Insulin is the most potent anabolic hormone known and is essential for appropriate tissue development, growth, and maintenance of whole-body glucose homeostasis. This hormone is secreted by the β cells of the pancreatic islets of Langerhans in response to increased circulating levels of glucose and amino acids after a meal. Insulin regulates glucose homeostasis at many sites, reducing hepatic glucose output (via decreased gluconeogenesis and glycogenolysis) and increasing the rate of glucose uptake, primarily into striated muscle and adipose tissue. In muscle and fat cells, the clearance of circulating glucose depends on the insulin-stimulated translocation of the glucose transporter GLUT4 isoform to the cell surface (see Shulman, this Perspective series, ref. 1). Insulin also profoundly affects lipid metabolism, increasing lipid synthesis in liver and fat cells, and attenuating fatty acid release from triglycerides in fat and muscle. Insulin resistance occurs when normal circulating concentrations of the hormone are insufficient to regulate these processes appropriately. Thus, by definition, insulin resistance is a defect in signal transduction.
The signaling mechanisms involved in the various biologic responses to insulin remain somewhat elusive, but recent progress has shed light on a few pathways that are critical for its regulation of glucose and lipid metabolism. Although insulin affects such diverse processes as cellular growth, differentiation, apoptosis, and lipid, protein, and glucose synthesis and breakdown, we focus here on the regulation of glucose transport as the rate-limiting step in glucose utilization and storage.
The insulin receptor
Insulin action is initiated through the binding to and activation of its cell-surface receptor, which consists of two α subunits and two β subunits that are disulfide linked into an α2β2 heterotetrameric complex. Insulin binds to the extracellular α subunits, transmitting a signal across the plasma membrane that activates the intracellular tyrosine kinase domain of the β subunit. The receptor then undergoes a series of intramolecular transphosphorylation reactions in which one β subunit phosphorylates its adjacent partner on specific tyrosine residues. Some evidence suggests that different tyrosine residues account for distinct functions. For example, phosphorylation of COOH-terminal tyrosines mediates the mitogenic actions of insulin. The phosphorylated tyrosines in the juxtamembrane domain may participate in substrate binding, whereas those found within the kinase domain regulate the catalytic activity of the insulin receptor β subunit.
Some forms of insulin resistance may involve the receptor itself. Alterations in insulin receptor expression, binding, phosphorylation state, and/or kinase activity could account for many insulin- resistance phenotypes. In addition, it is possible that the selected blockade of distinct phosphorylation sites selectively inhibits certain actions of insulin. In this regard, individuals have been identified with rare genetic defects in the insulin receptor that influence expression, ligand binding, and tyrosine kinase activity. These patients demonstrate severe insulin resistance, manifest as clinically diverse syndromes including the type A syndrome, leprechaunism, Rabson-Mendenhall syndrome, and lipoatropic diabetes (2, 3).
The mode of inheritance found in families afflicted with insulin receptor mutations offers insight into insulin receptor function. Most individuals with severe familial insulin resistance carry lesions in both insulin receptor (INSR) alleles, either as homozygotes or compound heterozygotes. In these individuals, the entire cellular complement of the insulin receptor is defective. However, in several reported cases of the type A syndrome of insulin resistance (characterized by polycystic ovarian disease, signs of virilization, acanthosis nigricans, and enhanced growth rate), affected individuals were apparently simple heterozygotes with only one defective allele. The substantial loss of insulin receptor function in these patients cannot be explained by a 50% decrease in insulin receptor levels, as such a reduction in the level of wild type receptor would not be expected to adversely influence insulin action.
Several mechanisms could account for the greater than expected degree of insulin resistance in these individuals. First, because the insulin receptor precursor can form hybrids, the mutant receptor might function in a dominant-interfering manner, inhibiting the function of the normal allele. However, an interesting alternative model has emerged from the study of Insr knockout mice. The developmental characteristics of homozygous insulin receptor null mice are different from those of the compound receptor mutations in humans, and these mice die shortly after birth owing to extreme insulin resistance (4, 5). Heterozygous mice, carrying only one disrupted Insr allele are phenotypically normal, with no apparent defects in insulin signaling. Similarly, heterozygous knockout mice lacking a single allele of the gene for the insulin receptor substrate protein IRS1 lack any significant phenotype, whereas homozygous disruption of the IRS1 gene results in a mild form of insulin resistance (6, 7). IRS1–/– mice do not become diabetic, presumably owing to pancreatic β−cell compensation. Nevertheless, mice that are doubly heterozygous for these null alleles (Insr–/+ IRS1–/+) develop both insulin resistance and diabetes (8), indicating that development of diabetes can be a polygenic, multihit process. At least in mice, then, mild defects in several genes can generate insulin resistance and diabetes. Although defects in the INSR gene are too rare in the general population to account for garden-variety insulin resistance, the possibility remains that a reduction in insulin receptor levels, which by itself has no effect, can interact with other downstream alterations to generate insulin resistance. In either case, these data strongly argue for a postinsulin receptor defect(s) as a primary site leading to peripheral insulin resistance.
In addition to tyrosine autophosphorylation, the insulin receptor is also subjected to β-subunit serine/threonine phosphorylation. Data from some experimental models suggest that this modification allows receptor function to be attenuated. Thus, in vitro studies show that the tyrosine kinase activity of the insulin receptor decreases as a consequence of serine/threonine phosphorylation. The chronic elevation in insulin levels that occurs as a result of insulin resistance might stimulate the relevant serine kinases, perhaps through the IGF-1 receptor, which can also be stimulated by high concentrations of insulin Such an interaction could provide a mechanism for a vicious cycle of insulin-induced insulin resistance. Similarly, counter-regulatory hormones and cytokines can activate serine kinases, particularly protein kinase C (PKC), which has been implicated in the development of peripheral insulin resistance. Several PKC isoforms are chronically activated in human and rodent models of insulin resistance (9–11). These kinases can catalyze the serine or threonine phosphorylation of the insulin receptor or its substrates. Pharmacologic inhibition of PKC activity or reduction in PKC expression enhances insulin sensitivity and insulin receptor tyrosine kinase activity (12).
A number of protein tyrosine phosphatases (PTPases) have also been described that can dephosphorylate the insulin receptor, reducing its kinase activity and thereby attenuating insulin action. Two PTPases have been implicated in the negative regulation of the insulin receptor, PTP1B and LAR. Elevated expression of each these phosphatases has been reported in insulin-resistant patients (13, 14). In cultured systems, increased expression of these enzymes prevents insulin receptor kinase activation and insulin signaling. More recently, a PTP1B mouse knockout resulted in enhanced insulin sensitivity, suggesting that the regulation of PTP1B function could represent an important target for insulin-sensitizing agents (15).
Proximal insulin receptor signaling events
Once activated, the insulin receptor phosphorylates a number of important proximal substrates on tyrosine, including members of the insulin receptor substrate family (IRS1/2/3/4), the Shc adapter protein isoforms, SIRP family members, Gab-1, Cbl, and APS. Tyrosine phosphorylation of the IRS proteins creates recognition sites for additional effector molecules containing Src homology 2 (SH2) domains. These include the small adapter proteins Grb2 and Nck, the SHP2 protein tyrosine phosphatase and, most importantly, the regulatory subunit of the type 1A phosphatidylinositol 3–kinase (PI 3-kinase).
Critical physiologic functions for both IRS1 and IRS2 have recently been established. As already described here, homozygous IRS1 knockout mice develop a mild state of insulin resistance (6, 7) but do not become diabetic, presumably owing to β-cell compensation. On the other hand, homozygous disruption of the IRS2 gene results in impaired insulin secretion, in addition to peripheral insulin resistance and diabetes (16). Given that skeletal muscle IRS2 does not appear to be necessary for insulin- or exercise-stimulated glucose transport, the insulin resistance observed in the IRS2 knockout animals most likely reflects secondary events occurring as a consequence of alterations in β-cell function or survival (17). This finding is consistent with recent studies on β cell–specific insulin receptor knockout mice. These animals develop both peripheral insulin resistance and diabetes, presumably due to alterations in the normal pattern of insulin secretion (18).
Downstream signaling events
At present, only one downstream signaling molecule is unequivocally essential for insulin-stimulated GLUT4 translocation, the type 1A PI 3-kinase. Multiple studies using various pharmacologic inhibitors, microinjection of blocking antibodies, and expression of dominant-interfering and constitutively active mutants are all consistent with a necessary role for PI 3-kinase activity in insulin-stimulated glucose uptake and GLUT4 translocation (19). Several studies have suggested that the interaction of IRS with PI 3-kinase is necessary for the appropriate activation and/or targeting of the enzyme to a critical intracellular site, perhaps including its association with GLUT4 vesicles. However, expression of the dominantly interfering IRS PH and PTB domains completely prevents insulin-stimulated IRS tyrosine phosphorylation and DNA synthesis but have no significant effect on GLUT4 translocation.
The targets of PI 3-kinase action are likewise controversial. Two classes of serine/threonine kinases are known to act downstream of PI 3-kinase, namely the serine/threonine kinase Akt, also known as protein kinase B (PKB), and the atypical protein kinase C isoforms ζ and λ (PKCζ/λ). Stable expression of a constitutively active, membrane-bound form of Akt in 3T3L1 adipocytes results in increased glucose transport and persistent localization of GLUT4 to the plasma membrane (20, 21). Conversely, expression of a dominant-interfering Akt mutant inhibits insulin-stimulated GLUT4 translocation (22, 23). Similarly, PKCζ is also activated by the formation polyphosphoinositides, which accumulate in insulin-treated cells; PKCζ is therefore also sensitive to pharmacologic PI 3-kinase inhibitors, such as wortmannin (24). Expression of PKCζ or PKCλ are also reported to induce GLUT4 translocation, whereas expression of a dominant-interfering PKCλ inhibited GLUT4 translocation (25, 26). Thus, although PI 3-kinase activation is essential, the protein kinase targets that mediate the effects of this pathway remain uncertain.
Several investigators have examined the role of Akt and PI 3-kinase in the regulation of peripheral insulin sensitivity. There appears to be a relative decrease in insulin-stimulated association of IRS proteins with PI 3-kinase and activation of Akt in insulin-resistant skeletal muscle (27, 28). Surprisingly however, patients with reduced insulin-stimulated PI 3-kinase maintain normal activation of Akt (29). Even though these studies involved a small number of patients, the data suggest that PI 3-kinase is in substantial excess, with only a relatively small activation necessary for the full expression of downstream signaling. These data further imply that defects in the pathway leading from IRS tyrosine phosphorylation to Akt activation may not be responsible for insulin resistance in patients with type II diabetes. Clearly, additional studies of Akt and/or PKCζ/λ activation and localization are required in both animal models and more insulin-resistant patient populations.
Although PI 3-kinase activity is clearly necessary for insulin-stimulated glucose uptake, additional signals are also required for the stimulation of GLUT4 translocation. Thus, activation of PI 3-kinase by stimulation with IL-4 or by engagement of certain integrins does not induce GLUT4 translocation (30, 31). Furthermore, two natural insulin receptor mutations that were fully capable of activating PI 3-kinase nevertheless failed to induce GLUT4 translocation and glucose uptake (32). The stimulation of endogenous PI 3-kinase activity must be distinguished from the overexpression of a constitutively active PI 3-kinase. Under the latter conditions, there are massive increases in polyphosphoinositide formation and serine/threonine protein phosphorylation, as well as marked changes in cellular morphology. Although this treatment can also induce GLUT4 translocation, it is questionable whether it does so through the normal insulin regulatory pathways or through a less-specific stress response.
The most compelling evidence for a required additional PI 3-kinase–independent pathway makes use of a cell-permeable analog of PI(3, 4, 5)P3 (33). In these experiments, addition of the PI(3, 4, 5)P3 analog had no effect on GLUT4 translocation. As expected, treatment of cells with wortmannin prevented insulin-stimulated translocation of GLUT4. However, treatment of adipocytes with wortmannin, insulin plus the PI(3, 4, 5)P3 analog, resulted in enhanced glucose uptake. These data suggest that although the PI 3-kinase pathway is necessary, there is at least one additional pathway that is independent of PI 3-kinase activation.
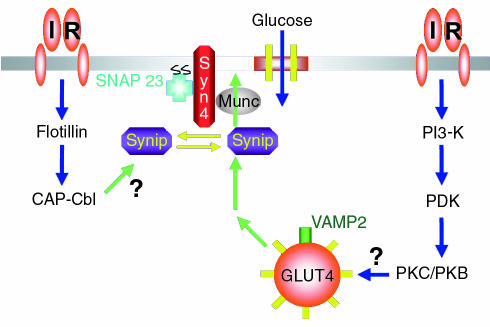
Recent studies have shown that insulin can also rapidly induce the tyrosine phosphorylation of the Cbl proto-oncoprotein, but only in insulin-responsive cells (34). This phosphorylation requires the presence of the adapter protein CAP, which associates with a proline-rich domain in Cbl through its COOH-terminal SH3 domain. CAP appears to be important in insulin signaling, as it is markedly induced during adipocyte differentiation and is transcriptionally regulated by the thiazolidinedione family of insulin-sensitizing PPARγ agonists (35). In support of this hypothesis, we have recently observed that expression of a dominant-interfering CAP mutant (CAPΔSH3) completely inhibited insulin-stimulated glucose uptake and GLUT4 translocation. This occurred through a marked reduction in the localization of tyrosine-phosphorylated Cbl in the plasma membrane lipid raft subdomain that is enriched in caveolin. Together, these data suggest that the insulin-dependent tyrosine phosphorylation and/or compartmentalization of CAP/Cbl complex may provide a necessary second signal that functions in parallel with the activation of the PI 3-kinase–dependent signaling pathway (Figure 1).
Figure 1.
Schematic model indicating the presence of two potential insulin receptor–dependent signal transduction pathways. In this model, insulin stimulation results in the activation of a PI 3-kinase–dependent pathway that is necessary but not sufficient to induce GLUT4 translocation. In parallel, the insulin receptor activates an additional pathway leading to Cbl tyrosine phosphorylation through its interaction with the CAP protein. Syn4, syntaxin 4; PI3-K, PI 3-kinase.
GLUT4 vesicle trafficking, docking, and fusion
The mechanisms by which upstream signaling pathways converge on the intracellular GLUT4-containing vesicles to translocate this protein to the cell surface remain obscure. In the basal state, GLUT4 continuously recycles between the cell-surface membrane and various intracellular compartments. After insulin stimulation, there is marked increase in the rate of GLUT4 vesicle exocytosis, with a small decrease in the rate of internalization. The insulin-stimulated exocytosis of GLUT4 resembles the regulated exocytosis of synaptic vesicles (36, 37). In particular, GLUT4 vesicles contain the v-SNARE proteins VAMP2 and VAMP3, which physically interact with their t-SNARE counterparts (syntaxin 4 and SNAP23) in the plasma membrane during GLUT4 vesicle translocation. Several lines of evidence have suggested that insulin specifically stimulates the translocation of the GLUT4 from VAMP2-containing compartments (38). Although these SNARE interactions are essential, none of these core proteins appear to be direct targets of insulin action. Similarly, although several important SNARE accessory proteins, such as Munc18c, Synip, and NSF, also appear required for the control of GLUT4 docking and fusion events, the molecular mechanism by which insulin regulates their function has yet to be elucidated. It is tempting to speculate that specific lesions in the SNARE protein complexes and/or the poorly defined signaling pathways that function in parallel with the PI 3-kinase pathway may also contribute to insulin resistance.
Summary and future directions
A number of different altered metabolic states, such as persistent elevation of circulating glucose, insulin, fatty acids, and cytokines, can lead to peripheral insulin resistance. Moreover, there is compelling evidence that susceptibility to insulin resistance is itself the result of a complex pattern of inheritance. The molecular targets and intracellular signaling systems that are modified during insulin resistance have received a great deal of attention, but there is still no evidence for a common mutation in any signaling pathway. Nevertheless, there has been significant progress in the identification of the signaling pathways leading to GLUT4 translocation and glucose uptake. A substantial body of evidence supports a reduction in the insulin receptor kinase itself, as well as a reduction in IRS protein tyrosine phosphorylation and PI 3-kinase association/activation in patients with type 2 diabetes. However, it is uncertain whether these changes in insulin receptor function represent primary lesions that cause insulin resistance or whether they occur secondary to hyperinsulinemia or hyperglycemia. Even if receptor-level defects can cause these phenotypes, whether attenuated insulin receptor function can account for the insulin-resistant phenotype present in the general patient population seems questionable. The relative contribution of defects in these signaling steps can only be resolved by increased population-based analysis and by more stringent quantitative determination of the extent of insulin receptor signaling in relationship to the biologic responsive end point, glucose uptake. Obviously, this approach can only be applied to known effector proteins and will necessitate similar analyses as other critical signal transduction proteins are identified.
Significant progress has also been made in our understanding of GLUT4 compartmentalization and SNARE protein interactions critical to insulin-stimulated GLUT4 translocation. At present, there is no evidence that the known components are defective or display aberrant function in insulin-resistant states. However, as we have only identified some of the core SNARE machinery, future studies will be necessary to focus on isolating additional regulatory proteins in the GLUT4 vesicle budding, trafficking, docking, and fusion events.
Finally, it must be considered that there may be no single or common defect that underlies peripheral insulin resistance. Most likely, insulin resistance is really a complex phenomenon in which several genetic defects combine with environmental stresses, such as obesity or infections, to generate the phenotype. Alternatively, it remains possible that there are no molecular defects in any signaling or effector system, but that several of these key molecules function at lower range of what is considered normal. Thus, the combination of several weakly coupling effectors that function within the normal range will result in poor signal transduction, insufficient to generate the full response of glucose uptake. The resolution of these issues will require a full understanding of the entire itinerary and functional consequences of insulin signaling and glucose transport regulation.
References
- 1.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor SI, Arioglu E. Syndromes associated with insulin resistance and acanthosis nigricans. J Basic Clin Physiol Pharmacol. 1998;9:419–439. doi: 10.1515/jbcpp.1998.9.2-4.419. [DOI] [PubMed] [Google Scholar]
- 3.Krook A, O’Rahilly S. Mutant insulin receptors in syndromes of insulin resistance. Baillieres Clin Endocrinol Metab. 1996;10:97–122. doi: 10.1016/s0950-351x(96)80330-2. [DOI] [PubMed] [Google Scholar]
- 4.Accili D, et al. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat Genet. 1996;12:106–109. doi: 10.1038/ng0196-106. [DOI] [PubMed] [Google Scholar]
- 5.Joshi RL, et al. Targeted disruption of the insulin receptor gene in the mouse results in neonatal lethality. EMBO J. 1996;15:1542–1547. [PMC free article] [PubMed] [Google Scholar]
- 6.Tamemoto H, et al. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature. 1994;372:182–186. doi: 10.1038/372182a0. [DOI] [PubMed] [Google Scholar]
- 7.Araki E, et al. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature. 1994;372:186–190. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- 8.Bruning JC, et al. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell. 1997;88:561–572. doi: 10.1016/s0092-8674(00)81896-6. [DOI] [PubMed] [Google Scholar]
- 9.Ishizuka T, et al. Alterations in insulin-induced postreceptor signaling in adipocytes of the Otsuka Long-Evans Tokushima fatty rat strain. J Endocrinol. 1998;156:1–13. doi: 10.1677/joe.0.1560001. [DOI] [PubMed] [Google Scholar]
- 10.Considine RV, et al. Protein kinase C is increased in the liver of humans and rats with non-insulin-dependent diabetes mellitus: an alteration not due to hyperglycemia. J Clin Invest. 1995;95:2938–2944. doi: 10.1172/JCI118001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avignon A, et al. Chronic activation of protein kinase C in soleus muscles and other tissues of insulin-resistant type II diabetic Goto-Kakizaki (GK), obese/aged, and obese/Zucker rats. A mechanism for inhibiting glycogen synthesis. Diabetes. 1996;45:1396–1404. doi: 10.2337/diab.45.10.1396. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly R, Qu X. Mechanisms of insulin resistance and new pharmacological approaches to metabolism and diabetic complications. Clin Exp Pharmacol Physiol. 1998;25:79–87. doi: 10.1111/j.1440-1681.1998.tb02181.x. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein BJ, Ahmad F, Ding W, Li PM, Zhang WR. Regulation of the insulin signalling pathway by cellular protein-tyrosine phosphatases. Mol Cell Biochem. 1998;182:91–99. [PubMed] [Google Scholar]
- 14.Drake PG, Posner BI. Insulin receptor-associated protein tyrosine phosphatase(s): role in insulin action. Mol Cell Biochem. 1998;182:79–89. [PubMed] [Google Scholar]
- 15.Elchebly M, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 16.Withers DJ, et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900–904. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]
- 17.Higaki Y, et al. Insulin receptor substrate-2 is not necessary for insulin- and exercise-stimulated glucose transport in skeletal muscle. J Biol Chem. 1999;274:20791–20795. doi: 10.1074/jbc.274.30.20791. [DOI] [PubMed] [Google Scholar]
- 18.Kulkarni RN, et al. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96:329–339. doi: 10.1016/s0092-8674(00)80546-2. [DOI] [PubMed] [Google Scholar]
- 19.Czech MP, Corvera S. Signaling mechanisms that regulate glucose transport. J Biol Chem. 1999;274:1865–1868. doi: 10.1074/jbc.274.4.1865. [DOI] [PubMed] [Google Scholar]
- 20.Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt ser/thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 21.Kohn AD, et al. Construction and characterization of a conditionally active version of the serine/threonine kinase Akt. J Biol Chem. 1998;273:11937–11943. doi: 10.1074/jbc.273.19.11937. [DOI] [PubMed] [Google Scholar]
- 22.Cong LN, et al. Physiological role of Akt in insulin-stimulated translocation of GLUT4 in transfected rat adipose cells. Mol Endocrinol. 1997;11:1881–1890. doi: 10.1210/mend.11.13.0027. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, et al. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol Cell Biol. 1999;19:4008–4018. doi: 10.1128/mcb.19.6.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bandyopadhyay G, et al. Activation of protein kinase C (alpha, beta, and zeta) by insulin in 3T3/L1 cells. Transfection studies suggest a role for PKC-zeta in glucose transport. J Biol Chem. 1997;272:2551–2558. doi: 10.1074/jbc.272.4.2551. [DOI] [PubMed] [Google Scholar]
- 25.Kotani K, et al. Requirement of atypical protein kinase clambda for insulin stimulation of glucose uptake but not for Akt activation in 3T3-L1 adipocytes. Mol Cell Biol. 1998;18:6971–6982. doi: 10.1128/mcb.18.12.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitamura T, et al. Requirement for activation of the serine-threonine kinase Akt (protein kinase B) in insulin stimulation of protein synthesis but not of glucose transport. Mol Cell Biol. 1998;18:3708–3717. doi: 10.1128/mcb.18.7.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cusi K, et al. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest. 2000;105:311–320. doi: 10.1172/JCI7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krook A, Roth RA, Jiang XJ, Zierath JR, Wallberg-Henriksson H. Insulin-stimulated Akt kinase activity is reduced in skeletal muscle from NIDDM subjects. Diabetes. 1998;47:1281–1286. doi: 10.2337/diab.47.8.1281. [DOI] [PubMed] [Google Scholar]
- 29.Kim YB, Nikoulina SE, Ciaraldi TP, Henry RR, Kahn BB. Normal insulin-dependent activation of Akt/protein kinase B, with diminished activation of phosphoinositide 3-kinase, in muscle in type 2 diabetes. J Clin Invest. 1999;104:733–741. doi: 10.1172/JCI6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isakoff SJ, et al. The inability of phosphatidylinositol 3-kinase activation to stimulate GLUT4 translocation indicates additional signaling pathways are required for insulin-stimulated glucose uptake. Proc Natl Acad Sci USA. 1995;92:10247–10251. doi: 10.1073/pnas.92.22.10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guilherme A, Czech MP. Stimulation of IRS-1-associated phosphatidylinositol 3-kinase and Akt/protein kinase B but not glucose transport by beta1-integrin signaling in rat adipocytes. J Biol Chem. 1998;273:33119–33122. doi: 10.1074/jbc.273.50.33119. [DOI] [PubMed] [Google Scholar]
- 32.Krook A, et al. Two naturally occurring insulin receptor tyrosine kinase domain mutants provide evidence that phosphoinositide 3-kinase activation alone is not sufficient for the mediation of insulin’s metabolic and mitogenic effects. J Biol Chem. 1997;272:30208–30214. doi: 10.1074/jbc.272.48.30208. [DOI] [PubMed] [Google Scholar]
- 33.Jiang T, et al. Membrane-permeant esters of phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:11017–11024. doi: 10.1074/jbc.273.18.11017. [DOI] [PubMed] [Google Scholar]
- 34.Ribon V, Saltiel AR. Insulin stimulates tyrosine phosphorylation of the proto-oncogene product of c-Cbl in 3T3-L1 adipocytes. Biochem J. 1997;324:839–845. doi: 10.1042/bj3240839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ribon V, Johnson JH, Camp HS, Saltiel AR. Thiazolidinediones and insulin resistance: peroxisome proliferatoractivated receptor gamma activation stimulates expression of the CAP gene. Proc Natl Acad Sci USA. 1998;95:14751–14756. doi: 10.1073/pnas.95.25.14751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pessin JE, Thurmond DC, Elmendorf JS, Coker KJ, Okada S. Molecular basis of insulin-stimulated GLUT4 vesicle trafficking. Location! Location! Location! J Biol Chem. 1999;274:2593–2596. doi: 10.1074/jbc.274.5.2593. [DOI] [PubMed] [Google Scholar]
- 37.Rea S, James DE. Moving GLUT4: the biogenesis and trafficking of GLUT4 storage vesicles. Diabetes. 1997;46:1667–1677. doi: 10.2337/diab.46.11.1667. [DOI] [PubMed] [Google Scholar]
- 38.Millar CA, Shewan A, Hickson GR, James DE, Gould GW. Differential regulation of secretory compartments containing the insulin-responsive glucose transporter 4 in 3T3-L1 adipocytes. Mol Biol Cell. 1999;10:3675–3688. doi: 10.1091/mbc.10.11.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]