Abstract
Vascular smooth muscle cells (VSMCs) at capacitance arteries of hypertensive individuals and animals undergo marked age- and blood pressure–dependent polyploidization and hypertrophy. We show here that VSMCs at capacitance arteries of rat models of hypertension display high levels of Akt1/PKB protein and activity. Gene transfer of Akt1 to VSMCs isolated from a normotensive rat strain was sufficient to abrogate the activity of the mitotic spindle cell–cycle checkpoint, promoting polyploidization and hypertrophy. Furthermore, the hypertrophic agent angiotensin II induced VSMC polyploidization in an Akt1-dependent manner. These results demonstrate that Akt1 regulates ploidy levels in VSMCs and contributes to vascular smooth muscle polyploidization and hypertrophy during hypertension.
Introduction
Aging is accompanied at the vascular system by changes in the morphology and hemodynamic properties of arterial vessels. These changes are specific for precise areas of the vascular tree. At capacitance arteries, the increase in mass and rigidity of the arterial wall contributes to systolic hypertension and constitutes an independent risk for left ventricular hypertrophy (1–3). Vascular smooth muscle cell (VSMC) hypertrophy with minimal hyperplasia accounts for the increase in smooth muscle mass in large arteries during hypertension (4, 5). VSMC hypertrophy is strongly associated with polyploidization (6–10). Polyploid VSMCs have up to fivefold the mass of diploid VSMCs (6) and, on a per cell basis, express higher levels of PDGF A, fibronectin, and collagen III than their diploid counterparts (11). Several stimuli, including catecholamines (12) and angiotensin II (13–17), have been shown to promote VSMC polyploidization. However, the molecular mechanisms that underlie the onset of VSMC polyploidization have not yet been characterized.
Mammalian cells are protected from polyploidization by the activity of the mitotic spindle cell–cycle checkpoint. This pathway prevents the onset of anaphase and the exit from mitosis until metaphase has been properly completed (18–20). The precise mechanism by which the mitotic checkpoint controls the progression and exit from mitosis (M phase) is not entirely understood, but it appears to involve a delay in the activation of the anaphase-promoting complex (APC). At the mitotic exit, the APC, also known as cyclosome, promotes the degradation of cyclin B by the mitotic proteosome, with the resulting inactivation of the M phase–promoting complex (MPF; a complex of cyclin B, Cdc2, and associated proteins) (21).
Here, we have investigated the role of Akt1, also known as PKB, in VSMC polyploidization. Akt1 is a serine/threonine protein kinase that contains a region homologous to a pleckstrin domain found in multiple signaling molecules (22, 23). Akt1 is stimulated by a number of receptor tyrosine kinases, including receptors for IGF, NGF, PDGF, VEGF, angiotensin, and insulin, by the action of phosphatidylinositol 3-kinase (PI 3-kinase) (22–26). Akt1 has diverse regulatory functions. It promotes glucose transport through translocation of GLUT1 and GLUT4 to the plasma membrane (27, 28) and controls glycogen synthesis by insulin-dependent phosphorylation and inactivation of GSK-3 (29). Akt1 has also been shown to inhibit apoptosis in multiple cell types (25, 30–35). The antiapoptotic activities of Akt1 are thought to be mediated by phosphorylation of the proapoptotic protein BAD (36–38), procaspase 9 (39), and a forkhead transcription factor (40–43). Akt1 also phosphorylates endothelial cell nitric oxide synthase, which regulates vasomotor tone (21).
We report here a new function of Akt1, the regulation of ploidy in vascular smooth muscle. We show that Akt1 protein levels and activity are elevated in VSMCs at capacitance arteries of hypertensive rats. Since Akt1 is well known to provide signals that promote cell growth (44–47), we hypothesized that an increase in Akt1 activity may be involved in the onset of VSMC polyploidization. To test this hypothesis, we generated populations of primary VSMCs overexpressing a wild-type or a catalytic inactive Akt1 mutant and investigated the ability of these cells to control the progression of mitosis. VSMCs with enhanced Akt1 expression underwent unscheduled cyclin B degradation and reentered the cell cycle at a tetraploid DNA content, generating polyploid and hypertrophic cells. Furthermore, angiotensin II, an activator of Akt1 in VSMCs (26, 48–50), induced Akt1-dependent VSMC polyploidization. These results demonstrate that Akt1 is a positive regulator of the progression of mitosis in VSMCs and implicate this protein in the mechanism of hypertension-related VSMC polyploidization and hypertrophy.
Methods
Animals and histology.
Spontaneously hypertensive rats (SHR) and Wistar Kyoto (WKY) rats were obtained from Charles River Laboratories (Wilmington, Massachusetts, USA). Zucker rats were obtained from Harland Sprague Dawley (Indianapolis, Indiana, USA). Two-kidney one-clip Goldblatt WKY rats were purchased from Taconic Farms (Germantown, New York, USA). Rats were sacrificed in accordance with American Veterinary Medicine Association recommendations and tissues rapidly processed for culture, histology, or extract preparation. Blood pressures were determined using tail cuffs and a programmed electrosphygmomanometer following the recommendations of the manufacturer (Narco Bio-Systems, Austin, Texas, USA). For histology, arteries were fixed in 10% formalin for a minimum of 12 hours and embedded in paraffin. Consecutive 5-μm tissue sections were deparaffinized in xylene, rehydrated in a series of increasingly diluted solutions of ethanol, and processed for Feulgen and periodic acid-Schiff–hematoxylin (PAS-hematoxylin) staining (51). Quantitative DNA analysis was carried out using a CAS200 microdensitometer (Becton Dickinson, San Jose, California, USA). Three hundred VSMC nuclei were quantified per sample. At our measurement conditions and using human bladder cells as standard, we regarded VSMCs with a DNA content of 6–9 pg DNA per nucleus as diploid cells, cells with 12–18 pg DNA per nucleus as tetraploid, and cells with a DNA content over 18 pg as octaploid (8, 52). Immunohistochemistry was carried out using Vectastain-ABC kits (Vector Laboratories, Burlingame, Massachusetts, USA) and Akt1 Ab (Santa Cruz Biotechnologies, Santa Cruz, California, USA) at a dilution of 1:50.
Isolation, infection, and culture of VSMCs.
VSMCs were isolated following the method described in detail by Owens and coworkers (53). Briefly, vessels were excised, placed in PBS, and adhering fat and connective tissue removed by dissection. Vessels were then preincubated at 37°C for 15 minutes in PBS with 1 mg/ml collagenase II (Worthington, Freehold, New Jersey, USA), 0.5 mg/ml elastase I (Worthington), 100 U/ml penicillin, and streptomycin (100 μg/ml; protease solution) in 5% CO2. Their adventitia was then carefully removed under the dissecting microscope. Vessels were subsequently opened longitudinally and the luminal endothelia scraped with forceps. The smooth muscle layer was then minced into 2-mm sections and digested in fresh protease solution for 2 hours, as above. The cellular suspension was filtered through a mess and cells were pelleted, washed three times in PBS, seeded at a density of 3,500 cells/cm2, and grown in DMEM media supplemented with 10% FBS and penicillin/streptomycin (Life Technologies Inc., Gaithersburg, Maryland, USA). The VSMC phenotype was verified by immunostaining using specific Ab’s against smooth muscle α-actin and smooth muscle myosin heavy chain, as described previously (53, 54). Population-doubling times were determined by plating VSMCs at low density and determining the cell number over several days. For that purpose, cell counts from several dishes were averaged daily using a Coulter model ZF cell counter beginning 1 day after plating.
Replication-defective adenovirus constructs expressing mouse Akt1 proteins under the control of the cytomegalovirus (CMV) promoter were as described previously (25, 35). The AA Akt1 construct contains the mutations T308A and S473A and cannot be activated by phosphorylation (55). Ad-β Gal expresses the bacterial β-galactosidase gene from the CMV promoter. Adenoviral constructs were amplified in 293 cells and purified by ultracentrifugation through a CsCl gradient. VSMCs were infected for 24 hours at a moi of 1–100 (25, 35).
Akt1 kinase assays.
Akt1 activity was measured using the method described by Tsichlis and coworkers (24). Briefly, vascular smooth muscle was disrupted using a Branson sonifier at setting 4 in lysis buffer (1% NP-40, 10% glycerol, 137 mM NaCl, 20 mM Tris-HCl at pH 7.4) containing 5 μg/ml of the protease inhibitors aprotinin and leupeptin, 1 mM PMSF, 1 mM NaF, 1 mM sodium pyrophosphate, and 1 mM sodium orthovanadate. Lysates (100 μg protein) were precleared by centrifugation and preabsorbed with protein A/G agarose slurry. Immunoprecipitation of Akt1 was carried out for 4 hours using a specific Ab (Santa Cruz). Immunoprecipitates were washed three times with lysis buffer, once with water, and once with kinase buffer. Kinase assays were performed in Akt kinase buffer (20 mM HEPES-NaOH, 10 mM magnesium chloride, and 10 mM manganese chloride). Kinase activity was assayed for 20 minutes at 30°C in a 30-μl volume of kinase buffer with 5 μM ATP, 100 μg/ml histone 2B, and 20 μCi [γ-32]ATP per sample. Reactions were stopped by an equal volume of 2× SDS buffer-PAGE sample buffer. The products of the in vitro kinase assay were analyzed by 15% SDS/PAGE and exposure to PhosphorImager screens.
VSMC fractionation in Percoll density gradients.
Cell fractionation was performed as described previously (56) using Percoll gradients (Pharmacia Biotech AB, Uppsala, Sweden) diluted in PBS, pH 7.4, containing 5 mg/ml BSA (Sigma Chemical Co., St. Louis, Missouri, USA). Thirty to seventy percent gradients were prepared fresh in 15-ml centrifuge tubes by layering from below, upwards 1 ml, 70%; 3 ml, 52%; 4 ml, 42%; and 5 ml, 30% Percoll. VSMCs (107 Akt1 wild-type transduced) were resuspended in DMEM media, loaded onto the gradients, and centrifuged at 20,000 g for 1 hour. Density bands were determined using Pharmacia Biotech AB marker beads. Low ploidy factions were obtained at the 1.08 g/l density band of exponentially growing VSMCs. High ploidy fractions were obtained at the 1.04 g/l density band of 1-week colcemid-treated (100 ng/ml) VSMCs. Cell fractions were isolated by pipetting, washed twice in DMEM media, and processed for flow cytometry or Western analysis.
Analysis of cell-cycle checkpoint status.
Matched sets of VSMC populations were incubated for a 1–2 population-doubling time periods in media with the mitotic spindle inhibitor colcemid (100 ng/ml). Cell-cycle distribution of DNA content was determined by flow cytometry as described previously (57, 58). When both total DNA content and newly synthesized DNA were determined, cells were labeled with 10 μM bromodeoxyuridine (BrdU) for 4 hours, trypsinized, counted, and fixed using 70% ethanol. Fixed cells were centrifuged and treated with 0.08% pepsin for the preparation of nuclei. The nuclear pellet was resuspended in 100 μl of a 1:5 dilution of anti-BrdU FITC-conjugated Ab (Becton Dickinson), incubated for 30 minutes, washed, stained with 50 μg/ml propidium iodide (Aldrich Chemical Co., Milwaukee, Wisconsin, USA), and analyzed by flow cytometry for cell-cycle distribution of DNA content. Flow cytometry was carried out using a Coulter Elite ESP flow cytometer and analyzed using CellQuest software (Becton Dickinson), and cells (104– 4 × 104 per sample) were analyzed.
For karyotyping (57), exponentially growing cells were exposed to 100 ng/ml colcemid for two population-doubling times, and then collected and incubated at room temperature for 30 minutes in hypotonic KCl plus sodium citrate, followed by fixation in methanol/acetic acid and staining with Giemsa; 16–39 spreads per VSMC population were examined.
Western blots.
For Western blot analysis, cells were harvested and lysed in 1 ml lysis buffer-PBS containing 1% Triton X-100, 0.1% SDS, 1mM DTT, 1mM PMSF, and 1 μg/ml of the protease inhibitors aprotinin, leupeptin, and pepstatin A, followed by centrifugation at 1500 g for 5 minutes. Equal amounts of proteins were assayed at each condition as determined by Bradford protein assay (Bio-Rad, Hercules, California, USA). Electrophoresis was carried out at 20 mA constant current in 15% PAGE (Bio-Rad). Proteins were transferred to Immobilon-P membranes (Millipore Corp., Bedford, Massachusetts, USA) and probed, following the recommendation of the manufacturers. Anti-Akt1 (Santa Cruz), anti-cyclin B (Santa Cruz), and anti-β actin (Sigma Chemical Co.) Ab’s were employed at dilution of 1:500, 1:500, and 1:10,000, respectively, in PBS-5% dry milk. Membranes were hybridized overnight at 4°C. For detection, membranes were incubated for 1 hour in 1:10,000 or 1:5,000 dilutions of horseradish peroxidase–linked (HRP-linked) IgG (Santa Cruz). HRP-luminescence reactions were carried out using the enhanced chemiluminescence (ECL) kit (Amersham Corp., Burlington, Massachusetts, USA). Membranes were exposed to Hyperfilm (Eastman Kodak Co. Scientific Imaging Systems, New Haven, Connecticut, USA) and protein bands detected by autoradiography. Low-exposure autoradiographs were scanned with a densitometer (LKB-Wallac, Stockholm, Sweden) to determine peak areas.
Determination of VSMC hypertrophy.
[3H] leucine incorporation was carried out as described (26). Briefly, VSMCs were made quiescent by incubation in 1% DMEM for 72 hours. Twenty-four hours before harvesting, [3H] leucine (1 μCi/ml) (NEN Life Science Products, Boston, Massachusetts, USA) was added to the media. VSMCs were washed twice in PBS, proteins precipitated in 5% trichloroacetic acid, and [3H] leucine incorporation determined using a LS 3801 scintillation counter (Beckman Instruments Inc., Fullerton, California, USA). Forward-scatter cell fluorescence and side-scatter cell fluorescence were determined using a Coulter Elite ESP flow cytometer and analyzed using CellQuest software (Becton Dickinson). VSMCs (4× 104 per sample) were analyzed.
Results
Akt1 protein and activity are upregulated in VSMCs of three animal models of hypertension.
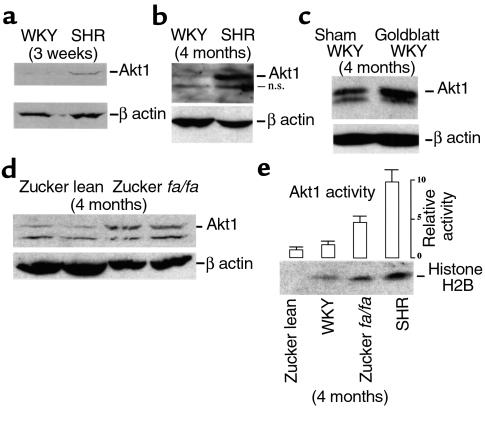
Akt1 protein expression was analyzed in VSMCs of hypertensive rats by Western blot analysis. Initial experiments compared SHR to normotensive WKY rats. SHR rates develop hypertension spontaneously and without exception by 7–15 weeks of age (59). Vascular smooth muscle extracts were prepared from the aortas of 3-week-old and 4-month-old WKY and SHR rats and processed for Western blot analysis. Markedly greater Akt1 protein levels, approximately fivefold, were found in the SHR strain at both stages (Figure 1, a and b). In the older animals, an additional faster-migrating band was observed. This band was reactive with our secondary Ab and was designated “nonspecific” (Figure 1, n.s.). To test whether genetic divergence might account for the differences in Akt1 protein expression between WKY and SHR rats, two additional models of experimental hypertension were investigated. In one of these models, the renin-angiotensin system was activated in WKY rats by clipping the left renal artery (referred to as Goldblatt’s operation), a procedure that has been shown to induce VSMC hypertrophy and polyploidization at capacitance arteries (17). Enhanced expression of Akt1 protein was observed in smooth muscle extracts from the aortas of Goldblatt rats relative to their mock-operated controls (Figure 1c). We also investigated the expression level of Akt1 protein in Zucker lean and fa/fa rats. The Zucker fa/fa rat is a model of obesity and moderate hypertension (60). Three-week-old animals expressed barely detectable Akt1 protein levels, with no differences between Zucker lean and fa/fa rats (not shown). However, threefold higher levels of Akt1 protein were found at 4 months in the hypertensive fa/fa group (Figure 1d and Table 1). Furthermore, the increase in Akt1 protein level was accompanied by enhanced Akt1 kinase activity. Akt1 assays were performed using vascular smooth muscle extracts from 4-month-old WKY, SHR, and Zucker rats. The results of these experiments, shown in Figure 1e, demonstrated that changes in Akt1 kinase activity in vivo correlate with those of Akt1 protein levels. Maximal Akt1 kinase activity was found in SHR rats, approximately fourfold higher than age-matched WKY controls.
Figure 1.
Akt1 protein levels and activity are upregulated in vascular smooth muscle of hypertensive rats. (a–d) Western blot analysis of Akt1 protein in aortic VSMCs freshly isolated from 3-week-old WKY and SHR rats (a), 4-month-old WKY and SHR rats (b), 4-month-old WKY and WKY-Goldblatt rats (c), and 4-month-old Zucker lean and fa/fa rats (d). As a control, blots were probed for β-actin expression. Each lane represents Akt1/β-actin levels in protein extracts of VSMCs isolated from the upper two-thirds of the thoracic aorta of nine animals; n.s., nonspecific, band reactive with secondary Ab. Western blotting was carried out as indicated in Methods. Three hundred fifty micrograms of protein extract was employed in gel in c and 200 μg in gels used in a, b, and d. Goldblatt-WKY rats underwent left renal artery constriction for 6 weeks (mean blood pressure [MBP] = 123 ± 4, sham-operated; MBP = 180 ± 12, Goldblatt). MBPs of WKY, SHR, and Zucker animals were within the range shown in Table 1. Parts a and b are representative of three preparations and c is representative of two preparations. (d) Two independent preparations of Zucker lean and fa/fa rats. (e) Akt1 activity in 4-month-old control (WKY and Zucker lean) and hypertensive rats (SHR and Zucker fa/fa). Activity was measured as histone H2B phosphorylation, as indicated in Methods. Bars indicate PhosphorImager scans of phosphorylated histone H2B bands in arbitrary units relative to Akt1 activity in extracts of Zucker lean animals. Data are representative of three independent experiments.
Table 1.
Frequency of polyploid VSMCs in sections of proximal aortas isolated from control and hypertensive rats
The development of hypertension in SHR and Goldblatt WKY rats is accompanied by VSMC polyploidization at capacitance arteries (6, 17), but this phenomenon has not been reported previously in Zucker fa/fa rats. Therefore, ploidy levels were determined in aortas of Zucker fa/fa rats using in situ DNA microdensitometry. WKY and Zucker lean rats were employed as negative controls and SHR rats as positive controls. Table 1 shows that hypertension in Zucker fa/fa rats is accompanied by VSMC polyploidization. Together, the results shown in Figure 1 and Table 1 demonstrate a correlation between hypertension, polyploidization, and elevated levels of Akt1 protein and activity in VSMCs.
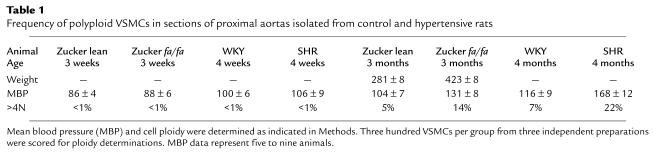
Previous studies have shown that the phenomena of VSMC polyploidization and hypertrophy are limited to vessels of capacitance function, such as the aorta and carotid arteries (52, 61–63). Thus, we also investigated whether Akt1 protein was upregulated differentially depending upon localization within the vascular tree. Consistent with a role in hypertension-related hypertrophy and polyploidization, high levels of Akt1 protein expression were detected by Western analysis in the aorta and carotid arteries of SHR (Figure 2). In contrast, low levels of Akt1 protein were detected at small arteries, such as III and IV branches of the superior mesenteric artery. Moreover, immunohistochemical assays demonstrated that Akt1 upregulation was restricted to the medial smooth muscle layer of capacitance arteries (upper aorta), with background staining at the vascular adventitia (Figure 2).
Figure 2.
Akt1 upregulation is specifically localized to capacitance arteries. (a) Levels of Akt1 protein in vascular smooth muscle of 3-week-old SHR rats. Western blot analysis was performed as indicated in Figure 1. Figure is representative of two experiments. (b) Akt1 immunohistochemistry in longitudinal sections of arterial vessels dissected from 3-week-old WKY and SHR rats. ×250. Immunohistochemistry was performed as indicated in Methods. Microphotographs are representative of at least ten slides per four animals.
Akt1 promotes VSMC endoreduplication.
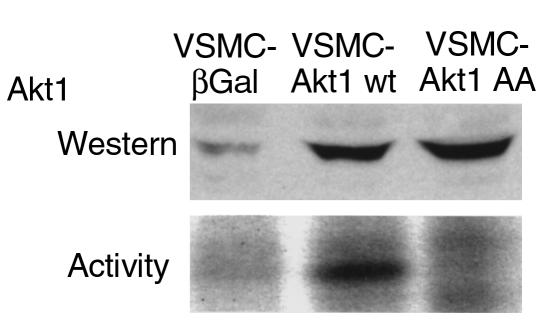
In view of these results and the reported pro-proliferative and antiapoptotic properties of Akt1 (64), we hypothesized that an increase in Akt1 activity in VSMCs of hypertensive animals could contribute to their polyploidization. To test this hypothesis, Akt1 was overexpressed in VSMCs isolated from the aorta of 3-week-old normotensive WKY rats using an adenovirus vector that encodes wild-type murine Akt1 (Akt1 wt) (25, 35). VSMCs were also infected with adenoviruses that encode the catalytic inactive Akt1 mutant T308A/S473A (Akt1 AA), or the β-galactosidase gene (β Gal). Overexpression of Akt1 was verified by Western blot analysis (Figure 3). As expected, we observed an increase in basal Akt1 activity in VSMC-overexpressing wild-type Akt1, but not in those overexpressing the catalytic inactive Akt1 AA mutant (Figure 3).
Figure 3.
Akt1 overexpression in VSMCs by adenovirus gene transfer. (Top) Western blot analysis demonstrating overexpression of Akt1 constructs in adenovirus-infected aortic VSMCs isolated from 3-week-old WKY rats. Adenovirus containing β Gal, wild-type Akt1, and double-mutant T308A/S473A Akt1 (Akt1 AA) are described in Methods. Data are representative of two experiments. Other details as in Figure 1. (Bottom) Akt1 activity in control (β Gal) and Akt1-expressing VSMC. Experimental details as in Figure 1. Data are representative of two experiments.
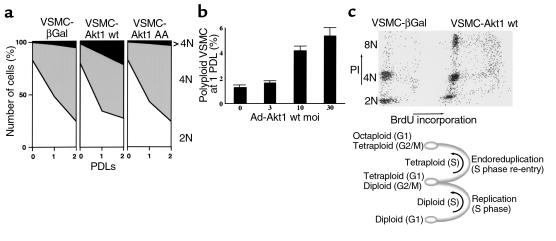
The adenovirus-infected VSMC populations were analyzed for their ability to control cell-cycle progression at the metaphase-to-anaphase transition, using the flow cytometry–based assay described previously (57). Incubation of wild-type Akt1–overexpressing VSMCs (VSMC-Akt1 wt cells) for two population-doubling times in the presence of a mitotic inhibitor resulted in dramatic progression to octaploid DNA content (4N, Figure 4a). In contrast, VSMCs expressing β-galactosidase or the catalytic inactive Akt1 AA mutant were able to arrest growth at 4N, with minor progression to 4N (Figure 4a). Moreover, when primary VSMCs were infected with increasing moi’s of wild-type Akt1 adenovirus, a direct correlation was observed between Akt1 transduction and VSMC polyploidization (Figure 4b). Furthermore, double staining of total DNA content (propidium iodide staining) versus newly synthesized DNA (BrdU incorporation) indicated that VSMC-Akt1 wt underwent cell-cycle reentry at 4N DNA content (Figure 4c). These results demonstrated that Akt1 overexpression promotes endoreduplication in VSMCs.
Figure 4.
Akt1-transduced VSMCs fail to arrest growth at mitosis. (a) Cell-cycle analysis of DNA content in control and Akt1-overexpressing VSMCs. Cells were exponentially growing (0 population-doubling time [PDL]) or incubated with 100 ng/ml of colcemid for one or two PDLs (1, 2 PDL). Graphs show 104 cells. PDLs were 63 (β Gal), 58 (Akt1 wt), and 62 (Akt1 AA) hours. VSMCs were fixed in 70% ethanol, nuclei isolated, stained with propidium iodide, and processed for flow cytometry, as indicated in Methods. Graphs represent the distribution in 2N, 4N, and greater than 4N DNA content of 104 VSMCs per time point. (b) Akt1 causes VSMC polyploidization. VSMCs (WKY aorta, 3 weeks old) were transduced with the indicated moi’s of Akt1 wt adenovirus. Forty-eight hours after infection, cells were passed and incubated in new media with 100 ng/ml of colcemid for 72 hours. DNA content was determined by flow cytometry, as above. The figure indicates the percentage of VSMCs with greater than 4N DNA content. Data are representative of three experiments. (c) Flow cytometry analysis of total DNA content (propidium iodide staining) and newly synthesized DNA (BrdU incorporation) of VSMC-Akt1 wt cells incubated for two population-doubling times in the presence of 100 ng/ml of colcemid. Other experimental details as indicated in Methods. Data are representative of three experiments.
Overexpression of Ak1 causes altered mitotic spindle cell–cycle checkpoint status in VSMCs.
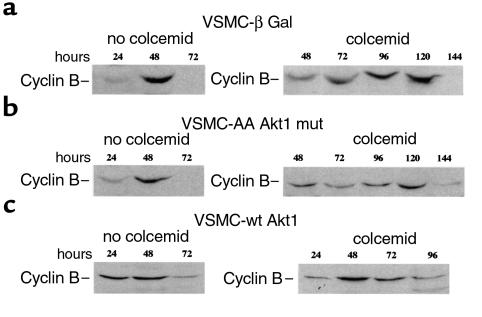
The fact that VSMCs overexpressing wild-type Akt1 may undergo endoreduplication indicates a loss of mitotic spindle cell–cycle checkpoint activity in these cells. The mitotic spindle cell-cycle checkpoint regulates exit from mitosis by controlling the metabolism of M-phase regulators, such as cyclin B (65). When cells cannot segregate their chromosomes appropriately, the degradation of cyclin B and, consequently, the progression from mitosis to a new G1-phase, is delayed (58). To determine the status of the mitotic checkpoint in Akt1-transduced VSMCs, we investigated the ability of these cells to regulate cyclin B turnover in response to the inhibition of their chromosomal segregation (58). Cells were made quiescent and then stimulated to enter the cell cycle synchronously in the presence or absence of colcemid. In the absence of microtubule inhibitor, cyclin B protein levels oscillated similarly in all cell groups. Cyclin B protein reached maximal levels at 48 hours and decreased to barely detectable levels at 72 hours, indicating entry and exit from mitosis (Figure 5, a–c; no colcemid). In wild-type Akt1-transduced cells, cyclin B expression was observed as early as 24 hours, suggesting shortening of G1 or S cell-cycle phases (Figure 5c; no colcemid), in agreement with previous reports (66). Importantly, a differential ability to regulate cyclin B degradation in response to colcemid was observed (Figure 5, a–c; colcemid). In β-Gal and Akt1 mutant–transduced cells, cyclin B levels remained elevated for up to 120 hours. This delay in the onset of cyclin B degradation indicated the activity of the mitotic spindle cell–cycle checkpoint and was similar in length to what has been observed previously in normal human fibroblasts (58). However, when wild-type Akt1-overexpressing VSMCs were incubated in colcemid, cyclin B levels declined between 48 hours and 72 hours. Thus, VSMCs with high Akt1 levels failed to maintain cyclin B levels in response to mitotic spindle depolymerization, demonstrating that overexpression of Akt1 alters the activity of the mitotic spindle cell–cycle checkpoint in VSMCs. Expression of a control protein, β-actin, in extracts from these cell groups was similar at all time points investigated (not shown).
Figure 5.
Unscheduled cyclin B degradation in Akt1 wt–transduced VSMCs. VSMCs infected with adenoviruses β Gal (a), T308A/S473A Akt1 mutant (b), AA Akt1 mutant, or wild-type Akt1 (c) were synchronized as above, then incubated at low density (1–2 × 104 cells/cm2) in 10% FBS in the absence or presence of 100 ng/ml colcemid and harvested at the indicated intervals. Colcemid was added at 16 hours after cell passage. Western blotting of cyclin B was carried out as indicated in Methods. Data are representative of three independent experiments.
Akt1 promotes VSMC polyploidization and hypertrophy.
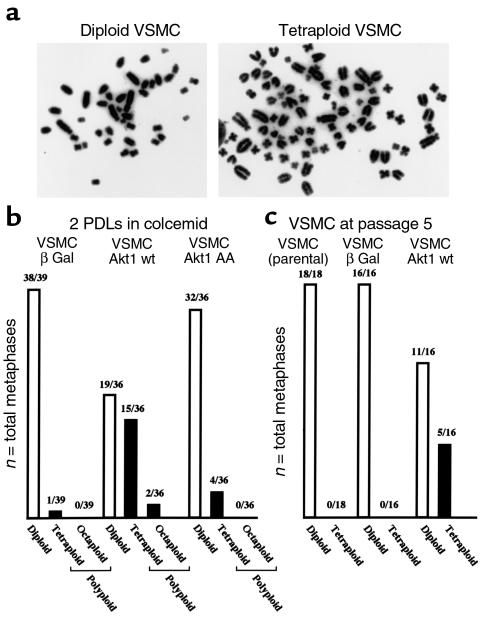
Although the results shown in Figures 4 and 5 demonstrate that Akt1 upregulation promotes endoreduplication in VSMCs, they do not provide formal proof of VSMC polyploidization. Thus, karyotypic analyses were performed to confirm whether or not overexpression of Akt1 is sufficient to induce polyploidization in VSMCs. Control and wild-type Akt1–transduced VSMC populations had a low number of polyploid cells (1–3%). However, when these cells were incubated in the presence of colcemid for two population-doubling time periods, a high polyploid fraction was observed in VSMCs transduced with wild-type Akt1, but not with Akt1 AA or βGal adenovirus (Figure 6b). Of note, chromosomal counts were multiples of the diploid number, supporting endoreduplication as the mechanism of polyploidization in VSMCs. Subsequent experiments, shown in Figure 6c, investigated the ploidy content of control and wild-type Akt1–transduced VSMCs after successive passage in culture. Higher ploidy fractions were found in VSMCs transduced with wild-type Akt1. Thus, cell populations overexpressing wild-type Akt1 may undergo spontaneous polyploidization in tissue culture, a phenomenon described previously in VSMCs isolated from hypertensive animals (67).
Figure 6.
Akt1 promotes VSMC polyploidization. (a) Microphotographs of a diploid and a tetraploid VSMC with enhanced Akt1 expression. (b) Karyotypic analysis of control and Akt1-overexpressing VSMC populations incubated for two population-doubling times in the presence of 100 ng/ml of colcemid. Figure represents G-banding and chromosome counts of 36–39 metaphase spreads. (c) Karyotypic analysis of control (β Gal) and Akt1-overexpressing (Akt1 wt) VSMC populations five tissue culture passages after viral transduction (15–20 days). Parental VSMCs were analyzed at the time of infection. VSMCs received 100 moi per week of the respective adenovirus. G-banding was performed after 4 hours’ incubation in colcemid.
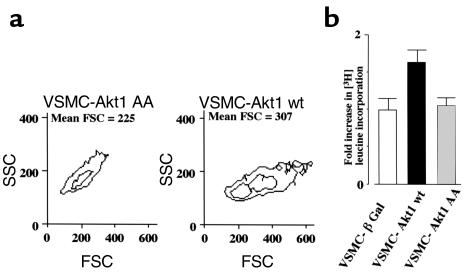
Since polyploid VSMCs are hypertrophic, we extended our observations to the analysis of the effects of Akt1 on VSMC size and protein synthesis. Forward-scatter cell (FSC) fluorescence flow cytometry demonstrated a 36% increase in cell size in wild-type Akt1–transduced VSMCs (Figure 7a). Furthermore, analysis of [3H]-leucine incorporation to protein demonstrated a 50% increase in protein synthesis in these cells (Figure 7b). Taken together, the results shown in Figures 6 and 7 demonstrate that overexpression of Akt1 is sufficient to induce polyploidization and hypertrophy in VSMCs.
Figure 7.
Akt1 promotes VSMC hypertrophy. (a) Flow cytometry of FSC fluorescence (cell size) versus side-scatter cell fluorescence (SSC; cell density) of control, wild-type, and mutant Akt1-expressing VSMCs incubated in 1% FBS media for 72 hours. Representative of two duplicate experiments. (b) [H3] leucine incorporation in control, wild-type, and mutant Akt1-expressing VSMC incubated in 1% FBS media for 72 hours. Data are expressed in arbitrary units relative to control (β Gal) incorporation. Represents three experiments. Other experimental details as indicated in Methods.
Akt1 function is required for angiotensin II–induced VSMC polyploidization.
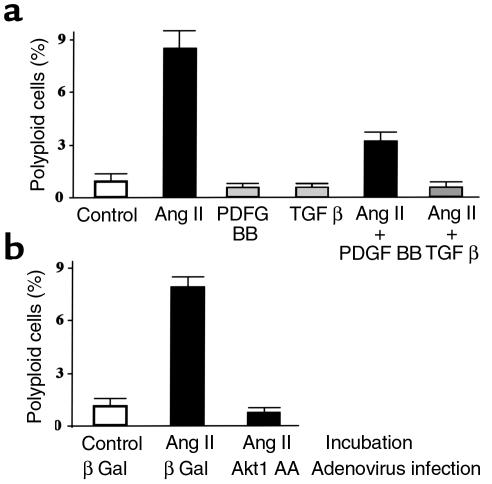
Multiple growth factors have been shown to induce Akt1 activity in VSMCs (26, 48–50, 68–71). We investigated the ability of angiotensin II (26, 48–50), PDGF BB (50, 69), and TGF-β (50), to modulate the onset of polyploidization in VSMCs. As shown in Figure 8a, angiotensin II, but not PDGF BB or TGF-β, promoted VSMC polyploidization. Furthermore, addition of PDGF BB or TGF-β decreased the level of angiotensin II–induced VSMC polyploidization (Figure 8a). To determine whether or not angiotensin II–induced polyploidization was mediated by Akt1, VSMCs were transduced with control or Akt1 AA mutant adenoviruses before stimulation. Expression of Akt1 AA blocked the polyploidization of VSMCs induced by angiotensin II (Figure 8b). Thus, these results demonstrated that Akt1 function is required for angiotensin II–induced VSMC polyploidization.
Figure 8.
Akt1 function is required for angiotensin II–induced VSMC polyploidization. (a) Effect of PDGF BB, angiotensin II, and TGF-β on VSMC ploidy. WKY VSMCs were incubated for 1 week in 1% FBS media with 100 ng/ml of colcemid and no additions (Control), 30 ng/ml PDGF BB, 0.1 μM angiotensin II (Ang), or 5 ng/ml TGF-β. After incubation, cells were harvested and processed for flow cytometry of DNA content as in Figure 4. Figure shows the percentage of VSMC with greater than 4N DNA content. Data represent three independent experiments. (b) Effect of Akt1 AA transduction on angiotensin II–induced VSMC polyploidization. WKY VSMCs were infected with 100 moi’s of β Gal or Akt1 AA adenovirus and incubated for 1 week in 1% FBS media with 100 ng/ml of colcemid and no additions (Control) or 0.1 μM angiotensin II (Ang). After incubation, cells were harvested and processed for flow cytometry of DNA content as in Figure 4. Figure shows the percentage of VSMCs with greater than 4N DNA content. Data represent three independent experiments.
Discussion
Most plants and animals generate tissue-specific subpopulations of polyploid cells that are larger than their diploid counterparts (72). However, little is known about the molecular events that promote tissue-specific hypertrophy and polyploidization. Here we provide evidence for a role of Akt1 in VSMC hypertrophy and polyploidization, phenomena that occur at capacitance arteries during hypertension (6–10, 17). High levels of Akt1 protein and activity were observed in aortas of hypertensive SHR, Goldblatt-WKY, and Zucker fa/fa rats, which also displayed polyploidization. The increase in basal Akt1 activity, four to fivefold higher in SHR related to age-matched WKY controls, correlates well with the elevation in Akt1 protein expression. These data provide correlative evidence that Akt1 is involved in VSMC polyploidization in several experimental models of hypertension. Consistent with these results, recent data from Jiang and coworkers demonstrate that the PI 3-kinase/Akt pathway is reduced in microvessels of Zucker fa/fa rats, whereas a moderate increase in activity was observed in the aorta (73).
To determine the functional significance of the elevated Akt1 levels in vascular smooth muscle, this protein was overexpressed in VSMCs isolated from the aortas of normotensive rats. Overexpression of wild-type Akt in VSMCs increased Akt1 activity approximately tenfold, within the range of induction observed in smooth muscle cells incubated in the presence of H2O2 or angiotensin II (26). Importantly, overexpression of wild-type, but not a catalytic inactive, Akt1 abrogated the activity of the mitotic spindle cell–cycle checkpoint in VSMCs, with corresponding perturbations in the cyclin B expression and DNA endoreduplication. Furthermore, enhanced expression of Akt1 was sufficient to induce polyploidization and hypertrophy in VSMCs. The fact that Akt1 promotes the progression of mitosis (M phase) parallels other growth-related properties of this protein. Akt1 has been shown to activate NF-κB activity (46) and c-fos (74), positive regulators of the progression through G1 cell-cycle phase (75, 76). Akt1 also activates E2F, a regulator of the G1/S transition (44). Collectively, these data demonstrate that Akt1 promotes cell growth by acting at multiple stages of the cell cycle. Akt1 appears to predispose VSMCs to polyploidization because no cell-cycle differences in Akt1 protein levels in VSMCs were found, nor were differences found in Akt levels between tetraploid versus diploid VSMCs (not shown). These data resemble our previous observations in patients with Li-Fraumeni syndrome who carry p53 mutations (57) and strongly suggest that Akt1 does not affect directly the chromosomal segregation machinery, but the pathways that regulate VSMC ploidy content.
Our results indicate that overexpression of Akt1 in VSMCs overrides the activity of the mitotic spindle checkpoint, facilitating unscheduled degradation of cyclin B, cell-cycle reentry (endoreduplication), and polyploidization. At least two molecular mechanisms can account for these effects of Akt1. First, Akt may directly modulate the expression of cell-cycle regulatory proteins. The metabolism of the cyclin B-Cdc2 complex is known to be regulated by its interaction with Cks(s) [in Homo sapiens, CKsHs(s)] (77, 78) and by spindle sensor proteins, such as MAD2 (79–81). Therefore, further investigation on the effects of Akt1 on these and other mitotic regulatory factors (82) may reveal the mechanism of cyclin B modulation by Akt1. In addition, polyploid VSMCs may be eliminated by the activity of the postmitotic checkpoint, an apoptotic pathway that target cells that escape the control of the mitotic spindle checkpoint (83, 84). Therefore, the antiapoptotic properties of Akt may be necessary to maintain the viability of polyploid VSMCs. In support of this hypothesis, Akt1 is a regulator of the bcl-2 family protein, BAD (37), and Bcl2 family members are known to promote polyploidization in mesenchymal cells (85).
As shown here, angiotensin II, but not PDGF BB or TGF-β, promoted Akt-dependent VSMC polyploidization. The fact that PDGF BB and TGF-β, activators of Akt signaling, fail to induce VSMC polyploidization may be related to the ability of these factors to activate additional signal-transduction pathways. For example, PDGF BB activates ERK and p38MAPK activities, which may oppose Akt signaling in smooth muscle (69). Importantly, Mek and p42 MAPK have been shown to trigger G2/M arrest in some systems (86). Likewise, TGF-β inhibits WKY VSMC polyploidization (87, 88), and this may be mediated by downregulation of Cks1 (89). Therefore, we propose that Akt signaling is both essential and sufficient for VSMC hypertrophy and polyploidization, but this activity can be overridden by factors that promote MAPK signaling and/or that modulate the expression of other cell-cycle regulatory proteins. Consistent with this hypothesis, we found that PDGF BB and TGF-β decrease angiotensin II–induced VSMC polyploidization.
In conclusion, we demonstrate that Akt1 protein levels and kinase activity are elevated in VSMCs that are predisposed to, or undergoing, hypertrophy in several animal models of hypertension. Although it is shown here that Akt1 is sufficient for the induction of VSMC hypertrophy and polyploidization, an unresolved issue is the nature of the causal relationship between polyploidization and hypertrophy. On one hand, hypertrophy, i.e., cell enlargement, may induce polyploidization. Alternatively, polyploidization may be an essential feature of the hypertrophic process. Evidence for the latter hypothesis comes from the recent finding of DNA ploidy–driven mechanisms of control of gene expression (90). In this study, an increase in cell size was explained by the longer G1 cell-cycle phase of polyploid yeast cells, which express low levels of G1 cyclins. Consistent with this hypothesis is the observation that tetraploid VSMCs exhibit longer population-doubling rates than their diploid counterparts (91, 92). Therefore, further investigation on the functions of Akt1 should provide additional information regarding the interrelationship between VSMC hypertrophy and polyploidization, and the molecular mechanisms that control these processes.
Acknowledgments
We thank K. Guo, E. R. Chan, P. N. Tsichlis, and A. Scarpa for reagents and suggestions. This work was supported in part by grants from Ohio Cancer Research Associates, the American Heart Association, and NIH to A. Gualberto, T. Hassold, and K. Walsh.
References
- 1.Lehmann ED, Watts GF, Fatemi-Langroudi B, Gosling RG. Aortic compliance in young patients with heterozygous familial hypercholesterolaemia. Clin Sci (Colch) 1992;83:717–721. doi: 10.1042/cs0830717. [DOI] [PubMed] [Google Scholar]
- 2.Lehmann ED, Gosling RG, Sonksen PH. Arterial wall compliance in diabetes. Diabet Med. 1992;9:114–119. doi: 10.1111/j.1464-5491.1992.tb01746.x. [DOI] [PubMed] [Google Scholar]
- 3.Toto-Moukouo JJ, Achimastos A, Asmar RG, Hugues CJ, Safar ME. Pulse wave velocity in patients with obesity and hypertension. Am Heart J. 1986;112:136–140. doi: 10.1016/0002-8703(86)90691-5. [DOI] [PubMed] [Google Scholar]
- 4.Owens GK, Rabinovitch PS, Schwartz SM. Smooth muscle cell hypertrophy versus hyperplasia in hypertension. Proc Natl Acad Sci USA. 1981;78:7759–7763. doi: 10.1073/pnas.78.12.7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olivetti G, Anversa P, Melissari M, Loud AV. Morphometry of medial hypertrophy in the rat thoracic aorta. Lab Invest. 1980;42:559–565. [PubMed] [Google Scholar]
- 6.Owens GK, Schwartz SM. Alterations in vascular smooth muscle mass in the spontaneously hypertensive rat. Role of cellular hypertrophy, hyperploidy, and hyperplasia. Circ Res. 1982;51:280–289. doi: 10.1161/01.res.51.3.280. [DOI] [PubMed] [Google Scholar]
- 7.Barrett TB, Sampson P, Owens GK, Schwartz SM, Benditt EP. Polyploid nuclei in human artery wall smooth muscle cells. Proc Natl Acad Sci USA. 1983;80:882–885. doi: 10.1073/pnas.80.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee RM, Conyers RB, Kwan CY. Incidence of multinucleated and polyploid aortic smooth muscle cells cultured from different age groups of spontaneously hypertensive rats. Can J Physiol Pharmacol. 1992;70:1496–1501. doi: 10.1139/y92-212. [DOI] [PubMed] [Google Scholar]
- 9.Conyers RB, Kwan CY, Lee RM. Alterations in beta-adrenoceptors and polyploidy in cultured aortic smooth muscle cells from different age groups of spontaneously hypertensive rats and Wistar-Kyoto rats. J Hypertens. 1995;13:507–515. doi: 10.1097/00004872-199505000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Dominiczak AF, et al. Vascular smooth muscle polyploidy and cardiac hypertrophy in genetic hypertension. Hypertension. 1996;27:752–759. doi: 10.1161/01.hyp.27.3.752. [DOI] [PubMed] [Google Scholar]
- 11.van Neck JW, et al. Effect of ploidy on transcription levels in cultured rat aortic smooth muscle cells. FEBS Lett. 1992;297:189–195. doi: 10.1016/0014-5793(92)80358-n. [DOI] [PubMed] [Google Scholar]
- 12.Yamori Y, Mano M, Nara Y, Horie R. Catecholamine-induced polyploidization in vascular smooth muscle cells. Circulation. 1987;75:I92–I95. [PubMed] [Google Scholar]
- 13.Black MJ, Adams MA, Bobik A, Campbell JH, Campbell GR. Effect of enalapril on aortic smooth muscle cell polyploidy in the spontaneously hypertensive rat. J Hypertens. 1989;7:997–1003. doi: 10.1097/00004872-198912000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Black MJ, Bertram JF, Campbell JH, Campbell GR. Angiotensin II induces cardiovascular hypertrophy in perindopril-treated rats. J Hypertens. 1995;13:683–692. doi: 10.1097/00004872-199506000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Devlin AM, et al. Vascular smooth muscle polyploidy in genetic hypertension: the role of angiotensin II. J Hum Hypertens. 1995;9:497–500. [PubMed] [Google Scholar]
- 16.Devlin AM, et al. The effects of perindopril on vascular smooth muscle polyploidy in stroke-prone spontaneously hypertensive rats. J Hypertens. 1995;13:211–218. [PubMed] [Google Scholar]
- 17.Owens GK, Schwartz SM. Vascular smooth muscle cell hypertrophy and hyperploidy in the Goldblatt hypertensive rat. Circ Res. 1983;53:491–501. doi: 10.1161/01.res.53.4.491. [DOI] [PubMed] [Google Scholar]
- 18.Murray AW, Mitchison TJ. Mitosis. Kinetochores pass the IQ test. Curr Biol. 1994;4:38–41. doi: 10.1016/s0960-9822(00)00007-5. [DOI] [PubMed] [Google Scholar]
- 19.Nicklas RB. How cells get the right chromosomes. Science. 1997;275:632–637. doi: 10.1126/science.275.5300.632. [DOI] [PubMed] [Google Scholar]
- 20.Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 21.Jaspersen SL, Charles JF, Kulberg RL, Tinker, Morgan DO. A late mitotic regulatory network controlling cyclin destruction in Saccharomyces cerevisiae. Mol Biol Cell. 1998;9:2803–2817. doi: 10.1091/mbc.9.10.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemmings BA. Akt signaling: linking membrane events to life and death decisions. Science. 1997;275:628–630. doi: 10.1126/science.275.5300.628. [DOI] [PubMed] [Google Scholar]
- 23.Hemmings BA. PtdIns(3, 4, 5)P3 gets its message across. Science. 1997;277:534. doi: 10.1126/science.277.5325.534. [DOI] [PubMed] [Google Scholar]
- 24.Franke TF, et al. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 25.Fujio Y, Walsh K. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem. 1999;274:16349–16354. doi: 10.1074/jbc.274.23.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ushio-Fukai M, et al. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J Biol Chem. 1999;274:22699–22704. doi: 10.1074/jbc.274.32.22699. [DOI] [PubMed] [Google Scholar]
- 27.Tanti JF, et al. Potential role of protein kinase B in glucose transporter 4 translocation in adipocytes. Endocrinology. 1997;138:2005–2010. doi: 10.1210/endo.138.5.5136. [DOI] [PubMed] [Google Scholar]
- 28.Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 29.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 30.Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 31.Dudek H, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 32.Kauffmann-Zeh A, et al. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy SG, et al. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 34.Kulik G, Klippel A, Weber MJ. Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol. 1997;17:1595–1606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujio Y, et al. Cell cycle withdrawal promotes myogenic induction of akt, a positive modulator of myocyte survival. Mol Cell Biol. 1999;19:5073–5082. doi: 10.1128/mcb.19.7.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 37.Datta SR, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 38.Yano S, Tokumitsu H, Soderling TR. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature. 1998;396:584–587. doi: 10.1038/25147. [DOI] [PubMed] [Google Scholar]
- 39.Cardone MH, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 40.Brunet A, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 41.Kops GJ, et al. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 42.Nakae J, Park BC, Accili D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a wortmannin-sensitive pathway. J Biol Chem. 1999;274:15982–15985. doi: 10.1074/jbc.274.23.15982. [DOI] [PubMed] [Google Scholar]
- 43.Tang ED, Nunez G, Barr FG, Guan KL. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- 44.Brennan P, et al. Phosphatidylinositol 3-kinase couples the interleukin-2 receptor to the cell cycle regulator E2F. Immunity. 1997;7:679–689. doi: 10.1016/s1074-7613(00)80388-x. [DOI] [PubMed] [Google Scholar]
- 45.Chang HW, et al. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science. 1997;276:1848–1850. doi: 10.1126/science.276.5320.1848. [DOI] [PubMed] [Google Scholar]
- 46.Kane LP, Shapiro VS, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 1999;9:601–604. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 47.Skorski T, et al. Transformation of hematopoietic cells by BCR/ABL requires activation of a PI-3k/Akt-dependent pathway. EMBO J. 1997;16:6151–6161. doi: 10.1093/emboj/16.20.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eguchi S, et al. Intracellular signaling of angiotensin II-induced p70 S6 kinase phosphorylation at Ser(411) in vascular smooth muscle cells. Possible requirement of epidermal growth factor receptor, Ras, extracellular signal-regulated kinase, and Akt. J Biol Chem. 1999;274:36843–36851. doi: 10.1074/jbc.274.52.36843. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi T, et al. Activation of Akt/protein kinase B after stimulation with angiotensin II in vascular smooth muscle cells. Am J Physiol. 1999;276:H1927–H1934. doi: 10.1152/ajpheart.1999.276.6.H1927. [DOI] [PubMed] [Google Scholar]
- 50.Higaki M, Shimokado K. Phosphatidylinositol 3-kinase is required for growth factor-induced amino acid uptake by vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:2127–2132. doi: 10.1161/01.atv.19.9.2127. [DOI] [PubMed] [Google Scholar]
- 51.Shabaik AS, Pow-Sang JM, Lockhart J, Nicosia SV. Role of DNA image cytometry in the follow-up of patients with urinary tract transitional cell carcinoma. Anal Quant Cytol Histol. 1993;15:115–123. [PubMed] [Google Scholar]
- 52.Owens GK, Schwartz SM, McCanna M. Evaluation of medial hypertrophy in resistance vessels of spontaneously hypertensive rats. Hypertension. 1988;11:198–207. doi: 10.1161/01.hyp.11.2.198. [DOI] [PubMed] [Google Scholar]
- 53.Owens GK, Loeb A, Gordon D, Thompson MM. Expression of smooth muscle-specific alpha-isoactin in cultured vascular smooth muscle cells: relationship between growth and cytodifferentiation. J Cell Biol. 1986;102:343–352. doi: 10.1083/jcb.102.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miano JM, Cserjesi P, Ligon KL, Periasamy M, Olson EN. Smooth muscle myosin heavy chain exclusively marks the smooth muscle lineage during mouse embryogenesis. Circ Res. 1994;75:803–812. doi: 10.1161/01.res.75.5.803. [DOI] [PubMed] [Google Scholar]
- 55.Alessi DR, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 56.Rajvanshi P, et al. Fractionation of rat hepatocyte subpopulations with varying metabolic potential, proliferative capacity, and retroviral gene transfer efficiency. Exp Cell Res. 1998;244:405–419. doi: 10.1006/excr.1998.4223. [DOI] [PubMed] [Google Scholar]
- 57.Gualberto A, Aldape K, Kozakiewicz K, Tlsty TD. An oncogenic form of p53 confers a dominant, gain-of-function phenotype that disrupts spindle checkpoint control. Proc Natl Acad Sci USA. 1998;95:5166–5171. doi: 10.1073/pnas.95.9.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hixon ML, Flores AI, Wagner MW, Gualberto A. Ectopic expression of cdc2/cdc28 kinase subunit homo sapiens 1 uncouples cyclin B metabolism from the mitotic spindle cell cycle checkpoint. Mol Cell Biol. 1998;18:6224–6237. doi: 10.1128/mcb.18.11.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamori, Y. 1994. Development of the spontaneously hypertensive rat (SHR), the stroke-prone SHR (SHRSP) and their various substrain models for hypertension-related cardiovascular diseases. In Experimental and genetic models of hypertension. Volume 16. D. Ganten and W. de Jong, editors. Elsevier. Amsterdam, The Netherlands. 346–364.
- 60.Kurtz TW, Morris RC, Pershadsingh HA. The Zucker fatty rat as a genetic model of obesity and hypertension. Hypertension. 1989;13:896–901. doi: 10.1161/01.hyp.13.6.896. [DOI] [PubMed] [Google Scholar]
- 61.Lombardi DM, Owens GK, Schwartz SM. Ploidy in mesenteric vessels of aged spontaneously hypertensive and Wistar-Kyoto rats. Hypertension. 1989;13:475–479. doi: 10.1161/01.hyp.13.5.475. [DOI] [PubMed] [Google Scholar]
- 62.Su EJ, Lombardi DM, Siegal J, Schwartz SM. Angiotensin II induces vascular smooth muscle cell replication independent of blood pressure. Hypertension. 1998;31:1331–1337. doi: 10.1161/01.hyp.31.6.1331. [DOI] [PubMed] [Google Scholar]
- 63.Black MJ, Campbell JH, Campbell GR. Does smooth muscle cell polyploidy occur in resistance vessels of spontaneously hypertensive rats? Blood Vessels. 1988;25:89–100. [PubMed] [Google Scholar]
- 64.Marte BM, Downward J. PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem Sci. 1997;22:355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- 65.Koepp DM, Harper JW, Elledge SJ. How the cyclin became a cyclin: regulated proteolysis in the cell cycle. Cell. 1999;97:431–434. doi: 10.1016/s0092-8674(00)80753-9. [DOI] [PubMed] [Google Scholar]
- 66.Gille H, Downward J. Multiple ras effector pathways contribute to G(1) cell cycle progression. J Biol Chem. 1999;274:22033–22040. doi: 10.1074/jbc.274.31.22033. [DOI] [PubMed] [Google Scholar]
- 67.Rosen EM, et al. Strain and site dependence of polyploidization of cultured rat smooth muscle. J Cell Physiol. 1986;128:337–344. doi: 10.1002/jcp.1041280228. [DOI] [PubMed] [Google Scholar]
- 68.Duan C, Liimatta MB, Bottum OL. Insulin-like growth factor (IGF)-I regulates IGF-binding protein-5 gene expression through the phosphatidylinositol 3-kinase, protein kinase B/Akt, and p70 S6 kinase signaling pathway. J Biol Chem. 1999;274:37147–37153. doi: 10.1074/jbc.274.52.37147. [DOI] [PubMed] [Google Scholar]
- 69.Hayashi K, et al. Changes in the balance of phosphoinositide 3-kinase/protein kinase B (Akt) and the mitogen-activated protein kinases (ERK/p38MAPK) determine a phenotype of visceral and vascular smooth muscle cells. J Cell Biol. 1999;145:727–740. doi: 10.1083/jcb.145.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Imai Y, Clemmons DR. Roles of phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathways in stimulation of vascular smooth muscle cell migration and deoxyribonucleic acid synthesis by insulin-like growth factor-I. Endocrinology. 1999;140:4228–4235. doi: 10.1210/endo.140.9.6980. [DOI] [PubMed] [Google Scholar]
- 71.Jiang ZY, et al. Endothelin-1 modulates insulin signaling through phosphatidylinositol 3-kinase pathway in vascular smooth muscle cells. Diabetes. 1999;48:1120–1130. doi: 10.2337/diabetes.48.5.1120. [DOI] [PubMed] [Google Scholar]
- 72.Hieter P, Griffiths T. Polyploidy: more is more or less. Science. 1999;285:210–211. doi: 10.1126/science.285.5425.210. [DOI] [PubMed] [Google Scholar]
- 73.Jiang ZY, et al. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest. 1999;104:447–457. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y, et al. Activation of the c-fos serum response element by phosphatidyl inositol 3-kinase and rho pathways in HeLa cells. Cell Growth Differ. 1998;9:513–522. [PubMed] [Google Scholar]
- 75.Cosenza SC, Owen TA, Soprano DR, Soprano KJ. Evidence that the time of entry into S is determined by events occurring in early G1. J Biol Chem. 1988;263:12751–12758. [PubMed] [Google Scholar]
- 76.Baldwin AS, Jr, Azizkhan JC, Jensen DE, Beg AA, Coodly LR. Induction of NF-kappa B DNA-binding activity during the G0-to-G1 transition in mouse fibroblasts. Mol Cell Biol. 1991;11:4943–4951. doi: 10.1128/mcb.11.10.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Basi, G., and Draetta, G. 1995. The cdc2 kinase: structure, activation, and its role at mitosis in vertebrate cells. In Cell cycle control. Volume 10. C. Hutchison and D.M. Glover, editors. Oxford University Press. Oxford, United Kingdom. 106–134.
- 78.Richardson HE, Stueland CS, Thomas J, Russell P, Reed SI. Human cDNAs encoding homologs of the small p34Cdc28/Cdc2-associated protein of Saccharomyces cerevisiae and Schizosaccharomyces pombe. Genes Dev. 1990;4:1332–1344. doi: 10.1101/gad.4.8.1332. [DOI] [PubMed] [Google Scholar]
- 79.Chen RH, Waters JC, Salmon ED, Murray AW. Association of spindle assembly checkpoint component XMAD2 with unattached kinetochores. Science. 1996;274:242–246. doi: 10.1126/science.274.5285.242. [DOI] [PubMed] [Google Scholar]
- 80.Li Y, Benezra R. Identification of a human mitotic checkpoint gene: hsMAD2. Science. 1996;274:246–248. doi: 10.1126/science.274.5285.246. [DOI] [PubMed] [Google Scholar]
- 81.Pangilinan F, Spencer F. Abnormal kinetochore structure activates the spindle assembly checkpoint in budding yeast. Mol Biol Cell. 1996;7:1195–1208. doi: 10.1091/mbc.7.8.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hixon ML, Gualberto A. The control of mitosis. Front Biosci. 2000;5:D50–D57. doi: 10.2741/hixon. [DOI] [PubMed] [Google Scholar]
- 83.Lanni JS, Jacks T. Characterization of the p53-dependent postmitotic checkpoint following spindle disruption. Mol Cell Biol. 1998;18:1055–1064. doi: 10.1128/mcb.18.2.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sherwood SW, Sheridan JP, Schimke RT. Induction of apoptosis by the anti-tubulin drug colcemid: relationship of mitotic checkpoint control to the induction of apoptosis in HeLa S3 cells. Exp Cell Res. 1994;215:373–379. doi: 10.1006/excr.1994.1354. [DOI] [PubMed] [Google Scholar]
- 85.Minn AJ, Boise LH, Thompson CB. Expression of Bcl-xL and loss of p53 can cooperate to overcome a cell cycle checkpoint induced by mitotic spindle damage. Genes Dev. 1996;10:2621–2631. doi: 10.1101/gad.10.20.2621. [DOI] [PubMed] [Google Scholar]
- 86.Walter SA, Guadagno TM, Ferrell JE., Jr Induction of a G2-phase arrest in Xenopus egg extracts by activation of p42 mitogen-activated protein kinase. Mol Biol Cell. 1997;8:2157–2169. doi: 10.1091/mbc.8.11.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Agrotis A, Saltis J, Dilley R, Bray P, Bobik A. Transforming growth factor-beta 1 and the development of vascular hypertrophy in hypertension. Blood Press Suppl. 1995;2:43–48. [PubMed] [Google Scholar]
- 88.Gibbons GH, Pratt RE, Dzau VJ. Vascular smooth muscle cell hypertrophy vs. hyperplasia. Autocrine transforming growth factor-beta 1 expression determines growth response to angiotensin II. J Clin Invest. 1992;90:456–461. doi: 10.1172/JCI115881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Simon KE, Cha HH, Firestone GL. Transforming growth factor beta down-regulation of CKShs1 transcripts in growth-inhibited epithelial cells. Cell Growth Differ. 1995;6:1261–1269. [PubMed] [Google Scholar]
- 90.Galitski T, Saldanha AJ, Styles CA, Lander ES, Fink GR. Ploidy regulation of gene expression. Science. 1999;285:251–254. doi: 10.1126/science.285.5425.251. [DOI] [PubMed] [Google Scholar]
- 91.Rosen EM, et al. Growth kinetics as a function of ploidy in diploid, tetraploid, and octaploid smooth muscle cells derived from the normal rat aorta. J Cell Physiol. 1985;125:512–250. doi: 10.1002/jcp.1041250322. [DOI] [PubMed] [Google Scholar]
- 92.Goldberg ID, et al. Isolation and culture of a tetraploid subpopulation of smooth muscle cells from the normal rat aorta. Science. 1984;226:559–561. doi: 10.1126/science.6494901. [DOI] [PubMed] [Google Scholar]