Abstract
Objective
The mechanism of infection-related mortality of pregnant rats and the intrauterine growth restriction (IUGR) are not understood. We assessed if nitric oxide (NO) has differential effects on infection with E. coli Dr/Afa mutants lacking either AfaE or AfaD invasins.
Material and Methods
Sprague-Dawley rats were intrauterinally infected with the clinical strain of E. coli AfaE+D+ or one of its isogenic mutants in the presence or absence of the NO synthesis inhibitor NG-nitro-L-arginine methyl ester (L-NAME). Maternal/fetal mortality, feto-placental weight, and the infection rate were evaluated.
Results
Maternal and/or fetal mortality was associated with the presence of at least one virulence factor (AfaE+D+>AfaE+D−>AfaE-D+) and was increased by L-NAME treatment. The fetal and placental weights were lower than controls and they were further reduced by L-NAME treatment.
Conclusions
These results demonstrate that NO enhanced AfaE and AfaD mediated virulence and play an important role in Dr/Afa+ E. coli gestational tropism.
Keywords: Afa-III adhesive structure, Escherichia coli, intrauterine infection, mortality, intrauterine growth restriction, Dr family, nitric oxide, L-NAME, rat
Introduction
Urinary tract infection (UTI) is a major cause of feto-maternal morbidity during pregnancy. Escherichia coli (E. coli) is the major etiologic agent of gestational UTI, accounting for 90% of infections 1–3 and is associated with multiple complications such as fetal growth restriction, preterm labor, cerebral palsy, septicemia or maternal death. We proposed that UTI complications develop in part due to gestational tropism of E. coli. Our laboratories pioneered studies on gestational tropism of E. coli-producing adhesins of the Dr family (Dr/Afa+ E. coli) and pyelonephritis 4–8. We established an E. coli experimental uterine infection model in pregnant rats, and showed that Dr adhesin is a key lethal factor in pregnant but not to non-pregnant rats 6;8. However, the impact of Dra E and Dra D, which together form Dr adhesin on lethality and fetal growth and the role of NO in the host defense are not well understood.
The strains of E. coli predominantly associated with gestational urogenital infection are those of the O75 serotype that frequently produced adhesins of the Dr family that play an essential and critical role in the infectious process and gestational virulence 9. Dr/Afa+ E. coli account for 40 % of pyelonephritis cases in the third trimester of pregnancy. The adhesins are encoded by very similar operons of at least five genes and are associated with fimbriae (such as Dr, Dr-II and F1845 adhesins) and small fibrils (such as AfaE-I and AfaE-III adhesins) 10. These adhesive structures are heterologous polymers composed of a Dra/AfaE fiber and a Dra/AfaD tip protein 11;12. We previously reported that decay accelerating factor (DAF) also called CD55 serves as a tissue receptor for Dr/Afa+ adhesins while the β1 integrin is recognized by the Dra/AfaD proteins 13;14;15;16 and that both E and D may contribute to invasion.
Nitric oxide (NO), a free gas molecule, is reported to be involved in a variety of physiological processes, including neurotransmission, vasodilatation, smooth muscle relaxation and infection 17;18. NO is constitutively generated from L-arginine by nitric oxide synthase (NOS), and three different isoforms of NOS (NOS I, NOS II and NOS III) have been identified in mouse, rat and human 19;20. Although the role of NO in the host defense is not well understood, reports using experimental animal models or clinical patients strongly suggest an anti-bacterial activity of NO. Inhibition of NO aggravates S. aureus arthritis in mice 21, reduces survival time after an Escherichia coli challenge in rats 22 and demonstrates a detrimental effect in the murine peritonitis model 23. It has been also shown that the NO donor compounds do not affect the viability and survival of bacteria themselves but enhance the neutrophil’s ability to kill E. coli, Proteus vulgaris and Salmonella Anatum 24. The findings from our laboratories further showed an inverse relationship between the severity of experimental pyelonephritis and NO production in C3H/HeJ mice 7. In addition, in clinical patients with infectious diseases, NO production appeared to be increased in macrophages and was correlated with better clinical outcome 25;26. NO production and three NOS isoforms were reported to be present in rat uterine tissues, and elevated NOS II expression was demonstrated to contribute to the increased NO production and consequent uterine quiescence during pregnancy 27;28. In our previous studies we reported that pregnancy modifies NO-associated host resistance mechanisms and alters the risk for genitourinary infection by Dr/Afa+ E. coli and its complications 4;29. Inhibition of NO synthesis during pregnancy doubled the mortality of the Dr/Afa+ E. coli infected rats 6, indicating that endogenous NO may play a role in the host’s ability to eliminate bacteria and lessen sepsis.
In this study we investigated both microbial and host factors involved in the mechanism of gestational tropism. We hypothesized that both Dr/Afa+ E. coli and NO contribute to the gestational tropism. It has been suggested that the invasive capacity of Dr/Afa+ E. coli depends on two virulence factors - Dra/AfaE and Dra/AfaD proteins, which are responsible for adhesion and internalization mediated by two different receptors 30. However, the contribution of either Dra/AfaE or Dra/AfaD subunit to in vivo infection is poorly understood. In our previous studies we showed that experimental intrauterine infection with Dr/Afa+ E. coli is lethal to pregnant but not to non-pregnant rats 6;8. In this study we sought to assess the influence of genes encoding the two proteins not only on maternal mortality but also on fetal and placental growth. All previous studies have analyzed NO-associated changes in infection caused by E. coli bearing intact Dr/Afa structure, which was assumed to be essential for the virulence of E. coli 4;6;31. In this study we investigated if the NO inhibitor, NG-nitro-L-arginine methyl ester (L-NAME), has differential effects on the maternal mortality and fetoplacental growth in rats with experimental intrauterine infection using AfaE and AfaD mutants of E. coli.
Material and Methods
Bacterial strains
The strains used in this study are listed in Table 1.
Table 1.
Characteristics of the bacterial strains used in the study. (MRHA – mannose-resistant hemagglutination).
| Bacterial strain | Relevant characteristics | MRHA | Ref. |
|---|---|---|---|
| A30 (AfaE+D+) | Cystitis-associated isolate carrying the afa-3 operon | + | [49] |
| AL858 (AfaE−D+) | Isogenic mutant of A30 with a mutated afaE3 gene | − | [48] |
| AL861 (AfaE+D−) | Isogenic mutant of A30 with a mutated afaD3 gene | + | [47] |
| AL863 (AfaE−D−) | Mutant of AL858 with a mutated afaD3 gene | − | [47] |
E. coli A30 is a clinical isolate expressing the afa-3 operon32. A30 derivatives containing mutated afaE3, afaD3 or both afaE3 and afaD3 genes were constructed by allelic exchange as previously described 15;33.
The growth rates were similar for all these strains.
Animal model
Timed pregnant Sprague-Dawley rats were obtained from Harlan Sprague Dawley, Houston, TX. Two days before experimental infection, each animal received one dose of streptomycin (7.0 mg/g of body weight) to eliminate possible sites of infection in the urogenital tract that may occur naturally. Twenty-four hours before infection, half of all animals in one group were implanted with Alzet osmotic minipumps (Alza Corporation, Palo Alto, CA; 10µl/hr) that contained the NO synthase inhibitor L-NAME (50 mg/day/300 g of body weight) and the other half were implanted with minipumps containing corresponding vehicle as controls.
Animals were infected with the clinical strain of E. coli A30 (AfaE+D+) or one of its isogenic mutants: AL861 (AfaE+D−), AL858 (AfaE−D+) and AL863 (AfaE−D−). An inoculum of E. coli in a volume of 200 µl was placed through the cervical os in the cavity of the left uterine horn of pregnant rats on day 17th of gestation using a blunted 16G animal feeding stainless steel needle (Popper & Sons, Inc. New Hyde Park, NY). In order to assess the effect of infection on both mother and fetus we used two different concentrations of bacteria: OD600 = 2.0 (5×1010 CFU/ml) to assess maternal and fetal mortality and OD600 = 0.8 (5×108 CFU/ml) to assess fetal and placental growth. The control groups were inoculated with fresh bacterial growth medium. Each study subgroup comprised of 8 animals.
The maternal mortality was evaluated at 24 hours after infection. The surviving animals were sacrificed, the weight of fetuses and placentas was assessed and the specimens of placentas and fetuses were collected for quantitative bacterial cultures. Two placentas or fetuses from each animal were homogenized in phosphate saline buffer, pH 7.2, 10-fold dilutions were prepared and 50 µl aliquots were cultured on L-agar and McConkey plates. The rate of colonization was expressed as the number of colony-forming units (CFU) per gram of tissue.
Statistical analysis
Statistical analysis was performed with GraphPad Prism4 program, GraphPad Software, Inc. La Jolla, CA, using Mann-Whitney test and chi2 test and p values of less than 0.05 were considered significant.
Results
Effects of L-NAME infusion on maternal and fetal mortality
We investigated the effects of NO in modifying the severity of intrauterine gestational infection, and the maternal and fetal mortality and morbidity in pregnant rats. Rats were treated with either vehicle alone or vehicle containing L-NAME and infected with Dr/Afa+ E. coli or its isogenic mutants lacking either one or both virulence factors (AfaE+D−, AfaE−D+ and AfaE−D−). We used two different concentrations of infecting agent (OD600=2.0 and OD600=0.8), since the higher concentration of infection agent (OD600=2.0; 5× 1010 CFU/ml) caused changes which made it impossible to assess fetal morbidity.
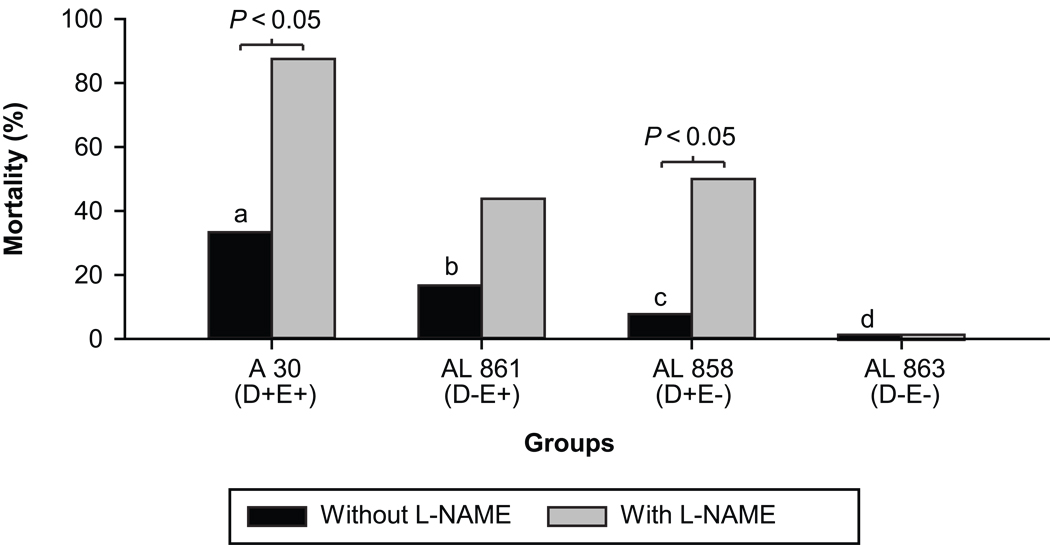
Maternal mortality was observed only in animals infected with a higher concentration of bacteria (OD600=2.0). L-NAME treatment to these animals caused an increase in maternal mortality in animals infected with strains of E. coli bearing at least one virulence factor. The differences between the animals with and without L-NAME treatment was significant in groups infected with A30 (AfaE+D+) and AL858 (AfaE−D+) (p<0.05). The increase was also observed in the group infected with AL861 (AfaE+D−) but was not significant (p=0.13). No mortality was observed in the group infected with the double mutant AL863 (AfaE−D−) either with or without L-NAME treatment (Figure 1).
Figure 1. E coli infection and L-NAME effects on maternal mortality.
Maternal mortality in rats infected (OD600=2.0) with clinical strain of E. coli or its isogenic mutants, with and without L-NAME treatment. Each group consists of 8 animals using chi-square test; bars with different letters differ significantly (P<0.05). Within the same E. Coli strain infected rats, effects of L-NAME are assessed and a P value <0.05 indicates significant differences.
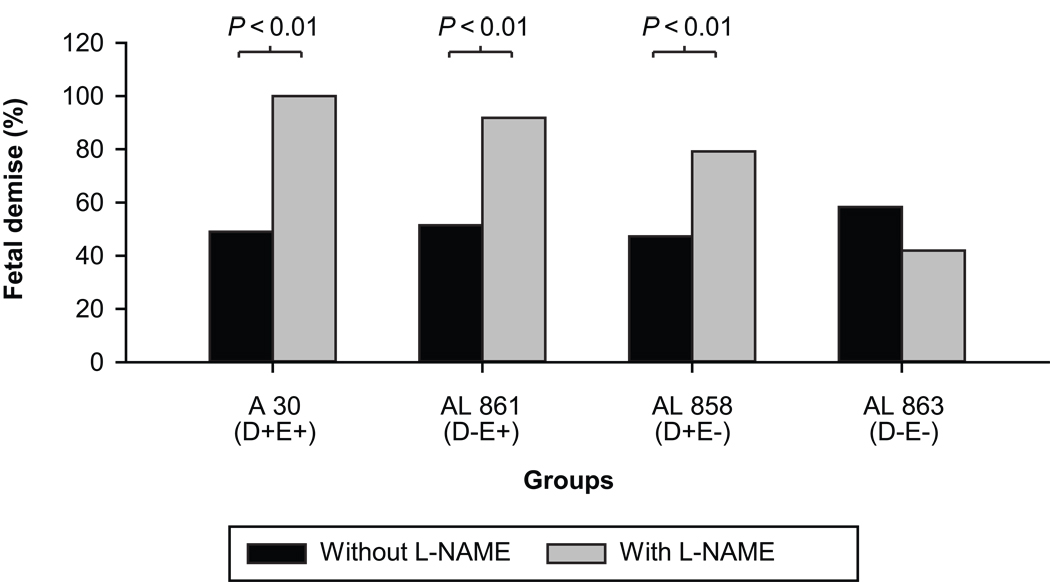
Fetal mortality was observed mainly in animals infected with a higher concentration (OD600=2.0) of bacteria and was similar in all infected groups (~50%). L-NAME treatment significantly increased fetal mortality with AfaE+ and AfaD+ strains (p<0.01) but not in the group infected with the double mutant AfaE−D− strain (AL863) (Figure 2). Fetuses which were dead were either edematic (large) or hemorrhagic (small). Their body weights were variable; therefore it was impossible to objectively assess growth restriction of the dead fetuses.
Figure 2. E coli infection and L-NAME effects on fetal mortality.
Fetal mortality in animals infected (OD600=2.0) with clinical strain of E. coli or its isogenic mutants, with and without L-NAME treatment. Each group consists of 8 animals using chi-square test; no significant differences among all bacterial strains. L-NAME increased the fetal mortality in all except the double mutant group.
Effects of L-NAME on fetal and placental growth
We assessed the effects of L-NAME and E. coli infection on fetal and placental growth, using a lower dose of infecting agent (OD600=0.8; 5 × 108 CFU/ml). In this set of experiments using this dose of E. coli, there were no maternal mortalities and the fetal demise rate was insignificant (~3%). Dead fetuses were excluded from data analysis.
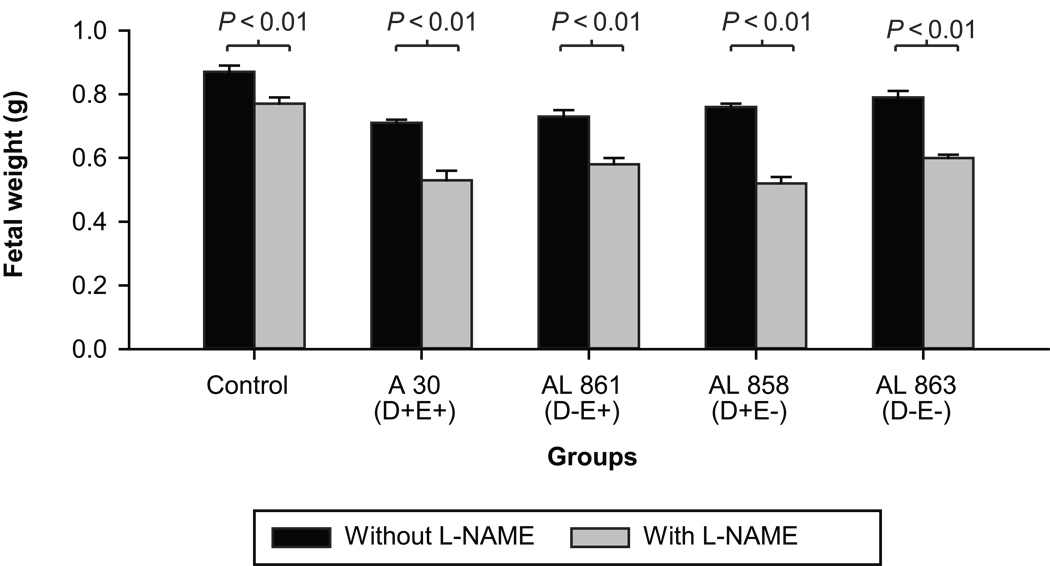
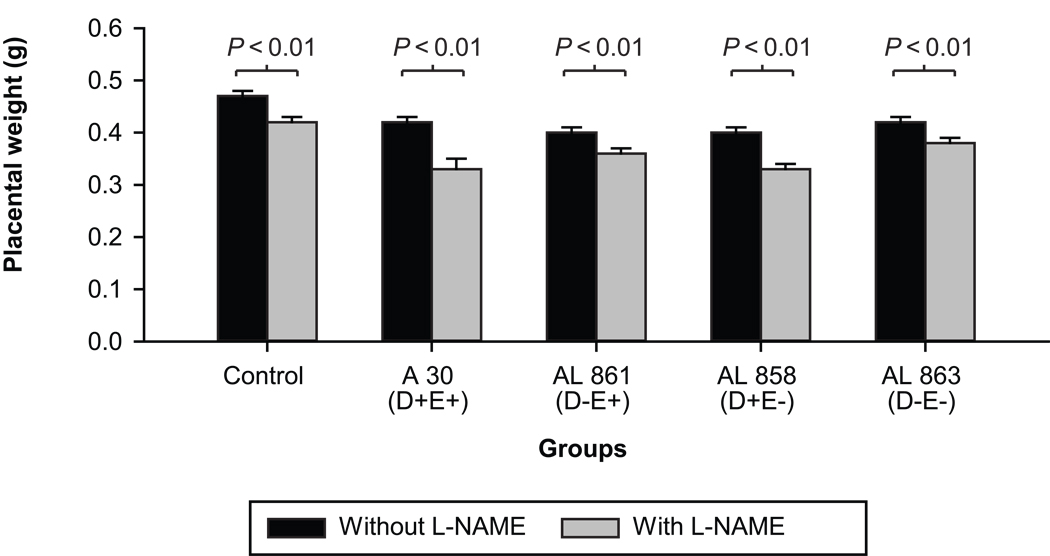
In all infected groups both fetal and placental weights were significantly reduced when compared to the control non-infected group (p<0.01) (Figures 3 and 4). The lowest fetal weight was observed in animals infected with the clinical AfaE+D+ E. coli strain and was significantly lower than in any other group (p<0.01). However differences in the placental weights between infected groups were not significant.
Figure 3. E coli infection and L-NAME effects on fetal weight.
Fetal weight in animals with and without L-NAME treatment, infected with the clinical strain of E. coli or its isogenic mutants (OD600=0.8; n= 8 in each subgroup); Mann-Whitney test: In each of the groups, treatment with L-NAME significantly decreased the fetal weights as indicated by the P values.
Figure 4. E coli infection and L-NAME effects on placental weight.
Placental weight in animals with and without L-NAME treatment, infected with the clinical strain of E. coli or its isogenic mutants (OD600=0.8; n= 8 in each subgroup); Mann-Whitney test: In each of the groups, treatment with L-NAME significantly decreased the fetal weights as indicated by the P values.
Both fetal and placental weights were decreased significantly with L-NAME treatment compared to control infected animals, as well as in animals infected with any of the bacterial strains (p<0.01). In addition there were also significant differences between animals treated with L-NAME only and animals receiving L-NAME and infection, irrespective of the infecting strain (p<0.01) on both placental (Figure 3) and fetal (Figure 4) weights. Although statistically not significant, the double mutant appeared to have less impact on placental weight reduction.
Analysis of tissue infection rate
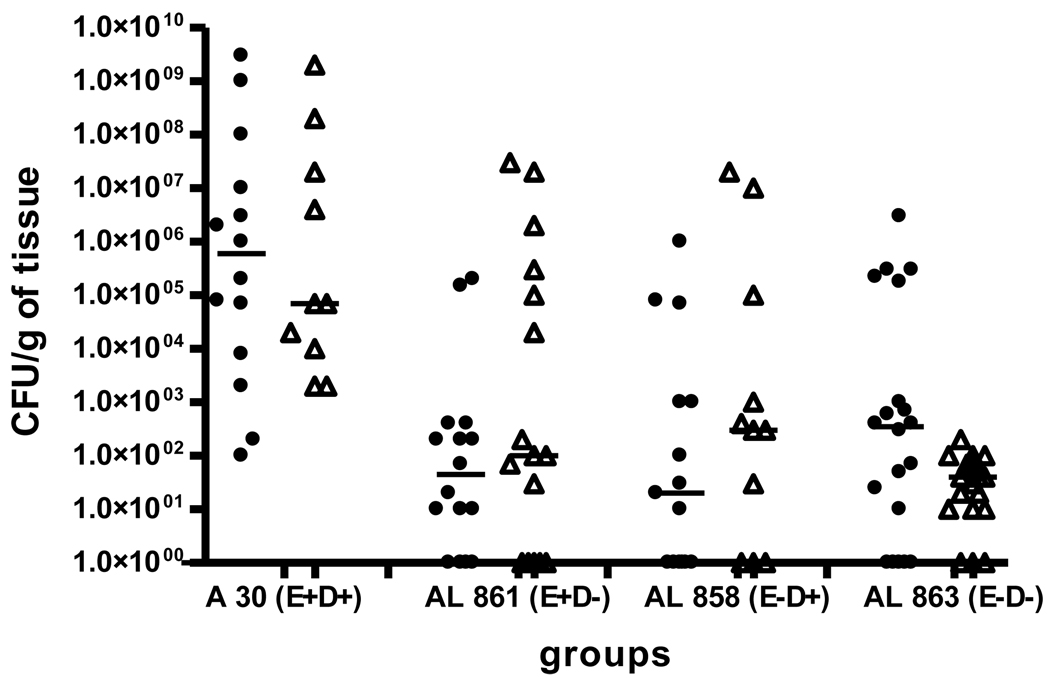
To better understand the role of NO in modifying the severity of infection caused by mutants of E. coli and its complications, we assessed the tissue infection rate in placentas (Figure 5) and fetuses (Figure 6) of the infected animals. Due to post-mortem effects on bacterial growth, quantitative tissue cultures of dead fetuses were not performed. Figures 5 and 6 show the tissue infection rates for placentas and fetuses respectively, in animals infected with E. coli of OD600=0.8. In animals infected with a higher dose of bacteria (OD600=2.0), the infection rates for both the tissues were approximately 2 logs higher, the distribution was similar and we did not observe significant differences (data not shown). There were significant differences in the infection rate of placental tissues between the clinical strain and the mutants (Figure 5); the CFU/g tissue was greater in clinical strain compared to the mutants and nearly 35% of placentas remained non-infected in the mutants groups, whereas all placentas in clinical strain group were infected. There were no significant differences in the CFU/g tissue in the placentas from animals with or without L-NAME treatment among different strains of E. coli used for infection.
Figure 5.
Infection rate in placentas of animals infected with the clinical strain of E. coli or its isogenic mutants at an OD600=0.8. (• - without L-NAME, Δ - with L-NAME)
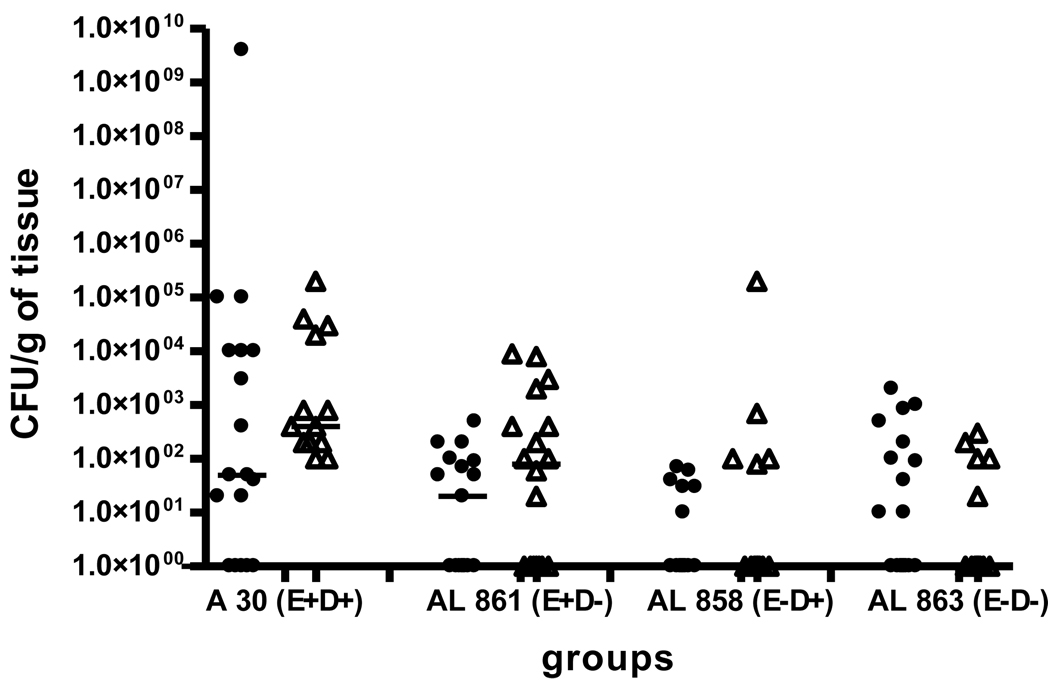
Figure 6.
Infection rate in fetuses of animals infected with the clinical strain of E. coli and its isogenic mutants at an OD600=0.8. (• - without L-NAME, Δ - with L-NAME)
In fetuses of animals infected with AfaE+D−, the infection rate was comparable to the animals infected with the clinical strain AfaE+D+. One the contrary, in groups infected with strains lacking AfaE (AfaE−D+ and AfaE−D−), the fetal infection rate was significantly lower (Figure 6). Again there was no L-NAME treatment effects in the CFU/g fetal tissue among different strains of E. coli used for infection.
Discussion
It has been proposed in many studies that NO plays an important role as a mediator of the host defense to infectious processes. In this report we show that an inhibitor of NO synthesis, L-NAME, significantly enhances feto-placental growth restriction in experimental infection caused by a Dra/Afa+ clinical E. coli strain and increases maternal and fetal mortality in the presence of E. coli bearing invasion factors AfaE and/or AfaD. These results indicate a modulatory role of NO in the resistance to infectious complications.
Three separate lines of evidence from our laboratories have suggested that higher levels of NO production by the urogenital tract in pregnancy protects against E. coli infection. First, we have shown that inhibition of NO with L-NAME in pregnant rats with intrauterine infection increased their sensitivity to infection and caused an increase in maternal mortality, whereas this did not influence the infection outcome in non-pregnant animals 6. Second, the sensitivity of the female rat or mouse urinary tract to E. coli infection was increased with inhibition of NO 6;7. Third, a spontaneous, localized increase in NO production and the expression of inducible NO synthase (NOS II) was observed in response to intrauterine infection29. Other investigators have also shown that intracellular NO is involved in the host defense mechanisms against bacterial infections 34. Interestingly a lack of bactericidal or static effect by NO donors in vitro, suggests an indirect inhibitory effect on infection, possibly by modification of epithelial cell function 24. Indeed our in vitro studies have shown that DAF expression in epithelial cells was increased after treatment with L-NAME, with a corresponding increase in Dr/Afa+ E. coli internalization 35.
In the current study we observed that maternal and fetal mortality and morbidity were significantly higher in animals infected with the clinical strain expressing both AfaE and AfaD, than in those infected with any of its mutants and further this effect was enhanced by L-NAME treatment. L-NAME also altered the maternal and fetal mortality and morbidity in animals infected with bacteria expressing either AfaE or AfaD only, suggesting that NO has a regulatory impact on pregnant rat tissue receptors.
AfaE, responsible for binding to DAF and bacterial internalization, seems to be the most critical virulence factor of Dr/Afa+ E. coli. In our previous studies we demonstrated that pregnant rats were more sensitive to complications with uterine infections caused by E. coli strains bearing – AfaE than to those caused by mutants lacking AfaE 8. This study confirms previous observations, however, it proposes a novel mechanism suggesting that Afa E mediated mortality and growth restriction is NO dependent. It was suggested before, that AfaD also mediates invasion of Dr/Afa+ E. coli using β1 integrin as a receptor 15. Therefore it is possible that this factor might also have some biological effect and its action might be modulated by NO.
It is worth noting that L-NAME did not have any impact on maternal and fetal mortality in animals infected with the double mutant strain which lost both virulence factors. We speculate that this might be due to deficit of invasive capacity of this strain compared to strains bearing either AfaE and/or AfaD. This is consistent with our hypothesis that L-NAME does not directly influence bacterial survival or action but rather modulates its invasion, changing the expression of DAF or related receptors. Thus, L-NAME cannot influence effects of bacteria which do not express factors responsible for binding to tissue receptors and resulting invasion.
Our results show that E. coli bearing Dr/Afa+ adhesive structures caused fetal and placental growth restriction. This effect seems to be an additional pathologic consequence of E. coli infection apart from the maternal mortality and morbidity which we observed in our previous studies 6;8. It is also consistent with the results of the study by Kaul et al. which showed that experimental infection with E. coli might be associated with low birth weight 36.
The mechanism of fetal and placental growth restriction in intrauterine infection by E. coli is not well understood. One possibility would be the transplacental transfer of virulent bacteria to the developing fetus and congenital bacterial infection in fetal organs. Such infection might cause poor organ development, growth restriction and with higher doses, it might lead to increased fetal death rate. This is consistent with the fact that both in placentas and in fetuses we observed a relatively high infection rate. On the other hand the intrauterine growth restriction may be also a result of infection-induced placental changes, such as thrombosis, necrosis and destruction of placental villi 37. This hypothesis is supported by clinical studies, which demonstrated that in 45–60% placentas of newborns with low birth weight, histological features of acute inflammatory process were present 38–40.
L-NAME enhanced feto-placental growth restriction, irrespective of the expression of virulence factors by infecting agents. We suggest that this might be due to the combined effects of infection and NO inhibition. NO inhibition by L-NAME alone also reduces feto-placental growth perhaps through increased placental vascular resistance. Both fetal and placental weights were lower in L-NAME treated animals compared to non-treated animals, and these observations are similar to those reported previously 41–44. Alternatively these results might suggest that apart from AfaE and AfaD, Dr/Afa+ E. coli may express another virulence factor(s), the action of which is also modulated by NO. Additionally, the fact that tissue infection rate in animals infected with the double mutant (AfaE−D−) was similar to those infected with single mutants (AfaE+D− and AfaE−D+) might suggest that immunocompromised pregnant rats are not able to eliminate E. coli even if they lack both virulence factors; this could lead to the other observed effects.
In summary, we demonstrated that inhibition of NO synthesis might enhance the pathologic effects caused by intrauterine infection, which is consistent with our previous studies and strongly suggests that NO plays an important role in host resistance to infection during pregnancy. Moreover, we have shown that the effect of NO depends on the presence of virulence factors in the infecting strain of E. coli. Our results confirm that maternal mortality and fetal mortality and morbidity in pregnant rats with Afa/Dr+ E. coli intrauterine infection, are mediated predominantly by its adhesin subunit AfaE and to much lesser extent by AfaD, whereas mutants negative for both proteins retain little virulence for pregnant rats although they have some pathological effects on fetuses. Thus, our study demonstrate that both NO and E. coli proteins AfaE and AfaD play an important role in Dr/Afa+ E. coli gestational tropism.
Acknowledgments
Financial support: is provided in part by grants HL58144 and HL72620 to C. Yallampalli, HD41687 to S. Nowicki, and DK42029 to B. Nowicki from the National Institute of Health. Authors express great appreciation to Dr Diana Marver for editorial comments
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Gabbe SG, Carpenter LB, Garrison EA. New strategies for glucose control in patients with type 1 and type 2 diabetes mellitus in pregnancy. Clin Obstet.Gynecol. 2007;50:1014–1024. doi: 10.1097/GRF.0b013e31815a6435. [DOI] [PubMed] [Google Scholar]
- 2.Nowicki B, Sledzinska A, Samet A, Nowicki S. Pathogenesis of gestational UTI: urinary obstruction vs. immune adaptation and microbial virulence. br. 2010 doi: 10.1111/j.1471-0528.2010.02706.x. In Press. [DOI] [PubMed] [Google Scholar]
- 3.OEledziñska A, Mielech A, Krawzcyk B, Samet A, Nowicki B, Nowicki S, Jankowski Z, Kur J. Fatal sepsis in a pregnant woman with pyelonephritis caused by Escherichia coli bearing Dr and P adhesins: diagnosis based on postmortem strain genotyping. br. 2010 doi: 10.1111/j.1471-0528.2010.02732.x. In Press. [DOI] [PubMed] [Google Scholar]
- 4.Fang L, Nowicki B, Yallampalli C. Differential expression of uterine NO in pregnant and nonpregnant rats with intrauterine bacterial infection. Am J Phsio Regul Integr Comp Physiol. 2001;280:R1356–R1363. doi: 10.1152/ajpregu.2001.280.5.R1356. [DOI] [PubMed] [Google Scholar]
- 5.Goluszko P, Popov V, Selvarangan R, Nowicki S, Pham T, Nowicki BJ. Dr fimbriae operon of uropathogenic Escherichia coli mediate microtubule-dependent invasion to the HeLa epithelial cell line. J Infect.Dis. 1997;176:158–167. doi: 10.1086/514018. [DOI] [PubMed] [Google Scholar]
- 6.Nowicki B, Fang L, Singhal J, Yallampalli C. Lethal outcome of uterine infection in pregnant but not in nonpregnant rats and increased death rate with inhibition of nitric oxide. Am J Reprod.Immunol. 1997;38:309–312. doi: 10.1111/j.1600-0897.1997.tb00521.x. [DOI] [PubMed] [Google Scholar]
- 7.Nowicki B, Singhal J, Fang L, Nowicki S, Yallampalli C. Inverse relationship between severity of experimental pyelonephritis and nitric oxide production in C3H/Hej mice. Infect Immun. 1999;67:2421–2427. doi: 10.1128/iai.67.5.2421-2427.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wroblewska-Seniuk K, Selvarangan R, Hart A, Pladzyk R, Goluszko P, Jafari A, et al. Dra/AfaE adhesin of uropathogenic Dr/Afa+ Escherichia coli mediates mortality in pregnant rats. Infect.Immun. 2005;73:7597–7601. doi: 10.1128/IAI.73.11.7597-7601.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hart A, Pham T, Nowicki S, Whorton EB, Jr, Martens MG, Anderson GD, et al. Gestational pyelonephritis--associated Escherichia coli isolates represent a nonrandom, closely related population. Am J Obstet Gynecol. 1996;174:983–989. doi: 10.1016/s0002-9378(96)70337-x. [DOI] [PubMed] [Google Scholar]
- 10.Le BC, Lalioui L, Du ML, Jouve M, Courcoux P, Bouzari S, et al. Characterization of AfaE adhesins produced by extraintestinal and intestinal human Escherichia coli isolates: PCR assays for detection of Afa adhesins that do or do not recognize Dr blood group antigens. J Clin Microbiol. 2001;39:1738–1745. doi: 10.1128/JCM.39.5.1738-1745.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson KL, Billington J, Pettigrew D, Cota E, Simpson P, Roversi P, et al. An atomic resolution model for assembly, architecture, and function of the Dr adhesins. Mol.Cell. 2004;15:647–657. doi: 10.1016/j.molcel.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Cota E, Jones C, Simpson P, Altroff H, Anderson KL, Du ML, et al. The solution structure of the invasive tip complex from Afa/Dr fibrils. Mol.Microbiol. 2006;62:356–366. doi: 10.1111/j.1365-2958.2006.05375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nowicki B, Nowicki B, Nowicki S, Selvarangan R. Family of Escherichia coli Dr adhesins: Decay-accelerating factor receptor recognition and invasiveness. J Infect.Dis. 2001;183:S24–S27. doi: 10.1086/318846. [DOI] [PubMed] [Google Scholar]
- 14.Nowicki B, Truong L, Moulds J, Hull R. Presence of the Dr receptor in normal human tissues and its possible role in the pathogenesis of ascending urinary tract infection. Am.J.Pathol. 1988;133:1–4. [PMC free article] [PubMed] [Google Scholar]
- 15.Plancon L, Du ML, Le FS, Gounon P, Jouve M, Guignot J, et al. Recognition of the cellular beta1-chain integrin by the bacterial AfaD invasin is implicated in the internalization of afa-expressing pathogenic Escherichia coli strains. Cell Microbiol. 2003;5:681–693. doi: 10.1046/j.1462-5822.2003.00308.x. [DOI] [PubMed] [Google Scholar]
- 16.Garcia MI, Jouve M, Nataro JP, Gounon P, Le BC. Characterization of the AfaD-like family of invasins encoded by pathogenic Escherichia coli associated with intestinal and extra-intestinal infections. FEBS Lett. 2000;479:111–117. doi: 10.1016/s0014-5793(00)01898-6. [DOI] [PubMed] [Google Scholar]
- 17.Miller RA, Britigan BE. Role of oxidants in microbial pathophysiology. Clin.Microbiol.Rev. 1997;10:1–18. doi: 10.1128/cmr.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N.Engl.J.Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 19.Forstermann U, Kleinert H, Gath I, Schwarz P, Closs EI, Dun NJ. Expression and expressional control of nitric oxide synthases in various cell types. Adv.Pharmacol. 1995;34:171–186. doi: 10.1016/s1054-3589(08)61085-6. [DOI] [PubMed] [Google Scholar]
- 20.Forstermann U, Gath I, Schwarz P, Closs EI, Kleinert H. Isoforms of nitric oxide synthase. Properties, cellular distribution and expressional control. Biochem.Pharmacol. 1995;50:1321–1332. doi: 10.1016/0006-2952(95)00181-6. [DOI] [PubMed] [Google Scholar]
- 21.Sakiniene E, Bremell T, Tarkowski A. Inhibition of nitric oxide synthase (NOS) aggravates Staphylococcus aureus septicaemia and septic arthritis. Clin Exp Immunol. 1997;110:370–377. doi: 10.1046/j.1365-2249.1997.4431456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukatsu K, Saito H, Fukushima R, Lin MT, Inoue T, Inaba T, et al. Effects of three inhibitors of nitric oxide synthase on host resistance to bacterial infection. Inflamm.Res. 1996;45:109–112. doi: 10.1007/BF02265161. [DOI] [PubMed] [Google Scholar]
- 23.Fukatsu K, Saito H, Fukushima R, Inoue T, Lin MT, Inaba T, et al. Detrimental effects of a nitric oxide synthase inhibitor (N-omega-nitro- L-arginine-methyl-ester) in a murine sepsis model. Arch Surg. 1995;130:410–414. doi: 10.1001/archsurg.1995.01430040072016. [DOI] [PubMed] [Google Scholar]
- 24.Klink M, Cedzynski M, St SA, Tchorzewski H, Sulowska Z. Involvement of nitric oxide donor compounds in the bactericidal activity of human neutrophils in vitro. J.Med.Microbiol. 2003;52:303–308. doi: 10.1099/jmm.0.04974-0. [DOI] [PubMed] [Google Scholar]
- 25.Ochoa JB, Udekwu AO, Billiar TR, Curran RD, Cerra FB, Simmons RL, et al. Nitrogen oxide levels in patients after trauma and during sepsis. Ann.Surg. 1991;214:621–626. doi: 10.1097/00000658-199111000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicholson S, Bonecini-Almeida M, Lapa e Silva JR, Nathan C, Xie QW, Mumford R, et al. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med. 1996;183:2293–2302. doi: 10.1084/jem.183.5.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yallampalli C, Dong YL, Gangula PRR, Fang L. Role and regulation of nitric oxide in the uterus during pregnancy and parturition. J Soc Gynecol Invest. 1998;5:58–67. doi: 10.1016/S1071-5576(97)00106-8. [DOI] [PubMed] [Google Scholar]
- 28.Yallampalli C, Garfield RE. Nitric oxide inhibits uterine contractility during pregnancy but not during delivery. In: Puri CP, VanHook PFA, editors. Current concepts in fertility regulation and reproduction. New Delhi: Wiley Eastern Limited Publishers; 1993. pp. 549–565. [Google Scholar]
- 29.Fang L, Nowicki BJ, Dong YL, Yallampalli C. Localized increase in nitric oxide production and the expression of nitric oxide synthase isoforms in rat uterus with experimental intrauterine infection. Am.J.Obstet.Gynecol. 1999;181:601–609. doi: 10.1016/s0002-9378(99)70499-0. [DOI] [PubMed] [Google Scholar]
- 30.Jouve M, Garcia MI, Courcoux P, Labigne A, Gounon P, Le BC. Adhesion to and invasion of HeLa cells by pathogenic Escherichia coli carrying the afa-3 gene cluster are mediated by the AfaE and AfaD proteins, respectively. Infect.Immun. 1997;65:4082–4089. doi: 10.1128/iai.65.10.4082-4089.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goluszko P, Moseley SL, Truong LD, Kaul A, Williford JR, Selvarangan R, et al. Development of experimental model of chronic pyelonephritis with Escherichia coli O75:K5:H-bearing Dr fimbriae: mutation in the dra region prevented tubulointerstitial nephritis. J Clin Invest. 1997;99:1662–1672. doi: 10.1172/JCI119329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labigne-Roussel A, Falkow S. Distribution and degree of heterogeneity of the afimbrial-adhesin-encoding operon (afa) among uropathogenic Escherichia coli isolates. Infect.Immun. 1988;56:640–648. doi: 10.1128/iai.56.3.640-648.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia MI, Gounon P, Courcoux P, Labigne A, Le BC. The afimbrial adhesive sheath encoded by the afa-3 gene cluster of pathogenic Escherichia coli is composed of two adhesins. Mol.Microbiol. 1996;19:683–693. doi: 10.1046/j.1365-2958.1996.394935.x. [DOI] [PubMed] [Google Scholar]
- 34.Fang FC. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest. 1997;99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang L, Nowicki BJ, Urvil P, Goluszko P, Nowicki S, Young SL, et al. Epithelial invasion by Escherichia coli bearing Dr fimbriae is controlled by nitric oxide-regulated expression of CD55. Infect.Immun. 2004;72:2907–2914. doi: 10.1128/IAI.72.5.2907-2914.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaul AK, Khan S, Martens MG, Crosson JT, Lupo VR, Kaul R. Experimental gestational pyelonephritis induces preterm births and low birth weights in C3H/HeJ mice. Infect.Immun. 1999;67:5958–5966. doi: 10.1128/iai.67.11.5958-5966.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Redline RW. Placental inflammation. Semin.Neonatol. 2004;9:265–274. doi: 10.1016/j.siny.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Redline RW, Wilson-Costello D, Hack M. Placental and other perinatal risk factors for chronic lung disease in very low birth weight infants. Pediatr.Res. 2002;52:713–719. doi: 10.1203/00006450-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 39.Odibo AO, Rodis JF, Sanders MM, Borgida AF, Wilson M, Egan JF, et al. Relationship of amniotic fluid markers of intra-amniotic infection with histopathology in cases of preterm labor with intact membranes. J.Perinatol. 1999;19:407–412. doi: 10.1038/sj.jp.7200210. [DOI] [PubMed] [Google Scholar]
- 40.Chellam VG, Rushton DI. Chorioamnionitis and funiculitis in the placentas of 200 births weighing less than 2.5 kg. Br.J.Obstet.Gynaecol. 1985;92:808–814. doi: 10.1111/j.1471-0528.1985.tb03050.x. [DOI] [PubMed] [Google Scholar]
- 41.Buhimschi I, Yallampalli C, Chwalisz K, Garfield RE. Pre-eclampsia-like conditions produced by nitric oxide inhibition: effects of L-arginine, D-arginine and steroid hormones. Hum Reprod. 1995;10:2723–2730. doi: 10.1093/oxfordjournals.humrep.a135775. [DOI] [PubMed] [Google Scholar]
- 42.Helmbrecht GD, Farhat MY, Lochbaum L, Brown HE, Yadgarova KT, Eglinton GS, et al. L-arginine reverses the adverse pregnancy changes induced by nitric oxide synthase inhibition in the rat. Am.J.Obstet.Gynecol. 1996;175:800–805. doi: 10.1016/s0002-9378(96)80002-0. [DOI] [PubMed] [Google Scholar]
- 43.Payne JA, Alexander BT, Khalil RA. Reduced endothelial vascular relaxation in growth-restricted offspring of pregnant rats with reduced uterine perfusion. Hypertension. 2003;42:768–774. doi: 10.1161/01.HYP.0000084990.88147.0C. [DOI] [PubMed] [Google Scholar]
- 44.Yallampalli C, Garfield RE. Inhibition of nitric oxide synthesis in rats during pregnancy produced signs similar to those of preeclampsia. Am.J.Obstet.Gynecol. 1993;169:1316–1320. doi: 10.1016/0002-9378(93)90299-x. [DOI] [PubMed] [Google Scholar]