Abstract
Understanding effects of estrogen on the medial prefrontal cortex (PFC) may help to elucidate the increased prevalence of depression and post-traumatic stress disorder in women of ovarian cycling age. Estrogen replacement in ovariectomized (OVX) young rats amplifies the detrimental effects of stress on working memory (a PFC-mediated task), but the mechanisms by which this occurs have yet to be identified. In male rats, stimulation of norepinephrine alpha-2 adrenoceptors protects working memory from stress-induced impairments. However, this effect has not been studied in females, and has not been examined for sensitivity to estrogen. The current study asked whether OVX females with estrogen replacement (OVX + Est) and without replacement (OVX + Veh) responded differently to stimulation of alpha-2 adrenoceptors after administration of the benzodiazepine inverse agonist FG7142, a pharmacological stressor. The alpha-2 agonist, guanfacine, protected working memory from the impairing effects of FG7142 in OVX + Veh, but not in OVX + Est rats. Western Blot analysis for alpha-2 receptors was performed on PFC tissue from each group, but no changes in expression were found, indicating that the behavioral effects observed were likely not due to changes in receptor expression. These findings point to possible mechanisms by which estrogen may enhance the stress response, and hold implications for the gender discrepancy in the prevalence of stress-related mental illness.
Keywords: Acute stress, estrogen, norepinephrine, prefrontal cortex, sex differences, working memory
Introduction
While mild stress exposure can enhance the function of brain regions like the hippocampus (Bowman et al. 2001; Shors 2001) and amygdala (McEwen 2000; Izquierdo et al. 2002), the cognitive functions of the prefrontal cortex (PFC) are markedly impaired by mild, uncontrollable stress (Arnsten and Goldman-Rakic 1990; Mizoguchi et al. 2000). Prolonged or traumatic stress exposure can result in pathologies known to involve PFC dysfunction, such as major depressive disorder (MDD; Dohrenwend et al. 1995; Mazure 1998; Botteron et al. 2002) and post-traumatic stress disorder (PTSD; Zubieta et al. 1999; Koenen et al. 2001). Work in primates and rodents have done much to outline the neurotransmitter systems and intracellular signaling pathways that mediate PFC dysfunction, which is manifest as a loss of working memory capabilities. Specifically, stress-induced working memory impairment arises in part from high levels of catecholamine release, stimulating dopamine (DA) D1 receptors (Murphy et al. 1996), which in turn activate the cAMP (Vijayraghavan et al. 2007) intracellular signaling cascade. Accordingly, intra-PFC infusions of a D1 agonist produce working memory impairment (Bushnell and Levin 1993; Birnbaum et al. 1999), while D1 receptor antagonists, as well as cAMP inhibitors, have been shown to reverse stress-related working memory impairments when infused into the PFC (Arnsten 2007). It has further been shown that stimulation of the norepinephrine (NE) alpha-2a receptor can protect working memory during stress, likely through its inhibition of the cAMP signaling pathway (Birnbaum et al. 2000).
This work, however, has only been done in male animals, and the mechanisms of stress-induced PFC dysfunction in females have only just begun to be explored. Initial research suggests that female rats with high estrogen levels are particularly sensitive to the PFC-impairing effects of both pharmacological stress (Shansky et al. 2004), and restraint stress (Shansky et al. 2006). Females rats with high levels of circulating estrogen [i.e., ovariectomized (OVX) rats with estrogen replacement (OVX + Est)] showed greater stress-induced working memory impairment than males or female rats with low circulating estrogen (OVX + Veh; Shansky et al. 2004). Similarly, variations in stress responsiveness were observed across the normal ovarian hormonal cycle, with females being more sensitive to stress when they had high circulating levels of estrogen (proestrus), than when they had low circulating estrogen (estrus; Shansky et al. 2004, 2006). These findings provide compelling evidence for a role of estrogen in modulating the stress response, but the mechanisms by which estrogen confers this sensitivity are unknown.
As noted above, stimulation of the NE alpha-2a receptor can reverse stress-induced working memory impairments, and there is evidence suggesting that estrogen can suppress activity at this receptor. In the frontal cortex, estradiol treatment has been shown to reduce both NE alpha-2 receptor binding and mRNA levels (Karkanias et al. 1997), while in the hypothalamus, estrogen prolongs phosphorylation of the NE alpha-2a receptor, uncoupling it from Gi and rendering it less effective (Ansonoff and Etgen 2001). Finally, estrogen has been shown to rapidly activate cAMP signaling in the hypothalamus (Kelly et al. 1999). Taken together, these studies suggest that estrogen can inhibit a signaling pathway that provides protection from stress-related working memory impairments, and may thus impart sensitivity to such impairments.
The current study examined whether estrogen can (1) alter the ability of the NE alpha-2 agonist guanfacine to reverse pharmacological stress-induced working memory impairment, and (2) up- or down-regulate PFC NE alpha-2a receptor protein expression in female rats.
Materials and methods
Subjects
Two-month old female (n = 24) Sprague–Dawley rats (Camm, Wayne, NJ, USA) were single-housed in a 12 h light/dark cycle (lights on 7–19 h) with all testing conducted during the light phase (between 13 and 15 h). The rats were fed Purina rat chow (15 g/rat per day) immediately following behavioral testing and water was available ad libitum. Rat weights increased from an average of 240 g at the beginning of the study to approximately 300 g by the end of the study (approximately 3 months in duration). All animal care and use was approved by the Yale University Animal Care and Use Committee, and done in accordance with the NIH Guide for Care and Use of Laboratory Animals.
Ovariectomy surgery
Rats were anesthetized with a ketamine (90 mg/kg) and xylazine (4 mg/kg) mixture (given by i.p. injection), supplemented with isoflurane inhalation, and bilaterally OVX. Twelve of the rats were implanted subcutaneously under the nape of the neck with a capsule of 10% 17-β estradiol 90% cholesterol (to mimic estrogen levels of proestrus, see Ref. McGinnis et al. (1981)), while the remaining 12 received a placebo capsule, containing cholesterol. Rats received subcutaneous Buprenex injections (0.03 mg/kg i.m. on the day on surgery) and for 2 days (0.03 mg/kg s.c.) following surgery, and were monitored for eating and drinking habits. Capsules were replaced after 2 months for the remainder of the study to ensure constant estrogen levels. Animals began T-maze habituation 2 weeks after surgery.
Cognitive testing
Working memory was measured by performance on the delayed alternation task. In addition to working memory, this task requires behavioral inhibition and sustained attention, and has been shown to be impaired in animals with ventromedial PFC lesions (Larsen and Divac 1978), and in rats administered the pharmacological stressor FG7142 (Birnbaum et al. 1999). Delayed alternation training and testing were performed in a T-maze (laquered plywood, w: 90 cm × l: 65 cm × h: 8 cm). Rats were habituated to the T-maze, until they were readily eating chocolate chips from the experimenter's hand. Following habituation, rats were trained on the delayed alternation task. A rat was placed in the start box of the T-maze and the gate was opened, allowing the rat to run to the choice point in the maze. On the first trial the rats were rewarded (fed a chocolate chip from the tester's hand) for entering either arm. The rat was then picked up and returned to the start box of the maze for the inter-trial delay. On all subsequent trials, the rat was rewarded only if it entered the maze arm that was not chosen on the immediately preceding trial. If the correct choice was made, the rat was given a reward and returned to the start box for the inter-trial delay. Following an incorrect choice, the rat was immediately returned to the start box for the inter-trial delay without reward. During each inter-trial delay, the maze was wiped with 75% ethanol to remove any olfactory cues. Each test session consisted of 10 trials. Rats were scored for accuracy of response and response time, or the time the start gate was lifted until the animal made its choice. Response time did not differ between groups, as reported in (Shansky et al. 2004). Rats were tested once daily, five times per week, for the duration of the study (approximately 6 months). Impairment on this task is reflected by performance of approximately 50% correct, which represents chance level of responding. A score lower than this indicates perseverance towards one arm of the maze.
The inter-trial delay was initially approximately 2 s, the minimal time needed to clean the choice point. Delays were increased by 5 s increments as needed in order to stabilize each rat's performance at approximately 70–80% correct. This score was used as a baseline in order to insure against ceiling effects, and so that either impairment or improvement could be observed after stress or drug treatment. After 60 days of testing, the rats did not differ between groups in level of delay. Animals were tested daily until a rat scored between 60 and 80% correct for 2 consecutive days. On the third day, the rats were administered drug. At least one week passed between drug sessions for each rat.
Pharmacological stress
The stress response was activated by the benzodiazepine inverse agonist, FG7142, an anxiogenic drug that reduces GABAergic transmission through the GABAa receptor. Similar to non-pharmacological stressors, FG7142 activates the HPA axis, stimulates corticosterone release (Mikkelsen et al. 2005) raises brain corticotropin releasing factor mRNA levels (Funk et al. 2006), increases catecholamine turnover in the PFC (Ida et al. 1991) and produces anxiety (Dorow et al. 1983). FG7142 has advantages over more commonly used “natural” stressors (such as tailshock, swim stress, and restraint stress) in that animals do not habituate to it, and there can be precise dosing. Thus, there is better control over the level of stress to which the animal is exposed, allowing a dose-response curve to be generated (Shansky et al. 2004). Furthermore, the animal performs the task while experiencing the stress, rather than after being released from stress, as in most other paradigms. Agents that reverse the cognitive effects of FG7142 similarly reverse the effects of noise stress (Murphy et al. 1996; Arnsten and Goldman-Rakic 1998), and restraint stress.
To ensure that the stress of receiving an injection did not influence task performance, the rats were habituated to the injection procedure before drug testing began. FG7142 (Tocris Cookson, St Louis, MO, USA) was suspended in a saline vehicle containing Tween 80, hydroxybetacyclodextrin and ethanol. The lowest impairing dose of FG7142 was found for each rat; this dose (ranging from 2 to 15 mg/kg) was given 30 min after guanfacine (GFC, 0.11 mg/ml in saline administered i.p.; Birnbaum et al. 2000) administration, and 30 min prior to testing. The experimenter testing the animal was blind to drug treatment conditions.
Western immunoblotting
At the conclusion of the behavioral studies, OVX + Est (n = 6) and OVX + Veh (n = 6) rats were anesthetized using isoflurane inhalation and killed by rapid decapitation. Brains were removed and the PFC dissected out and homogenized in 150 μl 20 mM Tris–HCl lysis buffer containing protease inhibitor [Complete Protease Inhibitor Cocktail tablet (Roche Pharmaceuticals, Indianapolis, IN, USA) dissolved in 10 ml buffer]. Protein levels were normalized using the Bradford Protein Assay, combined with 2 × sample buffer and boiled for 5 min at 95°C. Samples were then loaded onto a 20% SDS-page ready-gel (Bio-Rad, Philadelphia, PA, USA) and run at 100 V in 1 × Tris/Glycine/SDS running buffer (Bio-Rad) for 1 h. Protein was transferred onto a PVDF membrane at 100 V in 1 × Tris/Glycine/methanol transfer buffer for 1.5 h. The membrane was washed twice with TBST, and then blocked in 1% BSA-TBST for 1 h. After blocking, the membrane was rinsed briefly in TBST and then incubated overnight in primary antibody (alpha-2a AR 1:400, Sigma, St Louis, MO, USA; Milner et al. 1998) in 5% BSA-TBST. The membrane was then washed 3 × 10 min in TBST and incubated for 1 h in secondary antibody (1:2000 HRP-conjugated anti-rabbit, Vector Labs, Burlingame, CA, USA) in 5% BSA-TBST. Membranes were then washed 3 × 15 min in TBST and developed using an ECL detection kit (Amersham, Arlington Heights, IL, USA). After transfer, gels were stained with Coomassie dye to confirm equal lane loading. This technique was used rather than re-blotting for common loading control proteins like CREB because estrogen can alter the expression of such proteins (Lee et al. 2004).
Time course
The entire time course for the study (from rats arriving at the facility to terminal procedures) was approximately 10–12 weeks. This includes one week acclimation to the animal facility, OVX surgery and 2 weeks post-operative recovery, 2–3 weeks habituation and training on the T-maze, 1–2 weeks habituation to injection procedures, 2–3 weeks to find the lowest-impairing dose of FG7142 (rats could only receive one injection per week), and 1 week to do the co-injection experiment. All rats were killed on the same day to ensure equal duration of estrogen exposure.
Data analysis
Where appropriate, between- and within-subject comparisons were made with 2-way analysis of variance with repeated measures (ANOVA-R) using SPSS software. Western Blot band intensity was measured using NIH Image. Data are shown as group mean ± SEM.
Results
NE alpha-2-mediated reversal of FG7142 stress-induced PFC dysfunction
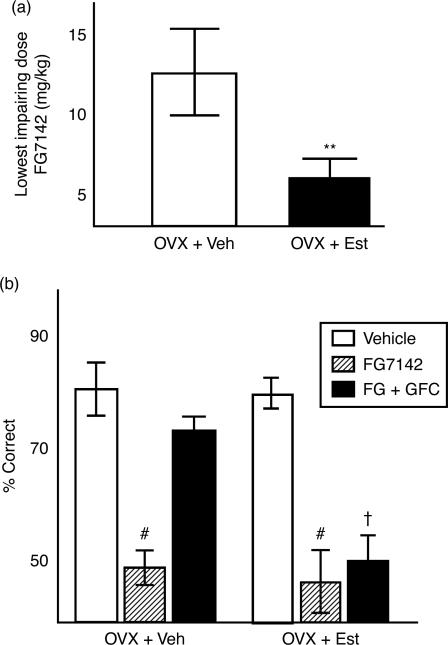
OVX + Est and OVX + Veh animals were stressed with an injection of FG7142, as in previous studies. The lowest possible impairing dose for each individual rat (determined previously) was administered in conjunction with 0.11 mg/kg GFC, a dose that successfully restored stress-induced PFC function in male rats. OVX + Veh rat required a higher dose of FG7142 to become impaired than did OVX + Est rats (Figure 1(a)), replicating previous results (Shansky et al. 2004). ANOVA revealed a significant between-subjects effect of estrogen (F[1,9] = 9.9, p = 0.01), a significant within subjects effect of FG7142 (F[2,18] = 59.9, p < 0.000001) and a significant interaction of estrogen and FG7142 (F[2,18] = 6.8, p < 0.007). Moreover, GFC reversed the stress-induced impairment in the OVX + Veh group, but did not have this effect in the OVX + E group (Figure 1(b)). A test of effects revealed! a significant effect of estrogen on the effects of GFC (F[1,9] = 36.4, p < 0.0002). Thus, even though they received a lower dose of FG7142, this dose of GFC was less effective in estrogen-exposed rats, while it completely reversed the memory impairments seen in the OVX + Veh group.
Figure 1.
(a) The lowest impairing dose of FG7142 for the OVX + Veh group was nearly three times greater than that for the OVX + Est group (10 ± 3.7 vs. 3.5 ± 1.2 mg/kg, **p < 0.007). (b) This dose of FG was then given in combination with 0.11 mg/kg GFC. The OVX + Veh group showed a reversal of working memory impairment, but the OVX + Est group did not (scores 74.3 ± 3.9 vs. 47.5 ± 5.5, respectively) #p < 0.000001 compared to performance with vehicle administration; †p < 0.0002 compared to OVX + Veh after FG7142 and GFC administration. Values are mean ± SEM, n = 12 rats per group.
The effects of estrogen on NE alpha-2a receptor protein levels in the PFC
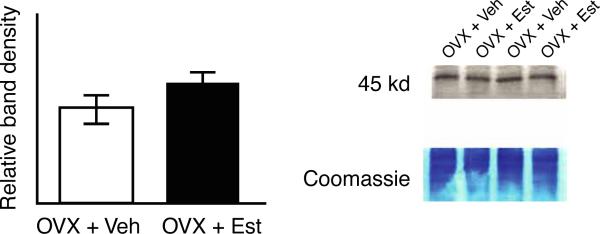
Western blot for NE alpha-2a receptor in the PFC of the OVX + Est and OVX + Veh groups are shown in Figure 2. No differences were seen in protein levels between groups. The mean band density for the OVX + Est group was 3031 ± 207 arbitrary units, while the mean band density for the OVX + Veh group was 2591 ± 220 arbitrary units, respectively, p > 0.05).
Figure 2.
Western blot for alpha-2a expression in the PFC. Examples are shown for two pairs of brains from the OVX + Veh and OVX + Est groups that were run in alternate lanes. No differences were seen between the OVX + Veh and OVX + Est groups. Values are mean ± SEM, n = 12 rats per group.
Discussion
The present studies demonstrate that the OVX Est and OVX + Veh groups are differentially responsive to a drug that acts as an agonist at alpha-2a receptors, without showing differences in the protein levels of these receptors. The OVX Est rats required almost a third of the dose of the pharmacological stressor FG7142 than the OVX + Veh rats to produce working memory deficits, replicating previous reports demonstrating that estrogen increases sensitivity to the working memory-impairing effects of pharmacological stress (Shansky et al. 2004), and of restraint stress (Shansky et al. 2006).
Estrogen-treated rats also were unresponsive to the restorative effects of the alpha-2a agonist GFC. A dose of GFC previously shown to reverse stress-related working memory impairment in male rats ameliorated this impairment in the OVX + Veh rats, but had no such effect in the OVX + Est rats. Notably, this absence of response to GFC occurred despite the fact that the OVX + Est rats received a relatively smaller dose of FG7142. This pattern of results suggests that the heightened sensitivity of the OVX + Est rats to the pharmacological stressor may be due to estrogen interfering with optimal alpha-2a activity.
The pharmacological stress and drug treatments used in this study were administered systemically, and thus can have widespread effects on the nervous system. However, it is likely that at least some of their effects occur in the medial PFC. The medial PFC is the brain region most needed for performance of the spatial delayed alternation task (Larsen and Divac 1978), and it is the region most sensitive to stress-induced increases in catecholamine release (Deutch and Roth 1990). Infusions of drug into the medial PFC in rats alter performance of the delayed alternation task: drugs that mimic stress-related catecholamine release (e.g., DA D1 agonists) impair performance (Zahrt et al. 1997), while infusions of guanfacine improve performance in this task (Ramos et al. 2006). Thus, if estrogen alters alpha-2a receptor signaling in the PFC, it would likely change task performance.
Against expectations, estrogen treatment had no effect on alpha-2a receptor protein levels in the medial PFC. These results are in contrast to those found by (Karkanias et al. 1997), who reported that 48 h of estradiol treatment reduced NE alpha-2 receptor binding in the frontal cortex, indicating a decrease in receptor number. The discrepancy may be due to the difference in duration of exposure, since in the present study the rats were exposed to estrogen for several months, rather than days, or due to methodological differences (binding assays vs. western blot). In particular, western blot analysis includes measures of internalized receptors that are less evident in binding assays. Another complication is that alpha-2a receptors are localized at multiple sites in the PFC, which likely have different or even opposing actions: e.g., presynaptically on NE axon terminals, post-synaptically on pyramidal cell spines, and on astrocytes (Aoki et al. 1998). Hence, estrogen might down-regulate alpha-2a receptor expression on neurons, while increasing expression in glia, but producing no change overall in crude measures such as western blotting. A more detailed understanding of the effects of estrogen on alpha-2a receptor expression in the PFC will likely require immuno-electron microscopy to visualize such changes.
The current findings of reduced efficacy of the alpha-2a agonist GFC, but maintained alpha-2a receptor protein expression indicate that modulation by estrogen of the behavioral response to adrenergic stimulation may be downstream of the receptor itself. Stimulation of post-synaptic alpha-2 receptors in the PFC engages Gi and inhibits cyclic AMP signaling (Wang et al. 2007). Estrogen is reported to uncouple alpha-2 receptors from Gi signaling in the hypothalamus (Ansonoff and Etgen 2001). Similar effects in the PFC would weaken the efficacy of GFC in blocking cognitive deficits during stress.
The ability of alpha-2a stimulation to rescue stress-induced working memory impairment is considered to arise from inhibition of cAMP signaling in the PFC. For example, infusion of either a D1 receptor or a cAMP agonist into the rat PFC can cause working memory deficits (Zahrt et al. 1996; Taylor et al. 1999), and the D1-mediated impairment is blocked by co-infusion of the cAMP antagonist, Rp-cAMPS. Conversely, the GFC effect is blocked by PFC-infusion of a cAMP agonist (Ramos et al. 2006). Similar effects are observed at the cellular level, whereby PFC neuronal firing is suppressed by agents that stimulate D1 receptors (Vijayraghavan et al. 2007) or increase cAMP level, while firing is rescued by agents that stimulate alpha-2a receptors or inhibit cAMP (Wang et al. 2007). Thus, it seems that the alpha-2a receptor offers its protective properties during stress by inhibiting cAMP production (Birnbaum et al. 2000; Ramos et al. 2006).
It cannot be ruled out that the current findings may be due to pre-synaptic alpha-2 receptor actions in locus coeruleus, the main source of NE for the PFC. Curtis et al. (2006) have demonstrated that the locus coeruleus shows greater stress-induced activity in females than in males, suggesting another possible mechanism by which sex differences in the cognitive response to stress may arise. However, this finding was observed to be independent of hormonal status, making it unlikely that the discrepancy was due to activational effects of estrogen.
Stress-related disorders like MDD and PTSD are often characterized by symptoms of PFC dysfunction, including working memory impairment. The current findings have important implications for how stress-related disorders might be treated in women, a group for whom the prevalence of such disorders is twice that reported for men (Weissman et al. 1996). A role for estrogen in promoting this discrepancy is supported by evidence that the difference in prevalence is observed to begin at puberty, and to disappear after the menopause (Halbreich and Lumley 1993). The influence of estrogen on the stress response has been studied with respect to many behaviors and biological measures, with interactions occurring in a region- and behavior-specific manner. Briefly, estrogen has been shown to protect or enhance cognitive performance during stress in tests of spatial ability (Bowman et al. 2002; Conrad et al. 2004), but to exacerbate stress-induced impairments on conditioning and working memory tasks (Wood and Shors 1998; Shansky et al. 2004). The mechanisms underlying these interactions are mostly unknown, but the present study suggests that estrogen can amplify the stress-induced working memory impairments by diminishing protective alpha-2a actions. Thus, it may be necessary to use drugs that suppress cAMP actions downstream from the alpha-2a receptor itself. Future pharmacological studies targeting intracellular signaling mechanisms in OVX ± Est animals will be crucial to furthering our understanding of the interactions between stress, estrogen and PFC function.
Acknowledgements
The authors would like to thank Tracy Sadlon, Lisa Civarella, Sam Johnson and Ellen Wittmack for their invaluable technical assistance. This work was done with support from a grant to AFTA from NIMH R21 070003.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden. The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ansonoff MA, Etgen AM. Receptor phosphorylation mediates estradiol reduction of alpha2-adrenoceptor coupling to G protein in the hypothalamus of female rats. Endocrine. 2001;14:165–174. doi: 10.1385/ENDO:14:2:165. [DOI] [PubMed] [Google Scholar]
- Aoki C, Venkatesan C, Go C-G, Forman R, Kurose H. Cellular and subcellular sites for noradrenergic action in the monkey dorsolateral prefrontal cortex as revealed by the immunocytochemical localization of noradrenergic receptors and axons. Cerebral Cortex. 1998;8:269–277. doi: 10.1093/cercor/8.3.269. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine and second messenger influences on prefrontal cortical networks of “representational knowledge”: A rational bridge between genetics and the symptoms of mental illness. Cereb Cortex. 2007;17(Suppl 1):i6–15. doi: 10.1093/cercor/bhm033. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Goldman-Rakic PS. Stress impairs prefrontal cortex cognitive function in monkeys: Role of dopamine. Soc Neurosci Abstr. 1990;16:164. [Google Scholar]
- Arnsten AFT, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys: Evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatry. 1998;55:362–369. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- Birnbaum SG, Gobeske KT, Auerbach J, Taylor JR, Arnsten AFT. A role for norepinephrine in stress-induced cognitive deficits: Alpha-1-adrenoceptor mediation in prefrontal cortex. Biol Psychiatry. 1999;46:1266–1274. doi: 10.1016/s0006-3223(99)00138-9. [DOI] [PubMed] [Google Scholar]
- Birnbaum SG, Podell DM, Arnsten AFT. Noradrenergic alpha-2 receptor agonists reverse working memory deficits induced by the anxiogenic drug, FG7142, in rats. Pharmacol Biochem Behav. 2000;67:397–403. doi: 10.1016/s0091-3057(00)00306-3. [DOI] [PubMed] [Google Scholar]
- Botteron KN, Raichle ME, Drevets WC, Heath AC, Todd RD. Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol Psychiatry. 2002;51:342–344. doi: 10.1016/s0006-3223(01)01280-x. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Zrull MC, Luine VN. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Res. 2001;904:279–289. doi: 10.1016/s0006-8993(01)02474-x. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Ferguson D, Luine VN. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 2002;113:401–410. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ, Levin ED. Effects of dopaminergic drugs on working and reference memory in rats. Pharmacol Biochem Behav. 1993;45:765–776. doi: 10.1016/0091-3057(93)90119-e. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Jackson JL, Wieczorek L, Baran SE, Harman JS, Wright RL, Korol DL. Acute stress impairs spatial memory in male but not female rats: Influence of estrous cycle. Pharmacol Biochem Behav. 2004;78:569–579. doi: 10.1016/j.pbb.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Bethea T, Valentino RJ. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31:544–554. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Roth RH. The determinants of stress-induced activation of the prefrontal cortical dopamine system. Prog Brain Res. 1990;85:367–403. doi: 10.1016/s0079-6123(08)62691-6. [DOI] [PubMed] [Google Scholar]
- Dohrenwend BP, Shrout PE, Link BG, Skodol AE, Stueve A. Life events and other possible risk factors for episodes of schizophrenia and major depression: A case-control study. In: Mazure Carolyn., editor. Does stress cause psychiatric illness? American Psychiatric Press; Washington, DC: 1995. [Google Scholar]
- Dorow R, Horowski R, Pashelke G, Amin M, Braestrup C. Severe anxiety induced by FG7142, a B-carboline ligand for benzodiazepine receptors. Lancet. 1983:98–99. doi: 10.1016/s0140-6736(83)90076-4. [DOI] [PubMed] [Google Scholar]
- Funk D, Li Z, Le AD. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: Relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138:235–243. doi: 10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Lumley LA. The multiple interactional biological processes that might lead to depression and gender differences in its appearance. J Affect Disord. 1993;29:159–173. doi: 10.1016/0165-0327(93)90030-n. [DOI] [PubMed] [Google Scholar]
- Ida Y, Elsworth J, Roth RH. Anxiogenic beta carboline FG7142 produces activation of NE neurons in specific brain regions of rats. Pharmacol Biochem Behav. 1991;39:791–793. doi: 10.1016/0091-3057(91)90166-y. [DOI] [PubMed] [Google Scholar]
- Izquierdo LA, Barros DM, Medina JH, Izquierdo I. Stress hormones enhance retrieval of fear conditioning acquired either one day or many months before. Behav Pharmacol. 2002;13:203–213. doi: 10.1097/00008877-200205000-00003. [DOI] [PubMed] [Google Scholar]
- Karkanias GB, Li CS, Etgen AM. Estradiol reduction of alpha-2-adrenoceptor binding in female rat cortex is correlated with decreases in alpha2A/D-adrenoceptor messenger RNA. Neuroscience. 1997;81:593–597. doi: 10.1016/s0306-4522(97)00359-x. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Lagrange AH, Wagner EJ, Ronnekleiv OK. Rapid effects of estrogen to modulate G protein-coupled receptors via activation of protein kinase A and protein kinase C pathways. Steroids. 1999;64:64–75. doi: 10.1016/s0039-128x(98)00095-6. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Driver KL, Oscar-Berman M, Wolfe J, Folsom S, Huang MT, Schlesinger L. Measures of prefrontal system dysfunction in posttraumatic stress disorder. Brain Cogn. 2001;45:64–78. doi: 10.1006/brcg.2000.1256. [DOI] [PubMed] [Google Scholar]
- Larsen JK, Divac I. Selective ablations within the prefrontal cortex of the rat and performance of delayed alternation. Physiol Psychol. 1978;6:15–17. [Google Scholar]
- Lee SJ, Camponmanes CR, Sikat PT, Greenfield AT, Allen PB, McEwen BS. Estrogen induces phosphorylation on cyclic AMP response element binding (pCREB) in primary hippocampal cells in a time-dependent manner. Neuroscience. 2004;124:549–560. doi: 10.1016/j.neuroscience.2003.11.035. [DOI] [PubMed] [Google Scholar]
- Mazure CM. Life stressors as risk factors in depression. Clin Psychol Sci Pract. 1998;5:291–313. [Google Scholar]
- McEwen BS. Effects of adverse experiences for brain structure and function. Biol Psychiatry. 2000;48:721–731. doi: 10.1016/s0006-3223(00)00964-1. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Krey LC, MacLusky NJ, McEwen BS. Steroid receptor levels in intact and ovariectomized estrogen-treated rats: An examination of quantitative, temporal and endocrine factors influencing the efficacy of an estradiol stimulus. Neuroendocrinology. 1981;33:158–165. doi: 10.1159/000123222. [DOI] [PubMed] [Google Scholar]
- Mikkelsen JD, Soderman A, Kiss A, Mirza N. Effects of benzodiazepines receptor agonists on the hypothalamic–pituitary–adrenocortical axis. Eur J Pharmacol. 2005;519:223–230. doi: 10.1016/j.ejphar.2005.06.049. [DOI] [PubMed] [Google Scholar]
- Milner TA, Lee A, Aicher SA, Rosin DL. Hippocampal alpha2a-adrenergic receptors are located predominantly presynaptically but are also found postsynaptically and in selective astrocytes. J Comp Neurol. 1998;395:310–327. [PubMed] [Google Scholar]
- Mizoguchi K, Yuzuihara M, Ishige A, Sasaki H, Chui D-H, Tabira T. Chronic stress induces impairment of spatial working memory due to prefrontal dopaminergic dysfunction. J Neurosci. 2000;20:1568–1575. doi: 10.1523/JNEUROSCI.20-04-01568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AFT, Jentsch JD, Roth RH. Dopamine and spatial working memory in rats and monkeys: Pharmacological reversal of stress-induced impairment. J Neurosci. 1996;16:7768–7775. doi: 10.1523/JNEUROSCI.16-23-07768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos BP, Stark D, Verduzco L, van Dyck CH, Arnsten AF. Alpha2A-adrenoceptor stimulation improves prefrontal cortical regulation of behavior through inhibition of cAMP signaling in aging animals. Learn Mem. 2006;13:770–776. doi: 10.1101/lm.298006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Glavis-Bloom C, Lerman D, McRae P, Benson C, Miller K, Cosand L, Horvath TL, Arnsten AFT. Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction. Mol Psychiatry. 2004;9:531–538. doi: 10.1038/sj.mp.4001435. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Rubinow K, Brennan A, Arnsten AF. The effects of sex and hormonal status on restraint-stress-induced working memory impairment. Behav Brain Funct. 2006;2:8. doi: 10.1186/1744-9081-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ. Acute stress rapidly and persistently enhances memory formation in the male rat. Neurobiol Learn Mem. 2001;75:10–29. doi: 10.1006/nlme.1999.3956. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Birnbaum SG, Ubriani R, Arnsten AFT. Activation of protein kinase A in prefrontal cortex impairs working memory performance. J Neurosci (Online) 1999;19:RC23. doi: 10.1523/JNEUROSCI.19-18-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley A, Nou E, Mazer JA, McCormick DA, Arnsten AF. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, Joyce PR, Karam EG, Lee CK, Lellouch J, Lepine JP, Newman SC, Rubio-Stipec M, Wells JE, Wickramaratne PJ, Wittchen H, Yeh EK. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276:293–299. [PubMed] [Google Scholar]
- Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci USA. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Arnsten AFT. Supranormal stimulation of dopamine D1 receptors in the prefrontal cortex impairs spatial working memory in rats. Soc Neurosci Abstr. 1996;22:1128. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AFT. Supranormal stimulation of dopamine D1 receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Chinitz JA, Lombardi U, Fig LM, Cameron OG, Liberzon I. Medial frontal cortex involvement in PTSD symptoms: A SPECT study. J Psychiatr Res. 1999;33:259–264. doi: 10.1016/s0022-3956(98)00060-0. [DOI] [PubMed] [Google Scholar]