Abstract
Background: Evidence suggests a relation between short sleep duration and obesity.
Objective: We assessed energy balance during periods of short and habitual sleep in normal-weight men and women.
Design: Fifteen men and 15 women aged 30–49 y with a body mass index (in kg/m2) of 22–26, who regularly slept 7–9 h/night, were recruited to participate in this crossover inpatient study. All participants were studied under short (4 h/night) and habitual (9 h/night) sleep conditions, in random order, for 5 nights each. Food intake was measured on day 5, and energy expenditure was measured with the doubly labeled water method over each period.
Results: Participants consumed more energy on day 5 during short sleep (2813.6 ± 593.0 kcal) than during habitual sleep (2517.7 ± 593.0 kcal; P = 0.023). This effect was mostly due to increased consumption of fat (20.7 ± 37.4 g; P = 0.01), notably saturated fat (8.7 ± 20.4 g; P = 0.038), during short sleep. Resting metabolic rate (short sleep: 1455.4 ± 129.0 kcal/d; habitual sleep: 1486.5 ± 129.5 kcal/d; P = 0.136) and total energy expenditure (short sleep: 2589.2 ± 526.5 kcal/d; habitual sleep: 2611.1 ± 529.0 kcal/d; P = 0.832) did not differ significantly between sleep phases.
Conclusions: Our data show that a reduction in sleep increases energy and fat intakes, which may explain the associations observed between sleep and obesity. If sustained, as observed, and not compensated by increased energy expenditure, the dietary intakes of individuals undergoing short sleep predispose to obesity. This trial is registered at clinicaltrials.gov as NCT00935402.
INTRODUCTION
Short sleep duration is a risk factor for weight gain in both adults and children (1–3). Along with other unhealthy behaviors, studies have shown that adults with short sleep duration, generally <7 h/night, have a greater odds of being obese than do those who sleep ≈7–8 h/night (2). These studies suggest that short sleep is related to obesity yet cannot prove a cause-effect relation.
Clinical studies of reductions in sleep duration in healthy young men have shown that hormones involved in appetite regulation, such as leptin and ghrelin, are altered by sleep duration (4–6). Such studies also suggest that short sleep duration may lead to a hormonal state that would predispose to overeating. However, this has not been universally observed (7, 8). Moreover, few studies have precisely measured food intake under periods of regular and reduced sleep duration. Two studies included men only and found either greater food intake after a single night of a 4-h reduction in sleep (9) or no difference after 2 nights of short or habitual sleep (8), whereas another study of longer duration, which included both men and women, found no effect on total energy intake over a 14-d period in men and women whose sleep was reduced by 2 h/night (7). However, data showed that energy intake from snacks was greater during the shorter sleep period. Another study in women only found that increasing sleep curtailment over a 4-night period led to increased energy intakes, body weight, and leptin and no change in energy expenditure (10).
The purpose of this study was to determine whether a reduction in sleep duration, by ≈3–4 h/night for 5 nights, affected food intake and energy expenditure, thereby altering energy balance in normal-weight men and women. We also assessed whether ratings of appetite and satiety were different between these periods of short (4 h/night) and habitual (9 h/night) sleep. We hypothesized that a short sleep duration would lead to greater food intake and less energy expenditure than would habitual sleep.
SUBJECTS AND METHODS
Participants
Participants were recruited through Internet advertisements. Inclusion criteria included age between 30 and 45 y, body mass index (BMI; in kg/m2) between 22 and 26, and regular sleep duration between 7 and 9 h/night without napping. Sleep duration was assessed during a 2-wk screening period with the use of actigraphy and sleep diaries. The average sleep duration over 14 nights was required to fall between 7 and 9 h/night, with ≥10 nights of sleep of ≥7 h and <4 nights with <6 h of sleep. Smokers and individuals with type 2 diabetes, a history of alcohol or substance abuse, excessive caffeine intakes, or eating, sleeping, or neurologic disorders were excluded from participating in this study. Participants were also excluded if they were shift workers, if they traveled across time zones within 4 wk of the study, or if their work required long-distance driving or operating heavy equipment. Thirty participants (15 men, 15 women) who met these criteria were enrolled in the study. Participants were enrolled in pairs, and each pair was randomly assigned to a sleep sequence. Each participant provided written informed consent before being enrolled in the study.
Overview
The study was a randomized inpatient study with 2 phases (short and habitual sleep), each of which lasted 6 d and differed in the amount of time spent in bed each night. During the short sleep phase, participants were permitted to go to bed at 0100 and were woken at 0500. During the habitual sleep phase, bedtimes were between 2200 and 0700. Sleep duration was confirmed by polysomnography (PSG). Naps were not permitted during the day, and study personnel ensured that all participants remained awake throughout the day. Food intake during the first 4 d of each phase was strictly controlled: participants ate a fixed diet at specific times. During the last 2 d, food intake was self-selected but weighed and recorded by study personnel. The protocol was approved by the Institutional Review Boards of St Luke's–Roosevelt Hospital Center and Columbia University (New York, NY).
Intervention
The participants were studied under each of 2 sleep phases: short and habitual. Each phase followed the same protocol and differed only in the amount of time participants spent in bed each night. Each sleep phase started 4 wk apart, providing 23 nights to recover from the previous sleep phase. Both inpatient phases took place at Clinilabs, a research sleep laboratory (New York, NY). Participants were inpatients but were permitted to leave the laboratory under study personnel supervision. This ensured that participants would not fall asleep during the day. Participants checked in on the morning of day 1 in the fasted state. At that time, they were weighed and had their vital signs taken. Urine and saliva samples were collected to determine baseline isotope enrichment for the doubly labeled water (DLW) measurement of energy expenditure. Then, participants were given a single oral dose of DLW consisting of 0.10 g 10 atom percent excess (APE) of 18O and 0.08 g 99.8 APE of 2H per kg body weight followed by a water rinse. During this time, postdose saliva was sampled at 3- and 4-h time points for determination of total body water (TBW) from 2H isotope dilution space. Then, on the mornings of days 2 and 6, enriched urine samples were collected separately to determine elimination rates for the 2 isotopes (2H and 18O). All samples were stored in a freezer (−80°C) until shipment to the Richardson Centre for Functional Foods and Nutraceuticals (Winnipeg, Manitoba) for isotopic analysis.
During the first 4 d of each phase, participants were fed a controlled diet based on their estimated energy requirements, estimated by the Harris-Benedict equation with an activity factor of 1.3 (11). Meals were served at 0800, 1200, and 1900, and a snack was provided at 1600. Each meal provided 30% of daily energy requirements; the snack supplied the remaining 10%. On day 4, during the last day of the controlled feeding period and before the start of the ad libitum feeding portion of the study, the participants filled out visual analog scales (VAS) assessing their feelings of appetite and satiety. The participants rated their feelings, on a scale of 0 to 10, to the following questions: 1) How hungry do you feel right now? 2) How satisfied do you feel right now? 3) How full do you feel right now? 4) How much do you think you could eat right now? 5) How energetic do you feel right now? 6) How sluggish do you feel right now? 7) How much would you like to eat something sweet right now? 8) How much would you like to eat something salty right now? 9) How much would you like to eat something savory right now? 10) How much would you like to eat fruits and vegetables right now? A rating of 0 corresponded to “not at all” and a rating of 10 corresponded to “very much so.” VAS questionnaires were filled out every hour from 0700 to 2200.
Starting on the morning of day 5 until discharge, at 2000 h on day 6, participants self-selected their food intake. Various foods were available at the research laboratory, and participants were also given a monetary allowance of US$25 to purchase foods and beverages of their choice. The only restrictions imposed on them were that nutrient information be available for the items purchased and that beverages be nonalcoholic. Caffeinated beverages were limited to one per day. Food intake during this period was weighed and recorded by study personnel and entered into Diet Analysis Plus Software version 8.0 (Wadsworth, Florence, KY). However, because the participants were discharged at 2000 on day 6, we did not have complete dietary intake data for that day. Food intake was therefore assessed from day 5 records only. We found that, after the habitual sleep phase, participants tended to go out with friends at discharge, whereas they went home and slept after the short sleep phase. Food intake from day 6 was therefore not reliable or representative of free-living short sleep-habitual sleep habits. Because the environment was consistent for both the short and habitual sleep phases on day 5, these data are more reliable. From food records, we also calculated eating occasions. Eating occasions that were ≥20 min apart were considered separate eating occasions.
Sleep and energy expenditure
Sleep duration was assessed by PSG (Aurora Recording Systems, Gamma, version 4.9; Grass Technologies, West Warwick, RI). All PSG recordings were scored manually for sleep staging, arousals, periodic leg movements, and respiratory events by using the AASM 2007 criteria (12) by a certified benchmark scorer. Total recording time and percentage time in each sleep stage (including sleep efficiency, sleep latencies, and arousal indexes) were calculated. Recordings from the first sleep phase were used to detect sleep disordered breathing (apnea/hypopnea index) and periodic leg movement disorder and exclude participants who do not fit the protocol inclusion or exclusion criteria (apnea/hypopnea index ≥ 10 for the average of the first 2 nights).
Resting metabolic rate (RMR) was measured in the fasted state on the morning of day 5 at the Body Composition Unit of St Luke's–Roosevelt Hospital. Each participant rested for ≥30 min before beginning the test. RMR was measured by using a ventilated-hood indirect calorimetry system (Delta-Trac II Metabolic Monitor; SensorMedics, Yorba Linda, CA) (13). On a same day test-retest, the CV for our system was 2.3%.
Actigraphy was measured in a subset of participants (n = 18) for 6 d by using GT3× ActiGraph Activity monitors worn around the waist (Actigraph LLC, Pensacola, FL). Peak activity, total activity, and average activity were calculated by using the ActiLife software. Although the participants were inpatients throughout each phase of the study, they were permitted to leave the premises under study personnel supervision. They also had free access to a 24-h sports club in the building and could use the facilities at their leisure.
DLW laboratory analysis and calculation
All isotope measurements were made with an automated High Temperature Conversion Elemental Analyzer (Thermo Finnigan TC/EA) in combination with an Isotope Ratio Mass Spectrometer (IRMS, Thermo Finnigan DELTAplus V; Brennen, Germany) with continuous helium flow (>90 mL/min). The ratios of 2H/1H and 18O/16O were calibrated against Vienna Standard Mean Ocean Water (V-SMOW), Greenland Ice Sheet Precipitation (GISP), 302A, and IA-R056 (highly enriched water) standards within a 48-h period. Each sample was measured >5 times depending on the extent of the memory effect. Only reasonable values with minimal memory effect were picked for mean calculations. Gathering all data, 2H dilution space (DSd) in kg was first calculated as follows (14):
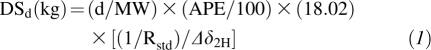 |
where d (in g) is the dose of 2H2O, MW is the molecular weight of 2H2O, APE is atom percent excess of 2H, Rstd is the ratio of 2H/H in the standard (1.5576 × 10−4), and Δδ2H is the enrichment change between pre- and postdose saliva samples in per mil units (‰). TBW (kg) was then calculated as DSd/1.041 (15).
Using the 2-point approach, elimination rates of 2H (kh) and 18O (ko) were computed from dividing changes in enrichment (Ef − Ei) by the corresponding time difference (tf − ti). Therefore, carbon dioxide production rates were calculated by using the formula (16):
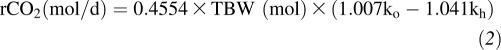 |
where ko and kh (day−1) are the elimination rates of 2H and 18O, respectively.
Finally, total energy expenditure (TEE; kcal/d) was determined from rCO2 and food quotient (FQ) assumed as 0.85 in the current study by using a modified version of the Weir formula (17):
Statistical analysis
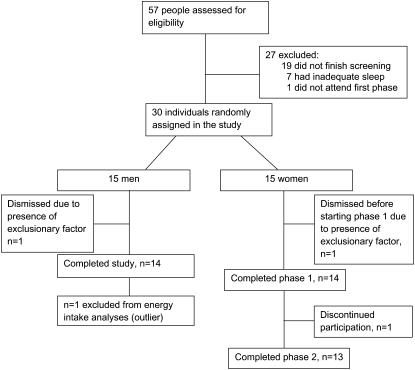
A total of 26 participants (13 men and 13 women) were included in the food intake analyses (Figure 1), and 26 were included in the RMR analysis (14 men and 12 women). One person who was an outlier for the food intake data remained in the RMR analysis because this measurement was taken before the free-living diet condition started. This man was considered an outlier for food intake measurement because his food intake during the habitual phase was almost twice his estimated energy requirements, and his consumption level was 3.6 times the SD of the intake of all other participants. One woman was not included in the RMR analysis because she went to the gym when she woke up on the morning of the measurement in the short sleep period. A total of 25 participants (13 men and 12 women) were included in the data analysis for DLW. One person was excluded from the TEE analysis because all delta values of his baseline urine sample were as high as his enriched samples, which was theoretically improbable. Actigraphy data were collected from a subset of 9 women and 9 men on days 2, 3, 5, and 6. Day 1 data were collected before the sleep intervention started, and day 4 was a day of very restricted activity because of catheter placement for frequent blood sampling. The percentage time spent in sedentary, light, moderate, heavy, and very heavy activity was determined based on the manufacturer's recommendations for activity counts. VAS data were analyzed for 11 women and 13 men because of too many missed ratings to obtain a meaningful area under the curve for the other participants. In pooled data, we had 71% power to detect a medium effect size (Cohen's f2 = 0.25) and 52% power to detect a low-medium effect size (Cohen's f2 = 0.2). When analyzing each sex separately, we had 42% power to detect a medium effect size (Cohen's f2 = 0.25) and 29% power to detect a low-medium effect size (Cohen's f2 = 0.2).
FIGURE 1.
Flow of participants throughout the study.
Mixed-effects analysis of variance was used to assess the effect of sleep on food intake variables after control for body weight, sex, and phase order. An interaction of sex × sleep was also assessed. RMR data were analyzed by using a mixed-effects model analysis of variance with control for age, sex, and body weight. Daily TEE was analyzed by using a mixed-effects model analysis of variance with control for sex, phase order, age, and baseline body weight. Actigraphy data were analyzed by using mixed-effects model analysis of variance with sleep, phase, and day as variables in the model. Data from the VAS were analyzed by using repeated-measures analysis of variance with compound symmetry and participant as a random variable. All models included phase order, sex, time, sleep, and the sleep × time interaction as independent variables. An interaction term of sleep × sex was also tested and removed from the model if it was not significant. If sleep × sex was significant, a 3-factor interaction with time (sleep × gender × time) was also explored. However, this interaction was not significant and was thus removed from those models. Eating occasions were compared by using a paired Student's t test and Wilcoxon's signed-rank test. Computations were performed by using Statistical Analysis Software version 9.2 (SAS Institute Inc, Cary, NC) and R version 2.9.2. Data are presented as adjusted means ± SD unless otherwise indicated. Statistical significance was defined as P < 0.05.
RESULTS
Baseline measures
Screening characteristics by sex and for all participants are presented in Table 1. Sleep duration during the 5 nights of short sleep was 226.2 ± 38.2 min (≈3 h 46 min) compared with 457.7 ± 38.2 min (≈7 h 38 min) for the habitual sleep phase (P < 0.0001). During the habitual sleep period, women slept significantly more than men (19.9 ± 52.4 min; P = 0.0033). Latency to first 10 min of sleep was significantly longer (P < 0.0001) during habitual sleep (21.1 ± 19.7 min) than during short sleep (7.1 ± 6.9 min). The percentage of time spent in all sleep stages was significantly longer during habitual sleep than during short sleep (stages 1, 2, and 3; P < 0.0001; rapid eye movement, P < 0.01) except for stage 4, which was shorter during habitual than during short sleep (P < 0.0001). The periodic limb movement index was not significantly different between the habitual and short sleep phases.
TABLE 1.
Participant characteristics1
| Characteristic | Men (n = 14) | Women (n = 13) |
| Age (y) | 36.6 ± 5.6 | 33.9 ± 4.3 |
| Weight (kg) | 77.5 ± 8.6 | 62.6 ± 5.4 |
| Height (cm) | 178.6 ± 6.4 | 164.8 ± 6.5 |
| BMI (kg/m2) | 24.1 ± 1.1 | 23.0 ± 1.1 |
| Estimated energy requirement (kcal/d) | 2309.9 ± 194.2 | 1805.2 ± 129.4 |
All values are means ± SDs.
Food intake measures
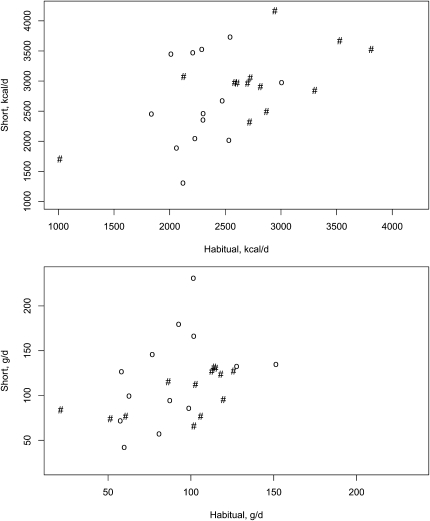
A main effect of sleep (P = 0.02) and sex (P = 0.02) on energy intake was observed (Table 2, Figure 2). No sex × sleep interaction for any of the nutrients analyzed was observed. Women had a 15.3% increase in energy intake during short sleep relative to habitual sleep (P = 0.07) and men had a 9.2% increase (P = 0.14). A main effect of sleep on fat (P = 0.01) and saturated fat (P = 0.04) intakes was observed. Women increased their intakes of fat and saturated fat by 39.0% (P = 0.007) and 61.7% (P = 0.02), respectively, compared with 10.3% (P = 0.32) and 9.6% (P = 0.57) for men. Carbohydrate intake was not affected by sleep duration (P = 0.19). Protein intakes tended to be higher during the period of short sleep than during the period of habitual sleep (P = 0.08). Fiber, sugar, and sodium intakes were not affected by sleep duration (P = 0.16, 0.24, and 0.27, respectively).
TABLE 2.
Energy and nutrients intakes of normal-weight men and women during a period of short or habitual sleep1
| Nutrient intakes | Short sleep | Habitual sleep | P value |
| Energy (kcal) | 2813.6 ± 593.0 | 2517.7 ± 593.0 | 0.02 |
| Fat (g) | 112.2 ± 34.7 | 91.5 ± 34.7 | 0.01 |
| Saturated fat (g) | 36.8 ± 17.8 | 28.1 ± 17.8 | 0.04 |
| Carbohydrates (g) | 402.1 ± 166.2 | 344.2 ± 166.2 | 0.19 |
| Protein (g) | 98.0 ± 20.9 | 88.1 ± 20.9 | 0.08 |
All values are means ± SDs and were adjusted for body weight, phase order, and sex. n = 26. The data were analyzed by using mixed-effects ANOVA.
FIGURE 2.
Energy (top) and fat (bottom) intakes during periods of short and habitual sleep in men (#) and women (○).
In all subjects together, the percentage of energy from fat was 35.8 ± 8.2% during short sleep compared with 32.5 ± 8.2% during habitual sleep (P = 0.10). Similarly, saturated fat intakes as a percentage of total energy intakes were slightly higher during short (11.3 ± 4.1%) than during habitual (10.0 ± 4.1%; P = 0.13) sleep. When we analyzed the data separately by sex, no difference in the proportion of energy coming from any of the macronutrients in men was observed (all P > 0.05). However, women tended to consume a greater percentage of their energy in the form of fat (38.5 ± 9.4% for short sleep and 32.8 ± 9.0% for habitual sleep; P = 0.05) and saturated fat (12.3 ± 5.0% for short sleep and 10.0 ± 5.0% for habitual sleep; P = 0.06). No main effect of sleep or sex or a sex × sleep interaction for percentage of energy from protein and carbohydrates was observed.
Participants ate significantly more often during short sleep than during habitual sleep (paired t test, P < 0.0001; Wilcoxon's signed-rank test, P = 0.00027). In general, the participants had 4.96 ± 1.2 eating occasions during habitual sleep and 6.08 ± 1.4 during short sleep. The number of eating occasions increased in 20 of the 26 participants, did not change in 3 participants, and decreased by 1 in 3 participants. This effect was observed in both men (P = 0.003) and women (P = 0.005). We also examined whether additional occasions during short sleep occurred past 2200—the bedtime for the habitual sleep phase. Of the 26 participants, 7 had an eating occasion past 2200 (1 of whom had no change in the number of eating occasions between the 2 sleep periods).
Energy expenditure measurements
In men and women combined, the RMR was 1455.4 ± 129.0 kcal/d after a period of 4 nights of short sleep and was 1486.5 ± 129.5 kcal/d after 4 nights of habitual sleep (P = 0.136). No difference in the respiratory quotient was found between the 2 sleep periods: 0.793 ± 0.043 for short sleep and 0.798 ± 0.043 for habitual sleep (P = 0.610).
Total and average activities measured by using actigraphy were not significantly different between the short and habitual sleep periods (total activity: 234,440 ± 190,868 and 251,332 ± 190,868 counts, respectively, P = 0.414; average activity: 162.7 ± 132.4 and 174.5 ± 132.4 counts/min, respectively, P = 0.410). There was a trend for a main effect of sleep on peak activity (P = 0.067), and peak activity tended to be higher during the habitual sleep period than during the short sleep period (948.4 ± 4358 counts). The percentage of time spent in sedentary activity was greater (P < 0.0001) during the habitual (84.9 ± 10.2%) than during the short (82.0 ± 10.2%) sleep period, whereas the time spent in light activity was less during the habitual (12.6 ± 9.3%) than during the short (16.0 ± 9.3%) sleep period (P < 0.0001). The percentage of time spent in moderate activity was not significantly different between sleep periods (habitual: 2.2 ± 1.7%; short: 1.9 ± 1.7%; P = 0.44). In contrast, the percentage of time spent in heavy (P = 0.018) and very heavy (P = 0.015) activity was significantly greater during the habitual (0.2 ± 0.6% and 0.1 ± 0.3% for heavy and very heavy activity, respectively) than during the short (0.04 ± 0.6% and 0.01 ± 0.3%, for heavy and very heavy, respectively) sleep period.
No effect of sleep on TEE was found (P = 0.832). Overall, participants expended 2611.1 ± 529.0 kcal/d during the period of habitual sleep compared with 2589.2 ± 526.5 kcal/d during the period of short sleep. We then calculated energy balance during periods of short and habitual sleep by subtracting TEE from total energy intakes over the 5-d period as follows:
 |
After control for phase order and sex, no effect of sleep on energy balance (habitual sleep: −1680.7 ± 2630.5 kcal; short sleep: −1501.9 ± 2662.5 kcal; P = 0.704) was found.
Visual analog scales
Data from the VAS showed no significant effect of sex or sleep or a sleep × time interaction on feelings of hunger, satiety, or fullness. However, an effect of time for those 3 questions and a sleep × sex interaction on fullness was found (P = 0.016). In general, men reported feeling less full during short sleep than during habitual sleep (P = 0.037). No sex or main effect of sleep on prospective food intake was found, but a significant effect of time (P < 0.0001) and a trend for a sleep × time interaction (P = 0.121) were found.
Significant main effects of sleep and time (all P < 0.0001) on feeling energetic and sluggish were found. A significant sleep × time interaction on feeling energetic (P < 0.0001) and a trend for feeling sluggish (P = 0.092) were found. A sleep × sex interaction on feeling sluggish (P = 0.003) was also found. In general, the participants felt less energetic (P < 0.0001) and more sluggish (P < 0.0001) during the period of short sleep than during the period of habitual sleep. Both men and women felt more sluggish during the short than during the habitual sleep period (both P < 0.0001). Finally, no effect of sex or sleep or a sleep × time interaction on the desire to eat fruit, vegetables, or something sweet, savory, or salty was found (all P > 0.05).
DISCUSSION
The main finding of our study was that sleep duration has a significant effect on ad libitum energy and fat intakes without affecting overall energy expenditure. The effect of sleep on food intake was observed in both men and women. Furthermore, we observed no compensatory rise in TEE during the period of short sleep. The increase in energy intakes beyond what is expanded during short sleep, if sustained, would lead to weight gain. Moreover, the increase in energy intake was mostly due to an increase in fat intake, particularly saturated fat intake. This altered eating pattern may lead to an increased risk of cardiovascular disease. In fact, saturated fat intakes were beyond the recommended saturated fat intakes of <10% of energy for healthy Americans (18).
Our data, which show that energy intake was ≈300 kcal/d greater during the period of short sleep than during the period of habitual sleep, are similar to the results observed by Nedeltcheva et al (7) over a 2-wk period. However, the difference in energy intakes observed between the short and habitual sleep periods was less than that observed by Brondel et al (9) after a single night of reduced sleep, from 8 to 4 h. It is important to note that our measurements were taken after 4 nights of short sleep, and the participants were free to self-select their foods and had free access to foods and beverages at any time. In contrast, previous studies had set meal times and set foods available for consumption (7, 9). In the current study, the participants had the added freedom of purchasing foods and beverages that they desired to eat, with the only restriction being the availability of nutrition information.
In the study by Nedeltcheva et al (7), the distribution of energy, macronutrients, and macronutrients in meals did not differ between periods of 5 and 7 h nights, but there were indications of altered nutrient distribution in snacks. Specifically, their data suggested greater intakes of carbohydrate-rich snacks lower in fat during short sleep than during habitual sleep. In the current study, fat (but not carbohydrate) intakes increased during the period of short sleep relative to the period of habitual sleep. The study by Nedeltcheva et al (7) also included both men and women and had too few participants (n = 11) to analyze the data separately by sex. It is possible that our larger sample size, with equal numbers of men and women, allowed us to detect differences between sleep periods that could not be discerned with fewer participants. Moreover, our results are consistent with data from the Women's Health Initiative, showing inverse correlations between actigraphy-measured total sleep time and self-reported energy and fat intakes (19).
Our data showing no clear effect of sleep duration on subjective feelings of hunger, appetite, and desire for something sweet, salty, or savory differ from previous reports (4, 9). On the basis of these previous studies, we expected participants to report greater feelings of hunger and appetite during the period of short sleep than during the period of habitual sleep. However, Schmid et al (6) also found no difference between fasting hunger ratings after single nights of 4.5 h sleep and 7 h sleep. Of note, studies have reported that short sleepers tend to consume more calories from snacks than do those who sleep longer (20) and a reduction in sleep duration increases snack intake relative to habitual sleep (7). These studies have suggested that short sleep duration may alter neuronal pathways that regulate reward behaviors. Studies are needed to examine brain reward pathways under periods of short and habitual sleep.
Differences between the results of our study and those of Spiegel et al (4) and Brondel et al (9) may be due to key differences in study design and participant characteristics. All previous studies reported data from men alone after a single night (6, 9) or 2 nights (4) of reduced sleep. In the current study, we included both men and women and obtained hunger and appetite ratings after 3 nights of short or habitual sleep. In addition, in the study by Brondel et al (9), only preprandial ratings were significantly different between short and habitual sleep. Food intake was not controlled; therefore, postprandial ratings could not be compared.
Spiegel et al (4) were the first to report increased hunger in men after 2 nights of short sleep relative to habitual sleep. However, it is important to note that, during each sleep period, the participants were infused intravenously with glucose, which may have amplified subjective ratings of hunger and appetite and the desire for sweet, salty, and starchy foods during the short sleep condition. The current study distinguished itself by having participants consume a completely controlled diet that was identical in both sleep periods. Our results, therefore, were not confounded by differences in meal timing, food choices, and energy intakes.
Our energy expenditure data differ somewhat from those of Schmid et al (8), who found that sleep restriction in men decreased physical activity measured by wrist accelerometry. Our actigraphy data showed no effect of sleep duration on total activity level, but the trend toward lower peak activity with short sleep than with habitual sleep is consistent with Schmid et al's findings of lower intensity levels during short sleep (8). However, our results agree with those of Nedeltcheva et al, who found no difference in TEE assessed by DLW over a 14-d period of short or long sleep (7). Jung et al (21) showed greater energy expenditure during a 24-h period of total sleep deprivation than during a 24-h period including 8 h of sleep. We found no effect of sleep on TEE, but our level of sleep deprivation was 4 h, compared with 8 h in the study by Jung et al. Moreover, sleep recovery (also 8 h of sleep) resulted in a lower TEE than did habitual (basal 8 h) sleep (21). In contrast, Benedict et al (22) found a lower resting and postprandial metabolic rate after one night of total sleep deprivation than after 8 h of sleep. On the basis of these data, we would not expect much change in TEE between sleep durations differing by 4 h/night.
Our study had some limitations worth noting. First, all of the participants were of normal weight and young. The age range was chosen based on data from NHANES, which showed a greater association between sleep duration and obesity in young adults (23). It is unknown whether a reduction in sleep in older or overweight participants would evoke the responses observed in the current study. We chose to include only normal-weight individuals specifically because of the known association between sleep duration and obesity (2). Our data show that a reduction in sleep could lead to weight gain and hence overweight or obesity and provide information on energy balance alone, not its hormonal controls. Our data, however, do not imply the reverse, ie, that an increase in sleep duration in overweight individuals would lead to reductions in food intake. We are also limited by our sample size, which prevented us from finding significant effects of sleep on RMR and food intake in men when we analyzed our data separately by sex.
Also, we had food intake data for only a single day, for a day immediately after a period of controlled feeding. This timing had the advantage that premeasurement intakes were identical between sleep periods, and our results were not due to serendipitous differences in premeasurement feeding status between periods. Our study could have benefited from additional days of food recording. However, multiple days of recording are typically necessary for self-recording by participants to account for day-to-day variability in food intakes. A controlled diet before ad libitum food intake recording helped reduce this daily variability, which allowed us to detect differences in energy and macronutrient intakes between sleep periods. In addition, we were confident in the quality of the food records because participants were under constant supervision and the study personnel were responsible for food provision and data recording.
In conclusion, we found that 4 nights of short sleep led to greater energy and fat consumption in young, healthy, normal-weight individuals than did habitual sleep without any compensatory effect on energy expenditure and possibly a slightly reduced RMR. Our data thus support a causal effect of sleep duration on the development of obesity via increases in energy intakes. More research is necessary to determine the role of sleep on the intensity of physical activity level.
Acknowledgments
We thank Jennifer Ahn (Columbia University, New York, NY), Andrew McReynolds (Columbia University), Sari Tepper (Columbia University), and Zalak Trivedi (Columbia University) for their help in conducting the study and collecting data; the staff at Clinilabs; and the research participants.
The authors’ responsibilities were as follows—M-PS-O: designed the research; ALR, MK, and MO: conducted the research; M-PS-O, ALR, MK, and MO: collected the data; JC and PJHJ: analyzed the DLW samples; M-PS-O and AR: analyzed the data; M-PS-O, ALR, MK, MO, and JC: wrote the manuscript; and M-PS-O: had primary responsibility for final content. All authors read and approved the final manuscript. The funding agencies played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. None of the authors had any conflicts of interest to disclose.
REFERENCES
- 1.Chen X, Beydoun MA, Wang Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity (Silver Spring) 2008;16:265–74 [DOI] [PubMed] [Google Scholar]
- 2.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol 2006;164:947–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004;141:846–50 [DOI] [PubMed] [Google Scholar]
- 5.Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab 2004;89:5762–71 [DOI] [PubMed] [Google Scholar]
- 6.Schmid SM, Hallschmid M, Jauch-Chara K, Born J, Schultes B. A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. J Sleep Res 2008;17:331–4 [DOI] [PubMed] [Google Scholar]
- 7.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr 2009;89:126–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmid SM, Hallschmid M, Jauch-Chara K, et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr 2009;90:1476–82 [DOI] [PubMed] [Google Scholar]
- 9.Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr 2010;91:1550–9 [DOI] [PubMed] [Google Scholar]
- 10.Bosy-Westphal A, Hinrichs S, Jauch-Chara K, et al. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes Facts 2008;1:266–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris JA, Benedict FG. A biometric study of basal metabolism in man. Washington, DC: Carnegie Institution of Washington, 1919 [Google Scholar]
- 12.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM manual for the scoring of sleep and associated events. Westchester, IL: American Academy of Sleep Medicine, 2007 [Google Scholar]
- 13.Jones A, Jr, Shen W, St-Onge MP, et al. Body-composition differences between African American and white women: relation to resting energy requirements. Am J Clin Nutr 2004;79:780–6 [DOI] [PubMed] [Google Scholar]
- 14.Schoeller DA, van Santen E, Peterson DW, Dietz W, Jaspan J, Klein PD. Total body water measurement in humans with 18O and 2H labeled water. Am J Clin Nutr 1980;33:2686–93 [DOI] [PubMed] [Google Scholar]
- 15.Racette SB, Schoeller DA, Luke AH, Shay K, Hnilicka J, Kushner RF. Relative dilution spaces of 2H- and 18O-labeled water in humans. Am J Physiol 1994;267:E585–90 [DOI] [PubMed] [Google Scholar]
- 16.Ebine N, Feng JY, Homma M, Saitoh S, Jones PJ. Total energy expenditure of elite synchronized swimmers measured by the doubly labeled water method. Eur J Appl Physiol 2000;83:1–6 [DOI] [PubMed] [Google Scholar]
- 17.Schoeller DA, Ravussin E, Schutz Y, Acheson KJ, Baertschi P, Jequier E. Energy expenditure by doubly labeled water: validation in humans and proposed calculation. Am J Physiol 1986;250:R823–30 [DOI] [PubMed] [Google Scholar]
- 18.US Department of Agriculture Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans, 2010. Washington, DC: US Department of Agriculture, 2010 [Google Scholar]
- 19.Grandner MA, Kripke DF, Naidoo N, Langer RD. Relationships among dietary nutrients and subjective sleep, objective sleep, and napping in women. Sleep Med 2010;11:180–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss A, Xu F, Storfer-Isser A, Thomas A, Ievers-Landis CE, Redline S. The association of sleep duration with adolescents’ fat and carbohydrate consumption. Sleep 2010;33:1201–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, Wright KP. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol 2011;589:235–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benedict C, Hallschmid M, Lassen A, et al. Acute sleep deprivation reduces energy expenditure in healthy men. Am J Clin Nutr 2011;93:1229–36 [DOI] [PubMed] [Google Scholar]
- 23.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep 2005;28:1289–96 [DOI] [PubMed] [Google Scholar]