Abstract
We sought to determine the molecular basis for the anticancer activities of 5,3′-dihydroxy-3,6,7,8,4′-pentamethoxyflavone (DH-PMF), isolated from Gardenia obtusifolia traditionally used in Thailand for a variety of ailments. As little as 1 μM DH-PMF inhibited the proliferation of prostate, colon, kidney, lung, head and neck, pancreas, breast, leukemia, and myeloma cancer cell lines. DH-PMF also suppressed the colony-forming ability of tumor cells, with 50% inhibition occurring at a dose less than 10 nM. DH-PMF induced G2/M and subG1 cell cycle arrest, increased the levels of p21WAF1/CIP1 and p27KIP1, and reduced the expression of cyclin D1, CDC2, and c-MYC. Furthermore, DH-PMF inhibited AKT and glycogen synthase kinase 3 beta (GSK3β) activation, reduced cell survival proteins, and induced apoptosis, as indicated by annexin V staining, TUNEL assay, and activation of caspase-8, -9 and -3. Overall, our results demonstrate that DH-PMF induces suppression of cell proliferation through modulation of AKT-GSK3β pathways and induction of cyclin-dependent kinase (CDK) inhibitors.
Keywords: AKT, GSK3beta, natural products, plant extract, CDK inhibitor
Although hundreds of agents have been suggested for the treatment of cancer, most are unsafe, expensive, and lack efficacy. Of the currently available anticancer agents approved for use over the past three decades (e.g. paclitaxel, doxorubicin, and camptothecin), up to 70% were directly or indirectly derived from natural sources (1).
As a means of identifying anticancer agents, this reverse pharmacology, or the ‘bedside-to-bench’ approach, involves studying medicinal plants that have been traditionally used to treat various ailments. Such an approach is thought to be advantageous for several reasons. Firstly, it provides a quick lead. Natural products are also usually multitargeted and thus ideal for chronic diseases such as cancer, which involves the dysregulation of multiple genes. Additionally, most natural products tend to have a low affinity, which is also preferred as cancer is due to overexpression of certain proteins. Thus, natural products are expected to exhibit less toxicity, as they do not completely inhibit (or knock out) a given protein.
One promising medicinal plant is Gardenia obtusifolia (of the Rubiaceae family), which is commonly used in Thailand. Extracts of this plant are used as inhibitors of implantation (2), ulcer suppressants (3), and antibacterial (4), analgesic (5), diuretic (5) and hypotensive (5) agents. Extracts also have antipyretic properties (6) and are used as larvicides (7). One of the compounds isolated from this plant, 5,3′-dihydroxy-3,6,7,8,4′-pentamethoxyflavone (DH-PMF) (Figure 1A), has been found to be cytotoxic to various cancer cell lines (8–10) and to exhibit anti-HIV activity (11). PMF isolated from another medicinal plant native to North America, Polanisia dodecandra, has also been found to exhibit anticancer activity by binding to tubulin and inhibiting its polymerization, with a 50% inhibitory concentration (IC50) of 0.83 μM (8–10).
Figure 1.
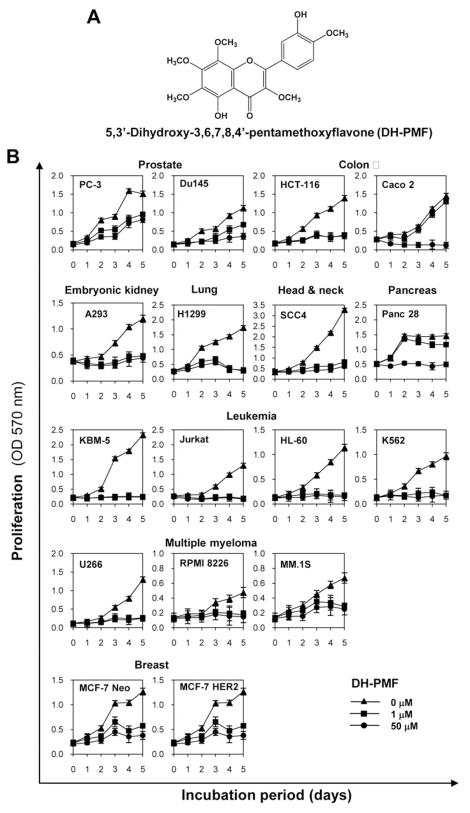
A. The chemical structure of DH-PMF. B: DH-PMF inhibits proliferation of various tumor cells. Cells (2,000 cells/well) were incubated at 37°C with DH-PMF for different durations, and the cell viability was assayed by MTT uptake as described in Materials and Methods. The results are shown as the mean±SD from triplicate cultures.
AKT, a serine (Ser)-threonine (Thr) kinase known as protein kinase B, is a central signaling molecule in the phosphatidylinositide-3-kinase (PI3K) pathway (12). Activation of PI3K/AKT signaling results in a decrease in activity of proapoptotic proteins or an increase in activity of antiapoptotic proteins (13). Akt is activated in response to various mitogens or growth factors and then phosphorylates glycogen synthase kinase 3 beta (GSK3β) at Ser residue 9, inactivating the kinase (14). GSK3β is involved in regulating cell fate and differentiation in a variety of organisms (15) and has a role in cell survival (16). Blockade of the PI3K/AKT/GSK3β pathway has been found to sensitize various tumor cell types to apoptotic cell death induced by a variety of chemotherapeutic agents (17), and thus, this pathway is an attractive target for the development of novel anticancer therapies.
Because it is not known which cell signaling pathways and proteins are modulated by DH-PMF in cancer cells, we investigated the effect of DH-PMF on cell signaling pathways involved in tumor cell survival, proliferation, and apoptosis.
Materials and Methods
Chemicals
Penicillin, streptomycin, RPMI-1640, Iscove’s modified Dulbecco’s medium (IMDM), and Dulbecco’s modified Eagle’s medium (DMEM) were obtained from Invitrogen (Carlsbad, CA, USA). Fetal bovine serum (FBS) was supplied by Atlanta Biologicals (Lawrenceville, GA, USA). Antibodies against cyclinD1, c-MYC, polyadenosine ribose polymerase (PARP), B-cell leukemia/lymphoma 2 (BCL-2), B-cell lymphoma-extra large (BCL-xL), myeloid cell leukemia 1 (MCL-1), caspase-3, caspase-8, caspase-9, CDC2, p21, BCL-2–associated protein X (BAX), AKT and PI3K were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Anti-survivin antibody was purchased from R&D Systems (Minneapolis, MN, USA). Antibodies against phosphorylated AKT (Ser 473), phosphorylated GSK3β (Ser-9), GSK3β, phosphorylated PI3K, and p27 were obtained from Cell Signaling Technology (Danvers, MA, USA). Anti-X-linked inhibitor of apoptosis protein (XIAP) antibodies was obtained from BD Biosciences (San Diego, CA, USA). Antibody against cellular FLICE inhibitory protein (cFLIP) was kindly provided by Imgenex (San Diego, CA, USA). Fine chemicals were obtained from Sigma (St. Louis, MO, USA).
Cell lines
The cell lines used in our studies included those established from chronic myelogenous leukemia (KBM-5), human lymphoblastic leukemia (Jurkat), human promyelocytic leukemia (HL-60), human chronic myelogenous leukemia (K562), human prostate cancer (PC-3, Du145), human colorectal cancer (HCT-116, Caco2), human embryonic kidney cells (A293), human non-small cell lung cancer (H1299), human head and neck cancer (SCC4), and human multiple myeloma (U266, RPMI8226, MM1S) were obtained from the American Type Culture Collection (Manassas, VA, USA). Panc-28 (pancreatic carcinoma) cell line was kindly provided by Dr. Shrikanth Reddy (The University of Texas M.D. Anderson Cancer Center, Houston, TX, USA). Breast cancer cell lines that express different levels of HER2, including stably transfected MCF7/HER2 and their vector control, were kindly provided by Dr. D. Yu (The University of Texas M. D. Anderson Cancer Center, Houston, TX). The mouse embryonic fibroblast (MEF) derived from GSK3β−/−C57Bl/6J mice and their wild type counterparts were kindly provided by Dr. James R. Woodgett (Ontario Cancer Institute, Toronto, Ontario, Canada). These cells have been well characterized and described (18). MEF/GSK3β+/+, MEF/GSK3β−/−, A293, Panc28, and HCT-116 cells were cultured in DMEM with 10% FBS; KBM-5 cells were cultured in IMDM with 15% FBS; MCF-7/Neo, MCF-7/HER2 cell lines were cultured in DMEM/F12 with 10% FBS and no antibiotics; SCC4 cell lines were cultured in DMEM with 1% sodium pyruvate and 10% FBS, and all other cell lines were cultured in RPMI-1640 with 10% FBS. All media were supplemented with 100 U/ml penicillin and 100 mg/ml streptomycin.
Extraction and isolation of DH-PMF
The leaves of Gardenia obtusifolia were collected from the Doi Suthep-Pui National Park, Chiang Mai, Thailand. Voucher herbarium specimen (No.18749) of the plant was identified by J.F. Maxwell, and deposited in the Chiang Mai University Herbarium, Chiang Mai, Thailand. The samples were washed, air-dried, and chopped into small pieces. They were oven-dried at temperature below 50°C and ground to powder. The dried powder was macerated with 95% ethanol. The ethanolic solutions were combined and evaporated at 50°C under reduced pressure to give a dark-brown residue. A portion of the crude extract was separated based on liquid-liquid partition procedure. These chloroform extracts exhibited the highest cytotoxic activity. Based on the bioassay-guide isolation, the crude chloroform extract was subjected to further isolation with column chromatography (CC) on SiO2. Gradient elution was performed with different compositions of a mobile phase as a gradient of increasing polarity. Separated fractions were evaluated by thin layer chromatography (TLC). Repeated separations were performed using CHCl3/ethyl acetate with increasing polarity up to a ratio of 5:5 to yield a pure fraction of DH-PMF. The purity and the structure of these yellow crystals was measured and identified by TLC, HPLC, MS and NMR analysis.
Cytotoxicity assay
Cytotoxicity was assayed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described previously (19).
Clonogenic assay
Clonogenic assay was performed as described previously (19). Briefly, 6-well dishes were seeded with HCT-116 cells (500 cells/well) in complete medium and allowed to grow for 24 h. The cells were then incubated in the presence or absence of different concentrations of DH-PMF for up to 24 h. The DH-PMF-containing medium was then removed, and the cells were washed in Dulbecco’s phosphate-buffered saline (DPBS) and incubated for an additional 9 days in complete medium. Each treatment was carried out in triplicate. The colonies obtained were stained in clonogenic reagent (50% methanol and 0.25% crystal violet) for 30 min at room temperature followed by washing with PBS twice. The colonies were counted and compared with those formed by untreated cells.
Flow cytometric analysis
Cells were pretreated with DH-PMF for the indicated times. Propidium iodide staining for DNA content analysis was carried out as described elsewhere (20).
Western blot analysis
To determine the levels of protein expression, we prepared extracts and fractionated them by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis, the proteins were electrotransferred to nitrocellulose membranes, blotted with the relevant antibody, and detected with an electrogenerated chemiluminescence reagent (GE Healthcare, Piscataway, NJ, USA).
Live/dead assay
To measure apoptosis, we also used the Live/Dead assay (Invitrogen Carlsbad, CA, USA), which determines intracellular esterase activity and plasma membrane integrity was used (19).
Annexin V assay
An early indicator of apoptosis is the rapid translocation and accumulation of the membrane phospholipid phosphatidylserine from the cytoplasmic interface of membrane to the extracellular surface. This loss of membrane asymmetry can be detected by using the binding properties of annexin V (19).
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay
Cytotoxicity using the terminal deoxynucleotidyl-transferase-mediated dUTP nick end-labeling (TUNEL) method with an in situ cell death detection reagent (Roche Molecular Biochemicals, Indianapolis, IN, USA). This assay was performed as described previously (19).
Statistical analysis
The statistical analysis was carried out using Student’s t-test using Microsoft Excel software.
Results
In the present studies, we investigated the effect of DH-PMF on cell proliferation and apoptosis in a wide variety of human tumor cell lines. The mechanisms by which DH-PMF modulates cell proliferation and apoptosis was then examined in human myeloid KBM-5 cells as these cells are well-characterized in our laboratory.
DH-PMF inhibits proliferation of different types of tumor cells
Whether or not DH-PMF affects the proliferation of various human tumor cell lines was examined using cell viability assays. DH-PMF inhibited the proliferation of prostate, colon, embryonic kidney, lung, head and neck, pancreas and breast carcinoma, and leukemia and multiple myeloma cell lines in a dose- and time-dependent manner (Figure 1B). As little as 1 μM DH-PMF suppressed the proliferation of most tumor cells lines completely. Leukemia and myeloma cells were the most sensitive to DH-PMF. Human breast cancer cells (MCF-7), irrespective of HER2 expression, were also sensitive to DH-PMF.
DH-PMF inhibits colony formation of human colon cancer cells in a long-term assay
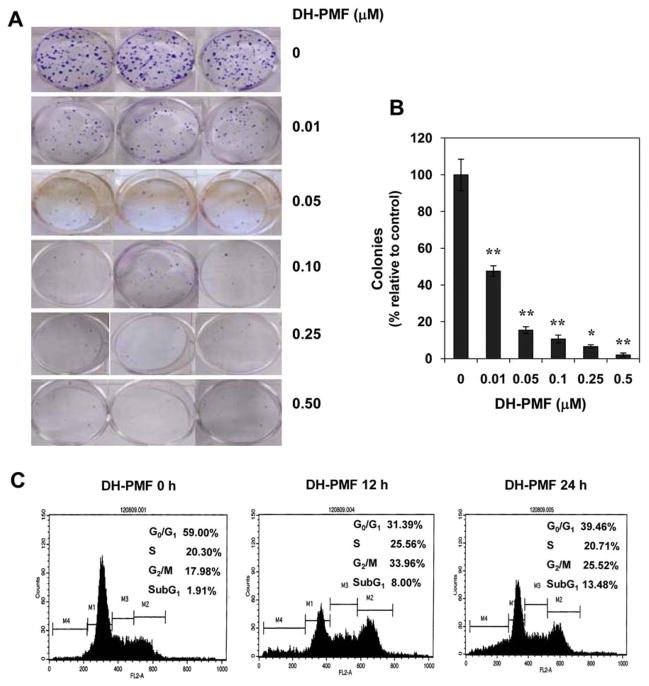
Tumors in vivo do not grow as monolayers but as colonies (21). We thus examined whether DH-PMF inhibits colony formation in human colon cancer HCT-116 cells in a long-term assay. We found that DH-PMF can inhibit colony formation of HCT-116 cells, and that 50% inhibition can be seen with as little as 0.01 μM of the flavone. These results thus suggest that flavone treatment is highly effective in suppressing the colony-forming ability of human colorectal HCT-116 cells (Figure 2A and 2B).
Figure 2.
DH-PMF inhibits cell growth as indicated by colony formation assays using HCT-116 cells. Cells were incubated with DH-PMF for 24 h and subsequently allowed to grow into colonies. After 9 days incubation, cells were stained with clonogenic reagent and photographed (A) and colonies were counted (B). Results are representative of three independent experiments. Data represent the mean of three measurements±S.D. **, p<0.005; *, p<0.01. C: DH-PMF arrests the cells at the G2/M and sub G1 phases of the cell cycle. KBM-5 cells (1×106/ml) were incubated in the absence or presence of 100 μM DH-PMF for different times. Thereafter, the cells were washed, fixed, stained with propidium iodide, and analyzed for DNA content by flow cytometric analyses (20).
DH-PMF arrests cells at the G2/M and subG1 phases of the cell cycle
To discover the mechanism by which DH-PMF inhibited the proliferation of cells, we investigated whether it affects the progression of these cells through the cell cycle. For this KBM-5 cells were treated with DH-PMF and cell cycle analysis was performed using flow cytometry. We found that DH-PMF induced a G2/M phase arrest after 12 h and that prolonged treatment led to apoptosis (sub G1 phase) at 24 h (Figure 2C).
DH-PMF modulates the expression of cell-cycle regulated proteins
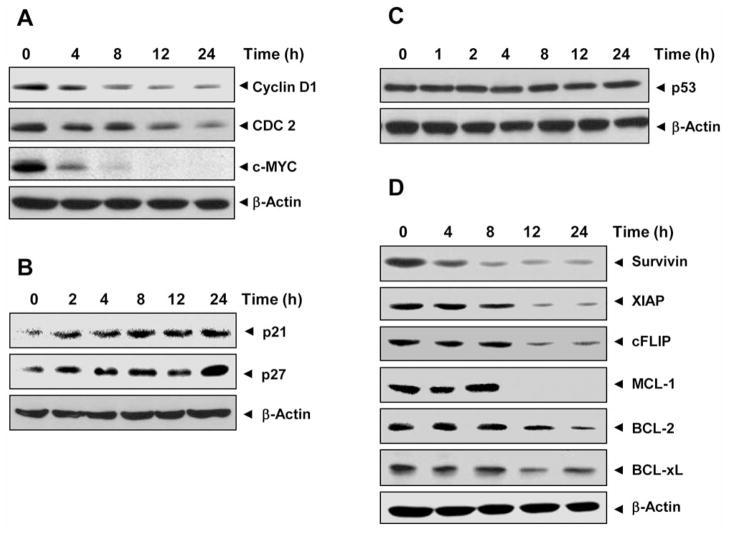
Because D-type cyclins and cyclin-dependent kinases (CDKs) are required for the progression of cells through the cell cycle (22), we determined the effect of DH-PMF on cyclin D1 and CDC2 expression in KBM-5 cells. We found that DH-PMF inhibited the expression of both cyclin D1 and CDC2 in a time-dependent manner (Figure 3A). DH-PMF also down-regulated the expression of c-MYC, which has been closely linked to growth modulation (23) (Figure 3A).
Figure 3.
DH-PMF modulates expression of cell cycle protein. KBM-5 cells (1×106/ml) were treated with DH-PMF (100 μM) for different times and then whole-cell extracts were prepared. Thirty micrograms of whole-cell extracts were resolved on 10% SDS-PAGE gel, electrotransferred onto a nitrocellulose membrane, and probed with antibodies against A: cyclin D1, CDC2, and c-MYC; B: p21 and p27; C: p53. The blots were stripped and reprobed with anti–β-actin antibody to show equal protein loading. D: DH-PMF inhibits the expression of antiapoptotic gene products. KBM-5 cells (1×106 cells/ml) were left untreated or incubated with 100 μM DH-PMF for different lengths of time. Whole-cell extracts were prepared, and 30 μg of the whole-cell lysate were analyzed by Western blot analysis using antibodies against survivin, XIAP, cFLIP, MCL-1, BCL-2 and BCL-xL. The blots were stripped and reprobed with anti–β-actin antibody to show equal protein loading.
The CDK inhibitors p21WAF1/CIP1 and p27KIP1 are prototypical members of the CIP/KIP family of CDK inhibitors. They negatively modulate cell-cycle progression by inhibiting the activity of cyclin/CDK2 complexes and block DNA replication by binding to CDK (24). DH-PMF induced the expression of both p21WAF1/CIP1 and p27 KIP1 in a time-dependent manner (Figure 3B).
Because of mutations in the p53 gene in most tumors, clinically useful antineoplastic agents are less potent and efficacious in the context of mutant p53. We next investigated whether the up-regulation of p21WAF1/CIP1 and p27 KIP1 was mediated through the up-regulation of p53. DH-PMF, however, was found to have no effect on the expression of p53 (Figure 3C), thus indicating the induction of p21WAF1/CIP1 and p27KIP1 by DH-PMF was independent of p53.
DH-PMF down-regulates the expression of cell survival proteins
Because the constitutive expressions of survivin, XIAP, cFLIP, MCL-1, BCL-2 and BCL-xL, which interfere with apoptotic pathways, has been implicated in cell survival and suppression of apoptosis (25–27), we examined the effect of DH-PMF on the constitutive expression of these proteins in KBM-5 cells. DH-PMF suppressed the expression of all these proteins in a time-dependent manner (Figure 3D). These results thus suggest that DH-PMF induces apoptosis.
DH-PMF induces apoptosis in tumor cells
Whether DH-PMF can induce apoptosis in KBM-5 cells was investigated by the esterase-staining method, which examined plasma membrane integrity. We found that DH-PMF induced apoptosis in a time-dependent manner from 5% to 71% within 72 h (Figure 4A).
Figure 4.
DH-PMF induces apoptosis. A: Cells were treated with 100 μM DH-PMF for different times, stained with Live/Dead reagent, and incubated at 37°C for 30 min. KBM-5 cells were analyzed under a fluorescence microscope. B: KBM-5 cells were treated with 100 μM DH-PMF for different lengths of time, incubated with anti-annexin V antibody conjugated with FITC, and analyzed with a flow cytometer for early apoptotic effects. C: KBM-5 cells were treated with different concentrations of DH-PMF for 48 h. Cells were fixed, stained with TUNEL assay reagent, and then analyzed with a flow cytometer for apoptotic effects.
To confirm these results, we used annexin V staining, which detects early-stage apoptosis. Our results confirmed that DH-PMF induced apoptosis in a time-dependent manner. The proportion of annexin V-positive cells increased from 8% to 75% within 72 h (Figure 4B).
TUNEL staining, which examined DNA integrity, also confirmed that DH-PMF induced apoptosis in a dose-dependent manner (Figure 4C).
DH-PMF induced BAX expression
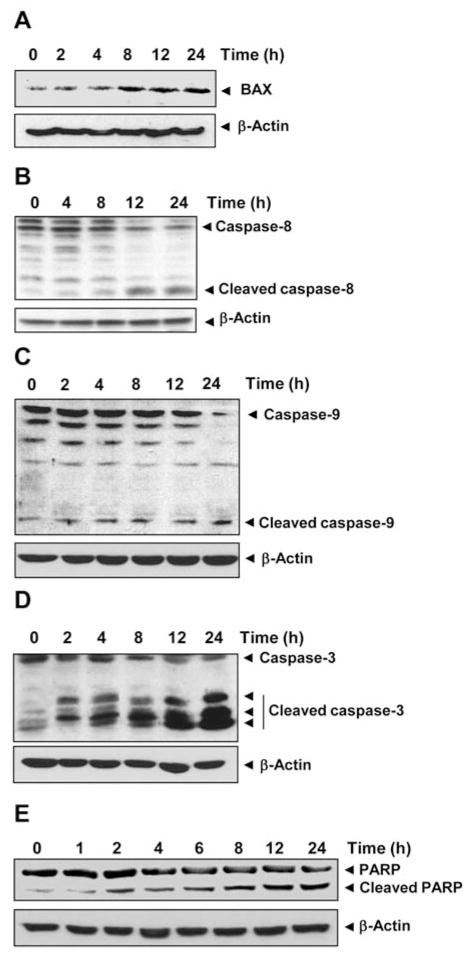
Members of the BCL-2 family of proteins are the most important regulators of apoptosis (28). BAX is one such protein that has been shown to promote apoptosis (29, 30). We therefore studied the effect of DH-PMF on BAX expression in KBM-5 cells. Western blot analysis showed that DH-PMF induced up-regulation of BAX expression, thus suggesting another mechanism by which DH-PMF may induce apoptosis (Figure 5A).
Figure 5.
DH-PMF induces BAX expression, caspase activation, and PARP cleavage. KBM-5 cells were treated with 100 μM DH-PMF for the different lengths of time. Whole-cell extracts were prepared and subjected to Western blot analysis using antibodies against A: BAX; B: caspase-8; C: caspase-9; D: caspase-3; E: PARP.
DH-PMF induces activation of upstream and terminal caspases
Procaspases-8 and -9 are upstream caspases, whereas procaspase-3 is a downstream caspase, all closely associated with the apoptotic cell death pathway (31, 32). Caspase-8 is stimulated in response to external stimuli and is negatively regulated by cFLIP. We examined whether this caspase was activated in DH-PMF-treated KBM-5 cells. Western blot analysis showed that DH-PMF activated caspase-8 by inducing the cleavage of procaspase-8 at 12 to 24 h, as seen by the disappearance of the procaspase-8 band and the appearance of its cleavage products (Figure 5B).
Caspase-9 is stimulated in response to internal stimuli and is negatively regulated by IAP and XIAP. We then examined whether this caspase was also activated in DH-PMF-treated KBM-5 cells. DH-PMF induced the cleavage of procaspase-9, as indicated by the disappearance of the procaspase-9 band and the appearance of its cleavage products at 12 to 24 h (Figure 5C).
Caspase-3 is a terminal caspase that is stimulated in response to activation of caspase-8 and -9. We thus examined whether this caspase is activated in DH-PMF-treated KBM-5 cells and found that treatment with DH-PMF also significantly induced the activation of caspase-3. The cleavage products of procaspase-3 were noted at as early as 2 h and increased gradually (Figure 5D).
Activation of caspase-3 has been shown to induce the degradation of multiple cellular proteins, including PARP, hence we investigated whether DH-PMF induced PARP cleavage as well. We noted the cleavage of PARP by caspase-3 at as early as 2 h, and it gradually increased thereafter (Figure 5E).
Overall, these results demonstrate that DH-PMF induced the activation of caspase-8, -9, and -3 that led to the cleavage of PARP.
DH-PMF suppresses the AKT pathway
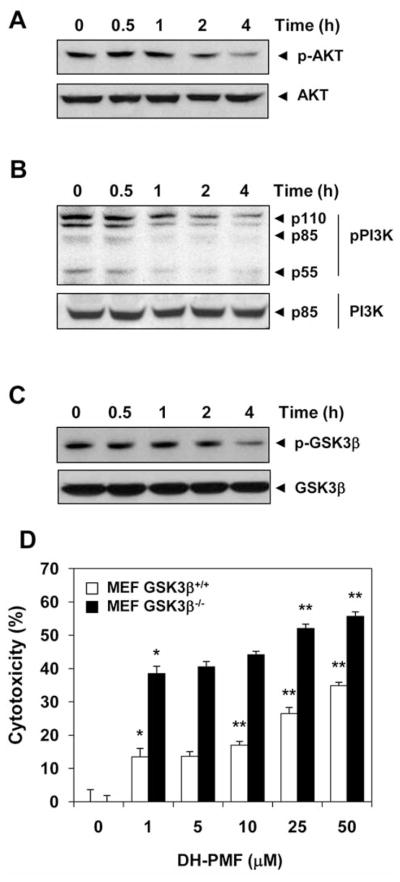
Because the PI3K/AKT pathway is an important regulator of tumor cell survival, inhibitors of this pathway can lead to suppression of tumor growth and induction of apoptosis (33). Thus we examined the effect of DH-PMF on AKT activation. As shown in Figure 6A, AKT was constitutively phosphorylated (i.e. activated) in KBM-5 cells, but DH-PMF treatment inhibited this activation. Inhibition can be seen at as early as 2 h after treatment; however, DH-PMF had no effect on the level of expression of the AKT protein itself.
Figure 6.
DH-PMF inhibits the AKT pathway. KBM-5 cells were treated with 100 μM DH-PMF for different lengths of time. Whole-cell extracts were prepared and analyzed by Western blot using antibodies against A: phosphorylated AKT and AKT; B: phosphorylated PI3K and PI3K; C: phosphorylated GSK3β (Ser-9) and GSK3β; D: GSK3β blocks DH-PMF-induced cytotoxicity. MEF/GSK3β−/− and wild-type, MEF/GSK3β+/+ cells (2000 cells/well) were incubated at 37°C with different concentrations of DH-PMF for 24 h, and the viable cell number was assayed by MTT uptake as described elsewhere (19). The results are shown as the mean±S.D. from triplicate cultures. Data represent the mean of three measurements±S.D. **p<0.001; *p<0.05.
We then investigated the effect of DH-PMF on the proteins both upstream (PI3K) and downstream (GSK3β) of the AKT signaling pathway. We found that DH-PMF inhibited the phosphorylation of PI3K, as shown in Figure 6B, without having any affect on the expression of the non-phosphorylated PI3K protein.
Whether DH-PMF can also affect AKT substrate GSK3β was next investigated. As can be noted in Figure 6C, KBM-5 cells expressed constitutively active GSK3β, and DH-PMF treatment led to the suppression of phosphorylation at Ser 9 of GSK3β.
GSK-3β abolishes DH-PMF-induced apoptosis
Because DH-PMF suppressed AKT activation and down-regulated GSK3β phosphorylatytion, we sought to determine the role of GSK3β in DH-PMF-induced apoptosis. We thus compared the cytotoxic effects of DH-PMF on MEF cells in which the GSK3β gene had been deleted. We found that DH-PMF was more highly cytotoxic to fibroblasts with GSK3β gene deletions (MEF/GSK3β−/−) than to wild-type murine fibroblasts (MEF/GSK3β+/+) (Figure 6D). These results once again suggest that GSK3β negatively controls DH-PMF-induced apoptosis. Thus, it is possible that suppression of tumor cell proliferation and induction of apoptosis by DH-PMF occurs in part through the modulation of the PI3K/AKT/GSK3β pathway.
Discussion
The killing of tumor cells through the induction of apoptosis is now recognized as a strategy for identifying anticancer drugs (34). In the present report, we sought to determine the mechanisms by which DH-PMF exerts its antiproliferative, proapoptotic, and anticancer properties on cancer cells. We confirmed that DH-PMF inhibits growth of a wide variety of cancer cells and concluded that DH-PMF induces apoptosis through modulation of antiapoptotic gene products and cell cycle proteins, activation of caspases, and inhibition of the PI3K/AKT/GSK3β pathway (see Figure 7).
Figure 7.
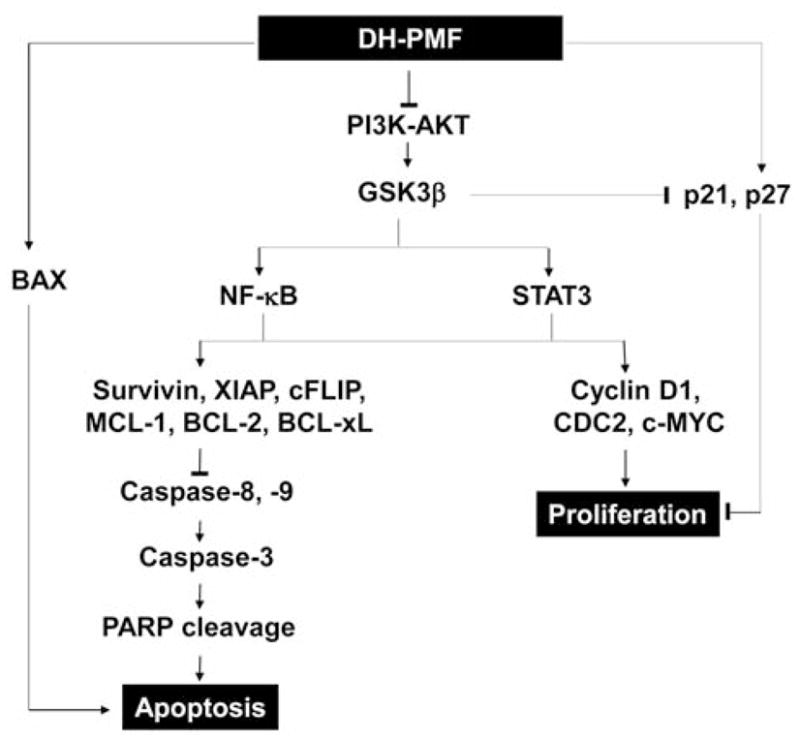
Schematic diagram showing how DH-PMF suppresses proliferation and induces apoptosis in cancer cells.
Numerous PMFs are found in the Citrus genus (35–37), including nobiletin (37, 38), tangeretin (38), artemetin (39), and sinensetin (40) but DH-PMF are present in G. obtusifolia, in the spices thyme and estragon (41), and in other plants (8, 9, 11). To our knowledge, our study is the first to demonstrate how DH-PMF inhibits proliferation in a wide variety of tumor cells, including those of prostate, colon, embryonic kidney, lung, head and neck, pancreas, leukemia, multiple myeloma, and breast.
DH-PMF was previously found to exhibit cytotoxic effects against different tumor cell lines (8, 9, 11). Moreover, numerous reports have shown that different analogs of PMF are cytotoxic to tumor cells (8, 9, 11, 36, 42, 43). Little is known, however, about the mechanism by which PMF exhibits its anticancer effects.
Although inhibition of tubulin polymerization has been suggested as one of the mechanisms by which DH-PMF mediates its cytotoxic effects, other mechanisms responsible are not known. We therefore sought to find out how DH-PMF suppresses cell proliferation and induces apoptosis in detail.
In our studies, at least six different pathways, connected either directly or indirectly, were identified by which PMF could manifest its affects. Firstly, by down-regulation of cell proliferation gene products (cyclin D1, CDC2, and c-MYC); secondly, by up-regulation of CDK inhibitors (p21WAF1/CIP1 and p27KIP1); thirdly, by down-regulation of cell survival proteins (survivin, XIAP, cFLIP, MCL-1, BCL-2, and BCL-xL); fourthly, by up-regulation of proapoptotic proteins (such as BAX); fifthly, by activation of caspases-3, -8, -9 and PARP cleavage; and finally, by inhibition of the PI3K/AKT/GSK3β pathway.
Indeed, our results suggest that DH-PMF blocks proliferation of tumor cells by arresting the cells in the G2/M and subG1 phases of the cell cycle. Cyclin D1, CDC2 and c-MYC are required for the progression of the cell cycle (22, 23). DH-PMF suppressed the expression of these proteins in a time-dependent manner. The expression of CDK inhibitors p21WAF1/CIP1 and p27KIP1, which block cell cycle progression by inhibiting the activity of cyclin/CDK2 complexes, was also up-regulated by DH-PMF. These observations are in agreement with reports where tangeretin (5,6,7,8,4′-pentamethoxyflavone) has been shown to induce G1 cell cycle arrest in colorectal cancer cells through up-regulation of p21WAF1/CIP1 and p27KIP1 (44) and inhibited growth of HL-60 cells through induction of apoptosis, without causing serious side-effects in immune cells (42). Moreover, in vivo studies found that consumption of 3′,4′,5′,5,7-pentamethoxyflavone by mice reduced Apc(Min) mouse adenoma cell proliferation (45).
Furthermore, we found that the expression of antiapoptotic gene products was suppressed by DH-PMF. These include survivin, XIAP, cFLIP, MCL-1, BCL-2, and BCL-xL. Our study also demonstrates that DH-PMF induces apoptosis in part through the activation of caspase-8, -9, and -3, followed by PARP cleavage. These results are in agreement with reports where monodemethylated PMFs were much more potent in growth inhibition, increasing the number of cells in the subG0/G1 phases of the cell cycles, and down-regulating oncogenic proteins, as well as in inducing apoptosis as evidenced by activation of caspase-3 and cleavage of PARP, than their permethoxylated counterparts (36). Furthermore, 5-hydroxy-6,7,8,3′,4′-pentamethoxyflavone and 5-hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone showed strong inhibitory activities against the proliferation and induced apoptosis of HL-60 cell lines (46). Likewise, hydroxylated PMFs were dramatically more active in inducing Ca2+-mediated apoptosis than were nonhydroxylated PMFs (43). However, whether flavones that are hydroxylated and methoxylated at different positions exhibit similar activities, is not known.
In addition, we found that DH-PMF suppressed the phosphorylation of AKT, which is closely linked with cell survival (47). Phosphorylation of the upstream kinase PI3K was also suppressed by DH-PMF. Suppression of AKT by PMF led to the suppression of phosphorylation of the AKT substrate GSK3β. We found that DH-PMF was more cytotoxic to GSK3β gene-deleted fibroblasts (MEF/GSK3β−/−) than to their wild-type counterparts. Thus, it is possible that the suppression of tumor cell proliferation and induction of apoptosis by DH-PMF occurs through the PI3K/AKT/GSK3β pathway.
PMF appears to potently inhibit tubulin polymerization, and therefore warrants further investigation as an antimitotic agent (9). It is well known that PI3K regulates tubulin polymerization via the AKT/GSK3β pathway (48). Inhibition of tubulin polymerization may induce apoptosis through inhibition of the PI3K/AKT pathway (49). It is therefore likely that PMF inhibits tubulin polymerization through the PI3K/AKT/GSK3β pathway.
It is also possible that the anti-HIV activity associated with DH-PMF (11) is caused by suppression of the PI3K/AKT pathway. T-Cells expressing wild-type AKT showed reduced glycoprotein 120 (gp120)-induced apoptosis and the expression of a dominant-negative mutant of AKT accelerated cell death as compared to the vector control (50). In addition, the inhibition of PI3K leads to enhanced gp120-induced apoptosis and identification of AKT-mediated signaling pathways may provide novel therapeutic targets to combat immune deficiency in AIDS.
On the other hand, various analogs of PMF have also been shown to inhibit the expression of the P-glycoprotein (P-gp) (35, 40, 51) that mediates chemoresistance. Because the PI3K/AKT pathway is involved in modulating P-gp-mediated multidrug resistance (52), it is thus possible that the down-regulation of P-gp by DH-PMF is caused by suppression of the PI3K/AKT pathway. Furthermore, since the suppression of multidrug resistance proteins also contributes to chemosensitization, this DH-PMF may contribute to chemosensitization in this way.
Overall, our results indicate that DH-PMF inhibits the growth of a wide variety of tumor cells and induces apoptosis through down-regulation of antiapoptotic gene products, modulation of cell cycle proteins, activation of caspases, and inhibition of the PI3K/AKT/GSK3β pathway. These results may provide the molecular basis for using DH-PMF as an anticancer agent.
Acknowledgments
We thank the Department of Scientific Publications for carefully editing the manuscript and providing valuable comments. Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research at The University of Texas M. D. Anderson Cancer Center. This work was supported by a grant from the Clayton Foundation for Research (B.B.A.), a program project grant from the National Institutes of Health (NIH CA124787-01A2), a grant from M.D. Anderson Cancer Center for Targeted Therapy, and grants from the Royal Golden Jubilee Ph.D. Program of Thailand.
Footnotes
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Liu EH, Qi LW, Wu Q, Peng YB, Li P. Anticancer agents derived from natural products. Mini Rev Med Chem. 2009;9:1547–1555. doi: 10.2174/138955709790361520. [DOI] [PubMed] [Google Scholar]
- 2.Luechtefeld NW, Cambre RC, Wang WL. Isolation of Campylobacter fetus subsp jejuni from zoo animals. J Am Vet Med Assoc. 1981;179:1119–1122. [PubMed] [Google Scholar]
- 3.Takase H, Imanishi K, Miura O, Yumioka E, Watanabe H. Features of the anti-ulcer effects of Oren-gedoku-to (a traditional Chinese medicine) and its component herb drugs. Jpn J Pharmacol. 1989;49:301–308. doi: 10.1254/jjp.49.301. [DOI] [PubMed] [Google Scholar]
- 4.Laurens A, Mboup S, Tignokpa M, Sylla O, Masquelier J. Antimicrobial activity of some medicinal species from the Dakar markets. Pharmazie. 1985;40:482–484. (in French) [PubMed] [Google Scholar]
- 5.Hussain MM, Sokomba EN, Shok M. Pharmacological effects of Gardenia erubescens in mice, rats and cats. Int J Pharmacogn. 1991;29:94–100. [Google Scholar]
- 6.el-Hamidi A. Drug plants of the Sudan Republic in native medicine. Planta Med. 1970;18:279–280. [PubMed] [Google Scholar]
- 7.Manson D. Malaria Inst India. 1939. pp. 85–93. [Google Scholar]
- 8.Lichius JJ, Thoison O, Montagnac A, Pais M, Gueritte-Voegelein F, Sevenet T, Cosson JP, Hadi AH. Antimitotic and cytotoxic flavonols from Zieridium pseudobtusifolium and Acronychia porteri. J Nat Prod. 1994;57:1012–1016. doi: 10.1021/np50109a024. [DOI] [PubMed] [Google Scholar]
- 9.Shi Q, Chen K, Li L, Chang JJ, Autry C, Kozuka M, Konoshima T, Estes JR, Lin CM, Hamel E. Antitumor agents, 154. Cytotoxic and antimitotic flavonols from Polanisia dodecandra. J Nat Prod. 1995;58:475–482. doi: 10.1021/np50118a001. [DOI] [PubMed] [Google Scholar]
- 10.Zhang SX, Bastow KF, Tachibana Y, Kuo SC, Hamel E, Mauger A, Narayanan VL, Lee KH. Antitumor agents. 196. Substituted 2-thienyl-1,8-naphthyridin-4-ones: their synthesis, cytotoxicity, and inhibition of tubulin polymerization. J Med Chem. 1999;42:4081–4087. doi: 10.1021/jm990208z. [DOI] [PubMed] [Google Scholar]
- 11.Tuchinda PPW, Reutrakul V, Pohmakotr M, Yoosook C, Kongyai N, Sophasan S, Sujarit K, Upathum SE, Santisuk T. Cytotoxic and anti-HIV-1 constituents of Gardenia obtusifolia and their modified compounds. Tetrahedron. 2002;58:8073–8086. [Google Scholar]
- 12.Testa JR, Bellacosa A. AKT plays a central role in tumorigenesis. Proc Natl Acad Sci USA. 2001;98:10983–10985. doi: 10.1073/pnas.211430998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. AKT phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 14.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 15.Kim L, Kimmel AR. GSK3, a master switch regulating cell-fate specification and tumorigenesis. Curr Opin Genet Dev. 2000;10:508–514. doi: 10.1016/s0959-437x(00)00120-9. [DOI] [PubMed] [Google Scholar]
- 16.Takada Y, Fang X, Jamaluddin MS, Boyd DD, Aggarwal BB. Genetic deletion of glycogen synthase kinase-3beta abrogates activation of IkappaBalpha kinase, JNK, AKT, and p44/p42 MAPK but potentiates apoptosis induced by tumor necrosis factor. J Biol Chem. 2004;279:39541–39554. doi: 10.1074/jbc.M403449200. [DOI] [PubMed] [Google Scholar]
- 17.Nicholson KM, Anderson NG. The protein kinase B/AKT signalling pathway in human malignancy. Cell Signal. 2002;14:381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 18.Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 19.Ahn KS, Sethi G, Aggarwal BB. Embelin, an inhibitor of X chromosome-linked inhibitor-of-apoptosis protein, blocks nuclear factor-kappaB (NF-kappaB) signaling pathway leading to suppression of NF-kappaB-regulated antiapoptotic and metastatic gene products. Mol Pharmacol. 2007;71:209–219. doi: 10.1124/mol.106.028787. [DOI] [PubMed] [Google Scholar]
- 20.Yodkeeree S, Sung B, Limtrakul P, Aggarwal BB. Zerumbone enhances TRAIL-induced apoptosis through the induction of death receptors in human colon cancer cells: Evidence for an essential role of reactive oxygen species. Cancer Res. 2009;69:6581–6589. doi: 10.1158/0008-5472.CAN-09-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramaniam D, May R, Sureban SM, Lee KB, George R, Kuppusamy P, Ramanujam RP, Hideg K, Dieckgraefe BK, Houchen CW, Anant S. Diphenyl difluoroketone: a curcumin derivative with potent in vivo anticancer activity. Cancer Res. 2008;68:1962–1969. doi: 10.1158/0008-5472.CAN-07-6011. [DOI] [PubMed] [Google Scholar]
- 22.Matsushime H, Roussel MF, Ashmun RA, Sherr CJ. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991;65:701–713. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- 23.Nieminen AI, Partanen JI, Klefstrom J. c-MYC blazing a trail of death: coupling of the mitochondrial and death receptor apoptosis pathways by c-MYC. Cell Cycle. 2007;6:2464–2472. doi: 10.4161/cc.6.20.4917. [DOI] [PubMed] [Google Scholar]
- 24.Luo Y, Hurwitz J, Massague J. Cell-cycle inhibition by independent CDK and PCNA binding domains in p21CIP1. Nature. 1995;375:159–161. doi: 10.1038/375159a0. [DOI] [PubMed] [Google Scholar]
- 25.Grumont RJ, Rourke IJ, Gerondakis S. Rel-dependent induction of A1 transcription is required to protect B-cells from antigen receptor ligation-induced apoptosis. Genes Dev. 1999;13:400–411. doi: 10.1101/gad.13.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 27.Zhu L, Fukuda S, Cordis G, Das DK, Maulik N. Anti-apoptotic protein survivin plays a significant role in tubular morphogenesis of human coronary arteriolar endothelial cells by hypoxic preconditioning. FEBS Lett. 2001;508:369–374. doi: 10.1016/s0014-5793(01)03084-8. [DOI] [PubMed] [Google Scholar]
- 28.Antonsson B, Montessuit S, Lauper S, Eskes R, Martinou JC. BAX oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem J. 2000;345(Pt 2):271–278. [PMC free article] [PubMed] [Google Scholar]
- 29.Chiu SM, Xue LY, Usuda J, Azizuddin K, Oleinick NL. Bax is essential for mitochondrion-mediated apoptosis but not for cell death caused by photodynamic therapy. Br J Cancer. 2003;89:1590–1597. doi: 10.1038/sj.bjc.6601298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu YT, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of BAX and Bcl-X(L) during apoptosis. Proc Natl Acad Sci USA. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anto RJ, Mukhopadhyay A, Denning K, Aggarwal BB. Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome c release: its suppression by ectopic expression of BCL-2 and BCL-xL. Carcinogenesis. 2002;23:143–150. doi: 10.1093/carcin/23.1.143. [DOI] [PubMed] [Google Scholar]
- 32.Hakem R, Hakem A, Duncan GS, Henderson JT, Woo M, Soengas MS, Elia A, de la Pompa JL, Kagi D, Khoo W, Potter J, Yoshida R, Kaufman SA, Lowe SW, Penninger JM, Mak TW. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1998;94:339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 33.Sethi G, Ahn KS, Sung B, Kunnumakkara AB, Chaturvedi MM, Aggarwal BB. SH-5, an AKT inhibitor potentiates apoptosis and inhibits invasion through the suppression of anti-apoptotic, proliferative and metastatic gene products regulated by IkappaBalpha kinase activation. Biochem Pharmacol. 2008;76:1404–1416. doi: 10.1016/j.bcp.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 34.Panchal RG. Novel therapeutic strategies to selectively kill cancer cells. Biochem Pharmacol. 1998;55:247–252. doi: 10.1016/s0006-2952(97)00240-2. [DOI] [PubMed] [Google Scholar]
- 35.Takanaga H, Ohnishi A, Yamada S, Matsuo H, Morimoto S, Shoyama Y, Ohtani H, Sawada Y. Polymethoxylated flavones in orange juice are inhibitors of P-glycoprotein but not cytochrome P450 3A4. J Pharmacol Exp Ther. 2000;293:230–236. [PubMed] [Google Scholar]
- 36.Xiao H, Yang CS, Li S, Jin H, Ho CT, Patel T. Mono-demethylated polymethoxyflavones from sweet orange (Citrus sinensis) peel inhibit growth of human lung cancer cells by apoptosis. Mol Nutr Food Res. 2009;53:398–406. doi: 10.1002/mnfr.200800057. [DOI] [PubMed] [Google Scholar]
- 37.Zheng G, Yang D, Wang D, Zhou F, Yang X, Jiang L. Simultaneous determination of five bioactive flavonoids in pericarpium Citri reticulatae from china by high-performance liquid chromatography with dual wavelength detection. J Agric Food Chem. 2009;57:6552–6557. doi: 10.1021/jf901225e. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Cheng C, Sun Q, Li F, Liu J, Zheng C. Isolation and purification of four flavonoid constituents from the flowers of Paeonia suffruticosa by high-speed counter-current chromatography. J Chromatogr A. 2005;1075:127–131. doi: 10.1016/j.chroma.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 39.Sertie JA, Basile AC, Panizza S, Matida AK, Zelnik R. Anti-inflammatory activity and sub-acute toxicity of artemetin. Planta Med. 1990;56:36–40. doi: 10.1055/s-2006-960879. [DOI] [PubMed] [Google Scholar]
- 40.Choi CH, Sun KH, An CS, Yoo JC, Hahm KS, Lee IH, Sohng JK, Kim YC. Reversal of P-glycoprotein-mediated multidrug resistance by 5,6,7,3′,4′-pentamethoxyflavone (Sinensetin) Biochem Biophys Res Commun. 2002;295:832–840. doi: 10.1016/s0006-291x(02)00755-6. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe J, Shinmoto H, Tsushida T. Coumarin and flavone derivatives from estragon and thyme as inhibitors of chemical mediator release from RBL-2H3 cells. Biosci Biotechnol Biochem. 2005;69:1–6. doi: 10.1271/bbb.69.1. [DOI] [PubMed] [Google Scholar]
- 42.Hirano T, Abe K, Gotoh M, Oka K. Citrus flavone tangeretin inhibits leukaemic HL-60 cell growth partially through induction of apoptosis with less cytotoxicity on normal lymphocytes. Br J Cancer. 1995;72:1380–1388. doi: 10.1038/bjc.1995.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sergeev IN, Li S, Colby J, Ho CT, Dushenkov S. Polymethoxylated flavones induce Ca(2+)-mediated apoptosis in breast cancer cells. Life Sci. 2006;80:245–253. doi: 10.1016/j.lfs.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 44.Pan MH, Chen WJ, Lin-Shiau SY, Ho CT, Lin JK. Tangeretin induces cell-cycle G1 arrest through inhibiting cyclin-dependent kinases 2 and 4 activities as well as elevating CDK inhibitors p21 and p27 in human colorectal carcinoma cells. Carcinogenesis. 2002;23:1677–1684. doi: 10.1093/carcin/23.10.1677. [DOI] [PubMed] [Google Scholar]
- 45.Cai H, Sale S, Schmid R, Britton RG, Brown K, Steward WP, Gescher AJ. Flavones as colorectal cancer chemopreventive agents--phenol-o-methylation enhances efficacy. Cancer Prev Res (Phila Pa) 2009;2:743–750. doi: 10.1158/1940-6207.CAPR-09-0081. [DOI] [PubMed] [Google Scholar]
- 46.Li S, Pan MH, Lai CS, Lo CY, Dushenkov S, Ho CT. Isolation and syntheses of polymethoxyflavones and hydroxylated polymethoxyflavones as inhibitors of HL-60 cell lines. Bioorg Med Chem. 2007;15:3381–3389. doi: 10.1016/j.bmc.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 47.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 48.Zhou FQ, Snider WD. Cell biology. GSK-3beta and microtubule assembly in axons. Science. 2005;308:211–214. doi: 10.1126/science.1110301. [DOI] [PubMed] [Google Scholar]
- 49.Iguchi T, Yachide-Noguchi T, Hashimoto Y, Nakazato S, Sagawa M, Ikeda Y, Kizaki M. Novel tubulin-polymerization inhibitor derived from thalidomide directly induces apoptosis in human multiple myeloma cells: possible anti-myeloma mechanism of thalidomide. Int J Mol Med. 2008;21:163–168. [PubMed] [Google Scholar]
- 50.Peng F, Dhillon NK, Yao H, Zhu X, Williams R, Buch S. Mechanisms of platelet-derived growth factor-mediated neuroprotection--implications in HIV dementia. Eur J Neurosci. 2008;28:1255–1264. doi: 10.1111/j.1460-9568.2008.06444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patanasethanont D, Nagai J, Matsuura C, Fukui K, Sutthanut K, Sripanidkulchai BO, Yumoto R, Takano M. Modulation of function of multidrug resistance associated-proteins by Kaempferia parviflora extracts and their components. Eur J Pharmacol. 2007;566:67–74. doi: 10.1016/j.ejphar.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 52.Han Z, Hong L, Han Y, Wu K, Han S, Shen H, Li C, Yao L, Qiao T, Fan D. Phospho Akt mediates multidrug resistance of gastric cancer cells through regulation of P-gp, BCL-2 and BAX. J Exp Clin Cancer Res. 2007;26:261–268. [PubMed] [Google Scholar]