Abstract
The primary cilium is a cellular antenna that detects and transmits chemical and mechanical cues in the environment through receptors and downstream signal proteins enriched along the ciliary membrane. While it is known that ciliary membrane proteins enter the cilium by way of vesicular and intraflagellar transport, less is known about how ciliary membrane proteins are retained in, and how apical membrane proteins are excluded from the cilium. Here, we review evidence for a membrane diffusion barrier at the base of the primary cilium, and highlight the recent finding of a septin cytoskeleton diffusion barrier. We also discuss candidate ciliopathy genes that may be involved in formation of the barrier, and the role of a diffusion barrier as a common mechanism for compartmentalizing membranes and lipid domains.
Keywords: primary cilium, ciliary membrane proteins, ciliopathy, diffusion barrier, basal body, ciliary pocket, ciliary necklace, transition zone, septins, GTPases
Introduction
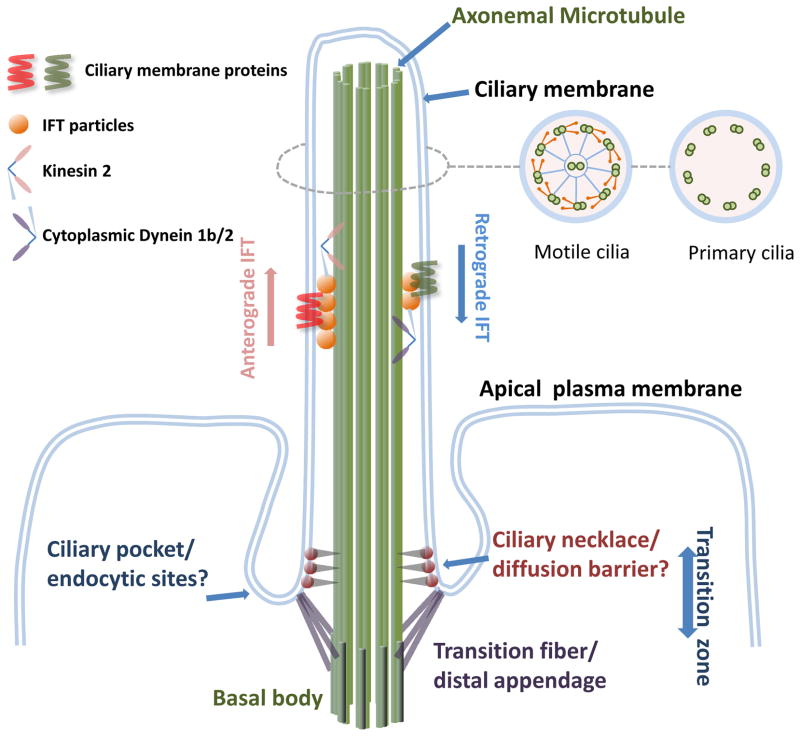
Cilia are rod-like membrane projections of several microns length on the apical surface of many, if not all cell types in vertebrates (Pazour and Bloodgood 2008). They are composed of a cylindrically organized microtubule axoneme that emanates from a centriole-derived structure called the basal body (Marshall 2008; Satir and Christensen 2007). The ciliary membrane surrounds the axoneme and is contiguous with the surrounding plasma membrane (Fig. 1).
Fig. 1. Scheme of a fully assembled primary cilium and ciliary base structures.
IFT particles move with their cargos (eg. ciliary membrane proteins) within the cilia by the molecular motors kinesin 2 (anterograde trafficking) and cytoplasmic dynein 1b/2 (retrograde trafficking). At the ciliary base, transition fiber/distal appendages link the basal body to the ciliary base, and together with ciliary necklace they form the transition zone and a diffusion barrier for ciliary proteins. Plasma membrane invagination (ciliary pocket) at the ciliary base appears to be sites for endocytosis. The illustration is adapted from (Rosenbaum and Witman 2002) and EM images from (Gilula and Satir 1972).
Cilia are assembled and maintained through a bidirectional transportation system called intraflagellar trafficking (IFT) mediated by IFT complexes and molecular motors moving along axonemal microtubules (Fig. 1) (Ishikawa and Marshall 2011; Pedersen and Rosenbaum 2008; Pigino et al. 2009; Rosenbaum and Witman 2002). IFT regulates cilia assembly, resorption and signaling, and defects in IFT proteins are found in a variety of cilium-related diseases (Pazour and Rosenbaum 2002; Pedersen and Rosenbaum 2008; Scholey and Anderson 2006; Snell et al. 2004; Wang et al. 2006).
Cilia are categorized into motile or multi-cilia (9 + 2 pattern of microtubule structure), and immotile or primary cilia (9 + 0 pattern of microtubule structure) based on the mobility and number of cilia, and the organization of axonemal microtubules (Fig. 1). Motile cilia in the respiratory airway clear mucus by beating constantly in coordinated waves (Shah et al. 2009), and nodal cilia generate flow to specify left-right asymmetry (Basu and Brueckner 2008; Nonaka et al. 1998). Sperm has a specialized cilium that is motile and drives sperm motility. This class of motile cilia will not be discussed further, and the reader is directed to several a recent review of this field (Salathe 2007).
The primary cilium in mammals was first identified over 100 years ago (Zimmermann 1898). However, it was considered a vestigial appendage of little importance and was largely ignored until the last decade when studies began to link ciliary dysfunction with genetic diseases such as polycystic kidney disease (PKD) (Bloodgood 2009; Pazour et al. 2000).
Recent studies have revealed that the primary cilium is the cell’s antenna which receives and transmits extracellular signals through specific receptors on the ciliary membrane that initiate cell signaling cascades critical for normal development and homeostasis (Eggenschwiler and Anderson 2007; Gerdes et al. 2009; Lancaster and Gleeson 2009; Marshall and Nonaka 2006; Pazour and Witman 2003; Singla and Reiter 2006; Sloboda and Rosenbaum 2007). Also mediated by the primary cilium (Eggenschwiler and Anderson 2007; Pazour and Bloodgood 2008) are mechano-reception (Malone et al. 2007; Nauli et al. 2003; Praetorius and Spring 2001; Schwartz et al. 1997), chemo-reception (McEwen et al. 2008; Mombaerts 1999), photo-reception (Besharse and Horst 1990), and extracellular signaling by Sonic Hedgehog (Shh) (Huangfu et al. 2003; Rohatgi et al. 2007; Wong and Reiter 2008) and Wnt (Gerdes and Katsanis 2008), Planar Cell Polarity (PCP) (Jones and Chen 2008; Ross et al. 2005), and PDGF-AA (Schneider et al. 2005).
Heritable diseases, called ciliopathies, are associated with ciliary dysfunction. They present clinically with a complex combination of phenotypes including cystic kidneys, retinal degeneration, hearing loss, situs inversus, and other defects: for example, PKD (Nauli et al. 2003; Pazour et al. 2000; Yoder et al. 2002), Bardet-Biedl syndrome (BBS) (Kulaga et al. 2004; Mykytyn and Sheffield 2004), Nephronophthisis (NPHP) (Hildebrandt et al. 2009), Meckel-Gruber syndrome (MKS) (Delous et al. 2007; Kyttala et al. 2006), Joubert syndrome (JBTS) (Baala et al. 2007; Parisi et al. 2007), Usher syndrome (Yan and Liu 2010). Each syndrome is caused by mutations in a number of genes, and in general the normal proteins encoded by those genes localize to the primary cilium or basal body (Badano et al. 2006; Fliegauf et al. 2007; Sharma et al. 2008).
Distinct protein and lipid compositions of the ciliary membrane and surrounding plasma membrane
The ciliary membrane is contiguous with the surrounding plasma membrane but retains a distinct composition of lipids and proteins required for cilia-mediated sensing/signaling events, ciliary membrane trafficking and ciliogenesis. However, mechanisms for retaining these proteins and lipids in the primary cilia are not clear.
In tissue culture cell lines, many proteins and signaling pathways are concentrated in the primary cilium including: the polycystic kidney disease-causing proteins polycystin-1, polycystin-2, cystin and polaris (Nauli et al. 2003; Pazour et al. 2002; Yoder et al. 2002); Shh signaling components Smoothened (Smo), Patched1 (Ptc1), Gli2, Gli3 and β-arrestin (Corbit et al. 2005; Haycraft et al. 2005; Kovacs et al. 2008; Rohatgi et al. 2007); platelet-derived growth factor receptor α (PDGFRα) (Schneider et al. 2005); the angiopoietin receptors tyrosine kinases Tie-1 and Tie-2 (Teilmann and Christensen 2005); and, melanin-concentrating hormone receptor 1 (Mchr1) (Berbari et al. 2008b). Specialized membrane signaling proteins are also found in olfactory cilia such as membrane and olfactory transduction proteins (Mayer et al. 2008), in the olfactory sensory neurons of C. elegans such as the odorant receptor cyclic nucleotide-gated channel CNGB1b (Jenkins et al. 2006), in motile cilia in airway epithelial such as sensory bitter taste receptors (Shah et al. 2009), and in the outer segment, a specialized cilium of rod photoreceptors that contain the photosensor, rhodopsin (Tam et al. 2000).
Small GTPases that mediate trafficking and biogenesis of ciliary membrane are also enriched in the primary cilium. Rab8a is present in primary cilia of cultured cells and coordinates with BBS proteins to promote ciliary membrane growth (Knödler et al. 2010; Nachury et al. 2007; Westlake et al. 2011). Proteomic analyses of photoreceptors and Chlamydomonas reinhardtii revealed that Rab subfamily members, ARF subfamily members, RAN, and SNARE proteins are present in the sensory cilium and flagellum, respectively (Kwok et al. 2008; Liu et al. 2007a; Pazour et al. 2005). The ADP ribosylation factor-like (ARL) family of small GTPases of the Ras superfamily are enriched in the cilium and mutations in Arl13b gives rise to PKD phenotype in Zebrafish (Duldulao et al. 2009). BBS proteins are present in primary cilium and assemble as a coat on vesicles that deliver membrane proteins to the cilium (Jin et al. 2010; Nachury et al. 2007). Some polarity protein complexes such as the transmembrane protein Crumbs3 localize to cilia of cultured MDCK cells, and are required for ciliogenesis (Fan et al. 2007; Sfakianos et al. 2007).
The ciliary membrane also maintains a lipid composition different from that of the apical plasma membrane. In quail oviduct, a high concentration of cholesterol was found on the shaft of ciliary membrane but not in the ciliary necklace enriched in intramembrane particles (Chailley and Boisvieux-Ulrich 1985). The trypanosome flagellar membrane is enriched in sterols and saturated fatty acids (Tyler et al. 2009). In addition, lipid-raft associated proteins such as palmitoylated and myristoylated proteins are targeted to the ciliary membranes (Emmer et al. 2010; Emmer et al. 2009; Janich and Corbeil 2007). Several studies have linked ciliopathy with defective phosphotidylinositol (PtdIns) signaling by inositol polyphosphate-5-phosphatase E (INPP5E), which mediates PtdIns metabolism and localizes in the primary cilia. Mutations of INPP5E were found in ciliopathy patients and impaired INPP5E phosphatase activity (Bielas et al. 2009; Jacoby et al. 2009). Therefore, cilia should be enriched in INPP5E product PI(4)P and PI(3,4)P2. A number of cilium-related proteins have been shown to bind phospholipids including Tubby-like protein 3 (TULP3) and BBSome proteins (Jin et al. 2010; Mukhopadhyay et al. 2010; Nachury et al. 2007).
Lipid rafts and lipid micro-domains may organize a micro-environment for signal transduction complexes (Simons and Toomre 2000). It remains to be determined if the primary ciliary membrane contains lipid rafts and if so, whether they play roles in cilia sensory functions. Difficulties in purifying primary cilia and the lack of tools to detect or manipulate lipids have impeded our understanding the function of lipids in the ciliary membrane (Mitchell et al. 2009). Nevertheless, the concentration and restricted distribution of proteins and lipids in the ciliary membrane indicate that the contents of the ciliary membrane and the surrounding plasma membrane are physically and functionally separated.
Early evidence of a membrane diffusion barrier in the primary cilium
Early studies suggested that the presence of a membrane diffusion barrier at the base of the primary cilium that physically and functionally separated the surrounding apical plasma membrane and ciliary membrane.
In Chlamydomonas reinhardii, glycoprotein agglutinins which mediate the adhesion of two algae during mating are segregated into two pools comprising an active fraction on flagella and an inactive fraction on the plasma membrane. However, cell body agglutinins move into the flagellum in response to mating signal, demonstrating that the function barrier can be opened by regulatory signals (Hunnicutt et al. 1990). In Chlamydomonas eugametos, the agglutination antigens present on the cell body are unable to diffuse into the flagellar/ciliary membrane, suggesting a physical barrier at the base of the ciliary membrane (Musgrave et al. 1986). In Madin–Darby canine kidney (MDCK) cells, Laurdan staining showed that the ciliary membrane has a condensed lipid zone of high lipid order at the base of primary cilium, regarded as the periciliary membrane domain (Pazour and Bloodgood 2008) and glycosylphosphatidylinositol (GPI) anchored proteins, while can diffuse in the surrounding plasma membrane, were excluded from the ciliary membrane in fixed cells (Vieira et al. 2006) although a recent study using live cell microscopy indicated that GPI-GFP is in the ciliary membrane of MDCK cells (Francis et al. 2011).
The outer segment of photoreceptor cells in the retina is a specialized primary cilium that concentrates the membrane protein rhodopsin (Besharse et al. 1977). In photoreceptor rod cells, rhodopsin is compartmentalized in the outer segment yet diffuses into the inner segment after breaching the connecting cilium, suggesting that the connecting cilium serves as a membrane diffusion barrier between the inner and outer segments (Spencer et al. 1988). Interestingly, in retinal rod photoreceptors the small soluble protein GFP is able to diffuse between the outer and inner segments across the connecting cilium, but it remains to be determined if larger soluble proteins have the same property (Calvert et al. 2010). It is possible, therefore, that mechanisms involved in retaining membrane proteins and soluble proteins in primary cilium are different. Nevertheless, these data suggest the presence of a membrane diffusion barrier surrounding the ciliary membrane.
Direct test of a membrane diffusion barrier in the primary cilium of mammalian cells
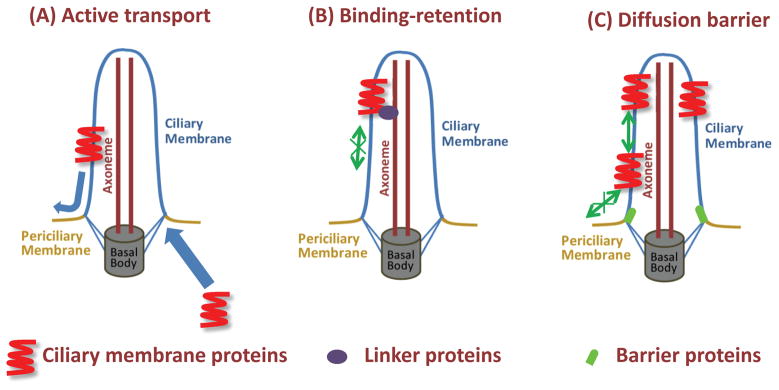
Ciliary membrane proteins can be targeted to the ciliary membrane through ciliary targeting sequences (CTS), and they become enriched in the cilum (Berbari et al. 2008a; Follit et al. 2009; Geng et al. 2006; Nachury et al. 2010; Pazour and Bloodgood 2008; Rohatgi et al. 2007; Tam et al. 2000; Tao et al. 2009). Several hypotheses have been put forward to explain the retention and enrichment of ciliary membrane proteins: active transport, binding-retention, and a diffusion barrier (Fig. 2) (Emmer et al. 2010; Nachury et al. 2010). The active transport hypothesis posits that newly synthesized proteins are actively transported into the primary cilia to offset the constant, free diffusion of proteins out of the primary cilia (Fig. 2A). In the binding-retention hypothesis, proteins once transported into the primary cilia are “fixed” in the primary cilia possibly by binding to a ciliary matrix or the microtubule axoneme (Fig. 2B). In diffusion barrier hypothesis, proteins are retained by a physical barrier that prevents proteins from diffusing from the ciliary membrane into the surrounding apical plasma membrane (Fig. 2C).
Fig. 2. Scheme of three hypotheses to retain ciliary membrane proteins.
A: Active transportation hypothesis. Ciliary membrane proteins are actively targeted and transported into the ciliary membrane and they are able to diffuse out of the ciliary membrane into plasma membrane or endocytosed from the plasma membrane into cytoplasm. The transportation rate into ciliary membrane is faster than the diffusion rate out of the cilium, thereby resulting in the enrichment of proteins in the cilium. B: Binding-retention hypothesis. Ciliary membrane proteins, once transported into the ciliary membrane, bind stably to the axoneme and are retained. C: Diffusion barrier hypothesis. Ciliary membrane proteins, once transported into the ciliary membrane are prevented from diffusing from the ciliary membrane by a physical barrier but may actively move within ciliary membrane.
The diffusion of ciliary membrane proteins in the ciliary membrane and surrounding apical plasma membrane of polarized epithelial cells were measured directly using fluorescence recovery after photobleaching (FRAP) (Hu et al. 2010). Photobleaching of the whole cilium or plasma membrane pool revealed that four membrane proteins in the apical plasma membrane and ciliary membrane are mobile, but do not exchange, indicating the presence of a physical barrier that blocks the free diffusion of those membrane proteins between these two adjacent plasma membrane compartments. It should be noted, however IFT88 shows high turnover and mobility suggesting IFT complexes may adopt the active-transportation mechanism to enter and be retained within the primary cilium (Hu et al. 2010). Interestingly, in Chlamydomonas, only a small portion of polycystic kidney disease 2 (PKD2) is mobile within the flagella suggesting that distinct mechanisms may be involved in retaining different ciliary membrane proteins in the ciliary membrane (Huang et al. 2007). Chlamydomonas flagellar proteome showed that a subset of membrane proteins are more enriched in the axonemal fraction than in the membrane and matrix fraction suggesting their anchorage to the axoneme (Pazour et al. 2005). Taken together, these results indicate that at least some membrane proteins are retained in the ciliary membrane by a diffusion barrier, and not by the active transportation or binding-retention hypothesis, whereas a subset of membrane proteins such as PKD2 may utilize the binding-retention mechanism to maintain ciliary localization.
Septins as a component of the membrane diffusion barrier in the ciliary membrane
Septins comprise a large, conserved family of GTPases that form linear heterotrimers (heterotetramers in budding yeast) which in turn assemble into apolar filaments, bundles and rings (Bertin et al. 2008; Sirajuddin et al. 2007; Versele and Thorner 2005). They play important roles in mitosis, cell migration, and cell morphogenesis by forming scaffolds and diffusion barriers (Caudron and Barral 2009; Hu et al. 2008; Joo et al. 2007; Kinoshita et al. 2002; Kremer et al. 2007; Oh and Bi 2011; Spiliotis et al. 2008; Spiliotis et al. 2005).
Studies in a several biological systems indicate that septins assemble into structures that regulate the distribution of membrane proteins between different compartments of cells, and hence have characteristics of a diffusion barrier. In budding yeast, septins assemble into hourglass shape rings and ordered protein “gauzes” at the mother-daughter neck. There, septins act as a scaffold to restrict the distribution of polarity and exocytosis factors (Faty et al. 2002; Gladfelter et al. 2001; Rodal et al. 2005) and form a diffusion barrier between the mother and daughter cells for plasma membrane proteins, the nuclear envelop and the endoplasmic reticulum (ER) to maintain asymmetric cell division (Barral et al. 2000; Dobbelaere and Barral 2004; Luedeke et al. 2005; Shcheprova et al. 2008). Disruption of the septin rings results in mislocalization of cortical proteins at the bud neck, and therefore a failure of cytokinesis (Dobbelaere and Barral 2004; Oh and Bi 2010; Versele and Thorner 2005). In mitotic mammalian cells, septins surround the midbody, and have been proposed to be a cortical barrier between the two daughter cells (Schmidt and Nichols 2004). In sperm, septin filaments encircle the cortical membrane between the middle and principle piece of sperm tail; septin gene knockout causes a defect in sperm motility due to cortical disorganization and dispersion of the membrane protein Basigin due to a loss of the septin-based diffusion barrier (Ihara et al. 2005; Kissel et al. 2005; Kwitny et al. 2010; Steels et al. 2007). In hippocampal neurons, septins localize at the membrane and at the base of dendrite spines; depletion of septins affects dendritic branch morphogenesis (Tada et al. 2007; Xie et al. 2007). In mouse epithelial cells, septins localize to the base of the primary cilia at the boundary between ciliary membrane and plasma membrane, and between the axoneme and distal/subdistal appendage proteins of basal body (Hu et al. 2010) (Fig. 3). In Xenopus epidermis, septins form ring-like structures at the base of cilia in multi-ciliated cells while exogenous, over-expressed SEPT2 localizes along the shaft of cilia suggesting septins may play a role in the axonemal matrix for ciliary function (Kim et al. 2010).
Fig. 3. SEPT2 forms a ring-like structure at the ciliary base of IMCD3 cells.
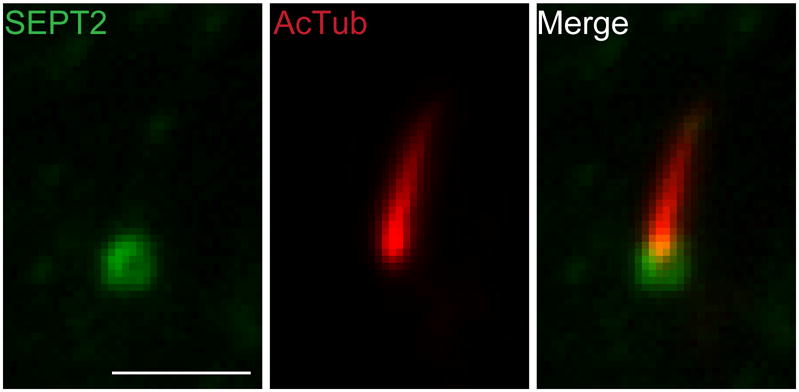
IMCD3 cells were fixed and stained with anti-SEPT2 antibody (green) and anti-acetylated tubulin antibody (red). The image shows the primary cilium on the apical membrane. Scale bar, ~2μm.
Depletion of septins in both mouse epithelial cells and Xenopus impairs the formation and organization of cilia. In epithelial cells with reduced levels of SEPT2, ciliary membrane proteins can diffuse across the barrier as measured using FRAP (Hu et al. 2010). Similarly, the enrichment of ciliary membrane proteins involved in Shh signaling is partially lost, resulting in a reduced Shh signaling. Thus, SEPT2 contributes to the formation of the diffusion barrier at the base of the primary cilium. In Xenopus, septins interact with PCP signaling proteins and Fritz (Kim et al. 2010). Septins and Fritz are in the same pathway to control convergent-extention and ciliogenesis in early Xenopus development. In addition, depletion of septins or Fritz impairs Shh signaling. Finally, mutations in the Fritz gene are found in patients with Meckel-Gruber and Bardet-Biedl syndromes, although it is not known whether septin mutations also exist in patients with those ciliopathies and whether mutations in Fritz in those patients are pathogenic. The finding that a septin cytoskeleton is localized to sites of many of these barriers for membrane compartmentalization indicates an evolutionarily conserved mechanism to organize and compartmentalize membrane structure.
Despite these recent advances, several important questions remain. First, it remains unknown how septins contribute to the formation of the membrane diffusion barrier; there are no data to explain how septins are specifically recruited to the diffusion barrier at the base of primary cilia, sperm annulus, bases of dendritic spins or midbody of mitotic cells. Septins can interact with phospholipid membranes and mediate their tubulation (Tanaka-Takiguchi et al. 2009; Zhang et al. 1999), and phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2) promotes the assembly of yeast septins in vitro (Bertin et al. 2010). It is possible that septins locally organize membrane lipids which in turn restrict the mobility of membrane proteins. Alternatively, septins may bind transmembrane proteins which serve as the diffusion barrier, although transmembrane proteins that interact with septins have not been identified.
Second, it is unknown whether the septin-mediated membrane diffusion barrier has selectivity for different membrane proteins, soluble (cytoplasmic) proteins of different molecular sizes, or peripheral membrane proteins that bind to different lipids.
Third, it is unknown whether and how ciliary diffusion barriers are regulated. For example, ciliary membrane proteins can be targeted and transported to the ciliary membrane during ciliogenesis indicating that the barrier either has not formed, or is permissive to the diffusion of ciliary membrane proteins. In another example, Shh binding to Ptc1 results in Ptc1 leaving the ciliary membrane causing Smo enrichment in the cilia (Corbit et al. 2005; Milenkovic et al. 2009; Rohatgi et al. 2007; Wong and Reiter 2008). How the ciliary barrier selectively gates these receptors remains unknown. In this context it is interesting to note that nuclear transport components RanGTP and importin-β2 mediate the shuttling of cytoplasmic kinesin-2 motor KIF17 into cilia (Dishinger et al. 2010; Hurd et al. 2011), but it is unknown how this complex by-passes the ciliary barrier.
Ultrastructure of the ciliary base
The diffusion barrier is localized at the boundary of apical plasma membrane and ciliary membrane (Hu et al. 2010) (Fig. 1–3). Ultrastructural studies of the ciliary base shed some light on the structural nature of the diffusion barrier. Freeze-fracture electron microscopy (EM) revealed a “ciliary necklace” surrounding the membrane at the base of cilia (Fig. 1) (Gilula and Satir 1972). The necklace is composed of rows of particles associated with the membrane that are connected to the basal body by appendages, and was proposed to form the membrane diffusion barrier or organize lipids at the transitional zone between the basal body and axonemal microtubules (Satir and Christensen 2007) (see Fig. 1). However, the molecular identity of the ciliary necklace remains to be determined.
Another membrane structure at the base of the ciliary membrane comprises the ciliary membrane pocket (Fig. 1). Cross sections of Elliptio lateral cilium revealed a pocket structure at the base of ciliary membrane with the ciliary necklace localized on the inner side of the pocket (Gilula and Satir 1972; Sorokin 1962). In Trypanosomatid, a protozoan parasite which uses a single flagellum as an invasion tool, a ciliary/flagellar pocket also exists and appears as a site for protein endocytosis and exocytosis (Gadelha et al. 2009; Gull 2003; Kohl et al. 2005; Overath et al. 1997). A cytoskeleton protein BILBO1 at the ciliary pocket was identified and plays an important role in ciliary pocket biogenesis (Bonhivers et al. 2008). A membrane pocket was also identified in Xenopus rod photoreceptor at the base of connecting cilum (Papermaster et al. 1985). Additional ultrastructural studies showed a similar structure in human retinal pigment epithelial (RPE) cells, mouse 3T3 cells and some mouse kidney epithelial (IMCD3) cells (Molla-Herman et al. 2010; Rohatgi and Snell 2010). Actin filaments were observed in the vicinity of ciliary pocket, possibly mediating the position of cilium. Remarkably, clathrin-coated pits and vesicles were also found exclusively at the ciliary pocket, further indicating that they are sites of endocytic targeting and recycling of ciliary membrane proteins (Molla-Herman et al. 2010).
Taken together, a ciliary diffusion barrier protein complex may be localized at the transition zone (TZ) consisting of the basal body, its accessory appendages (also called transition fibers) connected to the ciliary pocket, the proximal region of the axoneme, the ciliary necklace membrane region and the Y-connector between axonemal microtubules and ciliary necklace membrane (Fig. 1). This complex may be the main site for trafficking in and out of ciliary membrane and, as a diffusion barrier, regulate membrane and cytosolic protein diffusion (Nachury et al. 2010).
Additional candidates for the ciliary diffusion barrier
Several proteins, in addition to septins, have been identified at the ciliary diffusion barrier complex/transitional zone (Sharma et al. 2008) and some may constitute the diffusion barrier. Interestingly, many of them are proteins encoded by ciliopathy genes. In C. elegans and cilia of different mammalian tissues, the Nephronophthisis disease gene product NPHP1, NPHP2/Invesin, NPHP4/Nephroretinin, NPHP6/CEP290/MKS-4, NPHP8/RPGRIP1L/MKS-5, NPHP9/Nek8 and NPHP11/TMEM67/MKS-3/Meckelin are localized mainly at the transitional zone at the ciliary base (Delous et al. 2007; Fliegauf et al. 2006; Mollet et al. 2005; Otto et al. 2005; Otto et al. 2003; Otto et al. 2008; Sayer et al. 2006; Shiba et al. 2010; Simons et al. 2005; Valente et al. 2006; Winkelbauer et al. 2005). Remarkably, in Chlamydomonas reinhardtii, CEP290 is an integral component of Y-shape connector that links the microtubule doublets to the ciliary necklace at the TZ; significantly, cep290 mutant causes a loss of the Y-shape link and therefore the association between axonemal microtubules and ciliary membrane. Loss of CEPT290 leads to a reduction of IFT-associated and membrane proteins in the flagella (Craige et al. 2010). Thus, CEP290 appears to function as a gatekeeper to regulate the delivery and exit of flagellar proteins (Betleja and Cole 2010). Meckel-Gruber syndrome (MKS) proteins MKS-1, MKS-2/TMEM216, MKS-3/Meckelin/TMEM67 and their related proteins are also localized to the ciliary base and are required for ciliogenesis (Bialas et al. 2009; Dawe et al. 2009; Valente et al. 2010; Williams et al. 2009; Williams et al. 2008). A recent study systematically characterized the localization and function interaction of MKS and NPHP proteins at the TZ in C. elegans and demonstrated they establish the attachment between basal body/TZ and ciliary membrane and ciliary gating function (Williams et al. 2011). It would be of great interest to determine their ultrastructure localization and protein binding profiles. In Xenopus photoreceptors, the Usher syndrome gene products SANS (USH1G), Whirlin (USH2D), USH2b and VLGR1b are in a complex that forms a bridge between the connecting cilium and periciliary membrane (Liu et al. 2007b; Maerker et al. 2008); Usher syndrome is a disorder that causes combined deafness and blindness characterized with degeneration of retinal photoreceptors. Retinitis pigmentosa GTPase regulator (RPGR) localizes at the connecting cilium and maintains the polarized distribution of rhodopsin in the photoreceptor cells (He et al. 2008; Hong et al. 2001; Roepman et al. 2005); mutations in RPGR are a frequent cause of retinitis pigmentosa (RP), a retinal degeneration disease. Basal body distal appendage protein Cep164 forms a donut-shaped structure at the base of primary cilium (Graser et al. 2007). Oral-facial-digital type I (OFD1) syndrome gene product OFD1 has basal body and centrosomal localizations (Romio et al. 2004).
Although these proteins are candidates for forming the diffusion barrier, details of the fine structure localization and functions of these proteins networks at the transitional zone and at the base of the cilia remain to be determined. Immuno-gold labeling and super-resolution microscopy could be used to further pinpoint their localization at the ciliary base. Tandem affinity purification system coupled with sequential mass spectrometry has been used successfully to identify BBSomes and their associated proteins (Nachury et al. 2007), and this might be an application useful for the purification of protein complex in the transitional zone. Protein interactions could be verified subsequently by biochemical methods and genetic interaction studies. In-vitro purification, reconstitution, high resolution EM and crystal structures of those protein complexes would be helpful to study their structure-function relationships.
Conclusions and Perspectives
The ciliary diffusion barrier maintains the specific concentration of ciliary membrane proteins and associated signaling complexes within the cilium compared to the surrounding (apical) plasma membrane. The diffusion barrier appears to be localized to the TZ at the base of the ciliary membrane, and consist of ciliary necklace, the Y-link connecting ciliary necklace and axonemal microtubules, a septin cytoskeleton, and may include a complex protein network involving proteins encoded by ciliopathy genes.
Many important aspects of the diffusion barrier are poorly understood. First, selectivity of the diffusion barrier to different proteins remains to be determined. The diffusion of additional integral membrane proteins, peripheral proteins and IFT complex and associated proteins could be tested using FRAP. Soluble proteins of different sizes could be tagged with fluorophores and injected into or expressed in cells and their distribution observed in cilia. Interestingly, 10kDa fluorescently labeled dextrans can enter mammalian primary cilia while dextrans of 40kDa or larger are excluded from the ciliary compartment suggesting a size exclusion mechanism controlling the ciliary entry of soluble proteins (Kee et al. 2010). The underlying mechanisms involved in selectivity should be addressed. Second, the signaling cascades regulating the assembly, disassembly and permeability of the diffusion barrier are unknown. Third, the biochemical composition and structure of the diffusion barrier are unknown. Protein complexes encoded by ciliopathy genes at the TZ could be purified and identified, and their localization at the ciliary base could be pinpointed using high-resolution immuno-gold EM. Protein depletion or genetic knock-outs of single or multiple components are needed to test the structural and functional importance of specific proteins and combinations of proteins at the TZ of the diffusion barrier. Once the molecular nature of the diffusion barrier is defined, animal models can be established to explore the physiological relevance of the ciliary diffusion barrier in development, homeostasis and diseases.
Acknowledgments
Work from the Nelson laboratory was supported by a grant from the NIH (GM35527), and Q.H. was also supported by a US Department of Defense Breast Cancer Research Program Predoctoral Training Grant (BC083077). We thank Max V. Nachury, Hua Jin, Elias T. Spiliotis, and reviewers for helpful comments on the manuscript.
References
- Baala L, Romano Sp, Khaddour R, Saunier S, Smith UM, Audollent S, Ozilou C, Faivre L, Laurent N, Foliguet B, et al. The Meckel-Gruber Syndrome Gene, MKS3, Is Mutated in Joubert Syndrome. Am J Hum Genet. 2007;80(1):186–194. doi: 10.1086/510499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badano JL, Mitsuma N, Beales PL, Katsanis N. The Ciliopathies: An Emerging Class of Human Genetic Disorders. Annu Rev Genomics Hum Genet. 2006;7(1):125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- Barral Y, Mermall V, Mooseker MS, Snyder M. Compartmentalization of the Cell Cortex by Septins Is Required for Maintenance of Cell Polarity in Yeast. Mol Cell. 2000;5(5):841–851. doi: 10.1016/s1097-2765(00)80324-x. [DOI] [PubMed] [Google Scholar]
- Basu B, Brueckner M. Curr Top Dev Biol. Academic Press; 2008. Chapter 6 Cilia: Multifunctional Organelles at the Center of Vertebrate Left-Right Asymmetry; pp. 151–174. [DOI] [PubMed] [Google Scholar]
- Berbari NF, Johnson AD, Lewis JS, Askwith CC, Mykytyn K. Identification of Ciliary Localization Sequences within the Third Intracellular Loop of G Protein-coupled Receptors. Mol Biol Cell. 2008a;19(4):1540–1547. doi: 10.1091/mbc.E07-09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci U S A. 2008b;105(11):4242–4246. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin A, McMurray MA, Grob P, Park S-S, Garcia G, Patanwala I, Ng H-l, Alber T, Thorner J, Nogales E. Saccharomyces cerevisiae septins: Supramolecular organization of heterooligomers and the mechanism of filament assembly. Proc Natl Acad Sci U S A. 2008;105(24):8274–8279. doi: 10.1073/pnas.0803330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin A, McMurray MA, Thai L, Garcia G, III, Votin V, Grob P, Allyn T, Thorner J, Nogales E. Phosphatidylinositol-4,5-bisphosphate Promotes Budding Yeast Septin Filament Assembly and Organization. J Mol Biol. 2010;404(4):711–731. doi: 10.1016/j.jmb.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besharse JC, Hollyfield JG, Rayborn ME. Photoreceptor outer segments: accelerated membrane renewal in rods after exposure to light. Science. 1977;196(4289):536–538. doi: 10.1126/science.300504. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Horst CJ. The photoreceptor connecting cilium. A model for the transition zone. In: Bloodgood RA, editor. Ciliary and Flagellar Membranes. Plenum Press; New York: 1990. pp. 389–417. [Google Scholar]
- Betleja E, Cole DG. Ciliary Trafficking: CEP290 Guards a Gated Community. Curr Biol. 2010;20(21):R928–R931. doi: 10.1016/j.cub.2010.09.058. [DOI] [PubMed] [Google Scholar]
- Bialas NJ, Inglis PN, Li C, Robinson JF, Parker JDK, Healey MP, Davis EE, Inglis CD, Toivonen T, Cottell DC, et al. Functional interactions between the ciliopathy-associated Meckel syndrome 1 (MKS1) protein and two novel MKS1-related (MKSR) proteins. J Cell Sci. 2009;122(5):611–624. doi: 10.1242/jcs.028621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielas SL, Silhavy JL, Brancati F, Kisseleva MV, Al-Gazali L, Sztriha L, Bayoumi RA, Zaki MS, Abdel-Aleem A, Rosti RO, et al. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet. 2009;41(9):1032–1036. doi: 10.1038/ng.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood RA. Methods Cell Biol. Academic Press; 2009. From Central to Rudimentary to Primary: The History of an Underappreciated Organelle Whose Time Has Come. The Primary Cilium; pp. 2–52. [DOI] [PubMed] [Google Scholar]
- Bonhivers Ml, Nowacki S, Landrein N, Robinson DR. Biogenesis of the Trypanosome Endo-Exocytotic Organelle Is Cytoskeleton Mediated. PLoS Biol. 2008;6(5):e105. doi: 10.1371/journal.pbio.0060105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert PD, Schiesser WE, Pugh EN., Jr Diffusion of a soluble protein, photoactivatable GFP, through a sensory cilium. J Gen Physiol. 2010;135(3):173–196. doi: 10.1085/jgp.200910322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudron F, Barral Y. Septins and the Lateral Compartmentalization of Eukaryotic Membranes. Dev Cell. 2009;16(4):493–506. doi: 10.1016/j.devcel.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Chailley B, Boisvieux-Ulrich E. Detection of plasma membrane cholesterol by filipin during microvillogenesis and ciliogenesis in quail oviduct. J Histochem Cytochem. 1985;33(1):1–10. doi: 10.1177/33.1.3965567. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DYR, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437(7061):1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- Craige B, Tsao C-C, Diener DR, Hou Y, Lechtreck K-F, Rosenbaum JL, Witman GB. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J Cell Biol. 2010;190(5):927–940. doi: 10.1083/jcb.201006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe HR, Adams M, Wheway G, Szymanska K, Logan CV, Noegel AA, Gull K, Johnson CA. Nesprin-2 interacts with meckelin and mediates ciliogenesis via remodelling of the actin cytoskeleton. J Cell Sci. 2009;122(15):2716–2726. doi: 10.1242/jcs.043794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delous M, Baala L, Salomon R, Laclef C, Vierkotten J, Tory K, Golzio C, Lacoste T, Besse L, Ozilou C, et al. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet. 2007;39(7):875–881. doi: 10.1038/ng2039. [DOI] [PubMed] [Google Scholar]
- Dishinger JF, Kee HL, Jenkins PM, Fan S, Hurd TW, Hammond JW, Truong YN-T, Margolis B, Martens JR, Verhey KJ. Ciliary entry of the kinesin-2 motor KIF17 is regulated by importin-β2 and RanGTP. Nat Cell Biol. 2010;12(7):703–710. doi: 10.1038/ncb2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelaere J, Barral Y. Spatial Coordination of Cytokinetic Events by Compartmentalization of the Cell Cortex. Science. 2004;305(5682):393–396. doi: 10.1126/science.1099892. [DOI] [PubMed] [Google Scholar]
- Duldulao NA, Lee S, Sun Z. Cilia localization is essential for in vivo functions of the Joubert syndrome protein Arl13b/Scorpion. Development. 2009;136(23):4033–4042. doi: 10.1242/dev.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggenschwiler JT, Anderson KV. Cilia and Developmental Signaling. Annu Rev Cell Dev Biol. 2007;23(1):345–373. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmer BT, Maric D, Engman DM. Molecular mechanisms of protein and lipid targeting to ciliary membranes. J Cell Sci. 2010;123(4):529–536. doi: 10.1242/jcs.062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmer BT, Souther C, Toriello KM, Olson CL, Epting CL, Engman DM. Identification of a palmitoyl acyltransferase required for protein sorting to the flagellar membrane. J Cell Sci. 2009;122(6):867–874. doi: 10.1242/jcs.041764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S, Fogg V, Wang Q, Chen X-W, Liu C-J, Margolis B. A novel Crumbs3 isoform regulates cell division and ciliogenesis via importin β interactions. J Cell Biol. 2007;178(3):387–398. doi: 10.1083/jcb.200609096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faty M, Fink M, Barral Y. Septins: a ring to part mother and daughter. Curr Genet. 2002;41(3):123–131. doi: 10.1007/s00294-002-0304-0. [DOI] [PubMed] [Google Scholar]
- Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8(11):880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- Fliegauf M, Horvath J, von Schnakenburg C, Olbrich H, Müller D, Thumfart J, Schermer B, Pazour GJ, Neumann HPH, Zentgraf H, et al. Nephrocystin Specifically Localizes to the Transition Zone of Renal and Respiratory Cilia and Photoreceptor Connecting Cilia. J Am Soc Nephrol. 2006;17(9):2424–2433. doi: 10.1681/ASN.2005121351. [DOI] [PubMed] [Google Scholar]
- Follit JA, Li L, Vucica Y, Pazour GJ. The cytoplasmic tail of fibrocystin contains a ciliary targeting sequence. J Cell Biol. 2009;188(1):21–28. doi: 10.1083/jcb.200910096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis SS, Sfakianos J, Lo B, Mellman I. A hierarchy of signals regulates entry of membrane proteins into the ciliary membrane domain in epithelial cells. J Cell Biol. 2011;193(1):219–233. doi: 10.1083/jcb.201009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadelha C, Rothery S, Morphew M, McIntosh JR, Severs NJ, Gull K. Membrane domains and flagellar pocket boundaries are influenced by the cytoskeleton in African trypanosomes. Proc Natl Acad Sci U S A. 2009;106(41):17425–17430. doi: 10.1073/pnas.0909289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng L, Okuhara D, Yu Z, Tian X, Cai Y, Shibazaki S, Somlo S. Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J Cell Sci. 2006;119(7):1383–1395. doi: 10.1242/jcs.02818. [DOI] [PubMed] [Google Scholar]
- Gerdes JM, Davis EE, Katsanis N. The Vertebrate Primary Cilium in Development, Homeostasis, and Disease. Cell. 2009;137(1):32–45. doi: 10.1016/j.cell.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes JM, Katsanis N. Curr Top Dev Biol. Academic Press; 2008. Chapter 7 Ciliary Function and Wnt Signal Modulation; pp. 175–195. [DOI] [PubMed] [Google Scholar]
- Gilula NB, Satir P. THE CILIARY NECKLACE: A Ciliary Membrane Specialization. J Cell Biol. 1972;53(2):494–509. doi: 10.1083/jcb.53.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladfelter AS, Pringle JR, Lew DJ. The septin cortex at the yeast mother-bud neck. Curr Opin Microbiol. 2001;4(6):681–689. doi: 10.1016/s1369-5274(01)00269-7. [DOI] [PubMed] [Google Scholar]
- Graser S, Stierhof Y-D, Lavoie SB, Gassner OS, Lamla S, Le Clech M, Nigg EA. Cep164, a novel centriole appendage protein required for primary cilium formation. J Cell Biol. 2007;179(2):321–330. doi: 10.1083/jcb.200707181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gull K. Host-parasite interactions and trypanosome morphogenesis: a flagellar pocketful of goodies. Curr Opin Microbiol. 2003;6(4):365–370. doi: 10.1016/s1369-5274(03)00092-4. [DOI] [PubMed] [Google Scholar]
- Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 Localize to Cilia and Require the Intraflagellar Transport Protein Polaris for Processing and Function. PLoS Genet. 2005;1(4):e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Parapuram SK, Hurd TW, Behnam B, Margolis B, Swaroop A, Khanna H. Retinitis Pigmentosa GTPase Regulator (RPGR) protein isoforms in mammalian retina: Insights into X-linked Retinitis Pigmentosa and associated ciliopathies. Vision Res. 2008;48(3):366–376. doi: 10.1016/j.visres.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt F, Attanasio M, Otto E. Nephronophthisis: Disease Mechanisms of a Ciliopathy. J Am Soc Nephrol. 2009;20(1):23–35. doi: 10.1681/ASN.2008050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong D-H, Yue G, Adamian M, Li T. Retinitis Pigmentosa GTPase Regulator (RPGR)-interacting Protein Is Stably Associated with the Photoreceptor Ciliary Axoneme and Anchors RPGR to the Connecting Cilium. J Biol Chem. 2001;276(15):12091–12099. doi: 10.1074/jbc.M009351200. [DOI] [PubMed] [Google Scholar]
- Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, Nelson WJ. A Septin Diffusion Barrier at the Base of the Primary Cilium Maintains Ciliary Membrane Protein Distribution. Science. 2010;329(5990):436–439. doi: 10.1126/science.1191054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Nelson WJ, Spiliotis ET. Forchlorfenuron Alters Mammalian Septin Assembly, Organization, and Dynamics. J Biol Chem. 2008;283(43):29563–29571. doi: 10.1074/jbc.M804962200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, Diener DR, Mitchell A, Pazour GJ, Witman GB, Rosenbaum JL. Function and dynamics of PKD2 in Chlamydomonas reinhardtii flagella. J Cell Biol. 2007;179(3):501–514. doi: 10.1083/jcb.200704069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426(6962):83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- Hunnicutt GR, Kosfiszer MG, Snell WJ. Cell body and flagellar agglutinins in Chlamydomonas reinhardtii: the cell body plasma membrane is a reservoir for agglutinins whose migration to the flagella is regulated by a functional barrier. J Cell Biol. 1990;111(4):1605–1616. doi: 10.1083/jcb.111.4.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd TW, Fan S, Margolis BL. Localization of retinitis pigmentosa 2 to cilia is regulated by Importin β2. J Cell Sci. 2011;124(5):718–726. doi: 10.1242/jcs.070839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara M, Kinoshita A, Yamada S, Tanaka H, Tanigaki A, Kitano A, Goto M, Okubo K, Nishiyama H, Ogawa O, et al. Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev Cell. 2005;8(3):343–52. doi: 10.1016/j.devcel.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Marshall WF. Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol. 2011;12(4):222–234. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- Jacoby M, Cox JJ, Gayral Sp, Hampshire DJ, Ayub M, Blockmans M, Pernot E, Kisseleva MV, Compere P, Schiffmann SN, et al. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat Genet. 2009;41(9):1027–1031. doi: 10.1038/ng.427. [DOI] [PubMed] [Google Scholar]
- Janich P, Corbeil D. GM1 and GM3 gangliosides highlight distinct lipid microdomains within the apical domain of epithelial cells. FEBS Lett. 2007;581(9):1783–1787. doi: 10.1016/j.febslet.2007.03.065. [DOI] [PubMed] [Google Scholar]
- Jenkins PM, Hurd TW, Zhang L, McEwen DP, Brown RL, Margolis B, Verhey KJ, Martens JR. Ciliary Targeting of Olfactory CNG Channels Requires the CNGB1b Subunit and the Kinesin-2 Motor Protein, KIF17. Curr Biol. 2006;16(12):1211–1216. doi: 10.1016/j.cub.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Jin H, White SR, Shida T, Schulz S, Aguiar M, Gygi SP, Bazan JF, Nachury MV. The Conserved Bardet-Biedl Syndrome Proteins Assemble a Coat that Traffics Membrane Proteins to Cilia. Cell. 2010;141(7):1208–1219. doi: 10.1016/j.cell.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, Chen P. Curr Top Dev Biol. Academic Press; 2008. Chapter 8 Primary Cilia in Planar Cell Polarity Regulation of the Inner Ear; pp. 197–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo E, Surka MC, Trimble WS. Mammalian SEPT2 Is Required for Scaffolding Nonmuscle Myosin II and Its Kinases. Dev Cell. 2007;13(5):677–690. doi: 10.1016/j.devcel.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Kee H, McIntyre J, Jenkins P, Verhey K, Martens JR. Size Exclusion and Fatty Acylation Control Ciliary Protein Entry. Mol Biol Cell. 2010;21(suppl) Abstract No. 1108/B283. [Google Scholar]
- Kim SK, Shindo A, Park TJ, Oh EC, Ghosh S, Gray RS, Lewis RA, Johnson CA, Attie-Bittach T, Katsanis N, et al. Planar Cell Polarity Acts Through Septins to Control Collective Cell Movement and Ciliogenesis. Science. 2010;329(5997):1337–1340. doi: 10.1126/science.1191184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Field CM, Coughlin ML, Straight AF, Mitchison TJ. Self- and Actin-Templated Assembly of Mammalian Septins. Dev Cell. 2002;3(6):791–802. doi: 10.1016/s1534-5807(02)00366-0. [DOI] [PubMed] [Google Scholar]
- Kissel H, Georgescu MM, Larisch S, Manova K, Hunnicutt GR, Steller H. The Sept4 septin locus is required for sperm terminal differentiation in mice. Dev Cell. 2005;8(3):353–64. doi: 10.1016/j.devcel.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Knödler A, Feng S, Zhang J, Zhang X, Das A, Peränen J, Guo W. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci U S A. 2010;107(14):6346–6351. doi: 10.1073/pnas.1002401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl L, Bastin P, Kwang WJ. Int Rev Cytol. Academic Press; 2005. The Flagellum of Trypanosomes; pp. 227–285. [DOI] [PubMed] [Google Scholar]
- Kovacs JJ, Whalen EJ, Liu R, Xiao K, Kim J, Chen M, Wang J, Chen W, Lefkowitz RJ. b-Arrestin mediated Localization of Smoothened to the Primary Cilium. Science. 2008;320(5884):1777–1781. doi: 10.1126/science.1157983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer BE, Adang LA, Macara IG. Septins Regulate Actin Organization and Cell-Cycle Arrest through Nuclear Accumulation of NCK Mediated by SOCS7. Cell. 2007;130(5):837–850. doi: 10.1016/j.cell.2007.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulaga HM, Leitch CC, Eichers ER, Badano JL, Lesemann A, Hoskins BE, Lupski JR, Beales PL, Reed RR, Katsanis N. Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nat Genet. 2004;36(9):994–998. doi: 10.1038/ng1418. [DOI] [PubMed] [Google Scholar]
- Kwitny S, Klaus AV, Hunnicutt GR. The Annulus of the Mouse Sperm Tail Is Required to Establish a Membrane Diffusion Barrier That Is Engaged During the Late Steps of Spermiogenesis. Biol Reprod. 2010;82(4):669–678. doi: 10.1095/biolreprod.109.079566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok MCM, Holopainen JM, Molday LL, Foster LJ, Molday RS. Proteomics of Photoreceptor Outer Segments Identifies a Subset of SNARE and Rab Proteins Implicated in Membrane Vesicle Trafficking and Fusion. Mol Cell Proteomics. 2008;7(6):1053–1066. doi: 10.1074/mcp.M700571-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyttala M, Tallila J, Salonen R, Kopra O, Kohlschmidt N, Paavola-Sakki P, Peltonen L, Kestila M. MKS1, encoding a component of the flagellar apparatus basal body proteome, is mutated in Meckel syndrome. Nat Genet. 2006;38(2):155–157. doi: 10.1038/ng1714. [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Gleeson JG. The primary cilium as a cellular signaling center: lessons from disease. Curr Opin Genet Dev. 2009;19(3):220–229. doi: 10.1016/j.gde.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tan G, Levenkova N, Li T, Pugh EN, Rux JJ, Speicher DW, Pierce EA. The Proteome of the Mouse Photoreceptor Sensory Cilium Complex. Mol Cell Proteomics. 2007a;6(8):1299–1317. doi: 10.1074/mcp.M700054-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Bulgakov OV, Darrow KN, Pawlyk B, Adamian M, Liberman MC, Li T. Usherin is required for maintenance of retinal photoreceptors and normal development of cochlear hair cells. Proc Natl Acad Sci U S A. 2007b;104(11):4413–4418. doi: 10.1073/pnas.0610950104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedeke C, Frei SB, Sbalzarini I, Schwarz H, Spang A, Barral Y. Septin-dependent compartmentalization of the endoplasmic reticulum during yeast polarized growth. J Cell Biol. 2005;169(6):897–908. doi: 10.1083/jcb.200412143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maerker T, van Wijk E, Overlack N, Kersten FFJ, McGee J, Goldmann T, Sehn E, Roepman R, Walsh EJ, Kremer H, et al. A novel Usher protein network at the periciliary reloading point between molecular transport machineries in vertebrate photoreceptor cells. Hum Mol Genet. 2008;17(1):71–86. doi: 10.1093/hmg/ddm285. [DOI] [PubMed] [Google Scholar]
- Malone AMD, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, Jacobs CR. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci U S A. 2007;104(33):13325–13330. doi: 10.1073/pnas.0700636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WF. Curr Top Dev Biol. Academic Press; 2008. Chapter 1 Basal Bodies: Platforms for Building Cilia; pp. 1–22. [DOI] [PubMed] [Google Scholar]
- Marshall WF, Nonaka S. Cilia: Tuning in to the Cell’s Antenna. Curr Biol. 2006;16(15):R604–R614. doi: 10.1016/j.cub.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Mayer U, Ungerer N, Klimmeck D, Warnken U, Schnölzer M, Frings S, Möhrlen F. Proteomic Analysis of a Membrane Preparation from Rat Olfactory Sensory Cilia. Chem Senses. 2008;33(2):145–162. doi: 10.1093/chemse/bjm073. [DOI] [PubMed] [Google Scholar]
- McEwen DP, Jenkins PM, Martens JR. Curr Top Dev Biol. Academic Press; 2008. Chapter 12 Olfactory Cilia: Our Direct Neuronal Connection to the External World; pp. 333–370. [DOI] [PubMed] [Google Scholar]
- Milenkovic L, Scott MP, Rohatgi R. Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium. J Cell Biol. 2009;187(3):365–374. doi: 10.1083/jcb.200907126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KAP, Szabo G, Otero AdS. Methods Cell Biol. Academic Press; 2009. Methods for the Isolation of Sensory and Primary Cilia: An Overview; pp. 87–101. [DOI] [PubMed] [Google Scholar]
- Molla-Herman A, Ghossoub R, Blisnick T, Meunier A, Serres C, Silbermann F, Emmerson C, Romeo K, Bourdoncle P, Schmitt A, et al. The ciliary pocket: an endocytic membrane domain at the base of primary and motile cilia. J Cell Sci. 2010;123(10):1785–1795. doi: 10.1242/jcs.059519. [DOI] [PubMed] [Google Scholar]
- Mollet G, Silbermann F, Delous M, Salomon Rm, Antignac C, Saunier S. Characterization of the nephrocystin/nephrocystin-4 complex and subcellular localization of nephrocystin-4 to primary cilia and centrosomes. Hum Mol Genet. 2005;14(5):645–656. doi: 10.1093/hmg/ddi061. [DOI] [PubMed] [Google Scholar]
- Mombaerts P. MOLECULAR BIOLOGY OF ODORANT RECEPTORS IN VERTEBRATES. Annu Rev Neurosci. 1999;22(1):487–509. doi: 10.1146/annurev.neuro.22.1.487. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Wen X, Chih B, Nelson CD, Lane WS, Scales SJ, Jackson PK. TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes Dev. 2010;24(19):2180–2193. doi: 10.1101/gad.1966210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musgrave A, Wildt P, Etten I, Pijst H, Scholma C, Kooyman R, Homan W, Ende H. Evidence for a functional membrane barrier in the transition zone between the flagellum and cell body of Chlamydomonas eugametos gametes. Planta. 1986;167(4):544–553. doi: 10.1007/BF00391231. [DOI] [PubMed] [Google Scholar]
- Mykytyn K, Sheffield VC. Establishing a connection between cilia and Bardet-Biedl Syndrome. Trends Mol Med. 2004;10(3):106–109. doi: 10.1016/j.molmed.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peränen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, et al. A Core Complex of BBS Proteins Cooperates with the GTPase Rab8 to Promote Ciliary Membrane Biogenesis. Cell. 2007;129(6):1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- Nachury MV, Seeley ES, Jin H. Trafficking to the Ciliary Membrane: How to Get Across the Periciliary Diffusion Barrier? Annu Rev Cell Dev Biol. 2010;26(1):59–87. doi: 10.1146/annurev.cellbio.042308.113337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AEH, Lu W, Brown EM, Quinn SJ, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33(2):129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of Left-Right Asymmetry due to Loss of Nodal Cilia Generating Leftward Flow of Extraembryonic Fluid in Mice Lacking KIF3B Motor Protein. Cell. 1998;95(6):829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- Oh Y, Bi E. Septin structure and function in yeast and beyond. Trends Cell Biol. 2010 doi: 10.1016/j.tcb.2010.11.006. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh Y, Bi E. Septin structure and function in yeast and beyond. Trends Cell Biol. 2011;21(3):141–148. doi: 10.1016/j.tcb.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto EA, Loeys B, Khanna H, Hellemans J, Sudbrak R, Fan S, Muerb U, O’Toole JF, Helou J, Attanasio M, et al. Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior-Loken syndrome and interacts with RPGR and calmodulin. Nat Genet. 2005;37(3):282–288. doi: 10.1038/ng1520. [DOI] [PubMed] [Google Scholar]
- Otto EA, Schermer B, Obara T, O’Toole JF, Hiller KS, Mueller AM, Ruf RG, Hoefele J, Beekmann F, Landau D, et al. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet. 2003;34(4):413–420. doi: 10.1038/ng1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto EA, Trapp ML, Schultheiss UT, Helou J, Quarmby LM, Hildebrandt F. NEK8 Mutations Affect Ciliary and Centrosomal Localization and May Cause Nephronophthisis. J Am Soc Nephrol. 2008;19(3):587–592. doi: 10.1681/ASN.2007040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overath P, Stierhof Y-D, Wiese M. Endocytosis and secretion in trypanosomatid parasites -- Tumultuous traffic in a pocket. Trends Cell Biol. 1997;7(1):27–33. doi: 10.1016/S0962-8924(97)10046-0. [DOI] [PubMed] [Google Scholar]
- Papermaster DS, Schneider BG, Besharse JC. Vesicular transport of newly synthesized opsin from the Golgi apparatus toward the rod outer segment. Ultrastructural immunocytochemical and autoradiographic evidence in Xenopus retinas. Invest Ophthalmol Vis Sci. 1985;26(10):1386–404. [PubMed] [Google Scholar]
- Parisi MA, Doherty D, Chance PF, Glass IA. Joubert syndrome (and related disorders) (OMIM 213300) Eur J Hum Genet. 2007;15(5):511–521. doi: 10.1038/sj.ejhg.5201648. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. J Cell Biol. 2005;170(1):103–113. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Bloodgood RA. Curr Top Dev Biol. Academic Press; 2008. Chapter 5 Targeting Proteins to the Ciliary Membrane; pp. 115–149. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and Its Mouse Homologue, Polycystic Kidney Disease Gene Tg737, Are Required for Assembly of Cilia and Flagella. J Cell Biol. 2000;151(3):709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Rosenbaum JL. Intraflagellar transport and cilia-dependent diseases. Trends Cell Biol. 2002;12(12):551–555. doi: 10.1016/s0962-8924(02)02410-8. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol. 2002;12(11):R378–R380. doi: 10.1016/s0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Witman GB. The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol. 2003;15(1):105–110. doi: 10.1016/s0955-0674(02)00012-1. [DOI] [PubMed] [Google Scholar]
- Pedersen LB, Rosenbaum JL. Curr Top Dev Biol. Academic Press; 2008. Chapter 2 Intraflagellar Transport (IFT): Role in Ciliary Assembly, Resorption and Signalling; pp. 23–61. [DOI] [PubMed] [Google Scholar]
- Pigino G, Geimer S, Lanzavecchia S, Paccagnini E, Cantele F, Diener DR, Rosenbaum JL, Lupetti P. Electron-tomographic analysis of intraflagellar transport particle trains in situ. J Cell Biol. 2009;187(1):135–148. doi: 10.1083/jcb.200905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR. Bending the MDCK Cell Primary Cilium Increases Intracellular Calcium. J Membr Biol. 2001;184(1):71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- Rodal AA, Kozubowski L, Goode BL, Drubin DG, Hartwig JH. Actin and Septin Ultrastructures at the Budding Yeast Cell Cortex. Mol Biol Cell. 2005;16(1):372–384. doi: 10.1091/mbc.E04-08-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepman R, Letteboer SJF, Arts HH, van Beersum SEC, Lu X, Krieger E, Ferreira PA, Cremers FPM. Interaction of nephrocystin-4 and RPGRIP1 is disrupted by nephronophthisis or Leber congenital amaurosis-associated mutations. Proc Natl Acad Sci U S A. 2005;102(51):18520–18525. doi: 10.1073/pnas.0505774102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, Scott MP. Patched1 Regulates Hedgehog Signaling at the Primary Cilium. Science. 2007;317(5836):372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Snell WJ. The ciliary membrane. Curr Opin Cell Biol. 2010;22(4):541–546. doi: 10.1016/j.ceb.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romio L, Fry AM, Winyard PJD, Malcolm S, Woolf AS, Feather SA. OFD1 Is a Centrosomal/Basal Body Protein Expressed during Mesenchymal-Epithelial Transition in Human Nephrogenesis. J Am Soc Nephrol. 2004;15(10):2556–2568. doi: 10.1097/01.ASN.0000140220.46477.5C. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3(11):813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37(10):1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- Salathe M. Regulation of Mammalian Ciliary Beating. Annu Rev Physiol. 2007;69(1):401–422. doi: 10.1146/annurev.physiol.69.040705.141253. [DOI] [PubMed] [Google Scholar]
- Satir P, Christensen ST. Overview of Structure and Function of Mammalian Cilia. Annu Rev Physiol. 2007;69(1):377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- Sayer JA, Otto EA, O’Toole JF, Nurnberg G, Kennedy MA, Becker C, Hennies HC, Helou J, Attanasio M, Fausett BV, et al. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet. 2006;38(6):674–681. doi: 10.1038/ng1786. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Nichols BJ. A Barrier to Lateral Diffusion in the Cleavage Furrow of Dividing Mammalian Cells. Curr Biol. 2004;14(11):1002–1006. doi: 10.1016/j.cub.2004.05.044. [DOI] [PubMed] [Google Scholar]
- Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. PDGFRαα Signaling Is Regulated through the Primary Cilium in Fibroblasts. Curr Biol. 2005;15(20):1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Scholey JM, Anderson KV. Intraflagellar Transport and Cilium-Based Signaling. Cell. 2006;125(3):439–442. doi: 10.1016/j.cell.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Schwartz EA, Leonard ML, Bizios R, Bowser SS. Analysis and modeling of the primary cilium bending response to fluid shear. Am J Physiol. 1997;272(1):F132–F138. doi: 10.1152/ajprenal.1997.272.1.F132. [DOI] [PubMed] [Google Scholar]
- Sfakianos J, Togawa A, Maday S, Hull M, Pypaert M, Cantley L, Toomre D, Mellman I. Par3 functions in the biogenesis of the primary cilium in polarized epithelial cells. J Cell Biol. 2007;179(6):1133–1140. doi: 10.1083/jcb.200709111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile Cilia of Human Airway Epithelia Are Chemosensory. Science. 2009;325(5944):1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Berbari NF, Yoder BK. Curr Top Dev Biol. Academic Press; 2008. Chapter 13 Ciliary Dysfunction in Developmental Abnormalities and Diseases; pp. 371–427. [DOI] [PubMed] [Google Scholar]
- Shcheprova Z, Baldi S, Frei SB, Gonnet G, Barral Y. A mechanism for asymmetric segregation of age during yeast budding. Nature. 2008;454(7205):728–734. doi: 10.1038/nature07212. [DOI] [PubMed] [Google Scholar]
- Shiba D, Manning DK, Koga H, Beier DR, Yokoyama T. Inv acts as a molecular anchor for Nphp3 and Nek8 in the proximal segment of primary cilia. Cytoskeleton. 2010;67(2):112–119. doi: 10.1002/cm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1(1):31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37(5):537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla V, Reiter JF. The Primary Cilium as the Cell’s Antenna: Signaling at a Sensory Organelle. Science. 2006;313(5787):629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- Sirajuddin M, Farkasovsky M, Hauer F, Kuhlmann D, Macara IG, Weyand M, Stark H, Wittinghofer A. Structural insight into filament formation by mammalian septins. Nature. 2007;449(7160):311–315. doi: 10.1038/nature06052. [DOI] [PubMed] [Google Scholar]
- Sloboda RD, Rosenbaum JL. Making sense of cilia and flagella. J Cell Biol. 2007;179(4):575–582. doi: 10.1083/jcb.200709039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell WJ, Pan J, Wang Q. Cilia and Flagella Revealed: From Flagellar Assembly in Chlamydomonas to Human Obesity Disorders. Cell. 2004;117(6):693–697. doi: 10.1016/j.cell.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Sorokin S. CENTRIOLES AND THE FORMATION OF RUDIMENTARY CILIA BY FIBROBLASTS AND SMOOTH MUSCLE CELLS. J Cell Biol. 1962;15(2):363–377. doi: 10.1083/jcb.15.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer M, Detwiler PB, Bunt-Milam AH. Distribution of membrane proteins in mechanically dissociated retinal rods. Invest Ophthalmol Vis Sci. 1988;29(7):1012–1020. [PubMed] [Google Scholar]
- Spiliotis ET, Hunt SJ, Hu Q, Kinoshita M, Nelson WJ. Epithelial polarity requires septin coupling of vesicle transport to polyglutamylated microtubules. J Cell Biol. 2008;180(2):295–303. doi: 10.1083/jcb.200710039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiliotis ET, Kinoshita M, Nelson WJ. A mitotic septin scaffold required for Mammalian chromosome congression and segregation. Science. 2005;307(5716):1781–5. doi: 10.1126/science.1106823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steels JD, Estey MP, Froese CD, Reynaud Dn, Pace-Asciak C, Trimble WS. Sept12 is a component of the mammalian sperm tail annulus. Cell Motil Cytoskeleton. 2007;64(10):794–807. doi: 10.1002/cm.20224. [DOI] [PubMed] [Google Scholar]
- Tada T, Simonetta A, Batterton M, Kinoshita M, Edbauer D, Sheng M. Role of Septin cytoskeleton in spine morphogenesis and dendrite development in neurons. Curr Biol. 2007;17(20):1752–8. doi: 10.1016/j.cub.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam BM, Moritz OL, Hurd LB, Papermaster DS. Identification of an Outer Segment Targeting Signal in the Cooh Terminus of Rhodopsin Using Transgenic Xenopus laevis. J Cell Biol. 2000;151(7):1369–1380. doi: 10.1083/jcb.151.7.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka-Takiguchi Y, Kinoshita M, Takiguchi K. Septin-mediated uniform bracing of phospholipid membranes. Curr Biol. 2009;19(2):140–5. doi: 10.1016/j.cub.2008.12.030. [DOI] [PubMed] [Google Scholar]
- Tao B, Bu S, Yang Z, Siroky B, Kappes JC, Kispert A, Guay-Woodford LM. Cystin Localizes to Primary Cilia via Membrane Microdomains and a Targeting Motif. J Am Soc Nephrol. 2009;20(12):2570–2580. doi: 10.1681/ASN.2009020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teilmann SC, Christensen ST. Localization of the angiopoietin receptors Tie-1 and Tie-2 on the primary cilia in the female reproductive organs. Cell Biol Int. 2005;29(5):340–346. doi: 10.1016/j.cellbi.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Tyler KM, Fridberg A, Toriello KM, Olson CL, Cieslak JA, Hazlett TL, Engman DM. Flagellar membrane localization via association with lipid rafts. J Cell Sci. 2009;122(6):859–866. doi: 10.1242/jcs.037721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Logan CV, Mougou-Zerelli S, Lee JH, Silhavy JL, Brancati F, Iannicelli M, Travaglini L, Romani S, Illi B, et al. Mutations in TMEM216 perturb ciliogenesis and cause Joubert, Meckel and related syndromes. Nat Genet. 2010;42(7):619–625. doi: 10.1038/ng.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Silhavy JL, Brancati F, Barrano G, Krishnaswami SR, Castori M, Lancaster MA, Boltshauser E, Boccone L, Al-Gazali L, et al. Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat Genet. 2006;38(6):623–625. doi: 10.1038/ng1805. [DOI] [PubMed] [Google Scholar]
- Versele M, Thorner J. Some assembly required: yeast septins provide the instruction manual. Trends Cell Biol. 2005;15:414–424. doi: 10.1016/j.tcb.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira OV, Gaus K, Verkade P, Fullekrug J, Vaz WLC, Simons K. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-darby canine kidney (MDCK) cells. Proc Natl Acad Sci U S A. 2006;103(49):18556–18561. doi: 10.1073/pnas.0608291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Pan J, Snell WJ. Intraflagellar Transport Particles Participate Directly in Cilium-Generated Signaling in Chlamydomonas. Cell. 2006;125(3):549–562. doi: 10.1016/j.cell.2006.02.044. [DOI] [PubMed] [Google Scholar]
- Westlake CJ, Baye LM, Nachury MV, Wright KJ, Ervin KE, Phu L, Chalouni C, Beck JS, Kirkpatrick DS, Slusarski DC, et al. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci U S A. 2011;108(7):2759–2764. doi: 10.1073/pnas.1018823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Li C, Kida K, Inglis PN, Mohan S, Semenec L, Bialas NJ, Stupay RM, Chen N, Blacque OE, et al. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J Cell Biol. 2011;192(6):1023–1041. doi: 10.1083/jcb.201012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Masyukova SV, Yoder BK. Normal Ciliogenesis Requires Synergy between the Cystic Kidney Disease Genes MKS-3 and NPHP-4. J Am Soc Nephrol. 2009;21(5):782–793. doi: 10.1681/ASN.2009060597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Winkelbauer ME, Schafer JC, Michaud EJ, Yoder BK. Functional Redundancy of the B9 Proteins and Nephrocystins in Caenorhabditis elegans Ciliogenesis. Mol Biol Cell. 2008;19(5):2154–2168. doi: 10.1091/mbc.E07-10-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelbauer ME, Schafer JC, Haycraft CJ, Swoboda P, Yoder BK. The C. elegans homologs of nephrocystin-1 and nephrocystin-4 are cilia transition zone proteins involved in chemosensory perception. J Cell Sci. 2005;118(23):5575–5587. doi: 10.1242/jcs.02665. [DOI] [PubMed] [Google Scholar]
- Wong SY, Reiter JF. Curr Top Dev Biol. Academic Press; 2008. Chapter 9 The Primary Cilium: At the Crossroads of Mammalian Hedgehog Signaling; pp. 225–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Vessey JP, Konecna A, Dahm R, Macchi P, Kiebler MA. The GTP-binding protein Septin 7 is critical for dendrite branching and dendritic-spine morphology. Curr Biol. 2007;17(20):1746–51. doi: 10.1016/j.cub.2007.08.042. [DOI] [PubMed] [Google Scholar]
- Yan D, Liu XZ. Genetics and pathological mechanisms of Usher syndrome. J Hum Genet. 2010;55(6):327–335. doi: 10.1038/jhg.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder BK, Hou X, Guay-Woodford LM. The Polycystic Kidney Disease Proteins, Polycystin-1, Polycystin-2, Polaris, and Cystin, Are Co-Localized in Renal Cilia. J Am Soc Nephrol. 2002;13(10):2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kong C, Xie H, McPherson PS, Grinstein S, Trimble WS. Phosphatidylinositol polyphosphate binding to the mammalian septin H5 is modulated by GTP. Curr Biol. 1999;9(24):1458–67. doi: 10.1016/s0960-9822(00)80115-3. [DOI] [PubMed] [Google Scholar]
- Zimmermann KW. Beitrage zur Kenntniss einiger Drusen und epithelien. Arch Mikrosk Anat. 1898;52:552–706. [Google Scholar]