Summary
Unc93B1, a multi-transmembrane ER-resident protein, controls intracellular trafficking of endosomal Toll-like receptors. In this issue of Immunity, Fukui et al. (2011) revealed that Unc93B1 regulates differential transport of TLR7 and TLR9 into signaling endosomes to prevent autoimmunity.
Endosomal Toll-like receptors (TLRs) recognize viral nucleic acids and induce activation of antiviral genes. Upon endocytosis of virions, TLR9 binds to double stranded DNA (dsDNA) rich in unmethylated CpG motifs found in DNA viruses, and TLR7 recognizes single stranded RNA (ssRNA) with GU-rich sequences found in viral RNA. However, such molecular patterns associated with viral nucleic acids are not necessarily unique to viruses, as mammalian nucleic acids also share features that are recognized by these TLRs. Under certain circumstances, self nucleic acids can accidentally enter the endosome and trigger TLR7 and TLR9, which can lead to autoimmune diseases including psoriasis, arthritis and systemic lupus erythematosus (SLE) (Marshak-Rothstein and Rifkin, 2007). The innate immune system has in place multiple regulatory mechanisms to prevent recognition of self nucleic acids. One such mechanism involves regulation of TLR intracellular trafficking. At steady state, the majority of TLR7 and TLR9 are expressed in the endoplasmic reticulum (ER)(Figure 1). Upon viral infection or TLR signaling, TLR7 and TLR9 are mobilized to traffic from the ER to the endosomes where viral recognition takes place. A multi-transmembrane protein found in the ER at steady state, Unc93B1, controls trafficking of all endosomal TLRs, TLR3, 7, 8 and 9 (Kim et al., 2008). Subcellular localization of TLR7 and TLR9 is regulated such that these receptors are confined to the endosomes and are excluded from the plasma membrane, where self-nucleic acids are accessible. An additional level of control is provided by the fact that TLR7 and TLR9 are only active once they are cleaved in the acidified “signaling endosomes” by endosomal proteases (Barton and Kagan, 2009) (Figure 1). Once within the signaling endosomal compartment, TLR9 and TLR7 recruit the adaptor protein MyD88 and trigger signals leading to inflammatory cytokine expression through NF-kB activation. These receptors are further transported to lysosome related organelle by the adaptor protein-3 (AP-3) complex, enabling them to recruit interferon regulatory factor -7 (IRF7) and activate transcription of type I interferon (IFN) genes (Sasai et al., 2010). However, how the relative distribution of TLR7 and TLR9 in the signaling endosomes within the same responding cell is coordinated has remained unclear.
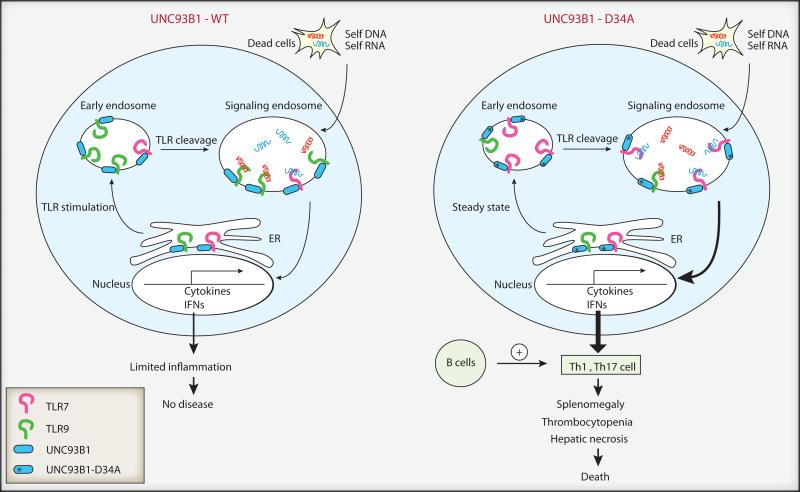
Figure 1. Unc93B1 control of TLR7 and TLR9 trafficking into signaling endosomes.
B cells, plasmacytoid DCs, DCs and macrophages express TLR7 and TLR9. At steady state, the majority of TLR7 and TLR9 reside in the ER in cells expressing WT Unc93B1. Upon TLR stimulation, TLR9 is preferentially transported through the early endosome to the acidified endosomes by Unc93B1, whereby it is cleaved to become competent for signaling. In contrast, D34A mutant Unc93B1 transports TLR7, but not TLR9, to the signaling endosome even in the absence of external stimuli. Stimulation of TLR7 by endogenous RNA ligands results in B cell-dependent Th1 and Th17 cell differentiation, which leads to splenomegaly with myeloproliferation, thrombocytopenia, hepatic necrosis and death.
The current study by Fukui et al. (Fukui et al., 2011) builds on their previous finding that TLR9 competes with TLR7 for Unc93B1-dependent transportation to signaling endosomes (Fukui et al., 2009), and reports an exuberant systemic inflammation that results from a dysregulation of this process in vivo. Previously, the same group used complementation cloning with a TLR7-unresponsive cell line and found that amino acid D34 in Unc93B1 repressed TLR7-mediated responses. Cells expressing D34A mutant Unc93B1 are hyperresponsive to TLR7 ligands but hyporesponsive to TLR9 ligands, due to preferential binding and trafficking of TLR7 into signaling endosomes (Fukui et al., 2009) (Figure 1). In the current study, the authors generated genetically targeted mice harboring a D34A mutation in Unc93B1. Remarkably, these mice developed severe lethal inflammatory disease. In the absence of any other autoimmune-predisposing mutations, half of the D34A homozygous genetically targeted mice died before 30 weeks of age due to liver necrosis, with no obvious necrosis in other organs including kidney, lung, heart and spleen. D34A mutant mice developed progressive splenomegaly with massive expansion of erythroblasts and myeloid cells, as well as severe thrombocytopenia. Moreover, autoantibody production was detected in some of these mice. To probe the pathogenesis, D34A genetically targeted mice were crossed to a variety of genetically ablated mice. TLR7 and MyD88 deficiency completely prevented disease development in D34A mice, while TLR9 deficiency only partially reversed the inflammatory phenotype. D34A genetically targeted mice spontaneously developed Th1 and Th17 cell responses, which were abrogated by TLR7 deficiency. Lymphocytes were required for the disease observed in D34A mice, since the D34A mutation on a Rag2-/- background completely eliminated inflammatory disease. Strikingly, B cells were required for the pathogenesis in D34A mice, as D34A mice crossed onto the B cell deficient Ighm-/- background did not suffer from inflammatory disease and lacked spontaneous activation of Th17 cell responses. Since antibody is not required for autoreactive T cell activation in MRL/lpr mice (Chan et al., 1999), and since only a mild increase in IgG2a and IgG2b amounts were observed in D34A genetically targeted mice (Fukui et al., 2011), the pathogenic role of B cells likely stems from their capacity to present autoantigens to T cells.
Intracellular distribution analysis of TLRs in D34A genetically targeted mice revealed that, while TLR9 remained excluded from lysosomal associated membrane protein-1 (LAMP1)+ compartment, TLR7 was confined almost exclusively to the LAMP1+ compartment in bone marrow derived DCs, even in the absence of any external stimulus. These data indicate that the D34A mutant Unc93B1 not only enables preferential trafficking of TLR7 to the endosome, but also overrides the requirement for TLR priming for such delivery (Figure 1). This could at least in part explain the severe autoimmune phenotype seen in D34A genetically targeted mice. On the scale of autoimmune diseases, D34A genetically targted mice rate at the severe end - as pathogenic as in TLR7 transgenic mice carrying 8-16 extra-copies of the TLR7 gene, which also develop splenomegaly, thrombocytopenia, liver inflammation and death (Deane et al., 2007). Even though TLR expression was unaltered in these mice, pathology in the D34A mutant mice was far beyond the SLE-like diseases seen in the Y-linked autoimmune accelerating (Yaa) model with TLR7 gene duplication, or MRL/lpr mice. These results indicate that not only the dosage, but also the subcellular location of TLR7, is strictly regulated to avoid autoimmune disease, in this case, by Unc93B1.
An obvious question that arises from these findings reported here (Fukui et al., 2011) is why TLR7, but not TLR9, stimulation results in autoimmune outcomes. This dichotomy is even more exaggerated in the case of autoimmune prone MRL/lpr mice, where TLR9 ameliorates disease progression (Christensen et al., 2006), and the protective role for TLR9 depends on its antagonism of TLR7 (Nickerson et al., 2010). First, although downstream signaling is assumed to be the same between TLR7 and TLR9, this has not been formally tested. If TLR7 and TLR9 can form heterodimers, downstream signaling can be regulated by the ratio of homo vs. heterodimers. Second, it is possible that endogenous TLR7 ligands are more abundant and/or potent in inducing signals compared to TLR9 ligands. Third, it is also possible to imagine a scenario in which two separate cell types are involved – eg, cells expressing TLR7 selectively induce transcription program that leads to overt inflammation in contrast to those expressing TLR9. However, the current study highlights that within the same responding cell, competition over Unc93B1 binding by TLR7 and TLR9 can have a profound consequence following recognition of endogenous nucleic acids.
These results also provide a platform with which to investigate the endogenous ligands responsible for activating TLR7, and the cell types responsible for pathogenesis of various autoimmune diseases. The pathogenic role of B cells in this regard is particularly interesting, and future studies are needed to probe whether cell intrinsic or extrinsic requirements for B cell TLR signaling leads to autoimmune T cell activation. In addition to B cells, involvement of plasmacytoid dendritic cells, dendritic cells and macrophages that also express these TLRs needs to be clarified. Even though the D34A mutation is not known to occur naturally in humans, whether other single nucleotide polymorphisms (SNPs) within the Unc93b1 gene or those that affect TLR trafficking in general are associated with autoimmune diseases in humans remains an intriguing question for future studies.
Finally, an evolutionarily relevant question that emerges from these findings is why viral-sensing TLRs and not bacterial TLR sensors need be co-regulated. Bacterial pathogen associated molecular patterns (PAMPs) have unique molecular signatures that are absent from mammalian cells, and TLRs that recognize these do not require an additional level of regulation among the receptors. In contrast, viral PAMPs are shared by endogenous nucleic acids, and require an additional level of regulation. Such co-regulation of TLR7 and TLR9 may have evolved to be optimized to avoid stimulation when they collectively sense the relative abundance of RNA to DNA from endogenous sources (i.e., dead cells), but can still engage robust activation when RNA or DNA predominates during a virus infection. In this regard, D34A mutant Unc93B1 also preferentially binds TLR8 and TLR13 compared to WT Unc93B1 (Fukui et al., 2009), indicating that co-regulated trafficking by Unc93B1 extends to RNA (TLR7, TLR8 and potentially TLR13) vs. DNA (TLR9) sensing TLRs. Therefore, the love triangle between Unc93B1, TLR7, and TLR9 may indeed involve more partners, and much remains to be seen how attraction between these nucleic acid sensing TLRs play out in keeping us out of fatal attraction.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009 doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. The Journal of experimental medicine. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Deane JA, Pisitkun P, Barrett RS, Feigenbaum L, Town T, Ward JM, Flavell RA, Bolland S. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui R, Saitoh S, Matsumoto F, Kozuka-Hata H, Oyama M, Tabeta K, Beutler B, Miyake K. Unc93B1 biases Toll-like receptor responses to nucleic acid in dendritic cells toward DNA- but against RNA-sensing. The Journal of experimental medicine. 2009;206:1339–1350. doi: 10.1084/jem.20082316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui R, Saitoh SI, Kanno A, Onji M, Shibata T, Ito A, Matsumoto M, Akira S, Yoshida N, Miyake K. Unc93B1 Restricts Systemic Lethal Inflammation by Orchestrating Toll-like Receptor 7 and 9 Trafficking. Immunity. 2011 doi: 10.1016/j.immuni.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452:234–238. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A, Rifkin IR. Immunologically active autoantigens: the role of toll-like receptors in the development of chronic inflammatory disease. Annual review of immunology. 2007;25:419–441. doi: 10.1146/annurev.immunol.22.012703.104514. [DOI] [PubMed] [Google Scholar]
- Nickerson KM, Christensen SR, Shupe J, Kashgarian M, Kim D, Elkon K, Shlomchik MJ. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J Immunol. 2010;184:1840–1848. doi: 10.4049/jimmunol.0902592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai M, Linehan MM, Iwasaki A. Bifurcation of Toll-like receptor 9 signaling by adaptor protein 3. Science. 2010;329:1530–1534. doi: 10.1126/science.1187029. [DOI] [PMC free article] [PubMed] [Google Scholar]