Abstract
Recently, we proposed a new mechanism for understanding the Warburg effect in cancer metabolism. In this new paradigm, cancer-associated fibroblasts undergo aerobic glycolysis, and extrude lactate to “feed” adjacent cancer cells, which then drives mitochondrial biogenesis and oxidative mitochondrial metabolism in cancer cells. Thus, there is vectorial transport of energy-rich substrates from the fibroblastic tumor stroma to anabolic cancer cells. A prediction of this hypothesis is that cancer-associated fibroblasts should express MCT4, a mono-carboxylate transporter that has been implicated in lactate efflux from glycolytic muscle fibers and astrocytes in the brain. To address this issue, we co-cultured MCF7 breast cancer cells with normal fibroblasts. Interestingly, our results directly show that breast cancer cells specifically induce the expression of MCT4 in cancer-associated fibroblasts; MCF7 cells alone and fibroblasts alone, both failed to express MCT4. We also show that the expression of MCT4 in cancer-associated fibroblasts is due to oxidative stress, and can be prevented by pre-treatment with the anti-oxidant N-acetyl-cysteine. In contrast to our results with MCT4, we see that MCT1, a transporter involved in lactate uptake, is specifically upregulated in MCF7 breast cancer cells when co-cultured with fibroblasts. Virtually identical results were also obtained with primary human breast cancer samples. In human breast cancers, MCT4 selectively labels the tumor stroma, e.g., the cancer-associated fibroblast compartment. Conversely, MCT1 was selectively expressed in the epithelial cancer cells within the same tumors. Functionally, we show that overexpression of MCT4 in fibroblasts protects both MCF7 cancer cells and fibroblasts against cell death, under co-culture conditions. Thus, we provide the first evidence for the existence of a stromal-epithelial lactate shuttle in human tumors, analogous to the lactate shuttles that are essential for the normal physiological function of muscle tissue and brain. These data are consistent with the “reverse Warburg effect,” which states that cancer-associated fibroblasts undergo aerobic glycolysis, thereby producing lactate, which is utilized as a metabolic substrate by adjacent cancer cells. In this model, “energy transfer” or “metabolic-coupling” between the tumor stroma and epithelial cancer cells “fuels” tumor growth and metastasis, via oxidative mitochondrial metabolism in anabolic cancer cells. Most importantly, our current findings provide a new rationale and novel strategy for anti-cancer therapies, by employing MCT inhibitors.
Key words: caveolin-1, oxidative stress, pseudohypoxia, lactate shuttle, MCT1, MCT4, metabolic coupling, tumor stroma, predictive biomarker, SLC16A1, SLC16A3, monocarboxylic acid transporter
Introduction
Lactate is a high-energy metabolite that is produced in excess when cells lack functional mitochondria and undergo aerobic glycolysis. Lactate can then be transferred to other cells that are undergoing oxidative mitochondrial metabolism. Two normal physiological examples of this “energy transfer” mechanism occur in skeletal muscle and the brain. Cells possess specialized transporters, termed mono-carboxylate transporters (MCTs), to transfer lactate from one cell-type to another. This is known as the “lactate shuttle.”1,2 For example, in skeletal muscle, fast-twitch fibers are glycolytic and extrude lactate, which is then taken up by slow-twitch fibers. Similarly, in the brain, astrocytes are glycolytic, and export lactate, which is then used as an energy source by adjacent neurons.3 In the brain, this is known as “neuron-glia metabolic coupling.”4–8
The vectorial transport of lactate from glycolytic cells (fast-twitch fibers and astrocytes) to oxidative cells (slow-twitch fibers and neurons) is achieved, in part, by the cell-type specific expression of MCT molecules.3,9 For example, MCT4 (which extrudes lactate10) is expressed by glycolytic cells. Expression of MCT4 is induced by hypoxia, and is a known HIF1-alpha target gene.3,11 In contrast, MCT1/2 transporters facilitate the uptake of lactate, consistent with their expression in slow-twitch muscle fibers (MCT1) and neurons (MCT2).3,12
In the present study, we set out to determine if a similar compartmentalization of lactate transporters occurs in a subset of human breast cancers, in which cancer-associated fibroblasts undergo aerobic glycolysis. This subset of breast cancers is defined by a loss of stromal Cav-1,13 which appears to be an indicator of hypoxia, oxidative stress and autophagy in the tumor stroma and creates a “lethal” tumor microenvironment.14–18 We have also shown that a loss of stromal Cav-1 predicts poor clinical outcome in breast cancer patients and is associated with tumor recurrence, metastasis and drug-resistance.14–18
Interestingly, our current results directly show that MCT4 is upregulated in cancer-associated fibroblasts via oxidative stress (pseudo-hypoxia). Conversely, we show that epithelial cancer cells express MCT1. Thus, a “lactate shuttle” may also exist in human tumors for transferring energy from the tumor stroma to epithelial cancer cells.13,19–21
Results
MCT4 is expressed in cancer-associated fibroblasts and its expression is induced by co-culture with cancer cells.
Previous studies have associated the expression of MCT4 with lactate “secretion/extrusion,” in both fast-twitch muscle fibers and astrocytes in the brain. Both of these cell types are focused on aerobic glycolysis and produce L-Lactate, so that it can be effectively transferred to slow-twitch muscle fibers and neurons, respectively.3,9 This energy transfer mechanism is known as the lactate-shuttle. In the brain, this mechanism is also known as “neuron-glia metabolic coupling.”6–8 In accordance with these observations, MCT4 is upregulated during hypoxia, and is a known HIF1-alpha target gene, supporting its association with a glycolytic metabolic state.11 However, the distribution of MCT4 in cancer-associated fibroblasts has never been formally evaluated.
To address this issue, we chose to evaluate the distribution of MCT4 in a novel co-culture system that we developed.16 In this co-culture system, MCF7 cells are co-incubated with normal immortalized fibroblasts for a period of up to 5 days.16 During this co-incubation, the MCF7 cells promote the conversion of normal fibroblasts into bonafide cancer-associated fibroblasts, that show upregulation of smooth muscle actin, with activated TGFβ signaling, behaving like tumor myo-fibroblasts.16 Interestingly, the cancer cells induce oxidative stress (pseudo-hypoxia) in adjacent fibroblasts to promote this conversion event, which can be blocked with anti-oxidants such as N-acetyl-cysteine.14,17,18 Also, it is well established that oxidative stress is sufficient to drive myo-fibroblast differentiation.22–24
Thus, we co-cultured MCF7 cells with fibroblasts and observed the distribution of MCT4 by fluorescence microscopy. Cultures of MCF7 cells alone or fibroblasts alone (monotypic cultures) were also processed in parallel.
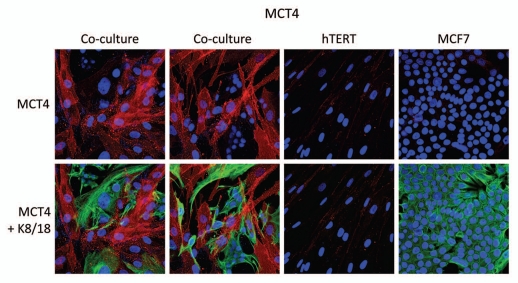
Interestingly, Figure 1 directly shows that MCF7 cells alone do not express significant amounts of MCT4. Similarly, fibroblasts alone do not express MCT4. However, when these two cell types are co-cultured, MCT4 is selectively upregulated in the cancer-associated fibroblasts. In this co-culture system, cancer cells are visualized by keratin staining. Note that there is little or no overlap between MCT4 staining and keratin staining, identifying MCT4 as a new marker of cancer-associated fibroblasts.
Figure 1.
MCF7 cancer cells induce the expression of MCT4 in cancer-associated fibroblasts. MCF7 cells were co-cultured with fibroblasts and then we observed the distribution of MCT4 (red) by fluorescence microscopy. Cultures of MCF7 cells alone or fibrobasts alone (monotypic cultures) were also processed in parallel. Note that MCF7 cells alone do not express significant amounts of MCT4. Similarly, fibroblasts alone do not express MCT4. However, when these two cell types are co-cultured, MCT4 is selectively upregulated in the cancer-associated fibroblasts. Epithelial cancer cells were visualized by keratin staining (green).
MCT4 expression in cancer-associated fibroblasts is upregulated by oxidative stress.
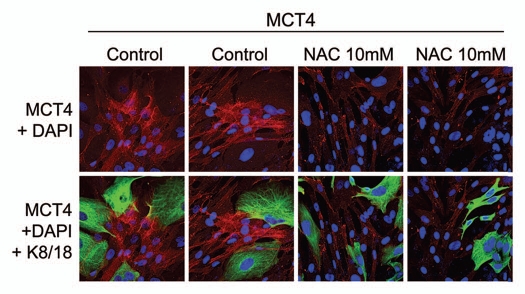
Next, we wanted to understand the possible mechanism(s) driving the upregulation of MCT4 in cancer-associated fibroblasts. As MCT4 expression is controlled by HIF1,11 and HIF1 is also activated by oxidative stress,25 we assessed the effects of anti-oxidants on this process. Figure 2 shows that treatment with N-acetyl-cysteine (NAC), a powerful anti-oxidant, is indeed sufficient to block the upregulation of MCT4 in cancer-associated fibroblasts. Thus, oxidative stress appears to induce MCT4 expression in cancer-associated fibroblasts.
Figure 2.
The induction of MCT4 in cancer-associated fibroblasts is due to oxidative stress and is prevented by antioxidants. MCF7 cells were co-cultured with fibroblasts and then we observed the distribution of MCT4 (red) by fluorescence microscopy. Since MCT4 expression is controlled by HIF1 and HIF1 is also activated by pseudo-hypoxia (oxidative stress), we assessed the effects of anti-oxidants on this process. Note that treatment with N-acetyl-cysteine (NAC; 10 mM), a powerful antioxidant, is sufficient to block that upregulation of MCT4 in cancer-associated fibroblasts, as predicted. Epithelial cancer cells were visualized by keratin staining (green).
MCT1 expression in MCF7 cancer cells is induced by co-culture with fibroblasts.
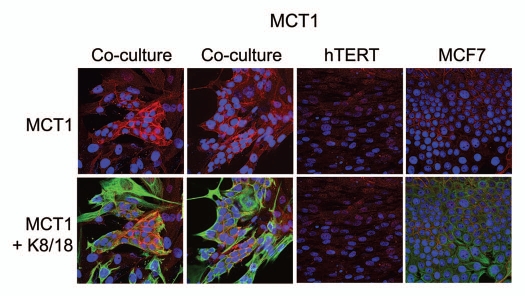
For comparison purposes, we assessed the distribution of MCT1 in this co-culture system. Unlike MCT4, which is designed to extrude L-Lactate, MCT1 has been implicated specifically in lactate uptake.10 Thus, according to our model, we would predict that MCT1 is localized within the cancer cells. Figure 3 shows that MCT1 is not well expressed in MCF7 cells or fibroblasts, when cultured individually. However, under conditions of co-culture, MCT1 is specifically induced in MCF7 cells. Thus, the compartmentalized distribution of MCT4 and MCT1 in different cell types (fibroblasts versus epithelial cancer cells) is consistent with the establishment of a lactate shuttle between the stroma (MCT4) and tumor cells (MCT1).
Figure 3.
Fibroblasts induce the expression of MCT1 in MCF7 cancer cells. MCF7 cells were co-cultured with fibroblasts and then we observed the distribution of MCT1 (red) by fluorescence microscopy. Cultures of MCF7 cells alone or fibrobasts alone (monotypic cultures) were also processed in parallel. Note that MCT1 is not well expressed in MCF7 cells or fibroblasts, when cultured individually. However, under conditions of co-culture, MCT1 is specifically induced in MCF7 cells. Epithelial cancer cells were visualized by keratin staining (green).
MDA-MB-231 cells also induce MCT4 in cancer-associated fibroblasts.
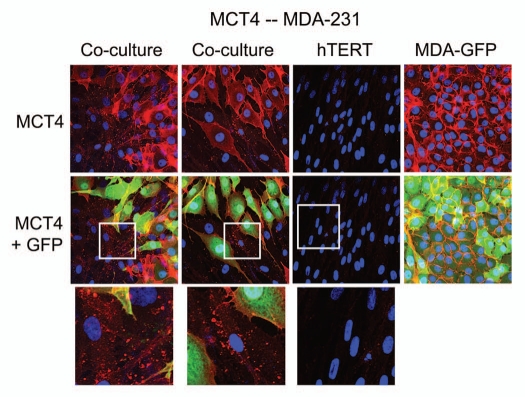
To assess the generality of this phenomenon, we also co-cultured MDA-MB-231 breast cancer cells with fibroblasts. Figure 4 shows that although MDA-MB-321 cells constitutively overexpress MCT4,26 they are also capable of inducing MCT4 expression in fibroblasts during co-culture.
Figure 4.
MDA-MB-231 cells also induce the expression of MCT4 in cancer-associated fibroblasts. MDA-MB-231 cells (expressing GFP) were co-cultured with fibroblasts and then we observed the distribution of MCT4 (red) by fluorescence microscopy. Cultures of MDA-MB-231 cells alone or fibrobasts alone (monotypic cultures) were also processed in parallel. Note that although MDA-MB-321 cells constitutively overexpress MCT4, they are also capable of inducing MCT4 expression in fibroblasts during co-culture (see also higher magnification insets). Epithelial cancer cells were visualized via GFP (green).
In human breast cancers, MCT4 is localized to the tumor stroma, while MCT1 is present selectively within epithelial cancer cells.
To assess the potential clinical relevance of our findings regarding the cell-type specific compartmentalization of MCT1 and MCT4, we next turned to the analysis of human breast cancer tumor samples. For this purpose, we selected cases in which there was a loss of stromal caveolin-1 (Cav-1), an established biomarker for hypoxia, oxidative stress and autophagy in the tumor stroma.14,17–20 Importantly, loss of stromal Cav-1 is associated with poor clinical outcome in the most common epithelial subtypes of breast cancer [ER(+), PR(+), HER2(+) and triple negative/basal].27–30
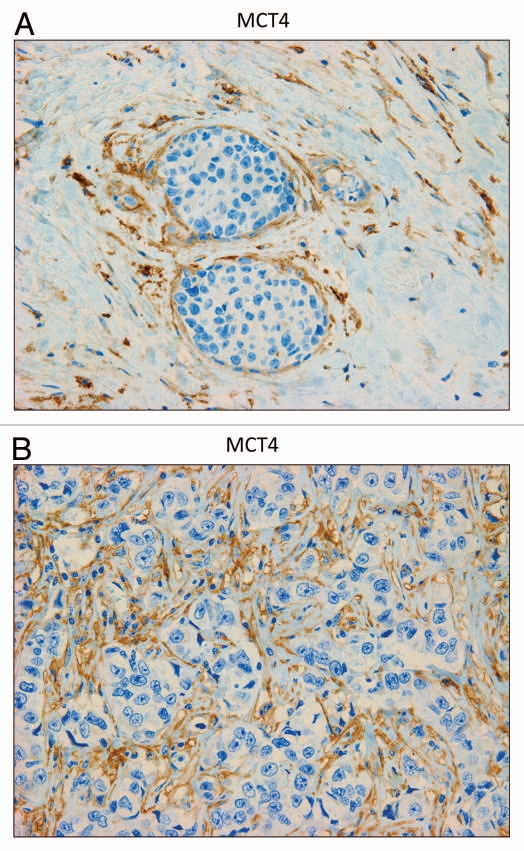
Figure 5A and B shows that MCT4 staining is selectively localized to the fibroblastic tumor stromal compartment. Two representative images are shown. Both images clearly show that MCT4 staining is absent from the tumor epithelial cells, but is present in the surrounding fibroblastic stroma. Figure 5B illustrates that MCT4 staining outlines the cancer-associated fibroblasts that surround or encircle nests of epithelial cancer cells. See also Supplemental Figure 1.
Figure 5.
MCT4 is expressed in the fibroblastic stromal compartment of human breast cancers. Note that MCT4 staining is selectively localized to the fibroblastic tumor stromal compartment of human breast cancers. Two representative images are shown. Both clearly show that MCT4 staining is absent from the tumor epithelial cells, but is present in the surrounding stroma. Panel (A) shows DCIS-like lesions and the surrounding MCT4(+) tumor stroma. Panel (B) shows that MCT4 staining outlines the cancer-associated fibroblasts that surround nests of epithelial cancer cells. Original magnification, 40×.
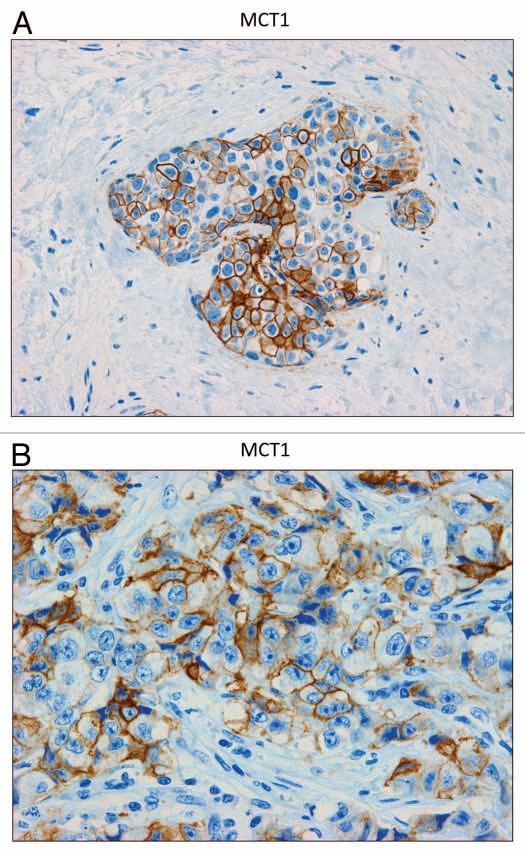
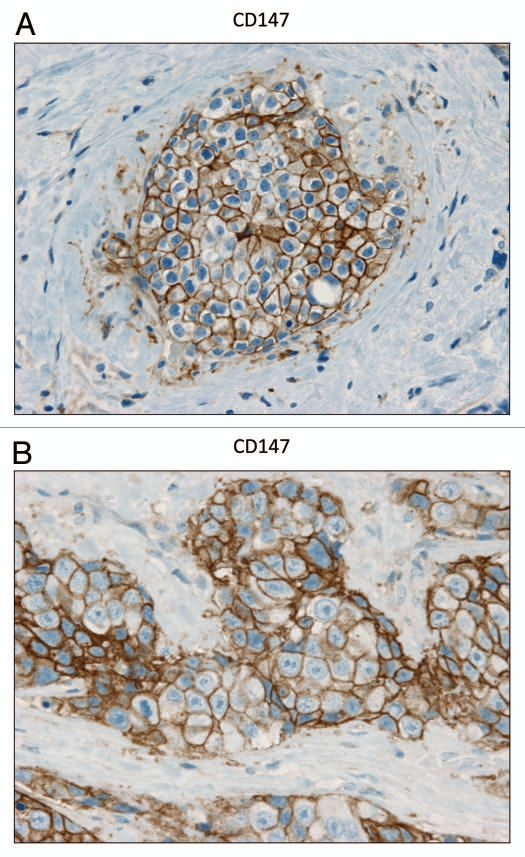
In contrast, as we observed with MCF7 cancer cells, we see that MCT1 expression is localized to tumor epithelial cells in human breast cancers (Fig. 6). However, MCT1 is not expressed in the tumor stroma, but instead is confined to epithelial cancer cells. Thus, the MCT1/4 compartmentalization we observed in MCF7-fibroblast co-cultures also occurs in human tumors lacking stromal Cav-1 (see also Sup. Fig. 2, for additional images). Similarly, the distribution of CD147, a known MCT1 interacting protein,31 was largely confined to the epithelial cancer cells (Fig. 7).
Figure 6.
MCT1 is expressed in the epithelial compartment of human breast cancers. Note that only epithelial cancer cells express MCT1 in human breast tumor samples. Two representative images are shown. Both clearly show that MCT1 staining is present in the tumor epithelial cells, but is absent in the surrounding stroma. Panel (A) shows DCIS-like lesions and the surrounding MCT1(+) epithelial cancer cells. Panel (B) shows that MCT1 staining identifies the epithelial cancer cells within the “cancer cell nests.” The original magnifications for (A and B) are 40× and 60×, respectively.
Figure 7.
CD147 is expressed in the epithelial compartment of human breast cancers. Note that epithelial cancer cells express CD147 in human breast tumor samples. Two representative images are shown. Both clearly show that CD147 staining is present in the tumor epithelial cells, but is largely absent in the surrounding stroma. Panel (A) shows DCIS-like lesions and the surrounding CD147(+) epithelial cancer cells. Panel (B) shows that CD147 staining identifies the epithelial cancer cells within the “cancer cell nests.” The original magnifications for both (A and B) are 60×.
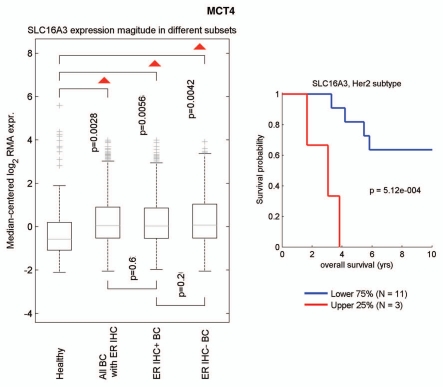
We next used informatics analysis to assess whether the mRNA transcripts for MCT1 and MCT4 are commonly upregulated in human breast cancer. Interestingly, Figure 8 shows that MCT4 (a.k.a., SLC16A3) is overexpressed in breast cancers (relative to normal breast tissue), including both ER(+) and ER(−) breast cancer sub-types. In the HER2(+) sub-type, we also observed an association with clinical outcome; increased MCT4 transcript levels were associated with decreased overall survival (n = 14 patients). However, a parallel analysis did not show any association between MCT1 (a.k.a., SLC16A1) transcript levels and breast cancer (not shown).
Figure 8.
Informatics analysis of the transcriptional levels of MCT4 in human breast cancers. We used informatics analysis to determine whether the mRNA transcript for MCT4 is commonly upregulated in human breast cancer. (Left) Note that MCT4 (SLC16A3) is overexpressed in all types of breast cancer (relative to normal breast tissue), including both ER(+) and ER(−) cancer sub-types. (Right) In the HER2(+) sub-type, we observed an association with clinical outcome; increased MCT4 transcript levels were associated with decreased overall survival (N = 14 patients).
In addition, we checked the expression MCT4 in a previously published transcriptional data set generated by Morag Park and colleagues,32 which was derived via laser capture micro-dissection of the tumor stroma of human breast cancers. Table 1 shows that MCT4 (an astrocyte marker in the brain) is overexpressed in the tumor stroma of human breast cancers, and is preferentially associated with tumor recurrence. The transcriptional expression of other “astrocyte markers” in breast cancer tumor stroma is shown for comparison. Interestingly, GFAP is a known marker of astrocytes, mesenchymal stem cells and myo-fibroblasts, consistent with the idea that these three cell types may be closely functionally related.
Table 1.
Overexpression of MCT4 and other “Astrocyte Markers” in the tumor stroma of human breast cancers
| Gene | Description | Tumor stroma | Recurrence-Stroma | Metastasis-Stroma |
| MCT4, an Astrocyte Marker and MCT Family Member | ||||
| Slc16a3 | solute carrier family 16 (monocarboxylic acid transporters), member 3 | 1.09E-03 | ||
| Other Glial Cell (Astrocyte) Associated Transcripts | ||||
| Gfap | glial fibrillary acidic protein | 1.64E-18 | 1.36E-03 | 2.28E-02 |
| Gfra2 | glial cell line derived neurotrophic factor receptor alpha2 | 2.28E-17 | 3.58E-02 | |
| Slc1a3 | solute carrier family 1 (glial high affinity glutamate transporter), member 3 | 4.22E-17 | 5.70E-03 | |
| Gfra3 | glial cell line derived neurotrophic factor receptor alpha3 | 2.97E-16 | ||
| Gdnf | glial cell line derived neurotrophic factor | 6.48E-14 | ||
| Gfra4 | glial cell line derived neurotrophic factor receptor alpha4 | 1.02E-02 | ||
p-values are as indicated. Gfap is a known marker for astrocytes, mesenchymal stem cells (MSCs), and myo-fibroblasts.
Thus, additional studies assessing the potential use of MCT4 as a biomarker for cancer-associated fibroblasts are warranted. In accordance with this assertion, Perou and colleagues have previously defined a “compact” hypoxia gene signature consisting of 13 genes, one of which was SLC16A3 (MCT4). This compact transcriptional hypoxia signature, containing SLC16A3, was associated with distant metastasis and poor clinical outcome in breast, lung and brain cancers (glioblastoma);33 however, they did not distinguish between stromal or tumor cell expression of this signature.
Overexpression of MCT4 in fibroblasts functionally protects both cancer cells and fibroblasts against cell death, under co-culture conditions.
Next, to assess the possible functional consequences of MCT4 expression in fibroblasts, we generated an hTERT-fibroblast cell line stably overexpressing MCT4. As critical controls, we also generated hTERT-fibroblast cell lines overexpressing MCT1, and the vector alone. Then, these three matched fibroblast cell lines were individually co-cultured with GFP-tagged MCF7 cells and cell death in both fibroblasts and cancer cells was monitored by FACS analysis, using Annexin-V and PI staining.
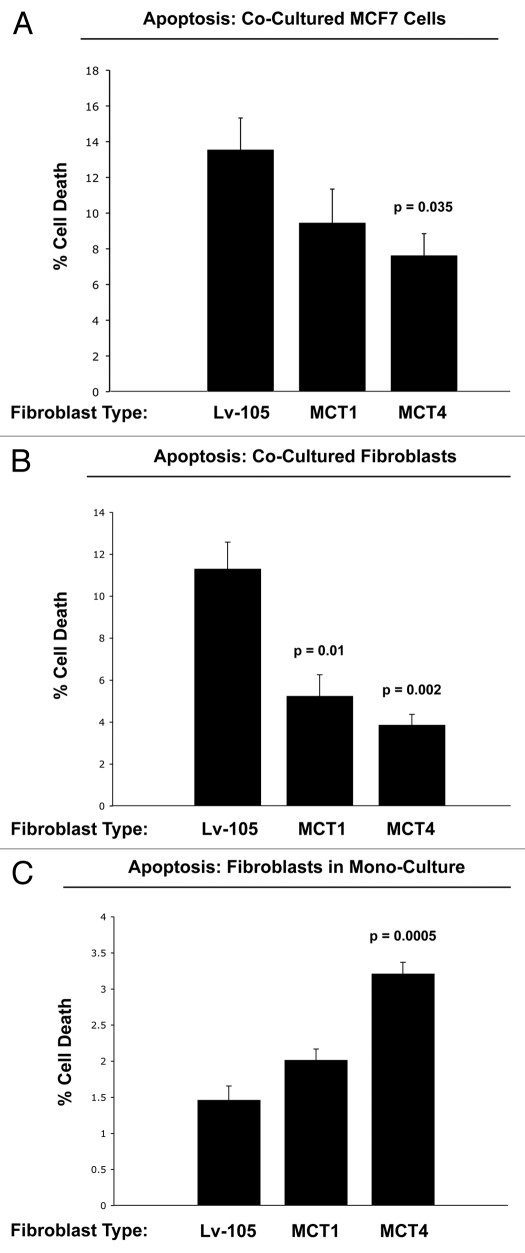
Figure 9A shows that co-culture with MCT4-expressing fibroblasts protects MCF7 cells against cell death by nearly 2-fold (p = 0.035). In contrast, the effects of MCT1-expressing fibroblasts on MCF7 cell death were not significant. Interestingly, co-cultured fibroblasts expressing MCT1 (p = 0.01) or MCT4 (p = 0.002) both showed a >2-fold protection against cell death (Fig. 9B). Thus, expression of MCT4 in fibroblasts functionally prolongs the life of both cancer cells and fibroblasts, under co-culture conditions.
Figure 9.
Overexpression of MCT4 in fibroblasts functionally protects both cancer cells and fibroblasts against cell death under co-culture conditions. To assess the possible functional consequences of MCT4 expression in fibroblasts, we generated an hTERT-fibroblast cell line stably overexpressing MCT4. Similarly, we also generated hTERT-fibroblast cell lines overexpressing MCT1, and the vector alone (Lv-105). Then, these three matched fibroblast cell lines were individually co-cultured with GFP-tagged MCF7 cells, and cell death in both fibroblasts and cancer cells was monitored by FACS analysis (See the Materials and Methods section). (A) Note that co-culture with MCT4-expressing fibroblasts protects MCF7 cells against cell death, by nearly 2-fold (p = 0.035). In contrast, the effects of MCT1-expressing fibroblasts on MCF7 cell death were not significant. (B) Note that co-cultured fibroblasts expressing MCT1 (p = 0.01) or MCT4 (p = 0.002) both showed >2-fold protection against cell death. (C) However, when MCT4 fibroblasts were cultured alone, in the absence of cancer cells, they showed a >2-fold increase in cell death (p = 0.005). Thus, expression of MCT4 in fibroblasts functionally prolongs the life of both cancer cells and fibroblasts, under co-culture conditions.
In contrast, when MCT4 fibroblasts were cultured alone, in the absence of cancer cells, they showed a >2-fold increase in cell death (p = 0.005) (Fig. 9C). This may explain why fibroblasts do not constitutively overexpress MCT4 when they are alone, but that MCT4 is induced in fibroblasts during their co-culture with cancer cells.
Taken together, these experimental observations are consistent with the known function of MCT4 as a means for lactate extrusion/secretion. Increased MCT4-mediated efflux of lactate under co-culture conditions would be predicted to benefit both fibroblasts and cancer cells alike, in the context of the “reverse Warburg effect.” However, increased MCT4-mediated extrusion of lactate in fibroblasts cultured alone, would be predicted to result in increased acidification of the culture media and subsequent cell death.
Discussion
Here, we assessed the distribution of MCT1 and MCT4 in human breast cancers, using two complementary approaches. First, we used an MCF7-fibroblast co-culture system,16 and observed that MCT4 was localized to the cancer-associated fibroblast compartment, while MCT1 stained the epithelial cancer cells. Both MCT1 and MCT4 were induced by the co-culture of fibroblasts with cancer cells, suggesting that both cell-types derived some sort of synergy from their compartmentalization. We also showed that the induction of MCT4 in cancer-associated fibroblasts was due to the onset of oxidative stress (pseudo-hypoxia), as treatment with N-acetyl-cysteine (NAC), an anti-oxidant, was indeed sufficient to prevent the upregulation of MCT4. Thus, accumulation of MCT4 in cancer-associated fibroblasts may be a marker of oxidative stress. Next, we assessed the distribution of MCT1 and MCT4 in human breast cancer tumor samples lacking stromal Cav-1. Our results indicate that MCT4 was selectively localized to the tumor stroma, specifically within cancer-associated fibroblasts that surround or encircle nests of cancer cells. In contrast, MCT1 was preferentially confined to the epithelial cancer cells. Taken together, these findings suggest that the localization of MCT1 and MCT4 in different cellular compartments may have a functional advantage for the survival of human tumors.
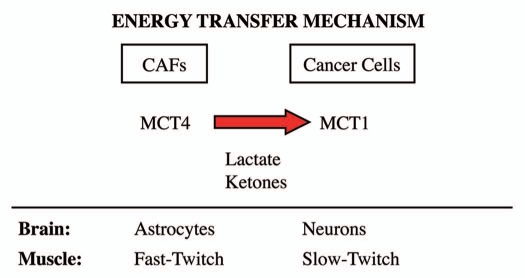
A survey of the literature indicates that MCT4 functions primarily as a transporter that extrudes lactate from cells that utilize aerobic glycolysis for energy metabolism and lack functional mitochondria.3,9 Two normal physiological examples of this are fast-twitch (glycolytic) fibers in skeletal muscle and astrocytes within the brain.3,9 In accordance with this idea, MCT4 expression is upregulated during hypoxia, and is a known HIF1 target gene.11 After lactate is extruded by MCT4, lactate is then taken up by other MCT transporters in adjacent cells, such as slow-twitch (mitochondrial-rich) fibers in muscle or neurons in the brain. To scavenge lactate from their micro-environment, slow-twitch muscle fibers use MCT1, while neurons use primarily MCT2. MCT1 and MCT2 are similar both structurally and functionally. This phenomenon has been referred to as “neuronglia metabolic coupling” in the brain and as the “lactate shuttle” in skeletal muscle.4–8 Thus, we believe that our current findings support the hypothesis that similar metabolic-coupling occurs between cancer-associated fibroblasts and adjacent tumor cells. In fact, we have previously coined the term, “the reverse Warburg effect,” to describe this symbiotic or parasitic relationship within tumors.13,15,19–21 These results are summarized schematically in Figure 10.
Figure 10.
The lactate shuttle: an energy transfer mechanism in normal tissue and human cancers. MCT4 functions primarily as a transporter that extrudes lactate from cells that are undergoing aerobic glycolysis and lack functional mitochondria. Two normal physiological examples of this are fast-twitch fibers in skeletal muscle and astrocytes within the brain. After lactate is extruded by MCT4, the lactate is then taken up by other MCT transporters in adjacent cells, such as slow-twitch (mitochondrial-rich) fibers in muscle or neurons in the brain. To accomplish the scavenging of lactate, slow-twitch muscle fibers use MCT1, while neurons use MCT2. In the brain, this phenomenon has been referred to as “neuron-glia metabolic coupling,” while in skeletal muscle it is known as the “lactate shuttle.” Our current studies support the hypothesis that similar metabolic-coupling occurs between cancer-associated fibroblasts and adjacent tumor cells.
The identification of the “reverse Warburg effect” was driven by a need to understand the powerful prognostic value of a stromal biomarker, namely caveolin-1 (Cav-1). More specifically, a loss of stromal Cav-1 in human breast cancers is associated with early tumor recurrence, metastasis and tamoxifen-resistance, driving poor clinical outcome.27–30,34 The prognostic value of stromal Cav-1 was also independent of epithelial marker status, as it was effective in ER(+), PR(+), HER2(+) and triple negative/basal patients.27–30 Similar results were also obtained via the analysis of cohorts of DCIS patients (a benign precursor lesion), as well as human prostate cancer patients.28,35 In both cases, a loss of stromal Cav-1 was associated with either tumor progression (in DCIS) or metastasis (in prostate cancer).28,35
To mechanistically understand how a loss of stromal Cav-1 confers a “lethal” tumor microenvironment, we used stromal cells derived from Cav-1 (−/−) null mice.13 Via a proteomics analysis, we observed that eight myofibroblast markers, eight glycolytic enzymes and two markers of oxidative stress were upregulated in Cav-1 (−/−) stromal cells.13 These results were later validated by genome-wide transcriptional profiling, which showed that a loss of Cav-1 drives the induction of gene expression profiles consistent with oxidative stress (pseudo-hypoxia) in fibroblasts, via the activation of two well-known transcription factors, namely HIF1-alpha and NFκB.19–21 Virtually identical results were obtained by co-culturing MCF7 cells with fibroblasts. Under these co-culture conditions, MCF7 cells specifically downregulated Cav-1 in adjacent fibroblasts and activated HIF1-alpha and NFκB, as seen by the use of luciferase-reporters in co-cultured fibroblasts.14,17 These phenotypic changes appeared to be driven by the induction of oxidative stress and autohagy/mitophagy in cancer-associated fibroblasts.15,16 Thus, we concluded that cancer cells use oxidative stress as a “weapon” to extract recycled nutrients from neighboring normal cells, such as stromal fibroblasts. We have coined the term the “Autophagic Tumor Stroma Model of Cancer Metabolism,”18,19 to expand this concept from the “reverse Warburg effect” to autophagy/mitophagy in the tumor stroma.
Thus, we believe that one of the major functions of the tumor stroma is to produce lactate and other high-energy nutrients (such as ketones and glutamine) to “fuel” oxidative mitochondrial metabolism in epithelial cancer cells. In accordance with this hypothesis, the intra-peritoneal (i.p.) injection of mouse xenografts with ketones or lactate is sufficient to significantly increase tumor growth (∼2.5-fold) and metastasis (∼10-fold), respectively.36 Furthermore, treatment of MCF7 cells with high-energy metabolites (such as lactate) is sufficient to promote mitochondrial biogenesis and phenocopies the positive effects of fibroblasts on cancer cells during co-culture.14,17 Finally, we have derived transcriptional gene signatures from MCF7 cells treated with either lactate or ketones, both of which are converted to Acetyl-CoA, so they can then enter the TCA cycle and be substrates for oxidative phosphorylation.37 Interestingly, these gene signatures indicate that lactate and ketones increase the “stemness” of cancer cells, based on an analysis of their transcriptional profiles.37 Most importantly, however, these lactate- and ketone-induced gene signatures can also be used to predict clinical outcome in human breast cancer patients, and are associated with recurrence, metastasis and significant decreases in overall survival.37 Thus, we have suggested that lactate and ketones both function as tumor promoters, via their ability to drive increased oxidative mitochondrial metabolism in cancer cells.37 Similarly, ketones are thought to be transported by the same MCT transporters that handle lactate transport.38,39
In summary, our current results directly support the “reverse Warburg effect,” which is driven by oxidative stress in cancer-associated fibroblasts. In accordance with this paradigm, the major lactate exporter, namely MCT4, was expressed in cancer-associated fibroblasts, and its expression was induced by oxidative stress. Conversely, MCT1, a major transporter involved in lactate uptake, was localized to epithelial cancer cells. Thus, a lactate/ketone shuttle may also exist in human tumors to facilitate energy transfer from the catabolic tumor stroma to anabolic “hungry” cancer cells. In further support of these assertions, (1) elevated tumor and serum lactate levels are a known predictor of poor clinical outcome for a variety of epithelial cancers;40–43 and (2) lactic acidosis is a key metabolic feature of patients with breast cancer metastasis.44–49
Materials and Methods
Materials.
Antibodies were obtained as follows: anti-cytokeratin 8/18 (cat #20R-CP004, Fitzgerald Industries International), anti-MCT4 (cat #sc50329, Santa Cruz Biotechnologies) and anti-CD147 (cat #10R-CD147AHU, Fitzgerald Industries International). Also, anti-MCT4 and anti-MCT1 isoform-specific antibodies were previously generated and characterized by Dr. Philp.26 Other reagents were as follows: 4,6-diamidino-2-phenylindole (DAPI) and Prolong Gold Antifade mounting reagent from Invitrogen, N-acetyl-cysteine (NAC) was from Sigma.
Cell culture.
Cell culture experiments were carried out as previously described, with minor modifications.16 Human skin fibroblasts immortalized with human telomerase reverse transcriptase (hTERT-BJ1) were purchased originally from Clontech, Inc. The breast cancer cell line, MCF7, was from ATCC. All cells were maintained in DMEM, with 10% Fetal Bovine Serum (FBS) and Penicillin 100 units/mL-Streptomycin 100 µg/mL (Invitrogen). Fibroblasts and MCF7 cells were co-plated or plated as a homotypic cell culture in 12-well plates in 1-ml of complete media on glass coverslips. In co-culture experiments, fibroblasts were plated first and MCF7 cells were plated within 2 hours of fibroblast plating. The total number of cells per well in co-culture was 1 × 105 cells. Co-culture experiments were carried out at a 5:1 fibroblast-to-epithelial cell ratio. As controls, homotypic cultures of fibroblasts and MCF7 cells were plated in parallel, using the same number of a given cell population as the corresponding co-cultures. The day after plating, the media was changed to DMEM with 10% NuSerum I (a low protein alternative to FBS; BD Biosciences; cat #355100) and Pen-Strep. Cells were maintained in this media for one to five days, until fixation. To study the effects of co-culture on MCT expression, fibroblasts and MCF7 cells were cultured in 10% Nu-Serum for five days prior to fixation. To study the effect of N-acetylcysteine on MCT4 expression, cells were cultured for 24 hours in Nu-Serum I prior to fixation. Both mono-cultures and co-cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Immunocytochemistry (ICC).
Cells were fixed after one to five days of culture. Then, the ICC protocol was performed as previously described, with minor modifications.54 Briefly, cells were fixed for 30 minutes at room temperature in 2% paraformaldehyde diluted in PBS, after which they were permeabilized with cold methanol at −20°C for 5 minutes. The cells were rinsed with PBS with 0.1 mM Calcium chloride and 1 mM Magnesium chloride (PBS/CM). Then, cells were incubated with NH4Cl in PBS to quench free aldehyde groups. Rinsing with PBS/CM was followed by blocking with immunofluorescence (IF) buffer (PBS, 1% BSA, 0.1% Tween 20) for 1 hour at room temperature. Primary antibodies were incubated in IF buffer for 1 hour at room temperature. After washing with IF buffer (3x, 10 minutes each), cells were incubated for 30 minutes at room temperature with fluorochrome-conjugated secondary antibodies diluted in IF buffer. Finally, slides were washed at room temperature with IF buffer (3x, 10 minutes each), rinsed with PBS/CM and counter-stained with DAPI (10 µg/ml) in PBS and mounted with Prolong Gold anti-fade Reagent.
Confocal microscopy.
Images were collected with a Zeiss LSM510 meta confocal system, using a 405-nm Diode excitation laser with a band pass filter of 420–480-nm, a 488-nm Argon excitation laser with a band pass filter of 505–530-nm, and a 543-nm He/Ne excitation laser with a 561–625-nm filter. Images were acquired with a 40x objective.
Immunohistochemistry (IHC).
Paraffin-embedded sections of human breast cancer samples were immuno-stained as previously described in reference 30. Briefly, sections were deparaffinized, rehydrated and washed in PBS. Antigen retrieval was performed in 10 mM sodium citrate, pH 6.0 for 10 min using a pressure cooker. After blocking with 3% hydrogen peroxide for 10 minutes, sections were incubated with 10% goat serum for 1 hour. Then, sections were incubated with primary antibodies overnight at 4°C. Antibody binding was detected using a biotinylated secondary (Vector Labs, Burlingame, CA) followed by streptavidin-HRP (Dako, Carpinteria, CA). Immunoreactivity was revealed using 3, 3′ diaminobenzidine.
Analysis of clinical outcome in human breast cancer patients.
A microarray dataset that was previously compiled from the public repositories Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo),55 and ArrayExpress (www.ebi.ac.uk/arrayexpress),56 was used to evaluate MCT4 expression in the context of clinical samples.57,58 In the BoxPlots (See Fig. 8), only samples with ER IHC data were selected for analysis, including 959 ER-positive and 323 ER-negative, for a total of 1,282 samples; in addition, 102 normal healthy breast tissue controls were used for comparison purposes. Samples were first analyzed in subsets based on their ER IHC status. Additional subsets were defined by classifying samples among five canonical breast cancer subtypes, including luminal A, luminal B, normal-like, basal and Her-2-overexpressing disease. Samples were classified by computing their correlation against five expression profile centroids representing these breast cancer subtypes and assigning them to the subtype with the highest corresponding correlation coefficient.59 Samples with a maximum correlation coefficient below 0.3 were considered unclassified. Differential expression of the averaged gene signature magnitude among these sample subsets was evaluated using a two-tailed t-test. Kaplan-meier analysis was used to evaluate survival trends within sample subsets, including 627 samples with metastasis-free survival time (507 ER-pos., 120 ER-neg.), 637 with relapse free survival time (517 ER-pos., 120 ER-neg.) and 329 with overall survival time (219 ER-pos., 110 ER-neg.). The Log-rank test was used to evaluate differences in survival curves for high vs. low signature-expressing populations.
Analysis of the expression of MCT4 and other astrocyte markers in the transcriptional profiles of human breast tumor stroma.
We obtained the transcriptional profiles of a large data set of human breast cancer patients32 whose tumors were subjected to laser-capture micro-dissection, to selectively isolate the tumor stroma. Based on this data set,32 we then generated three human breast cancer stromal genes lists:21
Tumor stroma vs. normal stroma list. Compares the transcriptional profiles of tumor stroma obtained from 53 patients to normal stroma obtained from 38 patients. Gene transcripts that were consistently upregulated in tumor stroma were selected and assigned a p-value, with a cut-off of p < 0.05 (contains 6,777 genes).21
Recurrence stroma list. Compares the transcriptional profiles of tumor stroma obtained from 11 patients with tumor recurrence to the tumor stroma of 42 patients without tumor recurrence. Gene transcripts that were consistently upregulated in the tumor stroma of patients with recurrence were selected and assigned a p-value, with a cut-off of p < 0.05 (contains 3,354 genes).21
Lymph-node (LN) metastasis stroma list. Compares the transcriptional profiles of tumor stroma obtained from 25 patients with LN metastasis to the tumor stroma of 25 patients without LN metastasis. Gene transcripts that were consistently upregulated in the tumor stroma of patients with LN metastasis were selected and assigned a p-value, with a cut-off of p < 0.05 (contains 1,182 genes).21
Stable overexpression of MCT1 or MCT4 in fibroblasts.
Vectors encoding the monocarboxylic acid transporters, MCT4 (EX-M0699-Lv105) and MCT1 (EX-C0751-Lv105), as well as the empty vector control (EX-Lv105), were all purchased from GeneCopoeia and lenti-viruses were prepared according to the manufacturer's protocols. Virus-containing media were centrifuged, filtered (0.45 µM PES low protein filter) and stored in 1 mL aliquots at −80°C. hTERT-fibroblasts (120,000 cells/well) were plated in 12 well dishes in growth media. After 24 hours, the media was removed and replaced with 250 µl DMEM + 5% FBS, 150 µl of virus-containing media and 5 µg/ml polybrene. 24 hours post-transduction, media containing virus was removed and replaced with DMEM with 10% FCS. After infection, fibroblasts stably overexpressing either MCT1 or MCT4, were selected with puromycin for 5 days.
Quantitation of cell death by FACS, with propidium iodide and annexin-V staining.
Cell death was quantified by flow cytometry using propidium iodide and Annexin-V-APC, as we previously described with minor modifications.14,17 Briefly, MCF7-GFP cells were plated in 12 well plates with hTERT fibroblasts, expressing MCT1, MCT4 or transfected with the vector alone control (Lv105). The day after, the media was changed to DMEM with 10% NuSerum. After 5 days, the cells were collected by trypsinization, and centrifugation and were re-suspended in 500 µL of Annexin-V Binding Buffer. Then, the annexin V-APC conjugate (BD Biosciences; cat # 550474) (4 µL) and propidium iodide (1 µL) was added and incubated in the dark at room temperature for 5 minutes. Cells were then analyzed by flow cytometry, using a GFP signal detector with an excitation wavelength of 488 nm and an emission of 530 nm (to detect MCF7-GFP cells), a PE Texas Red signal detector with excitation wavelength of 496 nm and emission of 615 and an APC signal detector with excitation wavelength of 650 nm and emission of 660 nm. We defined “cell death” as the population of cells that were annexin-V(−) and PI(+), corresponding to advanced apoptotic cell death.
Conclusions
It is now clear that both tumor cells and the surrounding stroma create the cancer microenvironment; however, previous studies have not examined whether these two cellular compartments are metabolically-coupled through a “lactate shuttle.”
The “lactate shuttle” is a proposed mechanism for metabolically coupling cells within normal tissue; lactate produced via aerobic glycolysis in one cell is utilized by another cell to fuel oxidative mitochondrial metabolism. Examples of metabolically-coupled cells via the lactate shuttle are found in the brain between astrocytes and neurons, in the retinal Muller cells and photoreceptor cells, and in skeletal muscle by the fast-twitch and slow-twitch fibers.1–7,12,50,51
In normal tissue, such as skeletal muscle and brain, it has been found that glycolytic and oxidative cells are metabolically-coupled, via monocarboxylate transporters (MCTs). Under aerobic conditions, fast-twich muscle fibers and astrocytes metabolize glucose through aerobic glycolysis, producing lactate that is extruded via MCT4. Lactate is taken-up by MCTs in slow-twitch fibers (MCT1) or neurons (MCT2), where it is oxidized to CO2 and H2O.
Recently, we have proposed that a “lactate shuttle” exists between stromal fibroblasts and adjacent epithelial cancer cells and have referred to this as the “reverse Warburg effect.”13 This model proposes that oxidative stress in stromal fibroblasts increases glycolytic metabolism, resulting in the extrusion of lactate and other metabolites.14 The increase in lactate in the tumor microenvironment stimulates an increase in lactate uptake and oxidative mitochondrial metabolism in cancer cells.17
Here, we show that antibodies directed against MCT4 selectively label the tumor stroma, e.g., the cancer-associated fibroblast compartment. Conversely, MCT1 was selectively expressed in the epithelial cancer cells, within the same tumors. Thus, we provide the first evidence for the existence of a “stromal-epithelial” lactate shuttle in human tumors.
Our current findings undoubtedly provide a new rationale and novel strategy for anti-cancer therapies, using MCT inhibitors. Fortunately, two clinically relevant MCT1 inhibitors (AR-C117977 and AR-C155858) have been recently developed.52,53
Acknowledgments
F.S. and her laboratory were supported by grants from the W.W. Smith Charitable Trust, the Breast Cancer Alliance (BCA) and a Research Scholar Grant from the American Cancer Society (ACS). M.P.L. was supported by grants from the NIH/NCI (R01-CA-080250; R01-CA-098779; R01-CA-120876; R01-AR-055660), and the Susan G. Komen Breast Cancer Foundation. A.K.W. was supported by a Young Investigator Award from the Breast Cancer Alliance, Inc., and a Susan G. Komen Career Catalyst Grant. R.G.P. was supported by grants from the NIH/NCI (R01-CA-70896, R01-CA-75503, R01-CA-86072 and R01-CA-107382) and the Dr. Ralph and Marian C. Falk Medical Research Trust. The Kimmel Cancer Center was supported by the NIH/NCI Cancer Center Core grant P30-CA-56036 (to R.G.P.). Funds were also contributed by the Margaret Q. Landenberger Research Foundation (to M.P.L.). This project is funded, in part, under a grant with the Pennsylvania Department of Health (to M.P.L. and F.S.). The Department specifically disclaims responsibility for any analyses, interpretations or conclusions. This work was also supported, in part, by a Centre grant in Manchester from Breakthrough Breast Cancer in the UK (to A.H.) and an Advanced ERC Grant from the European Research Council.
Supplementary Material
References
- 1.Gladden LB. A lactatic perspective on metabolism. Med Sci Sports Exerc. 2008;40:477–485. doi: 10.1249/MSS.0b013e31815fa580. [DOI] [PubMed] [Google Scholar]
- 2.Brooks GA. Lactate: link between glycolytic and oxidative metabolism. Sports Med. 2007;37:341–343. doi: 10.2165/00007256-200737040-00017. [DOI] [PubMed] [Google Scholar]
- 3.Bergersen LH. Is lactate food for neurons? Comparison of monocarboxylate transporter subtypes in brain and muscle. Neuroscience. 2007;145:11–19. doi: 10.1016/j.neuroscience.2006.11.062. [DOI] [PubMed] [Google Scholar]
- 4.Brooks GA. Lactate shuttles in nature. Biochem Soc Trans. 2002;30:258–264. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 5.Brooks GA. Current concepts in lactate exchange. Med Sci Sports Exerc. 1991;23:895–906. [PubMed] [Google Scholar]
- 6.Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J Exp Biol. 2006;209:2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- 7.Magistretti PJ. Role of glutamate in neuron-glia metabolic coupling. Am J Clin Nutr. 2009;90:875–880. doi: 10.3945/ajcn.2009.27462CC. [DOI] [PubMed] [Google Scholar]
- 8.Magistretti PJ, Pellerin L. The contribution of astrocytes to the 18F-2-deoxyglucose signal in PET activation studies. Mol Psychiatry. 1996;1:445–452. [PubMed] [Google Scholar]
- 9.Pierre K, Pellerin L. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem. 2005;94:1–14. doi: 10.1111/j.1471-4159.2005.03168.x. [DOI] [PubMed] [Google Scholar]
- 10.Dimmer KS, Friedrich B, Lang F, Deitmer JW, Broer S. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem J. 2000;350:219–227. [PMC free article] [PubMed] [Google Scholar]
- 11.Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is upregulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem. 2006;281:9030–9037. doi: 10.1074/jbc.M511397200. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto T, Masuda S, Taguchi S, Brooks GA. Immunohistochemical analysis of MCT1, MCT2 and MCT4 expression in rat plantaris muscle. J Physiol. 2005;567:121–129. doi: 10.1113/jphysiol.2005.087411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, et al. The reverse Warburg effect: Aerobic glycolysis in cancer-associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Outschoorn UE, Balliet RM, Rivadeneira DB, Chiavarina B, Pavlides S, Wang C, et al. Oxidative stress in cancer-associated fibroblasts drives tumorstroma co-Evolution: A new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle. 2010;9:3256–3276. doi: 10.4161/cc.9.16.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez-Outschoorn UE, Pavlides S, Howell A, Pestell RG, Tanowitz HB, Sotgia F, et al. Stromal-epithelial metabolic coupling in cancer: Integrating autophagy and metabolism in the tumor microenvironment. Int J Biochem Cell Biol. 2011 doi: 10.1016/j.biocel.2011.01.023. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-Outschoorn UE, Pavlides S, Whitaker-Menezes D, Daumer KM, Milliman JN, Chiavarina B, et al. Tumor cells induce the cancer-associated fibroblast phenotype via caveolin-1 degradation: Implications for breast cancer and DCIS therapy with autophagy inhibitors. Cell Cycle. 2010;9:2423–2433. doi: 10.4161/cc.9.12.12048. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Outschoorn UE, Trimmer C, Lin Z, Whitaker-Menezes D, Chiavarina B, Zhou J, et al. Autophagy in cancer-associated fibroblasts promotes tumor cell survival: Role of hypoxia, HIF1 induction and NFκB activation in the tumor stromal microenvironment. Cell Cycle. 2010;9:3515–3533. doi: 10.4161/cc.9.17.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Outschoorn UE, Whitaker-Menezes D, Pavlides S, Chiavarina B, Bonuccelli G, Casey T, et al. The autophagic tumor stroma model of cancer or “battery-operated tumor growth”: A simple solution to the autophagy paradox. Cell Cycle. 2010;9:4297–4306. doi: 10.4161/cc.9.21.13817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pavlides S, Tsirigos A, Migneco G, Whitaker-Menezes D, Chiavarina B, Flomenberg N, et al. The autophagic tumor stroma model of cancer: Role of oxidative stress and ketone production in fueling tumor cell metabolism. Cell Cycle. 2010;9:3485–3505. doi: 10.4161/cc.9.17.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavlides S, Tsirigos A, Vera I, Flomenberg N, Frank PG, Casimiro MC, et al. Loss of stromal caveolin-1 leads to oxidative stress, mimics hypoxia and drives inflammation in the tumor microenvironment, conferring the “reverse Warburg effect”: A transcriptional informatics analysis with validation. Cell Cycle. 2010;9:2201–2219. doi: 10.4161/cc.9.11.11848. [DOI] [PubMed] [Google Scholar]
- 21.Pavlides S, Tsirigos A, Vera I, Flomenberg N, Frank PG, Casimiro MC, et al. Transcriptional evidence for the “reverse Warburg effect” in human breast cancer tumor stroma and metastasis: similarities with oxidative stress, inflammation, Alzheimer's disease and “neuronglia metabolic coupling”. Aging. 2010;2:185–199. doi: 10.18632/aging.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, et al. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 23.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, et al. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toullec A, Gerald D, Despouy G, Bourachot B, Cardon M, Lefort S, et al. Oxidative stress promotes myofibroblast differentiation and tumour spreading. EMBO Mol Med. 2010;2:211–230. doi: 10.1002/emmm.201000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pouyssegur J, Mechta-Grigoriou F. Redox regulation of the hypoxia-inducible factor. Biol Chem. 2006;387:1337–1346. doi: 10.1515/BC.2006.167. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher SM, Castorino JJ, Wang D, Philp NJ. Monocarboxylate transporter 4 regulates maturation and trafficking of CD147 to the plasma membrane in the metastatic breast cancer cell line MDA-MB-231. Cancer Res. 2007;67:4182–4189. doi: 10.1158/0008-5472.CAN-06-3184. [DOI] [PubMed] [Google Scholar]
- 27.Witkiewicz AK, Casimiro MC, Dasgupta A, Mercier I, Wang C, Bonuccelli G, et al. Towards a new “stromal-based” classification system for human breast cancer prognosis and therapy. Cell Cycle. 2009;8:1654–1658. doi: 10.4161/cc.8.11.8544. [DOI] [PubMed] [Google Scholar]
- 28.Witkiewicz AK, Dasgupta A, Nguyen KH, Liu C, Kovatich AJ, Schwartz GF, et al. Stromal caveolin-1 levels predict early DCIS progression to invasive breast cancer. Cancer Biol Ther. 2009;8:1167–1175. doi: 10.4161/cbt.8.11.8874. [DOI] [PubMed] [Google Scholar]
- 29.Witkiewicz AK, Dasgupta A, Sammons S, Er O, Potoczek MB, Guiles F, et al. Loss of stromal caveolin-1 expression predicts poor clinical outcome in triple negative and basal-like breast cancers. Cancer Biol Ther. 2010;10:135–143. doi: 10.4161/cbt.10.2.11983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witkiewicz AK, Dasgupta A, Sotgia F, Mercier I, Pestell RG, Sabel M, et al. An absence of stromal caveolin-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancers. Am J Pathol. 2009;174:2023–2034. doi: 10.2353/ajpath.2009.080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weidle UH, Scheuer W, Eggle D, Klostermann S, Stockinger H. Cancer-related issues of CD147. Cancer Genomics Proteomics. 2010;7:157–169. [PubMed] [Google Scholar]
- 32.Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14:518–527. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- 33.Hu Z, Fan C, Livasy C, He X, Oh DS, Ewend MG, et al. A compact VEGF signature associated with distant metastases and poor outcomes. BMC Med. 2009;7:9. doi: 10.1186/1741-7015-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sloan EK, Ciocca D, Pouliot N, Natoli A, Restall C, Henderson M, et al. Stromal cell expression of caveolin-1 predicts outcome in breast cancer. Am J Pathol. 2009;174:2035–2043. doi: 10.2353/ajpath.2009.080924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Vizio D, Morello M, Sotgia F, Pestell RG, Freeman MR, Lisanti MP. An absence of stromal caveolin-1 is associated with advanced prostate cancer, metastatic disease and epithelial Akt activation. Cell Cycle. 2009;8:2420–2424. doi: 10.4161/cc.8.15.9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonuccelli G, Tsirigos A, Whitaker-Menezes D, Pavlides S, Pestell RG, Chiavarina B, et al. Ketones and lactate “fuel” tumor growth and metastasis: Evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle. 2010;9:3506–3514. doi: 10.4161/cc.9.17.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez-Outschoorn UE, Prisco M, Ertel A, Tsirigos A, Lin Z, Pavlides S, et al. Ketones and lactate increase cancer cell “stemness,” driving recurrence, metastasis and poor clinical outcome in breast cancer: Achieving personalized medicine via Metabolo-Genomics. Cell Cycle. 2011;10:1271–1286. doi: 10.4161/cc.10.8.15330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Levi AJ, Halestrap AP. Substrate and inhibitor specificities of the monocarboxylate transporters of single rat heart cells. Am J Physiol. 1996;270:476–484. doi: 10.1152/ajpheart.1996.270.2.H476. [DOI] [PubMed] [Google Scholar]
- 39.Muller F, Huber K, Pfannkuche H, Aschenbach JR, Breves G, Gabel G. Transport of ketone bodies and lactate in the sheep ruminal epithelium by monocarboxylate transporter 1. Am J Physiol Gastrointest Liver Physiol. 2002;283:1139–1146. doi: 10.1152/ajpgi.00268.2001. [DOI] [PubMed] [Google Scholar]
- 40.Brizel DM, Schroeder T, Scher RL, Walenta S, Clough RW, Dewhirst MW, et al. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51:349–353. doi: 10.1016/s0360-3016(01)01630-3. [DOI] [PubMed] [Google Scholar]
- 41.Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfor K, Rofstad EK, et al. High lactate levels predict likelihood of metastases, tumor recurrence and restricted patient survival in human cervical cancers. Cancer Res. 2000;60:916–921. [PubMed] [Google Scholar]
- 42.Walenta S, Mueller-Klieser WF. Lactate: Mirror and motor of tumor malignancy. Semin Radiat Oncol. 2004;14:267–274. doi: 10.1016/j.semradonc.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Walenta S, Salameh A, Lyng H, Evensen JF, Mitze M, Rofstad EK, et al. Correlation of high lactate levels in head and neck tumors with incidence of metastasis. Am J Pathol. 1997;150:409–415. [PMC free article] [PubMed] [Google Scholar]
- 44.Sculier JP, Nicaise C, Klastersky J. Lactic acidosis: A metabolic complication of extensive metastatic cancer. Eur J Cancer Clin Oncol. 1983;19:597–601. doi: 10.1016/0277-5379(83)90174-8. [DOI] [PubMed] [Google Scholar]
- 45.Varanasi UR, Carr B, Simpson DP. Lactic acidosis associated with metastatic breast carcinoma. Cancer Treat Rep. 1980;64:1283–1285. [PubMed] [Google Scholar]
- 46.McConnell AA, Parfitt VL, Walker PR. An unusual case of shock in a young woman. Postgrad Med J. 1989;65:120. doi: 10.1136/pgmj.65.760.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Warner E. Type B lactic acidosis and metastatic breast cancer. Breast Cancer Res Treat. 1992;24:75–79. doi: 10.1007/BF01832361. [DOI] [PubMed] [Google Scholar]
- 48.Evans TR, Stein RC, Ford HT, Gazet JC, Chamberlain GV, Coombes RC. Lactic acidosis. A presentation of metastatic breast cancer arising in pregnancy. Cancer. 1992;69:453–456. doi: 10.1002/1097-0142(19920115)69:2<453::aid-cncr2820690230>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 49.Cheng JC, Esparza SD, Knez VM, Sakamoto KM, Moore TB. Severe lactic acidosis in a 14-year-old female with metastatic undifferentiated carcinoma of unknown primary. J Pediatr Hematol Oncol. 2004;26:780–782. doi: 10.1097/00043426-200411000-00021. [DOI] [PubMed] [Google Scholar]
- 50.Philp NJ, Ochrietor JD, Rudoy C, Muramatsu T, Linser PJ. Loss of MCT1, MCT3 and MCT4 expression in the retinal pigment epithelium and neural retina of the 5A11/basigin-null mouse. Invest Ophthalmol Vis Sci. 2003;44:1305–1311. doi: 10.1167/iovs.02-0552. [DOI] [PubMed] [Google Scholar]
- 51.Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science. 2004;305:99–103. doi: 10.1126/science.1096485. [DOI] [PubMed] [Google Scholar]
- 52.Bueno V, Binet I, Steger U, Bundick R, Ferguson D, Murray C, et al. The specific monocarboxylate transporter (MCT1) inhibitor, AR-C117977, a novel immunosuppressant, prolongs allograft survival in the mouse. Transplantation. 2007;84:1204–1207. doi: 10.1097/01.tp.0000287543.91765.41. [DOI] [PubMed] [Google Scholar]
- 53.Ovens MJ, Davies AJ, Wilson MC, Murray CM, Halestrap AP. AR-C155858 is a potent inhibitor of monocarboxylate transporters MCT1 and MCT2 that binds to an intracellular site involving transmembrane helices 7–10. Biochem J. 2010;425:523–530. doi: 10.1042/BJ20091515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sotgia F, Del Galdo F, Casimiro MC, Bonuccelli G, Mercier I, Whitaker-Menezes D, et al. Caveolin-1−/− null mammary stromal fibroblasts share characteristics with human breast cancer-associated fibroblasts. Am J Pathol. 2009;174:746–761. doi: 10.2353/ajpath.2009.080658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barrett T, Troup DB, Wilhite SE, Ledoux P, Rudnev D, Evangelista C, et al. NCBI GEO: mining tens of millions of expression profiles—database and tools update. Nucl Acids Res. 2007;35:760–765. doi: 10.1093/nar/gkl887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brazma A, Parkinson H, Sarkans U, Shojatalab M, Vilo J, Abeygunawardena N, et al. ArrayExpress—a public repository for microarray gene expression data at the EBI. Nucleic Acids Res. 2003;31:68–71. doi: 10.1093/nar/gkg091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ertel A. Bimodal gene expression and biomarker discovery. Cancer Inform. 2010;9:11–14. doi: 10.4137/cin.s3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ertel A, Dean JL, Rui H, Liu C, Witkiewicz AK, Knudsen KE, et al. RB pathway disruption in breast cancer: differential association with disease subtypes, disease-specific prognosis and therapeutic response. Cell Cycle. 2010;9:4153–4163. doi: 10.4161/cc.9.20.13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu Z, Fan C, Oh DS, Marron JS, He X, Qaqish BF, et al. The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genomics. 2006;7:96. doi: 10.1186/1471-2164-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.