Abstract
The FoxO subfamily of forkhead transcription factors plays a critical role in a variety of physiological processes including metabolism, differentiation, proliferation, apoptosis and protection from stress. FoxO activity is inhibited by growth factors and the insulin signaling pathways and stimulated by nutrient depletion and a plethora of reactive oxygen species (ROS)-induced post-translational modifications. Recent studies have uncovered a fundamental role for FoxOs in skeletal homeostasis. In cells of the osteoblast lineage, FoxOs modulate redox balance, protein synthesis, and differentiation through the activation of specific gene programs and interaction with other transcription factors and co-factors such as β-catenin, ATF-4, and Runx2. FoxO activation also attenuates osteoclastogenesis through both cell autonomous and indirect mechanisms. In this review I discuss recent advances in the understanding of FoxO specific actions in osteoblast progenitors, osteoblasts, and osteoclast, as well as the implications of FoxO activation for age-related skeletal involution.
Keywords: aging, reactive oxygen species, Wnt signaling, osteoblasts, osteoclasts
1. Introduction
The forkhead box O (FoxO) transcription factors represent a subclass of a large family of forkhead proteins characterized by the presence of a winged-helix DNA binding domain called Forkhead box. In mammals this subclass comprises four members: FoxO1 (or FKHR), FoxO3 (or FKHRL1), FoxO4 (also called AFX) and FoxO6 [1]. Foxo 1, 3, and 4 are ubiquitously expressed and relatively abundant in bone and bone cells. FoxO6 expression is confined to the brain and, thus, has no relevance to bone [2]. FoxOs are master signaling integrators that translate environmental stimuli, like hormonal changes, inflammation, and oxidative stress, into dynamic gene expression programs involved in many physiological and pathological processes. Initial interest in FoxOs stemmed from the evidence that these transcription factors promote stress resistance and extend longevity in C. elegans and Drosophila [3, 4]. While the role of FoxOs in lifespan has yet to be established in mammals, recent genetic work in mice indicates that FoxOs can reduce the impact of age–related tissue damage and several pathologies including neurodegeneration, metabolic diseases, and cancer [5]. These studies have also established a fundamental role of FoxOs in defense against oxidative stress [6]. In line with this evidence, progressive oxidative damage is thought to represent a fundamental mechanism of age-associated functional decline and disease [7, 8]. Modest levels of cellular ROS—the radical forms of oxygen—are generated by the mitochondrial electron transport chain during normal metabolism. Progressive mitochondrial damage, however, results in excessive ROS production which, in turn, causes oxidative stress and damage to proteins, lipids, and DNA, leading to cell death [9–11].
A decline in bone mass and strength and, consequently, the rise in the incidence of bone fractures is one of the hallmarks of aging in humans. Nevertheless, despite its clinical relevance, the mechanisms underlying skeletal aging remain unclear. Studies by our group and others have demonstrated that rodents also lose bone mass and strength with age [12–15]. Similar to other tissues, the age-related decline in bone mass is associated with increased oxidative stress in the bone [12, 16]. In support of the contention that oxidative stress plays an important role in skeletal homeostasis, different antioxidants prevent the increased osteoclastogenesis and increased osteoblast and osteocyte apoptosis, as well as the loss of bone caused by gonadectomy in mice [12, 17, 18]. Administration of antioxidants also abrogates the age-related increase in osteoblast apoptosis [16]. Conversely, both osteoblast numbers and bone formation are decreased in mice treated with an inhibitor of the antioxidant glutathione [18], and murine models of premature aging associated with oxidative damage exhibit osteoporosis [19, 20]. Most strikingly, deletion of FoxOs in young adult mice increases oxidative stress in bone and recapitulates the adverse effect of aging on the skeleton [21, 22].
2. Modulation of FoxO activity
FoxOs are indispensable for a wide variety of cellular processes, including proliferation, differentiation, apoptosis, autophagy, metabolism, and stress resistance. FoxO family members promote cell cycle arrest at the G1-S and G2-M checkpoints [23, 24], which is critical for the cellular response to stress. FoxO-induced cell cycle arrest allows time for repair of damaged DNA and for detoxification of cells. To accomplish this, FoxO proteins stimulate the expression of several genes involved in DNA repair [24, 25] and scavenging of free radicals [25–27]. On the other hand, FoxOs can also induce apoptosis through the regulation of several pro-apoptotic genes [28, 29]. The role of FoxOs in cell death seems counterintuitive considering their role in protection against stress. Nonetheless, elimination of damaged or abnormal cells by FoxO-induced apoptosis evidently plays an important role in the ability of these transcription factors to promote tumor suppression [30]. FoxO activity is regulated by a variety of external stimuli including insulin, growth factors, hormones, cytokines, and oxidative stress, through a plethora of post-translational modification of FoxOs including phosphorylation, ubiquitination, acetylation, and methylation. These post-translational modifications affect FoxO protein levels, cellular localization, DNA binding potential, and transcriptional activity. FoxOs can also modulate gene transcription through the interaction with other transcription factors and co-factors [31, 32].
2.1. Inhibition by insulin and growth factors
Mammalian FoxO1, 3, and 4 are negatively regulated by insulin and growth factors via the serine/threonine kinase Akt [31]. Akt directly phosphorylates FoxOs on three conserved residues (e.g. Thr-32, Ser-253, and Ser-315 in FoxO3). This phosphorylation promotes the binding of FoxOs to the chaperone protein 14-3-3 in the nucleus, which in turn prevents the binding of FoxOs to DNA and causes FoxO nuclear export [33]. Therefore, Akt-mediated phosphorylation of FoxOs leads to cytoplasmic retention and inhibition of FoxO-mediated transcription (Fig. 1). Insulin and growth factors also block FoxO action by promoting proteasome-mediated FoxO degradation and, consequently, decreasing FoxO protein levels [34]. Specifically, Akt phosphorylated FoxO1 binds to the SCFSKP2 E3 ligase complex which induces the polyubiquitination and degradation of FoxO1. This mechanism of FoxO inhibition is particularly relevant in oncogenic transformation by PI3K/Akt. Activated extracellular signal-regulated kinase (ERK) also induce the phosphorylation of FoxOs at multiple residues [35, 36]. Moreover, phosphorylation of FoxO3 by ERK promotes the recruitment of the E3 ligase MDM2 and leads to the ubiquitination and proteasome degradation of FoxO3. Similar to the Akt/SKP2-, ERK/MDM2-mediated degradation of FoxO plays a critical role in promoting cell proliferation and tumorigenesis. Besides Akt and ERK, several other kinases including serum- and glucocorticoidinducible kinases (SGKs), IκB kinase (IKK), dual-specificity tyrosine-phosphorylated and regulated kinase (DYRK), as well as CDK2 phosphorylate FoxOs and inactivate transcription [1].
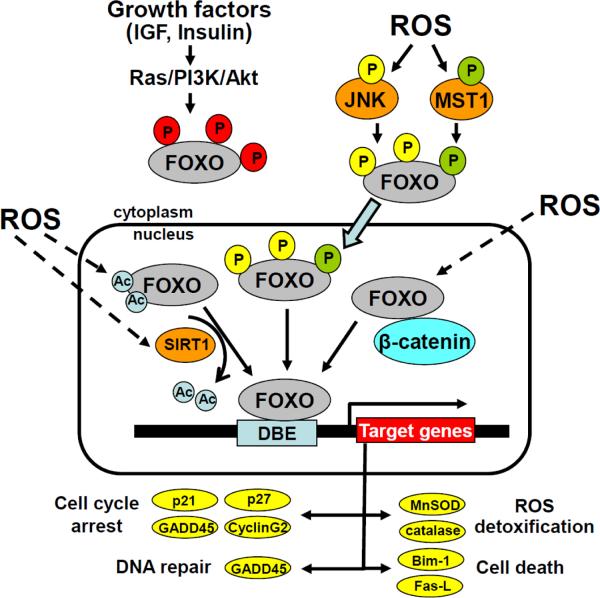
Figure 1. Activation of FoxO transcription factors by ROS.
Phosphorylation of FoxOs by Akt leads to the nuclear export of FoxOs and inhibition of FoxO-mediated transcription. ROS, via JNK or Mst1 activation, phosphorylate FoxOs at different residues, promote FoxOs nuclear translocation, and activation of transcription. ROS also promote FoxOs acetylation and deacetylation via Sirt1 activation. Finally, ROS promote the association between FoxOs and β-catenin which enhances FoxO-mediated transcription. Bim-1, Bcl-2-like protein 11; DBE, DAF-16 binding element; Fas-L, Fas ligand; GADD45, Growth Arrest and DNA Damage 45; MnSOD, Manganese Superoxide Dismutase.
Due to the tight control of FoxOs by phosphorylation, phosphatases also play an important role in the modulation of FoxO activity. Protein phosphatase 2A (PP2A) directly interacts with and dephosphorylates FoxOs [37, 38]. Inactivation of PP2A revealed that PP2A-mediated dephosphorylation of T32/S253 of FoxO3 is required for dissociation of 14-3-3, nuclear translocation, and transcriptional activation of FOXO3 when Akt is inhibited [37]. Inhibition of PP2A also prevented FOXO1-mediated increase in the expression of the pro-apoptotic protein BIM and apoptosis [38].
2.2. Activation by ROS
In contrast to growth factors, ROS leads to the retention of FoxOs in the nucleus and activation of transcription. ROS promote several post-translational modifications of FoxOs, including phosphorylation, monoubiquitination, acetylation/deacetylation and cysteine oxidation [39]. Work with different model organisms has revealed that in response to oxidative stress c-jun kinase (JNK) and mammalian sterile 20-like kinase-1 (Mst1) bind and phosphorylate FoxOs directly [40–43] (Fig. 1). JNK-mediated phosphorylation of FoxO4 at Thr447 and Thr451 causes the translocation of FoxO4 from the cytoplasm to the nucleus [40]. JNK also phosphorylate 14-3-3, which causes the release of FoxOs from this adaptor protein and further promote FoxOs nuclear localization [44]. Moreover, JNK, by phosphorylating the insulin receptor substrate 1 (IRS1) at Ser307, can inhibit its tyrosine phosphorylation by the insulin/IGF receptor and, thereby, prevent the Akt-mediated negative regulation of FoxO activity [45]. Mst1 phosphorylates FoxO1 and FoxO3 at Ser 112 and Ser207, respectively, which disrupts 14-3-3 binding and promotes FoxO translocation to the nucleus [41]. Oxidative stress activated Mst1/FoxO leads to neuronal cell apoptosis. Interestingly, JNK can enhance the Mst1-mediated pro-apoptotic signal by phosphorylating Mst1 at serine 82 [46]. In contrast, in naïve T cells the Mst1/FoxO signaling cascade exerts a protective pro-survival function, and in C. elegans extends lifespan and delays aging [41, 47]. Thus, stress-activated JNK and Mst1 and growth factor-activated Akt have opposing effects on the subcellular localization of FoxOs. Importantly, stress stimuli override the sequestration of FoxO in the cytoplasm by growth factors, both in mammalian cells and in Drosophila [42, 48].
ROS also induce the monoubiquitination of FoxO4 [49]. Interestingly, while MDM2 is responsible for the polyubiquitination and degradation of FoxO3 in response to growth factors, MDM2 also monoubiquitinates FoxO4 under conditions of oxidative stress which triggers its retention in the nucleus and stimulation of FoxO transcriptional activity [50].
In addition, ROS enhance the interaction of histone acetyltransferases, namely p300/CBP, with FoxOs leading to increased FoxO acetylation [48, 51, 52]. The role of acetylation in FoxO regulation is not clear, as most, but not all, studies indicate that acetylation inhibits FoxO transcriptional activity. Indeed, acetylation reduces FoxO DNA-binding affinity and increases Akt-mediated phosphorylation of FoxOs. Acetylation also prevents FoxO ubiquitination probably because these two post-translational modifications occur on lysine residues. Interestingly, in response to oxidative stress acetylated FoxO1 can suppress tumor growth by interacting with Atg7 and triggering autophagy - a catabolic process involving the degradation of the cell own components through the lysosomal machinery - in a transcription independent manner [53]. Recently, Dansen et al have shown that cysteines within FoxOs are subject to oxidation and disulfide formation when ROS levels are increased [54]. Importantly, cysteine oxidation is required for the binding of FoxO to CBP and p300 and FoxO acetylation.
Furthermore, oxidative stress promotes the interaction of the NAD-dependent deacetylase Sirt1 with FoxOs and consequent FoxO deacetylation (Fig. 1). Although the effects of Sirt1 on FoxO activity can vary, depending on which FoxO target gene is measured, the consensus from various studies is that Sirt1 plays a crucial role in promoting survival and stress resistance. The importance of Sirt1 as a FoxO co-activator as been recently highlighted by the fact that deletion of Sirt1 in osteoblasts or osteoclasts results in low bone mass [55]. Despite all the current knowledge, the precise mechanism by which different levels of stress promote different post-translational modifications of FoxO proteins and, in turn, how each post-translational modification modulates FoxO function remains elusive.
2.3. Protein-protein interaction
FoxOs can also interact with other transcription factors at gene promoters or with other co-factors [56]. Some of these interactions might be relevant for bone biology. For example, FoxOs cooperate with Smad3 and Smad4 in response to TGFβ to potentiate the expression of common target genes. Specifically, Smad3 and Smad4 form a complex with FoxO factors at the p21Cip1 promoter which is critical for the ability of TGFβ to promote cell cycle arrest in neuroepithelial and glioblastoma cells [57]. In addition, FoxOs are functionally connected to the Notch signaling. FoxO1 directly interacts with the Notch effector Cs1 and stimulates the transcription of Notch target genes like Hes1 by promoting co-repressor clearance from Csl [58]. This action promotes muscle cell differentiation and is independent of FoxO1 transcriptional function. Interaction between FoxOs and members of the nuclear receptor family, like peroxisome proliferator-activated receptor (PPAR) γ, estrogen receptor (ER) α, or androgen receptor (AR) inhibits FoxO-mediated transcription [56]. Indeed, PPARγ – a transcription factor that is indispensable for adipogenesis – binds to and prevents FoxO1 transcriptional activity [59]. FoxO1, in turn, antagonizes PPARγ activity by interacting with PPARγ bound to PPAR response elements on target genes promoters [59]. The FoxO1 transrepressional function is independent from FoxO1 DNA binding actions. Furthermore, in cultured adipocytes, FoxO1 binds to both PPARγ1 and PPARγ2 promoters to repress their transcriptional activity, leading to decreased PPARγ expression [60]. Importantly, via these mechanisms FoxO1 inhibits adipogenesis in preadipocytes and potentiates high fat diet induced diabetes in mice [61].
The AR directly associates with FoxOs and, in a ligand-dependent manner, decrease FoxO DNA-binding and activity [62, 63]. It has also been suggested that androgens can decrease FoxO1 levels by promoting the activity of an acidic cysteine protease and FOXO1 proteolytic degradation [64]. FoxOs, reciprocally, restrain AR activity by disrupting the interaction of the N-and C-terminal of the AR, an event that is critical for the biological actions of the receptor [65, 66]. FoxOs also prevent the recruitment of coactivators to AR N-terminal domain, resulting in suppression of androgen action. Importantly, via this mechanism FoxOs suppress androgen-induced proliferation in prostate cancer cells [66].
Several reports have suggested that FoxOs interact with the ER [67–70]. Similar to the case of AR the interaction between ER and FoxO members represses FoxO transcriptional activity [67]. In breast cancer cells, FOXO3 binding to the ER inhibits the transcriptional activity of this nuclear receptor and abrogates estradiol-induced cell growth [69, 70]. Accordingly, overexpression of FOXO3 in a breast cancer mouse model prevents estradiol-dependent tumourigenesis [69].
In osteoblasts FoxO1 binds to ATF4 and this association potentiates both FoxO1 and ATF4 transcriptional activity [22]. FoxO1 also binds to Runx2 in osteoblasts [71, 72], as well as in prostate cancer cells [73]. This binding requires the C-terminal region aa 360–456 of FoxO1 and the aa 242–258 region of Runx2 [71] and is independent of the DNA-binding activity of FoxO1 [73]. Interestingly, while Runx2 does not affect the binding of FoxO1 to DNA [71], FoxO1 can repress or stimulate Runx2 activity depending on the cell model used [71–73].
β-catenin, besides its important role in promoting T-cell cell factor (TCF)/Lymphoid enhancer binding factor-transcription, is an essential co-activator of the FoxO family of transcription factors [74, 75]. Oxidative stress, as opposed to insulin and growth factor signaling, promotes FoxO binding to β-catenin and activation of FoxO transcription. The interaction between β-catenin and FoxOs is evolutionary conserved as evidenced by the fact that in C. elegans the β-catenin ortholog, BAR-1, is required for oxidative stress-induced expression of the FoxO ortholog DAF-16 target gene sod-3, resistance to oxidative damage, and lifespan extension [74]. Recent discoveries in osteoblastic cells, discussed in detail below, suggest that the interaction between β-catenin and FoxOs might be vital for bone homeostasis and the mechanism of age related osteoporosis.
3. FoxOs are critical players in redox signaling and bone homeostasis
The role of mammalian FoxOs has been unraveled in the recent years, largely thanks to the generation of individual or combined mouse deletion mutants. Deletion of FoxO1, FoxO3, and FoxO4 individually has revealed that FoxO1 is indispensable during development, as FoxO1-null mice die at embryonic day 10.5 due to defects in angiogenesis [76, 77]. Studies using murine models of loss or gain of FoxO1 function in different tissues have revealed that this transcription factor plays a critical role in the regulation of glucose and lipid metabolism (reviewed in [78]). During stress conditions like hyperglycemia, FOXO1 protects pancreatic β-cells against oxidative stress-induced failure [52]. High concentrations of glucose lead to the accumulation of ROS within the β-cells islet and, consequently, to a decrease in the activity of transcription factors that are critical for the control of insulin expression [79]. In response to the oxidative insult FOXO1 enters the nuclei of b-cells and activates the expression of genes that preserve insulin secretion and promote cell survival [52]. Studies using FoxO3-null mice have revealed that FoxO3 is essential to maintain the hematopoietic stem cell pool by preventing oxidative stress [80]. Moreover, FoxO3-null mice die rapidly when exposed to oxidative stress because FoxO3 is essential for erythrocyte resistance to the deleterious effects of oxygen radicals [81]. FoxO4-deficient mice do not exhibit an overt phenotype [77]. Collectively, the studies with loss of function models have elucidated that, despite of sharing a similar DNA-binding domain and overlapping expression in multiple tissues, various FoxO proteins exert independent physiological functions. On the other hand, extensive in vitro studies have shown that FoxO1, FoxO3, and FoxO4 bind to the same DNA target sequence, regulate common target genes, and behave similarly in biochemical studies, suggesting that they exert redundant functions [82]. To address this issue the DePinho lab has generated mice with combined conditional deletion of FoxO1, 3, and 4. These murine models have revealed not only that FoxOs are lineage-restricted redundant tumor suppressors but also reinforced the notion that these transcription factors exert critical antioxidant functions [83, 84]. Indeed, combined deletion of FoxOs resulted in loss of hematopoietic stem cells due to increased ROS levels. Targeted combined deletion of FoxO1, 3, and 4, or FoxO3 alone, in the brain has further elucidated that similar to the hematopoietic stem cells, FoxOs exert antioxidant actions that are indispensable for the maintenance of neural stem cells [85, 86]. In addition, mice with combined loss of FoxO1 and 3 in cardiomyocytes have revealed that FoxOs play a critical role in limiting ROS production and cell dead in response to ischemia/reperfusion injury in the heart [87]. Accordingly, overexpression of Sirt1 or Sirt3 protects the heart from oxidative stress via increased expression of antioxidant FoxO-target genes [88, 89].
FoxO1, 3, and 4 are expressed at similar levels in bone and bone cells, including bone marrow derived osteoclasts, and osteoblastic cells derived from calvaria or bone marrow [21, 22]. In addition, work from our group has shown that combined global deletion of the three FoxOs at 3 months of age, for just 5 weeks, resulted in bone loss at both cancellous and cortical sites, and was associated with increased oxidative stress as determined by the phosphorylation of p66Shc in bone. P66Shc is activated by ROS and, within the mitochondria, amplifies H2O2 production by catalyzing the reduction of O2 to H2O2 through electron transfer from cytochrome c [90–92]. Importantly, p66Shc is required for ROS induced apoptosis in several cell types including osteoblasts [93]. Ex vivo studies performed with cells derived from the FoxO1, 3, and 4 deleted mice have demonstrated that FoxOs exert cell autonomous effects on osteoblasts and osteoclasts.
3.1. Effects on osteoblasts
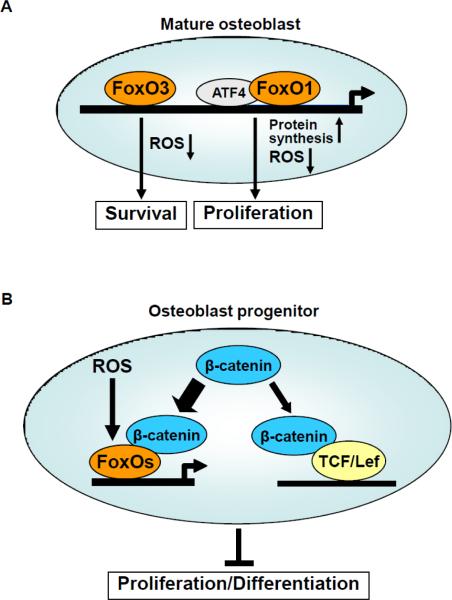
A role of FoxOs in cells of the osteoblast lineage was first revealed by the generation of murine models in which FoxO3 was overexpressed or FoxO1 was deleted in osteoblasts. Mice overexpressing FoxO3 under the control of the osteocalcin promoter exhibited increased vertebral bone mass, as well as decreased phosphorylation of p66Shc [21]. In addition, these mice had increased bone formation rate and osteoblast number and decreased osteoblast apoptosis. These findings, along with the evidence that loss of FoxOs leads to increased osteoblastic cell apoptosis, decreased osteoblast number and bone formation rate and decreased bone mass, favor the idea that FoxO3 reduce oxidative stress and promotes osteoblast survival (Fig. 2a). As in osteoblasts, FoxOs promote cell survival by attenuating oxidative stress in other cell types [94]. However, the pro-survival effect of FoxOs is highly dependent on the tissue and cell type. For example, loss of FoxO function promotes murine hematopoietic stem cell apoptosis but, in the same model, decreases the apoptosis of thymocytes or endothelial cells from the liver [83, 84]. In line with the antioxidant role of FoxO3 in osteoblasts, deletion of FoxO1 from collagen1a1 expressing cells leads to a decrease in osteoblast numbers and bone mass as a consequence of increased oxidative stress [22]. Nevertheless, in difference to the role of FoxO3 in the prevention of apoptosis, FoxO1 deletion in osteoblasts decreases cell proliferation but does not affect apoptosis. FoxO1 deletion also promoted a decrease in glutathione and collagen1 protein levels [22]. Nevertheless, unlike the bone phenotype, these changes were not prevented by the administration of the antioxidant N-acetyl-cysteine. In vitro studies have revealed that FoxO1 interacts with ATF4 – a transcription factor that promotes amino acid import and collagen synthesis in osteoblasts – and that this interaction potentiates both FoxO1 and ATF4 transcriptional activity. Thus, FoxO1 regulation of oxidative stress, osteoblast proliferation, and bone formation results, at least in part, from its ability to interact with ATF4 and promote amino acid import to favor the synthesis of glutathione and collagen1 (Fig. 2a). In contrast, in the muscle FoxO1 inhibits protein synthesis mediated by mammalian target of rapamycin (mTOR). This action is accomplished via FoxO1-induced up-regulation of eukaryotic initiation factor 4E-binding protein-1, which prevents the initiation of translation [95]. Despite the decrease in protein synthesis and osteoblast numbers, mice with targeted deletion of FoxO1 in osteoblasts exhibited a striking increase increase in the production of osteocalcin [96]. Mechanistic studies in osteoblastic cells have suggested that FoxO1 can inhibit osteocalcin expression via direct binding to the osteocalcin gene promoter, interaction with Runx2 and repression of its activity, or both [71, 96]. It has been recently suggested that osteoblasts control energy metabolism through the secretion of osteocalcin [97]. Indeed, the uncarboxylated form of osteocalcin acts as a hormone to promote β-cell proliferation, insulin secretion and sensitivity, as well as energy expenditure. Osteocalcin bioactivity is negatively regulated by the protein tyrosine phosphatase (OST-PTP), the product of the gene Esp, which is expressed in osteoblasts and promotes osteocalcin carboxylation. Deletion of FoxO1 in osteoblasts also led to a decrease in Esp as well as in increased pancreatic β-cell proliferation, insulin secretion and insulin sensitivity, suggesting that FoxO1 may be a key modulator of the ability of the skeleton to function as an endocrine organ regulating glucose metabolism [96].
Figure 2. Diverse role of FoxOs in osteoblast lineage cells.
(a) FoxO3 activity decreases ROS levels and promote osteoblast survival. FoxO1 associates with ATF4 to promote protein synthesis, decrease ROS levels and stimulate proliferation. (b) Activation of the canonical Wnt signaling by Wnt proteins prevents the proteasomal degradation of β-catenin and promotes its association with the TCF/Lef family of transcription factors and the expression of Wnt target genes. Activation of FoxO mediated transcription by ROS promotes the binding of FoxO to β-catenin, thus diverting the limited pool of from TCF/Lef to FoxO mediated transcription and thereby decreases osteoblastogenesis.
As discussed earlier, one of the main inhibitors of FoxO transcriptional activity is insulin. It has recently been shown that insulin signaling in osteoblasts is a positive regulator of postnatal bone acquisition [97]. Mice lacking the insulin receptor in osteoblasts had reduced trabecular bone volume secondary to decreased bone formation and lower osteoblast numbers. These findings, along with the evidence that FoxO activation in osteoblasts increases trabecular bone volume, bone formation, and osteoblast numbers, suggest that the positive actions of insulin on bone accrual do not involve suppression of FoxOs. In contrast, insulin signaling in osteoblasts increases osteocalcin secretion and promotes glucose homeostasis, at least in part, by attenuating FoxO activity and preventing the inhibitory actions of FoxO1 on Runx2 and osteocalcin expression [71, 97]. The actions of Sirt1 deacetylase in osteoblasts, similar to FoxOs, have a positive effect on bone mass [55]. In line with these findings, the Sirt1 activator resveratrol stimulates osteoblastogenesis and attenuates the bone loss that occurs with aging in mice [98, 99]. However, resveratrol also exerts Sirt1-independent effects and can affect bone cells for example via activation of the estrogen receptor [100].
Collectively, the results of the studies on the genetic manipulation of FoxOs in mature osteoblasts strongly suggest that these transcription factors are critical mediators of the cellular defense against oxidative stress, thereby contributing to skeletal homeostasis. However, old age or sex steroid deficiency leads to an increase in the prevalence of osteoblast and osteocyte apoptosis as a consequence of increased oxidative stress [12, 17]. The most likely explanation for the inability of FoxOs to prevent osteoblast apoptosis under these conditions is that the defense provided by these factors is overwhelmed by the elevated ROS. In addition, several other ROS-stimulated pathways can interfere with the activity of FoxOs. Indeed, increased phosphorylation of p66shc as well as increased activity of NF-kB are common features of old age and sex steroid deficiency [12, 101–103]. ROS-induced p66shc activation leads to FoxO inactivation via Akt-mediated FoxO phosphorylation [27, 104]. Moreover, ROS-induced stimulation of NF-kB may lead to IKK-mediated phosphorylation and inhibition of FoxO3 activity, at least in part by targeting it to ubiquitin-dependent degradation [105].
3.2. Effects on osteoblast progenitors
In aging mice, the expression of β-catenin/TCF-target genes decrease and FoxO-target genes increase in bone, along with an increase in markers of oxidative stress and decreased bone formation [12, 75]. Furthermore, in several cell types including osteoblasts, oxidative stress induces the association of FoxOs with β-catenin, and β-catenin is required for the stimulation of FoxO target genes. The interaction between β-catenin and FoxOs requires armadillo repeats 1 to 8 of β-catenin and the C-terminal half of FoxO [74] but it remains unknown whether any oxidative stress-induced post-translational modification of FoxOs promotes this interaction. Notably, oxidative stress induced by H2O2 promotes FoxO-mediated transcription at the expense of Wnt/TCF-mediated transcription and osteoblast differentiation [75] (Fig. 2b). The effect of H2O2 on TCF transcription is abrogated by increasing levels of β-catenin, strongly suggesting that a limited pool of active β-catenin is diverted from TCF to FoxO transcription under stress conditions. This cell intrinsic mechanism of Wnt signaling inhibition also occurs in colon cancer cells and hepatocytes [106, 107]. Interestingly, nutrient deprivation also favors the association of β-catenin with FoxOs, rather than with TCF, to regulate the hepatic gluconeogenic response [107]. In hepatocytes, β-catenin seems to favor the nuclear localization of FoxO1. In view of the role of β-catenin on cell proliferation/differentiation and the role of FoxOs in defense against stress, the ability of FoxOs to functionally interact with β-catenin might allow osteoblast progenitor cells to avoid proliferation under stress conditions, for example in the presence of high ROS levels or in the absence of growth factors. Activation of FoxOs might also decrease β-catenin/TCF-mediated transcription via alternative mechanisms. As shown in neural stem cells, FoxOs stimulate the transcription of the Wnt signaling inhibitors Sost, sFRP1, and sFRP2 [85]. However, the expression of these Wnt signaling inhibitors was unaffected in bones or osteoblastic cells from mice lacking FoxO1, 3, and 4 (Ambrogini and Almeida, unpublished data) suggesting that Sost, sFRP1, and sFRP2 are not FoxO target genes in bone cells.
Importantly, Wnt signaling inhibition may be also the mechanism by which lipid oxidation contributes to the decline in osteoblast number and bone formation that occurs with aging. In the process of lipid oxidation lipoxygenases oxidize polyunsaturated fatty acids to form products that bind to and activate PPARγ and generate pro-oxidants like 4-HNE [108]. Work from our group has shown that lipid oxidation increases with age in bone and that 4-HNE, similar to H2O2, activates FoxOs that in turn attenuate β-catenin/TCF-mediated transcription [109]. Because TCF-mediated transcription suppresses PPARγ expression [110, 111], inhibition of β-catenin/TCF transcriptional activity leads to an increase in PPARγ levels. Oxidized polyunsaturated fatty acids promote PPARγ association with β-catenin and induce β-catenin degradation [109, 112], thereby further decreasing β-catenin/TCF-mediated transcription. Consistent with the contention that FoxOs antagonize Wnt/TCF signaling, preliminary studies of ours indicate that deletion of FoxO1, 3, and 4 from committed osteoblast progenitors expressing osterix – the main target of the proosteogenic actions of β-catenin – increases bone mass [113]. Hence, diversion of the limited pool of β-catenin from TCF- to FoxO-mediated transcription represents an important cell-autonomous mechanism of β-catenin/TCF antagonism critical for skeletal homeostasis. Based on these findings it is tempting to speculate that the age-related bone loss might result, at least in part, from the suppressive effect of ROS on Wnt/β-catenin signaling via FoxO activation. In line with this idea, mice with targeted expression of Wnt10b in the bone marrow or mice carrying the LRP5 G171V mutation exhibit increased bone mass and no evidence of age-related loss of bone mass or strength, respectively [114, 115].
3.3. Effects on skeletogenesis and mesenchymal stem cells
Several studies indicate that FoxOs also play a role in the differentiation of mesenchymal cells, the early precursors of osteoblasts. Using microRNA technology Teixeira et al [72] downregulated FoxO1 expression in developing embryos to investigate the role of this protein in skeletogenesis. Silencing FoxO1 impaired skeletal development and reduced the size of the embryos, as well as the size of bones in the craniofacial area. Knockdown of FoxO1 expression in mouse embryonic tibiae ex vivo, similar to the results obtained in vivo, led to shorter and less mineralized bone. In C3H10T1–2 pluripotent cells, BMP-2 stimulated the binding of FoxOs to the promoter of Runx2, ALP, and osteocalcin. Moreover, similar to the case in osteoblasts, FoxO1 co-immunoprecipitated with Runx2 [22, 96]. However, in mesenchymal progenitors the association between Runx2 and FoxO1 enhanced the transcription of alkaline phosphatase and osteocalcin suggesting that, in contrast to the case in osteoblasts, the two transcription factors cooperate to activate the transcription of genes involved in osteoblast differentiation. The mechanism responsible for the opposite effect of FoxO1 on Runx2 activity, in mesenchymal progenitors versus osteoblasts, is currently unknown. In line with the defective skeletogenesis following FoxO1 downregulation in mesenchymal progenitor cells, global deletion of FoxO1, 3, and 4 in mice lead to the attenuation of the expression of Runx2, osterix, and alkaline phosphatase in ex-vivo cultures of osteoblastic progenitors [21]. In addition, the same mice exhibit a significant decrease in the number of colony-forming unit (CFU)-fibroblasts and CFU-osteoblasts present in the bone-marrow (Ambrogini and Almeida, unpublished data). Deletion of FoxOs also caused an increase of PPARγ — a nuclear receptor that stimulates adipogenesis [116] and represses osteoblastogenesis [117]. In line with these findings, FoxOs inhibit adipogenesis by repressing the activity of the PPARγ promoter and by antagonizing PPARγ ability to bind and transactivate target genes in pre-adipocytes [59, 60]. Consistent with the increase in PPARγ expression, cells from the FoxO deficient mice exhibit increased adipogenic potential in response to the PPARγ ligand rosiglitazone. Hence, increased adipocyte differentiation at the expense of osteoblast differentiation from their common mesenchymal progenitors may contribute to the decreased osteoblast number and bone formation in these mice.
While it is plausible that FoxOs have pro-osteogenic actions in early mesenchymal progenitors, it is also possible that, similar to other tissues, FoxOs preserve the integrity and homeostasis of the adult mesenchymal stem cell pool. Indeed, FoxOs maintain the homeostasis of the hematopoietic and neural stem cell compartments, via the regulation of genes controlling proliferation and intracellular ROS levels [83, 85, 86]. In contrast, as detailed above, FoxOs exert anti-osteogenic actions in committed osteoblast progenitors by inhibiting Wnt signaling. Taken together, these lines of evidence indicate that FoxOs exert multiple effects in osteoblastic cells depending on their stage of differentiation.
3.4. Effects on osteoclasts
ROS can be both damaging byproducts of aerobic metabolism as well as signaling molecules generated by cell surface receptors to propagate growth factor signals [118, 119]. Studies by the group of Greg Mundy in the 90's [120] followed by several other groups have shown that formation and activation of osteoclasts involves ROS generation. Indeed, RANKL increases ROS and in turn ROS is required for osteoclastogenesis (ref). The plasma membrane-associated NADPH oxidases Nox1 and Nox2 are an important source of ROS formation (ref). Indeed, RANKL increases ROS through TRAF6/Rac1/Nox1-dependent pathway in osteoclasts [121–125]. Conversely, decreased ROS levels by antioxidants, inhibition of Nox1, a dominant negative rac1, or overexpression of glutathione peroxidase prevents osteoclastogenesis by interfering with RANKL-induced JNK, p38, and ERKs activation [124, 125]. In agreement with the requirement of ROS for osteoclastogenesis, several different antioxidants prevent the increased bone resorption that follows loss of sex steroids [17]. Mitochondrial ROS production has also been implicated in osteoclast generation and activity. Ishii et al have shown that mitochondrial biogenesis and ROS production coupled with iron uptake through the transferrin receptor 1 and iron supply to mitochondrial respiratory proteins are essential for the bone resorbing function of osteoclasts and the bone loss caused by estrogen deficiency [126]. Moreover, the mitochondria-targeted antioxidant MitoQ suppresses osteoclastogenesis [127].
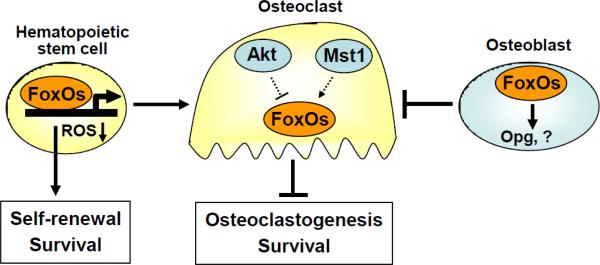
Combined deletion of FoxO1, 3, and 4 in mice resulted in myeloid lineage expansion and a marked decrease in the hematopoietic stem cell population. The later exhibited increased ROS levels and apoptosis, that correlated with decreased expression of genes that attenuate ROS including superoxide dismutases Sod1, Sod2 and Sod3, catalase, and glutathione peroxidase 1 [83]. Increased ROS enforced cell fate decisions, driving hematopoietic stem cells into the cell cycle and terminal differentiation at the expense of self-renewal. Thus, FoxO proteins play an essential role in the response to physiologic oxidative stress and thereby promote quiescence and survival of HSCs (Fig. 3). In line with the expansion of the myeloid lineage compartment, combined FoxO deletion increased the number of osteoclast progenitor cells [21].
Figure 3. FoxOs decrease osteoclast numbers and bone resorption.
FoxOs play an essential role in the response to physiologic oxidative stress and thereby promote survival and self-renewal of hematopoietic stem cells. In contrast, activation of FoxO in osteoclast precursor cells attenuates resorption and increases bone mass. In line with a cell autonomous inhibitory action of FoxOs on osteoclast generation and/or survival, Akt and Mst1 kinase activity promote and attenuate osteoclast number, respectively. However, it is unknown whether the actions Akt and Mst1 in osteoclastic cells are mediated by FoxOs. FoxO activity in osteoblast also decreases osteoclast number via increased Opg expression or other mechanisms.
To examine the role of FoxOs in osteoclastic cells, we have recently generated transgenic mice overexpressing FoxO3 in cells of the monocyte/macrophage lineage expressing lysozime M. Preliminary results indicate that these mice exhibit increased bone mass, along with decreased levels of several resorption markers, suggesting that FoxOs negatively affect osteoclast number via cell autonomous actions in osteoclast progenitors and/or mature osteoclasts [128]. These findings along with evidence that M-CSF and RANKL-induced Akt signaling in osteoclasts promotes proliferation and differentiation and attenuates apoptosis [129–131], suggest that phosphorylation and inhibition of FoxO activity might mediate the positive actions of Akt in osteoclasts. Mst1, a kinase that activates FoxOs, has been identified as a key intermediate in osteoclast apoptosis, whether activated by serum withdrawal, staurosporine, or bisphosphonates [132]. All these apoptosis-inducing treatments trigger caspase cleavage of Mst1 into an active 34-kDa species that maintains the catalytic domain. Caspase cleavage of Mst1 kinase is necessary for Mst1 to phosphorylate FoxOs [133]. Thus, in contrast to Akt, Mst1 might promote osteoclast apoptosis via activation of FoxO-mediated transcription (Fig. 3). Sirt1 activity in osteoclasts, like FoxO3, most likely decreases osteoclast number as indicated by the observation that targeted deletion of Sirt1 in osteoclasts results in low bone mass [55]. Accordingly, resveratrol inhibits osteoclastogenesis and attenuates the loss of bone following ovariectomy by decreasing NF-kB signaling and/or ROS generation in osteoclasts [134–137]. Whether FoxOs mediate the actions of Akt, Mst1, or Sirt1 on osteoclast generation and survival remains unknown.
FoxOs also decrease osteoblast number indirectly, via action in osteoblasts. Targeted overexpression of FoxO3 or deletion of FoxO1 in osteoblasts in mice led to a decrease or increase in osteoclast numbers, respectively [21, 22]. Thus, FoxO activation in osteoblasts also decreases osteoclast numbers. These actions of osteoblastic FoxOs were unexpected, because of the inhibitory effect of FoxOs on canonical Wnt signaling and evidence that β-catenin in osteoblasts increases osteoprotegerin (Opg) (a direct target of β-catenin/TCF transcriptional activity), decreases RANKL and, consequently, diminishes osteoclast numbers in bone [138, 139]. An explanation for this apparent discrepancy was provided by the findings that FoxO1 activity in osteoblasts increases the expression of Opg [140] (Fig. 3). Indeed, mice with targeted deletion of FoxO1 in osteoblasts exhibit diminished Opg expression in bone; and FoxO1 gain- or loss-of-function in osteoblastic cells, in vitro, increased or decreased Opg, respectively. In contrast, insulin signaling in osteoblasts favors bone resorption by preventing the stimulatory action of FoxO1 on Opg [140]. However, Opg levels were not altered in the bone of mice overexpressing FoxO3 in osteoblasts, suggesting that FoxO3 might control osteoclastogenesis independent of Opg [21]. For example, FoxO3 overexpression may decrease osteoclast number by reducing ROS in the bone microenvironment. Thus, FoxOs in mature osteoblasts may inhibit osteoclastogenesis independently of the crosstalk with the canonical Wnt signaling.
4. Conclusions
A series of recent discoveries indicate that FoxOs play an important role in skeletal homeostasis by exerting both ROS dependent and independent effects. In cells of the osteoblast lineage FoxOs regulate the expression of osteoblast-specific genes like osteocalcin and the function of other transcription factors vital for osteoblastogenesis like ATF4, Runx2, and β-catenin. Through these actions FoxOs promote osteoblastogenesis and/or maintain the pool of early mesenchymal progenitors, restrain the proliferation/differentiation of committed osteoblast precursors, and protect mature osteoblast from oxidative stress. Moreover, FoxOs through direct or indirect actions attenuate osteoclastogenesis. Future work is needed to elucidate FoxO target-gene populations, as well as the role of indirect FoxO action on other transcription factors in the control of bone cell biology. With advancing age an increase in ROS might decrease the number of osteoblasts via diversion of β-catenin from Wnt/Tcf- to FoxO-mediated transcription in osteoblast progenitors. While at present the role of FoxOs in skeletal aging is inferred from the phenotype of genetically modified young mice, aging studies using FoxO murine models should clarify the contribution of these transcription factors to the age-related skeletal involution. Given the importance of both FoxO and ROS in aging and bone biology, understanding the cellular events and molecular pathways that are controlled by FoxOs during aging may be vital to our understanding of the regulation of age-related osteoporosis.
Highlights
FoxO transcription factors are critical players in bone homeostasis.
In mature osteoblast FoxO1 and FoxO3 attenuate oxidative stress and increase bone mass.
In osteoblast progenitors FoxOs divert β-catenin form TCF- to FoxO-mediated transcription and decrease osteoblastogenesis.
FoxOs attenuate osteoclast generation via indirect and cell autonomous mechanisms.
Acknowledgements
The author is grateful to Stavros Manolagas and Charles O'Brien for reviewing the manuscript. The author's work was supported by NIH Grants R01 AR056679 (to M.A.) and P01 AG13918 (to Stavros Manolagas).
Abbreviations
- AR
Androgen receptor
- ER
Estrogen receptor
- FoxO
Forkhead box O
- Opg
osteoprotegerin
- PPAR
peroxisome proliferator-activated receptor
- ROS
reactive oxygen species
- TCF
T-cell specific transcription factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–25. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- [2].Jacobs FM, van der Heide LP, Wijchers PJ, Burbach JP, Hoekman MF, Smidt MP. FoxO6, a novel member of the FoxO class of transcription factors with distinct shuttling dynamics. J Biol Chem. 2003;278:35959–67. doi: 10.1074/jbc.M302804200. [DOI] [PubMed] [Google Scholar]
- [3].Arden KC. FOXO animal models reveal a variety of diverse roles for FOXO transcription factors. Oncogene. 2008;27:2345–50. doi: 10.1038/onc.2008.27. [DOI] [PubMed] [Google Scholar]
- [4].Puig O, Mattila J. Understanding Forkhead Box Class O Function: Lessons from Drosophila melanogaster. Antioxid Redox Signal. 2011;14:635–47. doi: 10.1089/ars.2010.3407. [DOI] [PubMed] [Google Scholar]
- [5].Partridge L, Bruning JC. Forkhead transcription factors and ageing. Oncogene. 2008;27:2351–63. doi: 10.1038/onc.2008.28. [DOI] [PubMed] [Google Scholar]
- [6].Sahin E, DePinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–8. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- [8].Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- [9].Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annu Rev Immunol. 1997;15:351–69. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- [10].Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–47. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- [11].Kenyon C. A conserved regulatory system for aging. Cell. 2001;105:165–8. doi: 10.1016/s0092-8674(01)00306-3. [DOI] [PubMed] [Google Scholar]
- [12].Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282:27285–97. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Syed FA, Modder UI, Roforth M, Hensen I, Fraser DG, Peterson JM, et al. Effects of chronic estrogen treatment on modulating age-related bone loss in female mice. J Bone Miner Res. 2010;25:2438–46. doi: 10.1002/jbmr.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Halloran BP, Ferguson VL, Simske SJ, Burghardt A, Venton LL, Majumdar S. Changes in bone structure and mass with advancing age in the male C57BL/6J mouse. J Bone Miner Res. 2002;17:1044–50. doi: 10.1359/jbmr.2002.17.6.1044. [DOI] [PubMed] [Google Scholar]
- [15].Iida H, Fukuda S. Age-related changes in bone mineral density, cross-sectional area and strength at different skeletal sites in male rats. J Vet Med Sci. 2002;64:29–34. doi: 10.1292/jvms.64.29. [DOI] [PubMed] [Google Scholar]
- [16].Jilka RL, Almeida M, Ambrogini E, Han L, Roberson PK, Weinstein RS, et al. Decreased oxidative stress and greater bone anabolism in the aged, as compared to the young, murine skeleton by parathyroid hormone. Aging Cell. 2010;9:851–67. doi: 10.1111/j.1474-9726.2010.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lean JM, Davies JT, Fuller K, Jagger CJ, Kirstein B, Partington GA, et al. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Invest. 2003;112:915–23. doi: 10.1172/JCI18859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jagger CJ, Lean JM, Davies JT, Chambers TJ. Tumor necrosis factor-alpha mediates osteopenia caused by depletion of antioxidants. Endocrinology. 2005;146:113–8. doi: 10.1210/en.2004-1058. [DOI] [PubMed] [Google Scholar]
- [19].Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- [20].De Boer J, Andressoo JO, de Wit J, Huijmans J, Beems RB, van Steeg H, et al. Premature aging in mice deficient in DNA repair and transcription. Science. 2002;296:1276–9. doi: 10.1126/science.1070174. [DOI] [PubMed] [Google Scholar]
- [21].Ambrogini E, Almeida M, Martin-Millan M, Paik J, dePinho R, Han L, et al. FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell Metab. 2010;11:136–46. doi: 10.1016/j.cmet.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rached MT, Kode A, Xu L, Yoshikawa Y, Paik JH, DePinho RA, et al. FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts. Cell Metab. 2010;11:147–60. doi: 10.1016/j.cmet.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–7. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- [24].Tran H, Brunet A, Grenier JM, Datta SR, Fornace AJ, Jr., DiStefano PS, et al. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science. 2002;296:530–4. doi: 10.1126/science.1068712. [DOI] [PubMed] [Google Scholar]
- [25].Ramaswamy S, Nakamura N, Sansal I, Bergeron L, Sellers WR. A novel mechanism of gene regulation and tumor suppression by the transcription factor FKHR. Cancer Cell. 2002;2:81–91. doi: 10.1016/s1535-6108(02)00086-7. [DOI] [PubMed] [Google Scholar]
- [26].Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–21. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- [27].Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–2. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- [28].Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- [29].Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the proapoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHRL1. Curr Biol. 2000;10:1201–4. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- [30].Greer EL, Brunet A. FOXO transcription factors in ageing and cancer. Acta Physiol (Oxf) 2008;192:19–28. doi: 10.1111/j.1748-1716.2007.01780.x. [DOI] [PubMed] [Google Scholar]
- [31].Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27:2276–88. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- [32].de Keizer P, Burgering B, Dansen TB. FOXO as a sensor, mediator and regulator of redox signaling. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2010.3403. [DOI] [PubMed] [Google Scholar]
- [33].Obsil T, Obsilova V. Structural basis for DNA recognition by FOXO proteins. Biochim Biophys Acta. 2010 doi: 10.1016/j.bbamcr.2010.11.025. Epub ahead of print. doi:10.1016/j.bbamcr.2010.11.025. [DOI] [PubMed] [Google Scholar]
- [34].Huang H, Tindall DJ. Regulation of FOXO protein stability via ubiquitination and proteasome degradation. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbamcr.2011.01.007. Epub ahead of print. doi:10.1016/j.bbamcr.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Asada S, Daitoku H, Matsuzaki H, Saito T, Sudo T, Mukai H, et al. Mitogen-activated protein kinases, Erk and p38, phosphorylate and regulate Foxo1. Cell Signal. 2007;19:519–27. doi: 10.1016/j.cellsig.2006.08.015. [DOI] [PubMed] [Google Scholar]
- [36].Yang JY, Zong CS, Xia W, Yamaguchi H, Ding Q, Xie X, et al. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol. 2008;10:138–48. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Singh A, Ye M, Bucur O, Zhu S, Tanya SM, Rabinovitz I, et al. Protein phosphatase 2A reactivates FOXO3a through a dynamic interplay with 14-3-3 and AKT. Mol Biol Cell. 2010;21:1140–52. doi: 10.1091/mbc.E09-09-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yan L, Lavin VA, Moser LR, Cui Q, Kanies C, Yang E. PP2A regulates the pro-apoptotic activity of FOXO1. J Biol Chem. 2008;283:7411–20. doi: 10.1074/jbc.M708083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].van der HA, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007;8:440–50. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- [40].Essers MA, Weijzen S, Vries-Smits AM, Saarloos I, de Ruiter ND, Bos JL, et al. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 2004;23:4802–12. doi: 10.1038/sj.emboj.7600476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villen J, Becker EB, et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- [42].Wang MC, Bohmann D, Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell. 2005;121:115–25. doi: 10.1016/j.cell.2005.02.030. [DOI] [PubMed] [Google Scholar]
- [43].Oh SW, Mukhopadhyay A, Svrzikapa N, Jiang F, Davis RJ, Tissenbaum HA. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc Natl Acad Sci U S A. 2005;102:4494–9. doi: 10.1073/pnas.0500749102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sunayama J, Tsuruta F, Masuyama N, Gotoh Y. JNK antagonizes Akt-mediated survival signals by phosphorylating 14-3-3. J Cell Biol. 2005;170:295–304. doi: 10.1083/jcb.200409117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lee YH, Giraud J, Davis RJ, White MF. c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. J Biol Chem. 2003;278:2896–902. doi: 10.1074/jbc.M208359200. [DOI] [PubMed] [Google Scholar]
- [46].Bi W, Xiao L, Jia Y, Wu J, Xie Q, Ren J, et al. c-Jun N-terminal kinase enhances MST1-mediated pro-apoptotic signaling through phosphorylation at serine 82. J Biol Chem. 2010;285:6259–64. doi: 10.1074/jbc.M109.038570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Choi J, Oh S, Lee D, Oh HJ, Park JY, Lee SB, et al. Mst1-FoxO signaling protects Naive T lymphocytes from cellular oxidative stress in mice. PLoS ONE. 2009;4:e8011. doi: 10.1371/journal.pone.0008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–5. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- [49].van der HA, Vries-Smits AM, Brenkman AB, van Triest MH, van den BN, Colland F, et al. FOXO4 transcriptional activity is regulated by monoubiquitination and USP7/HAUSP. Nat Cell Biol. 2006;8:1064–73. doi: 10.1038/ncb1469. [DOI] [PubMed] [Google Scholar]
- [50].Brenkman AB, de Keizer PL, van den Broek NJ, Jochemsen AG, Burgering BM. Mdm2 induces mono-ubiquitination of FOXO4. PLoS ONE. 2008;3:e2819. doi: 10.1371/journal.pone.0002819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Frescas D, Valenti L, Accili D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem. 2005;280:20589–95. doi: 10.1074/jbc.M412357200. [DOI] [PubMed] [Google Scholar]
- [52].Kitamura YI, Kitamura T, Kruse JP, Raum JC, Stein R, Gu W, et al. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–63. doi: 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- [53].Zhao Y, Yang J, Liao W, Liu X, Zhang H, Wang S, et al. Cytosolic FoxO1 is essential for the induction of autophagy and tumour suppressor activity. Nat Cell Biol. 2010;12:665–75. doi: 10.1038/ncb2069. [DOI] [PubMed] [Google Scholar]
- [54].Dansen TB, Smits LM, van Triest MH, de Keizer PL, van Leenen D, Koerkamp MG, et al. Redox-sensitive cysteines bridge p300/CBP-mediated acetylation and FoxO4 activity. Nat Chem Biol. 2009;5:664–72. doi: 10.1038/nchembio.194. [DOI] [PubMed] [Google Scholar]
- [55].Edwards JR, Zainabadi K, Lwin ST, Elefteriou E, Munoz S, Moore MM, Guarente L, Mundy GR. The longevity gene SIRT-1 independently controls both osteoblast and osteoclast function. J Bone Miner Res. 2008;23:S28. [Google Scholar]
- [56].van der Vos KE, Coffer PJ. FOXO-binding partners: it takes two to tango. Oncogene. 2008;27:2289–99. doi: 10.1038/onc.2008.22. [DOI] [PubMed] [Google Scholar]
- [57].Seoane J, Le HV, Shen L, Anderson SA, Massague J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell. 2004;117:211–23. doi: 10.1016/s0092-8674(04)00298-3. [DOI] [PubMed] [Google Scholar]
- [58].Kitamura T, Kitamura YI, Funahashi Y, Shawber CJ, Castrillon DH, Kollipara R, et al. A Foxo/Notch pathway controls myogenic differentiation and fiber type specification. J Clin Invest. 2007;117:2477–85. doi: 10.1172/JCI32054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Dowell P, Otto TC, Adi S, Lane MD. Convergence of peroxisome proliferator-activated receptor gamma and Foxo1 signaling pathways. J Biol Chem. 2003;278:45485–91. doi: 10.1074/jbc.M309069200. [DOI] [PubMed] [Google Scholar]
- [60].Armoni M, Harel C, Karni S, Chen H, Bar-Yoseph F, Ver MR, et al. FOXO1 represses peroxisome proliferator-activated receptor-gamma1 and -gamma2 gene promoters in primary adipocytes. A novel paradigm to increase insulin sensitivity. J Biol Chem. 2006;281:19881–91. doi: 10.1074/jbc.M600320200. [DOI] [PubMed] [Google Scholar]
- [61].Nakae J, Kitamura T, Kitamura Y, Biggs WH, III, Arden KC, Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell. 2003;4:119–29. doi: 10.1016/s1534-5807(02)00401-x. [DOI] [PubMed] [Google Scholar]
- [62].Li P, Lee H, Guo S, Unterman TG, Jenster G, Bai W. AKT-independent protection of prostate cancer cells from apoptosis mediated through complex formation between the androgen receptor and FKHR. Mol Cell Biol. 2003;23:104–18. doi: 10.1128/MCB.23.1.104-118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Fan W, Yanase T, Morinaga H, Okabe T, Nomura M, Daitoku H, et al. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem. 2007;282:7329–38. doi: 10.1074/jbc.M610447200. [DOI] [PubMed] [Google Scholar]
- [64].Huang H, Muddiman DC, Tindall DJ. Androgens negatively regulate forkhead transcription factor FKHR (FOXO1) through a proteolytic mechanism in prostate cancer cells. J Biol Chem. 2004;279:13866–77. doi: 10.1074/jbc.M314143200. [DOI] [PubMed] [Google Scholar]
- [65].Ikonen T, Palvimo JJ, Janne OA. Interaction between the amino- and carboxyl-terminal regions of the rat androgen receptor modulates transcriptional activity and is influenced by nuclear receptor coactivators. J Biol Chem. 1997;272:29821–8. doi: 10.1074/jbc.272.47.29821. [DOI] [PubMed] [Google Scholar]
- [66].Ma Q, Fu W, Li P, Nicosia SV, Jenster G, Zhang X, et al. FoxO1 mediates PTEN suppression of androgen receptor N- and C-terminal interactions and coactivator recruitment. Mol Endocrinol. 2009;23:213–25. doi: 10.1210/me.2008-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Schuur ER, Loktev AV, Sharma M, Sun Z, Roth RA, Weigel RJ. Ligand-dependent interaction of estrogen receptor-alpha with members of the forkhead transcription factor family. J Biol Chem. 2001;276:33554–60. doi: 10.1074/jbc.M105555200. [DOI] [PubMed] [Google Scholar]
- [68].Zhao HH, Herrera RE, Coronado-Heinsohn E, Yang MC, Ludes-Meyers JH, Seybold-Tilson KJ, et al. Forkhead homologue in rhabdomyosarcoma functions as a bifunctional nuclear receptor-interacting protein with both coactivator and corepressor functions. J Biol Chem. 2001;276:27907–12. doi: 10.1074/jbc.M104278200. [DOI] [PubMed] [Google Scholar]
- [69].Zou Y, Tsai WB, Cheng CJ, Hsu C, Chung YM, Li PC, et al. Forkhead box transcription factor FOXO3a suppresses estrogen-dependent breast cancer cell proliferation and tumorigenesis. Breast Cancer Res. 2008;10:R21. doi: 10.1186/bcr1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Morelli C, Lanzino M, Garofalo C, Maris P, Brunelli E, Casaburi I, et al. Akt2 inhibition enables the forkhead transcription factor FoxO3a to have a repressive role in estrogen receptor alpha transcriptional activity in breast cancer cells. Mol Cell Biol. 2010;30:857–70. doi: 10.1128/MCB.00824-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Yang S, Xu H, Yu S, Cao H, Fan J, Ge C, et al. FOXO1 mediates IGF1/insulin regulation of osteocalcin expression by antagonizing RUNX2 in osteoblasts. J Biol Chem. 2011 doi: 10.1074/jbc.M110.197905. Epub ahead of print. doi/10.1074/jbc.M110.197905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Teixeira CC, Liu Y, Thant LM, Pang J, Palmer G, Alikhani M. Foxo1, a novel regulator of osteoblast differentiation and skeletogenesis. J Biol Chem. 2010;285:31055–65. doi: 10.1074/jbc.M109.079962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zhang H, Pan Y, Zheng L, Choe C, Lindgren B, Jensen ED, et al. FOXO1 Inhibits Runx2 Transcriptional Activity and Prostate Cancer Cell Migration and Invasion. Cancer Res. 2011;71:3257–67. doi: 10.1158/0008-5472.CAN-10-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Essers MA, Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science. 2005;308:1181–4. doi: 10.1126/science.1109083. [DOI] [PubMed] [Google Scholar]
- [75].Almeida M, Han L, Martin-Millan M, O'Brien CA, Manolagas SC. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J Biol Chem. 2007;282:27298–305. doi: 10.1074/jbc.M702811200. [DOI] [PubMed] [Google Scholar]
- [76].Furuyama T, Kitayama K, Shimoda Y, Ogawa M, Sone K, Yoshida-Araki K, et al. Abnormal angiogenesis in Foxo1 (Fkhr)-deficient mice. J Biol Chem. 2004;279:34741–9. doi: 10.1074/jbc.M314214200. [DOI] [PubMed] [Google Scholar]
- [77].Hosaka T, Biggs WH, III, Tieu D, Boyer AD, Varki NM, Cavenee WK, et al. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci U S A. 2004;101:2975–80. doi: 10.1073/pnas.0400093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Cheng Z, White MF. Targeting Forkhead box O1 from the concept to metabolic diseases: lessons from mouse models. Antioxid Redox Signal. 2011;14:649–61. doi: 10.1089/ars.2010.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Robertson RP, Harmon JS. Diabetes, glucose toxicity, and oxidative stress: A case of double jeopardy for the pancreatic islet beta cell. Free Radic Biol Med. 2006;41:177–84. doi: 10.1016/j.freeradbiomed.2005.04.030. [DOI] [PubMed] [Google Scholar]
- [80].Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–12. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- [81].Marinkovic D, Zhang X, Yalcin S, Luciano JP, Brugnara C, Huber T, et al. Foxo3 is required for the regulation of oxidative stress in erythropoiesis. J Clin Invest. 2007;117:2133–44. doi: 10.1172/JCI31807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Obsil T, Obsilova V. Structure/function relationships underlying regulation of FOXO transcription factors. Oncogene. 2008;27:2263–75. doi: 10.1038/onc.2008.20. [DOI] [PubMed] [Google Scholar]
- [83].Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–39. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- [84].Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–23. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Paik JH, Ding Z, Narurkar R, Ramkissoon S, Muller F, Kamoun WS, et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. 2009;5:540–53. doi: 10.1016/j.stem.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Renault VM, Rafalski VA, Morgan AA, Salih DA, Brett JO, Webb AE, et al. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. 2009;5:527–39. doi: 10.1016/j.stem.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sengupta A, Molkentin JD, Paik JH, DePinho RA, Yutzey KE. FoxO transcription factors promote cardiomyocyte survival upon induction of oxidative stress. J Biol Chem. 2011;286:7468–78. doi: 10.1074/jbc.M110.179242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, et al. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122:2170–82. doi: 10.1161/CIRCULATIONAHA.110.958033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–71. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–33. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- [91].Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–13. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- [92].Trinei M, Berniakovich I, Beltrami E, Migliaccio E, Fassina A, Pelicci P, et al. P66Shc signals to age. Aging (Albany NY) 2009;1:503–10. doi: 10.18632/aging.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Almeida M, Han L, Ambrogini E, Bartell SM, Manolagas SC. Oxidative Stress Stimulates Apoptosis and Activates NF-kappaB in Osteoblastic Cells via a PKCbeta/p66shc Signaling Cascade: Counter Regulation by Estrogens or Androgens. Mol Endocrinol. 2010;24:2030–7. doi: 10.1210/me.2010-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Tothova Z, Gilliland DG. FoxO transcription factors and stem cell homeostasis: insights from the hematopoietic system. Cell Stem Cell. 2007;1:140–52. doi: 10.1016/j.stem.2007.07.017. [DOI] [PubMed] [Google Scholar]
- [95].Southgate RJ, Neill B, Prelovsek O, El Osta A, Kamei Y, Miura S, et al. FOXO1 regulates the expression of 4E-BP1 and inhibits mTOR signaling in mammalian skeletal muscle. J Biol Chem. 2007;282:21176–86. doi: 10.1074/jbc.M702039200. [DOI] [PubMed] [Google Scholar]
- [96].Rached MT, Kode A, Silva BC, Jung DY, Gray S, Ong H, et al. FoxO1 expression in osteoblasts regulates glucose homeostasis through regulation of osteocalcin in mice. J Clin Invest. 2010;120:357–68. doi: 10.1172/JCI39901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Clemens TL, Karsenty G. The osteoblast: An insulin target cell controlling glucose homeostasis. J Bone Miner Res. 2010;26:677–80. doi: 10.1002/jbmr.321. [DOI] [PubMed] [Google Scholar]
- [98].Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–68. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Backesjo CM, Li Y, Lindgren U, Haldosen LA. Activation of Sirt1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells. J Bone Miner Res. 2006;21:993–1002. doi: 10.1359/jbmr.060415. [DOI] [PubMed] [Google Scholar]
- [100].Mizutani K, Ikeda K, Kawai Y, Yamori Y. Resveratrol stimulates the proliferation and differentiation of osteoblastic MC3T3-E1 cells. Biochem Biophys Res Commun. 1998;253:859–63. doi: 10.1006/bbrc.1998.9870. [DOI] [PubMed] [Google Scholar]
- [101].Biswas DK, Singh S, Shi Q, Pardee AB, Iglehart JD. Crossroads of estrogen receptor and NF-kappaB signaling. Sci STKE. 2005;2005:e27. doi: 10.1126/stke.2882005pe27. [DOI] [PubMed] [Google Scholar]
- [102].Adler AS, Sinha S, Kawahara TL, Zhang JY, Segal E, Chang HY. Motif module map reveals enforcement of aging by continual NF-kappaB activity. Genes Dev. 2007;21:3244–57. doi: 10.1101/gad.1588507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].De Bosscher K, Vanden Berghe W, Haegeman G. Cross-talk between nuclear receptors and nuclear factor kappaB. Oncogene. 2006;25:6868–86. doi: 10.1038/sj.onc.1209935. [DOI] [PubMed] [Google Scholar]
- [104].Smith WW, Norton DD, Gorospe M, Jiang H, Nemoto S, Holbrook NJ, et al. Phosphorylation of p66Shc and forkhead proteins mediates Abeta toxicity. J Cell Biol. 2005;169:331–9. doi: 10.1083/jcb.200410041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–37. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- [106].Hoogeboom D, Essers MA, Polderman PE, Voets E, Smits LM, Burgering BM. Interaction of FOXO with beta-catenin inhibits beta-catenin/T cell factor activity. J Biol Chem. 2008;283:9224–30. doi: 10.1074/jbc.M706638200. [DOI] [PubMed] [Google Scholar]
- [107].Liu H, Fergusson MM, Wu JJ, Rovira II, Liu J, Gavrilova O, et al. Wnt signaling regulates hepatic metabolism. Sci Signal. 2011;4:ra6. doi: 10.1126/scisignal.2001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Schneider C, Porter NA, Brash AR. Routes to 4-hydroxynonenal: fundamental issues in the mechanisms of lipid peroxidation. J Biol Chem. 2008;283:15539–43. doi: 10.1074/jbc.R800001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Almeida M, Ambrogini E, Han L, Manolagas SC, Jilka RL. Increased lipid oxidation causes oxidative stress, increased PPAR{gamma} expression and diminished pro-osteogenic Wnt signaling in the skeleton. J Biol Chem. 2009;284:27438–48. doi: 10.1074/jbc.M109.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Kang S, Bennett CN, Gerin I, Rapp LA, Hankenson KD, MacDougald OA. Wnt Signaling Stimulates Osteoblastogenesis of Mesenchymal Precursors by Suppressing CCAAT/Enhancer-binding Protein α and Peroxisome Proliferator-activated Receptor γ. J Biol Chem. 2007;282:14515–24. doi: 10.1074/jbc.M700030200. [DOI] [PubMed] [Google Scholar]
- [111].Okamura M, Kudo H, Wakabayashi K, Tanaka T, Nonaka A, Uchida A, et al. COUP-TFII acts downstream of Wnt/beta-catenin signal to silence PPARgamma gene expression and repress adipogenesis. Proc Natl Acad Sci U S A. 2009;106:5819–24. doi: 10.1073/pnas.0901676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Sharma C, Pradeep A, Wong L, Rana A, Rana B. Peroxisome proliferator-activated receptor gamma activation can regulate beta-catenin levels via a proteasome-mediated and adenomatous polyposis coli-independent pathway. J Biol Chem. 2004;279:35583–94. doi: 10.1074/jbc.M403143200. [DOI] [PubMed] [Google Scholar]
- [113].Ambrogini E, Han L, Bartell S, Warren A, Shelton R, Vyas K, Deloose A, Weinstein R, O'Brien C, Manolagas S, Almeida M. Deletion of the FoxO1, 3 a nd 4 genes from commited osteoblast progenitors expressing osterix increases Wnt signaling and bone mass. J Bone Min Res. 2010;25:S23. [Google Scholar]
- [114].Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A. 2005;102:3324–9. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Babij P, Zhao W, Small C, Kharode Y, Yaworsky PJ, Bouxsein ML, et al. High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res. 2003;18:960–74. doi: 10.1359/jbmr.2003.18.6.960. [DOI] [PubMed] [Google Scholar]
- [116].Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- [117].Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–55. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–9. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- [119].Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG. Inactivation of peroxiredoxin I by phosphorylation allows localized H(2)O(2) accumulation for cell signaling. Cell. 2010;140:517–28. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- [120].Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest. 1990;85:632–9. doi: 10.1172/JCI114485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Levasseur R, Barrios R, Elefteriou F, Glass DA, Lieberman MW, Karsenty G. Reversible skeletal abnormalities in gamma-glutamyl transpeptidase-deficient mice. Endocrinology. 2003;144:2761–4. doi: 10.1210/en.2002-0071. [DOI] [PubMed] [Google Scholar]
- [122].Bai XC, Lu D, Liu AL, Zhang ZM, Li XM, Zou ZP, et al. Reactive oxygen species stimulates receptor activator of NF-kappaB ligand expression in osteoblast. J Biol Chem. 2005;280:17497–506. doi: 10.1074/jbc.M409332200. [DOI] [PubMed] [Google Scholar]
- [123].Lean JM, Jagger CJ, Kirstein B, Fuller K, Chambers TJ. Hydrogen peroxide is essential forestrogen-deficiency bone loss and osteoclast formation. Endocrinology. 2005;146:728–35. doi: 10.1210/en.2004-1021. [DOI] [PubMed] [Google Scholar]
- [124].Ha H, Kwak HB, Lee SW, Jin HM, Kim HM, Kim HH, et al. Reactive oxygen species mediate RANK signaling in osteoclasts. Exp Cell Res. 2004;301:119–27. doi: 10.1016/j.yexcr.2004.07.035. [DOI] [PubMed] [Google Scholar]
- [125].Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, et al. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 2005;106:852–9. doi: 10.1182/blood-2004-09-3662. [DOI] [PubMed] [Google Scholar]
- [126].Ishii KA, Fumoto T, Iwai K, Takeshita S, Ito M, Shimohata N, et al. Coordination of PGC-1beta and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat Med. 2009;15:259–66. doi: 10.1038/nm.1910. [DOI] [PubMed] [Google Scholar]
- [127].Srinivasan S, Koenigstein A, Joseph J, Sun L, Kalyanaraman B, Zaidi M, et al. Role of mitochondrial reactive oxygen species in osteoclast differentiation. Ann N Y Acad Sci. 2010;1192:245–52. doi: 10.1111/j.1749-6632.2009.05377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Ambrogini E, Bartell S, Han L, Zhao H, Warren A, Shelton R, Qiu X, Goellner J, O'Brien C, Almeida Maria, Manolagas S. Gain of FoxO function in osteoclast precursors and their progeny decreases osteoclastogenesis and increases BMD in mice. J Bone Min Res. 2010;25:S15. [Google Scholar]
- [129].Sugatani T, Hruska KA. Akt1/Akt2 and mammalian target of rapamycin/Bim play critical roles in osteoclast differentiation and survival, respectively, whereas Akt is dispensable for cell survival in isolated osteoclast precursors. J Biol Chem. 2005;280:3583–9. doi: 10.1074/jbc.M410480200. [DOI] [PubMed] [Google Scholar]
- [130].Kawamura N, Kugimiya F, Oshima Y, Ohba S, Ikeda T, Saito T, et al. Akt1 in osteoblasts andosteoclasts controls bone remodeling. PLoS ONE. 2007;2:e1058. doi: 10.1371/journal.pone.0001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Wong BR, Besser D, Kim N, Arron JR, Vologodskaia M, Hanafusa H, et al. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol Cell. 1999;4:1041–9. doi: 10.1016/s1097-2765(00)80232-4. [DOI] [PubMed] [Google Scholar]
- [132].Reszka AA, Halasy-Nagy JM, Masarachia PJ, Rodan GA. Bisphosphonates act directly on the osteoclast to induce caspase cleavage of mst1 kinase during apoptosis. A link between inhibition of the mevalonate pathway and regulation of an apoptosis-promoting kinase. J Biol Chem. 1999;274:34967–73. doi: 10.1074/jbc.274.49.34967. [DOI] [PubMed] [Google Scholar]
- [133].Jang SW, Yang SJ, Srinivasan S, Ye K. Akt phosphorylates MstI and prevents its proteolytic activation, blocking FOXO3 phosphorylation and nuclear translocation. J Biol Chem. 2007;282:30836–44. doi: 10.1074/jbc.M704542200. [DOI] [PubMed] [Google Scholar]
- [134].Boissy P, Andersen TL, Abdallah BM, Kassem M, Plesner T, Delaisse JM. Resveratrol inhibits myeloma cell growth, prevents osteoclast formation, and promotes osteoblast differentiation. Cancer Res. 2005;65:9943–52. doi: 10.1158/0008-5472.CAN-05-0651. [DOI] [PubMed] [Google Scholar]
- [135].He X, Andersson G, Lindgren U, Li Y. Resveratrol prevents RANKL-induced osteoclast differentiation of murine osteoclast progenitor RAW 264.7 cells through inhibition of ROS production. Biochem Biophys Res Commun. 2010;401:356–62. doi: 10.1016/j.bbrc.2010.09.053. [DOI] [PubMed] [Google Scholar]
- [136].Shakibaei M, Buhrmann C, Mobasheri A. Resveratrol-mediated SIRT-1 Interactions with p300 Modulate Receptor Activator of NF-{kappa}B Ligand (RANKL) Activation of NF-{kappa}B Signaling and Inhibit Osteoclastogenesis in Bone-derived Cells. J Biol Chem. 2011;286:11492–505. doi: 10.1074/jbc.M110.198713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Su JL, Yang CY, Zhao M, Kuo ML, Yen ML. Forkhead proteins are critical for bone morphogenetic protein-2 regulation and anti-tumor activity of resveratrol. J Biol Chem. 2007;282:19385–98. doi: 10.1074/jbc.M702452200. [DOI] [PubMed] [Google Scholar]
- [138].Glass DA, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, et al. Canonical wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell. 2005;8:751–64. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- [139].Holmen SL, Zylstra CR, Mukherjee A, Sigler RE, Faugere MC, Bouxsein ML, et al. Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem. 2005;280:21162–8. doi: 10.1074/jbc.M501900200. [DOI] [PubMed] [Google Scholar]
- [140].Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]