Abstract
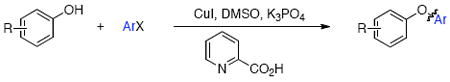
Cu-catalyzed O-arylation of phenols with aryl iodides and bromides can be performed under mild condition in DMSO/K3PO4 using picolinic acid as the ligand for copper. This method tolerates a variety of functional groups and is effective in the synthesis of hindered diaryl ethers and heteroaryl ethers.
The diaryl ether linkage is present in a range of important compounds including a number of potential pharmaceuticals,1-4 commercially available engineering thermoplastics5,6 and herbicides (Scheme 1).7-9 This motif also appears in biologically active natural products, notably in the mammalian hormone thyroxine10 and the vancomycin family of antibiotics.11 There has been recent interest in the synthesis of atropisomeric diaryl ethers12,13 as these may have application as molecular gears.14
Scheme 1.
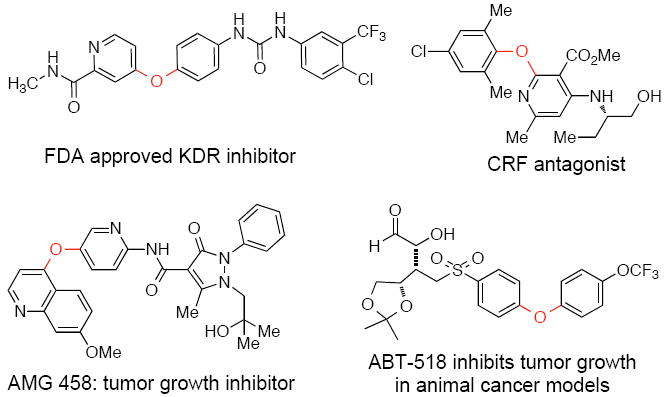
Selected biologically active diaryl ethers
Diaryl ethers are classically made by the Ullmann reaction15 of phenols with aryl halides promoted by stoichiometric or greater quantities of copper at high temperatures (125-300 °C) in polar solvents (typically pyridine or DMF), conditions which are unsuitable for the construction of complex molecules.16-21
In an important advance, Lam,22 Chan23 and Evans24 developed the Cu-catalyzed coupling of arylboronic acids with phenols.16,25 The ability to use stable, and in some cases commercially available, boronic acids in these reactions was a considerable step forward and these reactions have been applied in the synthesis of a number of complex natural products.16,19 Despite the advantages of this method a number of limitations remain, typically an excess of the boronic acid component is required for optimal yields and the use of heterocyclic substrates and ortho substituted coupling partners in intermolecular reactions is rare. Furthermore, the required boronic acids, when commercially available, can be expensive. The diaryl ether linkage can also be forged by an SNAr reaction between a phenol and an activated aryl fluoride.26 This method holds promise as it can be performed in the presence of a weak base and as such has also seen application in complex molecule synthesis. Unfortunately, suitable aryl fluoride substrates are not always readily available and the reaction lacks generality as it is limited to the coupling of electron-rich or electron-neutral phenols with highly activated aryl fluorides.
As a result of these problems, efforts continue to find a general method for formation of diaryl ethers. Much interest has focused on the metal-catalyzed coupling of phenols with aryl halides due to the low cost and ready availability of the starting materials. Pd-catalyzed methods hold considerable promise, especially in allowing economically attractive aryl chlorides to be used as substrates, however, a number of limitations remain.27-32
In 1997 it was shown that the Cu-catalyzed Ullmann-type coupling of phenols and aryl halides can be performed in the presence of the weak base Cs2CO3 in non-polar solvents and in some cases naphthoic acid was found to promote the reaction.33 Since this discovery a number of efficient Cu/ligand systems have been described and the high functional group tolerance and low air- and moisture-sensitivity has prompted ongoing interest in these reactions. 16-21,34-48 Unfortunately, despite this effort, little progress has been made in ameliorating some of the key limitations of these reactions, namely the difficulty in coupling heterocyclic compounds and the fact that ortho-substituted coupling partners are often challenging. We set out to attempt to address these issues and to move closer to a general set of reaction conditions for the synthesis of diaryl ethers.
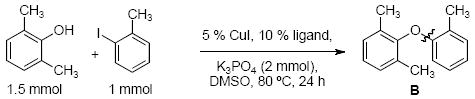
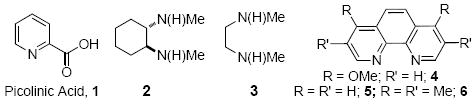
We have recently shown that a catalyst system composed of CuI and picolinic acid in combination with K3PO4/DMSO permits the selective O-arylation of aminophenols,49 and we discovered that this system is also expedient in the coupling of 2,6-dimethylphenol with 2-iodotoluene (Table 1), a cross-coupling reaction that has not previously been reported with a Cu catalyst. Screening a range of base/solvent combinations showed K3PO4/DMSO to be much more efficacious than the more commonly used Cs2CO3/1,4-dioxane system (yields 100% and 27% respectively).17-19,39-46 Using this base/solvent combination pyrrole-2-carboxylic acid and N,N-dimethylglycine also proved to be effective ligands, however, we elected to pursue the use of picolinic acid as it is economically more attractive.50
Table 1.
Comparison of various ligands in the coupling of 2,6-dimethylphenol with 2-iodotoluene
 | ||
|---|---|---|
| entry | ligand | GC-yield B(%) |
| 1 | 1 | 100 |
| 2 | 2 | 17 |
| 3 | 3 | 15 |
| 4 | 4 | 40 |
| 5 | 5 | 14 |
| 6 | 6 | 15 |
| 7 | 7 | 73 |
| 8 | 8 | 12 |
| 9 | 9 | 78 |
| 10 | 10 | 97 |
| 11 | 11 | 100 |
| ||
| ||
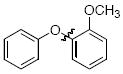
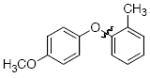
The scope of the reaction was explored (Table 2) with a range of ortho-substituted phenols and aryl halides which are usually difficult substrates for Cu-catalyzed methods (in contrast to Pd-catalyzed reactions). By using picolinic acid 1 as ligand, o-cresol and 2,6-dimethylphenol could be coupled with a variety of ortho-substituted aryl halides (entries 1-3; 4 and 5). 2-Methoxyphenol also coupled effectively with 4-iodotoluene (entry 6) as well as with 2-bromotoluene (entry 7). Note that the reactions of aryl bromides were slower than those of the analogous aryl iodides and required higher catalyst loading.
Table 2.
 | |||
|---|---|---|---|
| entry | product | Conditions | yield (%) |
| 1 |
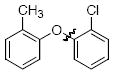
|
A | 68 |
| 2 |
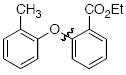
|
A | 79 |
| 3 |
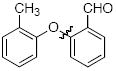
|
A | 85 |
| 4 |
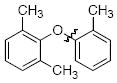
|
A | 89 |
| 5 |
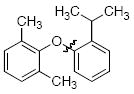
|
A | 74d |
| 6 |
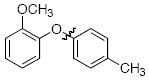
|
A | 92b |
| 7 |
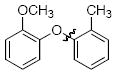
|
B | 83c |
| 8 |
|
A | 85b |
| 9 |
|
B | 78 |
Isolated yield, average of two runs.
90 °C, 10 mol% CuI, 20 mol% 1.
105 °C.
10 mol% CuI, 20 mol% 1.
Cross-coupling reactions between phenols and heteroaryl halides were also investigated (Table 3).35-38,51 Employing our standard protocol with 1, we were able to obtain heteroaryl ethers from the reaction of substituted phenols and 3-bromo-2-formylbenzothiophene (entry 1), 3-iodothiophene (entry 2), 5-bromopyrimidine52 (entry 3) and 2- and 3-iodopyridine (entries 4 and 5) in good yield (Table 3). Heteroaryl halides such as 3-bromoquinolines (entry 6), 5-bromoisoquinolines (entry 7) and 4-bromoisoquinolines (entry 8) could be coupled with electron-deficient, -neutral and hindered phenols (Table 3).53 Cu-catalyzed etherification can also be challenging when electron-withdrawing groups are present on the phenol component. An excellent yield of the desired diaryl ether could, however, be obtained when 4-cyanophenol (entry 9), methyl 4-hydroxybenzoate (entry 10) and 4-bromophenol (entry 11) were used as the nucleophile. We note, however, that 5-membered ring heteroaryl halides containing 2 heteroatoms such as 4-bromoisoxazole (entry 12) and 4-bromo-1,3,5-trimethylpyrazole (entry 13) did not provide any of the desired product under these reaction conditions.
Table 3.
 | |||
|---|---|---|---|
| entry | product | Conditions | yield (%) |
| 1 |
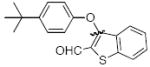
|
B | 69 |
| 2 |
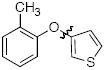
|
A | 71 |
| 3 |
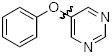
|
B | 70 |
| 4 |
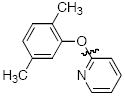
|
A | 88 |
| 5 |
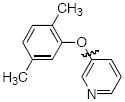
|
A | 85 |
| 6 |
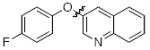
|
B | 91 |
| 7 |
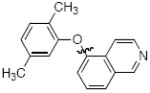
|
B | 89 |
| 8 |
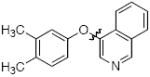
|
B | 69 |
| 9 |
|
B | 92 |
| 10 |
|
B | 91 |
| 11 |
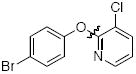
|
B | 87 |
| 12 |
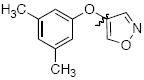
|
B | 0 |
| 13 |
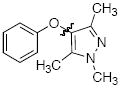
|
B | 0 |
Isolated yield, average of two runs.
Next we studied the synthesis of diaryl ethers possessing a heteroaryl moiety on both the nucleophilic and electrophilic components (Table 4). The construction of such diaryl ethers by metal-catalyzed cross-coupling is rare.51,55 We found that by applying our standard protocol based on CuI and 1, 3-hydroxypyridines were successfully coupled with a range of aryl halides (entries 1, 2 and 3). Furthermore, 6-hydroxyquinoline could be arylated with a bromopyridine even in the presence of free N-H groups (entry 4).49 The O-arylation of 8-hydroxyquinoline (entry 5) with a substituted pyridine also proceeded smoothly even though this compound has previously been employed as an effective ligand for Cu-catalyzed arylation of phenols.56
Table 4.
 | |||
|---|---|---|---|
| entry | product | Conditions | yield (%) |
| 1 |
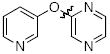
|
A | 84 |
| 2 |
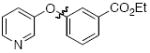
|
A | 78 |
| 3 |
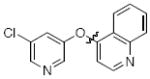
|
B | 78 |
| 4 |
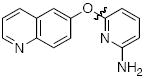
|
B | 95 |
| 5 |
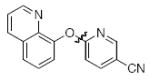
|
B | 96 |
Isolated yield, average of two runs.
In summary, we have devised an efficient, experimentally simple, and economically attractive method for Cu-catalyzed O-arylation of phenols with aryl iodides and bromides. This method tolerates a variety of functional groups and provides a considerable advance in the ability to synthesize hindered and heteroaryl diaryl ethers by Cu-catalyzed etherification.
Experimental Procedure
General procedure for synthesis of diaryl ether
An oven-dried screw cap test tube was charged with a magnetic stirbar, copper(I) iodide (9.5 mg, 0.05 mmol, 5 mol%), picolinic acid, 1 (12.3 mg, 0.10 mmol, 10 mol%), aryl halide (if solid; 1.0 mmol), ArOH (1.2 mmol) and K3PO4 (424 mg, 2.0 mmol). The tube was then evacuated and back-filled with argon. The evacuation/backfill sequence was repeated two additional times. Under a counterflow of argon, remaining liquid reagents were added, followed by dimethylsulfoxide (2.0 mL) by syringe. The tube was placed in a preheated oil bath at 80 °C and the reaction mixture was stirred vigorously for 24 hr. The reaction mixture was cooled to room temperature. Ethyl acetate (10 mL) and H2O (1 mL) were added and the mixture was stirred. The organic layer was separated and the aqueous layer was extracted twice more with ethyl acetate (10 mL). Combined organic layer was dried over Na2SO4 and filtered through the pad of silica gel. The filtrate was concentrated and the resulting residue was purified via the Biotage SP4 (silica- packed SNAP cartridge, KP-Sil, 10 g) using hexane: ethyl acetate (3:1).
Supplementary Material
Acknowledgments
This activity is supported by an educational donation provided by Amgen and by funds from the National Institutes of Health (Grant GM-58160). We are grateful to Dr. David Surry for comments and help with this manuscript. The NMR instruments used for this study were furnished by funds from the National Science Foundation (CHE 9808061 and DBI 9729592).
Footnotes
Supporting Information Available: Procedural and spectral data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Hodous BL, Geuns-Meyer SD, Hughes PE, Albrecht BK, Bellon S, Bready J, Caenepeel S, Cee VJ, Chaffee SC, Coxon A, Emery M, Fretland J, Gallant P, Gu Y, Hoffman D, Johnson RE, Kendall R, Kim JL, Long AM, Morrison M, Olivieri PR, Patel VF, Polverino A, Rose P, Tempest P, Wang L, Whittington DA, Zhao HL. J Med Chem. 2007;50:611–626. doi: 10.1021/jm061107l. [DOI] [PubMed] [Google Scholar]
- 2.Caron S, Do NM, Sieser JE, Whritenour DC, Hill PD. Org Process Res Dev. 2009;13:324–330. [Google Scholar]
- 3.Liu LB, Siegmund A, Xi N, Kaplan-Lefko P, Rex K, Cheti A, Lin J, Moriguchi J, Berry L, Huang L, Teffera Y, Yang YI, Zhang YH, Bellon SF, Lee M, Shimanovich R, Bak A, Dominguez C, Norman MH, Harmange JC, Dussault I, Kimt TS. J Med Chem. 2008;51:3688–3691. doi: 10.1021/jm800401t. [DOI] [PubMed] [Google Scholar]
- 4.Wada CK, Holms JH, Curtin ML, Dai Y, Florjancic AS, Garland RB, Guo Y, Heyman HR, Stacey JR, Steinman DH, Albert DH, Bouska JJ, Elmore IN, Goodfellow CL, Marcotte PA, Tapang P, Morgan DW, Michaelides MR, Davidsen SK. J Med Chem. 2002;45:219–232. doi: 10.1021/jm0103920. [DOI] [PubMed] [Google Scholar]
- 5.Laskoski M, Dominguez DD, Keller TM. J Polym Sci Part A: Polym Chem. 2006;44:4559–4565. [Google Scholar]
- 6.Labadie JW, Hedrick JL, Ueda M. Am Chem Soc Symp Ser. 1996;624:210. [Google Scholar]
- 7.Draper WM, Casida JE. J Agric Food Chem. 1983;31:227–231. doi: 10.1021/jf00116a011. [DOI] [PubMed] [Google Scholar]
- 8.Draper WM, Casida JE. J Agric Food Chem. 1983;31:1201–1207. doi: 10.1021/jf00120a015. [DOI] [PubMed] [Google Scholar]
- 9.Scrano L, Bufo SA, D’Auria M, Meallier P, Behechti A, Shramm KW. J Environ Qual. 2002;31:268–274. doi: 10.2134/jeq2002.2680. [DOI] [PubMed] [Google Scholar]
- 10.Tan ES, Miyakawa M, Bunzow JR, Grandy DK, Scanlan TS. J Med Chem. 2007;50:2787–2798. doi: 10.1021/jm0700417. [DOI] [PubMed] [Google Scholar]
- 11.Nicolaou KC, Boddy CNC, Brase S, Winssinger N. Angew Chem Int Ed. 1999;38:2097–2152. doi: 10.1002/(sici)1521-3773(19990802)38:15<2096::aid-anie2096>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12.Betson MS, Clayden J, Worrall CR, Peace S. Angew Chem, Int Ed. 2006;45:5803–5807. doi: 10.1002/anie.200601866. [DOI] [PubMed] [Google Scholar]
- 13.Clayden J, Worrall CP, Moran WJ, Helliwell M. Angew Chem, Int Ed. 2008;47:3234–3237. doi: 10.1002/anie.200705660. [DOI] [PubMed] [Google Scholar]
- 14.Fuji K, Oka T, Kawabata T, Kinoshita T. Tetrahedron Lett. 1998;39:1373–1376. [Google Scholar]
- 15.Ullmann F. Ber Dtsch Chem Ges. 1904;37:853–854. [Google Scholar]
- 16.Ley SV, Thomas AW. Angew Chem, Int Ed. 2003;42:5400–5449. doi: 10.1002/anie.200300594. [DOI] [PubMed] [Google Scholar]
- 17.Frlan R, Kikelj D. Synthesis. 2006:2271–2285. [Google Scholar]
- 18.Sawyer JS. Tetrahedron. 2000;56:5045–5065. [Google Scholar]
- 19.Evano G, Blanchard N, Toumi M. Chem Rev. 2008;108:3054–3131. doi: 10.1021/cr8002505. [DOI] [PubMed] [Google Scholar]
- 20.Lindley J. Tetrahedron. 1984;40:1433–1456. [Google Scholar]
- 21.Beletskaya IP, Cheprakov AV. Coord Chem Rev. 2004;248:2337–2364. [Google Scholar]
- 22.Lam PYS, Clark CG, Saubern S, Adams J, Winters MP, Chan DMT, Combs A. Tetrahedron Lett. 1998;39:2941–2944. [Google Scholar]
- 23.Chan DMT, Monaco KL, Wang RP, Winters MP. Tetrahedron Lett. 1998;39:2933–2936. [Google Scholar]
- 24.Evans DA, Katz JL, West TR. Tetrahedron Lett. 1998;39:2937–2940. [Google Scholar]
- 25.Theil F. Angew Chem Int Ed. 1999;38:2345–2347. doi: 10.1002/(sici)1521-3773(19990816)38:16<2345::aid-anie2345>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Zhu JP. Synlett. 1997:133. [Google Scholar]
- 27.Burgos CH, Barder TE, Huang XH, Buchwald SL. Angew Chem, Int Ed. 2006;45:4321–4326. doi: 10.1002/anie.200601253. [DOI] [PubMed] [Google Scholar]
- 28.Shelby Q, Kataoka N, Mann G, Hartwig JF. J Am Chem Soc. 2000;122:10718–10719. [Google Scholar]
- 29.Mann G, Incarvito C, Rheingold AL, Hartwig JF. J Am Chem Soc. 1999;121:3224–3225. [Google Scholar]
- 30.Schwarz N, Pews-Davtyan A, Alex K, Tillack A, Beller M. Synthesis. 2007:3722–3730. [Google Scholar]
- 31.Harkal S, Kumar K, Michalik D, Zapf A, Jackstell R, Rataboul F, Riermeier T, Monsees A, Beller M. Tetrahedron Lett. 2005;46:3237–3240. [Google Scholar]
- 32.Hu T, Schulz T, Torborg C, Chen X, Wang J, Beller M, Huang J. Chem Comm. 2009 doi: 10.1039/b915249k. [DOI] [PubMed] [Google Scholar]
- 33.Marcoux JF, Doye S, Buchwald SL. J Am Chem Soc. 1997;119:10539–10540. [Google Scholar]
- 34.Xia N, Taillefer M. Chem-Eur J. 2008;14:6037–6039. doi: 10.1002/chem.200800436. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Q, Wang DP, Wang XY, Ding K. J Org Chem. 2009;74:7187–7190. doi: 10.1021/jo9012157. [DOI] [PubMed] [Google Scholar]
- 36.D’Angelo ND, Peterson JJ, Booker SK, Fellows I, Dominguez C, Hungate R, Reider PJ, Kim TS. Tetrahedron Lett. 2006;47:5045–5048. [Google Scholar]
- 37.Ouali A, Spindler JF, Cristau HJ, Taillefer M. Adv Synth Catal. 2006;348:499–505. [Google Scholar]
- 38.Lipshutz BH, Unger JB, Taft BR. Org Lett. 2007;9:1089–1092. doi: 10.1021/ol0700409. [DOI] [PubMed] [Google Scholar]
- 39.Monnier F, Taillefer M. Angew Chem, Int Ed. 2009;48:6954–6971. doi: 10.1002/anie.200804497. [DOI] [PubMed] [Google Scholar]
- 40.Cai Q, Zou BL, Ma DW. Angew Chem, Int Ed. 2006;45:1276–1279. doi: 10.1002/anie.200503538. [DOI] [PubMed] [Google Scholar]
- 41.Cai Q, He G, Ma DW. J Org Chem. 2006;71:5268–5273. doi: 10.1021/jo0606960. [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi D, Asano I, Osakada K. J Org Chem. 2006;71:8614–8617. doi: 10.1021/jo0609982. [DOI] [PubMed] [Google Scholar]
- 43.Ma DW, Cai QA. Acc Chem Res. 2008;41:1450–1460. doi: 10.1021/ar8000298. [DOI] [PubMed] [Google Scholar]
- 44.Ma DW, Cai Q. Org Lett. 2003;5:3799–3802. doi: 10.1021/ol0350947. [DOI] [PubMed] [Google Scholar]
- 45.Chen YJ, Chen HH. Org Lett. 2006;8:5609–5612. doi: 10.1021/ol062339h. [DOI] [PubMed] [Google Scholar]
- 46.Schareina T, Zapf A, Cotte A, Muller N, Beller M. Synthesis. 2008:3351–3355. [Google Scholar]
- 47.Lv X, Bao WL. J Org Chem. 2007;72:3863–3867. doi: 10.1021/jo070443m. [DOI] [PubMed] [Google Scholar]
- 48.For a full list of copper-catalyzed diaryl ether formation, see Supporting Informations.
- 49.Maiti D, Buchwald SL. J Am Chem Soc. 131:17423–17429. doi: 10.1021/ja9081815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.At current prices from Sigma-Aldrich: picolinic acid $26.4/mol, pyrrole-2-carboxylic acid $2346/mol and N,N-dimethylglycine $996/mol.
- 51.Corbett JW, Rauckhorst MR, Qian F, Hoffman RL, Knauer CS, Fitzgerald LW. Bioorg Med Chem Lett. 2007;17:6250–6256. doi: 10.1016/j.bmcl.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 52.Cherng YH. Tetrahedron. 2002;58:887–890. [Google Scholar]
- 53.The control experiments without catalyst were performed to confirm these products are not generated by SNAr reaction, but by picolinic acid-ligated copper-catalyzed C-O bond forming reaction.
- 54.We found that the reduction of aryl halide (Ar-X to Ar-H) was obtained as the major side reaction. Thus in Table 2, ethylbenzoate (5 %, entry 6) and benzaldehyde (2 %, entry 7) were detected. Similarly, isoquinoline (10 %, entry 1; 5%, entry 2), benzo[b]thiophene-2-carbaldehyde (entry 4) and pyridine (1 %, entry 7) were detected in Table 3 as were quinoline (2 %, entry 1) and trace of nicotinonitrile in Table 4, entry 5.
- 55.Altman RA, Buchwald SL. Org Lett. 2007;9:643–646. doi: 10.1021/ol062904g. [DOI] [PubMed] [Google Scholar]
- 56.Fagan PJ, Hauptman E, Shapiro R, Casalnuovo A. J Am Chem Soc. 2000;122:5043–5051. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


