomic phage library. The first 206-bp were amplified by PCR and a BamHI site was introduced for cloning. A targeting vector was constructed by inserting the neomycin resistance cassette (neo) into the BamHI restriction site of that amplified exon. Standard procedures were used to generate Ncx1+/− mice (12). Except as otherwise stated, experimental mice were 2 to 6 months old, of both sexes, and had F2 genetic backgrounds from 129/Sv and CD1 mice. Ncx1+/+ mice consisted of age-matched littermates with two wild-type (WT) alleles at the Ncx1 locus (Ncx1+/+).
Intracellular Ca2+ concentration measurements.
The measurement of intracellular Ca2+ concentration using fura-2 was done, as previously described (11,13), using a camera-based image analysis system (MetaFluor, Universal Imaging, Ypsilanti, MI).
Time-frequency analysis of spectral densities of the Ca2+ oscillations was computed using Ackqnowledge Software, (Biopac Systems, Goleta, CA), with a Hamming window. Data were segmented in successive epochs of 1 min. We computed the power spectral density (PSD), the integral below the power spectrum for the frequency band 0–0.17 Hz, and the crest factor for the same frequency band (ratio amplitude of the power spectra/integral 0–0.17 Hz).
45Ca uptake, insulin content, and insulin release from isolated islets.
The methods used to measure 45Ca uptake in intact islets and insulin release from perifused islets are described in detail elsewhere (11,13–15). Insulin was assayed using an ELISA kit (Mercodia, Uppsala, Sweden). For the measurement of insulin content, the islets were homogenized in water in the absence of glucose.
Measurement of glucose metabolism and morphology.
The methods used to measure d-[5-3H]glucose metabolism and d-[U-14C]glucose oxidation in intact islets, have been previously described (16,17).
Golgi proinsulin/β-cell area was evaluated on slides stained for proinsulin with immunoperoxidase (18) digitized through a JVC KY-F58 color digital camera (Japan Victor, Brussels, Belgium). Tissues sections (5-µm thick) were prepared according to Jacoby et al. (19) and processed for hematoxylin-eosin staining.
For electron microscopy, groups of 30 islets were fixed, epoxide-embedded, and analyzed as described previously (20).
Measurement of β-cell proliferation, apoptosis, mass, and size.
To measure β-cell proliferation, mice were injected intraperitoneally with 5-bromo-2-deoxyuridine (BrdU, Sigma-Aldrich, St. Louis, MO; 100 mg/kg body wt) 6 h before euthanasia, as previously described (21). An alternative method to measure β-cell replication was the Ki67 labeling method (rabbit monoclonal anti-Ki67, Vector Laboratories, Brussels, Belgium) with a method similar to the BrdU immunofluorescence labeling (22).
To measure β-cell apoptosis, the terminal deoxynucleotidyltransferase mediated dUTP nick-end labeling (TUNEL) method was used, with the In Situ Cell Death Detection Kit (Roche Diagnostics, Vilvoorde, Belgium). The method for β-cell labeling and counting was similar to that for β-cell proliferation.
Cell viability in vitro was measured using Hoechst 33342 (Ho342) and propidium iodide (PI) (23). The percent viability in Ncx1+/+ single β-cells and islets (not exposed to thapsigargin or cyclopiazonic acid) was 65% to 70% and 85% to 95%, respectively. In some experiments, cytokines were used at the following concentrations: human IL-1β: 50 units/mL (R&D Systems, Oxon, UK); mouse interferon-γ: 1000 units/mL (tebu-bio, Boechout, Belgium).
Quantification of β-cell mass was performed by point-counting morphometry of insulin-peroxidase immunostained pancreatic sections, as previously described (24). Individual β-cell size was measured using the calibrated ImageJ (National Institutes of Health, Bethesda, MD) image analysis program. The β-cell area of the pancreatic section was divided by the number of β-cell nuclei identified in the area.
In vitro hypoxia studies.
In vitro hypoxia studies were as previously described (25). The duration of hypoxia was 6 h. Viability of β cells was measured as described above.
Glucose metabolism, insulin sensitivity, serum glucagon, growth hormone, and glucagon-like peptide 1 measurement in vivo.
The measurement of glucose metabolism and insulin sensitivity in vivo were done as previously described (26,27). Serum glucagon, growth hormone, and glucagon-like peptide 1 (GLP-1) were measured using Glucagon Human/Mouse/Rat ELISA (Alpco, Salem, NH), Rat/Mouse Growth Hormone ELISA Kit (Millipore, St. Charles, MO), and Mouse GLP-1 ELISA kit (Antibodies-online.com, Aachen, Germany).
Diabetes induction and islets transplantation.
Diabetes was induced in 10- to 12-week-old C57BL6N mice using a single intravenous injection of alloxan (90 mg/kg; Sigma) (25,26). Grafts of 50 to 400 islets from Ncx1+/+ or Ncx1+/− mice were transplanted under the kidney capsule in diabetic mice. Thereafter, the nonfasting blood glucose levels were measured in each animal up to 100 days, using a Glucometer (Abbott, Diegem, Belgium). Islet grafts were considered functional when the nonfasting blood glucose returned to normoglycemic levels (<220 mg/dL). In some animals, the graft-bearing kidney was removed to confirm islet graft function.
Statistics.
The results are expressed as means ± SEM. The statistical significance of differences between data was assessed by using ANOVA, followed by the Tukey post-test.
RESULTS
Generation of Ncx1−/− mice.
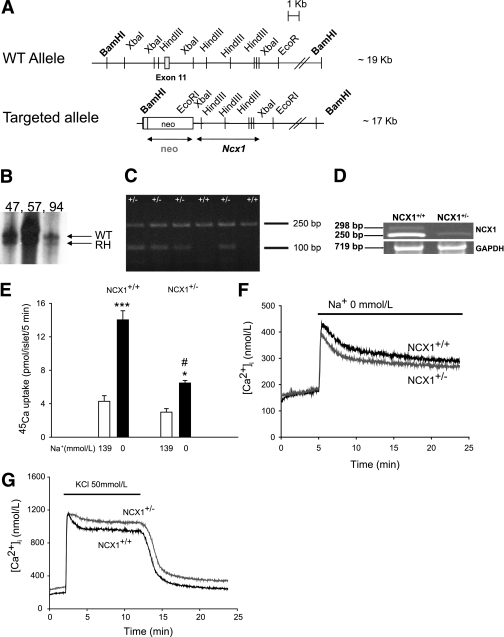
A mutant allele was constructed as described in research design and methods (Fig. 1A). After electroporation with the targeting vector, the recombinant embryonic stem cell clones were identified by DNA hybridization and used to produce chimeric mice (Fig. 1B). Transmission of the mutant allele produced Ncx1 heterozygous mice that were mated to generate Ncx1 null mutants (Ncx1−/−). The Ncx1−/− mice were not viable and died during embryogenesis as described (28). Heterozygous and WT mice genotyping from tail biopsy specimen DNA revealed an Ncx1 WT amplicon of ∼250 bp and an Ncx1 recombinant allele of ∼100 bp, which were simultaneously amplified by PCR (Fig. 1C). NCX1 mRNA levels in batches of islets from Ncx1+/+ and Ncx1+/− mice, measured using RT-PCR, showed a clear decrease in Ncx1+/− mice (Fig. 1D). Ncx1+/− mice were viable, fertile, had a normal body weight, and showed no macro- or microscopic abnormalities at the level of the brain, heart, lung, liver, spleen, stomach, intestine, and thymus (Supplementary Fig. 1). Likewise, serum levels of glucagon, growth hormone (GH) in the fasting state and GLP-1 in the fed state were comparable in Ncx1+/+ and Ncx1+/− mice at 12 weeks (0.48 ± 0.04 vs. 0.41 ± 0.14 ng/mL, 2.4 ± 0.44 vs. 1.67 ± 0.44 ng/mL, and 3.20 ± 0.28 vs. 2.86 ± 0.15 pg/ml, respectively; n = 4 in each case, except for GH where n = 9–11, respectively, P > 0.05).
FIG. 1.
Targeted disruption of the Ncx1 gene and Na/Ca exchange activity in Ncx1+/+ and Ncx1+/− islets. A: Structure of the WT and the targeted alleles. The eleventh exon, the neomycin resistance cassette, and the probes used in DNA and RNA hybridization analysis (bars underneath the targeted allele) are represented. B: DNA hybridization analysis of BamHI digested genomic DNA isolated from embryonic stem cell clones using the depicted probe. RH, recombinant homolog. C: PCR analysis for genotyping. D: Ncx1 mRNA levels in batches of islets from Ncx1+/+ and Ncx1+/− mice. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. E: 45Ca uptake in the presence and the absence of extracellular Na+. Means ± SEM of four experiments, comprising four to five replicates each. *P < 0.05, ***P < 0.001 vs. Na+ 139 mmol/L, #P < 0.001 vs. Ncx1+/+. F: Effect of extracellular Na+ removal on [Ca2+]i in Ncx1+/+ and Ncx1+/− islets. The period of exposure to Na+-free medium is indicated by a bar above the curves. The curves shown are the mean of seven traces in each case. G: Effect of KCl (50 mmol/L) on [Ca2+]i in Ncx1+/+ and Ncx1+/− islets. The period of exposure to KCl is indicated by a bar above the curves. The curves shown are the mean of 13 traces in each case.
Na/Ca exchange activity in Ncx1+/− islets.
To evaluate the functional consequences of Ncx1 heterozygous inactivation, the activity of the Na/Ca exchanger was evaluated by measuring the effect of extracellular Na+ removal on 45Ca uptake and cytosolic free Ca2+ concentration ([Ca2+]i), and of K+-induced membrane depolarization on [Ca2+]i. Removal of extracellular Na+ increased 45Ca uptake in Ncx1+/+ and Ncx1+/− islets (Fig. 1E), the uptake in Ncx1+/− islets recorded in the absence of extracellular Na+ being half of that measured in Ncx1+/+ islets. Removal of extracellular Na+ induced a sustained increase in [Ca2+]i (Fig. 1F), that again was reduced in Ncx1+/− compared with Ncx1+/+ islets. Thus, the increase in [Ca2+]i induced by the absence of extracellular Na+, measured as the area under the curve over the baseline value during a 5- to 25-min period was reduced by 24% in Ncx1+/− compared with Ncx1+/+ islets (P < 0.05). K+ increased [Ca2+]i in both types of islets (Fig. 1G), but [Ca2+]i was always slightly higher in Ncx1+/− than in Ncx1+/+ mice. The difference in mean concentration was higher (36%) during the period of K+ exposure (min 2–12) and of K+ removal (65%; min 12–24) than during the baseline period (min 0–2; P < 0.001), indicating that Ca2+ extrusion was mainly impaired during the period of K+ stimulation and recovery. Taken as a whole, these data indicate that partial Ncx1 heterozygous inactivation reduces Na/Ca exchange activity in islet cells both in its forward and reverse mode. We then examined the effect of Ncx1 heterozygous inactivation on islet function.
Islet function in Ncx1+/− islets
Insulin release.
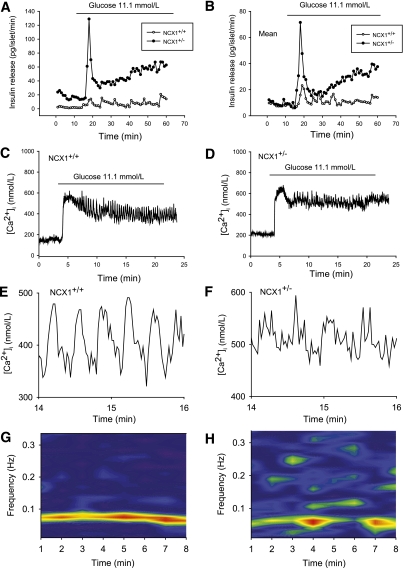
Figure 2A and B shows the effect of an increase in glucose concentration from 2.8 to 11.1 mmol/L on insulin release from perifused islets (A: representative experiment, B: mean of 4–6 experiments). In Ncx1+/+ islets, glucose induced an oscillatory increase in insulin release. In Ncx1+/− islets, glucose induced a marked first-phase insulin release, followed by a progressive increase with less clear oscillations. The amount of insulin released during the entire period of stimulation (16–60 min) was about 2.4-times higher in Ncx1+/− than in Ncx1+/+ islets (P < 0.02). A similar observation was made at 16.7 mmol/L glucose, where the fold-increase was 1.7 (P < 0.05), or by perifusing a single islet, where the fold-increase in response to 11.1 mmol/L glucose was 2.6 (P < 0.05, Supplementary Figs. 2 and 3, respectively).
FIG. 2.
Effect of Ncx1 heterozygous inactivation on glucose-induced insulin release and [Ca2+]i oscillations. Effect of 11.1 mmol/L glucose on insulin release (A and B) and [Ca2+]i (C and D) from groups of 20 islets: representative experiment (A), mean of four (Ncx1+/+) and six (Ncx1+/−) individual experiments (B). C and D show typical individual traces observed in Ncx1+/+ and Ncx1+/− islets, respectively. The curves are representative of seven and eight individual traces, respectively. The period of exposure to glucose 11.1 mmol/L is indicated by a bar above the curves. In both cases, the islets were from different mice. E and F present the same traces as in panels C and D, showing the [Ca2+]i oscillations between min 14 and 16 on a larger scale. G and H: Power spectral analysis of the oscillations observed in typical individual traces. (A high-quality digital representation of this figure is available in the online issue.)
Glucose-induced increase in [Ca2+]i.
Oscillatory insulin release is associated with oscillatory [Ca2+]i increases (29,30). Figure 2C and D shows the effect of 11.1 mmol/L glucose on [Ca2+]i oscillations in intact islets. Ncx1+/+ islets showed a rapid increase in [Ca2+]i with an initial phase, followed by a second phase displaying regular oscillations. Ncx1+/− islets also showed a rapid increase in [Ca2+]i but the initial phase was more distinct, separated from the second phase, which displayed disrupted [Ca2+]i oscillations. Figure 2E and F shows at a larger scale the oscillations during the period 14–16 min. The number of oscillations is easily determined and counted in Ncx1+/+ islets, at variance with Ncx1+/− islets, where the oscillations are irregular and difficult to identify. PSD analysis of the oscillations showed that Ncx1+/+ islets displayed a single peak with a very stable generator in frequency and magnitude (Fig. 2G), whereas Ncx1+/− islets displayed an unstable dominant frequency and numerous peak intrusions above the frequency of 0.10 Hz, illustrating the irregularity of the oscillations (Fig. 2H). Supplementary Fig. 4A and B also shows that there was a significant difference both in the magnitude of the peak of power spectra (Max PSD) and in the crest factor (ratio Max/Integral) between Ncx1+/+ and Ncx1+/− islets.
Glucose metabolism.
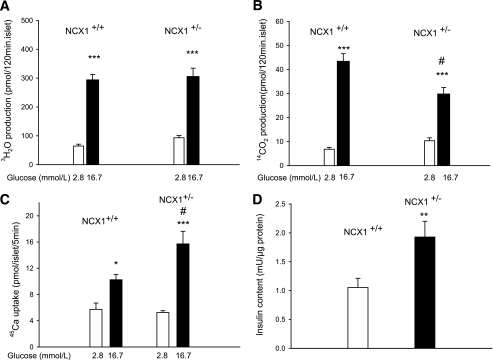
To stimulate insulin release, glucose must be metabolized by the pancreatic β-cell (31). In Ncx1+/+ and Ncx1+/− islets, the utilization of d-[5-3H] glucose increased as a result of the rise in the glucose concentration from 2.8 to 16.7 mmol/L (Fig. 3A). At the low hexose concentration, 3H2O generation from d-[5-3H]glucose was slightly higher in Ncx1+/− than in Ncx1+/+ cells.
FIG. 3.
Effect of Ncx1 heterozygous inactivation on glucose metabolism, 45Ca uptake, and insulin content. Metabolism of d-[5H]glucose (A) and d-[U-14C]glucose (B) is shown in Ncx1+/+ and Ncx1+/− islets. Mean ± SEM values are shown for 12 to 25 individual determinations. A: ***P < 0.001 vs. 2.8 mmol/L. B: ***P < 0.01 vs. 2.8 mmol/L; #P < 0.01 vs. Ncx1+/+ islets at 16.7 mmol/L glucose. C: Effect of glucose on 45Ca uptake in Ncx1+/+ and Ncx1+/− islets. Mean ± SEM values are shown for four experiments, comprising four to six replicates in each case. *P < 0.05; ***P < 0.001 vs. 2.8 mmol/L; #P < 0.01 vs. Ncx1+/+ islets at 16.7 mmol/L glucose. D: Insulin content of batches of 10 islets (means ± SEM of 14 and 7 individual determinations). **P < 0.01 vs. Ncx1+/+ islets.
Generation of 14CO2 from d-[U-14C]glucose was also increased in response to the rise in glucose concentration in both type of islets (Fig. 3B). However, although oxidation tended to be higher in Ncx1+/− islets at the low glucose concentration, glucose oxidation was significantly lower in Ncx1+/− than in Ncx1+/+ cells at the high glucose concentration. As a result of these differences, the paired ratio between d-[U-14C]glucose oxidation and d-[5-3H]glucose utilization increased with the rise in glucose concentration in Ncx1+/+ cells (P < 0.05), but it did not change in Ncx1+/− cells. Compared with Ncx1+/+ cells, ATP generation tended to be increased in Ncx1+/− islets at low (53%) but reduced at high glucose concentration (−20%, P < 0.05, Supplementary Table 1).
Uptake of 45Ca in response to glucose.
Glucose-induced insulin release is preceded by Ca2+ uptake in the β-cell (32). In Ncx1+/+ and Ncx1+/− islets, 16.7 mmol/L glucose stimulated 45Ca uptake (Fig. 3C). Whereas at low glucose, there was no significant difference in 45Ca uptake between the two types of islet; the increase in uptake induced by 16.7 mmol/L glucose was twice as high in Ncx1+/− than in Ncx1+/+ islets.
Insulin content.
In view of the major increase in insulin release in Ncx1+/− islets, the insulin content of the islets was measured and was about double that of Ncx1+/+ islets (Fig. 3D).
Morphology.
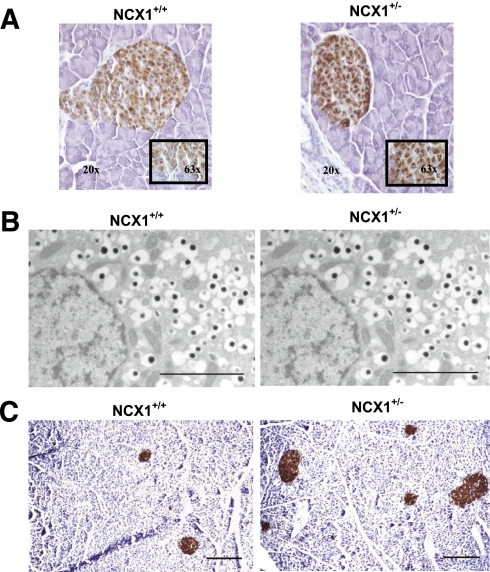
There were no differences in islet morphology or in the position of β-, α-, and δ-cells between Ncx1+/+ and Ncx1+/− islets (data not shown). Proinsulin staining, however, was increased in Ncx1+/− compared with Ncx1+/+ islets (Fig. 4A) of adult mice (12–14 weeks), a finding in line with the increase in insulin content. Electron microscopic analysis revealed no difference in the features of pancreatic β-cells, including secretory granules, nucleus, mitochondria, ER, and Golgi apparatus of Ncx1+/+ and Ncx1+/− adult mice (Fig. 4B). Quantitative analysis of relative volume of organelles demonstrated no significant difference between Ncx1+/+ and Ncx1+/− β-cells (data not shown).
FIG. 4.
Effect of Ncx1 heterozygous inactivation on islet morphology. A: Immunohistochemistry: proinsulin labeling (immunoperoxidase in brown) is stronger in Ncx1+/− than in Ncx1+/+ islets. Representative images of six 12-week-old mice pancreas sections in each case. Inset: Same image at higher magnification (original magnification ×63). B: Transmission electron microscopy. Groups of 30 islets from Ncx1+/+ and Ncx1+/− mice were fixed, and three sections in each block demonstrating an islet were cut at randomly chosen depths. The characteristic ultrastructural features of pancreatic β-cells were comparable in both types of islets. Representative images are shown of β-cells from 12-week-old mice Ncx1+/+ (left panel) and Ncx1+/− islets (right panel). Scale bar = 2 µm. C: Representative images of pancreatic β-cells immunolabeled with immunoperoxidase (brown) using a polyclonal anti-insulin antibody, with hematoxylin used for the counterstain. The sections were used to evaluate the β-cell mass by counting morphometry. The photomicrographs represent 12-week-old mice pancreatic sections of Ncx1+/+ (left panel) and Ncx1+/− islets (right panel). Scale bar = 0.2 µm. (A high-quality digital representation of this figure is available in the online issue.)
Taken as a whole, the data so far presented show that Ncx1 heterozygous inactivation strongly increases glucose-induced insulin production and release.
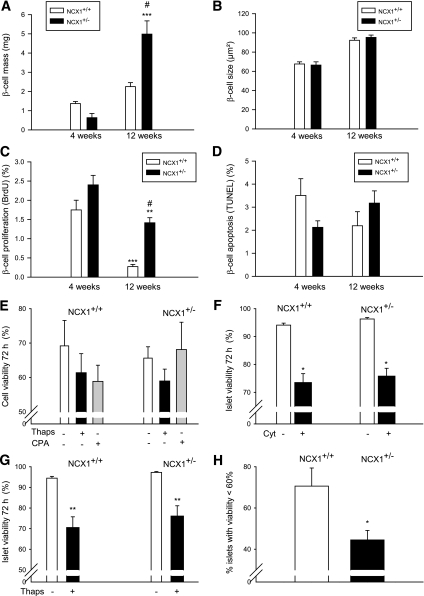
β-Cell mass, size, proliferation, and viability.
β-Cell mass, size, and proliferation was measured in young (4 weeks) and adult (12 weeks) mice (Fig. 5A–C). As expected, β-cell mass was increased at 12 weeks in both types of islet, although the increase was of much larger magnitude in Ncx1+/− than in Ncx1++ islets (8.8- vs. 1.6-fold increase, respectively, P < 0.001; Fig. 4C). This increase was not due to β-cell or islet hypertrophy because no change in β-cell and islet size was observed between Ncx1+/+ and Ncx1+/− mice (Fig. 5B and data not shown); rather, it was due to an increase in the β-cell proliferation rate. As expected, β-cell proliferation, as measured using BrdU, was decreased at 12 weeks compared with 4 weeks (Fig. 5C), although the decrease was of a lower magnitude in Ncx1+/− than in Ncx1+/+ islets (−40 vs. −85%, P < 0.01). As a result, a 5.25-times higher proliferation rate was observed at 12 weeks in Ncx1+/− compared with Ncx1+/+ mice (Fig. 5C and Supplementary Fig. 5). We also observed an increase in proliferation rate of the exocrine pancreatic cells that was lower than in the endocrine pancreas (1.7- vs. 5.25-fold increase, respectively; P < 0.02, Supplementary Fig. 5). Ki67 staining showed an increased labeling in Ncx1+/− compared with Ncx1+/+ islets, with the number of positive nuclei averaging 2.75 ± 0.31 vs. 1.58 ± 0.28 per islet, respectively (P < 0.01).
FIG. 5.
Effect of Ncx1 heterozygous inactivation is shown on β-cell mass (A), size (B), proliferation rate (C), and apoptosis (D) between weeks 4 and 12 in Ncx1+/+ and Ncx1+/− mice. Mean ± SEM values are shown from five and six pancreas specimens, respectively. A: ***P < 0.001 vs. values at 4 weeks; #P < 0.001 vs. Ncx1+/+ β-cells at 12 weeks. C: **P < 0.01; ***P < 0.001 vs. respective value at 4 weeks; #P < 0.01 vs. Ncx1+/+ islets at 12 weeks. Cell viability was measured in isolated islet cells (E) and in intact islets (F, G, H) using Ho342 and PI staining in the absence or after 72-h exposure to thapsigargin (Thaps) or cyclopiazonic acid (CPA) (E and G), cytokines (Cyt; F), and after 6-h exposure to hypoxia (H). Mean ± SEM values are shown from four individual experiments. F: *P < 0.05 vs. control in the absence of cytokines. G: **P < 0.01 vs. respective Ncx1 islets in the absence of Thaps. H: *P < 0.05 vs. Ncx1+/+ islets.
Apoptosis of β-cells was also measured, but no difference could be found between Ncx1+/− and Ncx1+/+ islets. Thus, whether using the TUNEL method at 4 and 12 weeks under basal conditions (Fig. 5D), or Ho342 and PI staining of islet cells (Fig. 5E–G) under basal or stimulated conditions created by the absence or presence of sarcoendoplasmic reticulum Ca2+ ATPase (SERCA) inhibitors (Fig. 5E), and 72-h exposure of intact islets to cytokines (Fig. 5F) or thapsigargin (Fig. 5G), no difference could be found between both types of islets.
Hypoxia studies.
To test their resistance to physiologic stress, the islets were then subjected to hypoxia for 6 h, cell death being measured using Ho342 and PI staining of intact islets. Figure 5H shows that 71% of Ncx1+/+ islets showed a decrease in viability <60% compared with 45% of Ncx1+/− islets.
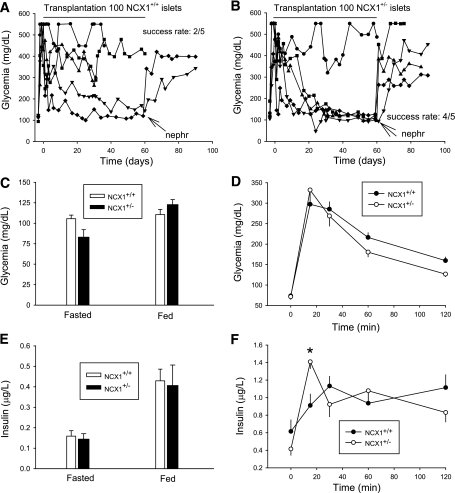
Transplantation.
We then transplanted Ncx1+/− islets under the kidney capsule of alloxan-diabetic mice to examine their performances compared with Ncx1+/+ islets. First, we had to determine the minimum number of Ncx1+/+ islets to transplant to cure diabetes, which must be ∼300 islets. Indeed, all transplantations with 400 islets were successful, with nephrectomy leading to a reincrease in glycemia (Supplementary Fig. 6A), whereas the rate of success of transplantation of 200 Ncx1+/+ islets was only 2/5 (Supplementary Fig. 6B). In comparison, the rate of success of transplantation of 100 Ncx1+/− islets was 4/5 (Supplementary Fig. 6C), whereas the rate of success of transplantation of 50 Ncx1+/− islets was 2/3 (Supplementary Fig. 5D). This suggests that the Ncx1+/− islets are at least four- to seven-times more efficient to cure diabetes than the Ncx1+/+ islets. Finally, we performed a last series of parallel transplantations of 100 islets (Fig. 6A and B). The success rate was 4/5 and 2/5 for Ncx1+/− and Ncx1+/+ islets, indicating that the Ncx1+/− islets are at least twice as efficient in curing diabetes than Ncx1+/+ islets.
FIG. 6.
Effect of Ncx1 heterozygous inactivation on islets transplantation and glucose metabolism is shown in vivo. Effect of transplanting grafts of 100 islets from Ncx1+/+ (A) or Ncx1+/− (B) mice is shown on nonfasting blood glucose levels. The success rate of diabetes cure (blood glucose level <220 mg/dL) was 2/5 for Ncx1+/+ and 4/5 for Ncx1+/− islets. Nephr, nephrectomy. C and E: Glycemia and insulin levels measured in the fasting and the fed state of Ncx1+/+ and Ncx1+/− mice (n = 5 in each case). D and F: Glycemia and insulin levels are shown during an intraperitoneal glucose tolerance test in Ncx1+/+ and Ncx1+/− mice (n = 8 in each case). *P < 0.05. Data are shown as the means ± SEM.
Morphometric analysis of the islet grafts after nephrectomy of mice who received a transplant of 100 islets showed that the relative volume of islet grafts was about seven-times higher in cured than in noncured animals (4.26 ± 1.09% vs. 0.61 ± 0.39%, respectively [n = 4–6]; P < 0.05), confirming that diabetes cure is attributable to islet transplantation.
Glucose metabolism and insulin sensitivity in vivo.
Despite the major increase in insulin release seen in vitro, plasma glucose and insulin levels were comparable in Ncx1+/− and Ncx1+/+ mice, both in the fasted and the fed state (Fig. 6C and E). However, the glucose-tolerance test showed an increased and earlier initial peak of insulin release (Fig. 6D and F), with a subsequent faster decrease in glucose levels during the ensuing 45 min (−152 ± 20 compared with −81 ± 2 mg/dL in Ncx1+/− and Ncx1++ mice, respectively, P < 0.01). The intraperitoneal insulin sensitivity test showed no difference between the mice (Supplementary Fig. 7).
ER Ca2+ stores.
In a previous study we showed that NCX1 overexpression increased β-cell apoptosis and decreased β-cell proliferation by depleting ER Ca2+ stores (11). Therefore, we wondered whether the opposite changes seen in the current study in Ncx1+/− mice were not due to an increase in ER Ca2+ stores. To test this hypothesis, the effect of the SERCA inhibitor thapsigargin on [Ca2+]i in pancreatic islets was examined (Supplementary Fig. 8). In Ncx1+/+ islets, thapsigargin induced an important but transient increase in [Ca2+]i, a phenomenon that was increased by about 60% in Ncx1+/− islets (P < 0.05), indicating that ER Ca2+ stores were increased in Ncx1+/− compared with Ncx1+/+ islets.
DISCUSSION
We generated a mouse strain with heterozygous inactivation of the gene coding for NCX1, the isoform of the Na/Ca exchanger that is expressed in the pancreatic β-cell (33). RT-PCR studies confirmed heterozygous inactivation of the exchanger in Ncx1+/− mice and the reduction of its expression in β-cells. The latter reduction led to a decrease in NCX1 activity as assessed at the physiologic level, with inhibition of both modes of the exchanger (forward and reverse). The reduction in Na/Ca exchange activity in Ncx1+/− β-cells had multiple consequences:
First, as expected from cells from which Ca2+ extrusion is reduced, the rise in [Ca2+]i induced by membrane depolarization and the uptake of 45Ca induced by glucose were increased.
Second, the [Ca2+]i oscillations induced by the sugar were disrupted. This was not unexpected, because the exchanger generates an inward current when working in its forward mode that contributes to the duration of the bursts of electrical activity (34) that underlie [Ca2+]i oscillations (35,36). Hence, the reduction of the NCX1 inward current in Ncx1+/− cells led to the shortening and disorganization of the oscillations. However, in view of the decreased rate of ATP generation at high glucose in Ncx1+/− compared with Ncx1+/+ islets, the disruption of the Ca2+ oscillations could also be due to an attenuation of the metabolic oscillations that underlie the slow electrical and Ca2+ oscillations via a relief of phosphofructokinase inhibition by ATP (37).
Third, glucose-induced insulin release was markedly increased. Both phases of insulin release were enhanced, which could also be related to the reduction in Ca2+ extrusion with subsequent increase in cellular Ca2+ content as objectified at the ER level. In view of this major increase in insulin release, we next examined insulin content, islet morphology, and glucose metabolism.
Insulin content was doubled in Ncx1+/− compared with Ncx1+/+ β-cells, but no change in morphology was found between the cell types except for an increase in proinsulin staining in Ncx1+/− β-cells, a finding compatible with the increase in insulin content. In Ncx1+/− β-cells, there was a tendency toward an increase in glucose utilization at low glucose concentration and a significant decrease in glucose oxidation at high glucose concentration, making it unlikely that the observed increase in glucose-induced insulin release in these islets is explained by an augmented mitochondrial glucose metabolism. The increase in the insulin secretory rate may result instead from increased insulin production and cellular Ca2+ content.
We next looked at β-cell mass, size, proliferation, and death. At 12 weeks (adult mice), β-cell mass had increased 100% in Ncx1+/− compared with Ncx1+/+ mice, a phenomenon that was not due to an increase in β-cell size but rather to an increase in β-cell proliferation, the rate of which was five-times higher in Ncx1+/− mice than in Ncx1+/+ mice. The view that a decrease in β-cell death could also contribute to this observation was examined, but the results obtained were negative except in the case of hypoxia. Indeed, although measurement by the TUNEL method showed no difference in the rate of apoptosis under basal conditions and no difference in viability between single cells or islets of Ncx1+/+ and Ncx1+/− mice under basal and stimulated conditions, Ncx1+/− islets showed a 37% protection against hypoxia compared with Ncx1+/+ islets.
The increase in β-cell function, growth, and resistance to the physiologic stress of Ncx1+/− islets was confirmed in transplantation studies, the Ncx1+/− islets being at least twice as efficient in curing diabetes than the Ncx1+/+ islets. In this respect, it is important to know that islets transplantation represents a valuable approach in the treatment of diabetes. However, its applicability is limited by the need to transplant a high number of islets from two or more donors. In clinical islet transplantation, it has been estimated that up to 70% of the transplanted β-cell mass is destroyed in the early post-transplant period due to non–immune-mediated physiologic stress, namely prolonged hypoxia during the revascularization process (38).
The increase in insulin content, β-cell proliferation, and mass seen in Ncx1+/− islets could result from the activation by raised cellular Ca2+ of the calcineurin/nuclear factor of activated T-cell (NFAT) signaling pathway. Calcineurin is a calmodulin-dependent serine/threonine phosphatase that dephosphorylates the cytoplasmic subunits of NFAT (NFATc) upon activation by Ca2+. Dephosphorylation of NFATc allows its rapid translocation to the nucleus, with subsequent activation of insulin transcription and promotion of β-cell proliferation by increasing the expression of cell cycle promoters such as cyclin D1, cyclin D2, and cyclin-dependent kinase 4 (CDK4) (39). β-Cell–specific calcineurin inactivation in knockout mice impaired insulin transcription and cyclins expression, leading to markedly decreased β-cell proliferation and mass and to diabetes onset (40). Interestingly, calcineurin inactivation did not increase β-cell apoptosis (39), a finding compatible with the absence of a decrease in β-cell apoptosis observed in this study.
Last, except for a higher initial peak of insulin release during the glucose tolerance test, no difference was found in in vivo glucose metabolism or in insulin resistance between both mice.
In conclusion, heterozygous inactivation of a single gene (Ncx1) leads to an increase in insulin release, β-cell proliferation and mass, and to an increase in resistance to β-cell death, namely, to various changes in β-cell function that are opposite to the major abnormalities seen in type 2 diabetes. Moreover, it increased the success rate of islet transplantation in diabetic animals. Downregulation of the β-cell Na/Ca exchanger is thus a unique model providing a novel concept for the prevention and treatment of type 2 diabetes and to improve the applicability of islet transplantation.
ADDENDUM
During the process of submitting the present work for publication, another study showed that KB-R7943, an NCX1 inhibitor, enhanced glucose-induced insulin release, a finding confirming part of our results (41).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Belgian Fund for Scientific Research (FRSM 3.4593.04, 3.4527.08), the European Foundation for the Study of Diabetes/Novo Nordisk Programme in Diabetes Research (2005/6), the Juvenile Diabetes Research Foundation International (1-2008-536), the Communauté Française de Belgique–Actions de Recherche Concertées (ARC), the European Union (Integrated Project Naimit [FP7] of the European Community), and the Belgium Program on Interuniversity Poles of Attraction initiated by the Belgium State (IUAP P6/40). CIBERDEM is an initiative of Instituto de Salud Carlos III (Spain). A.K.C. is a Research Associate, and J.-M.V. is a Research Director of the Fonds National de la Recherche Scientifique. F.A. was supported by a fellowship from the “Crédit de Relations Internationales de l’Université Libre de Bruxelles”. G.J. was supported by a grant from the Fundació Privada Institut d’Investigació Biomedica de Bellvitge. No other potential conflicts of interest relevant to this article were reported.
E.N., S.So., S.B., N.P., and E.D. researched data and contributed to discussion. F.A., J.-M.V., and A.S. researched data, contributed to discussion, and reviewed and edited the manuscript. M.M., M.D., J.M., and G.J. researched data and contributed to discussion. E.M., J.R., A.K.C., and D.L.E. researched data, contributed to discussion, and reviewed and edited the manuscript. S.Sc. researched data and contributed to discussion. A.H. researched data, contributed to discussion, and wrote the manuscript.
The authors thank A. Van Praet, A. Iabkriman, and M.P. Berghmans (Laboratory of Pharmacology, Université Libre de Bruxelles) for excellent technical support.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-0924/-/DC1.
S.So. is currently affiliated with the University of California–Los Angeles School of Nursing, Los Angeles, California.
S.Sc. is currently affiliated with Unité de Biochimie, Département des Sciences Fonctionnelles, Faculté de Médecine Vétérinaire, Groupe Interdisciplinaire de Génoprotéomique Appliquée Research, Université de Liège, Liège, Belgium.
REFERENCES
- 1.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care 1998;21:1414–1431 [DOI] [PubMed] [Google Scholar]
- 2.Yang W, Lu J, Weng J, et al. ; China National Diabetes and Metabolic Disorders Study Group Prevalence of diabetes among men and women in China. N Engl J Med 2010;362:1090–1101 [DOI] [PubMed] [Google Scholar]
- 3.Utzschneider KM, Kahn SE. β-cell dysfunction in type 2 diabetes. In International Textbook of Diabetes Mellitus. DeFronzo RA, Ferrannini E, Keen H, Zimmet P, Eds. Hoboken, NJ, John Wiley & Sons, Ltd, 2003, p. 375–388
- 4.Porte D., Jr Banting lecture 1990. Beta-cells in type II diabetes mellitus. Diabetes 1991;40:166–180 [DOI] [PubMed] [Google Scholar]
- 5.Kahn SE, Zraika S, Utzschneider KM, Hull RL. The beta cell lesion in type 2 diabetes: there has to be a primary functional abnormality. Diabetologia 2009;52:1003–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacLean N, Ogilvie RF. Quantitative estimation of the pancreatic islet tissue in diabetic subjects. Diabetes 1955;4:367–376 [DOI] [PubMed] [Google Scholar]
- 7.Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab 2008;10(Suppl. 4):32–42 [DOI] [PubMed] [Google Scholar]
- 8.Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 2005;54(Suppl. 2):S97–S107 [DOI] [PubMed] [Google Scholar]
- 9.Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev 1999;79:763–854 [DOI] [PubMed] [Google Scholar]
- 10.Philipson KD, Nicoll DA, Ottolia M, et al. The Na+/Ca2+ exchange molecule: an overview. Ann N Y Acad Sci 2002;976:1–10 [DOI] [PubMed] [Google Scholar]
- 11.Diaz-Horta O, Kamagate A, Herchuelz A, Van Eylen F. Na/Ca exchanger overexpression induces endoplasmic reticulum-related apoptosis and caspase-12 activation in insulin-releasing BRIN-BD11 cells. Diabetes 2002;51:1815–1824 [DOI] [PubMed] [Google Scholar]
- 12.Sokolow S, Manto M, Gailly P, et al. Impaired neuromuscular transmission and skeletal muscle fiber necrosis in mice lacking Na/Ca exchanger 3. J Clin Invest 2004;113:265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Eylen F, Lebeau C, Albuquerque-Silva J, Herchuelz A. Contribution of Na/Ca exchange to Ca2+ outflow and entry in the rat pancreatic beta-cell: studies with antisense oligonucleotides. Diabetes 1998;47:1873–1880 [DOI] [PubMed] [Google Scholar]
- 14.Plasman PO, Lebrun P, Herchuelz A. Characterization of the process of sodium-calcium exchange in pancreatic islet cells. Am J Physiol 1990;259:E844–E850 [DOI] [PubMed] [Google Scholar]
- 15.Herchuelz A, Malaisse WJ. Regulation of calcium fluxes in pancreatic islets: dissociation between calcium and insulin release. J Physiol 1978;283:409–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malaisse WJ, Sener A. Hexose metabolism in pancreatic islets. Feedback control of d-glucose oxidation by functional events. Biochim Biophys Acta 1988;971:246–254 [DOI] [PubMed] [Google Scholar]
- 17.Hutton JC, Sener A, Malaisse WJ. The metabolism of 4-methyl-2-oxopentanoate in rat pancreatic islets. Biochem J 1979;184:291–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sempoux C, Guiot Y, Dahan K, et al. The focal form of persistent hyperinsulinemic hypoglycemia of infancy: morphological and molecular studies show structural and functional differences with insulinoma. Diabetes 2003;52:784–794 [DOI] [PubMed] [Google Scholar]
- 19.Jacoby M, Cox JJ, Gayral S, et al. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat Genet 2009;41:1027–1031 [DOI] [PubMed] [Google Scholar]
- 20.Mast J, Nanbru C, van den Berg T, Meulemans G. Ultrastructural changes of the tracheal epithelium after vaccination of day-old chickens with the La Sota strain of Newcastle disease virus. Vet Pathol 2005;42:559–565 [DOI] [PubMed] [Google Scholar]
- 21.Téllez N, Montolio M, Biarnés M, Castaño E, Soler J, Montanya E. Adenoviral overexpression of interleukin-1 receptor antagonist protein increases beta-cell replication in rat pancreatic islets. Gene Ther 2005;12:120–128 [DOI] [PubMed] [Google Scholar]
- 22.Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of β-cells in aged adult mice. Diabetes 2005;54:2557–2567 [DOI] [PubMed] [Google Scholar]
- 23.Cardozo AK, Ortis F, Storling J, et al. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic β-cells. Diabetes 2005;54:452–461 [DOI] [PubMed] [Google Scholar]
- 24.Biarnés M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes 2002;51:66–72 [DOI] [PubMed] [Google Scholar]
- 25.Emamaullee JA, Rajotte RV, Liston P, et al. XIAP overexpression in human islets prevents early posttransplant apoptosis and reduces the islet mass needed to treat diabetes. Diabetes 2005;54:2541–2548 [DOI] [PubMed] [Google Scholar]
- 26.Brüning JC, Winnay J, Bonner-Weir S, Taylor SI, Accili D, Kahn CR. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell 1997;88:561–572 [DOI] [PubMed] [Google Scholar]
- 27.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008;51:216–226 [DOI] [PubMed] [Google Scholar]
- 28.Koushik SV, Wang J, Rogers R, et al. Targeted inactivation of the sodium-calcium exchanger (Ncx1) results in the lack of a heartbeat and abnormal myofibrillar organization. FASEB J 2001;15:1209–1211 [DOI] [PubMed] [Google Scholar]
- 29.Gilon P, Shepherd RM, Henquin JC. Oscillations of secretion driven by oscillations of cytoplasmic Ca2+ as evidences in single pancreatic islets. J Biol Chem 1993;268:22265–22268 [PubMed] [Google Scholar]
- 30.Bergsten P, Grapengiesser E, Gylfe E, Tengholm A, Hellman B. Synchronous oscillations of cytoplasmic Ca2+ and insulin release in glucose-stimulated pancreatic islets. J Biol Chem 1994;269:8749–8753 [PubMed] [Google Scholar]
- 31.Malaisse WJ, Sener A, Herchuelz A, Hutton JC. Insulin release: the fuel hypothesis. Metabolism 1979;28:373–386 [DOI] [PubMed] [Google Scholar]
- 32.Malaisse-Lagae F, Malaisse WJ. The stimulus-secretion coupling of glucose-induced insulin release. 3. Uptake of 45calcium by isolated islets of Langerhans. Endocrinology 1971;88:72–80 [DOI] [PubMed] [Google Scholar]
- 33.Van Eylen F, Svoboda M, Herchuelz A. Identification, expression pattern and potential activity of Na/Ca exchanger isoforms in rat pancreatic B-cells. Cell Calcium 1997;21:185–193 [DOI] [PubMed] [Google Scholar]
- 34.Gall D, Gromada J, Susa I, Rorsman P, Herchuelz A, Bokvist K. Significance of Na/Ca exchange for Ca2+ buffering and electrical activity in mouse pancreatic β-cells. Biophys J 1999;76:2018–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez-Armendariz E, Atwater I. Glucose-evoked changes in [K+] and [Ca2+] in the intercellular spaces of the mouse islet of Langerhans. Adv Exp Med Biol 1986;211:31–51 [DOI] [PubMed] [Google Scholar]
- 36.Henquin JC, Jonas JC, Gilon P. Functional significance of Ca2+ oscillations in pancreatic beta cells. Diabetes Metab 1998;24:30–36 [PubMed] [Google Scholar]
- 37.Lenzen S, Lerch M, Peckmann T, Tiedge M. Differential regulation of [Ca2+]i oscillations in mouse pancreatic islets by glucose, α-ketoisocaproic acid, glyceraldehyde and glycolytic intermediates. Biochim Biophys Acta 2000;1523:65–72 [DOI] [PubMed] [Google Scholar]
- 38.Emamaullee JA, Shapiro AM. Perspectives in diabetes. Interventional strategies to prevent β-cell apoptosis in islet transplantation. Diabetes 2006;55:1907–1914 [DOI] [PubMed] [Google Scholar]
- 39.Heit JJ. Calcineurin/NFAT signaling in the beta-cell: From diabetes to new therapeutics. Bioessays 2007;29:1011–1021 [DOI] [PubMed] [Google Scholar]
- 40.Heit JJ, Apelqvist AA, Gu X, et al. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature 2006;443:345–349 [DOI] [PubMed] [Google Scholar]
- 41.Hamming KSC, Soliman D, Webster NJ, et al. Inhibition of β-cell sodium-calcium exchange enhances glucose-dependent elevations in cytoplasmic calcium and insulin secretion. Diabetes 2010;59:1686–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.