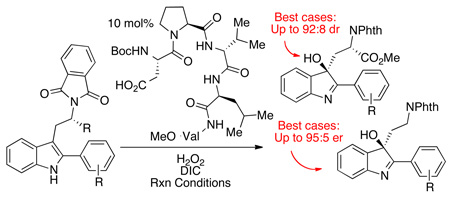
Abstract
Catalytic enantioselective indole oxidation is a process of particular relevance to the chemistry of complex alkaloids, as it has been implicated in their biosynthesis. In the context of synthetic methodology, catalytic enantioselective indole oxidation allows a rapid and biomimetic entry into several classes of alkaloid natural products. Despite this potentially high utility in the total synthesis, reports of catalytic enantioselective indole oxidation remain sparse. Here we report a highly chemoselective catalytic system for the indole oxidation that delivers 3-hydroxy-indolenines with good chemical yields and moderate to high levels of enantio- and diastereoselectivity (up to 95:5 er and up to 92:8 dr. These results represent, to our knowledge, the most selective values yet reported in the literature for catalytic asymmetric indole oxidation). Furthermore, the utility of enantioenriched hydroxy-indolenines in stereospecific rearrangements is demonstrated.
Introduction
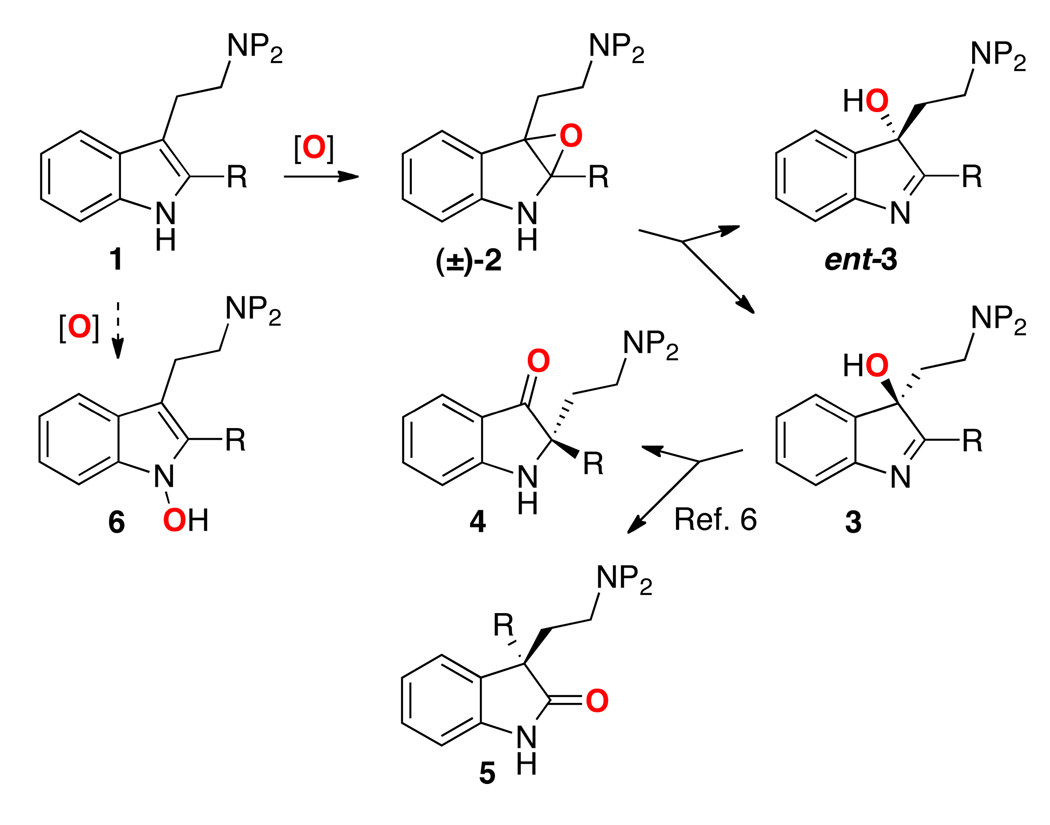
The catalytic enantioselective oxidation of indoles is a significant challenge, and one of particular relevance to the total synthesis of complex, naturally occurring alkaloids.1 At the same time, enzymatic oxidation of indoles is well-recognized as a key step in indole alkaloid biosynthesis,2 rendering oxidation approaches to their chemical synthesis inherently biomimetic.3 Chemically, tryptamine derivatives such as 1 may be chemoselectively oxidized to afford hydroxy-indolenines (3), which derive from the intermediate indole oxides (2). These compounds, in turn may be subjected to highly stereoselective rearrangements. Notably, in a condition-dependent fashion, hydroxy-indolenines of type 3 may be converted to either the 3-oxindole regioisomer 4 or the 2-oxindole regioisomer 5.4,5,6 These oxidative isomerizations have played a critical role in a number of elegant natural product syntheses for introduction of challenging stereochemical arrays.4
Selective indole oxidation presents a myriad of challenges to the goal of developing catalytic enantioselective approaches. Presently, asymmetric catalytic approaches to the preparation of optically enriched 3-hydroxy-indolenines (3), from achiral starting materials, are quite limited. Application of the venerable extant asymmetric epoxidation catalysts7 to these highly electron-rich heterocycles has been reported, but with only limited success.8 Among the challenges, in addition to stereocontrol, is the issue of chemoselectivity. Reactivity may be diverted from the desired formation of 3 to the observation of N-hydroxy-indoles (6) and indole-ring hydroxylation.9 Thus, complications are often encountered with a variety of metal-catalyzed, dioxirane- or oxaziridine-mediated approaches.
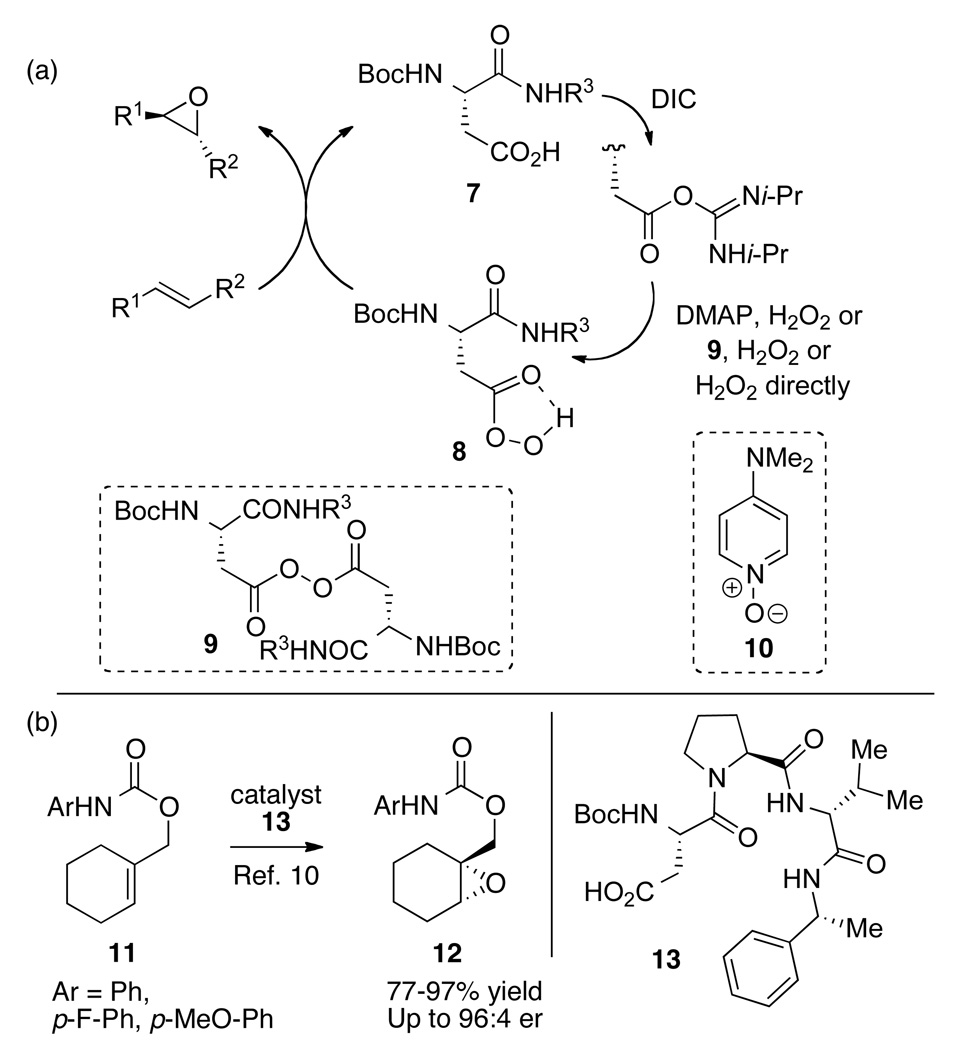
In contrast, classical peracid-mediated oxidation of indoles provides an opportunity to assess alternative approaches to the chemo- and enantioselective oxidation of indoles. Indeed, the classical and highly general oxidation potential of m-chloroperbenzoic acid (MCPBA) recently inspired the development of a catalytic cycle for asymmetric epoxidation based on the transient generation of aspartic peracids in peptide-based catalysts (Figure 2a).10 For example, it is now known that the aspartic acid residue (e.g., 7) can function as a catalyst following carboxyl activation with carbodiimide reagents. In the presence of hydrogen peroxide, the putative peracid 8 is formed, which then delivers an O-atom to an olefin to form an epoxide, regenerating the free acid. Mechanistic studies have documented aspects of this catalytic cycle, including the observation of off-cycle intermediates such as 9.11,12 Nonetheless, these may reinsert into the catalytic cycle under the aegis of nucleophilic catalysts such a N,N-dimethylaminopyridine (DMAP), or the corresponding N-oxide (10). Moreover, as shown in Figure 2b, this approach was rendered enantioselective for the epoxidation of substrates like 11, which could be converted to epoxide 12 in up to 96:4 enantiomeric ratio (er; 92% ee), with application of as little as 5 mol% of catalyst 13. Given the generality of MCPBA in the effective oxidation of a wide range of olefinic compounds, we wished to explore whether or not the aspartyl peptide-based oxidation catalysts might be appropriate for the effective oxidation of indolic compounds of relevance to natural product synthesis. We report herein our studies, which allow us to conclude that this approach is indeed appropriate for the efficient chemoselective oxidation of many indoles to the corresponding hydroxy-indolenines. Moreover, we describe our studies of stereoselective indole oxidation, including the first reports of significant levels of enantioselectivity and indeed diastereoselectivity in catalytic asymmetric indole oxidation reactions.
Figure 2.
Aspartyl peptide-based catalytic enantioselective oxidation. (a) The development of a peracid-based catalytic cycle. (b) The development of enantioselective variants of the process. DMAP = N,N-dimethyl-4-aminopyridine, DIC = diisopropylcarbodiimide.
Results and Discussion
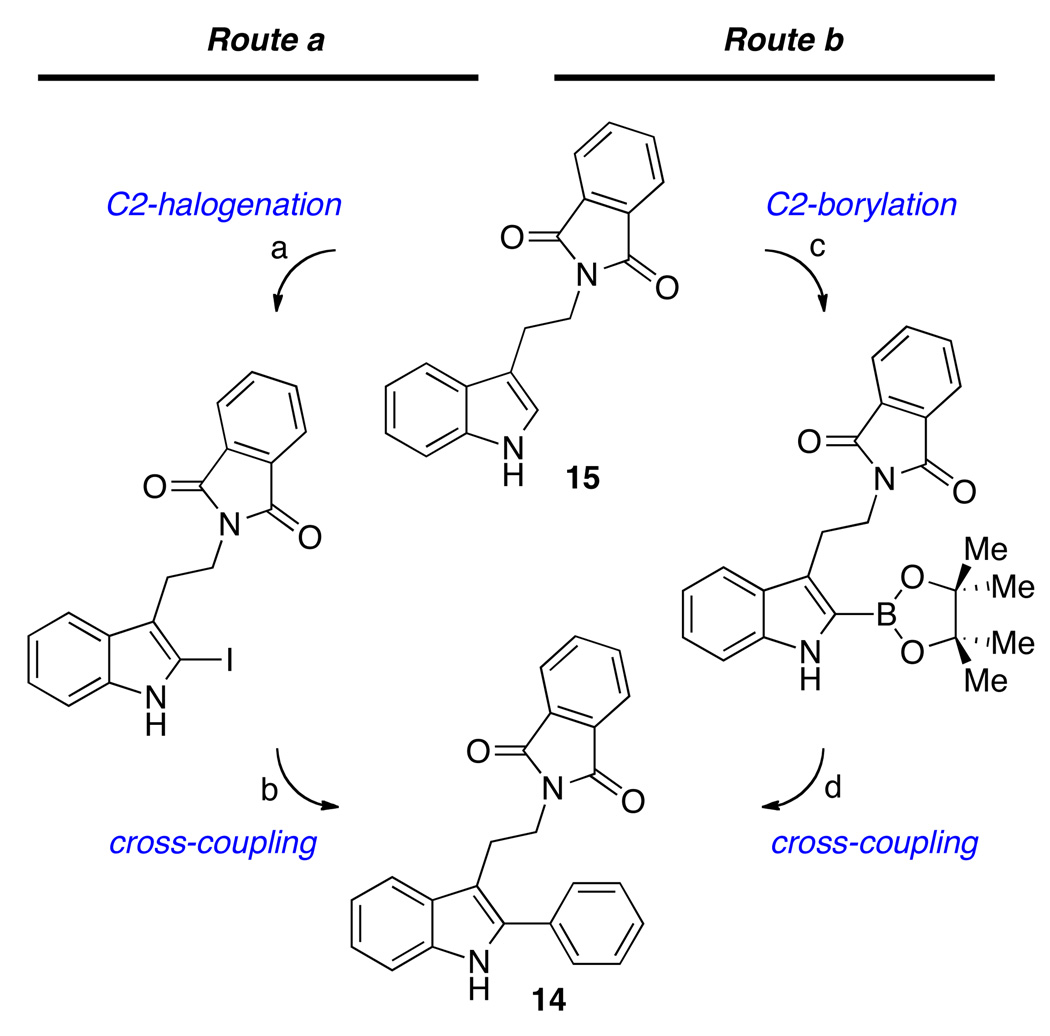
Our studies began with tryptamine 14 (Scheme 1) as a superb substrate for development of the chemistry aimed at a variety of complex indole alkaloids containing cyclotryptamine and cyclotryptophan substructures. Substrates such as 14 may be prepared by two concise and equally versatile synthetic routes as illustrated in Scheme 1. The first route involves C2-halogenation of tryptamine derivatives such as 15, followed by Suzuki-Miyaura coupling with aryl boronates to afford 2-aryltryptamines (Scheme 1, Route a). An alternative route utilizes iridium-catalyzed13 C2-borylation of tryptamines, followed by palladium-catalyzed cross-coupling with aryl halides (Scheme 1, Route b).14,15
Scheme 1.
Two synthetic strategies used for preparation of substrates in this study from simple tryptamines. Route a-Conditions: (a) I2 (1.1 equiv), AgOTf (1.1 equiv), THF, −78→23 °C, 15 min, 96%. (b) Pd2(dba)3 (2.5 mol%), XPhos (10 mol%), K3PO4 (2.0 equiv), toluene, water, 110 °C, 2 h, PhB(OH)2 (1.5 equiv), 75%. Route b-Conditions: (c) [Ir(OMe)(COD)]2 (3 mol%), dtbpy (6 mol%), (Bpin)2 (2.0 equiv), CH2Cl2, 65 °C, 3 h, 78%. (d) Pd2(dba)3 (2.5 mol%), XPhos (10 mol%), K3PO4 (2.0 equiv), toluene, water, 110 °C, 2 h, PhI (1.0 equiv), 82%. dtbpy = 4,4'-di-tert-butyl-2,2'-bipyridine.
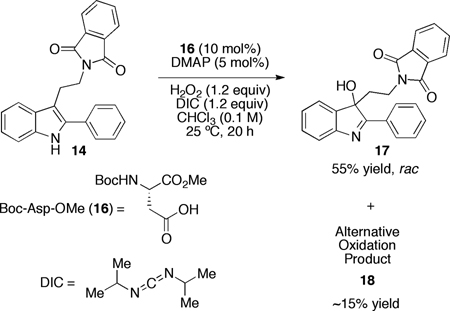
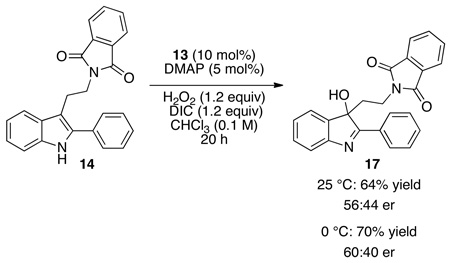
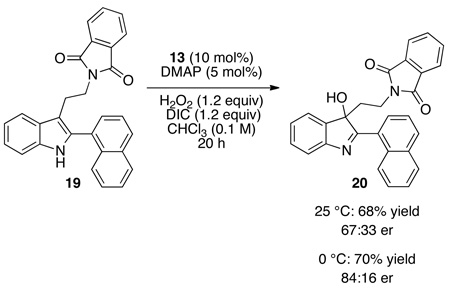
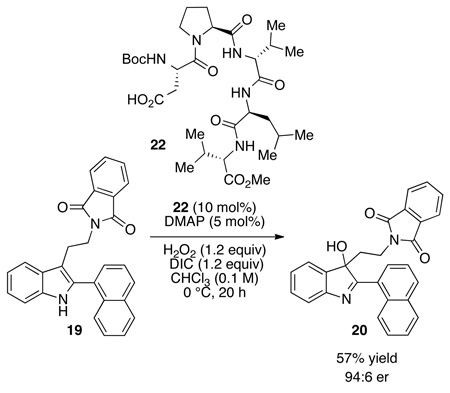
We then turned our attention to the oxidation chemistry, employing the simple aspartic acid derivative 16 (equation 1) as a catalyst. As outlined in equation 1, performing the oxidation at 25 °C, the indole substructure of 14 was oxidized to the corresponding 3-hydroxy-indolenine derivative 17, isolated in 55% yield. In addition to 17, approximately 15% of an alternative oxidation product 18 had formed, and this side product could be removed by column chromatography. In terms of enantioselectivity, we noted minimal enantioselectivity when the simple catalyst 16 was employed. However, employing catalyst 13 (Figure 2), discovered during the original aspartyl-peptide epoxidation study,10 we were pleased to observe that 17 could be formed in 64% yield, with an er of 56:44 under analogous conditions (10 mol%, 25 °C; eq 2). A preliminary screen of reaction conditions revealed that maintaining the temperature at 0 °C is beneficial for selectivity, and that at this temperature 3-hydroxy-indolenine 17 is formed with 60:40 er (70% yield). These effects are amplified with the 2-naphthyl-substituted indole 19 (eq 3). At 25 °C, indole 19 is converted to 20 with a 67:33 er. At 0 °C, hydroxyindolenine 20 is formed with an encouraging er of 84:16 (70% yield). Notably, under these conditions the formation of the byproduct is also suppressed. We attributed this behavior to the presence of an alternative and non-selective oxidation pathway at higher temperatures, perhaps via DIC–H2O2 adduct,16 which seems to be mostly supressed at 0 °C. In terms of reaction efficiency, and in line with earlier results,10 a strong dependence of the conversion on the presence of nucleophilic co-catalyst N,N-dimethyl-4-aminopyridine is observed.
 |
(1) |
 |
(2) |
 |
(3) |
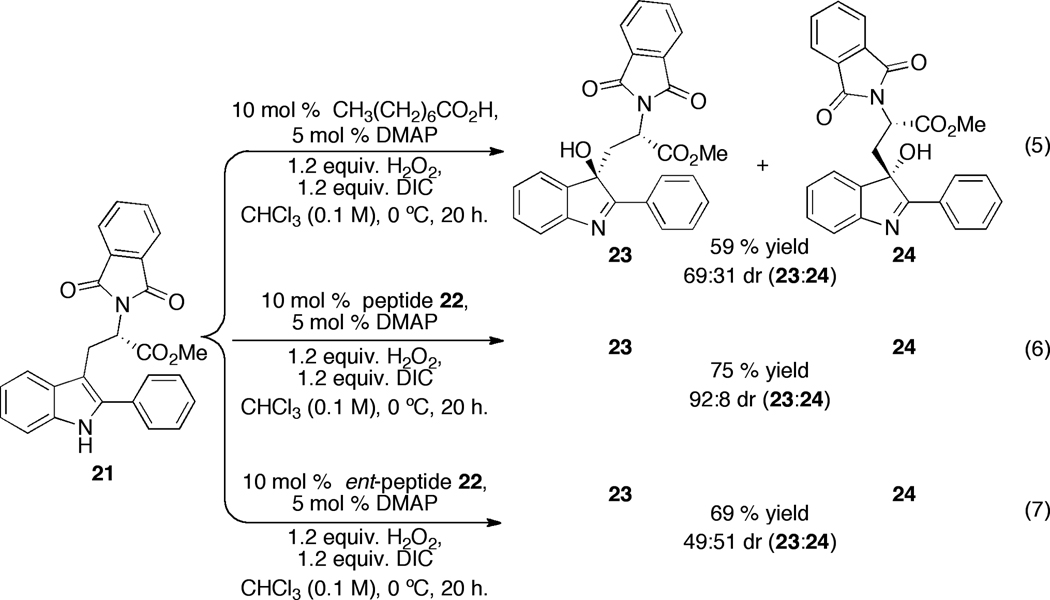
With encouraging levels of chemoselectivity and a preliminary observation of enantioselectivity in the aspartyl peptide-catalyzed oxidation of indoles, we then turned our attention to the issue of stereoselectivity. We describe here our initial efforts in the area of diastereoselective oxidation of indoles. We chose to study this type of stereoselective process for several reasons. First, the notion of using chiral catalysts as tools for the selective modification of complex architectures is a challenging frontier for the field of asymmetric catalysis.17 Such approaches are also of potential value in the total synthesis of stereochemically complex molecules, including complex indole alkaloids.4 In addition, the study of double diastereodifferentiating reactions often provides valuable data of a fundamental nature in the development of asymmetric processes.18 Thus, we began with an examination of chiral aspartyl peptides that might affect selective oxidation of tryptophan derivatives such as 21 (Figure 3). In order to proceed with an appropriate catalyst, it was important to benchmark its inherent selectivity with an achiral substrate. Thus, we examined a library of peptides based on a historically valuable β-turn scaffold19 that incorporated a catalytic Asp residue as the N-terminal residue. (See Supporting Information for more details). This study was initially conducted with substrate 19, an achiral analog that was thought to mimic more elaborated bis(indolyl) substrates of interest (to be discussed below). Through this process, peptide 22 emerged as a promising catalyst for oxidation of simple indoles such as 19 (94:6 er; 57% yield; eq 4).
 |
(4) |
Figure 3.
Studies of double asymmetric induction in the chemoselective catalytic oxidation of 21.
With catalyst 22 in hand, we turned our attention to oxidation of chiral substrates derived from tryptophan, such as compound 21. It is well precedented that the presence of a chiral center in the indole side chain renders the oxidation diastereoselective.6,20 Thus, unsurprisingly, use of octanoic acid as an achiral catalyst for the oxidation of 21 we observed formation of diastereomers 23 and 24 in the diastereomeric ratio of 69:31 (59% combined yield; Figure 3, eq 5). A similar diastereomeric ratio (dr) value is observed in the oxidation of 21 using stoichiometric quantities of DMDO.6 On the other hand, a significant amplification of diastereoselectivity of the reaction is observed when peptide 22 is used as a catalyst. In this case, a striking “matched” case of double asymmetric induction is observed, with the formation of 23 and 24 as a 92:8 mixture of diastereomers (75% combined yield; Figure 3, eq 6).21 Significantly, use of the enantiomer of peptide-catalyst 22 led to a nonselective, “mismatched” case, and a 49:51 mixture of diastereomers 23 and 24 was observed (69% combined yield; Figure 3, eq 7). We note parenthetically that major compound 23 possesses the correct stereochemical array relative to many naturally occurring cyclotryptamines.6
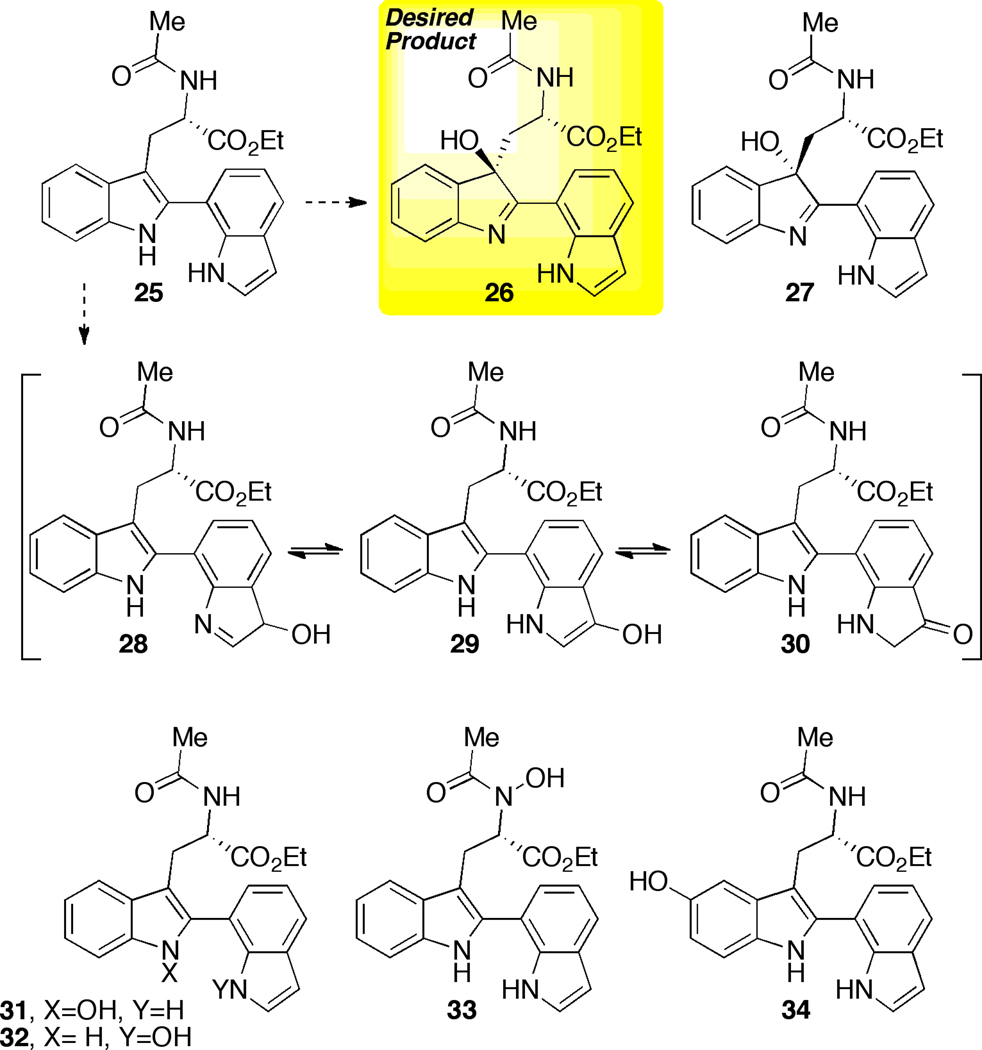
We then wished to assess the viability of aspartyl peptide-based oxidation catalysis in increasingly complex settings. Thus, we wished to examine the challenge of chemoselective oxidation in the face of substrates that present two indolyl N-H groups, each a potential culprit in terms of undesired N-H oxidation side reactions. Notably, substrate 25 also possesses an acetamide, with an additional potentially vulnerable N-H function. Given the presence of a second highly electron rich aromatic system, one can envision formation of multiple regioisomeric indole oxidation products emanating from 25 (e.g., hydroxy-indolenines (26 and 27); hydroxy-indole tautomers 28–30; distinct N-OH indoles (31 and 32); N-hydroxy acetamide 33, distal indole ring oxidation products such as 34; Figure 4.)
Figure 4.
Various hypothetical oxidation products derived from 25.
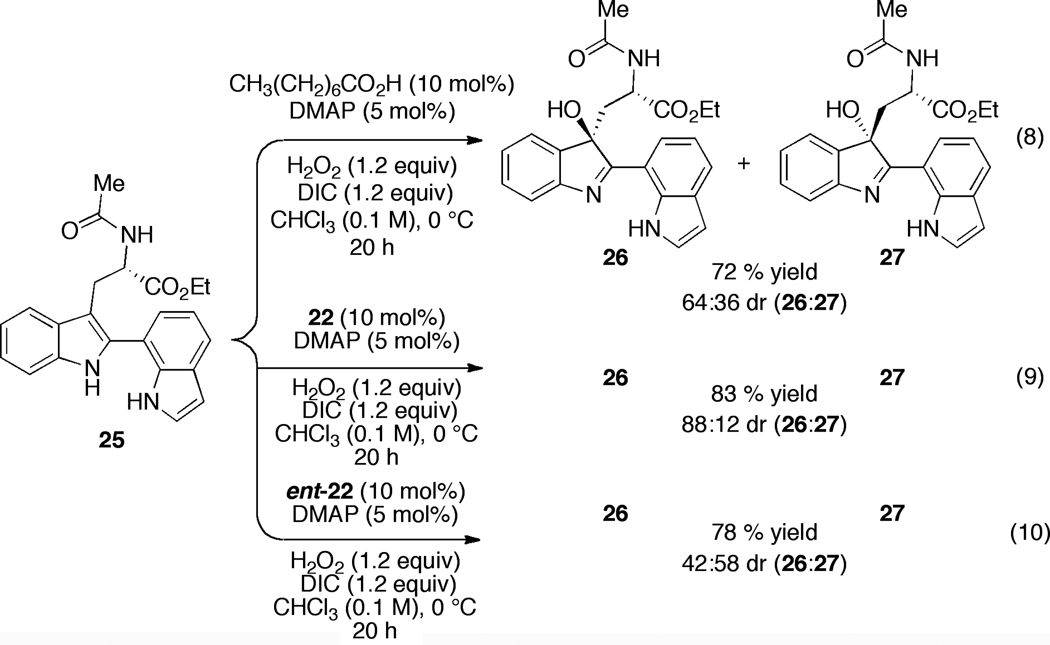
In addition to these chemoselectivity challenges, we wished to assess whether catalyst 22 was portable to this new substrate class in terms of affording comparable levels of diastereoselectivity in the specific formation of 26 and 27. Thus, the bis(indole) compound 25 became our next focus for study. Remarkably, as shown in Figure 5, we found that substrate 25 was an excellent substrate for chemoselective oxidation to the corresponding hydroxy-indolenines 26 and 27. Indeed, these compounds could be isolated in 72–83% chemical yield under the catalytic conditions. Competing N-H oxidation reactions were not significant side reactions. Moreover, only the desired tryptaminyl indole oxidation products were isolated, with no perturbation of second indole moiety detected. The inherent diastereoselectivity of bis(indole) 25 was defined as a 64:36 ratio, employing octanoic acid as the catalyst (72% yield; Figure 5, eq 8). The results in terms of peptide-based control of stereochemistry were also encouraging. As shown in Figure 5, diastereomers 26 and 27 were obtained in an 88:12 diastereomeric ratio when catalyst 22 was employed (10 mol%), a manifestation of a “matched” case of double asymmetric induction (83% yield; Figure 5, eq 9).22 Moreover, hydroxy-indolenines 26 and 27 are readily separated by flash column chromatography. In the mismatched case, the enantiomer of catalyst 22 delivered 26/27 as a 42:58 diastereomeric mixture (78% isolated yield; Figure 4, eq 10). Of note, the reversal of diastereoselectivity reveals that the catalyst overrides, albeit modestly, the inherent stereoselectivity preference of the substrate.
Figure 5.
Studies of double asymmetric induction in the chemoselective catalytic oxidation of 25.
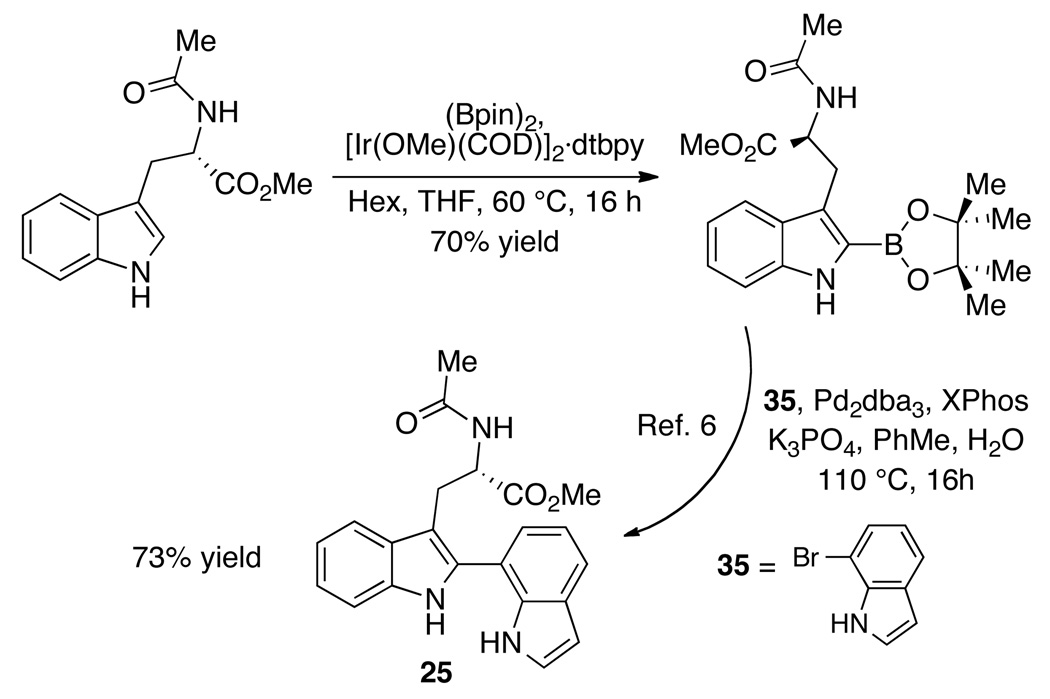
Most notably, under the present conditions, none of the products arising from the oxidation of the second indole moiety or from the indole N-oxidation could be detected, thus obviating the necessity for the introduction of protecting groups into the synthetic schemes. The higher reactivity of the double bond of the more substituted indole may be correlated to the corresponding higher HOMO energy resulting from a partial orbital overlap with the aromatic system of the second indole.23 Compound 25 is readily accessible in two steps (Scheme 2) from a commercially available tryptophan derivative consistent with the conditions discussed in Scheme 1, and therefore these results represent a step efficient entry into optically pure tryptophan based 3-hydroxy-indolenines. 13, 14
Scheme 2.
Preparation of the chiral bis-indole 25 via indole borylation/Suzuki-Miyaura coupling protocol. pin = pinacol, dtbpy = 4,4'-di-tert-butyl-2,2'-bipyridine, XPhos = 2-dicyclohexylphosphino-2',4',6'-triisopropylbiphenyl.
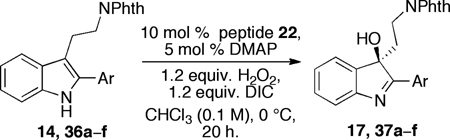
Having defined a highly chemoselective indole oxidation process employing a mild and catalytic process, we next wished to determine if these diastereoselective reactions might be extendable to enantioselective processes. Since catalytic enantioselective reactions that convert indoles to the corresponding hydroxy-idolenines are so rare, we wished to document the scope of catalyst 22 for the highly challenging arena of enantioselective, electon rich heterocycle oxidation. Thus, our studies began by examining a range of different tryptamine derivatives with monocyclic aryl substitution at the indolic 2-position (Table 1). As noted above (eq 2), the parent compound 14 undergoes a smooth chemoselective oxidation with peptide 13 employed as the catalyst (60:40 er; 65% yield). Employing catalyst 22, the er is enhanced to 77:23 (entry 1). Moreover, we found that substrates containing ortho-substituents on the 2-aryl substructure were subject to quite selective reactions with catalyst 22. Thus, o-CF3 compound 36a undergoes catalytic enantioselective oxidation to give 37a with a 94:6 er (93% isolated yield; entry 2). The o-NO2–substituted compound 36b is also a good substrate for catalyst 22, although the er and yield are slightly attenuated for product 37b (89:11 er, 79% yield, entry 3). A substrate bearing o-CH3 group (36c) performs similarly (37c: 88:12 er, 76% yield, entry 4). On the other hand, for reasons that are at present unclear, the o-MeO–containing compound 36d is resistant to efficient oxidation (entry 5). The p-substitued compounds 36e and 36f are oxidized with reasonable yields (37e and 37f, 64% and 69%, respectively), but exhibit modest er (~3:1, entries 6 and 7). Thus, we found that 2-phenyl substituents bearing an electron withdrawing or an electron neutral substituent ortho relative to the indole attachment site (e.g. substrates 36a–c) resulted in the formation of products (37a–c) with the highest er values. The high er’s and yields obtained with these compounds additionally attest to their position as good substrates for peptide catalyst 22, and they also represent perhaps the highest enantioselectivities yet reported for the catalytic enantioslective oxidation of indolic compounds.
Table 1.
Oxidation of simple 2-arylindoles with peptide 22 as a catalyst.
 | |||||
|---|---|---|---|---|---|
| entry | indole | hydroxy-indoleninea | Ar | yield (%) | er |
| 1 | 14 | 17 |  |
59 | 77:23 |
| 2 | 36a | 37ab |  |
93 | 94:6 |
| 3 | 36b | 37b | 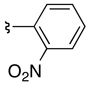 |
78 | 89:11 |
| 4 | 36c | 37c | 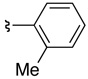 |
76 | 88:12 |
| 5 | 36d | 37dc | 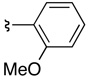 |
--d | -- |
| 6 | 36e | 37e | 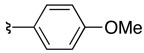 |
64 | 75:25 |
| 7 | 36f | 37fb | 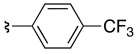 |
69 | 72:28 |
Reaction conditions for indole oxidation: indole (1.0 equiv), peptide (10 mol%), and DMAP (5 mol%) in CHCl3 (0.1 M) at 0 °C, then add aq. H2O2 (1.2 equiv), and DIC (1.2 equiv).
Concentration of indole in these reactions was increased to 0.5 M.
Concentration of indole in this reaction was increased to 0.2 M.
DMAP = N,N-dimethyl-4-aminopyridine, DIC = diisopropylcarbodiimide, Phth = phthaloyl.
A sluggish reaction and complex mixture of product is observed.
The presence of an ortho-substituent stimulated an examination of additional substrates that possessed analogous substitution, including elaborated arene substitution. As shown in Table 2, compounds 19 and 38a–c proved interesting in this regard. In particular, the 2-napthyl-subsituted tryptamine 19 proved to be a good substrate for catalyst 22, with 20 delivered with an er of 94:6 (57% isolated yield, entry 1). The anthracenyl compound 38a performed similarly, with 39a obtained as a 95:5 er (88% isolated yield, entry 2). In the case of both product 20 and 39a, recrystallization afforded material that was essentially enantiopure (er >98:2). On the other hand, the alternative substitution pattern of the athracenyl moiety (9- versus 1-position), defines substrate 38b as a less optimal case for catalyst 22, with 39b obtained with an 84:16 er, but in only 7% isolated yield (entry 3). It appears that a compound possessing aryl rings fused on both sides of the 2-phenyl-substituent engenders unfavorable interactions with the catalyst, thus preventing its efficient processing. Alleviating these interactions, as in the remotely substituted naphthalene derivative 38c allows reaction to occur (39c is obtained in 74% yield), although this ortho-unsubstituted compound exhibits modest enantioselectivity (67:33 er, entry 4).
Table 2.
Oxidation of indoles incorporating an elaborate arene with peptide 22 as a catalyst.
 | |||||
|---|---|---|---|---|---|
| entry | indole | hydroxy-indoleninea | Ar | yield (%) | er |
| 1 | 19 | 20 | 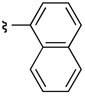 |
57 | 94:6 (>98:2)b |
| 2 | 38a | 39a | 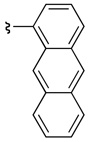 |
88 | 95:5 (>99:1)c |
| 3 | 38b | 39b | 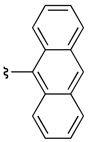 |
7 | 84:16 |
| 4 | 38c | 39c | 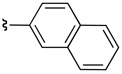 |
74 | 67:33 |
Reaction conditions for indole oxidation: indole (1.0 equiv), peptide (10 mol%), and DMAP (5 mol%) in CHCl3 (0.1 M) at 0 °C, then add aq. H2O2 (1.2 equiv), and DIC (1.2 equiv).
Recrystallized to >98:2 er from 95/5 2-propanol/ethanol mixture (yield after recrystallization 45%).
Recrystallized to >99:1 er from dichloromethane (yield after recrystallization 71%).
DMAP = N,N-dimethyl-4-aminopyridine, DIC = diisopropylcarbodiimide, Phth = phthaloyl.
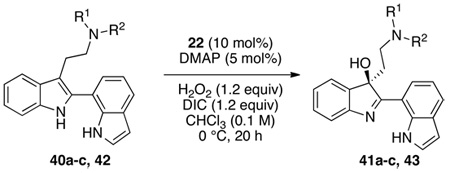
In addition, we wished to study the enantioselective indole oxidation substrate scope in structural arenas that would allow for an increasingly demanding assessment of chemoselectivity. Thus, we explored a class of compounds containing a second indole moiety, as well as additional oxidation-sensitive N-H functional groups. Bis(indole) substrates 40a–c (Table 3) were generally24 prepared by a Suzuki-Miyaura coupling of the 2-borylated tryptamines with the corresponding aryl halides as eluded to in Scheme 1.6 As discussed earlier, oxidation products derived from these compounds (41a–c) could find a direct application in the synthesis of complex alkaloids, and similar intermediates are often invoked in the biosynthesis of these natural products.2 At the onset of our investigations, we confirmed that 2-(7-indolyl)-tryptamines (40a–c) undergo highly chemoselective oxidation with oxygen delivery at the site of the 1,2-disubstituted indole, rather than to the competing functional groups. Notably, in all cases (Table 3), chemoselectivity proved very high, as is reflected in the isolated chemical yields. In cases such as 40a–b, selective indole oxidation occurs in the presence of two indoles, as well as additional amide functionality. For the former case, sulfonamide 41a is obtained as an 86:14 er, in 77% isolated yield (entry 1), while the latter pivaloyl amide 41b is obtained in 87% yield, with a 83:17 er (entry 2). These are in comparison to the parent bis(indole) 40c, which is converted to 41c with an 82:18 er, isolated in 89% yield (entry 3). While the phthalate compound 41c had formed with slightly lower er value as compared to the other analogs tested, this oxidation product was readily recrystallized to the enantiomeric ratio of 95:5, and the single crystals thus obtained allowed us to unambiguously assign the sense of the asymmetric induction through X-ray diffraction analysis (vide infra). In all cases, the observed chemoselectivity is exceptionally high.
Table 3.
Oxidation of simple bis(indoles) with peptide 22 as a catalyst.
 | ||||||
|---|---|---|---|---|---|---|
| entry | bis-indole | hydroxy-indoleninea | R1 | R2 | yield (%) | er |
| 1 | 40a | 41a |  |
H | 77 | 86:14 |
| 2 | 40b | 41b | 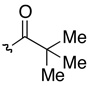 |
H | 87 | 83:17 |
| 3 | 40c | 41c | 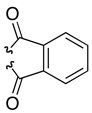 |
89 | 82:18 (95:5)b | |
| 4 | 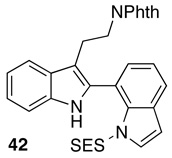 |
 |
70 | 93:7 | ||
Reaction conditions for indole oxidation: indole (1.0 equiv), peptide (10 mol%), and DMAP (5 mol%) in CHCl3 (0.1 M) at 0 °C, then add aq. H2O2 (1.2 equiv), and DIC (1.2 equiv).
Recrystallized to 95:5 er from 95/5 2-propanol/ethanol mixture (yield after recrystallization 47%).
DMAP = N,N-dimethyl-4-aminopyridine, DIC = diisopropylcarbodiimide, Phth = phthaloyl, SES = 2-(trimethylsilyl)ethylsulfonyl.
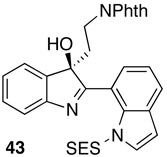
For purposes of comparison to the results obtained with bis(indoles) with both indolyl-N atoms unprotected, we also elected to examine the enantioselective oxidation of SES-protected indole 42. Interestingly, catalyst 22 mediates formation of 43 in 70% isolated yield, with a 93:7 er (Table 3, entry 9). This result suggests that enantioselectivities may be enhanced, at least in some cases, through the use of protecting groups, or the incorporation of bulky substituents. Moreover, we suspect that studies of catalyst optimization could well lead to higher inherent enantioselectivities for the unprotected bis(indoles) found in Table 3 (e.g., 40a–c). On the other hand, preliminary investigations of compounds that bear 2,3-dialkyl substitution afford the product of overoxidation as the dominant product.25 The current catalyst and conditions are thus well tuned for selective oxidation of 2-aryl-/indolyl-3-alkyl substrates, substrates of great interest in the context of the assembly of oligocyclotryptamine natural products.
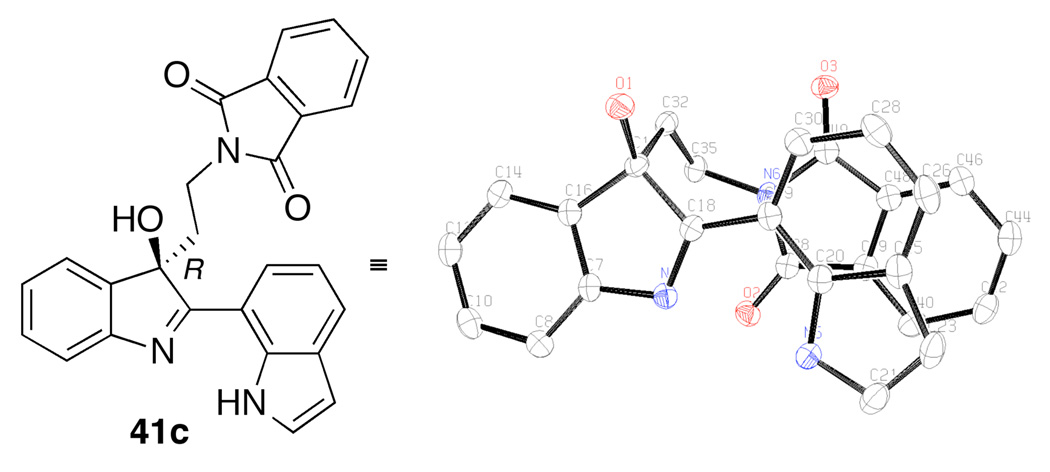
As noted above, several of the indole oxidation products were highly crystalline. A representative of the bis(indole) product class, compound 41c was subjected to X-ray diffraction analysis and the stereochemistry of the C-3 alcohol was assigned the R absolute configuration, as depicted in Figure 6. The assignment was confirmed through additional diffraction analyses, as well as through comparison with a literature data pertaining to a related system.6
Figure 6.
Determination of absolute stereochemistry through X-ray diffraction analysis.
Finally, the application of catalytic asymmetric indole oxidation creates important opportunities for manipulation of the products to elaborated, enantioenriched materials. In this vein,6 we have demonstrated that hydroxy-indolenines such as 23 and 24 may be rearranged with a high level of stereoselectivity. As shown in equation 11, we now report that compound 39a and 39c may be rearranged with complete preservation of the enantiomeric ratio. We project that this transformation, in conjunction with the catalytic enantioselective indole oxidation will find applications in synthesis of complex, naturally occurring alkaloids.
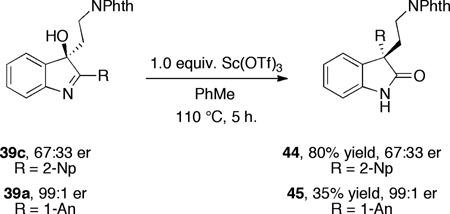 |
(11) |
Conclusions
We have described a highly chemoselective and mild catalytic system for the asymmetric oxidation of 2-aryl tryptamine derivatives. Noteworthy features of this chemistry include broad substrate scope and functional group tolerance and a highly versatile catalyst design that allows for adjustment and optimization for key substrate classes of interest. While a single catalyst, optimized for substrate 14, was used in this study for uniformity and direct comparison, the practical nature of catalyst tunning combined with the overall high level of stereoinduction in all cases holds great promise for application in complex setting. For example, as illustrated in Figures 3 and 5, catalyst 22, optimized for substrate 14, demonstrated an outstanding level of diastereoselection in the oxidation of substrates 21 and 25 in matched cases while still reversing the inherent substrate selectivity in the corresponding mismatch cases. Moreover, this unique catalyst was found to afford high er’s for several substrates of interest. Application of this catalyst system to oxidation of highly functional tryptamine and tryptophan derivatives in more challenging settings in the context of asymmetric synthesis of complex natural products is ongoing and will be the subject of upcoming reports.
Experimental Section
General indole oxidation protocol
The oxidation of indoles was carried out in a reaction flask mounted to a cryogenic cooler with temperature control of ±0.1 °C. Reactions were set up on the benchtop without exclusion of air or moisture. In a typical experiment, indole (0.1 mmol) was dissolved in 890 µL of chloroform (for deviations from the standard concentration see Tables 1–3) and peptide 22 was added as a chloroform solution (typically 100 µL of 0.1 M solution, 0.01 mmol). To this solution, a chloroform solution of N,N-dimethyl-4-aminopyridine was added (typically 10 µL of 0.5 M solution, 5 µmol), followed by 12.2 µL of aqueous 30% hydrogen peroxide (0.12 mmol). The biphasic mixture was stirred at 0 °C for 5 minutes, and the reaction initiated by the addition 18.6 uL of diisopropylcarbodiimide (0.12 mmol). After 20 hours, the reaction mixture was diluted with 2.0 mL of chloroform and directly purified by flash chromatography or preparative TLC.
Full details may be found in the Supporting Information.
Supplementary Material
Figure 1.
Oxidation of indoles and rearrangements.
ACKNOWLEDGMENT
This work is supported by National Institutes of Health, National Institute of General Medical Sciences (GM096403) to SJM and (GM089732) to MM. MM also wishes to thank Amgen and DuPont for additional financial support. MM, MNN, and MT thank Dr. Cristina M. Nieto-Oberhuber and Ms. Yuki Nakamura of the Movassaghi group for helpful discussions.
Footnotes
Supporting Information Available. All experimental procedures and compound characterization may be found on the World Wide Web at http://pubs.acs.org.
REFERENCES and NOTES
- 1.(a) Williams RM, Kwast E, Coffman H, Glinka T. J. Am. Chem. Soc. 1989;111:3064–3065. [Google Scholar]; (b) Sanz-Cervera JF, Glinka T, Williams RM. Tetrahedron. 1993;49:8471–8482. [Google Scholar]; (c) Schmidt MA, Movassaghi M. Synlett. 2008;3:313–324. [Google Scholar]
- 2.Williams RM, Stocking EM, Sanz-Cervera JF. In: Biosynthesis: Aromatic Polyketides, Isoprenoids, Alkaloids (Topics in Current Chemistry) Leeper FJ, Vederas JC, editors. Springer; 2000. [Google Scholar]
- 3.(a) Schkeryantz JM, Woo JCG, Siliphaivanh P, Depew KM, Danishefsky SJ. J Am. Chem Soc. 1999;121:11964–11975. [Google Scholar]; (b) Wang H, Ganesan A. J. Org. Chem. 2000;65:4685–4693. doi: 10.1021/jo000306o. [DOI] [PubMed] [Google Scholar]; (c) Baran PS, Corey EJ. J. Am. Chem. Soc. 2002;124:7904–7905. doi: 10.1021/ja026663t. [DOI] [PubMed] [Google Scholar]; (d) Marti C, Carreira EM. Eur. J. Org. Chem. 2003;12:2209–2219. [Google Scholar]; (e) Williams RM, Cox RJ. Acc. Chem. Res. 2003;36:127–139. doi: 10.1021/ar020229e. [DOI] [PubMed] [Google Scholar]
- 4.For representative examples of the utility of hydroxyindolenine intermediates in complex synthesis, see: Finch N, Taylor WI. J. Am. Chem. Soc. 1962;84:3871. Finch N, Gemenden CW, Hsu IH, Kerr A, Sim GA, Taylor WI. J. Am. Chem. Soc. 1965;87:2229–2235. doi: 10.1021/ja01088a024. Hutchison AJ, Kishi Y. J. Am. Chem. Soc. 1979;101:6786–6788. Williams RM, Glinka T, Kwast E, Coffman H, Stille JK. J. Am. Chem. Soc. 1990;112:808–821. Jossang A, Jossand P, Hadi HA, Sevenet T, Bodo B. J. Org. Chem. 1991;56:6527–6530. Gueller R, Borschberg H. Helv. Chim. Acta. 1993;76:1847–1862. Stoermer D, Heathcock CH. J. Org. Chem. 1993;58:564–568. Gueller R, Borschberg H. Tetrahedron Lett. 1994;35:865–868. Cushing TD, Sanz-Cervera JF, Williams RM. J. Am. Chem. Soc. 1996;118:557–579. Takayama H, Kurihara M, Subhadhirasakul S, Kitajima M, Aimi N, Sakai S. Heterocycles. 1996;42:87–92. Ito M, Clark CW, Mortimore M, Goh JB, Martin SF. J. Am. Chem. Soc. 2001;123:8003. doi: 10.1021/ja010935v. Baran PS, Corey EJ. J. Am. Chem. Soc. 2002;124:7904–7905. doi: 10.1021/ja026663t. Williams RM, Cox RJ. Acc. Chem. Res. 2003;36:127. doi: 10.1021/ar020229e. and references cited therein Liu Y, McWhorter WW, Jr, Hadden CE. Org. Lett. 2003;5:333–335. doi: 10.1021/ol0202417. Baran PS, Richter JM. J. Am. Chem. Soc. 2005;127:15394–15396. doi: 10.1021/ja056171r. Poriel C, Lachia M, Wilson C, Davies JR, Moody CJ. J. Org. Chem. 2007;72:2978–2987. doi: 10.1021/jo062627r. Pettersson M, Knueppel D, Martin SF. Org. Lett. 2007;9:4623–4626. doi: 10.1021/ol702132v. Miller KA, Tsukamoto S, Williams RM. Nature Chemistry. 2009;1:63–68. doi: 10.1038/nchem.110.
- 5.For examples of aryl migration, see: Witkop B, Ek A. J. Am. Chem. Soc. 1951;73:5664–5669. Evans FJ, Lyle GG, Watkins J, Lyle RE. J. Org. Chem. 1962;27:1553–1557. Hishmat OH, Abd-El Rahman AH, El-Ebrashi NMA, Ishmail MMF. Chem. Ind. 1986;4:142–143. Liu Y, McWhorter WW., Jr J. Org. Chem. 2003;68:2618–2622. doi: 10.1021/jo020715f. For key related studies, see: Witkop B, Patrick JB. J. Am. Chem. Soc. 1951:2188–2195. Acheson RM, Snaith RW, Vernon JM. J. Chem. Soc. 1964:3229–3223. Walser A, Blount JF, Fryer RI. J. Org. Chem. 1973;38:3077–3084. Wenkert E, Shi Y. Synth. Commun. 1989;19:1071–1079. Zhang X, Foote CS. J. Am. Chem. Soc. 1993;115:8867–8868. Tany M, Matsumoto S, Aida Y, Arikawa S, Nakane A, Yokoyama Y, Murakami Y. Chem. Pharm. Bull. 1994;42:443–453. Lachia M, Poriel C, Slawin AMZ, Moody CJ. Chem. Commun. 2007:286–288. doi: 10.1039/b613716d. Lindel T, Bräuchle L, Golz G, Böhrer P. Org. Lett. 2007;9:283–286. doi: 10.1021/ol0627348.
- 6.Movassaghi M, Schmidt MA, Ashenhurst JA. Org. Lett. 2008;10:4009–4012. doi: 10.1021/ol8015176. [DOI] [PubMed] [Google Scholar]
- 7.For selected reviews and recent advances, see: Johnson RA, Sharpless KB. In: Comprehensive Organic Synthesis. Trost BM, editor. Vol. 7. New York: Pergamon; 1991. p. 389. Jacobsen EN, Wu MH. In: Comprehensive Asymmetric Catalysis II. Jacobsen EN, Pfaltz A, Yamamoto H, editors. Berlin: Springer-Verlag; 1999. p. 649. Aggarwal VK, Winn CL. Acc. Chem. Res. 2004;37:611–620. doi: 10.1021/ar030045f. Xia QH, Ge HQ, Liu ZM, Su KX. Chem. Rev. 2005;105:1603–1662. doi: 10.1021/cr0406458. Kelly DR, Roberts SM. Biopolymers. 2006;84:74–89. doi: 10.1002/bip.20373. Berkessel A, Koch B, Toniolo C, Rainaldi M, Broxterman QB, Kaptein B. Biopolymers. 2006;84:90–96. doi: 10.1002/bip.20413. Wong OA, Shi Y. Chem. Rev. 2008;108:3958–3987. doi: 10.1021/cr068367v. Li Z, Zhang W, Yamamoto H. Angew. Chem. Int. Ed. 2008;47:7520–7522. doi: 10.1002/anie.200802523.
- 8.For an interesting and promising example, see Reference 4q.
- 9.(a) Taniguchi M, Anjiki T, Nakagawa M, Hino T. Chem. Pharm. Bull. 1984;32:2544–2554. [Google Scholar]; (b) Berrier C, Jacquesy JC, Jouannetaud MP, Renoux A. New Journal of Chemistry. 1987;11:611–615. [Google Scholar]; (c) Duflos A, Redoules F, Fahy J, Jacquesy J, Jouannetaud M. J. Nat. Prod. 2001;64:193–195. doi: 10.1021/np000425z. [DOI] [PubMed] [Google Scholar]; (d) Lakatosh SA, Balzarini J, Andrei G, Snoeck R, De Clercq E, Preobrazhenskaya MN. Journal of Antibiotics. 2002;55:768–773. doi: 10.7164/antibiotics.55.768. [DOI] [PubMed] [Google Scholar]; (e) Beyer M, Fritscher J, Feresin E, Schiemann O. J. Org. Chem. 2003;68:2209–2215. doi: 10.1021/jo026742n. [DOI] [PubMed] [Google Scholar]
- 10.Peris G, Jakobsche CE, Miller SJ. J. Am. Chem. Soc. 2007;129:8710–8711. doi: 10.1021/ja073055a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Greene FD, Kazan J. J. Org. Chem. 1963;28:2168–2171. [Google Scholar]; (b) Rebek J, McCready R, Wolf S, Mossman A. J. Org. Chem. 1979;44:1485–1493. [Google Scholar]; (c) Spantulescu MD, Jain RP, Derksen DJ, Vederas JC. Org. Lett. 2003;5:2963–2965. doi: 10.1021/ol035125y. [DOI] [PubMed] [Google Scholar]
- 12.Jakobsche CE, Peris G, Miller SJ. Angew. Chem. Int. Ed. 2008;47:6707–6711. doi: 10.1002/anie.200802223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Takagi J, Sato K, Hartwig JF, Ishiyama T, Miyaura N. Tetrahedron Lett. 2002;43:5649–5651. [Google Scholar]; (b) Ishiyama T, Takagi J, Nobuta Y, Miyaura N. Org. Synth. 2005;82:126–131. [Google Scholar]
- 14. Billingsley K, Buchwald SL. J. Am. Chem. Soc. 2007;129:3358–3366. doi: 10.1021/ja068577p. (b) See also: reference 6.
- 15.Synthesis details may be found in the Supporting Information.
- 16.(a) Majetich G, Hicks R. Synlett. 1996:649–651. [Google Scholar]; (b) Murray RW, Iyanar K. J. Org. Chem. 1998;63:1730–1731. [Google Scholar]; (c) Majetich G, Hicks R, Sun G, McGill P. J. Org. Chem. 1998;63:2564–2573. doi: 10.1021/jo972026n. [DOI] [PubMed] [Google Scholar]
- 17.(a) Lewis CA, Miller SJ. Angew. Chem. Int. Ed. 2006;45:5616–5619. doi: 10.1002/anie.200601490. [DOI] [PubMed] [Google Scholar]; (b) Balskus EP, Jacobsen EN. Science. 2007;317:1736–1740. doi: 10.1126/science.1146939. [DOI] [PubMed] [Google Scholar]
- 18.Masamune S, Choy W, Petersen JS, Sita LR. Angew. Chem. Int. Ed. 1985;24:1–76. [Google Scholar]
- 19.(a) Rose GD, Gierasch LM, Smith JA. Adv. Protein Chem. 1985;37:1–109. doi: 10.1016/s0065-3233(08)60063-7. [DOI] [PubMed] [Google Scholar]; (b) Haque TS, Little JC, Gellman SH. J. Am. Chem. Soc. 1996;118:6975–6985. [Google Scholar]; (c) Miller SJ. Acc. Chem. Res. 2004;37:601–610. doi: 10.1021/ar030061c. [DOI] [PubMed] [Google Scholar]
- 20.(a) Kametani T, Kanaya N, Ihara M. J. Am. Chem. Soc. 1980;102:3974–3975. [Google Scholar]; (b) Plate R, Akkerman MAJ, Ottenheijm HCJ. J. Chem. Soc. Perkin Trans. I. 1987:2481–2490. [Google Scholar]; (c) Sanz-Cervera JF, Glinka T, Williams RM. Tetrahedron. 1993;49:8471–8482. [Google Scholar]; (d) Schkeryantz JM, Woo JCG, Siliphaivanh P, Depew KM, Danishefsky SJ. J Am. Chem Soc. 1999;121:11964–11975. [Google Scholar]
- 21.The stereochemical assignments for the products are discussed below and in the Supporting Information.
- 22.Catalytic reactions involving substrate 25 have been performed on up to 770 mg (2 mmol) of the substrate with excellent consistency and reproducibility.
- 23.Calculations at the B3LYP/6-31G(d) suggests the HOMO of (N-phthalimido)-2-phenyltryptamine is higher than (N-phthalimido)-tryptamine by 2.9 kcal/mol.
- 24.Substrates 40a–b were prepared by deprotection of compound 40c followed by amine acylation or sulfonylation.
- 25.Oxidation of 2,3-dimethylindole and 3-(2-N,N-phthaloylaminoethyl)-2-(3-oxobutyl)-1H-indole under the standard conditions delivers primarily the corresponding overoxidation products. See Supporting Information.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.