Abstract
The development and demise of the corpus luteum (CL) are accompanied by angiogenic and angioregressive processes; however, the mediators of these processes have not been fully identified and characterized. Transcriptional profiling studies revealed the upregulation of cysteine-rich 61 (CYR61) in the CL, about which nothing was previously known. In the present study, we found that over a 12-h period following a single injection of prostaglandin F2alpha (PGF2alpha), RT-PCR revealed the upregulation of CYR61 at 0.5 and 1 h, after which it declined. We also determined that luteal-derived endothelial cells as well as luteal steroidogenic cells are sources of CYR61. Treatment with PGF2alpha in vitro had no effect on CYR61 expression in luteal-derived endothelial cells, but it increased CYR61 expression in luteal steroidogenic cells. During the estrous cycle, CYR61/CYR61 (transcript/protein) was increased in the Day 4 but not in the Day 10 and Day 16 CL, suggesting that it may be associated with the switch to the angiogenic phenotype. In addition, the specific but transient upregulation of CYR61 by PGF2alpha in vivo, and in luteal steroidogenic cells but not endothelial cells in vitro, may be part of the mechanism underlying the previously reported transient increase in blood flow during the early onset of luteolysis. This is supported by our preliminary finding that CYR61 transiently inhibited endothelial cell expression of endothelin-converting enzyme 1 mRNA but not endothelin 1. Collectively, the increased expression of CYR61 in the Day 4 CL and its transient increase by PGF2alpha in Day 6, Day 10, and Day 16 CL indicate that CYR61 may play a role in regulating angiogenesis over the life span of the CL.
Keywords: angiogenesis, corpus luteum, CYR61, endothelial cells, luteal regression, PGF2α
Elevated expression of CYR61 in the developing Day 4 corpus luteum and its transient increase by PGF2α in early (Day 6), midcycle (Day 10), and late stage (Day 16) corpora lutea indicate that CYR61 may play a role in regulating angiogenesis over the life span of the corpus luteum.
INTRODUCTION
Although it is a hallmark of a variety of human pathologies, angiogenesis rarely occurs under normal circumstances. One exception to this rule is found in the female reproductive system. We and others [1–3] previously suggested that the corpus luteum (CL), an ephemeral gland of the ovary, might be a useful model for studying the mechanisms underlying angiogenesis induction and regression of angiogenesis during the reproductive cycle. Breakdown of the basement membrane that previously separated follicular granulosa and theca cells commences following the ovulatory gonadotropin surge [4, 5]. In addition, endothelial cells from the theca interna proliferate and migrate across the disrupted basement membrane to form new capillary vessels in the granulosa layer [4, 6, 7]. As a result, the latter undergoes a striking transition from an avascular to a vascular compartment, as shown in the rat [5] and in the rabbit [8], much like the “switch” to the angiogenic phenotype that occurs during tumor progression [9, 10]. This angiogenic switch provides the blood supply for the differentiated steroidogenic cells of rabbit and rat CL [8, 11]. and it initiates luteal formation and development during the early stages of the estrous cycle in the cow [1]. At midcycle, ongoing angiogenesis or angiomaintenance is necessary to sustain the increased steroidogenesis by the CL [12, 13], one of the most vascularized tissues in the body, as shown in the cow [14]. At the end of the estrous cycle, inhibition of angiogenesis along with the regression of preexisting blood vessels accompany the functional and structural demise of the CL [15, 16]. Thus, the development, function, and demise of the CL are intimately linked to angiogenesis, angiomaintenance, and angioregression [17, 18].
We and others [3, 13, 19, 20] have utilized the CL model to identify and study expression patterns of molecular determinants such as matrix metalloproteinases (MMP) and tissue inhibitors of metalloproteinases (TIMPs) associated with angiogenesis in the mammalian CL. Angiogenesis is a dynamic and complex biological process that is orchestrated by a variety of angiogenic factors. In addition to the MMPs and their endogenous inhibitors, vascular endothelial growth factor (VEGF) [21–24], basic fibroblast growth factor (bFGF; official symbol FGF2) in the human [22] and cow [25], endocrine gland-VEGF (EG-VEGF; official symbol PROK1) in the human [26], and angiopoietins (ANGPT1 [Ang-1] and ANGPT2 [Ang-2]) [27] have been studied in the CL. These angiogenic factors show distinct expression patterns during the life span of the CL in the human [26], bovine [28], and rat [27]. This suggests that different angiogenic factors have a variety of functions during luteal angiogenesis, angiomaintenance, and angioregression.
In a nonfertile cycle, angiogenesis is most intense during the early development of the CL and declines thereafter. When the cycle ends, much less is known about the mechanisms/processes associated with angioregression during luteolysis [18]. As part of our ongoing initiative to profile the molecular determinants that switch angiogenesis on and off in the bovine CL, we performed transcriptional profiling studies on CL collected 0.5 h following injection of prostaglandin F2α (PGF2α), the natural luteolysin in ruminants. We identified and confirmed the differential expression of cysteine-rich 61 (CYR61), an angiogenic inducer. CYR61 is a member of the connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family of proteins, which consists of extracellular matrix (ECM)-associated signaling proteins including CYR61, connective tissue growth factor (CTGF), nephroblastoma overexpressed gene, and WNT1-induced secreted proteins (WISPs 1, 2, and 3) [29]. CYR61 is a secreted, ECM-associated, heparin-binding protein that promotes endothelial cell adhesion, migration, and growth factor (FGF2)-induced DNA synthesis [30]. Whereas CTGF has been described in the pig follicle and CL [31], to our knowledge no reports of CYR61 in the CL have appeared. In the present study, we investigated the temporal regulation of CYR61 by PGF2α in vivo and in vitro. Furthermore, we determined the pattern of CYR61 expression over the estrous cycle and its cellular localization in bovine CL.
MATERIALS AND METHODS
Overview of Experimental Design
Corpora lutea were collected from cows on Days 6, 10, and 16 of the estrous cycle 0.5 h after PGF2α or saline injection. Transcriptional profiling was performed on pooled Day 6 CL from PGF2α- and saline-treated cows. The upregulation of the CYR61 gene was confirmed by semiquantitative RT-PCR analysis of each CL collected as described above. Temporal expression of the CYR61 gene was further defined by injecting Day 10 cows with PGF2α and collecting CL at 0.5, 1, 2, 4, 8, and 12 h after treatment for analysis by RT-PCR. Furthermore, luteal tissue from Day 10 CL was processed for immunofluorescence and dissociated to investigate expression of and regulation by CYR61 in vitro. For the in vitro experiments, luteal steroidogenic cells (LSCs) and luteal-derived endothelial cells (LDECs) were treated with PGF2α, and expression of the CYR61 transcript over 24 h was determined by RT-PCR. In addition, LDECs were treated with recombinant CYR61 to study its effects on the endothelin system. Lastly, CL at Days 4, 10, and 16 of the estrous cycle were collected and analyzed for CYR61 gene and protein expression. Animal treatments and tissue collection for transcriptional profiling, CYR61 expression over the estrous cycle, LSC cultures, and immunofluorescence were conducted at the University of New Hampshire, whereas animal treatments and tissue collection for the temporal expression of CYR61 after PGF2α injection were conducted at the Ohio State University. All sample analyses, LDEC cultures, and expression and purification of recombinant CYR61 were conducted at Children's Hospital Boston.
Animals and Tissue Collection
All animal procedures in the present study were performed according to protocols approved by the Institutional Animal Care and Use Committees at the University of New Hampshire and the Ohio State University. CL were collected from cyclic, nonlactating dairy cows housed at the University of New Hampshire's Fairchild Dairy Teaching and Research Center and at the Krauss Dairy Center at the Ohio Agricultural Research and Development Center using previously reported procedures [32, 33]. To profile gene expression during the early onset of luteal regression, PGF2α (25 mg) or saline (control) was injected intramuscularly into cows on Day 6, 10, or 16 of the estrous cycle (Day 0 = estrus; n = 3 for each stage). CL were collected 0.5 h after PGF2α or saline injection and stored at −80°C until later analysis.
To determine CYR61 expression over the estrous cycle, Day 4 (early stage; n = 3), Day 10 (midcycle; n = 6), and Day 16 (late stage; n = 5) CL were collected. For Day 4 CL, the ovary was removed by colpotomy from animals under epidural anesthesia (2% [w/v] mepivacaine hydrochloride, 0.01 ml/kg body wt; Upjohn). The CL was then dissected from the ovarian stroma. The Days 10 and 16 CL were removed from the ovary by enucleation. Additionally, to further define the temporal gene expression pattern during luteal regression, PGF2α (25 mg) was injected intramuscularly into cows on Day 10 of the estrous cycle, and CL (n = 4 per time point) were collected 0.5, 1, 2, 4, 8, and 12 h after treatment.
Transcriptional Profiling
To profile gene expression patterns at the early onset of luteal regression, total RNA was extracted from pooled CL obtained 0.5 h after PGF2α injection into cows on Day 6 of the estrous cycle. Gene profiling analysis was performed at the Microarray Core Facility, Children's Hospital Boston. Briefly, cDNA was synthesized from RNA and then transcribed into RNA labeled with biotin before processing and hybridization onto the Affymetrix GeneChip U133A probe array. After scanning, hybridization profiles between Day 6 saline-injected control and PGF2α-treated luteal samples were then analyzed using the Affymetrix Suit software. Genes with expression that exhibited at least a 2-fold difference compared to the controls were then selected for further verification and analysis.
Cell Culture
Corpora lutea (n = 4) were collected from cows on Day 10 of the estrous cycle. LSCs were dissociated as previously described [34]. Briefly, luteal tissues were minced before placement into a spinner flask containing 25 ml of Ham F-12 with 1% bovine serum albumin (BSA) and type I collagenase (2000 U/g tissue; Worthington Biochemical) for 1 h at 37°C. During this period, tissues were triturated every 10 min. After 1 h, dissociated cells were centrifuged sequentially at 190 × g, 110 × g, and 80 × g to remove collagenase, connective tissues, and other tissue debris. Cell viability and number were determined by trypan blue exclusion and counting with a hemocytometer, respectively.
Luteal steroidogenic cells were seeded into six-well plates (4 × 105 cells/well) containing 2 ml of Ham F-12 culture medium supplemented with insulin/selenium/transferrin (5 μg/5 μg/5 ng/ml; Sigma) and gentamicin (30 μg/ml; GIBCO-BRL). After an overnight incubation, unattached cells were removed by rinsing with fresh Ham F-12 medium. LSCs were then treated with 1 μM PGF2α for 0.5, 2, 12, and 24 h. Cells were enumerated before and after treatment at each time. Cells were then processed for total RNA extraction according to the method described in the next section.
Luteal-derived endothelial cells (generously provided by Dr. Bo Rueda, Massachusetts General Hospital, Boston, MA) were isolated from Day 10 bovine CL as previously described [35]. LDECs (passages 4–9) were seeded into six-well plates (1 × 105 cells/well) and cultured in endothelial cell growth medium (EBM-2) containing growth factors and 10% fetal bovine serum (BioWhittaker, Inc.) for 2 days. After starvation with growth factor-free, serum-free EBM-2 medium for 6 h, cells were treated with 1 μM PGF2α in serum-free EBM-2 medium for 0.5, 2, 12, and 24 h and subsequently processed for total RNA extraction according to the method described in the next section. To study the effect of CYR61 on the endothelin system in the CL, LDECs were treated with 10 or 1000 ng/ml of CYR61 for 1 h. The LDECs from three different passages were used to perform three replicate experiments, and total RNA was then extracted from cells for RT-PCR analysis.
Semiquantitative RT-PCR
Total RNA was isolated from luteal tissue or from cultured luteal steroidogenic and endothelial cells using RNeasy Spin Columns (Qiagen). One microgram of total RNA was reverse transcribed to first-strand cDNA using Supertranscriptase III (Invitrogen). PCR was accomplished using primers for detecting CYR61 (forward, 5′-AAGACCCACAGGAGGAGAAG-3′; reverse, 5′-CCACAGCATCCAGGT TATCAG-3′), endothelin 1 (EDN1; forward, 5′-TGCCAAGCAGGAAAAGAACT-3′; reverse, 5′-GTGGACGAGGAGCTTCAGAC-3′), and endothelin-converting enzyme 1 (ECE1; forward, 5′-ACACAACCAAGCCATCATCA-3′; reverse, 5′-CAGGGTGTCCTGGAAGTTGT-3′) with “hot start” at 94°C for 3 min, followed by 28 cycles of 94°C for 30 sec, 54°C for 30 sec, and 72°C for 30 sec. Bovine glyceraldehyde phosphate dehydrogenase (GAPDH; forward, 5′-TGTTCCAGTATGATTCCACCC-3′; reverse, 5′-GTCTTCTGGGTGGCAGTGAT-3′) was run as an internal control. The PCR products were fractionated on 3% agarose gels, and the intensities of PCR bands were determined using the UN-SCAN-IT gel automated digitizing system (Silk Scientific Corporation). The CYR61, EDN1, and ECE1 were expressed as a ratio of their individual intensities to GAPDH.
Western Blot Analysis
Total protein was extracted from luteal tissues and luteal steroidogenic and endothelial cells as previously described [19, 20], and equal amounts of proteins were subjected to Western blot analysis. Proteins were separated on 4%–12% SDS-PAGE under reducing conditions and then electrophoretically transferred onto nitrocellulose membranes (Invitrogen). Nonspecific binding sites were blocked by incubating the membranes with 5% (w/v) nonfat dry milk in TBST buffer (10 mM Tris-HCl, 150 mM NaCl, and 0.05% [v/v] Triton X-100; pH 8.0). Membranes were then incubated with rabbit anti-human CYR61 antibody (1:250; Abcam). After extensive washes with TBST buffer (five times, 15 min per wash), horseradish peroxidase-conjugated anti-rabbit-immunoglobulin (Ig) G (1:10 000) was applied onto membranes as a secondary antibody. Signals were detected with the SuperSignal West Pico Chemiluminescent Substrate (Pierce) according to the manufacturer's instructions. Human recombinant CYR61 protein expressed from the Baculovirus-sf9 cell system was used as positive control.
Immunofluorescence
Sections (thickness, 6 μm) were prepared from Day 4, Day 10, and Day 16 CL frozen in OCT (Sakura Finetek) using a Cryotome (Shandon). Sections were fixed in cold acetone for 5 min. Nonspecific sites were blocked by 10% (w/v) BSA before incubating the slides with mouse anti-human CYR61 antibody (1:25; Abcam), which was followed by incubation with Alexa Fluor 594-conjugated goat anti-mouse IgG secondary antibody (Molecular Probes). LSCs were identified by staining with a rabbit anti-rat cytochrome p450 side-chain cleavage (SCC) enzyme antibody (1:200; Chemicon). Slides were visualized after incubation with Alexa Fluor 488-conjugated goat anti-rabbit IgG antibody (Molecular Probes). For each tissue sample, a consecutive section placed on the same slide and demarcated by circling with a hydrophobic barrier pen was used as a negative control, with IgG substituting for the primary antibody.
Expression and Purification of Human Recombinant CYR61
We used human recombinant CYR61 to further investigate the regulatory effect of this protein on endothelial cells. Human CYR61 cDNA (a generous gift from Dr. Lester Lau, University of Illinois at Chicago College of Medicine) was fused with 6×HIS Tag at the C-terminus and then inserted into the Baculovirus Transfer Vector pVL1393. The recombinant Baculovirus was generated by cotransfection of the recombinant transfer vector and BaculoGold DNA into sf9 cells according to the manufacturer's instructions (BD Pharminogen). Recombinant CYR61 protein was expressed by monolayer sf9 cells for 4 days after infection. The conditioned medium was then run through an Ni-NTA Agarose column (Qiagen). The purified CYR61 protein was confirmed by SDS-PAGE and Western blot analysis (data not shown). To investigate the regulatory effect of CYR61 on endothelial cells, recombinant CYR61 protein (10 or 1000 ng/ml) was incubated with LDECs for 1 h.
Data Analysis
Densitometric intensities of bands on PCR gels and Western blots were determined using the UN-SCAN-IT gel automated digitizing system. Data were then analyzed by ANOVA followed by Tukey test of pairwise comparisons to determine differences between groups.
RESULTS
Upregulation of CYR61 by PGF2α at the Onset of Luteal Regression
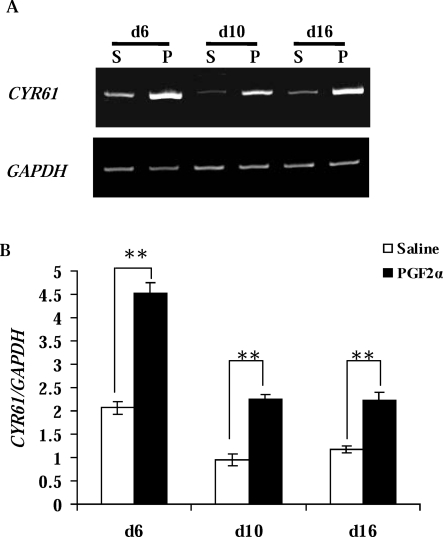
To characterize gene expression during the early onset of luteal regression, we injected PGF2α into cows on Day 6 of the estrous cycle. CL were collected 0.5 h later. Gene expression profiling was achieved using Affymetrix GeneChip U133A. Those genes with changes above 2-fold were selected for further verification and characterization. Among the 50 differentially expressed genes, CYR61, a cysteine-rich angiogenic inducer, was increased by 2.4-fold, which was confirmed by RT-PCR (Fig. 1A). This was a gene of significant interest because of its direct association with angiogenesis. In the Day 10 and Day 16 CL, PGF2α also significantly stimulated (P < 0.01) CYR61 expression when compared to their respective contemporaneous saline-injected controls (Fig. 1B).
FIG. 1.
Stimulation of CYR61 expression during the early onset of luteal regression. A) Representative semiquantitative RT-PCR results from CL collected 0.5 h after PGF2α (P) or saline (S; control) administration to cows on Day 6 (d6), Day 10 (d10), and Day 16 (d16) of the estrous cycle. Bovine GAPDH was the internal, loading control. B) Densitometric analysis of CYR61/GAPDH in CL collected from all animals (n = 3 at each stage; open bar, saline; black bar, PGF2α). Error bars are mean ± SD; **P < 0.01.
Transient Stimulation of CYR61 In Vivo Following PGF2α Administration
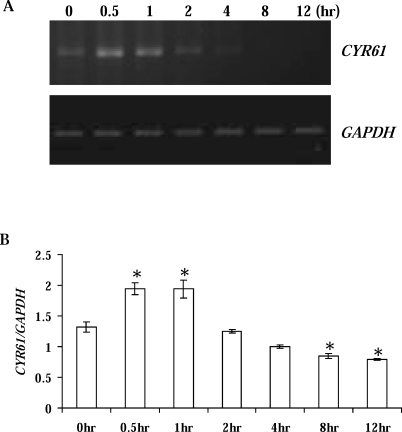
To further define the temporal expression of CYR61 during luteal regression, CL were collected 0.5, 1, 2, 4, 8, and 12 h after PGF2α administration on Day 10 of the estrous cycle. Similar to the gene microarray analysis, CYR61 mRNA was dramatically increased (P < 0.05) relative to untreated controls at 0.5 and 1 h after PGF2α (Fig. 2). CYR61 then declined and was significantly decreased (P < 0.05) at 8 and 12 h (Fig. 2).
FIG. 2.
Temporal expression of CYR61 during PGF2α-induced luteal regression in vivo. A) Representative semiquantitative RT-PCR results from midcycle Day 10 CL collected 0.5, 1, 2, 4, 8, and 12 h after PGF2α. Bovine GAPDH was the internal, loading control. B) Densitometric analysis of CYR61/GAPDH in CL collected from all animals (n = 4 for each time point). Error bars are mean ± SD; *P < 0.05.
Localization of CYR61 in LSCs
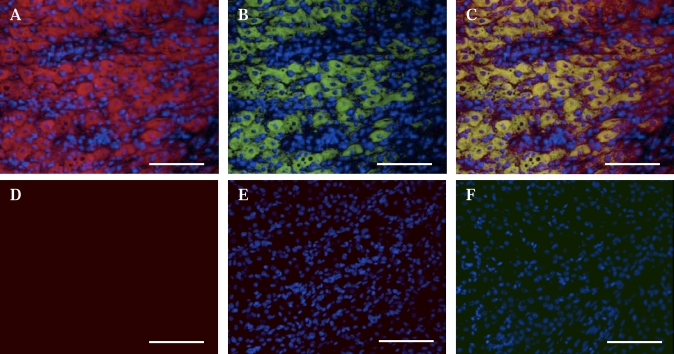
To identify the cellular source(s) of CYR61 expression, tissue sections were dually stained with CYR61 antibody and the steroidogenic cell marker cytochrome p450 SCC enzyme, respectively. Dual staining of CYR61 and p450 SCC enzyme was conducted on midcycle, Day 10 CL (Fig. 3, A and B), a time when progesterone biosynthesis is very high. The colocalization of CYR61 and cytochrome p450 SCC enzyme indicated that LSCs are a source of CYR61 (Fig. 3C).
FIG. 3.
Immunofluorescence staining of CYR61 protein and cytochrome p450 SCC (p450SCC) enzyme in the midcycle Day 10 bovine CL. Frozen sections of midcycle Day 10 CL were probed with CYR61 (A) and p450SSC enzyme (B) antibodies. Nuclei were counterstained with 4′,6′-diamidino-2-phenylindole. Overlay of CYR61 and p450SSC staining is shown in C. A representative image of autofluorescence is shown in D, which was taken with the highest exposure time among the three filter settings used. E and F are representative images stained with Alexa Fluor 594- and Alexa Fluor 488-conjugated secondary antibodies, respectively. Original magnification ×20; bar = 100 μm.
Regulation of CYR61 in LSCs But Not in LDECs by PGF2α In Vitro
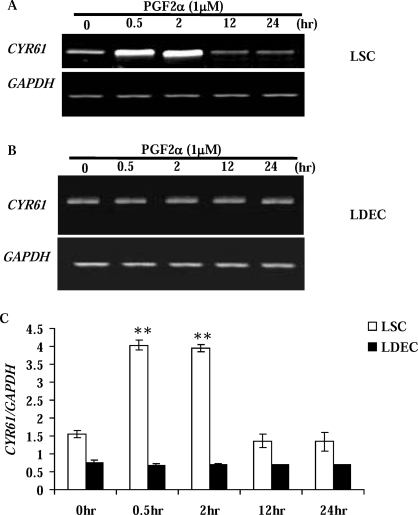
To study the regulation of CYR61 expression by PGF2α in vitro, luteal steroidogenic and endothelial cells were isolated and treated with PGF2α for 0.5, 2, 12, and 24 h. Although PGF2α had no effect (P > 0.05) on CYR61 expression in LDECs (Fig. 4, B and C), it significantly stimulated the CYR61 transcript in LSCs at 0.5 and 2 h posttreatment (Fig. 4, A and C). This temporal regulatory effect of PGF2α on CYR61 in LSCs is similar to its in vivo expression profile during luteal regression, suggesting that the LSCs, rather than endothelial cells, were the primary cell population in the CL contributing to the PGF2α-induced increase of CYR61 expression.
FIG. 4.
Regulation of CYR61 by PGF2α in luteal steroidogenic (A) and endothelial (B) cells in vitro. LSCs (A) from midcycle Day 10 CL (n = 4) and LDECs (B) were incubated without (0 h) or with 1 μM PGF2α for 0.5, 2, 12, and 24 h. Bovine GAPDH was the internal, loading control. C) Densitometric analysis of CYR61/GAPDH in LSC (open bar) and LDEC (black bar) following treatment with PGF2α. Error bars are mean ± SD; **P < 0.01 vs. 0-h control.
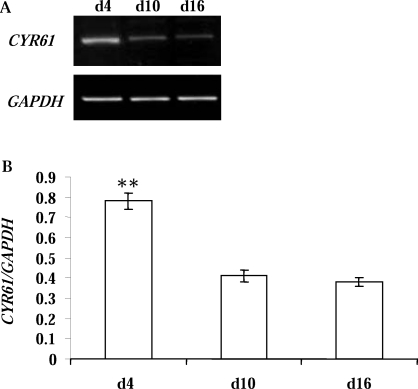
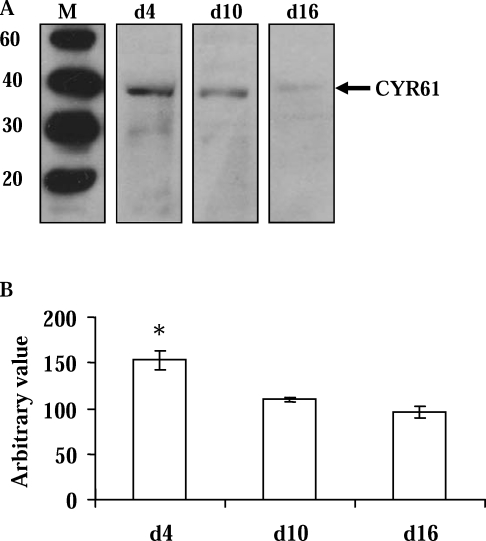
Differential Expression of CYR61 Transcript and Protein in CL Obtained Over the Estrous Cycle
Over the course of the estrous cycle, the CL undergoes angiogenesis, angiomaintenance, and angioregression. We demonstrated that the CYR61 transcript was highly expressed in the early stage, young, developing Day 4 CL but was dramatically decreased (P < 0.01) in the Day 10 and Day 16 CL (Fig. 5). Similar to other angiogenic factors (e.g., VEGF and FGF), CYR61 is a secreted protein and associated with the ECM. Using total protein extracts from luteal tissues, Western blot analysis detected CYR61-immunoreactive protein (Fig. 6A), which showed the highest (P < 0.05) concentration in the day 4 CL, and less in the Day 10 and Day 16 CL (Fig. 6B).
FIG. 5.
Expression of CYR61 mRNA in the bovine CL during the estrous cycle. A) Representative semiquantitative RT-PCR results from Day 4 (d4; n = 3), Day 10 (d10; n = 6), and Day 16 (d16; n = 5) CL of the estrous cycle. Bovine GAPDH was the internal, loading control. B) Densitometric analysis of CYR61/GAPDH in Day 4 (d4; n = 3), Day 10 (d10; n = 6), and Day 16 (d16; n = 5) CL. Error bars are mean ± SD; **P < 0.01.
FIG. 6.
Expression of CYR61 protein in the bovine CL during the estrous cycle. A) Representative immunoblot showing the detection of an approximately 40-kDa CYR61 protein (arrow) in Day 4 (d4), Day 10 (d10), and Day 16 (d16) CL (M = Mr × 10−3; molecular weight [relative molecular mass; Mr × 10−3]). B) Densitometric analysis of CYR61 in Day 4 (d4; n = 3), Day 10 (d10; n = 6), and Day 16 (d16; n = 5) CL. Error bars are mean ± SD; *P < 0.05.
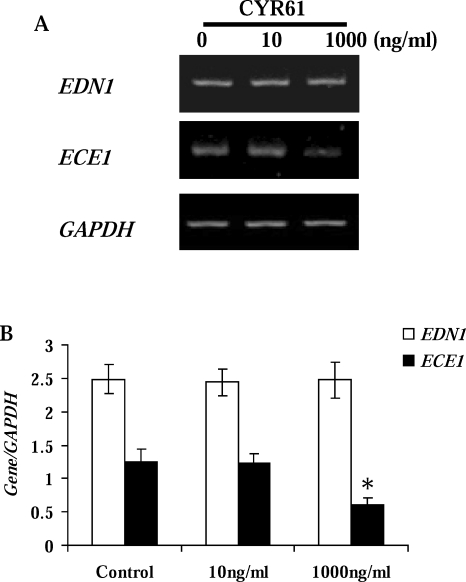
Reduction of ECE1 Expression by CYR61 in LDECs
Because LDECs were unresponsive to PGF2α (with respect to CYR61), we investigated whether CYR61 of LSC origin had a possible role in luteal regression by studying its effect on the endothelin system in LDECs. Given the rapid upregulation of CYR61 in vivo, LDECs were treated with 10 or 1000 ng/ml of CYR61 for 1 h. Analysis by RT-PCR demonstrated that neither concentration of CYR61 affected the expression of EDN1 (Fig. 7). However, the expression of ECE1 was reduced (P < 0.05) by the higher, but not by the lower, concentration of CYR61 (Fig. 7).
FIG. 7.
Regulation of the endothelin system in LDECs by CYR61. A) Representative semiquantitative RT-PCR results for EDN1 and ECE1. Endothelial cells were isolated from midcycle Day 10 CL and incubated with 10 or 1000 ng/ml of CYR61 for 1 h. Bovine GAPDH was the internal, loading control. B) Densitometric analysis of Gene/GAPDH (Gene [EDN1], open bar; ECE1, black bar) in LDEC. Error bars are mean ± SD; *P < 0.05 vs. control.
DISCUSSION
Angiogenesis is a hallmark of CL development and function [2]. It is switched on during the folliculoluteal transition and then switched off during angioregression. Although several angiogenic factors, including VEGF [22, 27, 28], FGF2 in the cow [14, 25], and PROK1 in the human [26] are expressed by the CL, the interplay between them is not fully understood. In the present study, we identified CYR61, a cysteine-rich angiogenic inducer, in the bovine CL and found that during the early onset of luteal regression in vivo, CYR61 was transiently stimulated by 0.5 and 1 h following administration of PGF2α, returning to control concentrations at 2 h. Afterward, CYR61 fell below control levels and remained there for the duration of the treatment period. This response to PGF2α is quicker than that observed with a benign human endometrial cell line, in which CYR61 mRNA was increased at 2 h after treatment and not before [36]. Because multiple PGF2α pulses are necessary to complete luteolysis in the cow [37], we propose that CYR61, as an early response gene, may play a role in maintaining blood vessel integrity, which is necessary for the transportation of cell debris during the early onset of luteal regression. This is most likely achieved in cooperation with FGF1 and FGF2. The immediate response of CYR61 at 0.5 h to PGF2α, followed by its decline at 2 h, precedes expression of both FGF1 and FGF2. Specifically, FGF1 and FGF2 increase later than CYR61 (i.e., between 2 and 12 h before decreasing at 48 h after PGF2α administration in the cow [38]). Because CYR61 works synergistically with FGF2 to increase endothelial cell proliferation [39], the sequential expression of CYR61 and FGFs during the early onset of luteal regression may serve to promote endothelial cell survival and maintain the vasculature. This is consistent with other studies showing that bovine CL at the late stage (Days 18–20), compared to bovine CL at the early (Days 1–4) and midcycle (Days 5–17) stages, has the greatest angiogenic activity [2, 40].
It is worth noting that single injections of PGF2α into cows on or before Day 5 of the estrous cycle do not cause luteal regression, whereas single injections administered starting on Day 6 of the estrous cycle do [41, 42]. One explanation for the lack of sensitivity to PGF2α by bovine CL younger than Day 5 may be a lack of a well-developed vascular system, despite the fact that angiogenesis is at a high rate at this time. This is consistent with the findings of Shirasuna et al. [43], who reported that the distribution of capillaries and arteriolovenous blood vessels change in cow CL between Day 4 and Day 10. However, this alone may not fully explain the differential sensitivity to PGF2α between early (younger than Day 5) and midcycle (Days 5–17) bovine CL. This differential sensitivity is not due to a lack of PGF2α receptors, because high-affinity PGF2α receptors are expressed by the Day 4 CL [44]. Instead, other researchers suggest that differences in expression of postreceptor signal transduction pathways or regulatory molecules may account for the resistance to PGF2α by CL younger than Day 5. For example, the mRNA expression of the steroidogenic acute regulatory protein (STAR) is decreased in the midcycle (Day 11) but not in the early (Day 4) bovine CL after PGF2α treatment in vivo [44]. In contrast, PGF2α increases progesterone production in vitro by the midcycle (Days 8–12) microdialyzed bovine CL or dispersed luteal cells [45, 46]. Furthermore, PGF2α treatment fails to increase prostaglandin endoperoxide synthase 2 and, subsequently, intraluteal PGF2α production in the Day 4 bovine CL [44], but it increases 15-hydroxyprostaglandin dehydrogenase, which breaks down PGF2α, in the Day 4 ovine CL [47]. In addition, whereas PGF2α decreases expression of VEGF and FGF2 in the Day 10 midcycle bovine CL, it increases them in the Day 4 CL [43]. Collectively, distinct pathways appear to regulate the responses to PGF2α by the early (Day 4) and midcycle bovine CL.
We also found that LSCs and LDECs are sources of CYR61, but that only LSCs responded to PGF2α in vitro by upregulating CYR61 expression. A lack of response by bovine LDECs could be explained by the absence of PGF2α receptors [48, 49], although others [50, 51] have reported that these receptors are present. This discrepancy may be due to methodological differences in receptor detection and culture conditions or to the fact that subpopulations of microvascular endothelial cells are resident in the bovine CL [52]. We conclude, based on the present data, that PGF2α regulated CYR61 in steroidogenic, but not endothelial, cells in the bovine CL.
Given the lack of response by LDECs to PGF2α, cross-talk between LSCs and LDECs may exist, whereby PGF2α acts on LSCs to upregulate CYR61, which in turn acts on LDECs. Similar cellular interactions within the bovine CL have been suggested by Townson [53] and Liptak et al. [54] between immune and endothelial cells. To test our hypothesis, we performed a preliminary experiment, which showed that CYR61 decreased expression of ECE1, but not of EDN1, by LDEC. Endothelin 1 is a very potent vasoconstrictive agent. It is synthesized as an inactive, 203-amino-acid preproendothelin. After proteolytic processing of preproendothelin to big endothelin, it is converted to an active, 21-amino-acid peptide by ECE1 [55, 56]. PGF2α elevates EDN1 expression in vitro and in vivo [57, 58] in the cow. Whereas endothelial cells are the major source of EDN1, both bovine luteal steroidogenic and endothelial cells also synthesize ECE1 [59]. Given our present findings and those published previously, we propose that CYR61 may locally regulate the activity of EDN1 through ECE1. Following PGF2α, EDN1 secretion from endothelial cells is elevated. At the same time, LSCs increase CYR61, which acts on endothelial cells to reduce ECE1 expression. This results in decreased availability of bioactive EDN1 in the endothelium compartment, which contributes to blood vessel integrity during the early onset of luteal regression and could therefore explain the previously observed transient and acute increase in luteal blood flow in the cow that occurs 0.5–2 h after PGF2α [60].
Our data also showed that CYR61/CYR61 was highly expressed in the young, developing Day 4 bovine CL, consistent with its function as an angiogenic inducer. Knockout of the CYR61 gene leads to a defect in vessel bifurcation and, consequently, an undervascularized placenta, causing embryonic death [61]. CYR61 promotes endothelial cell adhesion [62, 63], stimulates chemotaxis in human microvascular endothelial cells [64], and is a potent stimulator of angiogenesis in vivo [65]. Another angiogenic regulator, VEGF, is expressed by the bovine CL [28], and CYR61 is capable of stimulating VEGF expression [66, 67]. VEGF also upregulates CYR61 in human umbilical vein endothelial cells [68]; therefore, the coincident expression of these two potent angiogenic factors, along with FGF2, may work synergistically to promote the high rate of angiogenesis known to occur during the early formation of the CL [1]. With CYR61 and VEGF being localized to granulosa-derived luteal cells, they may serve as chemoattractants to induce the directed migration of endothelial cell from the theca layer into the previously avascular cavity of the ruptured follicle. This migration through the ECM is accomplished by locally produced MMPs (e.g., in the cow [3, 32, 69]), which facilitate detachment of endothelial cells from the basement membrane.
Similar to VEGF, an interaction may also exist between CYR61 and FGF2. Even though CYR61 alone does not stimulate endothelial cell proliferation, it augments the mitogenic activity of FGF2 on endothelial cell proliferation. As a heparin-binding protein, CYR61 is capable of displacing FGF2 from the ECM and, consequently, increasing its local bioavailability [39], resulting in increased proliferation of endothelial cells during the early stage of the estrous cycle.
In summary, we have identified CYR61 as a potential molecular mediator of angiogenesis in the CL. Luteal steroidogenic and endothelial cells are sources of CYR61. Its transient upregulation by PGF2α in vivo suggests a possible role for CYR61 in endothelial cell survival and maintenance of the vasculature during the early onset of luteolysis. Furthermore, the luteal steroidogenic and endothelial cells may interact such that PGF2α-stimulated CYR61 from steroidogenic cells decreases ECE1 expression by endothelial cells, which may play a role in the transient increase in blood flow observed during the early onset of luteolysis in cows. The high expression of CYR61 in the developing Day 4 bovine CL affirms its role in the angiogenesis that accompanies luteal formation. Taken together, these results suggest that CYR61 may be a pivotal regulator of the angiogenic switch during the life span of the CL.
ACKNOWLEDGMENTS
We extend a special thanks to the staff at the University of New Hampshire's Fairchild Dairy Teaching and Research Center and at the Krauss Dairy Center at the Ohio Agricultural Research and Development Center for their expert assistance and support of this work.
Footnotes
Supported by NIH P01CA045548 and the Breast Cancer Research Foundation to M.A.M. and the Multistate Northeast Regional Projects NE-1007 and NE-1027 to P.C.W.T. and J.L.P. This is Scientific Contribution 2427 from the New Hampshire Agricultural Experiment Station.
These authors contributed equally to this work.
REFERENCES
- Augustin HG, Braun K, Telemenakis I, Modlich U, Kuhn W. Ovarian angiogenesis. Phenotypic characterization of endothelial cells in a physiological model of blood vessel growth and regression. Am J Pathol 1995; 147: 339 351. [PMC free article] [PubMed] [Google Scholar]
- Reynolds LP, Grazul-Bilska AT, Redmer DA. Angiogenesis in the corpus luteum. Endocrine 2000; 12: 1 9. [DOI] [PubMed] [Google Scholar]
- Zhang B, Yan L, Tsang PC, Moses MA. Matrix metalloproteinase-2 (MMP-2) expression and regulation by tumor necrosis factor α (TNFα) in the bovine corpus luteum. Mol Reprod Dev 2005; 70: 122 132. [DOI] [PubMed] [Google Scholar]
- Murdoch WJ, Gottsch ML. Proteolytic mechanisms in the ovulatory folliculo-luteal transformation. Connect Tissue Res 2003; 44: 50 57. [PubMed] [Google Scholar]
- Pederson E. Histogenesis of luteal tissue of the albino rat. Am J Anat 1951; 88: 397 427. [DOI] [PubMed] [Google Scholar]
- Parry DM, Willcox DL, Thorburn GD. Ultrastructural and cytochemical study of the bovine corpus luteum. J Reprod Fertil 1980; 60: 349 357. [DOI] [PubMed] [Google Scholar]
- Cavender JL, Murdoch WJ. Morphological studies of the microcirculatory system of periovulatory ovine follicles. Biol Reprod 1988; 39: 989 997. [DOI] [PubMed] [Google Scholar]
- Wiltbank MC, Dysko RC, Gallagher KP, Keyes PL. Relationship between blood flow and steroidogenesis in the rabbit corpus luteum. J Reprod Fertil 1988; 84: 513 520. [DOI] [PubMed] [Google Scholar]
- Fang J, Shing Y, Wiederschain D, Yan L, Butterfield C, Jackson G, Harper J, Tamvakopoulos G, Moses MA. Matrix metalloproteinase-2 is required for the switch to the angiogenic phenotype in a tumor model. Proc Natl Acad Sci U S A 2000; 97: 3884 3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2000; 2: 737 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmarajan AM, Bruce NW, Meyer GT. Quantitative ultrastructural characteristics relating to transport between luteal cell cytoplasm and blood in the corpus luteum of the pregnant rat. Am J Anat 1985; 172: 87 99. [DOI] [PubMed] [Google Scholar]
- Jablonka-Shariff A, Grazul-Bilska AT, Redmer DA, Reynolds LP. Growth and cellular proliferation of ovine corpora lutea throughout the estrous cycle. Endocrinology 1993; 133: 1871 1879. [DOI] [PubMed] [Google Scholar]
- Smith MF, McIntush EW, Smith GW. Mechanisms associated with corpus luteum development. J Anim Sci 1994; 72: 1857 1872. [DOI] [PubMed] [Google Scholar]
- Zheng J, Redmer DA, Reynolds LP. Vascular development and heparin-binding growth factors in the bovine corpus luteum at several stages of the estrous cycle. Biol Reprod 1993; 49: 1177 1189. [DOI] [PubMed] [Google Scholar]
- Duncan WC. The human corpus luteum: remodeling during luteolysis and maternal recognition of pregnancy. Rev Reprod 2000; 5: 12 17. [DOI] [PubMed] [Google Scholar]
- McCracken JA, Custer EE, Lamsa JC. Luteolysis: a neuroendocrine-mediated event. Physiol Rev 1999; 79: 263 323. [DOI] [PubMed] [Google Scholar]
- Davis JS, Rueda BR, Spanel-Borowski K. Microvascular endothelial cells of the corpus luteum. Reprod Biol Endocrinol 2003; 1: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser HM, Wulff C. Angiogenesis in the corpus luteum. Reprod Biol Endocrinol 2003; 1: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Yan L, Moses MA, Tsang PC. Bovine membrane-type 1 matrix metalloproteinase: molecular cloning and expression in the corpus luteum. Biol Reprod 2002; 67: 99 106. [DOI] [PubMed] [Google Scholar]
- Zhang B, Moses MA, Tsang PC. Temporal and spatial expression of tissue inhibitors of metalloproteinases 1 and 2 (TIMP-1 and −2) in the bovine corpus luteum. Reprod Biol Endocrinol 2003; 1: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Weich HA, Bernart W, Breckwoldt M, Neulen J. Vascular endothelial growth factor (VEGF) messenger ribonucleic acid (mRNA) expression in luteinized human granulosa cells in vitro. J Clin Endocrinol Metab 1993; 77: 1723 1725. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Konishi I, Tsuruta Y, Nanbu K, Mandai M, Kuroda H, Matsushita K, Hamid AA, Yura Y, Mori T. Expression of vascular endothelial growth factor (VEGF) during folliculogenesis and corpus luteum formation in the human ovary. Gynecol Endocrinol 1997; 11: 371 381. [DOI] [PubMed] [Google Scholar]
- Laitinen M, Ristimaki A, Honkasalo M, Narko K, Paavonen K, Ritvos O. Differential hormonal regulation of vascular endothelial growth factors VEGF, VEGF-B, and VEGF-C messenger ribonucleic acid levels in cultured human granulosa-luteal cells. Endocrinology 1997; 138: 4748 4756. [DOI] [PubMed] [Google Scholar]
- Geva E, Jaffe RB. Role of vascular endothelial growth factor in ovarian physiology and pathology. Fertil Steril 2000; 74: 429 438. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D, Cheng J, Lui GM, Baird A, Esch F, Bohlen P. Corpus luteum angiogenic factor is related to fibroblast growth factor. Endocrinology 1985; 117: 2383 2391. [DOI] [PubMed] [Google Scholar]
- Fraser HM, Bell J, Wilson H, Taylor PD, Morgan K, Anderson RA, Duncan WC. Localization and quantification of cyclic changes in the expression of endocrine gland vascular endothelial growth factor in the human corpus luteum. J Clin Endocrinol Metab 2005; 90: 427 434. [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997; 277: 55 60. [DOI] [PubMed] [Google Scholar]
- Berisha B, Schams D, Kosmann M, Amselgruber W, Einspanier R. Expression and tissue concentration of vascular endothelial growth factor, its receptors, and localization in the bovine corpus luteum during estrous cycle and pregnancy. Biol Reprod 2000; 63: 1106 1114. [DOI] [PubMed] [Google Scholar]
- Perbal B. CCN proteins: multifunctional signaling regulators. Lancet 2004; 363: 62 64. [DOI] [PubMed] [Google Scholar]
- Lau LF, Lam SC. The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res 1999; 248: 44 57. [DOI] [PubMed] [Google Scholar]
- Wandji SA, Gadsby JE, Barber JA, Hammond JM. Messenger ribonucleic acids for MAC25 and connective tissue growth factor (CTGF) are inversely regulated during folliculogenesis and early luteogenesis. Endocrinology 2000; 141: 2648 2657. [DOI] [PubMed] [Google Scholar]
- Goldberg MJ, Moses MA, Tsang PC. Identification of matrix metalloproteinases and metalloproteinase inhibitors in bovine corpora lutea and their variation during the estrous cycle. J Anim Sci 1996; 74: 849 857. [DOI] [PubMed] [Google Scholar]
- Cannon MJ, Pate JL. Expression and regulation of interferon gamma-inducible proteasomal subunits LMP7 and LMP10 in the bovine corpus luteum. Biol Reprod 2003; 68: 1447 1454. [DOI] [PubMed] [Google Scholar]
- Pate JL, Condon WA. Effects of serum and lipoproteins on steroidogenesis in cultured bovine luteal cells. Mol Cell Endocrinol 1982; 28: 551 562. [DOI] [PubMed] [Google Scholar]
- Pru JK, Lynch MP, Davis JS, Rueda BR. Signaling mechanisms in tumor necrosis factor α-induced death of microvascular endothelial cells of the corpus luteum. Reprod Biol Endocrinol 2003; 1: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gashaw I, Stiller S, Boing C, Kimmig R, Winterhager E. Premenstrual regulation of the pro-angiogenic factor CYR61 in human endometrium. Endocrinology 2008; 149: 2261 2269. [DOI] [PubMed] [Google Scholar]
- Ginther OJ, Araujo RR, Palhao MP, Rodrigues BL, Beg MA. Necessity of sequential pulses of prostaglandin F2α for complete physiologic luteolysis in cattle. Biol Reprod 2009; 80: 641 648. [DOI] [PubMed] [Google Scholar]
- Neuvians TP, Berisha B, Schams D. Vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) expression during induced luteolysis in the bovine corpus luteum. Mol Reprod Dev 2004; 67: 389 395. [DOI] [PubMed] [Google Scholar]
- Kolesnikova TV, Lau LF. Human CYR61-mediated enhancement of bFGF-induced DNA synthesis in human umbilical vein endothelial cells. Oncogene 1998; 16: 747 754. [DOI] [PubMed] [Google Scholar]
- Redmer DA, Grazul AT, Kirsch JD, Reynolds LP. Angiogenic activity of bovine corpora lutea at several stages of luteal development. J Reprod Fertil 1988; 82: 627 634. [DOI] [PubMed] [Google Scholar]
- Beal WE, Milvae RA, Hansel W. Estrous cycle length and plasma progesterone concentrations following administration of prostaglandin F-2α early in the bovine estrous cycle. J Reprod Fertil 1980; 59: 393 396. [DOI] [PubMed] [Google Scholar]
- Inskeep EK. Potential uses of prostaglandins in control of reproductive cycles of domestic animals. J Anim Sci 1973; 36: 1149 1157. [DOI] [PubMed] [Google Scholar]
- Shirasuna K, Sasahara K, Matsui M, Shimizu T, Miyamoto A. Prostaglandin F2α differentially affects mRNA expression relating to angiogenesis, vasoactivation and prostaglandins in the early and mid corpus luteum in the cow. J Reprod Dev 2010; 56: 428 436. [DOI] [PubMed] [Google Scholar]
- Tsai SJ, Wiltbank MC. Prostaglandin F2α regulates distinct physiological changes in early and mid-cycle bovine corpora lutea. Biol Reprod 1998; 58: 346 352. [DOI] [PubMed] [Google Scholar]
- Miyamoto A, von Lutzow H, Schams D. Acute actions of prostaglandin F2α, E2, and I2 in microdialyzed bovine corpus luteum in vitro. Biol Reprod 1993; 49: 423 430. [DOI] [PubMed] [Google Scholar]
- Okuda K, Uenoyama Y, Lee KW, Sakumoto R, Skarzynski D. Progesterone stimulation by prostaglandin F2α involves the protein kinase C pathway in cultured bovine luteal cells. J Reprod Dev 1998; 44: 79 84. [Google Scholar]
- Silva PJ, Juengel JL, Rollyson MK, Niswender GD. Prostaglandin metabolism in the ovine corpus luteum: catabolism of prostaglandin F2α (PGF2α) coincides with resistance of the corpus luteum to PGF2α. Biol Reprod 2000; 63: 1229 1236. [DOI] [PubMed] [Google Scholar]
- Cavicchio VA, Pru JK, Davis BS, Davis JS, Rueda BR, Townson DH. Secretion of monocyte chemoattractant protein-1 by endothelial cells of the bovine corpus luteum: regulation by cytokines but not prostaglandin F2α. Endocrinology 2002; 143: 3582 3589. [DOI] [PubMed] [Google Scholar]
- Anderson LE, Wu YL, Tsai SJ, Wiltbank MC. Prostaglandin F2α receptor in the corpus luteum: recent information on the gene, messenger ribonucleic acid, and protein. Biol Reprod 2001; 64: 1041 1047. [DOI] [PubMed] [Google Scholar]
- Mamluk R, Chen D, Greber Y, Davis JS, Meidan R. Characterization of messenger ribonucleic acid expression for prostaglandin F2α and luteinizing hormone receptors in various bovine luteal cell types. Biol Reprod 1998; 58: 849 856. [DOI] [PubMed] [Google Scholar]
- Meidan R, Levy N, Kisliouk T, Podlovny L, Rusiansky M, Klipper E. The yin and yang of corpus luteum-derived endothelial cells: balancing life and death. Domest Anim Endocrinol 2005; 29: 318 328. [DOI] [PubMed] [Google Scholar]
- Spanel-Borowski K, van der Bosch J. Different phenotypes of cultured microvessel endothelial cells obtained from bovine corpus luteum. Study by light microscopy and by scanning electron microscopy (SEM). Cell Tissue Res 1990; 261: 35 47. [DOI] [PubMed] [Google Scholar]
- Townson D. Immune cell-endothelial cell interactions in the bovine corpus luteum. Integrative and Comparative Biology 2006; 46: 1055 1059. [DOI] [PubMed] [Google Scholar]
- Liptak AR, Sullivan BT, Henkes LE, Wijayagunawardane MP, Miyamoto A, Davis JS, Rueda BR, Townson DH. Cooperative expression of monocyte chemoattractant protein 1 within the bovine corpus luteum: evidence of immune cell-endothelial cell interactions in a coculture system. Biol Reprod 2005; 72: 1169 1176. [DOI] [PubMed] [Google Scholar]
- Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A 1989; 86: 2863 2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opgenorth TJ, Wu-Wong JR, Shiosaki K. Endothelin-converting enzymes. FASEB J 1992; 6: 2653 2659. [DOI] [PubMed] [Google Scholar]
- Girsh E, Wang W, Mamluk R, Arditi F, Friedman A, Milvae RA, Meidan R. Regulation of endothelin-1 expression in the bovine corpus luteum: elevation by prostaglandin F 2α. Endocrinology 1996; 137: 5191 5196. [DOI] [PubMed] [Google Scholar]
- Wright MF, Sayre B, Inskeep EK, Flores JA. Prostaglandin F2α regulation of the bovine corpus luteum endothelin system during the early and midluteal phase. Biol Reprod 2001; 65: 1710 1717. [DOI] [PubMed] [Google Scholar]
- Levy N, Gordin M, Mamluk R, Yanagisawa M, Smith MF, Hampton JH, Meidan R. Distinct cellular localization and regulation of endothelin-1 and endothelin-converting enzyme-1 expression in the bovine corpus luteum: implications for luteolysis. Endocrinology 2001; 142: 5254 5260. [DOI] [PubMed] [Google Scholar]
- Acosta TJ, Yoshizawa N, Ohtani M, Miyamoto A. Local changes in blood flow within the early and midcycle corpus luteum after prostaglandin F2α injection in the cow. Biol Reprod 2002; 66: 651 658. [DOI] [PubMed] [Google Scholar]
- Mo FE, Muntean AG, Chen CC, Stolz DB, Watkins SC, Lau LF. CYR61 (CCN1) is essential for placental development and vascular integrity. Mol Cell Biol 2002; 22: 8709 8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kireeva ML, Mo FE, Yang GP, Lau LF. CYR61, a product of a growth factor-inducible immediate-early gene, promotes cell proliferation, migration, and adhesion. Mol Cell Biol 1996; 16: 1326 1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kireeva ML, Lam SC, Lau LF. Adhesion of human umbilical vein endothelial cells to the immediate-early gene product Cyr61 is mediated through integrin αvβ3. J Biol Chem 1998; 273: 3090 3096. [DOI] [PubMed] [Google Scholar]
- Babic AM, Kireeva ML, Kolesnikova TV, Lau LF. CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc Natl Acad Sci U S A 1998; 95: 6355 6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fataccioli V, Abergel V, Wingertsmann L, Neuville P, Spitz E, Adnot S, Calenda V, Teiger E. Stimulation of angiogenesis by CYR61 gene: a new therapeutic candidate. Hum Gene Ther 2002; 13: 1461 1470. [DOI] [PubMed] [Google Scholar]
- Tsai MS, Bogart DF, Castaneda JM, Li P, Lupu R. CYR61 promotes breast tumorigenesis and cancer progression. Oncogene 2002; 21: 8178 8185. [DOI] [PubMed] [Google Scholar]
- Chen CC, Mo FE, Lau LF. The angiogenic factor CYR61 activates a genetic program for wound healing in human skin fibroblasts. J Biol Chem 2001; 276: 47329 47337. [DOI] [PubMed] [Google Scholar]
- Abe M, Sato Y. cDNA microarray analysis of the gene expression profile of VEGF-activated human umbilical vein endothelial cells. Angiogenesis 2001; 4: 289 298. [DOI] [PubMed] [Google Scholar]
- Tsang PC, Poff JP, Boulton EP, Condon WA. Four-day-old bovine corpus luteum: progesterone production and identification of matrix metalloproteinase activity in vitro. Biol Reprod 1995; 53: 1160 1168. [DOI] [PubMed] [Google Scholar]