Abstract
Kaposi’s sarcoma herpesvirus (KSHV), also known as human herpesvirus 8 (HHV-8), is the most recently identified human tumor virus, and is associated with the pathogenesis of Kaposi’s sarcoma and two lymphoproliferative disorders known to occur frequently in AIDS patients—primary effusion lymphoma and multicentric Castleman disease. In the 15 years since its discovery, intense studies have demonstrated an etiologic role for KSHV in the development of these malignancies. Here, we review the recent advances linked to understanding KSHV latent and lytic life cycle and the molecular mechanisms of KSHV-mediated oncogenesis in terms of transformation, cell signaling, cell growth and survival, angiogenesis and immune invasion, and highlight the potential therapeutic targets for blocking KSHV tumorigenesis.
I. GENERAL BACKGROUND
A. Discovery of KSHV/HHV-8
Kaposi’s sarcoma (KS) was first described by a Hungarian dermatologist, Moritz K. Kaposi, in 1872 (Kaposi, 1872). Kaposi published the case histories of elder male patients in Vienna with “idiopathic multiple pigmented sarcoma of the skin” which is now referred to as classic KS (Antman and Chang, 2000). Prior to the acquired immunodeficiency syndrome (AIDS) epidemic, KS was thought of as a rare, slow progressing neoplasm which affects mainly elderly men of Mediterranean and Eastern European region. However, the AIDS epidemic triggered KS to become the most aggressive AIDS-associated cancer which can present with lymphoadenopathy rather than skin lesions (Oettle, 1962). Unlike classic KS, this endemic KS often can be rapidly fatal and is associated with significant morbidity and mortality, and is now recognized as one of the leading cancers in African children with HIV (Bayley, 1984; Downing et al., 1984). Importantly, KS is also known to develop after organ transplantation (posttransplant or iatrogenic KS; Penn, 1983). The fact that the immunosuppressed patients born in countries where classic KS occurs continues to develop KS after a transplant suggests that there is a genetic predisposition or an infectious agent transmitted and responsible for KS development. Studies of AIDS case surveillance showing that KS occurs predominantly in gay and bisexual men with AIDS instead of AIDS patients with hemophilad or intravenous drug users further support the existence of an infectious cofactor (Beral et al., 1990).
According to the epidemiological data, several groups set out to identify a “KS agent” in the early 1990s. In 1994, Chang and Moore’s group successfully identified the infectious cause of KS as a new herpesvirus called Kaposi’s sarcoma-associated herpesvirus (KSHV) using polymerase chain reaction-based subtractive analysis between the KS lesions and unaffected skin from the same patient (Chang et al., 1994). Analysis of conserved herpesviral genes showed that KSHV belongs to a clade of primate herpesviruses within the gamma2 sublineage, and it is ranked eighth known human herpesvirus (HHV-8) which is closely related to rhesus rhadinovirus (RRV) in nonhuman primates and was with their hosts ~25 million years ago (McGeoch and Davison, 1999).
B. Diseases associated with KSHV
In the several years since the original identification of KSHV from KS lesions by Chang and Moore, KSHV sequence has been described in an array of disease entities. They are included marrow hypoplasia, haemophagocytic syndrome (HPS), multiple myeloma, sarcoidosis, angio-immunoblasticlymphoma, and most recently primary pulmonary hypertension (PPH; Cool et al., 2003; Low et al., 1998; Pastore et al., 2000; Schulz, 2006). Since these results are largely based upon PCR analysis for detection of KSHV DNA which can be froth with false positivity, and none of these findings have been confirmed by several other investigating groups, it is still yet to be accepted that KSHV plays a significant role in any of these diseases. However, KSHV has been demonstrated to be present in endothelial/spindle cells and the cells that appear to constitute the primary derivation of the tumor (Boshoff et al., 1995). In addition to KS, two B-lymphocyte disorders: multicentric Castleman’s disease (MC; Soulier et al., 1995) and primary effusion lymphoma (PEL; Cesarman et al., 1995) are consistently linked with KSHV infection.
1. Kaposi’s sarcoma
KS is an unusual multifocal neoplasm characterized by dark purple lesions, which differs from most other common tumors in that the lesions contain multiple cell types (Boshoff et al., 1997). KS lesions contain extensive neoangiogenesis, infiltrating inflammatory cells, erythrocyte extravasation, endothelial cells, and “spindle” cells typical for KS. There are four distinct clinical variants of KS which is based on the extent of immunosuppression and severity of infection. These include classic KS, endemic KS, iatrogenic KS, and AIDS-associated KS (Boshoff and Chang, 2001). The form of KS that was originally described by Kaposi is now referred to as classic KS. Classic KS most commonly presents in HIV-negative elderly male patients of Mediterranean and Eastern European decent and is relatively indolent (Franceschi and Serraino, 1995). Endemic KS is the prevalent form of the disease in Equatorial, Eastern, and Southern Africa, and is a substantially more aggressive form than classic KS (Wabinga et al., 1993). Unlike classic KS, endemic KS often presents in HIV-negative children as a lymphadenopathy rather than skin lesions (Ziegler and Katongole-Mbidde, 1996). Latrogenic or posttransplant KS is developed in patients undergoing immunosuppressive therapy to prevent graft rejection after organ transplantation (Regamey et al., 1998). The most aggressive form of KS is the AIDS-associated KS, which is the most common neoplastic manifestation of AIDS in the United States and Europe (Martin et al., 1993). This form of KS is most commonly presented in gay and bisexual men, suggesting that the disease transmission is likely through high-risk sexual routes (Gao et al., 1996b; Kedes et al., 1996; Simpson et al., 1996). Distinct from the classic form of KS localized to the lower extremities; AIDS-KS commonly occurs throughout the body, which includes skin of face, torso, the extremities, and mucous membranes of the oral cavity (Cheung, 2004). However, despite these different clinical manifestations of KS, the histology of KS lesions from skin, lymph nodes, respiratory tract, and intestines are very similar.
2. Primary effusion lymphoma
PEL, also referred to as body cavity-based lymphoma (BCBL), is a rare, rapidly fatal lymphoma associated with KSHV infection and commonly found in HIV-infected patients (Cesarman, 2002). PEL is a unique form of NHL found more commonly in immunocompromised AIDS patients and unlike KS, PEL is generally presented as a pleural or pericardial effusion without a detectable tumor mass (Carbone and Gaidano, 1997), or can present as a solid mass in the lymph nodes, lungs, or the gastrointestinal tract (Arvanitakis et al., 1996). Due to the presence of hypermutated immunoglobulin genes and markers of late stage B cell differentiation like CD30 and CD138, PEL cells are thought to be usually monoclonal and originated from postgerminal center B cells (Arvanitakis et al., 1996; Carbone et al., 1997; Gaidano et al., 1996). In PEL cells, it has frequently been seen that KSHV presents as single positive or KSHV/Epstein Barr Virus (EBV) double positive, and that the KSHV genome is maintained at a relatively high copy number (50–150 per cell; Arvanitakis et al., 1996; Cesarman et al., 1995; Renne et al., 1996a).
3. Multicentric Castleman’s disease
Multicentric Castleman’s disease (MCD) is a usual lymphoproliferative disorder characterized by expanded germinal centers with B cell proliferation and vascular proliferation. KSHV-positive MCD is now recognized as a distinct subset of MCD, called plasmablastic MCD, which contains large plasmablastic cells (Dupin et al., 2000). Dysregulated IL-6 levels are considered a likely contributor to the clinicpathophysiology of MCD (Parravicini et al., 1997). Like KS and PEL, KSHV genomes are detectable in almost all HIV-seropositive MCD cases and approximately 50% HIV-seronegative MCD cases (Dupin et al., 1999; Soulier et al., 1995). However, different from PEL cells, coinfection of EBV with KSHV has not been detected in MCD plasmablasts.
C. Epidemiology of KSHV infection
Seroepidemiologic studies have clearly shown that KSHV is prevalent throughout the world, although there appears to be striking variation in local seroprevalence. The association between KS prevalence and KSHV seroprevalence is high. In an attempt to understand the divergence and preference of KSHV in certain human populations, several groups have been working on the pattern of KSHV variability (Alagiozoglou et al., 2000; Biggar et al., 2000; Lacoste et al., 2000; Poole et al., 1999). Unlike other herpesvirus, KSHV contains a highly variable gene K1 which is located at the region next to the terminal repeat (TR) in viral genome. This makes it be a good marker to trace KSHV variants that are associated with particular populations. Based on the phylogenetic analysis of K1 gene, KSHV has been classified into five main branches (termed A–E) which appears to relate with different human populations (see Table 1). Generally, the sequence variation between these different clades is very low and less than 3% at nucleotide level in most regions of the viral genome. In view of the fact that KSHV is highly coevolved with the human population, the geographical distribution would be interesting to further investigate. In 1996, the antibodies produced by recombinant capsid protein or the latent nuclear antigen (LANA) made it possible to study the distribution of KSHV seroprevalence among the different risk groups (Gao et al., 1996a,b; Kedes et al., 1996; Lennette et al., 1996; Simpson et al., 1996). The epidemiology data showed that KSHV remains widespread in sub-Saharan Africa, where KSHV is found in more than 50% of adults (Simpson et al., 1996), and relatively frequent in countries from the Mediterranean region which ranges from 3% of northern Italy to 30% in Sicily (Calabro et al., 1998; Whitby et al., 1998). In contrast, seroprevalence rates are lower than 5% in northern Europe, Asia, and most parts of North America. In endemic areas like Africa, it has been found that transmission from the mother to the child or among siblings or transmission through sexual contact is the most important route (Eltom et al., 2002; Martin et al., 1998; Simpson et al., 1996). However, in nonendemic areas, KSHV is commonly found in persons who have multiple sexual partners (especially homosexual men) or who immigrate from endemic areas (Dukers et al., 2000; Melbye et al., 1998). Although KSHV transmission by blood transfusion or transplanted organs is documented, based on cost-analysis most countries do not yet routinely screen blood or organ donors for KSHV infection (the epidemiological studies related to KSHV transmission are reviewed elsewhere; Corey et al., 2002; Henke-Gendo and Schulz, 2004).
TABLE 1.
World-wide distribution and divergence of KSHV subtype infection
| KSHV subtype | Populations related | References |
|---|---|---|
| A | Northern European, Americans, Asian | Lacoste et al. (2000), Poole et al. (1999) |
| B | African | Biggar et al. (2000), Cook et al. (1999), Kakoola et al. (2001), Lacoste et al. (2000), Meng et al. (2001), Poole et al. (1999) |
| C | Northern European, Americans, Asian | Lacoste et al. (2000), Poole et al. (1999) |
| D | Old Asian, Polynesian (old pacific island) | Biggar et al. (2000), Cook et al. (1999), Kakoola et al. (2001), Lacoste et al. (2000), Meng et al. (2001), Poole et al. (1999) |
| E | Brazilian Amerindian | Biggar et al. (2000), Cook et al. (1999), Kakoola et al. (2001), Lacoste et al. (2000), Meng et al. (2001), Poole et al. (1999) |
| Z | Small cohort of Zambian children | Lacoste et al. (2000), Poole et al. (1999) |
According to K1 differ by 0.4–44%.
II. LIFE CYCLE OF KSHV
KSHV, like other herpesviruses, exhibits a biphasic life cycle with predominant lifelong latent infection and a typical short-lived lytic reactivation cycle. Latent infection is the quiescent state of the viral life cycle, which is characterized by the expression of a limited number of viral genes with no production of virus particles. Majority of the herpesviruses other than the members of gamamaherpesvirus (KSHV and EBV) family do not cause any obvious pathology during latent infection. Members of the gammaherpesvirus have the ability to drive cell proliferation and transformation and are broadly referred to as oncogenic viruses. Kaposi’s sarcoma-associated herpesvirus is the latest member of the human herpesviruses, which belongs to the gammaherpesvirus subfamily, rhadinovirus genera and have a significant genetic similarity with EBV, a member of the lymphocryptovirus genera (see reviewed in Damania, 2004). Full genome sequence of KSHV virus from KS biopsy samples and PEL cells reveal that its genome is approximately 165 kbp in size with a central region of low GC (L-DNA) flanked by GC-rich (H-DNA) TR units (Neipel et al., 1997b; Russo et al., 1996). The L-DNA is the viral protein-coding region, which encodes for at least 90 ORFs, some with homology to cellular genes (Neipel et al., 1997a,b; Russo et al., 1996; Fig. 1). These viral homologs seem to be the pirated copies of the human genome, which were acquired during the process of evolution by the viruses to have growth and immune evasion advantages (Moore and Chang, 1998a,b; Neipel et al., 1997a,b). The past 15 years of research have certainly advanced our understanding of the biology and pathogenesis of KSHV.
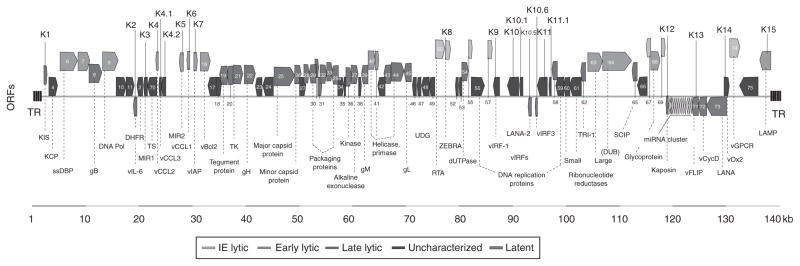
FIGURE 1.
A schematic of the KSHV genome consisting of 145 kb long unique, gene-coding region flanked with terminal repeat units. The coding regions contain over 90 open reading frames (ORFs; Russo et al., 1996). The gene-encoded proteins are labeled in the bottom from left to right. ORFs unique to the KSHV are given the prefix K. The miRNA cluster encoding for 12 microRNAs (yellow) is located between K12 (Kaposin) and ORF71 (vFLIP). The blocks representing the ORFs are also labeled in color according to gene class [latent, immediate early (IE), early and late lytic, and unclassified] (based on previous reviews of Coscoy, 2007; Moore and Chang, 2001). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this chapter.)
The KSHV genome exists as linear dsDNA copy in the virion particle. It is delivered into the infected cells by a mechanism which is likely to be a multistep process which is yet to be completely resolved (Chandran, 2010). The virion particle attaches to the host cell surface by temporal interaction of multiple cellular receptors with surface glycoproteins. Attachment is followed by penetration of the viral capsid into the cytosol, achieved by either direct fusion of the virion envelope with the plasma membrane or by internalization through endocytosis through fusion of viral envelope with endosomal membrane (reviewed in Chandran, 2010). Virion capsid delivered to the cytoplasm is transported to the nuclear periphery, which is followed by disassembly of virion capsid and release of viral DNA into the nucleus (Naranatt et al., 2004, 2005; Raghu et al., 2007). The entry of virion into the targets’ cells also brings cellular and viral proteins as well as RNA (Bechtel et al., 2005a,b; Zhu et al., 2005). These proteins include replication and transcription activator (RTA), ORF8, ORF21, ORF24, ORF25, ORF26, ORF33, ORF75, and heat shock proteins 70 and 90 (Bechtel et al., 2005b; Zhu et al., 2005). These proteins are considered to be important in establishing early infection. Since RTA is an immediate early protein, the incoming RTA may boost lytic replication and increase the viral copy number. A quantitative real-time PCR analysis of the early transcripts detected RTA as early as 2 h postinfection, which declines sharply at 24 h postinfection (Krishnan et al., 2004). In contrast, expression of ORF73 encoded LANA is detected at very low levels within 6 h postinfection but the levels increase significantly 24 h postinfection (Cai et al., 2007; Lan et al., 2005). Newly synthesized and virally delivered RTA promotes expression of LANA by binding to the LANA promoter (Lan et al., 2005). Expression of LANA subsequently blocks the expression of lytic proteins and pushes the cells to enter into latent phase. Fine tuning of LANA and RTA expression levels decides the fate of the virus which undergoes the latent to lytic cycle (Lan et al., 2005). The distinct viral gene expression profiles during latent and lytic phase of the viral life cycle will be discussed in following sections.
A. Latent infection
Latency is characterized by the expression of subset of viral transcripts and persistence of the viral genome as a circular episome attached to the host chromatin. Another characteristic feature of latency is that the cells harboring the viral genome downregulate cell surface makers which can be typically detected by the host immune surveillance. Importantly, viral-encoded latent genes have been shown to play an important role in modulating viral and cellular gene expression to successfully establish latent infection.
1. KSHV latent genes/transcripts
Due to the lack of a true latency animal model for studying KSHV infection, cell lines derived from KSHV-infected patients have proved useful in characterizing the expression profiles of latency-associated genes (Cannon et al., 2000; Flore et al., 1998; Foreman et al., 1997; Renne et al., 1996b). PEL cells, which maintain high levels (98–99%) of latently infected cells (a fraction of KSHV-infected cells can undergo spontaneous lytic reactivation) restrict transcription to specific viral genes, including the latency-associated nuclear antigen encoded by ORF73, viral cyclin D encoded by ORF72, vFLIP encoded by ORF71, Kaposin encoded by K12, and viral miRNA (Burysek and Pitha, 2001; Dittmer et al., 1998; Kedes et al., 1997; Rainbow et al., 1997; Sadler et al., 1999). Another latent protein that consistently detected in KSHV-induced tumors is viral interferon regulatory factor (vIRF; Russo et al., 1996). Genes encoded by ORF71, ORF72, and ORF73 are expressed from the same locus in polycistronic, differentially spliced mRNAs whose transcription is regulated by a common promoter upstream of LANA (Dittmer et al., 1998; Grundhoff and Ganem, 2001). Interestingly, LANA promoter is bidirectional and also controls the transcription of ORF73, 72, and 71 during latency and a bicistronic transcript encoding the expression of K14 and ORF74/vGPCR during lytic cycle (Chiou et al., 2002). Intriguingly, the bicistronic transcripts for ORF74-K14 were detected in some KS lesions and latent biopsy samples. However, it is somewhat enigmatic that ORF74-K14 can be expressed in latently infected cells that coexpress LANA–vCyclin–vFLIP from the same locus ( Jeong et al., 2001; Nador et al., 2001). In addition, the Kaposin is expressed from the promoter present downstream of ORF73 (Li et al., 2002). Among these latent proteins, LANA is the most consistently detected antigen in KSHV-infected cells of KS, PELs, and MCD origins and is a hallmark of KSHV genome persistence (Ballestas et al., 1999; Cotter and Robertson, 1999; Dupin et al., 1999). LANA is a large (220–230 kDa) heavily posttranslationally modified nuclear protein detected as specific punctate dots in immunofluorescence assays (Lennette et al., 1996). Colocalization of these LANA punctate dots with the hybridization signals for the KSHV genome by immnuo-FISH (fluorescence in situ hybridization) assay in the chromosomes of the infected cells suggested a role for LANA in viral genome attachment to the host chromatin (Cotter and Robertson, 1999). Studies from a number of laboratories later identified LANA binding sites (LBS) in the KSHV genome, which mapped to the TR units (Ballestas and Kaye, 2001; Cotter et al., 2001). In addition, binding of LANA to the TR DNA was mapped to the DNA-binding domain of LANA at the carboxyl terminus (Cotter et al., 2001; Hu and Renne, 2005).
2. KSHV latent replication and persistence
Linear KSHV genome circularizes to make episomal DNA through a not-yet-known mechanism upon entry into the nucleus following infection. The viral genome persists as a circular episome in the form of highly ordered chromatin structure during latent infection (Stedman et al., 2004, 2008). LANA has been shown to be important for attaching the viral episomal structure to the host chromatin. The amino-terminal domain of LANA has a chromatin binding sequence (5–22 aa) that attaches to chromatin surface by binding to a multiprotein complex including histones on the cellular chromatin (Barbera et al., 2006; Cotter and Robertson, 1999; Krithivas et al., 2002; Lim et al., 2003; Matsumura et al., 2010; Ottinger et al., 2006). The carboxyl terminal domain of LANA directly binds to the LANA binding sequence (LBS) located in the TRs of the viral genome (Cotter et al., 2001; Hu and Renne, 2005; Komatsu et al., 2004; Srinivasan et al., 2004). Binding of LANA at the TR is critical to efficient initiation of latent replication at the TRs as demonstrated by short-term replication assays using a construct with the TR cloned onto a plasmid (Hu et al., 2002; Komatsu et al., 2004; Lim et al., 2002; Verma et al., 2006). In an attempt to identify the minimal DNA element required for replication at the TR, Hu et al. performed short-term replication assays with the deletion mutants of the TR and mapped a 71-bp unit of the TR as the minimal replicator element (Hu and Renne, 2005). This unit comprises LBS1/2 and an adjacent 29- to 32-bp-long GC-rich element referred to as replication element (RE) upstream of the LBSs (Hu and Renne, 2005). We recently identified a latent replication origin in the long unique region of the viral genome, which initiates replication independent of viral proteins in trans, suggesting that this is an autonomously replicating element in the LUR (Verma et al., 2007a). In contrast to the LANA-dependent replication origin of TR, this autonomously replicating element is high in AT content and can recruit the host cellular replication machinery to initiate replication (Verma et al., 2007a). Although LANA does not seem to have any enzymatic activity for replication function, it is essential for TR-mediated replication primarily because it recruits the required cellular replication machinery to the RE element of the TR (Stedman et al., 2004; Verma et al., 2006). The primary replication protein, origin recognition complexes (ORCs), which serves as the launching pad for the recruitment of other proteins including MCMs gets recruited by LANA at the RE site of the TR (Verma et al., 2006). Chromatin immunoprecipitation assay with hyper-acetylated histone H3 and H4 antibodies primarily detects the TR region suggesting a loose chromatin structure at the TR which is indicative of replicative origin (Stedman et al., 2004). LANA-mediated TR replication as well as replications mediated by the autonomous elements was done on a plasmid-based assay. Therefore, we cannot conclusively state that these sites are firing during replication of the viral genome. A comprehensive study to demonstrate the usage of these replication sites would be important in understanding the mechanism of latent viral DNA replication.
KSHV-infected PEL cells maintain similar number of copies of the viral episome over multiple rounds of cell division suggesting a faithful mechanism of viral genome segregation after each cell division (Skalsky et al., 2007). As discussed earlier, LANA associates with the KSHV genome in infected cells and colocalizes with the viral genome at inter-phase nuclei and on mitotic chromosome as a punctuate pattern (Ballestas et al., 1999; Cotter and Robertson, 1999). The ability of LANA to tether viral episomes to the host chromatin is important for the establishment of latent infection. Studies with a KSHV genome deleted for LANA ORF cloned in a bacterial artificial chromosome showed loss of viral episomes from the infected cells without establishment of latency (Ye et al., 2004). Another study where LANA expression was depleted using the shRNA strategy in PEL cells showed reduction of viral episomes to 20% compared to the control shRNA treated cells (Godfrey et al., 2005). These studies clearly indicate that the presence of LANA in the host cells is required for persistence of the viral DNA. LANA binding to the host chromatin at nucleosomal surface through histones and other cellular proteins throughout the cell cycle confirms that the LANA: cellular proteins interactions are critical in maintaining the viral genome (Barbera et al., 2006; Cotter and Robertson, 1999; Krithivas et al., 2002; Lim et al., 2003; Matsumura et al., 2010; Si et al., 2008; Verma et al., 2007b; Viejo-Borbolla et al., 2005; You et al., 2006). A yeast-2-hybrid assay using the LANA-N terminus as bait identified the Nuclear Mitotic Apparatus (NuMA) protein as a LANA interacting protein (Si et al., 2008). NuMA is an essential protein for cellular genome segregation, which interacts with a number of essential mitotic components including microtubule proteins dynein and dynactin during mitosis to efficiently segregate the daughter chromatids (Du et al., 2002; Merdes et al., 1996). Depletion of NuMA by siRNA and dominant-negative approach to block NuMA function has resulted in decreased copies of latently maintained KSHV epiosome (Si et al., 2008). Proteomic analysis of LANA binding proteins from KSHV-infected cells identified a centromeric protein F, a component of multiprotein complex that assembles on centromeric DNA and links the chromosome to microtubule during mitosis and associates with the kinetochore (Cheeseman et al., 2005; Liao et al., 1995). LANA associates with CENP-F and a kinetochore protein, Bub1 in KSHV-infected cells. Interestingly, depletion of Bub1 by shRNA dramatically reduced the number of latently persisting KSHV genomes (Xiao et al., 2010). These studies suggest that KSHV episomal DNA segregates in a fashion similar to the case of the host genome at the same time usurping the cellular segregation mechanism.
B. Lytic replication
Lytic cycle is characterized by the expression of most of the viral genes in an orderly fashion (immediate early, early, and late) and the production of infectious virion particles. Gene expression profiles of KSHV have been studied in biopsies from KS tissue, PELs, and MCD, and also in in vitro setting by de novo infection of the cultured cells. These studies show that the majority of tumor cells in the KS biopsies contain KSHV viral DNA and express viral latent transcripts, and that a subpopulation (1–3%) of tumor cells undergo lytic reactivation as demonstrated by the expression of early and late viral transcripts (Staskus et al., 1997; Sun et al., 1999). These lytic transcripts include viral macrophage inflammatory protein-I, viral interleukin 6, viral Bcl-2 homolog, and polyadenylated nuclear RNA (PAN RNA). The subpopulation of cells undergoing lytic reactivation also express the late viral transcripts major capsid protein (MCP) and the small viral capsid (sVCA) which indicates production of virion particles and potentially go on to infect the surrounding cells. Additionally, the cells undergoing lytic reactivation produce cellular cytokines, which may create a favorable microenvironment to enhance the growth of latently infected cells and contribute to tumor progression. In order to study the full-blown lytic reactivation, PEL cells (BC-1, BC-3, and BCBL-1) can be treated with chemical agents such as phorbol esters or sodium butyrate (NaB) to induce the cascade of lytic cycle genes and the detection of viral transcripts shows immediate early, early, and late gene patterns like other herpesvirus (Gradoville et al., 2000; Renne et al., 1996b; Sarid et al., 1998; Zhong et al., 1996).
1. KSHV lytic genes
The lytic genes are classified into three major groups, immediate early (IE), early (E), and late (L) genes. The immediate early genes are the first group of genes expressed during lytic replication whose transcription generally does not require de novo protein synthesis. The immediate early genes encode for proteins with regulatory functions in activating cascade of downstream genes and also modulating the host cell environment for viral replication. PEL cells treated with TPA and NaB to activate lytic a cascade as well as primary cells, TIME, HFF, and 293 cells infected with the KSHV virus have identified a number of immediate early genes which include ORF50, ORF45, ORF K4.2 (Gradoville et al., 2000; Krishnan et al., 2004; Lukac et al., 1999; Sarid et al., 1998; Sharma-Walia et al., 2005). ORF50, also called RTA is the best-characterized immediate early gene. RTA is 691aa long, which has an N-terminal DNA binding and dimerization domain, C-terminal activation domain, and two nuclear localization signals (Lukac et al., 1998; Sun et al., 1998). The molecular weight (110 kDa) of RTA is higher than the predicted (73.3 kDa) size because of various posttranslational modifications (Lukac et al., 1999; Song et al., 2002). Studies from various laboratories have shown that RTA serves as a molecular switch from latent to lytic cycle and ectopic expression of RTA into PEL cells induces the cascade of lytic gene expression including vIL-6, PAN RNA, ORF59, ORF65, and K8.1 and production of DNase-resistant encapsidated viral DNA, providing proof that RTA is capable of driving complete viral lytic cycle (Gradoville et al., 2000; Lukac et al., 1998; Sun et al., 1998).
The expression of RTA is controlled by various cellular and viral proteins, including, RTA itself representing an important strategy used by KSHV to express sufficient amounts of RTA to activate the lytic cycle (Gradoville et al., 2000; Lan et al., 2004). RTA activates its promoter by binding to the Oct-1 transcription factor and RBP-Jκ, a known cellular partner of RTA bound to the RTA promoter (Deng et al., 2000; Liang et al., 2002). Even though RTA has a DNA-binding domain, direct binding of RTA to its promoter is not required for it autoactivation as Oct-1–RTA complex was not detected in gel shift assay and a defective DNA-binding RTA mutant was capable of autoactivating through binding with RBPJκ (Chang et al., 2005; Liang et al., 2002; Sakakibara et al., 2001). Latent protein, LANA also controls RTA expression and suppress lytic reactivation by repressing basal RTA promoter activity as well as RTA-mediated autoactivation (Lan et al., 2004). LANA-mediated suppression of RTA promoter is dependent on RBP-Jk, which is also involved in RTA auto-activation suggests that LANA may be suppressing RTA autoactivation by competing with RTA for binding to RBP-Jk (Lan et al., 2005). Since RBP-Jκ is one of the key molecules in both positive and negative regulation of RTA expression, the levels of LANA and RTA stringently controls the latent to lytic switch. Additionally, RTA upregulates LANA expression to suppress lytic reactivation and thus acts as a negative feedback regulator of RTA (Lan et al., 2005; Matsumura et al., 2005).
The early (E) genes generally encode proteins that are involved in nucleic acid metabolism, modulation of cellular functions, and their expression is controlled by the IE genes. RTA activates a number of early lytic genes by either direct or indirect mechanisms and they include PAN RNA, Kaposin, ORF57, K-bZIP (K8), vIL-6, K5, K9, K14, K15, ORF6, ORF59, ORF21, and ORF74 (Chang et al., 2000; Chen et al., 2000; Haque et al., 2000; Jeong et al., 2001; Lukac et al., 2001; Wong and Damania, 2006; Zhang et al., 1998).
Among the early gene transcripts, PAN RNA is most abundant transcript comprising approximately 80% of the total polyadenylated RNA in the infected cells. PAN RNA is a novel 1.1–1.2-kb noncoding polyadenylated transcript whose expression is controlled by RTA (Song et al., 2001). The function of PAN RNA in lytic reactivation and pathogenesis is yet to be resolved. Kaposin, which is expressed during latent infection but strongly induced by lytic reactivation, has the ability to drive cell transformation (Kliche et al., 2001; Muralidhar et al., 1998). RTA controls the expression of Kaposin (E) through RRE (RTA response element) site within the Kaposin promoter (Song et al., 2003). ORF57 encodes a post-transcriptional regulator, a conserved protein in herpesviruses, which is upregulated by RTA expression (Duan et al., 2001; Kirshner et al., 1999). ORF57 promotes the accumulation (stabilization) and export of viral intronless RNA transcripts by a mechanism, which is yet to be clearly defined. vMIP-1, a virus encoded chemokine homologue, is also expressed during early lytic cycle and its expression is controlled by RBP-Jκ and RTA protein–protein interaction and formation of a macromolecular complex at the RBP-Jκ binding site at the vMIP promoter (Chang et al., 2005).
KSHV encodes vIL-6 (encoded by ORFK2) which is an early protein of lytic cycle. vIL-6 has 25% amino acid similarity with the human homologue (IL-6) and promotes growth and proliferation of IL-6-dependent human B cells similar to the human IL-6 (Moore et al., 1996; Nicholas et al., 1997). The immediate early gene, RTA strongly induces the expression of vIL-6 by binding to the RRE site making vIL-6 one of the most abundant transcripts in PEL cells during lytic reactivation (Deng et al., 2002; Sun et al., 1999). KSHV-encoded G protein-coupled receptor (vGPCR) is also an early gene which plays an important role in angiogenesis. vGPCR is expressed from a bicistronic RNA with K14 at the 5′ end and vGPCR at the 3′ end (Nador et al., 2001). The promoter controlling K14/vGPCR is highly responsive to RTA, which binds to RBP-Jκ to upregulate the transcription of this K14/vGPCR transcript (Liang and Ganem, 2004). K-bZIP (K8), a basic leucine zipper protein, plays an important role in lytic viral DNA replication, and is an early protein whose expression is controlled by RTA (Lin et al., 2003). RTA also controls the expression of other early and late gene by either directly binding to the RTA response elements or through binding with other cellular factors (West and Wood, 2003).
2. KSHV reactivation
KSHV establishes lifelong latency with the persistence of viral genome as chromatin with the expression of only latency-associated genes. These genes maintain latency by blocking the expression of the immediate early gene. However, certain physiological conditions including hypoxia and pharmaceutical agents may trigger the expression of RTA (Cai et al., 2006a; Chen et al., 2001; Haque et al., 2003). During latency, viral genomes are assembled into a nucleosomal structure with DNA wrapped around histones (Shinohara et al., 2002; Stedman et al., 2004). Tail modifications of histones play an important role in determining chromatin structure and condensation, both of which are important in regulating transcriptional activity. Acetylation of core histone tail by histone acetyltransferases (HATs) leads to the loosening of the chromatin and thus makes it transcriptionally active (Niedermeier et al., 2006). In contrast, deacetylation of histone tails by histone deacetylases (HDACs) condenses the chromatin making it transcriptionally inactive (Lu et al., 2003; Stedman et al., 2004). HDACs associate with the RTA promoter during latency resulting in hypoacetylated histones and an inactive promoter (Lu et al., 2003). Treatment of latently infected PEL cells with HDAC inhibitors, NaB, and TPA leads to hyperacetylation of histones with the recruitment of HATs and expression of RTA and other lytic genes (Lu et al., 2003).
Methylation of DNA also plays an important role in controlling lytic reactivation as the inhibitor of DNA methyltransferases, 5-azacytidine (5-AzaC) stimulates KSHV lytic cycle (Chen et al., 2001). KSHV genome does not seem to have extensive methylation but promoters of specific genes like RTA and LANA have been shown to be controlled by methylation (Chen et al., 2001). BCBL-1 cells showed extensive methylation at the RTA promoter in latent cells and treatment with 5-AzaC results in demethylation of ORF50 promoter and expression of ORF50 and early (vIRF) and late gene (K8.1; Chen et al., 2001).
As indicated above, chromatin remodeling due to histone modification can modulate transcriptional activity. The HAT inducer, TPA can induce lytic cycle by activating and enhancing the DNA-binding activity of transcription factors (Wang et al., 2004c). TPA can induce the expression of C/EBPalpha transcription factor and enhance its transactivation activity. Since RTA promoter has C/EBPalpha binding site, expression is enhanced by TPA (Wang et al., 2004c). TPA can also enhance the binding of the AP-1 transcription factor at the RTA promoter to induce RTA expression (Wang et al., 2004c). A number of KSHV proteins including vIRF, LANA, RTA, and K-bZIP have been shown to interact with the transcriptional coactivators p300 and CBP (CREB-binding protein; Huang et al., 2001; Lim et al., 2001; Seo et al., 2000). CBP/p300 has intrinsic HAT activity and RTA binding positively regulates HAT activity (Gwack et al., 2001a). However, binding of LANA, vIRF, and K-bZIP leads to a reduction in protein’s HAT activity (Hwang et al., 2001; Lim et al., 2001; Wang et al., 2003). NaB-mediated activation of ORF50 transcription is due to the binding of the Sp1 transcription factor at the RTA promoter (Ye et al., 2005). LANA regulates ORF50 activity by directly binding to ORF50 promoter and binds to Sp1 during latency (Lu et al., 2006; Verma et al., 2004). Treatment of PEL cells with NaB results in acetylation of LANA resulting in the disruption of LANA from ORF50 promoter bound to Sp1 (Lu et al., 2006). Therefore, it can be speculated that removal of LANA from the ORF50 promoter and Sp1 may allow ORF50 and Sp1 to form a complex which enhances transcription.
Posttranslational modification including sumoylation, phosphorylation, and ADP-ribosylation of viral proteins, primarily RTA, plays an important role in viral reactivation. Poly(ADP-ribose) polymerase 1 (PARP-1) and a kinase, hKFC interacts with RTA to ribosylate and phosphorylate which reduces the transcriptional activity of RTA by abolishing binding to RRE (Gwack et al., 2003). Sumoylation of K-bZIP also plays a role in modulating k-bZIP-mediated RTA activation of KSHV-specific promoters (Izumiya et al., 2005). These studies propose that posttranslation modifications of viral proteins are required to regulate KSHV lytic replication.
III. KSHV PRIMARY INFECTION
Studies for characterization of KSHV primary infection totally relies on the development of systems with high infection efficiency. KSHV produced from PEL and sometimes KS lesions has been showed to infect various cell types but with limited primary infection efficiency or failure of long-term infection. Based on all the systems examined so far, KSHV was found to eventually establish latency after primary infection (Fig. 2). However, depending on cell types or infection conditions, it has been shown that KSHV either enters latency immediately or starts the full productive replication phase followed by establishment of latency infection (Dezube et al., 2002; Foglieni et al., 2005; Gao et al., 2003). Interestingly, the first description of KSHV primary infection system was tested in 293 cells (Foreman et al., 1997). However, due to the direct involvement of endothelial cells in KS tumors, many groups have mainly focused on investigation of KSHV infection in human primary endothelial cells (Ciufo et al., 2001; Flore et al., 1998; Lagunoff et al., 2002; Moses et al., 1999). Initially, it was reported that KSHV infected only a small number of human primary endothelial cells in vitro, like bone marrow microvascular endothelial cells and human umbilical vein endothelial cells (HUVEC; Flore et al., 1998). Subsequent study showed that KSHV was also able to infect primary human dermal microvascular endothelial cells (DMVEC; Moses et al., 1999). The KSHV-infected cells present a typical KS spindle morphology and are able to survive for many months while the uninfected cells go to senescence within a few weeks of been in culture (Flore et al., 1998). The efficiency of primary cell infection in these systems remains very low, although some studies are suggesting strategies for improvement. To further investigate whether the other genetic factors contribute to KSHV primary infection, several similar studies were performed in the E6/E7 or telomerase-immortalized DMVEC culture (Moses et al., 1999). Surprisingly, KSHV infection in the telomerase-immortalized DMVEC shows that it is high. However, the growth of the virus in these cells is not sustained in long-term culture or reactivation to induce lytic replication (Moses et al., 1999). Overall, the limitations in the systems mentioned above have restricted their use to be able to use them for further characterization of KSHV infection. Fortunately, in contrast to the systems above, the recombinant KSHV Bac36 is able to efficiently infect HUVEC cultures and produces large amounts of infectious virion as well as can establish latency at a later stage of infection (Gao et al., 2003). The latently infected cells can be induced to lytic replication by TPA and NaB. This provides a possible path for examining KSHV latent and lytic replication via primary infection (Gao et al., 2003).
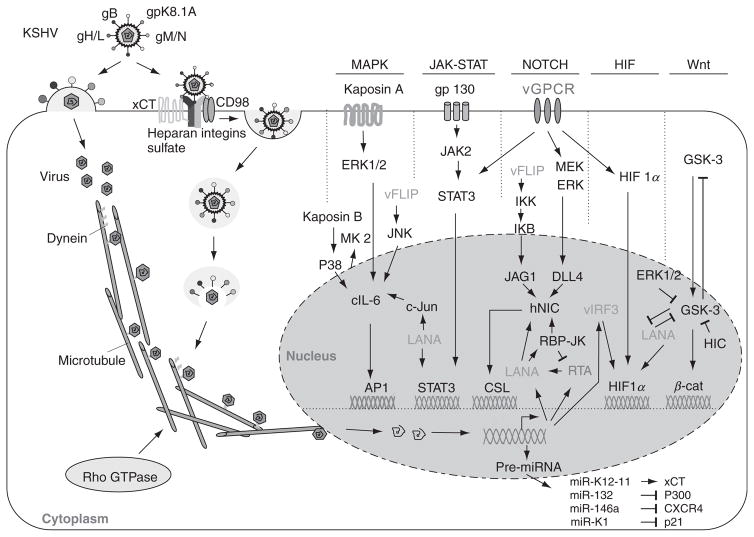
FIGURE 2.
KSHV primary infection and the cell signaling pathways targeted. Infection is initiated by cell fusion or endocytosis. For cell fusion, KSHV fuses with the plasma membrane by gB, gH, gL, gM, gN, and gpK8.1A, then release of virus particles to the cell plasma. For endocytosis, KSHV binds to the cell surface via interactions with heparan sulfate (HS) followed by temporal interactions with integrins and xCT (CD98) molecules, then formation of endocytic vesicles, virus entry, movement in the cytoplasm, release of virus particles, delivery into the nucleus by dynein along the microtubule. Viral gene expression interferes with the cellular signaling pathways such as MAPK, JAK–STAT, Notch, HIF, Wnt, and miRNA, consequently reprograms host cell gene expression.
A. KSHV entry and internalization
Similar to other herpesvirus, the process of KSHV primary infection includes two steps: attachment (or binding) and entry (Spear and Longnecker, 2003). The attachment allows viral proteins to contact with host cell coreceptors, and then stimulate the entry step by either a fusion event between envelope and cell membrane, or receptor-mediated endocytosis (Spear and Longnecker, 2003). KSHV encodes several transmembrane glycoproteins that are involved in attachment and entry into target cells. Some of them are conserved among the herpesvirus like gB (ORF8), gH (ORF22), gL (ORF47), gM (ORF39), and gN (ORF53; Koyano et al., 2003; Krishnan et al., 2005). Some are unique and share no significant homology with glycoproteins of other herpesvirus like K1, K8.1A, K8.1B, and vOX-2(K14) (see Table 2; Chandran et al., 1998; Chung et al., 2002; Li et al., 1999; Luna et al., 2004). KSHV was also shown to attach to the cell surface molecules heparin sulfate (Akula et al., 2001a,b), integrin α3β1 (Akula et al., 2002), and DC-SIGN (dendritic cell-specific ICAM-3-3-grabbing nonintegrin; Rappocciolo et al., 2006).
TABLE 2.
Major cellular homologs encoded by KSHV
| Gene product | ORF | Function | Expression pattern |
|---|---|---|---|
| vIL-6 | K2 | Constitutively activate gp130 independently of IL-6R; B cell proliferation; auto/paracrine growth factors; angiogenic | Productive |
| vCCL1 | K6 | CCR5 and CCR8 agonists; chemoattraction of Th2 cells and monocytes; angiogenic | Productive |
| vCCL2 | K4 | CCR3 and CCR8 agonists; chemoattraction of Th2 cells and monocytes; angiogenic | Productive |
| vCCL3 | K4.1 | CCR4 agonist; chemoattraction of Th2 cells; induction of VEGF-A and angiogenesis | Productive |
| vIAP | K7 | Inhibitor of apoptosis; inhibition of vGPCR expression and function | Productive |
| vBcl-2 | ORF16 | Inhibition of Bax-mediated and virally induced apoptosis | Productive |
| vIRF1 | K9 | Transformation; inhibition of p300, p53, and TGF-β; inhibition of type I interferon | Productive |
| vIRF2 | K11.5 | Inhibition of type I interferon and NF-kB; inhibition of Fas-mediated apoptosis via inhibition of CD95L expression | Productive |
| LANA-2 | K10.5 | Inhibition of type I interferon production; inhibition of PKR- and caspase 3-mediated apoptosis | Productive |
| vIRF3 | K10.6 | Inhibition of p53 and NF-kB; inhibition of Fas-mediated apoptosis via inhibition CD95L expression | Productive |
| vFLIP | K13/ORF71 | Transactivator of NF-kB; antiapoptotic activity via FADD and TRADD binding; transformation | Latent |
| vCyclin | ORF72 | ||
| Constitutively activate Cdk6; resistant to Cdk inhibitors, destabilizes p27 | Latent | ||
| vOX-2 | K14 | Downregulation of myeloid cell activation; regulation of inflammatory cytokine production (IL-1β, TNF-α, IL-8, IFN-γ, and IL-6) | Productive |
| vGPCR | ORF74 | Constitutively active; induces VEGF secretion; transformation | Latent/productive |
After attachment, KSHV enters the target cell via fusion at the plasma membrane or via endocytosis. KSHV fuses with the plasma membrane to enter target cells as shown for 293 cells and MVDECs (Dezube et al., 2002; Inoue et al., 2003; Pertel, 2002). KSHV envelope proteins, gB, gH, gL, gM, gN, and gpK8.1A, are important and play key roles in the cell–cell fusion process (Chandran, 2010; Pertel, 2002). Endocytosis can occur through four major pathways which include clathrin-mediated endocytosis, caveolae, macropinocytosis, and novel nonclathrin, noncaveolae pathways (Kirchhausen, 2000; McPherson et al., 2001; Sieczkarski and Whittaker, 2002). KSHV entry is via interactions with heparan sulfate (HS) followed by temporal interactions with integrins and xCT (CD98) molecules (Kaleeba and Berger, 2006), followed by formation of endocytic vesicles. A recent report showed that KSHV infection induces RhoA GTPase as well as rearrangements of microtubules and the actin cytoskeleton by clathrin-mediated endocytosis pathway in endothelial cells (Sharma-Walia et al., 2004). Thus, the actin dynamics play a pivotal role in internalization and endosomal sorting/trafficking of KSHV and clathrin-mediated endocytosis in HUVEC cells (Greene and Gao, 2009). KSHV also utilizes the actin polymerization-dependent macropinocytic pathway that involves a Rob34 GTPase-dependent late endosome and low-pH environment to entry into HMVEC-d and HUVEC cells (Raghu et al., 2009). KSHV virions are seen in large endocytic vesicles within 5 min of HMVEC-d and HFF cell infection and fusion of the virions envelope with endocytic vesicles (Akula et al., 2003; Raghu et al., 2009).
B. KSHV interaction with cellular signaling pathways
1. MAPK signaling
The activation of the MEK/ERK, JNK, and p38 mitogen-activated protein kinase (MAPK) pathways is pivotal at several stages during KSHV infection. Their activation immediately following infection enables successful establishment of KSHV infection (Pan et al., 2006; Sharma-Walia et al., 2005). Subsequently, MAPK pathways are activated during reactivation of latent infection (Ford et al., 2006; Xie et al., 2008; Yu et al., 2007). LANA is a major activator of the serum response element and MAPK pathways via interactions with a mediator complex (Roupelieva et al., 2010). Extracellular heat shock protein 90 localizes to the cells surface (csHsp90) and is a cofactor for MAPK activation and latent viral gene expression during de novo infection by KSHV (Qin et al., 2010a). These studies suggest that KSHV may utilize MAPK pathways to regulate viral infection and switch from viral latency to lytic replication.
2. JAK–STAT signaling
Cytokine-mediated JAK–STAT signaling controls a number of important biological processes like immune response, cell growth, and differentiation. It has been shown that KSHV infection constitutively activates receptor-associated Janus tyrosine kinases (JAKs) and thereby results in the subsequent phosphorylation of signal transducers and activators of transcription (STATs). For instance, KSHV infection upregulates gp130 receptor expression and leads to constitutive phosphorylation of JAK2/STAT3 activation (Morris et al., 2008; Punjabi et al., 2007). Further studies have indicated that both LANA and vGPCR play a role in regulation of JAK2/STAT3 signaling to produce angiogenic factors (Burger et al., 2005; Muromoto et al., 2006). Recent studies showed that not only is the IL-6 induced by the Tat protein of HIV-1which is dependent on activated STAT3 signaling, but also that IL-4/STAT6 signaling contributes to KSHV lytic replication (Chen et al., 2009; Zeng et al., 2007). This is further confirmed by our findings that KSHV LANA plays a role in inhibition of IL-4-mediated STAT6 phosphorylation for maintenance of latency and response to apoptosis stress (Cai et al., 2010a).
3. Notch signaling
KSHV infection is essential for the development of Kaposi sarcoma (KS). Notch signaling is also known to play a pivotal role in KS cell survival and entry of KSHV into the lytic phase. KSHV-encoded RTA binds to RBP-Jκ and is a major end point of the Notch signal transduction pathway (Liang et al., 2002; Persson and Wilson, 2010). Moreover, the KSHV-encoded LANA protein can stabilize activated forms of the Notch receptor by targeting the Sel10 protein (Lan et al., 2007). KSHV also manipulates the Notch signaling pathway by directly increasing the expression of two Notch ligands (JAG1 and DLL4) through two KSHV genes expressed during latent and lytic infection, respectively (Emuss et al., 2009). These results showed that KSHV infection can manipulate the Notch signaling pathway to influence cell proliferation and differentiation.
4. HIF signaling
Hypoxia-inducible factor (HIF) is a ubiquitously expressed transcriptional regulator that involves an induction of numerous genes associated with angiogenesis and tumor growth. HIF is a heterodimer which composes of inducible α subunit and a constitutively expressed β subunit. It has been demonstrated that there are at least three isoforms of HIFα (HIF1α, HIF2α, and HIF3α) in human cells. In the presence of oxygen, HIFα is hydroxylated and ubiquitylated for proteasomal degradation (Maxwell et al., 1999; Ravi et al., 2000). However, under hypoxic conditions, HIFα hydroxylation is blocked and become stable to activate large number of downstream genes associated with angiogenesis, erythropoiesis, and glycolysis (Lee et al., 2004; Seagroves et al., 2001). Due to the powerful activation of HIF signaling, it has been demonstrated that HIFα is aberrantly overexpressed in many cancers and there is a striking correlation with tumor grade and vascularization (Zagzag et al., 2000; Zhong et al., 1999). In the KSHV-associated cancers, we and other groups have found that HIF1α and HIF2α are overexpressed in KSHV latently infected cells and tissues (Cai et al., 2007; Carroll et al., 2006). Furthermore, both latent antigens LANA and vIRF3 play a role on the HIF1 α stabilization via protein–protein interaction (Cai et al., 2006b, 2007; Shin et al., 2008). Recent analysis of clinical patient samples further supported a role for LANA in stabilization of HIF1α (Long et al., 2009). Interestingly, the lytic antigen vGPCR has also been shown to upregulate VEGF expression through activation of HIF1α expression (Sodhi et al., 2000). These results suggest that HIF1α is stringently targeted by KSHV during both latent and lytic replication.
5. Wnt signaling
KSHV uses components of Wnt pathways to regulate their own viral gene expression and additionally alter cell gene expression through mimicry or manipulation of downstream pathway responses after entry the cells. LANA stabilizes β-catenin by interacting with GSK-3β and inducing its nuclear translocation, thereby preventing phosphorylation of β-catenin in the cytoplasm and stimulation of TCF/LEF-dependent transcription (Fujimuro and Hayward, 2003, 2004). And the I-mfa domain proteins, HIC (human I-mfa domain-containing protein), and I-mfa (inhibitor of MyoD family) interact with LANA and negatively regulate LANA-mediated activation of Wnt signaling-dependent transcription (Kusano and Eizuru, 2010). These data suggest that LANA-mediated dysregulation of β-catenin can play an important role in KSHV-mediated transformation after primary infection.
6. miRNA
Like all herpesviruses, KSHV has a large, double-stranded DNA genome (~160 kb; Russo et al., 1996). KSHV encodes more than 85 protein-coding genes, and at least 12 pre-miRNAs that give rise to at least 17 different miRNAs (16 different 5p or 3p miRNAs, and a single-nucleotide-edited miRNA) that are highly conserved (Lin et al., 2010; Marshall et al., 2007). These viral miRNAs interact with cellular factors important for establishment and/or maintenance of KSHV latent infection. For instance, miR-K12-11 upregulates xCT expression in both KSHV-infected macrophages and endothelial cells via suppression of BACH-1 (Qin et al., 2010b). miR-132 regulates the innate antiviral immunity by inhibiting expression of the p300 transcriptional coactivator (Lagos et al., 2010). KSHV-encoded viral FLICE inhibitory protein vFLIP suppresses CXCR4 expression by upregulating miR-146a (Punj et al., 2010). KSHV-encoded miRNAs target the leucine zipper transcription factor MAF (musculoaponeurotic fibrosarcoma oncogene homolog) and downregulates its expression during primary KSHV infection (Hansen et al., 2010). MicroRNA miR-K1 inhibits p21 expression and attenuates p21-mediated cell-cycle arrest (Gottwein and Cullen, 2010). miR-K1 also regulates NF-κB inhibitor IκBα and viral replication by targeting the 3′UTR of its transcript (Lei et al., 2010), and KSHV miRNA cluster can derepress the ORF50 (RTA) transcription (Lu et al., 2010). These studies suggest that viral miRNAs play a critical role in establishment and/or maintenance of KSHV latent infection.
C. Animal and virus models
To date, many animal and virus models have been developed to investigate the in vivo behavior of KSHV-related malignancies. For animal models, BCBL-1 and infected PEL cells were injected alone or with human peripheral blood mononuclear cells (PBMCs) into SCID mice (Boshoff et al., 1998; Picchio et al., 1997). SCID-Hu Thy/Liv mice were utilized to study viral transcription as well as the susceptibility of the mice to infection with BCBL-1-derived KSHV (Dittmer et al., 1999; Parsons et al., 2006). Similarly, injection of KSHV in human skin engrafted on SCID mice induces KS-like lesions (Foreman et al., 2001). FVB/N transgenic mouse lines that express constitutively active Rac1 (V12 mutant or RacCA) under the control of the α-smooth muscle actin (α-SMA) promoter can develop tumors resembling KS (Ma et al., 2009). Recently, one study reported the successful zoonotic transmission of KSHV into common marmosets (Callithrix jacchus, Cj), a New World primate. Marmosets infected with the recombinant KSHV rapidly seroconvert and maintain a vigorous anti-KSHV antibody response. KSHV DNA and LANA were readily detected in the PBMCs and tissues of the infected marmosets (Chang et al., 2009). Recently, Lossos group developed a direct xenograft model, UM-PEL-1, by transferring freshly isolated human PEL cells into the peritoneal cavities of NOD/SCID mice without in vitro cell growth to avoid the changes in KSHV gene expression evident in cultured cells, showing that bortezomib induces PEL remission and extends overall survival of mice bearing lymphomatous effusions (Sarosiek et al., 2010).
For virus models, rhesus monkey rhadinovirus (RRV) infection results in the development of abnormal cellular proliferations and can coinfect rhesus macaques with simian immunodeficiency virus. This has been suggested as an excellent primate model to investigate KSHV-like pathogenesis (Orzechowska et al., 2008; Wong et al., 1999). Another homolog of KSHV—retroperitoneal fibromatosis-associated herpesviruses (RFHV), is its ability to develop a malignancy closely resembling KS and retroperitoneal fibromatosis in animal that become immunodeficient after infection with a simian virus (Bruce et al., 2006).
Murine gammaherpesvirus 68 (MHV-68) is a small mouse model, but its infection is not associated with KS-like and related diseases (Flach et al., 2009; Virgin et al., 1997). Herpesvirus saimiri (HVS) mainly infects New World primates and results in T-lymphoproliferative disorder ( Jung et al., 1999). Additionally, two types of cell lines carrying KSHV are able to generate KSHV-associated tumors. One is based on HUVECs that express telomerase (TIVE-LTC; An et al., 2006; Sadagopan et al., 2009), and the other is based on normal mouse bone marrow endothelial lineage cells (Meck36) transfected with the KSHV-infectious bacterial artificial chromosome (KSHV-Bac36; An et al., 2006; Mutlu et al., 2007).
IV. KSHV-MEDIATED ONCOGENESIS
Due to the extensive association of KSHV with two different human malignancies (KS and PEL), KSHV is considered to be a human oncogenic virus (Brooks et al., 1997; Cathomas, 2003; Ensoli and Sirianni, 1998). Unlike other oncogenic viruses, KSHV is a complex DNA virus and infection not only leads to cell (endothelial) morphology changes, growth rate, and extended life span, but also provokes deregulated angiogenesis, inflammation, and modulation of immune system in favor of tumor growth (Fig. 3; Dagna et al., 2005; Ensoli and Sturzl, 1998). However, in most experimental systems in vitro infection of endothelial cells with KSHV did not fully result in neoplastic transformation. Moreover, although KSHV encodes oncogenic genes that could potentially induce all KS-related malignant phenotype, the evidences which link KSHV infection to the development of KS mostly occurs in AIDS or immunosuppressed patients, but rarely in general population. This indicates that the presence of KSHV DNA alone in healthy individuals is not sufficient to cause clinical KS, and that the existence of cofactors like HIV infection or drug-induced immunosuppression are important for KSHV-associated disease progression (Cathomas, 2003; Goedert, 2000).
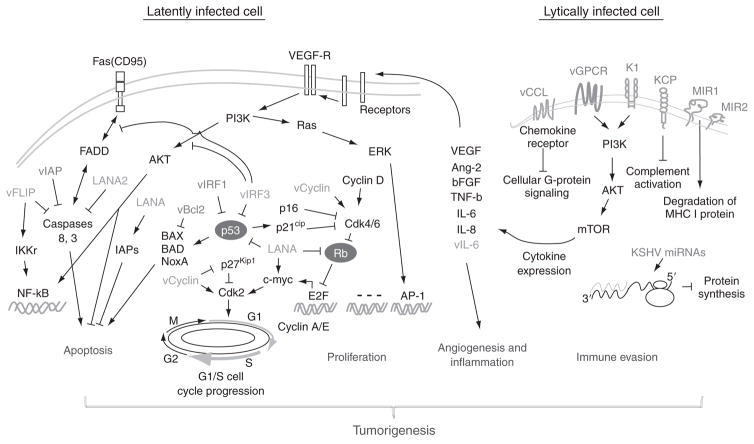
FIGURE 3.
Putative mechanisms of KSHV-mediated regulation of angiogenesis, cell growth and survival, and immune evasion. Cell cycle progression in G1 is controlled by cyclin D/Cdk4/6 complexes which phophorylates Rb. Phosphorylation of Rb leads to Rb inactivation and release transcription factor E2F and transcription of S-phase genes, like Cyclin E and A (Malumbres and Barbacid, 2005). Additionally, p53-mediated Cdk2 and Bax activation and Fas-mediated Caspase activation individually induces cell-cycle arrest and apoptosis. In the latently infected cells, KSHV-encoded latent antigens (green) like LANA and vCyclin drive cell proliferation by targeting two cell-cycle checkpoints: (1) Inactivate Rb to release E2F; and (2) Block p53/p27Kip1-mediated cell-cycle arrest (Cai et al., 2006a,b; Friborg et al., 1999; Jarviluoma et al., 2006; Mann et al., 1999). Meanwhile, LANA cooperates with vFLIP to block Caspase and Bax-mediated apoptosis (Sarid et al., 1999). However, in a small amount of lytically infected cells, KSHV encodes some early lytic proteins (orange) like vGPCR vIRF and MIR1/2 or miRNA which dysregulate immune system to produce certain cytokines and help viral infection by angiogenesis and inflammation (Wang et al., 2004).
In view of the fact that the vast majority of KS spindle cells are latently infected with KSHV, it has been documented that latent infection plays an essential role in KSHV-induced malignancy and pathogenesis (Deng et al., 2004; Fakhari et al., 2006; Staudt and Dittmer, 2003). Nevertheless, a small percentage of infected cells were also found to undergo lytic replication leading to production of mature virus and cell lysis. This indicates that KSHV lytic replication may also be important for KS development (Fig. 3). This notion is further supported by the facts that some drugs targeting KSHV replication have been shown to be effective in inhibiting KS tumor growth in vivo (Mocroft et al., 1996; Robles et al., 1999), and that there was a strong correlation between viral load and progression of KS tumor (Brown et al., 2005; Duprez et al., 2005; Polstra et al., 2004).
A. Induction of cellular growth and survival
Extensive studies have shown that KSHV targets multiple pathways to induce cell proliferation and survival for promoting tumor development. One line of evidence is that genetic instability is found to be commonly seen in KS tumors and PEL cells (Delli Bovi et al., 1986; Gaidano et al., 1997; Popescu et al., 1996), and that KSHV infection is sufficient to induce chromosome instability (Pan et al., 2004). This requires at least five KSHV genes—LANA-1 (or LANA; Cai et al., 2006b; Friborg et al., 1999; Radkov et al., 2000; Si and Robertson, 2006), RTA (Gwack et al., 2001b), k-ZIP, LANA-2 (Rivas et al., 2001), and vIRF-1 (Nakamura et al., 2001; Seo et al., 2001; Shin et al., 2006), which have been shown to interact with and suppress the function of tumor suppressor p53 and Rb resulting in suppression of their activities. Loss of p53 and Rb function leads to inhibition of the DNA damage repair, cell death, and cell-cycle checkpoint which contribute to KSHV-induced oncogenesis. Furthermore, to accelerate cellular proliferation, KSHV encodes vCyclin to promote cell-cycle progression from G1 to S phase by interaction with phosphorylated cyclin-dependent kinase 6 (Cdk6; Chang et al., 1996; Child and Mann, 2001; Godden-Kent et al., 1997; Li et al., 1997; Sarek et al., 2006).
Due to constant selection pressure of favoring cell survival, viruses have evolved different strategies to avoid apoptosis to promote tumor growth and survival by dysregulating cellular signaling pathways. For instance, KSHV encodes vFLIP (K13/ORF71), which like its cellular homolog FLIP, contains the DED domain to inhibit apoptosis by blocking signaling through the death receptor (Belanger et al., 2001; Djerbi et al., 1999). Nevertheless, a recent report utilizing a transgenic mouse model questions the ability of vFLIP to inhibit Fas-mediated apoptosis (Chugh et al., 2005). Several studies have suggested that the antiapoptosis ability of vFLIP is primarily associated with the activation of NF-kB pathway which is essential for cell survival (Chaudhary et al., 1999; Field et al., 2003; Guasparri et al., 2006; Keller et al., 2000, 2006; Liu et al., 2002; Matta and Chaudhary, 2004; Matta et al., 2003;Sun et al., 2005, 2006). Consistently, NF-kB activation by vFLIP leads to cellular transformation and an increased incidence of lymphoma in the transgenic vFLIP mice (Chugh et al., 2005; Sun et al., 2003). It is also important to note that KSHV constitutively activates the NF-kB pathway by encoding vGPCR to produce several cytokines and chemokines (Bais et al., 1998; Couty et al., 2001, 2009; Grisotto et al., 2006; Montaner et al., 2001; Munshi et al., 1999; Schwarz and Murphy, 2001) and vIRF1–3 (Burysek et al., 1999;Flowers et al., 1998; Gao et al., 1997; Kirchhoff et al., 2002; Li et al., 1998; Lubyova and Pitha, 2000; Seo et al., 2002). Treatment with inhibitors of the NF-kB pathway has been shown to completely repress PEL tumors in a mouse model and in vitro tissue culture (Keller et al., 2006; Wang and Damania, 2008). In addition, KSHV was found to promote cell proliferation through autocrine and/or paracrine signaling by encoding or inducing the secretion of various growth factors such as vIL-6 (Molden et al., 1997; Nicholas et al., 1997), IL-6 (Xie et al., 2005), IL-8 (Cerimele et al., 2001), VEGF (Masood et al., 2002), and basic fibroblast growth factor (bFGF; Naranatt et al., 2004; Wang et al., 2004a). It is believed that a variety of cellular growth factors and cytokines regulated by KSHV all play pivotal roles in the development and progression of KS. In addition, cell death is shown to be deregulated by KSHV-encoded antiapoptotic proteins. For instance, KSHV-encoded cellular Bcl-2 homolog vBcl-2 (ORF16) is able to protect cells from Bax-mediated apoptosis (Cheng et al., 1997; Polster et al., 2004; Sarid et al., 1997), and the viral homolog of human survivin also referred to as inhibitor of apoptosis protein (vIAP) encoded by KSHV ORF K7 was shown to inhibit caspase 3 activity and apoptosis by forming a bridge between cellular Bcl-2 and active caspase 3 (Mahotka et al., 1999; Wang et al., 2002).
Another critical strategy is that KSHV encodes a large nuclear antigen called LANA (ORF73), which has no cellular homologs. It has been documented that LANA is essential for many viral functions including gene expression, DNA replication, and episomal maintenance of KSHV genome (Ballestas et al., 1999; Barbera et al., 2006; Cotter and Robertson, 1999; Garber et al., 2002; Hu et al., 2002; Lim et al., 2002). In addition to tethering the KSHV DNA to host chromosome during mitosis, LANA not only plays a role in the maintenance of latency by repressing the transcriptional activity of RTA (a lytic reactivator of KSHV; Lan et al., 2004), but also induce oncogenesis by disrupting p53 and Rb function on cell-cycle checkpoint (Cai et al., 2006b; Friborg et al., 1999; Radkov et al., 2000; Si and Robertson, 2006). Our recent studies suggested that LANA is also able to directly induce the level of cellular IAP expression to enhance the life span and proliferation of KSHV-infected cells (Lu et al., 2009).
B. Regulation of angiogenesis
The typical tumor cell in KS biopsies is a spindle-shaped cell expressing endothelial cell markers with some markers for smooth muscle cells, macrophages, and dentric cells (Flore, 2004). Recent findings have shown that KS is a highly angiogenic neoplasm with dense and irregular shaped blood vessels, and that KSHV infection is involved in angiogenesis and lymphangiogenesis (Carroll et al., 2004; Hong et al., 2004; Wang et al., 2004a). However, different from the angiogenesis in wound healing and female reproduction, pathological angiogenesis is correlated with tumor growth and metastasis (Carmeliet, 2005; Folkman, 2006). Although the mechanisms of angiogenesis in KS tumor development remain to be further elucidated, it has been demonstrated that KSHV-induced angiogenic factors and inflammatory cytokines play essential roles in driving the late stages of KS tumor development. For example, in an SCID mouse model with human skin grafts, neutralization of VEGF blocks the early-stage KS cells growing into KS-like tumors (Masood et al., 2002; Samaniego et al., 2002). Many other angiogenic cytokines including bFGF, IL-6, IL-8, TNF-β, and ephrin B2 have also been shown to be targeted by KSHV (Masood et al., 2005; Naranatt et al., 2004; Wang et al., 2004a; Xie et al., 2005). In clinical samples, higher levels of serum VEGF and mRNA levels of angiopoietins (Ang-1 and Ang-2) were also seen in the AIDS patients with KS than without KS (Brown et al., 2000; Weindel et al., 1992). Moreover, cyclooxygenase-2 (Cox-2) and heme oxygenase-1 induced by KSHV infection were also shown to play an important role in angiogenesis (McAllister et al., 2004; Sharma-Walia et al., 2006, 2010a,b). In addition to the host factor, a number of KSHV-encoded proteins like vIL-6, vGPCR, vCCL-1, and vCCL-II has been shown to act in concert with vCyclin, vFLIP, and vIRF1 to stimulate hematopoiesis and promote angiogenesis by regulating the paracine secretion of angiogenesis-related growth factors and proinflammatory molecules through different signaling pathways (Aoki et al., 1999; Lagos et al., 2007; Montaner et al., 2001, 2003, 2006; Sodhi et al., 2000; Stine et al., 2000). Moreover, recent studies have identified the small GTP-binding protein Rac1 as a key mediator of vGPCR-mediated paracine neoplasia. Prevention of the vGPCR-induced activation of Rac1 efficiently blocks the activation of a series of key transcription factors including NF-kB, AP-1, and NF-AT, and inhibition of cytokine secretion and sarcomagenesis in vitro and in vivo (Montaner et al., 2004).
C. Immune evasion
It has been demonstrated that the immune evasion strategies exploited by KSHV leads to uncontrolled cell proliferation and thereby promote tumorigenesis (Choi et al., 2001; Moore and Chang, 2003; Ploegh, 1998). With the exception of modulation of immune response, KSHV encodes multiple viral encoded proteins which are directly involved in the inhibition of host innate and adaptive immunity (Choi et al., 2001; Means et al., 2002). These include the viral proteins that interfere with interferon signaling, complement system, cytokine secretion, and antigen processing and presentation (Fig. 3).
1. Interference of interferon signaling
Interferon response is the first line of host immune response against viral infection (Fenner et al., 2006). Host cells start to produce and secrete interferon α/β upon virus infection. It has been shown that the interferon response is regulated by cellular interferon factors (cIRFs) at the transcriptional level. To interfere with this response, KSHV encodes four viral homologs of IRF (vIRF1–4; Means et al., 2002; Moore and Chang, 2003). Among these, vIRF1 functions as a repressor of cellular IFN-mediated signal transduction by directly binding to the IFN-stimulated response DNA element. Sequestration of p300/CBP provides another strategy for vIRF1 to broadly inhibit IFN-mediated gene expression (Li et al., 2000). The observation of vIRF1-induced cell transformation and tumorigenesis in nude mice, suggests that vIRF1 plays a potential role in oncogenesis (Gao et al., 1997; Li et al., 1998). Another IRF encoded by KSHV is vIRF3 also called LANA-2 (Esteban et al., 2003; Rivas et al., 2001). It has been demonstrated that vIRF3 is a B cell-specific viral latent protein without DNA-binding ability and is able to inhibit dsRNA-activated protein PKR and p53-dependent apoptosis (Esteban et al., 2003; Rivas et al., 2001). Another mechanism for KSHV to evade the effect of interferon response is expressing viral IL-6. By using the different binding receptor from the cellular homolog, vIL-6 directly binds gp130 independent of gp80, and activates STAT1 phosphorylation and MAPK serine/threonine kinase pathways (Chatterjee et al., 2002; Miles et al., 1990; Molden et al., 1997).
2. Dysregulation of complement system
Another strategy in the first defense mechanism against virus attack is to deregulate the complement system. Like many other viruses, KSHV also interferes with complement and this is targeted by the KSHV-encoded ORF4 also called complement control protein (KCP) which has homology to human complement regulators (Mark et al., 2004, 2006). Through four conserved element termed short consensus repeats (SCRs), KCP disrupts the progression of the complement cascade (Mark et al., 2004, 2007; Mullick et al., 2003; Spiller et al., 2003, 2006). The disruption of complement activation in a mouse model can lead to both acute viral infection and establishment of latency has elucidated the importance of complement inhibition during virus infection (Kapadia et al., 2002).
3. Viral induction of cytokine secretion
Cytokines are the signaling molecules that are used extensively in cellular communication for immune response. Normally, cytokines are unstable and short-lived, and this property is used to prevent too strong and detrimental a response from the host defense system. To promote cytokine stabilization, KSHV expresses the latent protein Kaposin B which activates the p38-MK2 pathway, and leads to increased expression of cytokines including cellular IL-6 and granulocyte-macrophage colony-stimulating factor (GM-CSF; McCormick and Ganem, 2005, 2006; Wang et al., 2004a). Another strategy of KSHV modulation of cytokine signaling is by directly expressing signaling ligands and receptors. For instance, the transmembrane receptor KIS encoded by KSHV K1 gene is constitutively activated through its cytoplasmic immunoreceptor tyrosine-based activation motif (IATM; Lagunoff et al., 1999; Lee et al., 1998a). Upon stimulation, K1 IATM is tyrosine phosphorylated. The K1 signaling activates the PI3K/Akt pathway and in turn a series of downstream cellular transcription factors including AP-1, NF-AT, and NF-kB which lead to expression of a number of inflammatory cytokines, such as IL-6, IL-8, IL-10, and VEGF (Lee et al., 1998b; Samaniego et al., 2001; Wang et al., 2004b). Further studies have confirmed that the K1 protein is an oncogenic protein which is able to transform primary HUVEC (Lee et al., 1998b; Wang and Damania, 2008; Wang et al., 2006). Besides Kaposin B and KIS, the latent protein vFLIP has also been shown to induce IL-8 and IL-6 expression via NF-kB and JNK/AP-1 pathways (An et al., 2003; Sun et al., 2006). Taken together, in the latently infected cells, KSHV-induced cytokine secretion can function in both autocrine and paracrine fashion, and may contribute to the development of KSHV-associated neoplasms.
4. Viral disruption of antigen processing and presentation
Antigen processing and presentation through the major histocompatibility complex (MHC) is a critical step in initiating effective cell-mediated adaptive immune response against pathogens. Downregulation of the cell surface MHC class I molecules is a key viral immune evasion strategy. KSHV encodes two zinc finger membrane proteins MIR1 and MIR2 (also called K3 and K5) which are E3 ubiquitin ligases for modulation by ubiquitylation of MHC I molecules on the infected cell surfaces (Coscoy and Ganem, 2000, 2001). The ubiquitylated MHC I molecules then undergoes endocytosis and is degraded in the lysosome (Coscoy et al., 2001). Interestingly, it has also been demonstrated that MIR1 is able to act as an E3 ubiquitin ligase on ubiquitylation of lysineless molecules (Cadwell and Coscoy, 2005, 2008; Coscoy and Ganem, 2003). Additionally, MIR2 dow-regulates ICAM-1 and B7-2 which are ligands for NK cell-mediated cytotoxicity receptors (Ishido et al., 2000). The second strategy for KSHV to evade the adaptive immune system is bu encoding several viral chemokines (vCCL). At least three vCCLs have been identified and known to inhibit Th1 cell-mediated immune responses by binding with cellular chemokine receptors on Th1 helper cells which result in blocking signal transduction of G-proteins (Means et al., 2002; Moore and Chang, 2003). Additionally, KSHV also encodes its own versions of the chemokine receptors like vGPCR (Sodhi et al., 2004a). vGPCR is a relative of cellular IL-8 receptors CXCR1 and CXCR2 (Sodhi et al., 2004a). Similar to K1, vGPCR is also constitutively active and induces an array of proinflammatory cytokines and growth factors such as IL-1β, IL-6, IL-8, TNF-α, VEGF, and bFGF through AP-1, NF-kB, and HIF1 pathways (Montaner et al., 2004; Schwarz and Murphy, 2001; Sodhi et al., 2000). However, vGPCR activates several downstream kinases including Lyn, JNK, Akt, and p38 not only by constitutive activation but also by certain induction of chemokines like IL-8 (Montaner et al., 2001; Sodhi et al., 2000, 2004b).
D. Response to microenvironmental stress
As the tumor progresses, the cancer cells and its surrounding tissues form a microenvironment with characterizations of both hypoxia and oxidative. Many observations have showed that premalignant cells progress differently in different microenvironments, and hinder the effectiveness of antitumor treatments such as radiation therapy and chemotherapy (Bissell and Radisky, 2001; Liotta and Kohn, 2001). This hostile microen-vironmental stress not only promotes tumor growth and protects it from immune attack, but also affect the host’s susceptibility to pathogens (Bissell and Radisky, 2001; Liotta and Kohn, 2001). KSHV-associated KS lesions are usually found in the lower extremity of the human body like the feet and hands where there is lower oxygen supply (hypoxia). Investigation of the relationship between the host microenvironment and viral tumor cells will provide new insights into the mechanisms of tumorigen-esis and will be a great value in development of therapeutic strategies against clinically relevant viral diseases (Fig. 4).
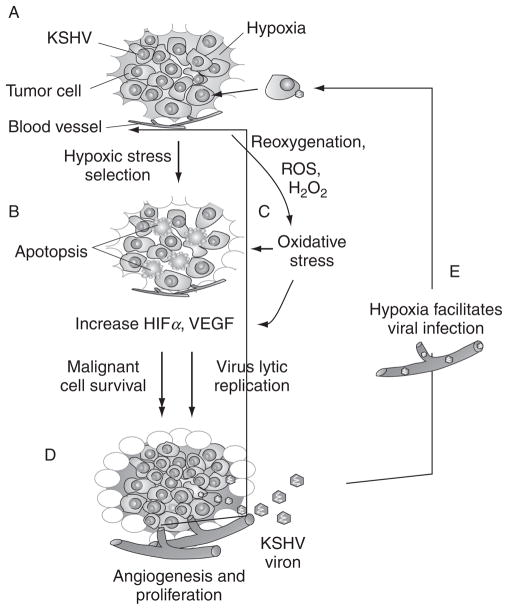
FIGURE 4.
Microenvironmental stress promotes the development of KSHV-mediated malignancy. (A) Proliferation of KSHV-infected tumor cells initiates the formation of hypoxia microenvironment; (B) Hypoxic stress induces cell selection by apoptosis and mutation and increases HIFα and VEGF levels; meanwhile, (C) Oxidative stress caused by reoxygenation or overproduction of ROS also induces similar response as hypoxic stress, and thereby (D) selects the survival of malignant tumor cells to promote angiogenesis and tumor progression. On the other hand, (E) the microenvironmental stresses reactivate KSHV from latent infection to produce new virion particles and help viral primary infection.
1. Hypoxia stress
The rapid progression of the primary tumor usually generates a hypoxic microenvironment inside the tumor lesion. To investigate the effect of hypoxic stress on KSHV-infected cells, we and others have demonstrated that KSHV is able to mimic hypoxic stress to establish latent infection in these cells (Cai et al., 2007; Carroll et al., 2006). Our studies further showed that the EC5S (Elongin BC-Cul5-SOSC-box) E3 ubiquitin complex is recruited by KSHV latent antigen LANA to degrade the HIF1α negative regulators p53 and VHL (Cai et al., 2006b, 2007). A recent study also indicated that another latent antigen vIRF3 can play a role in stabilization of HIF1α and production of VEGF (Shin et al., 2008). Interestingly, several studies have focused on the life cycle of KSHV and discovered that the KSHV genome contains multiple HIF1α-binding DNA elements, and that hypoxic stress induces KSHV lytic replication (Cai et al., 2006a; Davis et al., 2001; Haque et al., 2003, 2006). In addition, hypoxia was also shown to increase the cell toxicity of ganciclovir and azidothymidine in PEL cells in vitro (Davis et al., 2007; Long et al., 2009), as well as inactivate the function of tumor suppressor VHL and so contribute to viral infection (Cai et al., 2010b).
2. Oxidative stress
Oxidative stress represents an imbalance between the production of reactive oxygen species (ROS) and the cellular ability to remove ROS and repair cellular damage. When the concentrations of ROS exceed the ability of the cell to turn over these species, oxidative stress will result and lead to the widespread oxidation and damage of biomolecules including DNA and proteins (Berlett and Stadtman, 1997; Finkel and Holbrook, 2000). There is accumulating evidence which shows that oxidative stress can affect the interaction between the host and viral pathogens, and hence viral pathogenesis (Beck et al., 2000). Recent studies have indicated that herpesvirus infections like HSV-1 and RRV can induce oxidative stress in cells and in tissues through induction of oxidized proteins which are enriched in the VICE (virus-induced chaperone enriched domains) foci in the nucleus (Mathew et al., 2010). However, the biological consequences of virus-induced oxidative stress have not been fully characterized. For KSHV, it is speculated that the redox status may affect replication and pathogenicity of KSHV (Wang et al., 2004d). Most recently, in transgenic Rac1 mice, it has been showed that constitutive activation of Rac1 is sufficient to cause KS-like tumors, and the treatment of antioxidants contributes to inhibition of KS-like tumor growth (Ma et al., 2009). Additionally, it has been reported that KSHV-encoded miRNA upregulates xCT (the inducible subunit of a membrane-bound amino acid transporter) which facilitates KSHV dissemination and persistence in the environment of oxidative stress (Qin et al., 2010b). This indicates that oxidative stress can play a causal role in carcinogenesis mediated by chronic viral infection and inflammation and might be a potential target for therapeutic interventions.
V. POTENTIAL THERAPIES AGAINST KSHV-ASSOCIATED MALIGNANCES
Since the discovery of the virus, understanding of the molecular biology, pathogenesis, and tumorigenesis of KSHV have been increasing and lead to the development of rational therapeutic trials and drug design (Dittmer and Krown, 2007). Although the introduction of HAART (Highly active antiretroviral therapy) has effectively reduced the incidence of AIDS-KS in the USA (Nguyen et al., 2008), it still remains a clinical problem with only half of patients achieving resolution (Vanni et al., 2006). In addition to HAART, the current treatment regiments include radiotherapy and conventional chemotherapy (like liposomal daunorubicin and taxanes) are used to treat the symptoms (Vanni et al., 2006). However, these therapies do not prevent new KS lesions from developing (Sullivan et al., 2006, 2009). Several promising approaches are ongoing with preclinical or clinical trials. These aim to interrupt the angiogenesis process (VEGF/VEGFR, NF-kB/vFLIP, angiopoietin-2, DLL4, and MMP), cell proliferation (cKit/PDGFR, PI3K/AKT/mTOR, and Notch), or both (Rac/Ros; see reviewed elsewhere Dezube et al., 2006; Dittmer and Krown, 2007; Mesri et al., 2010; Sullivan et al., 2006). Moreover, through targeting KSHV lytic replication, interferon-α is now used as an approved treatment for KS (Grundhoff and Ganem, 2004). Using lentivirus-mediated RNA interference to inhibit viral latent gene expression, has provided another feasible approach for treatment of established lymphomas in murine model (Godfrey et al., 2005). Along with the discovery of the unique mechanisms used by viral pathogen, we believe that the use of antiviral agents and small molecules that specifically target the signaling pathways of KSHV-infected tumor cells will be effective therapies with fewer side effects in the treatment of KSHV-associated malignancies.
Acknowledgments
Due to space restrictions, we regret that we had to omit many important references. The work in authors’ laboratory is support from the National Cancer Institute, including NCI CA072510 and CA091792, NIDCR DE014136, NIAID AI067037, and DE17338 (E. S. R.). E. S. R. is a scholar of the Leukemia and Lymphoma Society of America. S. C. V. is supported by the NIH pathways to independence award, CA126182.
References
- Akula SM, Pramod NP, Wang FZ, Chandran B. Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology. 2001a;284:235. doi: 10.1006/viro.2001.0921. [DOI] [PubMed] [Google Scholar]
- Akula SM, Wang FZ, Vieira J, Chandran B. Human herpesvirus 8 interaction with target cells involves heparan sulfate. Virology. 2001b;282:245. doi: 10.1006/viro.2000.0851. [DOI] [PubMed] [Google Scholar]
- Akula SM, Pramod NP, Wang FZ, Chandran B. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell. 2002;108:407. doi: 10.1016/s0092-8674(02)00628-1. [DOI] [PubMed] [Google Scholar]
- Akula SM, Naranatt PP, Walia NS, Wang FZ, Fegley B, Chandran B. Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) infection of human fibroblast cells occurs through endocytosis. J Virol. 2003;77:7978. doi: 10.1128/JVI.77.14.7978-7990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagiozoglou L, Sitas F, Morris L. Phylogenetic analysis of human herpesvirus-8 in South Africa and identification of a novel subgroup. J Gen Virol. 2000;81:2029. doi: 10.1099/0022-1317-81-8-2029. [DOI] [PubMed] [Google Scholar]
- An J, Sun Y, Sun R, Rettig MB. Kaposi’s sarcoma-associated herpesvirus encoded vFLIP induces cellular IL-6 expression: The role of the NF-kappaB and JNK/AP1 pathways. Oncogene. 2003;22:3371. doi: 10.1038/sj.onc.1206407. [DOI] [PubMed] [Google Scholar]
- An FQ, Folarin HM, Compitello N, Roth J, Gerson SL, McCrae KR, Fakhari FD, Dittmer DP, Renne R. Long-term-infected telomerase-immortalized endothelial cells: A model for Kaposi’s sarcoma-associated herpesvirus latency in vitro and in vivo. J Virol. 2006;80:4833. doi: 10.1128/JVI.80.10.4833-4846.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antman K, Chang Y. Kaposi’s sarcoma. N Engl J Med. 2000;342(14):1027–1038. doi: 10.1056/NEJM200004063421407. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Jaffe ES, Chang Y, Jones K, Teruya-Feldstein J, Moore PS, Tosato G. Angiogenesis and hematopoiesis induced by Kaposi’s sarcoma-associated herpesvirus-encoded interleukin-6. Blood. 1999;93:4034. [PubMed] [Google Scholar]
- Arvanitakis L, Mesri EA, Nador RG, Said JW, Asch AS, Knowles DM, Cesarman E. Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood. 1996;88:2648. [PubMed] [Google Scholar]
- Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka EG, Gutkind JS, Asch AS, Cesarman E, Gershengorn MC, Mesri EA. G-protein-coupled receptor of Kaposi’s sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391:86. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- Ballestas ME, Kaye KM. Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mediates episome persistence through cis-acting terminal repeat (TR) sequence and specifically binds TR DNA. J Virol. 2001;75:3250. doi: 10.1128/JVI.75.7.3250-3258.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballestas ME, Chatis PA, Kaye KM. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science. 1999;284:641. doi: 10.1126/science.284.5414.641. [DOI] [PubMed] [Google Scholar]
- Barbera AJ, Chodaparambil JV, Kelley-Clarke B, Joukov V, Walter JC, Luger K, Kaye KM. The nucleosomal surface as a docking station for Kaposi’s sarcoma herpesvirus LANA. Science. 2006;311:856. doi: 10.1126/science.1120541. [DOI] [PubMed] [Google Scholar]
- Bayley AC. Aggressive Kaposi’s sarcoma in Zambia, 1983. Lancet. 1984;1:1318. doi: 10.1016/s0140-6736(84)91818-x. [DOI] [PubMed] [Google Scholar]
- Bechtel J, Grundhoff A, Ganem D. RNAs in the virion of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2005a;79:10138. doi: 10.1128/JVI.79.16.10138-10146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtel JT, Winant RC, Ganem D. Host and viral proteins in the virion of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2005b;79:4952. doi: 10.1128/JVI.79.8.4952-4964.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck MA, Handy J, Levander OA. The role of oxidative stress in viral infections. Ann NY Acad Sci. 2000;917:906. doi: 10.1111/j.1749-6632.2000.tb05456.x. [DOI] [PubMed] [Google Scholar]
- Belanger C, Gravel A, Tomoiu A, Janelle ME, Gosselin J, Tremblay MJ, Flamand L. Human herpesvirus 8 viral FLICE-inhibitory protein inhibits Fas-mediated apoptosis through binding and prevention of procaspase-8 maturation. J Hum Virol. 2001;4:62. [PubMed] [Google Scholar]
- Beral V, Peterman TA, Berkelman RL, Jaffe HW. Kaposi’s sarcoma among persons with AIDS: A sexually transmitted infection? Lancet. 1990;335:123. doi: 10.1016/0140-6736(90)90001-l. [DOI] [PubMed] [Google Scholar]
- Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- Biggar RJ, Whitby D, Marshall V, Linhares AC, Black F. Human herpesvirus 8 in Brazilian Amerindians: A hyperendemic population with a new subtype. J Infect Dis. 2000;181:1562. doi: 10.1086/315456. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshoff C, Chang Y. Kaposi’s sarcoma-associated herpesvirus: A new DNA tumor virus. Annu Rev Med. 2001;52:453. doi: 10.1146/annurev.med.52.1.453. [DOI] [PubMed] [Google Scholar]
- Boshoff C, Schulz TF, Kennedy MM, Graham AK, Fisher C, Thomas A, McGee JO, Weiss RA, O’Leary JJ. Kaposi’s sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat Med. 1995;1:1274. doi: 10.1038/nm1295-1274. [DOI] [PubMed] [Google Scholar]
- Boshoff C, Endo Y, Collins PD, Takeuchi Y, Reeves JD, Schweickart VL, Siani MA, Sasaki T, Williams TJ, Gray PW, Moore PS, Chang Y, et al. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science. 1997;278:290. doi: 10.1126/science.278.5336.290. [DOI] [PubMed] [Google Scholar]
- Boshoff C, Gao SJ, Healy LE, Matthews S, Thomas AJ, Coignet L, Warnke RA, Strauchen JA, Matutes E, Kamel OW, Moore PS, Weiss RA, et al. Establishing a KSHV+ cell line (BCP-1) from peripheral blood and characterizing its growth in Nod/SCID mice. Blood. 1998;91:1671. [PubMed] [Google Scholar]
- Brooks LA, Wilson AJ, Crook T. Kaposi’s sarcoma-associated herpesvirus (KSHV)/human herpesvirus 8 (HHV8)–a new human tumour virus. J Pathol. 1997;182:262. doi: 10.1002/(SICI)1096-9896(199707)182:3<262::AID-PATH836>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Brown LF, Dezube BJ, Tognazzi K, Dvorak HF, Yancopoulos GD. Expression of Tie1, Tie2, and angiopoietins 1, 2, and 4 in Kaposi’s sarcoma and cutaneous angiosarcoma. Am J Pathol. 2000;156:2179. doi: 10.1016/S0002-9440(10)65088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EE, Whitby D, Vitale F, Fei PC, Del Carpio C, Marshall V, Alberg AJ, Serraino D, Messina A, Gafa L, Goedert JJ. Correlates of human herpesvirus-8 DNA detection among adults in Italy without Kaposi sarcoma. Int J Epidemiol. 2005;34:1110. doi: 10.1093/ije/dyi131. [DOI] [PubMed] [Google Scholar]
- Bruce AG, Bakke AM, Bielefeldt-Ohmann H, Ryan JT, Thouless ME, Tsai CC, Rose TM. High levels of retroperitoneal fibromatosis (RF)-associated herpesvirus in RF lesions in macaques are associated with ORF73 LANA expression in spindleoid tumour cells. J Gen Virol. 2006;87:3529. doi: 10.1099/vir.0.82339-0. [DOI] [PubMed] [Google Scholar]
- Burger M, Hartmann T, Burger JA, Schraufstatter I. KSHV-GPCR and CXCR2 transforming capacity and angiogenic responses are mediated through a JAK2-STAT3-dependent pathway. Oncogene. 2005;24:2067. doi: 10.1038/sj.onc.1208442. [DOI] [PubMed] [Google Scholar]
- Burysek L, Pitha PM. Latently expressed human herpesvirus 8-encoded interferon regulatory factor 2 inhibits double-stranded RNA-activated protein kinase. J Virol. 2001;75:2345. doi: 10.1128/JVI.75.5.2345-2352.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burysek L, Yeow WS, Lubyova B, Kellum M, Schafer SL, Huang YQ, Pitha PM. Functional analysis of human herpesvirus 8-encoded viral interferon regulatory factor 1 and its association with cellular interferon regulatory factors and p300. J Virol. 1999;73:7334. doi: 10.1128/jvi.73.9.7334-7342.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadwell K, Coscoy L. Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase. Science. 2005;309:127. doi: 10.1126/science.1110340. [DOI] [PubMed] [Google Scholar]
- Cadwell K, Coscoy L. The specificities of Kaposi’s sarcoma-associated herpesvirus-encoded E3 ubiquitin ligases are determined by the positions of lysine or cysteine residues within the intracytoplasmic domains of their targets. J Virol. 2008;82:4184. doi: 10.1128/JVI.02264-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Lan K, Verma SC, Si H, Lin D, Robertson ES. Kaposi’s sarcoma-associated herpesvirus latent protein LANA interacts with HIF-1 alpha to upregulate RTA expression during hypoxia: Latency control under low oxygen conditions. J Virol. 2006a;80:7965. doi: 10.1128/JVI.00689-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai QL, Knight JS, Verma SC, Zald P, Robertson ES. EC5S ubiquitin complex is recruited by KSHV latent antigen LANA for degradation of the VHL and p53 tumor suppressors. PLoS Pathog. 2006b;2:e116. doi: 10.1371/journal.ppat.0020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Murakami M, Si H, Robertson ES. A potential alpha-helix motif in the amino terminus of LANA encoded by Kaposi’s sarcoma-associated herpesvirus is critical for nuclear accumulation of HIF-1alpha in normoxia. J Virol. 2007;81:10413. doi: 10.1128/JVI.00611-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Verma SC, Choi J-Y, Ma M, Robertson ES. Kaposi’s sarcoma herpesvirus inhibits IL-4-mediated STAT6 phosphorylatiion to regulate apoptosis and maintain latency. J Virol. 2010a doi: 10.1128/JVI.01293-10. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Verma SC, Kumar P, Ma M, Robertson ES. Hypoxia inactivates the VHL tumor suppressor through PIASy-mediated SUMO modification. PLoS ONE. 2010b;5(3):e9720. doi: 10.1371/journal.pone.0009720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabro ML, Sheldon J, Favero A, Simpson GR, Fiore JR, Gomes E, Angarano G, Chieco-Bianchi L, Schulz TF. Seroprevalence of Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 in several regions of Italy. J Hum Virol. 1998;1:207. [PubMed] [Google Scholar]
- Cannon JS, Ciufo D, Hawkins AL, Griffin CA, Borowitz MJ, Hayward GS, Ambinder RF. A new primary effusion lymphoma-derived cell line yields a highly infectious Kaposi’s sarcoma herpesvirus-containing supernatant. J Virol. 2000;74:10187. doi: 10.1128/jvi.74.21.10187-10193.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone A, Gaidano G. HHV-8-positive bodycavity-based lymphoma: A novel lymphoma entity. Br J Haematol. 1997;97:515. doi: 10.1046/j.1365-2141.1997.00064.x. [DOI] [PubMed] [Google Scholar]
- Carbone A, Cilia AM, Gloghini A, Canzonieri V, Pastore C, Todesco M, Cozzi M, Perin T, Volpe R, Pinto A, Gaidano G. Establishment of HHV-8-positive and HHV-8-negative lymphoma cell lines from primary lymphomatous effusions. Int J Cancer. 1997;73:562. doi: 10.1002/(sici)1097-0215(19971114)73:4<562::aid-ijc18>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- Carroll PA, Brazeau E, Lagunoff M. Kaposi’s sarcoma-associated herpesvirus infection of blood endothelial cells induces lymphatic differentiation. Virology. 2004;328:7. doi: 10.1016/j.virol.2004.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll PA, Kenerson HL, Yeung RS, Lagunoff M. Latent Kaposi’s sarcoma-associated herpesvirus infection of endothelial cells activates hypoxia-induced factors. J Virol. 2006;80:10802. doi: 10.1128/JVI.00673-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathomas G. Kaposi’s sarcoma-associated herpesvirus (KSHV)/human herpesvirus 8 (HHV-8) as a tumour virus. Herpes. 2003;10:72. [PubMed] [Google Scholar]
- Cerimele F, Curreli F, Ely S, Friedman-Kien AE, Cesarman E, Flore O. Kaposi’s sarcoma-associated herpesvirus can productively infect primary human keratinocytes and alter their growth properties. J Virol. 2001;75:2435. doi: 10.1128/JVI.75.5.2435-2443.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesarman E. The role of Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) in lymphoproliferative diseases. Recent Results Cancer Res. 2002;159:27. doi: 10.1007/978-3-642-56352-2_4. [DOI] [PubMed] [Google Scholar]
- Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related bodycavity-based lymphomas. N Engl J Med. 1995;332:1186. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- Chandran B. Early events in Kaposi’s sarcoma-associated herpesvirus infection of target cells. J Virol. 2010;84:2188. doi: 10.1128/JVI.01334-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran B, Bloomer C, Chan SR, Zhu L, Goldstein E, Horvat R. Human herpesvirus-8 ORF K8.1 gene encodes immunogenic glycoproteins generated by spliced transcripts. Virology. 1998;249:140. doi: 10.1006/viro.1998.9316. [DOI] [PubMed] [Google Scholar]
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- Chang Y, Moore PS, Talbot SJ, Boshoff CH, Zarkowska T, Godden K, Paterson H, Weiss RA, Mittnacht S. Cyclin encoded by KS herpesvirus. Nature. 1996;382:410. doi: 10.1038/382410a0. [DOI] [PubMed] [Google Scholar]
- Chang J, Renne R, Dittmer D, Ganem D. Inflammatory cytokines and the reactivation of Kaposi’s sarcoma-associated herpesvirus lytic replication. Virology. 2000;266:17. doi: 10.1006/viro.1999.0077. [DOI] [PubMed] [Google Scholar]
- Chang PJ, Shedd D, Miller G. Two subclasses of Kaposi’s sarcoma-associated herpesvirus lytic cycle promoters distinguished by open reading frame 50 mutant proteins that are deficient in binding to DNA. J Virol. 2005;79:8750. doi: 10.1128/JVI.79.14.8750-8763.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Wachtman LM, Pearson CB, Lee JS, Lee HR, Lee SH, Vieira J, Mansfield KG, Jung JU. Non-human primate model of Kaposi’s sarcoma-associated herpesvirus infection. PLoS Pathog. 2009;5:e1000606. doi: 10.1371/journal.ppat.1000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M, Osborne J, Bestetti G, Chang Y, Moore PS. Viral IL-6-induced cell proliferation and immune evasion of interferon activity. Science. 2002;298:1432. doi: 10.1126/science.1074883. [DOI] [PubMed] [Google Scholar]
- Chaudhary PM, Jasmin A, Eby MT, Hood L. Modulation of the NF-kappa B pathway by virally encoded death effector domains-containing proteins. Oncogene. 1999;18:5738. doi: 10.1038/sj.onc.1202976. [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, MacLeod I, Yates JR, 3rd, Oegema K, Desai A. The CENP-F-like proteins HCP-1 and HCP-2 target CLASP to kinetochores to mediate chromosome segregation. Curr Biol. 2005;15:771. doi: 10.1016/j.cub.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Chen J, Ueda K, Sakakibara S, Okuno T, Yamanishi K. Transcriptional regulation of the Kaposi’s sarcoma-associated herpesvirus viral interferon regulatory factor gene. J Virol. 2000;74:8623. doi: 10.1128/jvi.74.18.8623-8634.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Ueda K, Sakakibara S, Okuno T, Parravicini C, Corbellino M, Yamanishi K. Activation of latent Kaposi’s sarcoma-associated herpesvirus by demethylation of the promoter of the lytic transactivator. Proc Natl Acad Sci USA. 2001;98:4119. doi: 10.1073/pnas.051004198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Cheng L, Jia X, Zeng Y, Yao S, Lv Z, Qin D, Fang X, Lei Y, Lu C. Human immunodeficiency virus type 1 Tat accelerates Kaposi sarcoma-associated herpesvirus Kaposin A-mediated tumorigenesis of transformed fibroblasts in vitro as well as in nude and immunocompetent mice. Neoplasia. 2009;11:1272. doi: 10.1593/neo.09494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng EH, Nicholas J, Bellows DS, Hayward GS, Guo HG, Reitz MS, Hardwick JM. A Bcl-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc Natl Acad Sci USA. 1997;94:690. doi: 10.1073/pnas.94.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung TW. AIDS-related cancer in the era of highly active antiretroviral therapy (HAART): A model of the interplay of the immune system, virus, and cancer. “On the offensive–the Trojan Horse is being destroyed”–Part A: Kaposi’s sarcoma. Cancer Invest. 2004;22:774. doi: 10.1081/cnv-200032788. [DOI] [PubMed] [Google Scholar]
- Child ES, Mann DJ. Novel properties of the cyclin encoded by human herpesvirus 8 that facilitate exit from quiescence. Oncogene. 2001;20:3311. doi: 10.1038/sj.onc.1204447. [DOI] [PubMed] [Google Scholar]
- Chiou CJ, Poole LJ, Kim PS, Ciufo DM, Cannon JS, ap Rhys CM, Alcendor DJ, Zong JC, Ambinder RF, Hayward GS. Patterns of gene expression and a transactivation function exhibited by the vGCR (ORF74) chemokine receptor protein of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2002;76:3421. doi: 10.1128/JVI.76.7.3421-3439.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Means RE, Damania B, Jung JU. Molecular piracy of Kaposi’s sarcoma associated herpesvirus. Cytokine Growth Factor Rev. 2001;12:245. doi: 10.1016/s1359-6101(00)00029-0. [DOI] [PubMed] [Google Scholar]
- Chugh P, Matta H, Schamus S, Zachariah S, Kumar A, Richardson JA, Smith AL, Chaudhary PM. Constitutive NF-kappaB activation, normal Fas-induced apoptosis, and increased incidence of lymphoma in human herpes virus 8 K13 transgenic mice. Proc Natl Acad Sci USA. 2005;102:12885. doi: 10.1073/pnas.0408577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YH, Means RE, Choi JK, Lee BS, Jung JU. Kaposi’s sarcoma-associated herpesvirus OX2 glycoprotein activates myeloid-lineage cells to induce inflammatory cytokine production. J Virol. 2002;76:4688. doi: 10.1128/JVI.76.10.4688-4698.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciufo DM, Cannon JS, Poole LJ, Wu FY, Murray P, Ambinder RF, Hayward GS. Spindle cell conversion by Kaposi’s sarcoma-associated herpesvirus: Formation of colonies and plaques with mixed lytic and latent gene expression in infected primary dermal microvascular endothelial cell cultures. J Virol. 2001;75:5614. doi: 10.1128/JVI.75.12.5614-5626.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook PM, Whitby D, Calabro ML, Luppi M, Kakoola DN, Hjalgrim H, Ariyoshi K, Ensoli B, Davison AJ, Schulz TF. Variability and evolution of Kaposi’s sarcoma-associated herpesvirus in Europe and Africa. International Collaborative Group. AIDS. 1999;13:1165. doi: 10.1097/00002030-199907090-00004. [DOI] [PubMed] [Google Scholar]
- Cool CD, Rai PR, Yeager ME, Hernandez-Saavedra D, Serls AE, Bull TM, Geraci MW, Brown KK, Routes JM, Tuder RM, Voelkel NF. Expression of human herpesvirus 8 in primary pulmonary hypertension. N Engl J Med. 2003;349:1113. doi: 10.1056/NEJMoa035115. [DOI] [PubMed] [Google Scholar]
- Corey L, Brodie S, Huang ML, Koelle DM, Wald A. HHV-8 infection: A model for reactivation and transmission. Rev Med Virol. 2002;12:47. doi: 10.1002/rmv.341. [DOI] [PubMed] [Google Scholar]
- Coscoy L. Immune evasion by Kaposi’s sarcoma-associated herpesvirus. Nat Rev Immunol. 2007;7(5):391–401. doi: 10.1038/nri2076. [DOI] [PubMed] [Google Scholar]
- Coscoy L, Ganem D. Kaposi’s sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc Natl Acad Sci USA. 2000;97:8051. doi: 10.1073/pnas.140129797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coscoy L, Ganem D. A viral protein that selectively downregulates ICAM-1 and B7-2 and modulates T cell costimulation. J Clin Invest. 2001;107:1599. doi: 10.1172/JCI12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coscoy L, Ganem D. PHD domains and E3 ubiquitin ligases: Viruses make the connection. Trends Cell Biol. 2003;13:7. doi: 10.1016/s0962-8924(02)00005-3. [DOI] [PubMed] [Google Scholar]
- Coscoy L, Sanchez DJ, Ganem D. A novel class of herpesvirus-encoded membrane-bound E3 ubiquitin ligases regulates endocytosis of proteins involved in immune recognition. J Cell Biol. 2001;155:1265. doi: 10.1083/jcb.200111010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter MA, Robertson ES. The latency-associated nuclear antigen tethers the Kaposi’s sarcoma-associated herpesvirus genome to host chromosomes in body cavity-based lymphoma cells. Virology. 1999;264:254. doi: 10.1006/viro.1999.9999. [DOI] [PubMed] [Google Scholar]
- Cotter MA, 2nd, Subramanian C, Robertson ES. The Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen binds to specific sequences at the left end of the viral genome through its carboxyterminus. Virology. 2001;291:241. doi: 10.1006/viro.2001.1202. [DOI] [PubMed] [Google Scholar]
- Couty JP, Geras-Raaka E, Weksler BB, Gershengorn MC. Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor signals through multiple pathways in endothelial cells. J Biol Chem. 2001;276:33805. doi: 10.1074/jbc.M104631200. [DOI] [PubMed] [Google Scholar]
- Couty JP, Lupu-Meiri M, Oron Y, Gershengorn MC. Kaposi’s sarcoma-associated herpesvirus-G protein-coupled receptor-expressing endothelial cells exhibit reduced migration and stimulated chemotaxis by chemokine inverse agonists. J Pharmacol Exp Ther. 2009;329:1142. doi: 10.1124/jpet.108.147686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagna L, Broccolo F, Paties CT, Ferrarini M, Sarmati L, Praderio L, Sabbadini MG, Lusso P, Malnati MS. A relapsing inflammatory syndrome and active human herpesvirus 8 infection. N Engl J Med. 2005;353:156. doi: 10.1056/NEJMoa042850. [DOI] [PubMed] [Google Scholar]
- Damania B. Oncogenic gamma-herpesviruses: Comparison of viral proteins involved in tumorigenesis. Nat Rev Microbiol. 2004;2(8):656–668. doi: 10.1038/nrmicro958. [DOI] [PubMed] [Google Scholar]
- Davis DA, Rinderknecht AS, Zoeteweij JP, Aoki Y, Read-Connole EL, Tosato G, Blauvelt A, Yarchoan R. Hypoxia induces lytic replication of Kaposi sarcoma-associated herpesvirus. Blood. 2001;97:3244. doi: 10.1182/blood.v97.10.3244. [DOI] [PubMed] [Google Scholar]
- Davis DA, Singer KE, Reynolds IP, Haque M, Yarchoan R. Hypoxia enhances the phosphorylation and cytotoxicity of ganciclovir and zidovudine in Kaposi’s sarcoma-associated herpesvirus infected cells. Cancer Res. 2007;67:7003. doi: 10.1158/0008-5472.CAN-07-0939. [DOI] [PubMed] [Google Scholar]
- Delli Bovi P, Donti E, Knowles DM, 2nd, Friedman-Kien A, Luciw PA, Dina D, Dalla-Favera R, Basilico C. Presence of chromosomal abnormalities and lack of AIDS retrovirus DNA sequences in AIDS-associated Kaposi’s sarcoma. Cancer Res. 1986;46:6333. [PubMed] [Google Scholar]
- Deng H, Young A, Sun R. Auto-activation of the rta gene of human herpesvirus-8/Kaposi’s sarcoma-associated herpesvirus. J Gen Virol. 2000;81:3043. doi: 10.1099/0022-1317-81-12-3043. [DOI] [PubMed] [Google Scholar]
- Deng H, Chu JT, Rettig MB, Martinez-Maza O, Sun R. Rta of the human herpesvirus 8/Kaposi sarcoma-associated herpesvirus up-regulates human interleukin-6 gene expression. Blood. 2002;100:1919. doi: 10.1182/blood-2002-01-0015. [DOI] [PubMed] [Google Scholar]
- Deng JH, Zhang YJ, Wang XP, Gao SJ. Lytic replication-defective Kaposi’s sarcoma-associated herpesvirus: Potential role in infection and malignant transformation. J Virol. 2004;78:11108. doi: 10.1128/JVI.78.20.11108-11120.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezube BJ, Zambela M, Sage DR, Wang JF, Fingeroth JD. Characterization of Kaposi sarcoma-associated herpesvirus/human herpesvirus-8 infection of human vascular endothelial cells: Early events. Blood. 2002;100:888. doi: 10.1182/blood.v100.3.888. [DOI] [PubMed] [Google Scholar]
- Dezube BJ, Sullivan R, Koon HB. Emerging targets and novel strategies in the treatment of AIDS-related Kaposi’s sarcoma: Bidirectional translational science. J Cell Physiol. 2006;209:659. doi: 10.1002/jcp.20795. [DOI] [PubMed] [Google Scholar]
- Dittmer DP, Krown SE. Targeted therapy for Kaposi’s sarcoma and Kaposi’s sarcoma-associated herpesvirus. Curr Opin Oncol. 2007;19:452. doi: 10.1097/CCO.0b013e3281eb8ea7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer D, Lagunoff M, Renne R, Staskus K, Haase A, Ganem D. A cluster of latently expressed genes in Kaposi’s sarcoma-associated herpesvirus. J Virol. 1998;72:8309. doi: 10.1128/jvi.72.10.8309-8315.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer D, Stoddart C, Renne R, Linquist-Stepps V, Moreno ME, Bare C, McCune JM, Ganem D. Experimental transmission of Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) to SCID-hu Thy/Liv mice. J Exp Med. 1999;190:1857. doi: 10.1084/jem.190.12.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djerbi M, Screpanti V, Catrina AI, Bogen B, Biberfeld P, Grandien A. The inhibitor of death receptor signaling, FLICE-inhibitory protein defines a new class of tumor progression factors. J Exp Med. 1999;190:1025. doi: 10.1084/jem.190.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing RG, Eglin RP, Bayley AC. African Kaposi’s sarcoma and AIDS. Lancet. 1984;1:478. doi: 10.1016/s0140-6736(84)92850-2. [DOI] [PubMed] [Google Scholar]
- Du Q, Taylor L, Compton DA, Macara IG. LGN blocks the ability of NuMA to bind and stabilize microtubules. A mechanism for mitotic spindle assembly regulation. Curr Biol. 2002;12:1928. doi: 10.1016/s0960-9822(02)01298-8. [DOI] [PubMed] [Google Scholar]
- Duan W, Wang S, Liu S, Wood C. Characterization of Kaposi’s sarcoma-associated herpesvirus/human herpesvirus-8 ORF57 promoter. Arch Virol. 2001;146:403. doi: 10.1007/s007050170185. [DOI] [PubMed] [Google Scholar]
- Dukers NH, Renwick N, Prins M, Geskus RB, Schulz TF, Weverling GJ, Coutinho RA, Goudsmit J. Risk factors for human herpesvirus 8 seropositivity and seroconversion in a cohort of homosexual men. Am J Epidemiol. 2000;151:213. doi: 10.1093/oxfordjournals.aje.a010195. [DOI] [PubMed] [Google Scholar]
- Dupin N, Fisher C, Kellam P, Ariad S, Tulliez M, Franck N, van Marck E, Salmon D, Gorin I, Escande JP, Weiss RA, Alitalo K, et al. Distribution of human herpesvirus-8 latently infected cells in Kaposi’s sarcoma, multicentric Castleman’s disease, and primary effusion lymphoma. Proc Natl Acad Sci USA. 1999;96:4546. doi: 10.1073/pnas.96.8.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupin N, Diss TL, Kellam P, Tulliez M, Du MQ, Sicard D, Weiss RA, Isaacson PG, Boshoff C. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood. 2000;95:1406. [PubMed] [Google Scholar]
- Duprez R, Kassa-Kelembho E, Plancoulaine S, Briere J, Fossi M, Kobangue L, Minsart P, Huerre M, Gessain A. Human herpesvirus 8 serological markers and viral load in patients with AIDS-associated Kaposi’s sarcoma in Central African Republic. J Clin Microbiol. 2005;43:4840. doi: 10.1128/JCM.43.9.4840-4843.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltom MA, Mbulaiteye SM, Dada AJ, Whitby D, Biggar RJ. Transmission of human herpesvirus 8 by sexual activity among adults in Lagos, Nigeria. AIDS. 2002;16:2473. doi: 10.1097/00002030-200212060-00014. [DOI] [PubMed] [Google Scholar]
- Emuss V, Lagos D, Pizzey A, Gratrix F, Henderson SR, Boshoff C. KSHV manipulates Notch signaling by DLL4 and JAG1 to alter cell cycle genes in lymphatic endothelia. PLoS Pathog. 2009;5:e1000616. doi: 10.1371/journal.ppat.1000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensoli B, Sirianni MC. Kaposi’s sarcoma pathogenesis: A link between immunology and tumor biology. Crit Rev Oncog. 1998;9:107. doi: 10.1615/critrevoncog.v9.i2.20. [DOI] [PubMed] [Google Scholar]
- Ensoli B, Sturzl M. Kaposi’s sarcoma: A result of the interplay among inflammatory cytokines, angiogenic factors and viral agents. Cytokine Growth Factor Rev. 1998;9:63. doi: 10.1016/s1359-6101(97)00037-3. [DOI] [PubMed] [Google Scholar]
- Esteban M, Garcia MA, Domingo-Gil E, Arroyo J, Nombela C, Rivas C. The latency protein LANA2 from Kaposi’s sarcoma-associated herpesvirus inhibits apoptosis induced by dsRNA-activated protein kinase but not RNase L activation. J Gen Virol. 2003;84:1463. doi: 10.1099/vir.0.19014-0. [DOI] [PubMed] [Google Scholar]
- Fakhari FD, Jeong JH, Kanan Y, Dittmer DP. The latency-associated nuclear antigen of Kaposi sarcoma-associated herpesvirus induces B cell hyperplasia and lymphoma. J Clin Invest. 2006;116:735. doi: 10.1172/JCI26190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner JE, Starr R, Cornish AL, Zhang JG, Metcalf D, Schreiber RD, Sheehan K, Hilton DJ, Alexander WS, Hertzog PJ. Suppressor of cytokine signaling 1 regulates the immune response to infection by a unique inhibition of type I interferon activity. Nat Immunol. 2006;7(1):33–39. doi: 10.1038/ni1287. [DOI] [PubMed] [Google Scholar]
- Field N, Low W, Daniels M, Howell S, Daviet L, Boshoff C, Collins M. KSHV vFLIP binds to IKK-gamma to activate IKK. J Cell Sci. 2003;116:3721. doi: 10.1242/jcs.00691. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Flach B, Steer B, Thakur NN, Haas J, Adler H. The M10 locus of murine gammaherpesvirus 68 contributes to both the lytic and the latent phases of infection. J Virol. 2009;83:8163. doi: 10.1128/JVI.00629-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flore O. Kaposi’s sarcoma. Lancet. 2004;364:740. doi: 10.1016/S0140-6736(04)16952-3. [DOI] [PubMed] [Google Scholar]
- Flore O, Rafii S, Ely S, O’Leary JJ, Hyjek EM, Cesarman E. Transformation of primary human endothelial cells by Kaposi’s sarcoma-associated herpesvirus. Nature. 1998;394:588. doi: 10.1038/29093. [DOI] [PubMed] [Google Scholar]
- Flowers CC, Flowers SP, Nabel GJ. Kaposi’s sarcoma-associated herpesvirus viral interferon regulatory factor confers resistance to the antiproliferative effect of interferon-alpha. Mol Med. 1998;4:402. [PMC free article] [PubMed] [Google Scholar]
- Foglieni C, Scabini S, Belloni D, Broccolo F, Lusso P, Malnati MS, Ferrero E. Productive infection of HUVEC by HHV-8 is associated with changes compatible with angiogenic transformations. Eur J Histochem. 2005;49:273. doi: 10.4081/954. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- Ford PW, Bryan BA, Dyson OF, Weidner DA, Chintalgattu V, Akula SM. Raf/MEK/ERK signalling triggers reactivation of Kaposi’s sarcoma-associated herpesvirus latency. J Gen Virol. 2006;87:1139. doi: 10.1099/vir.0.81628-0. [DOI] [PubMed] [Google Scholar]
- Foreman KE, Friborg J, Jr, Kong WP, Woffendin C, Polverini PJ, Nickoloff BJ, Nabel GJ. Propagation of a human herpesvirus from AIDS-associated Kaposi’s sarcoma. N Engl J Med. 1997;336:163. doi: 10.1056/NEJM199701163360302. [DOI] [PubMed] [Google Scholar]
- Foreman KE, Friborg J, Chandran B, Katano H, Sata T, Mercader M, Nabel GJ, Nickoloff BJ. Injection of human herpesvirus-8 in human skin engrafted on SCID mice induces Kaposi’s sarcoma-like lesions. J Dermatol Sci. 2001;26:182. doi: 10.1016/s0923-1811(01)00087-1. [DOI] [PubMed] [Google Scholar]
- Franceschi S, Serraino D. Kaposi’s sarcoma and KSHV. Lancet. 1995;346:1360. [PubMed] [Google Scholar]
- Friborg J, Jr, Kong W, Hottiger MO, Nabel GJ. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature. 1999;402:889. doi: 10.1038/47266. [DOI] [PubMed] [Google Scholar]
- Fujimuro M, Hayward SD. The latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus manipulates the activity of glycogen synthase kinase-3beta. J Virol. 2003;77:8019. doi: 10.1128/JVI.77.14.8019-8030.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimuro M, Hayward SD. Manipulation of glycogen-synthase kinase-3 activity in KSHV-associated cancers. J Mol Med. 2004;82:223. doi: 10.1007/s00109-003-0519-7. [DOI] [PubMed] [Google Scholar]
- Gaidano G, Cechova K, Chang Y, Moore PS, Knowles DM, Dalla-Favera R. Establishment of AIDS-related lymphoma cell lines from lymphomatous effusions. Leukemia. 1996;10:1237. [PubMed] [Google Scholar]
- Gaidano G, Pastore C, Gloghini A, Capello D, Tirelli U, Saglio G, Carbone A. Microsatellite instability in KSHV/HHV-8 positive bodycavity-based lymphoma. Hum Pathol. 1997;28:748. doi: 10.1016/s0046-8177(97)90187-8. [DOI] [PubMed] [Google Scholar]
- Gao SJ, Kingsley L, Hoover DR, Spira TJ, Rinaldo CR, Saah A, Phair J, Detels R, Parry P, Chang Y, Moore PS. Seroconversion to antibodies against Kaposi’s sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi’s sarcoma. N Engl J Med. 1996a;335:233. doi: 10.1056/NEJM199607253350403. [DOI] [PubMed] [Google Scholar]
- Gao SJ, Kingsley L, Li M, Zheng W, Parravicini C, Ziegler J, Newton R, Rinaldo CR, Saah A, Phair J, Detels R, Chang Y, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996b;2:925. doi: 10.1038/nm0896-925. [DOI] [PubMed] [Google Scholar]
- Gao SJ, Boshoff C, Jayachandra S, Weiss RA, Chang Y, Moore PS. KSHV ORF K9 (vIRF) is an oncogene which inhibits the interferon signaling pathway. Oncogene. 1997;15:1979. doi: 10.1038/sj.onc.1201571. [DOI] [PubMed] [Google Scholar]
- Gao SJ, Deng JH, Zhou FC. Productive lytic replication of a recombinant Kaposi’s sarcoma-associated herpesvirus in efficient primary infection of primary human endothelial cells. J Virol. 2003;77:9738. doi: 10.1128/JVI.77.18.9738-9749.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber AC, Hu J, Renne R. Latency-associated nuclear antigen (LANA) cooperatively binds to two sites within the terminal repeat, and both sites contribute to the ability of LANA to suppress transcription and to facilitate DNA replication. J Biol Chem. 2002;277:27401. doi: 10.1074/jbc.M203489200. [DOI] [PubMed] [Google Scholar]
- Godden-Kent D, Talbot SJ, Boshoff C, Chang Y, Moore P, Weiss RA, Mittnacht S. The cyclin encoded by Kaposi’s sarcoma-associated herpesvirus stimulates cdk6 to phosphorylate the retinoblastoma protein and histone H1. J Virol. 1997;71:4193. doi: 10.1128/jvi.71.6.4193-4198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey A, Anderson J, Papanastasiou A, Takeuchi Y, Boshoff C. Inhibiting primary effusion lymphoma by lentiviral vectors encoding short hairpin RNA. Blood. 2005;105:2510. doi: 10.1182/blood-2004-08-3052. [DOI] [PubMed] [Google Scholar]
- Goedert JJ. The epidemiology of acquired immunodeficiency syndrome malignancies. Semin Oncol. 2000;27:390. [PubMed] [Google Scholar]
- Gottwein E, Cullen BR. A human herpesvirus microRNA inhibits p21 expression and attenuates p21-mediated cell cycle arrest. J Virol. 2010;84:5229. doi: 10.1128/JVI.00202-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradoville L, Gerlach J, Grogan E, Shedd D, Nikiforow S, Metroka C, Miller G. Kaposi’s sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic cycle in the HH-B2 primary effusion lymphoma cell line. J Virol. 2000;74:6207. doi: 10.1128/jvi.74.13.6207-6212.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene W, Gao SJ. Actin dynamics regulate multiple endosomal steps during Kaposi’s sarcoma-associated herpesvirus entry and trafficking in endothelial cells. PLoS Pathog. 2009;5:e1000512. doi: 10.1371/journal.ppat.1000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisotto MG, Garin A, Martin AP, Jensen KK, Chan P, Sealfon SC, Lira SA. The human herpesvirus 8 chemokine receptor vGPCR triggers autonomous proliferation of endothelial cells. J Clin Invest. 2006;116:1264. doi: 10.1172/JCI26666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundhoff A, Ganem D. Mechanisms governing expression of the v-FLIP gene of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2001;75:1857. doi: 10.1128/JVI.75.4.1857-1863.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundhoff A, Ganem D. Inefficient establishment of KSHV latency suggests an additional role for continued lytic replication in Kaposi sarcoma pathogenesis. J Clin Invest. 2004;113:124. doi: 10.1172/JCI200417803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasparri I, Wu H, Cesarman E. The KSHV oncoprotein vFLIP contains a TRAF-interacting motif and requires TRAF2 and TRAF3 for signalling. EMBO Rep. 2006;7:114. doi: 10.1038/sj.embor.7400580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwack Y, Byun H, Hwang S, Lim C, Choe J. CREB-binding protein and histone deacetylase regulate the transcriptional activity of Kaposi’s sarcoma-associated herpesvirus open reading frame 50. J Virol. 2001a;75:1909. doi: 10.1128/JVI.75.4.1909-1917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwack Y, Hwang S, Byun H, Lim C, Kim JW, Choi EJ, Choe J. Kaposi’s sarcoma-associated herpesvirus open reading frame 50 represses p53-induced transcriptional activity and apoptosis. J Virol. 2001b;75:6245. doi: 10.1128/JVI.75.13.6245-6248.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwack Y, Nakamura H, Lee SH, Souvlis J, Yustein JT, Gygi S, Kung HJ, Jung JU. Poly(ADP-ribose) polymerase 1 and Ste20-like kinase hKFC act as transcriptional repressors for gamma-2 herpesvirus lytic replication. Mol Cell Biol. 2003;23:8282. doi: 10.1128/MCB.23.22.8282-8294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen A, Henderson S, Lagos D, Nikitenko L, Coulter E, Roberts S, Gratrix F, Plaisance K, Renne R, Bower M, Kellam P, Boshoff C. KSHV-encoded miRNAs target MAF to induce endothelial cell reprogramming. Genes Dev. 2010;24:195. doi: 10.1101/gad.553410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque M, Chen J, Ueda K, Mori Y, Nakano K, Hirata Y, Kanamori S, Uchiyama Y, Inagi R, Okuno T, Yamanishi K. Identification and analysis of the K5 gene of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2000;74:2867. doi: 10.1128/jvi.74.6.2867-2875.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque M, Davis DA, Wang V, Widmer I, Yarchoan R. Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) contains hypoxia response elements: Relevance to lytic induction by hypoxia. J Virol. 2003;77:6761. doi: 10.1128/JVI.77.12.6761-6768.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque M, Wang V, Davis DA, Zheng ZM, Yarchoan R. Genetic organization and hypoxic activation of the Kaposi’s sarcoma-associated herpesvirus ORF34-37 gene cluster. J Virol. 2006;80:7037. doi: 10.1128/JVI.00553-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke-Gendo C, Schulz TF. Transmission and disease association of Kaposi’s sarcoma-associated herpesvirus: Recent developments. Curr Opin Infect Dis. 2004;17:53. doi: 10.1097/00001432-200402000-00011. [DOI] [PubMed] [Google Scholar]
- Hong YK, Foreman K, Shin JW, Hirakawa S, Curry CL, Sage DR, Libermann T, Dezube BJ, Fingeroth JD, Detmar M. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat Genet. 2004;36:683. doi: 10.1038/ng1383. [DOI] [PubMed] [Google Scholar]
- Hu J, Renne R. Characterization of the minimal replicator of Kaposi’s sarcoma-associated herpesvirus latent origin. J Virol. 2005;79:2637. doi: 10.1128/JVI.79.4.2637-2642.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Garber AC, Renne R. The latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus supports latent DNA replication in dividing cells. J Virol. 2002;76:11677. doi: 10.1128/JVI.76.22.11677-11687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LM, Chao MF, Chen MY, Shih H, Chiang YP, Chuang CY, Lee CY. Reciprocal regulatory interaction between human herpesvirus 8 and human immunodeficiency virus type 1. J Biol Chem. 2001;276:13427. doi: 10.1074/jbc.M011314200. [DOI] [PubMed] [Google Scholar]
- Hwang S, Gwack Y, Byun H, Lim C, Choe J. The Kaposi’s sarcoma-associated herpesvirus K8 protein interacts with CREB-binding protein (CBP) and represses CBP-mediated transcription. J Virol. 2001;75:9509. doi: 10.1128/JVI.75.19.9509-9516.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue N, Winter J, Lal RB, Offermann MK, Koyano S. Characterization of entry mechanisms of human herpesvirus 8 by using an Rta-dependent reporter cell line. J Virol. 2003;77:8147. doi: 10.1128/JVI.77.14.8147-8152.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishido S, Choi JK, Lee BS, Wang C, DeMaria M, Johnson RP, Cohen GB, Jung JU. Inhibition of natural killer cell-mediated cytotoxicity by Kaposi’s sarcoma-associated herpesvirus K5 protein. Immunity. 2000;13:365. doi: 10.1016/s1074-7613(00)00036-4. [DOI] [PubMed] [Google Scholar]
- Izumiya Y, Ellison TJ, Yeh ET, Jung JU, Luciw PA, Kung HJ. Kaposi’s sarcoma-associated herpesvirus K-bZIP represses gene transcription via SUMO modification. J Virol. 2005;79:9912. doi: 10.1128/JVI.79.15.9912-9925.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarviluoma A, Child ES, Sarek G, Sirimongkolkasem P, Peters G, Ojala PM, Mann DJ. Phosphorylation of the cyclin-dependent kinase inhibitor p21Cip1 on serine 130 is essential for viral cyclin-mediated bypass of a p21Cip1-imposed G1 arrest. Mol Cell Biol. 2006;26(6):2430–2440. doi: 10.1128/MCB.26.6.2430-2440.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J, Papin J, Dittmer D. Differential regulation of the overlapping Kaposi’s sarcoma-associated herpesvirus vGCR (orf74) and LANA (orf73) promoters. J Virol. 2001;75:1798. doi: 10.1128/JVI.75.4.1798-1807.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JU, Choi JK, Ensser A, Biesinger B. Herpesvirus saimiri as a model for gammaherpesvirus oncogenesis. Semin Cancer Biol. 1999;9:231. doi: 10.1006/scbi.1998.0115. [DOI] [PubMed] [Google Scholar]
- Kakoola DN, Sheldon J, Byabazaire N, Bowden RJ, Katongole-Mbidde E, Schulz TF, Davison AJ. Recombination in human herpesvirus-8 strains from Uganda and evolution of the K15 gene. J Gen Virol. 2001;82:2393. doi: 10.1099/0022-1317-82-10-2393. [DOI] [PubMed] [Google Scholar]
- Kaleeba JA, Berger EA. Kaposi’s sarcoma-associated herpesvirus fusion-entry receptor: Cystine transporter xCT. Science. 2006;311(5769):1921–1924. doi: 10.1126/science.1120878. [DOI] [PubMed] [Google Scholar]
- Kapadia SB, Levine B, Speck SH, Virgin HWT. Critical role of complement and viral evasion of complement in acute, persistent, and latent gamma-herpesvirus infection. Immunity. 2002;17:143. doi: 10.1016/s1074-7613(02)00369-2. [DOI] [PubMed] [Google Scholar]
- Kaposi M. Idiopathisches multiples Pigementsarkom der Haut. Arch Dermatol Syph. 1872:265. [Google Scholar]
- Kedes DH, Operskalski E, Busch M, Kohn R, Flood J, Ganem D. The seroepidemiology of human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus): Distribution of infection in KS risk groups and evidence for sexual transmission. Nat Med. 1996;2:918. doi: 10.1038/nm0896-918. [DOI] [PubMed] [Google Scholar]
- Kedes DH, Lagunoff M, Renne R, Ganem D. Identification of the gene encoding the major latency-associated nuclear antigen of the Kaposi’s sarcoma-associated herpesvirus. J Clin Invest. 1997;100:2606. doi: 10.1172/JCI119804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SA, Schattner EJ, Cesarman E. Inhibition of NF-kappaB induces apoptosis of KSHV-infected primary effusion lymphoma cells. Blood. 2000;96:2537. [PubMed] [Google Scholar]
- Keller SA, Hernandez-Hopkins D, Vider J, Ponomarev V, Hyjek E, Schattner EJ, Cesarman E. NF-kappaB is essential for the progression of KSHV-and EBV-infected lymphomas in vivo. Blood. 2006;107:3295. doi: 10.1182/blood-2005-07-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T. Three ways to make a vesicle. Nat Rev Mol Cell Biol. 2000;1:187. doi: 10.1038/35043117. [DOI] [PubMed] [Google Scholar]
- Kirchhoff S, Sebens T, Baumann S, Krueger A, Zawatzky R, Li-Weber M, Meinl E, Neipel F, Fleckenstein B, Krammer PH. Viral IFN-regulatory factors inhibit activation-induced cell death via two positive regulatory IFN-regulatory factor 1-dependent domains in the CD95 ligand promoter. J Immunol. 2002;168:1226. doi: 10.4049/jimmunol.168.3.1226. [DOI] [PubMed] [Google Scholar]
- Kirshner JR, Staskus K, Haase A, Lagunoff M, Ganem D. Expression of the open reading frame 74 (G-protein-coupled receptor) gene of Kaposi’s sarcoma (KS)-associated herpesvirus: Implications for KS pathogenesis. J Virol. 1999;73:6006. doi: 10.1128/jvi.73.7.6006-6014.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliche S, Nagel W, Kremmer E, Atzler C, Ege A, Knorr T, Koszinowski U, Kolanus W, Haas J. Signaling by human herpesvirus 8 kaposin A through direct membrane recruitment of cytohesin-1. Mol Cell. 2001;7:833. doi: 10.1016/s1097-2765(01)00227-1. [DOI] [PubMed] [Google Scholar]
- Komatsu T, Ballestas ME, Barbera AJ, Kelley-Clarke B, Kaye KM. KSHV LANA1 binds DNA as an oligomer and residues N-terminal to the oligomerization domain are essential for DNA binding, replication, and episome persistence. Virology. 2004;319:225. doi: 10.1016/j.virol.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Koyano S, Mar EC, Stamey FR, Inoue N. Glycoproteins M and N of human herpesvirus 8 form a complex and inhibit cell fusion. J Gen Virol. 2003;84:1485. doi: 10.1099/vir.0.18941-0. [DOI] [PubMed] [Google Scholar]
- Krishnan HH, Naranatt PP, Smith MS, Zeng L, Bloomer C, Chandran B. Concurrent expression of latent and a limited number of lytic genes with immune modulation and antiapoptotic function by Kaposi’s sarcoma-associated herpesvirus early during infection of primary endothelial and fibroblast cells and subsequent decline of lytic gene expression. J Virol. 2004;78:3601. doi: 10.1128/JVI.78.7.3601-3620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan HH, Sharma-Walia N, Zeng L, Gao SJ, Chandran B. Envelope glycoprotein gB of Kaposi’s sarcoma-associated herpesvirus is essential for egress from infected cells. J Virol. 2005;79:10952. doi: 10.1128/JVI.79.17.10952-10967.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krithivas A, Fujimuro M, Weidner M, Young DB, Hayward SD. Protein interactions targeting the latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus to cell chromosomes. J Virol. 2002;76:11596. doi: 10.1128/JVI.76.22.11596-11604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano S, Eizuru Y. Human I-mfa domain proteins specifically interact with KSHV LANA and affect its regulation of Wnt signaling-dependent transcription. Biochem Biophys Res Commun. 2010;396:608. doi: 10.1016/j.bbrc.2010.04.111. [DOI] [PubMed] [Google Scholar]
- Lacoste V, Judde JG, Briere J, Tulliez M, Garin B, Kassa-Kelembho E, Morvan J, Couppie P, Clyti E, Forteza Vila J, Rio B, Delmer A, et al. Molecular epidemiology of human herpesvirus 8 in Africa: Both B and A5 K1 genotypes, as well as the M and P genotypes of K14.1/K15 loci, are frequent and widespread. Virology. 2000;278:60. doi: 10.1006/viro.2000.0629. [DOI] [PubMed] [Google Scholar]
- Lagos D, Trotter MW, Vart RJ, Wang HW, Matthews NC, Hansen A, Flore O, Gotch F, Boshoff C. Kaposi sarcoma herpesvirus-encoded vFLIP and vIRF1 regulate antigen presentation in lymphatic endothelial cells. Blood. 2007;109:1550. doi: 10.1182/blood-2006-05-024034. [DOI] [PubMed] [Google Scholar]
- Lagos D, Pollara G, Henderson S, Gratrix F, Fabani M, Milne RS, Gotch F, Boshoff C. miR-132 regulates antiviral innate immunity through suppression of the p300 transcriptional co-activator. Nat Cell Biol. 2010;12:513. doi: 10.1038/ncb2054. [DOI] [PubMed] [Google Scholar]
- Lagunoff M, Majeti R, Weiss A, Ganem D. Deregulated signal transduction by the K1 gene product of Kaposi’s sarcoma-associated herpesvirus. Proc Natl Acad Sci USA. 1999;96:5704. doi: 10.1073/pnas.96.10.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunoff M, Bechtel J, Venetsanakos E, Roy AM, Abbey N, Herndier B, McMahon M, Ganem D. De novo infection and serial transmission of Kaposi’s sarcoma-associated herpesvirus in cultured endothelial cells. J Virol. 2002;76:2440. doi: 10.1128/jvi.76.5.2440-2448.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan K, Kuppers DA, Verma SC, Robertson ES. Kaposi’s sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen inhibits lytic replication by targeting Rta: A potential mechanism for virus-mediated control of latency. J Virol. 2004;78:6585. doi: 10.1128/JVI.78.12.6585-6594.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan K, Kuppers DA, Verma SC, Sharma N, Murakami M, Robertson ES. Induction of Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen by the lytic transactivator RTA: A novel mechanism for establishment of latency. J Virol. 2005;79:7453. doi: 10.1128/JVI.79.12.7453-7465.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan K, Verma SC, Murakami M, Bajaj B, Kaul R, Robertson ES. Kaposi’s sarcoma herpesvirus-encoded latency-associated nuclear antigen stabilizes intracellular activated Notch by targeting the Sel10 protein. Proc Natl Acad Sci USA. 2007;104:16287. doi: 10.1073/pnas.0703508104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Guo J, Li M, Choi JK, DeMaria M, Rosenzweig M, Jung JU. Identification of an immunoreceptor tyrosine-based activation motif of K1 transforming protein of Kaposi’s sarcoma-associated herpesvirus. Mol Cell Biol. 1998a;18:5219. doi: 10.1128/mcb.18.9.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Veazey R, Williams K, Li M, Guo J, Neipel F, Fleckenstein B, Lackner A, Desrosiers RC, Jung JU. Deregulation of cell growth by the K1 gene of Kaposi’s sarcoma-associated herpesvirus. Nat Med. 1998b;4:435. doi: 10.1038/nm0498-435. [DOI] [PubMed] [Google Scholar]
- Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1)alpha: Its protein stability and biological functions. Exp Mol Med. 2004;36:1. doi: 10.1038/emm.2004.1. [DOI] [PubMed] [Google Scholar]
- Lei X, Bai Z, Ye F, Xie J, Kim CG, Huang Y, Gao SJ. Regulation of NF-kappaB inhibitor IkappaBalpha and viral replication by a KSHV microRNA. Nat Cell Biol. 2010;12:193. doi: 10.1038/ncb2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennette ET, Blackbourn DJ, Levy JA. Antibodies to human herpesvirus type 8 in the general population and in Kaposi’s sarcoma patients. Lancet. 1996;348:858. doi: 10.1016/S0140-6736(96)03240-0. [DOI] [PubMed] [Google Scholar]
- Li M, Lee H, Yoon DW, Albrecht JC, Fleckenstein B, Neipel F, Jung JU. Kaposi’s sarcoma-associated herpesvirus encodes a functional cyclin. J Virol. 1997;71:1984. doi: 10.1128/jvi.71.3.1984-1991.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Lee H, Guo J, Neipel F, Fleckenstein B, Ozato K, Jung JU. Kaposi’s sarcoma-associated herpesvirus viral interferon regulatory factor. J Virol. 1998;72:5433. doi: 10.1128/jvi.72.7.5433-5440.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, MacKey J, Czajak SC, Desrosiers RC, Lackner AA, Jung JU. Identification and characterization of Kaposi’s sarcoma-associated herpesvirus K8.1 virion glycoprotein. J Virol. 1999;73:1341. doi: 10.1128/jvi.73.2.1341-1349.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Damania B, Alvarez X, Ogryzko V, Ozato K, Jung JU. Inhibition of p300 histone acetyltransferase by viral interferon regulatory factor. Mol Cell Biol. 2000;20:8254. doi: 10.1128/mcb.20.21.8254-8263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Komatsu T, Dezube BJ, Kaye KM. The Kaposi’s sarcoma-associated herpesvirus K12 transcript from a primary effusion lymphoma contains complex repeat elements, is spliced, and initiates from a novel promoter. J Virol. 2002;76:11880. doi: 10.1128/JVI.76.23.11880-11888.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Ganem D. RBP-J (CSL) is essential for activation of the K14/vGPCR promoter of Kaposi’s sarcoma-associated herpesvirus by the lytic switch protein RTA. J Virol. 2004;78:6818. doi: 10.1128/JVI.78.13.6818-6826.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Chang J, Lynch SJ, Lukac DM, Ganem D. The lytic switch protein of KSHV activates gene expression via functional interaction with RBP-Jkappa (CSL), the target of the Notch signaling pathway. Genes Dev. 2002;16:1977. doi: 10.1101/gad.996502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H, Winkfein RJ, Mack G, Rattner JB, Yen TJ. CENP-F is a protein of the nuclear matrix that assembles onto kinetochores at late G2 and is rapidly degraded after mitosis. J Cell Biol. 1995;130:507. doi: 10.1083/jcb.130.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C, Gwack Y, Hwang S, Kim S, Choe J. The transcriptional activity of cAMP response element-binding protein-binding protein is modulated by the latency associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus. J Biol Chem. 2001;276:31016. doi: 10.1074/jbc.M102431200. [DOI] [PubMed] [Google Scholar]
- Lim C, Sohn H, Lee D, Gwack Y, Choe J. Functional dissection of latency-associated nuclear antigen 1 of Kaposi’s sarcoma-associated herpesvirus involved in latent DNA replication and transcription of terminal repeats of the viral genome. J Virol. 2002;76:10320. doi: 10.1128/JVI.76.20.10320-10331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C, Lee D, Seo T, Choi C, Choe J. Latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus functionally interacts with heterochromatin protein 1. J Biol Chem. 2003;278:7397. doi: 10.1074/jbc.M211912200. [DOI] [PubMed] [Google Scholar]
- Lin CL, Li H, Wang Y, Zhu FX, Kudchodkar S, Yuan Y. Kaposi’s sarcoma-associated herpesvirus lytic origin (ori-Lyt)-dependent DNA replication: Identification of the ori-Lyt and association of K8 bZip protein with the origin. J Virol. 2003;77:5578. doi: 10.1128/JVI.77.10.5578-5588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YT, Kincaid RP, Arasappan D, Dowd SE, Hunicke-Smith SP, Sullivan CS. Small RNA profiling reveals antisense transcription throughout the KSHV genome and novel small RNAs. RNA. 2010;16:1540. doi: 10.1261/rna.1967910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta LA, Kohn EC. The microenvironment of the tumour–host interface. Nature. 2001;411:375. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- Liu L, Eby MT, Rathore N, Sinha SK, Kumar A, Chaudhary PM. The human herpes virus 8-encoded viral FLICE inhibitory protein physically associates with and persistently activates the Ikappa B kinase complex. J Biol Chem. 2002;277:13745. doi: 10.1074/jbc.M110480200. [DOI] [PubMed] [Google Scholar]
- Long E, Ilie M, Hofman V, Havet K, Selva E, Butori C, Lacour JP, Nelson AM, Cathomas G, Hofman P. LANA-1, Bcl-2, Mcl-1 and HIF-1alpha protein expression in HIV-associated Kaposi sarcoma. Virchows Arch. 2009;455:159. doi: 10.1007/s00428-009-0791-1. [DOI] [PubMed] [Google Scholar]
- Low P, Neipel F, Rascu A, Steininger H, Manger B, Fleckenstein B, Kalden JR, Harrer T. Suppression of HHV-8 viremia by foscarnet in an HIV-infected patient with Kaposi’s sarcoma and HHV-8 associated hemophagocytic syndrome. Eur J Med Res. 1998;3:461. [PubMed] [Google Scholar]
- Lu F, Zhou J, Wiedmer A, Madden K, Yuan Y, Lieberman PM. Chromatin remodeling of the Kaposi’s sarcoma-associated herpesvirus ORF50 promoter correlates with reactivation from latency. J Virol. 2003;77:11425. doi: 10.1128/JVI.77.21.11425-11435.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Day L, Gao SJ, Lieberman PM. Acetylation of the latency-associated nuclear antigen regulates repression of Kaposi’s sarcoma-associated herpesvirus lytic transcription. J Virol. 2006;80:5273. doi: 10.1128/JVI.02541-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Verma SC, Murakami M, Cai Q, Kumar P, Xiao B, Robertson ES. Latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus (KSHV) upregulates survivin expression in KSHV-Associated B-lymphoma cells and contributes to their proliferation. J Virol. 2009;83:7129. doi: 10.1128/JVI.00397-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Stedman W, Yousef M, Renne R, Lieberman PM. Epigenetic regulation of Kaposi’s sarcoma-associated herpesvirus latency by virus-encoded microRNAs that target Rta and the cellular Rbl2-DNMT pathway. J Virol. 2010;84:2697. doi: 10.1128/JVI.01997-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubyova B, Pitha PM. Characterization of a novel human herpesvirus 8-encoded protein, vIRF-3, that shows homology to viral and cellular interferon regulatory factors. J Virol. 2000;74:8194. doi: 10.1128/jvi.74.17.8194-8201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukac DM, Renne R, Kirshner JR, Ganem D. Reactivation of Kaposi’s sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology. 1998;252:304. doi: 10.1006/viro.1998.9486. [DOI] [PubMed] [Google Scholar]
- Lukac DM, Kirshner JR, Ganem D. Transcriptional activation by the product of open reading frame 50 of Kaposi’s sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J Virol. 1999;73:9348. doi: 10.1128/jvi.73.11.9348-9361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukac DM, Garibyan L, Kirshner JR, Palmeri D, Ganem D. DNA binding by Kaposi’s sarcoma-associated herpesvirus lytic switch protein is necessary for transcriptional activation of two viral delayed early promoters. J Virol. 2001;75:6786. doi: 10.1128/JVI.75.15.6786-6799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna RE, Zhou F, Baghian A, Chouljenko V, Forghani B, Gao SJ, Kousoulas KG. Kaposi’s sarcoma-associated herpesvirus glycoprotein K8.1 is dispensable for virus entry. J Virol. 2004;78:6389. doi: 10.1128/JVI.78.12.6389-6398.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Cavallin LE, Yan B, Zhu S, Duran EM, Wang H, Hale LP, Dong C, Cesarman E, Mesri EA, Goldschmidt-Clermont PJ. Antitumorigenesis of antioxidants in a transgenic Rac1 model of Kaposi’s sarcoma. Proc Natl Acad Sci USA. 2009;106:8683. doi: 10.1073/pnas.0812688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahotka C, Wenzel M, Springer E, Gabbert HE, Gerharz CD. Survivin-deltaEx3 and survivin-2B: Two novel splice variants of the apoptosis inhibitor survivin with different antiapoptotic properties. Cancer Res. 1999;59:6097. [PubMed] [Google Scholar]
- Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem Sci. 2005;30(11):630–641. doi: 10.1016/j.tibs.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Mann DJ, Child ES, Swanton C, Laman H, Jones N. Modulation of p27 (Kip1) levels by the cyclin encoded by Kaposi’s sarcoma-associated herpesvirus. EMBO J. 1999;18 (3):654–663. doi: 10.1093/emboj/18.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark L, Lee WH, Spiller OB, Proctor D, Blackbourn DJ, Villoutreix BO, Blom AM. The Kaposi’s sarcoma-associated herpesvirus complement control protein mimics human molecular mechanisms for inhibition of the complement system. J Biol Chem. 2004;279:45093. doi: 10.1074/jbc.M407558200. [DOI] [PubMed] [Google Scholar]
- Mark L, Lee WH, Spiller OB, Villoutreix BO, Blom AM. The Kaposi’s sarcoma-associated herpesvirus complement control protein (KCP) binds to heparin and cell surfaces via positively charged amino acids in CCP1-2. Mol Immunol. 2006;43:1665. doi: 10.1016/j.molimm.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Mark L, Spiller OB, Villoutreix BO, Blom AM. Kaposi’s sarcoma-associated herpes virus complement control protein: KCP–complement inhibition and more. Mol Immunol. 2007;44:11. doi: 10.1016/j.molimm.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Marshall V, Parks T, Bagni R, Wang CD, Samols MA, Hu J, Wyvil KM, Aleman K, Little RF, Yarchoan R, Renne R, Whitby D. Conservation of virally encoded microRNAs in Kaposi sarcoma–associated herpesvirus in primary effusion lymphoma cell lines and in patients with Kaposi sarcoma or multicentric Castleman disease. J Infect Dis. 2007;195:645. doi: 10.1086/511434. [DOI] [PubMed] [Google Scholar]
- Martin RW, Hood AF, Farmer ER. Kaposi sarcoma. Medicine (Baltimore) 1993;72:245. doi: 10.1097/00005792-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Martin JN, Ganem DE, Osmond DH, Page-Shafer KA, Macrae D, Kedes DH. Sexual transmission and the natural history of human herpesvirus 8 infection. N Engl J Med. 1998;338:948. doi: 10.1056/NEJM199804023381403. [DOI] [PubMed] [Google Scholar]
- Masood R, Cesarman E, Smith DL, Gill PS, Flore O. Human herpesvirus-8-transformed endothelial cells have functionally activated vascular endothelial growth factor/vascular endothelial growth factor receptor. Am J Pathol. 2002;160:23. doi: 10.1016/S0002-9440(10)64344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masood R, Xia G, Smith DL, Scalia P, Still JG, Tulpule A, Gill PS. Ephrin B2 expression in Kaposi sarcoma is induced by human herpesvirus type 8: Phenotype switch from venous to arterial endothelium. Blood. 2005;105:1310. doi: 10.1182/blood-2004-03-0933. [DOI] [PubMed] [Google Scholar]
- Mathew SS, Bryant PW, Burch AD. Accumulation of oxidized proteins in herpesvirus infected cells. Free Radic Biol Med. 2010;49:383. doi: 10.1016/j.freeradbiomed.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura S, Fujita Y, Gomez E, Tanese N, Wilson AC. Activation of the Kaposi’s sarcoma-associated herpesvirus major latency locus by the lytic switch protein RTA (ORF50) J Virol. 2005;79:8493. doi: 10.1128/JVI.79.13.8493-8505.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura S, Persson LM, Wong L, Wilson AC. The latency-associated nuclear antigen interacts with MeCP2 and nucleosomes through separate domains. J Virol. 2010;84:2318. doi: 10.1128/JVI.01097-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta H, Chaudhary PM. Activation of alternative NF-kappa B pathway by human herpes virus 8-encoded Fas-associated death domain-like IL-1 beta-converting enzyme inhibitory protein (vFLIP) Proc Natl Acad Sci USA. 2004;101:9399. doi: 10.1073/pnas.0308016101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta H, Sun Q, Moses G, Chaudhary PM. Molecular genetic analysis of human herpes virus 8-encoded viral FLICE inhibitory protein-induced NF-kappaB activation. J Biol Chem. 2003;278:52406. doi: 10.1074/jbc.M307308200. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- McAllister SC, Hansen SG, Ruhl RA, Raggo CM, DeFilippis VR, Greenspan D, Fruh K, Moses AV. Kaposi sarcoma-associated herpesvirus (KSHV) induces heme oxygenase-1 expression and activity in KSHV-infected endothelial cells. Blood. 2004;103:3465. doi: 10.1182/blood-2003-08-2781. [DOI] [PubMed] [Google Scholar]
- McCormick C, Ganem D. The kaposin B protein of KSHV activates the p38/MK2 pathway and stabilizes cytokine mRNAs. Science. 2005;307:739. doi: 10.1126/science.1105779. [DOI] [PubMed] [Google Scholar]
- McCormick C, Ganem D. Phosphorylation and function of the kaposin B direct repeats of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2006;80:6165. doi: 10.1128/JVI.02331-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch DJ, Davison AJ. The descent of human herpesvirus 8. Semin Cancer Biol. 1999;9:201. doi: 10.1006/scbi.1999.0093. [DOI] [PubMed] [Google Scholar]
- McPherson PS, Kay BK, Hussain NK. Signaling on the endocytic pathway. Traffic. 2001;2:375. doi: 10.1034/j.1600-0854.2001.002006375.x. [DOI] [PubMed] [Google Scholar]
- Means RE, Choi JK, Nakamura H, Chung YH, Ishido S, Jung JU. Immune evasion strategies of Kaposi’s sarcoma-associated herpesvirus. Curr Top Microbiol Immunol. 2002;269:187. doi: 10.1007/978-3-642-59421-2_12. [DOI] [PubMed] [Google Scholar]
- Melbye M, Cook PM, Hjalgrim H, Begtrup K, Simpson GR, Biggar RJ, Ebbesen P, Schulz TF. Risk factors for Kaposi’s-sarcoma-associated herpesvirus (KSHV/HHV-8) seropositivity in a cohort of homosexual men, 1981–1996. Int J Cancer. 1998;77:543. doi: 10.1002/(sici)1097-0215(19980812)77:4<543::aid-ijc12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Meng YX, Sata T, Stamey FR, Voevodin A, Katano H, Koizumi H, Deleon M, De Cristofano MA, Galimberti R, Pellett PE. Molecular characterization of strains of human herpesvirus 8 from Japan, Argentina and Kuwait. J Gen Virol. 2001;82:499. doi: 10.1099/0022-1317-82-3-499. [DOI] [PubMed] [Google Scholar]
- Merdes A, Ramyar K, Vechio JD, Cleveland DW. A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell. 1996;87:447. doi: 10.1016/s0092-8674(00)81365-3. [DOI] [PubMed] [Google Scholar]
- Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma herpesvirus/human herpesvirus-8, and the oncogenesis of Kaposi’s sarcoma. Nat Rev Cancer. 2010 doi: 10.1038/nrc2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles SA, Rezai AR, Salazar-Gonzalez JF, Vander Meyden M, Stevens RH, Logan DM, Mitsuyasu RT, Taga T, Hirano T, Kishimoto T, et al. AIDS Kaposi sarcoma-derived cells produce and respond to interleukin 6. Proc Natl Acad Sci USA. 1990;87:4068. doi: 10.1073/pnas.87.11.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocroft A, Youle M, Gazzard B, Morcinek J, Halai R, Phillips AN. Anti-herpesvirus treatment and risk of Kaposi’s sarcoma in HIV infection. Royal Free/Chelsea and Westminster Hospitals Collaborative Group. Aids. 1996;10:1101. [PubMed] [Google Scholar]
- Molden J, Chang Y, You Y, Moore PS, Goldsmith MA. A Kaposi’s sarcoma-associated herpesvirus-encoded cytokine homolog (vIL-6) activates signaling through the shared gp130 receptor subunit. J Biol Chem. 1997;272:19625. doi: 10.1074/jbc.272.31.19625. [DOI] [PubMed] [Google Scholar]
- Montaner S, Sodhi A, Pece S, Mesri EA, Gutkind JS. The Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor promotes endothelial cell survival through the activation of Akt/protein kinase B. Cancer Res. 2001;61:2641. [PubMed] [Google Scholar]
- Montaner S, Sodhi A, Molinolo A, Bugge TH, Sawai ET, He Y, Li Y, Ray PE, Gutkind JS. Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi’s sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell. 2003;3:23. doi: 10.1016/s1535-6108(02)00237-4. [DOI] [PubMed] [Google Scholar]
- Montaner S, Sodhi A, Servitja JM, Ramsdell AK, Barac A, Sawai ET, Gutkind JS. The small GTPase Rac1 links the Kaposi sarcoma-associated herpesvirus vGPCR to cytokine secretion and paracrine neoplasia. Blood. 2004;104:2903. doi: 10.1182/blood-2003-12-4436. [DOI] [PubMed] [Google Scholar]
- Montaner S, Sodhi A, Ramsdell AK, Martin D, Hu J, Sawai ET, Gutkind JS. The Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor as a therapeutic target for the treatment of Kaposi’s sarcoma. Cancer Res. 2006;66:168. doi: 10.1158/0008-5472.CAN-05-1026. [DOI] [PubMed] [Google Scholar]
- Moore PS, Chang Y. Kaposi’s sarcoma-associated herpesvirus-encoded oncogenes and oncogenesis. J Natl Cancer Inst Monogr. 1998a:65. doi: 10.1093/oxfordjournals.jncimonographs.a024176. [DOI] [PubMed] [Google Scholar]
- Moore PS, Chang Y. Antiviral activity of tumorsuppressor pathways: Clues from molecular piracy by KSHV. Trends Genet. 1998b;14:144. doi: 10.1016/s0168-9525(98)01408-5. [DOI] [PubMed] [Google Scholar]
- Moore PS, Chang Y. Molecular virology of Kaposi’s sarcoma-associated herpesvirus. Philos Trans R Soc Lond B Biol Sci. 2001;356(1408):499–516. doi: 10.1098/rstb.2000.0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PS, Chang Y. Kaposi’s sarcoma-associated herpesvirus immunoevasion and tumorigenesis: Two sides of the same coin? Annu Rev Microbiol. 2003;57:609. doi: 10.1146/annurev.micro.57.030502.090824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PS, Boshoff C, Weiss RA, Chang Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science. 1996;274:1739. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- Morris VA, Punjabi AS, Lagunoff M. Activation of Akt through gp130 receptor signaling is required for Kaposi’s sarcoma-associated herpesvirus-induced lymphatic reprogramming of endothelial cells. J Virol. 2008;82:8771. doi: 10.1128/JVI.00766-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses AV, Fish KN, Ruhl R, Smith PP, Strussenberg JG, Zhu L, Chandran B, Nelson JA. Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. J Virol. 1999;73:6892. doi: 10.1128/jvi.73.8.6892-6902.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullick J, Bernet J, Singh AK, Lambris JD, Sahu A. Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) open reading frame 4 protein (kaposica) is a functional homolog of complement control proteins. J Virol. 2003;77:3878. doi: 10.1128/JVI.77.6.3878-3881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi N, Ganju RK, Avraham S, Mesri EA, Groopman JE. Kaposi’s sarcoma-associated herpesvirus-encoded G protein-coupled receptor activation of c-jun amino-terminal kinase/stress-activated protein kinase and lyn kinase is mediated by related adhesion focal tyrosine kinase/proline-rich tyrosine kinase 2. J Biol Chem. 1999;274:31863. doi: 10.1074/jbc.274.45.31863. [DOI] [PubMed] [Google Scholar]
- Muralidhar S, Pumfery AM, Hassani M, Sadaie MR, Kishishita M, Brady JN, Doniger J, Medveczky P, Rosenthal LJ. Identification of kaposin (open reading frame K12) as a human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus) transforming gene. J Virol. 1998;72:4980. doi: 10.1128/jvi.72.6.4980-4988.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muromoto R, Okabe K, Fujimuro M, Sugiyama K, Yokosawa H, Seya T, Matsuda T. Physical and functional interactions between STAT3 and Kaposi’s sarcoma-associated herpesvirus-encoded LANA. FEBS Lett. 2006;580:93. doi: 10.1016/j.febslet.2005.11.057. [DOI] [PubMed] [Google Scholar]
- Mutlu AD, Cavallin LE, Vincent L, Chiozzini C, Eroles P, Duran EM, Asgari Z, Hooper AT, La Perle KM, Hilsher C, Gao SJ, Dittmer DP, et al. In vivo-restricted and reversible malignancy induced by human herpesvirus-8 KSHV: A cell and animal model of virally induced Kaposi’s sarcoma. Cancer Cell. 2007;11:245. doi: 10.1016/j.ccr.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nador RG, Milligan LL, Flore O, Wang X, Arvanitakis L, Knowles DM, Cesarman E. Expression of Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor monocistronic and bicistronic transcripts in primary effusion lymphomas. Virology. 2001;287:62. doi: 10.1006/viro.2001.1016. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Li M, Zarycki J, Jung JU. Inhibition of p53 tumor suppressor by viral interferon regulatory factor. J Virol. 2001;75:7572. doi: 10.1128/JVI.75.16.7572-7582.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranatt PP, Krishnan HH, Svojanovsky SR, Bloomer C, Mathur S, Chandran B. Host gene induction and transcriptional reprogramming in Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8)-infected endothelial, fibroblast, and B cells: Insights into modulation events early during infection. Cancer Res. 2004;64:72. doi: 10.1158/0008-5472.can-03-2767. [DOI] [PubMed] [Google Scholar]
- Naranatt PP, Krishnan HH, Smith MS, Chandran B. Kaposi’s sarcoma-associated herpesvirus modulates microtubule dynamics via RhoA-GTP-diaphanous 2 signaling and utilizes the dynein motors to deliver its DNA to the nucleus. J Virol. 2005;79:1191. doi: 10.1128/JVI.79.2.1191-1206.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neipel F, Albrecht JC, Ensser A, Huang YQ, Li JJ, Friedman-Kien AE, Fleckenstein B. Human herpesvirus 8 encodes a homolog of interleukin-6. J Virol. 1997a;71:839. doi: 10.1128/jvi.71.1.839-842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neipel F, Albrecht JC, Fleckenstein B. Cell-homologous genes in the Kaposi’s sarcoma-associated rhadinovirus human herpesvirus 8: Determinants of its pathogenicity? J Virol. 1997b;71:4187. doi: 10.1128/jvi.71.6.4187-4192.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HQ, Magaret AS, Kitahata MM, Van Rompaey SE, Wald A, Casper C. Persistent Kaposi sarcoma in the era of highly active antiretroviral therapy: Characterizing the predictors of clinical response. AIDS. 2008;22:937. doi: 10.1097/QAD.0b013e3282ff6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas J, Ruvolo VR, Burns WH, Sandford G, Wan X, Ciufo D, Hendrickson SB, Guo HG, Hayward GS, Reitz MS. Kaposi’s sarcoma-associated human herpesvirus-8 encodes homologues of macrophage inflammatory protein-1 and interleukin-6. Nat Med. 1997;3:287. doi: 10.1038/nm0397-287. [DOI] [PubMed] [Google Scholar]
- Niedermeier A, Talanin N, Chung EJ, Sells RE, Borris DL, Orenstein JM, Trepel JB, Blauvelt A. Histone deacetylase inhibitors induce apoptosis with minimal viral reactivation in cells infected with Kaposi’s sarcoma-associated herpesvirus. J Invest Dermatol. 2006;126(11):2516–2524. doi: 10.1038/sj.jid.5700438. [DOI] [PubMed] [Google Scholar]
- Oettle AG. Geographical and racial differences in the frequency of Kaposi’s sarcoma as evidence of environmental or genetic causes. Acta Unio Int Contra Cancrum. 1962;18:330. [PubMed] [Google Scholar]
- Orzechowska BU, Powers MF, Sprague J, Li H, Yen B, Searles RP, Axthelm MK, Wong SW. Rhesus macaque rhadinovirus-associated non-Hodgkin lymphoma: Animal model for KSHV-associated malignancies. Blood. 2008;112:4227. doi: 10.1182/blood-2008-04-151498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottinger M, Christalla T, Nathan K, Brinkmann MM, Viejo-Borbolla A, Schulz TF. Kaposi’s sarcoma-associated herpesvirus LANA-1 interacts with the short variant of BRD4 and releases cells from a BRD4- and BRD2/RING3-induced G1 cell cycle arrest. J Virol. 2006;80:10772. doi: 10.1128/JVI.00804-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Zhou F, Gao SJ. Kaposi’s sarcoma-associated herpesvirus induction of chromosome instability in primary human endothelial cells. Cancer Res. 2004;64:4064. doi: 10.1158/0008-5472.CAN-04-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Xie J, Ye F, Gao SJ. Modulation of Kaposi’s sarcoma-associated herpesvirus infection and replication by MEK/ERK, JNK, and p38 multiple mitogen-activated protein kinase pathways during primary infection. J Virol. 2006;80:5371. doi: 10.1128/JVI.02299-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parravicini C, Corbellino M, Paulli M, Magrini U, Lazzarino M, Moore PS, Chang Y. Expression of a virus-derived cytokine, KSHV vIL-6, in HIV-seronegative Castleman’s disease. Am J Pathol. 1997;151:1517. [PMC free article] [PubMed] [Google Scholar]
- Parsons CH, Adang LA, Overdevest J, O’Connor CM, Taylor JR, Jr, Camerini D, Kedes DH. KSHV targets multiple leukocyte lineages during long-term productive infection in NOD/SCID mice. J Clin Invest. 2006;116:1963. doi: 10.1172/JCI27249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore RD, Chadburn A, Kripas C, Schattner EJ. Novel association of haemophagocytic syndrome with Kaposi’s sarcoma-associated herpesvirus-related primary effusion lymphoma. Br J Haematol. 2000;111:1112. doi: 10.1046/j.1365-2141.2000.02478.x. [DOI] [PubMed] [Google Scholar]
- Penn I. Kaposi’s sarcoma in immunosuppressed patients. J Clin Lab Immunol. 1983;12:1. [PubMed] [Google Scholar]
- Persson LM, Wilson AC. Wide-scale use of Notch signaling factor CSL/RBP-Jkappa in RTA-mediated activation of Kaposi’s sarcoma-associated herpesvirus lytic genes. J Virol. 2010;84:1334. doi: 10.1128/JVI.01301-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertel PE. Human herpesvirus 8 glycoprotein B (gB), gH, and gL can mediate cell fusion. J Virol. 2002;76:4390. doi: 10.1128/JVI.76.9.4390-4400.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picchio GR, Sabbe RE, Gulizia RJ, McGrath M, Herndier BG, Mosier DE. The KSHV/HHV8-infected BCBL-1 lymphoma line causes tumors in SCID mice but fails to transmit virus to a human peripheral blood mononuclear cell graft. Virology. 1997;238:22. doi: 10.1006/viro.1997.8822. [DOI] [PubMed] [Google Scholar]
- Ploegh HL. Viral strategies of immune evasion. Science. 1998;280:248. doi: 10.1126/science.280.5361.248. [DOI] [PubMed] [Google Scholar]
- Polster BM, Pevsner J, Hardwick JM. Viral Bcl-2 homologs and their role in virus replication and associated diseases. Biochim Biophys Acta. 2004;1644:211. doi: 10.1016/j.bbamcr.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Polstra AM, Cornelissen M, Goudsmit J, van der Kuyl AC. Retrospective, longitudinal analysis of serum human herpesvirus-8 viral DNA load in AIDS-related Kaposi’s sarcoma patients before and after diagnosis. J Med Virol. 2004;74:390. doi: 10.1002/jmv.20192. [DOI] [PubMed] [Google Scholar]
- Poole LJ, Zong JC, Ciufo DM, Alcendor DJ, Cannon JS, Ambinder R, Orenstein JM, Reitz MS, Hayward GS. Comparison of genetic variability at multiple loci across the genomes of the major subtypes of Kaposi’s sarcoma-associated herpesvirus reveals evidence for recombination and for two distinct types of open reading frame K15 alleles at the right-hand end. J Virol. 1999;73:6646. doi: 10.1128/jvi.73.8.6646-6660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu NC, Zimonjic DB, Leventon-Kriss S, Bryant JL, Lunardi-Iskandar Y, Gallo RC. Deletion and translocation involving chromosome 3 (p14) in two tumorigenic Kaposi’s sarcoma cell lines. J Natl Cancer Inst. 1996;88:450. doi: 10.1093/jnci/88.7.450. [DOI] [PubMed] [Google Scholar]
- Punj V, Matta H, Schamus S, Tamewitz A, Anyang B, Chaudhary PM. Kaposi’s sarcoma-associated herpesvirus-encoded viral FLICE inhibitory protein (vFLIP) K13 suppresses CXCR4 expression by upregulating miR-146a. Oncogene. 2010;29:1835. doi: 10.1038/onc.2009.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punjabi AS, Carroll PA, Chen L, Lagunoff M. Persistent activation of STAT3 by latent Kaposi’s sarcoma-associated herpesvirus infection of endothelial cells. J Virol. 2007;81:2449. doi: 10.1128/JVI.01769-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, DeFee M, Isaacs JS, Parsons C. Extracellular Hsp90 serves as a co-factor for MAPK activation and latent viral gene expression during de novo infection by KSHV. Virology. 2010a;403:92. doi: 10.1016/j.virol.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, Freitas E, Sullivan R, Mohan S, Bacelieri R, Branch D, Romano M, Kearney P, Oates J, Plaisance K, Renne R, Kaleeba J, et al. Upregulation of xCT by KSHV-encoded microRNAs facilitates KSHV dissemination and persistence in an environment of oxidative stress. PLoS Pathog. 2010b;6:e1000742. doi: 10.1371/journal.ppat.1000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radkov SA, Kellam P, Boshoff C. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nat Med. 2000;6:1121. doi: 10.1038/80459. [DOI] [PubMed] [Google Scholar]
- Raghu H, Sharma-Walia N, Veettil MV, Sadagopan S, Caballero A, Sivakumar R, Varga L, Bottero V, Chandran B. Lipid rafts of primary endothelial cells are essential for Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8-induced phosphatidylinositol 3-kinase and RhoA-GTPases critical for microtubule dynamics and nuclear delivery of viral DNA but dispensable for binding and entry. J Virol. 2007;81:7941. doi: 10.1128/JVI.02848-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu H, Sharma-Walia N, Veettil MV, Sadagopan S, Chandran B. Kaposi’s sarcoma-associated herpesvirus utilizes an actin polymerization-dependent macropinocytic pathway to enter human dermal microvascular endothelial and human umbilical vein endothelial cells. J Virol. 2009;83:4895. doi: 10.1128/JVI.02498-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbow L, Platt GM, Simpson GR, Sarid R, Gao SJ, Stoiber H, Herrington CS, Moore PS, Schulz TF. The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by orf73 and is a component of the latency-associated nuclear antigen. J Virol. 1997;71:5915. doi: 10.1128/jvi.71.8.5915-5921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappocciolo G, Jenkins FJ, Hensler HR, Piazza P, Jais M, Borowski L, Watkins SC, Rinaldo CR., Jr DC-SIGN is a receptor for human herpesvirus 8 on dendritic cells and macrophages. J Immunol. 2006;176:1741. doi: 10.4049/jimmunol.176.3.1741. [DOI] [PubMed] [Google Scholar]
- Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q, Dillehay LE, Madan A, Semenza GL, Bedi A. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 2000;14:34. [PMC free article] [PubMed] [Google Scholar]
- Regamey N, Tamm M, Wernli M, Witschi A, Thiel G, Cathomas G, Erb P. Transmission of human herpesvirus 8 infection from renal-transplant donors to recipients. N Engl J Med. 1998;339:1358. doi: 10.1056/NEJM199811053391903. [DOI] [PubMed] [Google Scholar]
- Renne R, Lagunoff M, Zhong W, Ganem D. The size and conformation of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) DNA in infected cells and virions. J Virol. 1996a;70:8151. doi: 10.1128/jvi.70.11.8151-8154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Lytic growth of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med. 1996b;2:342. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- Rivas C, Thlick AE, Parravicini C, Moore PS, Chang Y. Kaposi’s sarcoma-associated herpesvirus LANA2 is a B-cell-specific latent viral protein that inhibits p53. J Virol. 2001;75:429. doi: 10.1128/JVI.75.1.429-438.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles R, Lugo D, Gee L, Jacobson MA. Effect of antiviral drugs used to treat cytomegalovirus endorgan disease on subsequent course of previously diagnosed Kaposi’s sarcoma in patients with AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:34. doi: 10.1097/00042560-199901010-00005. [DOI] [PubMed] [Google Scholar]
- Roupelieva M, Griffiths SJ, Kremmer E, Meisterernst M, Viejo-Borbolla A, Schulz T, Haas J. Kaposi’s sarcoma-associated herpesvirus Lana-1 is a major activator of the serum response element and mitogen-activated protein kinase pathways via interactions with the mediator complex. J Gen Virol. 2010;91:1138. doi: 10.1099/vir.0.017715-0. [DOI] [PubMed] [Google Scholar]
- Russo JJ, Bohenzky RA, Chien MC, Chen J, Yan M, Maddalena D, Parry JP, Peruzzi D, Edelman IS, Chang Y, Moore PS. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadagopan S, Sharma-Walia N, Veettil MV, Bottero V, Levine R, Vart RJ, Chandran B. Kaposi’s sarcoma-associated herpesvirus upregulates angiogenin during infection of human dermal microvascular endothelial cells, which induces 45S rRNA synthesis, antiapoptosis, cell proliferation, migration, and angiogenesis. J Virol. 2009;83 (7):3342–3364. doi: 10.1128/JVI.02052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler R, Wu L, Forghani B, Renne R, Zhong W, Herndier B, Ganem D. A complex translational program generates multiple novel proteins from the latently expressed kaposin (K12) locus of Kaposi’s sarcoma-associated herpesvirus. J Virol. 1999;73:5722. doi: 10.1128/jvi.73.7.5722-5730.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara S, Ueda K, Chen J, Okuno T, Yamanishi K. Octamer-binding sequence is a key element for the autoregulation of Kaposi’s sarcoma-associated herpesvirus ORF50/Lyta gene expression. J Virol. 2001;75:6894. doi: 10.1128/JVI.75.15.6894-6900.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaniego F, Pati S, Karp JE, Prakash O, Bose D. Human herpesvirus 8 K1-associated nuclear factor-kappaB-dependent promoter activity: Role in Kaposi’s sarcoma inflammation? J Natl Cancer Inst Monogr. 2001:65. doi: 10.1093/oxfordjournals.jncimonographs.a024252. [DOI] [PubMed] [Google Scholar]
- Samaniego F, Young D, Grimes C, Prospero V, Christofidou-Solomidou M, DeLisser HM, Prakash O, Sahin AA, Wang S. Vascular endothelial growth factor and Kaposi’s sarcoma cells in human skin grafts. Cell Growth Differ. 2002;13:387. [PubMed] [Google Scholar]
- Sarek G, Jarviluoma A, Ojala PM. KSHV viral cyclin inactivates p27KIP1 through Ser10 and Thr187 phosphorylation in proliferating primary effusion lymphomas. Blood. 2006;107:725. doi: 10.1182/blood-2005-06-2534. [DOI] [PubMed] [Google Scholar]
- Sarid R, Sato T, Bohenzky RA, Russo JJ, Chang Y. Kaposi’s sarcoma-associated herpesvirus encodes a functional bcl-2 homologue. Nat Med. 1997;3:293. doi: 10.1038/nm0397-293. [DOI] [PubMed] [Google Scholar]
- Sarid R, Flore O, Bohenzky RA, Chang Y, Moore PS. Transcription mapping of the Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1) J Virol. 1998;72:1005. doi: 10.1128/jvi.72.2.1005-1012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarid R, Wiezorek JS, Moore PS, Chang Y. Characterization and cell cycle regulation of the major Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) latent genes and their promoter. J Virol. 1999;73(2):1438–1446. doi: 10.1128/jvi.73.2.1438-1446.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarosiek KA, Cavallin LE, Bhatt S, Toomey NL, Natkunam Y, Blasini W, Gentles AJ, Ramos JC, Mesri EA, Lossos IS. Efficacy of bortezomib in a direct xenograft model of primary effusion lymphoma. Proc Natl Acad Sci USA. 2010;107:13069. doi: 10.1073/pnas.1002985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TF. The pleiotropic effects of Kaposi’s sarcoma herpesvirus. J Pathol. 2006;208:187. doi: 10.1002/path.1904. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Murphy PM. Kaposi’s sarcoma-associated herpesvirus G protein-coupled receptor constitutively activates NF-kappa B and induces proinflammatory cytokine and chemokine production via a C-terminal signaling determinant. J Immunol. 2001;167:505. doi: 10.4049/jimmunol.167.1.505. [DOI] [PubMed] [Google Scholar]
- Seagroves TN, Ryan HE, Lu H, Wouters BG, Knapp M, Thibault P, Laderoute K, Johnson RS. Transcription factor HIF-1 is a necessary mediator of the pasteur effect in mammalian cells. Mol Cell Biol. 2001;21:3436. doi: 10.1128/MCB.21.10.3436-3444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo T, Lee D, Lee B, Chung JH, Choe J. Viral interferon regulatory factor 1 of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) binds to, and inhibits transactivation of, CREB-binding protein. Biochem Biophys Res Commun. 2000;270:23. doi: 10.1006/bbrc.2000.2393. [DOI] [PubMed] [Google Scholar]
- Seo T, Park J, Lee D, Hwang SG, Choe J. Viral interferon regulatory factor 1 of Kaposi’s sarcoma-associated herpesvirus binds to p53 and represses p53-dependent transcription and apoptosis. J Virol. 2001;75:6193. doi: 10.1128/JVI.75.13.6193-6198.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo T, Lee D, Shim YS, Angell JE, Chidambaram NV, Kalvakolanu DV, Choe J. Viral interferon regulatory factor 1 of Kaposi’s sarcoma-associated herpesvirus interacts with a cell death regulator, GRIM19, and inhibits interferon/retinoic acid-induced cell death. J Virol. 2002;76:8797. doi: 10.1128/JVI.76.17.8797-8807.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma-Walia N, Naranatt PP, Krishnan HH, Zeng L, Chandran B. Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 envelope glycoprotein gB induces the integrin-dependent focal adhesion kinase-Src-phosphatidylinositol 3-kinase-rho GTPase signal pathways and cytoskeletal rearrangements. J Virol. 2004;78:4207. doi: 10.1128/JVI.78.8.4207-4223.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma-Walia N, Krishnan HH, Naranatt PP, Zeng L, Smith MS, Chandran B. ERK1/2 and MEK1/2 induced by Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) early during infection of target cells are essential for expression of viral genes and for establishment of infection. J Virol. 2005;79:10308. doi: 10.1128/JVI.79.16.10308-10329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma-Walia N, Raghu H, Sadagopan S, Sivakumar R, Veettil MV, Naranatt PP, Smith MM, Chandran B. Cyclooxygenase 2 induced by Kaposi’s sarcoma-associated herpesvirus early during in vitro infection of target cells plays a role in the maintenance of latent viral gene expression. J Virol. 2006;80:6534. doi: 10.1128/JVI.00231-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma-Walia N, Paul AG, Bottero V, Sadagopan S, Veettil MV, Kerur N, Chandran B. Kaposi’s sarcoma associated herpes virus (KSHV) induced COX-2: A key factor in latency, inflammation, angiogenesis, cell survival and invasion. PLoS Pathog. 2010a;6:e1000777. doi: 10.1371/journal.ppat.1000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma-Walia N, Paul AG, Bottero V, Sadagopan S, Veettil MV, Kerur N, Chandran B. Kaposi’s sarcoma associated herpes virus (KSHV) induced COX-2: A key factor in latency, inflammation, angiogenesis, cell survival and invasion. PLoS Pathog. 2010b;6:e1000777. doi: 10.1371/journal.ppat.1000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YC, Nakamura H, Liang X, Feng P, Chang H, Kowalik TF, Jung JU. Inhibition of the ATM/p53 signal transduction pathway by Kaposi’s sarcoma-associated herpesvirus interferon regulatory factor 1. J Virol. 2006;80:2257. doi: 10.1128/JVI.80.5.2257-2266.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YC, Joo CH, Gack MU, Lee HR, Jung JU. Kaposi’s sarcoma-associated herpesvirus viral IFN regulatory factor 3 stabilizes hypoxia-inducible factor-1 alpha to induce vascular endothelial growth factor expression. Cancer Res. 2008;68:1751. doi: 10.1158/0008-5472.CAN-07-2766. [DOI] [PubMed] [Google Scholar]
- Shinohara H, Fukushi M, Higuchi M, Oie M, Hoshi O, Ushiki T, Hayashi J, Fujii M. Chromosome binding site of latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus is essential for persistent episome maintenance and is functionally replaced by histone H1. J Virol. 2002;76(24):12917–12924. doi: 10.1128/JVI.76.24.12917-12924.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si H, Robertson ES. Kaposi’s sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen induces chromosomal instability through inhibition of p53 function. J Virol. 2006;80:697. doi: 10.1128/JVI.80.2.697-709.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si H, Verma SC, Lampson MA, Cai Q, Robertson ES. Kaposi’s sarcoma-associated herpesvirus-encoded LANA can interact with the nuclear mitotic apparatus protein to regulate genome maintenance and segregation. J Virol. 2008;82:6734. doi: 10.1128/JVI.00342-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieczkarski SB, Whittaker GR. Dissecting virus entry via endocytosis. J Gen Virol. 2002;83:1535. doi: 10.1099/0022-1317-83-7-1535. [DOI] [PubMed] [Google Scholar]
- Simpson GR, Schulz TF, Whitby D, Cook PM, Boshoff C, Rainbow L, Howard MR, Gao SJ, Bohenzky RA, Simmonds P, Lee C, de Ruiter A, et al. Prevalence of Kaposi’s sarcoma associated herpesvirus infection measured by antibodies to recombinant capsid protein and latent immunofluorescence antigen. Lancet. 1996;348:1133. doi: 10.1016/S0140-6736(96)07560-5. [DOI] [PubMed] [Google Scholar]
- Skalsky RL, Hu J, Renne R. Analysis of viral cis elements conferring Kaposi’s sarcoma-associated herpesvirus episome partitioning and maintenance. J Virol. 2007;81:9825. doi: 10.1128/JVI.00842-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi A, Montaner S, Patel V, Zohar M, Bais C, Mesri EA, Gutkind JS. The Kaposi’s sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1alpha. Cancer Res. 2000;60:4873. [PubMed] [Google Scholar]
- Sodhi A, Montaner S, Gutkind JS. Does dysregulated expression of a deregulated viral GPCR trigger Kaposi’s sarcomagenesis? FASEB J. 2004a;18:422. doi: 10.1096/fj.03-1035hyp. [DOI] [PubMed] [Google Scholar]
- Sodhi A, Montaner S, Patel V, Gomez-Roman JJ, Li Y, Sausville EA, Sawai ET, Gutkind JS. Akt plays a central role in sarcomagenesis induced by Kaposi’s sarcoma herpesvirus-encoded G protein-coupled receptor. Proc Natl Acad Sci USA. 2004b;101:4821. doi: 10.1073/pnas.0400835101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MJ, Brown HJ, Wu TT, Sun R. Transcription activation of polyadenylated nuclear rna by rta in human herpesvirus 8/Kaposi’s sarcoma-associated herpesvirus. J Virol. 2001;75:3129. doi: 10.1128/JVI.75.7.3129-3140.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MJ, Li X, Brown HJ, Sun R. Characterization of interactions between RTA and the promoter of polyadenylated nuclear RNA in Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8. J Virol. 2002;76:5000. doi: 10.1128/JVI.76.10.5000-5013.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MJ, Deng H, Sun R. Comparative study of regulation of RTA-responsive genes in Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8. J Virol. 2003;77:9451. doi: 10.1128/JVI.77.17.9451-9462.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d’Agay MF, Clauvel JP, Raphael M, Degos L, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86:1276. [PubMed] [Google Scholar]
- Spear PG, Longnecker R. Herpesvirus entry: An update. J Virol. 2003;77:10179. doi: 10.1128/JVI.77.19.10179-10185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller OB, Blackbourn DJ, Mark L, Proctor DG, Blom AM. Functional activity of the complement regulator encoded by Kaposi’s sarcoma-associated herpesvirus. J Biol Chem. 2003;278:9283. doi: 10.1074/jbc.m211579200. [DOI] [PubMed] [Google Scholar]
- Spiller OB, Mark L, Blue CE, Proctor DG, Aitken JA, Blom AM, Blackbourn DJ. Dissecting the regions of virion-associated Kaposi’s sarcoma-associated herpesvirus complement control protein required for complement regulation and cell binding. J Virol. 2006;80:4068. doi: 10.1128/JVI.80.8.4068-4078.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan V, Komatsu T, Ballestas ME, Kaye KM. Definition of sequence requirements for latency-associated nuclear antigen 1 binding to Kaposi’s sarcoma-associated herpesvirus DNA. J Virol. 2004;78:14033. doi: 10.1128/JVI.78.24.14033-14038.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskus KA, Zhong W, Gebhard K, Herndier B, Wang H, Renne R, Beneke J, Pudney J, Anderson DJ, Ganem D, Haase AT. Kaposi’s sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J Virol. 1997;71:715. doi: 10.1128/jvi.71.1.715-719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt MR, Dittmer DP. Viral latent proteins as targets for Kaposi’s sarcoma and Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) induced lymphoma. Curr Drug Targets Infect Disord. 2003;3:129. doi: 10.2174/1568005033481150. [DOI] [PubMed] [Google Scholar]
- Stedman W, Deng Z, Lu F, Lieberman PM. ORC, MCM, and histone hyperacetylation at the Kaposi’s sarcoma-associated herpesvirus latent replication origin. J Virol. 2004;78(22):12566–12575. doi: 10.1128/JVI.78.22.12566-12575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman W, Kang H, Lin S, Kissil JL, Bartolomei MS, Lieberman PM. Cohesins localize with CTCF at the KSHV latency control region and at cellular c-myc and H19/Igf2 insulators. EMBO J. 2008;27:654. doi: 10.1038/emboj.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine JT, Wood C, Hill M, Epp A, Raport CJ, Schweickart VL, Endo Y, Sasaki T, Simmons G, Boshoff C, Clapham P, Chang Y, et al. KSHV-encoded CC chemokine vMIP-III is a CCR4 agonist, stimulates angiogenesis, and selectively chemoat-tracts TH2 cells. Blood. 2000;95:1151. [PubMed] [Google Scholar]
- Sullivan R, Dezube BJ, Koon HB. Signal transduction targets in Kaposi’s sarcoma. Curr Opin Oncol. 2006;18:456. doi: 10.1097/01.cco.0000239884.05914.13. [DOI] [PubMed] [Google Scholar]
- Sullivan RJ, Pantanowitz L, Dezube BJ. Targeted therapy for Kaposi sarcoma. BioDrugs. 2009;23:69. doi: 10.2165/00063030-200923020-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R, Lin SF, Gradoville L, Yuan Y, Zhu F, Miller G. A viral gene that activates lytic cycle expression of Kaposi’s sarcoma-associated herpesvirus. Proc Natl Acad Sci USA. 1998;95:10866. doi: 10.1073/pnas.95.18.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R, Lin SF, Staskus K, Gradoville L, Grogan E, Haase A, Miller G. Kinetics of Kaposi’s sarcoma-associated herpesvirus gene expression. J Virol. 1999;73:2232. doi: 10.1128/jvi.73.3.2232-2242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Zachariah S, Chaudhary PM. The human herpes virus 8-encoded viral FLICE-inhibitory protein induces cellular transformation via NF-kappaB activation. J Biol Chem. 2003;278:52437. doi: 10.1074/jbc.M304199200. [DOI] [PubMed] [Google Scholar]
- Sun Q, Matta H, Chaudhary PM. Kaposi’s sarcoma associated herpes virus-encoded viral FLICE inhibitory protein activates transcription from HIV-1 long terminal repeat via the classical NF-kappaB pathway and functionally cooperates with Tat. Retrovirology. 2005;2:9. doi: 10.1186/1742-4690-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Matta H, Lu G, Chaudhary PM. Induction of IL-8 expression by human herpesvirus 8 encoded vFLIP K13 via NF-kappaB activation. Oncogene. 2006;25:2717. doi: 10.1038/sj.onc.1209298. [DOI] [PubMed] [Google Scholar]
- Vanni T, Sprinz E, Machado MW, Santana Rde C, Fonseca BA, Schwartsmann G. Systemic treatment of AIDS-related Kaposi sarcoma: Current status and perspectives. Cancer Treat Rev. 2006;32:445. doi: 10.1016/j.ctrv.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Verma SC, Borah S, Robertson ES. Latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus up-regulates transcription of human telomerase reverse transcriptase promoter through interaction with transcription factor Sp1. J Virol. 2004;78:10348. doi: 10.1128/JVI.78.19.10348-10359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma SC, Choudhuri T, Kaul R, Robertson ES. Latency-associated nuclear antigen (LANA) of Kaposi’s sarcoma-associated herpesvirus interacts with origin recognition complexes at the LANA binding sequence within the terminal repeats. J Virol. 2006;80:2243. doi: 10.1128/JVI.80.5.2243-2256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma SC, Lan K, Choudhuri T, Cotter MA, Robertson ES. An autonomous replicating element within the KSHV genome. Cell Host Microbe. 2007a;2:106. doi: 10.1016/j.chom.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma SC, Lan K, Robertson E. Structure and function of latency-associated nuclear antigen. Curr Top Microbiol Immunol. 2007b;312:101. doi: 10.1007/978-3-540-34344-8_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viejo-Borbolla A, Ottinger M, Bruning E, Burger A, Konig R, Kati E, Sheldon JA, Schulz TF. Brd2/RING3 interacts with a chromatin-binding domain in the Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen 1 (LANA-1) that is required for multiple functions of LANA-1. J Virol. 2005;79:13618. doi: 10.1128/JVI.79.21.13618-13629.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin HWT, Latreille P, Wamsley P, Hallsworth K, Weck KE, Dal Canto AJ, Speck SH. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J Virol. 1997;71:5894. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wabinga HR, Parkin DM, Wabwire-Mangen F, Mugerwa JW. Cancer in Kampala, Uganda, in 1989–91: Changes in incidence in the era of AIDS. Int J Cancer. 1993;54:26. doi: 10.1002/ijc.2910540106. [DOI] [PubMed] [Google Scholar]
- Wang L, Damania B. Kaposi’s sarcoma-associated herpesvirus confers a survival advantage to endothelial cells. Cancer Res. 2008;68:4640. doi: 10.1158/0008-5472.CAN-07-5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HW, Sharp TV, Koumi A, Koentges G, Boshoff C. Characterization of an anti-apoptotic glycoprotein encoded by Kaposi’s sarcoma-associated herpesvirus which resembles a spliced variant of human survivin. EMBO J. 2002;21:2602. doi: 10.1093/emboj/21.11.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SE, Wu FY, Yu Y, Hayward GS. CCAAT/enhancer-binding protein-alpha is induced during the early stages of Kaposi’s sarcoma-associated herpesvirus (KSHV) lytic cycle reactivation and together with the KSHV replication and transcription activator (RTA) cooperatively stimulates the viral RTA, MTA, and PAN promoters. J Virol. 2003;77:9590. doi: 10.1128/JVI.77.17.9590-9612.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HW, Trotter MW, Lagos D, Bourboulia D, Henderson S, Makinen T, Elliman S, Flanagan AM, Alitalo K, Boshoff C. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat Genet. 2004a;36:687. doi: 10.1038/ng1384. [DOI] [PubMed] [Google Scholar]
- Wang L, Wakisaka N, Tomlinson CC, DeWire SM, Krall S, Pagano JS, Damania B. The Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) K1 protein induces expression of angiogenic and invasion factors. Cancer Res. 2004b;64:2774. doi: 10.1158/0008-5472.can-03-3653. [DOI] [PubMed] [Google Scholar]
- Wang SE, Wu FY, Chen H, Shamay M, Zheng Q, Hayward GS. Early activation of the Kaposi’s sarcoma-associated herpesvirus RTA, RAP, and MTA promoters by the tetradecanoyl phorbol acetate-induced AP1 pathway. J Virol. 2004c;78:4248. doi: 10.1128/JVI.78.8.4248-4267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YF, Chen CY, Chung SF, Chiou YH, Lo HR. Involvement of oxidative stress and caspase activation in paclitaxel-induced apoptosis of primary effusion lymphoma cells. Cancer Chemother Pharmacol. 2004d;54:322. doi: 10.1007/s00280-004-0831-0. [DOI] [PubMed] [Google Scholar]
- Wang L, Dittmer DP, Tomlinson CC, Fakhari FD, Damania B. Immortalization of primary endothelial cells by the K1 protein of Kaposi’s sarcoma-associated herpesvirus. Cancer Res. 2006;66:3658. doi: 10.1158/0008-5472.CAN-05-3680. [DOI] [PubMed] [Google Scholar]
- Weindel K, Marme D, Weich HA. AIDS-associated Kaposi’s sarcoma cells in culture express vascular endothelial growth factor. Biochem Biophys Res Commun. 1992;183:1167. doi: 10.1016/s0006-291x(05)80313-4. [DOI] [PubMed] [Google Scholar]
- West JT, Wood C. The role of Kaposi’s sarcoma-associated herpesvirus/human herpesvirus-8 regulator of transcription activation (RTA) in control of gene expression. Oncogene. 2003;22:5150. doi: 10.1038/sj.onc.1206555. [DOI] [PubMed] [Google Scholar]
- Whitby D, Luppi M, Barozzi P, Boshoff C, Weiss RA, Torelli G. Human herpesvirus 8 seroprevalence in blood donors and lymphoma patients from different regions of Italy. J Natl Cancer Inst. 1998;90:395. doi: 10.1093/jnci/90.5.395. [DOI] [PubMed] [Google Scholar]
- Wong EL, Damania B. Transcriptional regulation of the Kaposi’s sarcoma-associated herpesvirus K15 gene. J Virol. 2006;80:1385. doi: 10.1128/JVI.80.3.1385-1392.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SW, Bergquam EP, Swanson RM, Lee FW, Shiigi SM, Avery NA, Fanton JW, Axthelm MK. Induction of B cell hyperplasia in simian immunodeficiency virus-infected rhesus macaques with the simian homologue of Kaposi’s sarcoma-associated herpesvirus. J Exp Med. 1999;190:827. doi: 10.1084/jem.190.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Verma SC, Cai Q, Kaul R, Lu J, Saha A, Robertson ES. Bub1 and CENP-F can contribute to KSHV genome persistence by targeting LANA to kinetochores. J Virol. 2010 doi: 10.1128/JVI.00713-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Pan H, Yoo S, Gao SJ. Kaposi’s sarcoma-associated herpesvirus induction of AP-1 and interleukin 6 during primary infection mediated by multiple mitogen-activated protein kinase pathways. J Virol. 2005;79:15027. doi: 10.1128/JVI.79.24.15027-15037.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Ajibade AO, Ye F, Kuhne K, Gao SJ. Reactivation of Kaposi’s sarcoma-associated herpesvirus from latency requires MEK/ERK, JNK and p38 multiple mitogen-activated protein kinase pathways. Virology. 2008;371:139. doi: 10.1016/j.virol.2007.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye FC, Zhou FC, Yoo SM, Xie JP, Browning PJ, Gao SJ. Disruption of Kaposi’s sarcoma-associated herpesvirus latent nuclear antigen leads to abortive episome persistence. J Virol. 2004;78:11121. doi: 10.1128/JVI.78.20.11121-11129.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Shedd D, Miller G. An Sp1 response element in the Kaposi’s sarcoma-associated herpesvirus open reading frame 50 promoter mediates lytic cycle induction by butyrate. J Virol. 2005;79:1397. doi: 10.1128/JVI.79.3.1397-1408.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J, Srinivasan V, Denis GV, Harrington WJ, Jr, Ballestas ME, Kaye KM, Howley PM. Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen interacts with bromodomain protein Brd4 on host mitotic chromosomes. J Virol. 2006;80:8909. doi: 10.1128/JVI.00502-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Harada JN, Brown HJ, Deng H, Song MJ, Wu TT, Kato-Stankiewicz J, Nelson CG, Vieira J, Tamanoi F, Chanda SK, Sun R. Systematic identification of cellular signals reactivating Kaposi sarcoma-associated herpesvirus. PLoS Pathog. 2007;3:e44. doi: 10.1371/journal.ppat.0030044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagzag D, Zhong H, Scalzitti JM, Laughner E, Simons JW, Semenza GL. Expression of hypoxia-inducible factor 1alpha in brain tumors: Association with angiogenesis, invasion, and progression. Cancer. 2000;88:2606. [PubMed] [Google Scholar]
- Zeng Y, Zhang X, Huang Z, Cheng L, Yao S, Qin D, Chen X, Tang Q, Lv Z, Zhang L, Lu C. Intracellular Tat of human immunodeficiency virus type 1 activates lytic cycle replication of Kaposi’s sarcoma-associated herpesvirus: Role of JAK/STAT signaling. J Virol. 2007;81:2401. doi: 10.1128/JVI.02024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chiu J, Lin JC. Activation of human herpesvirus 8 (HHV-8) thymidine kinase (TK) TATAA-less promoter by HHV-8 ORF50 gene product is SP1 dependent. DNA Cell Biol. 1998;17:735. doi: 10.1089/dna.1998.17.735. [DOI] [PubMed] [Google Scholar]
- Zhong W, Wang H, Herndier B, Ganem D. Restricted expression of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi sarcoma. Proc Natl Acad Sci USA. 1996;93:6641. doi: 10.1073/pnas.93.13.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830. [PubMed] [Google Scholar]
- Zhu FX, Chong JM, Wu L, Yuan Y. Virion proteins of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2005;79:800. doi: 10.1128/JVI.79.2.800-811.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler JL, Katongole-Mbidde E. Kaposi’s sarcoma in childhood: An analysis of 100 cases from Uganda and relationship to HIV infection. Int J Cancer. 1996;65:200. doi: 10.1002/(SICI)1097-0215(19960117)65:2<200::AID-IJC12>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]