Abstract
Kaposi sarcoma herpesvirus (KSHV)–associated multicentric Castleman disease (MCD) is a lymphoproliferative disorder most commonly observed in HIV-infected patients. It is characterized by KSHV-infected plasmablasts that frequently express lytic genes. Patients manifest inflammatory symptoms attributed to overproduction of KSHV viral IL-6, human IL-6, and human IL-6. There is no standard therapy and no established response criteria. We investigated an approach targeting 2 KSHV lytic genes, ORF36 and ORF21, the protein of which, respectively, phosphorylate ganciclovir and zidovudine to toxic moieties. In a pilot study, 14 HIV-infected patients with symptomatic KSHV-MCD received high-dose zidovudine (600 mg orally every 6 hours) and the oral prodrug, valganciclovir (900 mg orally every 12 hours). Responses were evaluated using new response criteria. A total of 86% of patients attained major clinical responses and 50% attained major biochemical responses. Median progression-free survival was 6 months. With 43 months of median follow-up, overall survival was 86% at 12 months and beyond. At the time of best response, the patients showed significant improvements in C-reactive protein, albumin, platelets, human IL-6, IL-10, and KSHV viral load. The most common toxicities were hematologic. These observations provide evidence that therapy designed to target cells with lytic KSHV replication has activity in KSHV-MCD. This trial was registered at www.clinicaltrials.gov as #NCT00099073.
Introduction
Multicentric Castleman disease (MCD) is a rare disorder characterized by inflammatory symptoms that include fever, splenomegaly, lymphadenopathy, respiratory symptoms, edema, effusions, cytopenias, hypoalbuminemia, and elevated inflammatory markers.1,2 Kaposi sarcoma-herpesvirus (KSHV), also called human herpesvirus-8,3 is the cause of a sizable subset of MCD,4,5 especially MCD arising in immunosuppressed patients, such as those infected with HIV. KSHV is also the etiologic agent of Kaposi sarcoma (KS) and primary effusion lymphoma (PEL); not uncommonly, patients manifest more than 1 of these disorders.
KSHV-associated MCD (KSHV-MCD) is diagnosed pathologically, usually through a lymph node biopsy demonstrating hypocellular germinal centers with vascularized core and proliferation of perifollicular monotypic plasmablastic cells that usually express IgM and λ.6 A number of these plasmablastic cells are KSHV-infected, expressing KSHV latency associated nuclear antigen.7,8 A sizable subset of these cells express a KSHV-encoded viral interleukin-6 (vIL-6),5 a gene whose mRNA is sometimes detectable in latently infected cells, and is significantly up-regulated with lytic activation.5,9–12 KSHV-MCD patients often have detectable serum vIL-6, and inflammatory symptoms in this condition are attributed in part to vIL-6.13 Elevated serum human IL-6 (hIL-6) and IL-10 may also contribute to KSHV-MCD symptomatology.14 In addition, KSHV-MCD is unusual in that a substantial proportion of the KSHV-infected plasmablasts have lytic KSHV gene expression.9
Clinically, KSHV-MCD often waxes and wanes, but without treatment, it is generally fatal within 2 years.1 Causes of death include multiorgan failure, progressive KS, immunoblastic lymphomas, or intercurrent infections. A pooled evaluation of 86 published cases between 1985 and 2006 reported median survival of approximately 12 months, although fatality rate was less in patients who received highly active antiretroviral therapy (HAART).15 There is evidence that KSHV-MCD incidence is increasing with widespread use of HAART.16
There is no standard treatment for KSHV-MCD. Furthermore, there are currently no standardized response criteria for MCD, making it difficult to compare results of different therapeutic approaches. Successful treatment has been reported with various chemotherapeutic agents, including vincristine, vinblastine, etoposide, cyclophosphamide, and doxorubicin, alone or in combination1,15; as well as with ganciclovir,17 IFN-α,18,19 and thalidomide.20,21 Steroids have been described to reduce inflammatory symptoms in MCD not associated with KSHV.22 With all of these modalities, relapse is common. The best-studied agent in KSHV-MCD is the monoclonal anti-CD20 antibody rituximab, either as first-line monotherapy23 or after chemotherapy.24 Whereas rituximab was often effective in the management of symptoms and correction of biochemical abnormalities, KS flared in 35% to 67% of patients. Disease-free survival at 1 to 2 years was 77% to 79%. Thus, more effective therapies for KSHV-MCD are needed.
Our group is exploring selectively targeting KSHV-MCD plasmablasts based on their expression of KSHV lytic genes.25 One KSHV lytic gene, ORF36, encodes a phosphotransferase that activates ganciclovir to a toxic triphosphate moiety, and another, ORF21, encodes a thymidine kinase that phosphorylates zidovudine (AZT).25–27 We previously showed that PEL cells in which KSHV was lytically activated produced increased amounts of AZT and ganciclovir triphosphate moieties. AZT and ganciclovir had synergistic toxicity at doses attainable in patients, in these PEL lines.25 We hypothesized that the combination of AZT and ganciclovir would selectively target the KSHV-infected plasmablasts in KSHV-MCD and have utility in the treatment of symptomatic disease. To translate these findings, we conducted a pilot study of high-dose AZT and valganciclovir (VGC), an orally available prodrug of ganciclovir, in patients with symptomatic KSHV-MCD. In conducting this study, we also prospectively defined clinical, biochemical, and radiographic criteria to assess responses to KSHV-MCD, and piloted the use of these criteria in assessing responses.
Methods
Study population
Starting in 2004, patients with pathologically confirmed KSHV-associated MCD were enrolled in a clinical research protocol to investigate the natural history of the disease and assess virus-activated cytotoxic therapy with AZT/VGC. Patients with symptomatic MCD, defined as at least 1 clinical symptom (Table 1) and at least 1 laboratory abnormality (elevated C-reactive protein [CRP], hyponatremia, hypoalbuminemia, thrombocytopenia, or anemia) attributed to KSHV-MCD, were eligible for treatment with AZT/VGC. There was no limitation on number of prior therapies. Per protocol, AZT/VGC was used as initial therapy in symptomatic patients, except those for whom this approach did not appear appropriate; patients with advanced KS, diffuse large B-cell lymphoma, or with life-threatening disease (ie, Eastern Cooperative Oncology Group performance status of 4 or end-organ damage) received alternate biochemotherapy regimens instead of AZT/VGC. The protocol (#NCT00099073) was approved by the National Cancer Institute Institutional Review Board. All patients gave written informed consent in accordance with the Declaration of Helsinki.
Table 1.
Baseline patient characteristics
| Characteristic | Value |
|---|---|
| Sex | |
| Men | 13 |
| Women | 1 |
| Race/ethnicity | |
| African | 3 |
| Black | 3 |
| White | 7 |
| Hispanic (Puerto Rican) | 1 |
| Median age, y (range) | 41 (28-56) |
| Median ECOG Performance Status (range) | 2 (1-3) |
| Median time since HIV diagnosis, y (range) | 6.5 (0.3-18) |
| Median time on antiretroviral therapy, y (range) | 3.5 (2 wk to 13 y) |
| HIV viral load < 50 copies/mL, no. (%) of patients | 9 (64) |
| Median CD4, cells/mL (range) | 270 (67-1319) |
| Median time since MCD diagnosis, mo (range) | 1.5 (0.5-45) |
| History of KS, no. (%) of patients | 8 (57) |
| History of KSHV-associated lymphoma, no. (%) of patients | 1 (7) |
| Prior therapies* | |
| Median no. (range) | 1 (0-7) |
| Prior therapy, no. (%) of patients | 8 (57) |
| Clinical symptoms, no. (%) of patients | |
| Fever/night sweats | 13 (93) |
| Fatigue | 13 (93) |
| Weight loss | 12 (86) |
| Respiratory symptoms | 12 (86) |
| Gastrointestinal symptoms | 11 (79) |
| Neuropathy | 5 (36) |
| Headache | 5 (36) |
| Edema | 3 (21) |
| Rash | 3 (21) |
| Myalgia | 2 (14) |
| Median biochemical parameters (range) | |
| CRP, mg/dL | 7.5 (1-35.9)† |
| Albumin, g/dL | 2.8 (1.5-3.5) |
| Sodium, mEq/L | 134 (127-142) |
| Platelets, × 103/μL | 115 (16-347) |
| Hemoglobin, g/dL | 10.1 (7.7-12.7) |
| Radiographic parameters | |
| Median spleen size, cm (range) | 18.5 (10-26.5) |
| Enlarged lymph nodes, no. of patients | 12 |
Number of different regimens, including steroids, used to treat MCD.
CRP was assayed using Siemens Dimension Vista platform (Siemens AG); from study inception until May 2009 a standard sensitivity assay (sCRP) was used, which was subsequently replaced by a high sensitivity (hsCRP) assay. Correction of CRP for values obtained after May 2009 was performed using a formula: [sCRP = (hsCRP − 0.56)/0.93], which was validated by the National Institutes of Health Clinical Center Department of Laboratory Medicine. Normal CRP < 0.04 mg/dL.
Treatment regimen
Treatment consisted of AZT 600 mg orally every 6 hours combined with VGC 900 mg orally every 12 hours. Substitution with AZT 300 mg intravenously every 6 hours combined with ganciclovir 5 mg/kg intravenous every 12 hours was allowed for inpatients not able to tolerate or absorb oral medications. Patients initially received AZT/VGC for 7 days, with the option of continuing for up to 21 days during the first cycle if patients had substantial symptoms after the first 7 days. Patients with severe MCD symptoms and laboratory abnormalities could receive corticosteroids during initial therapy, generally consisting of a single dose or a short steroid taper at the beginning of the first cycle.
Given the risk of hematotoxicity with prolonged dosing high-dose AZT/VGC, subsequent 21-day cycles generally consisted of 7 days of therapy followed by 14 days off therapy, although the protocol allowed for a longer durations of treatment at investigator discretion. Concurrent antiretroviral therapy was generally adjusted during the periods of treatment with AZT/VGC to avoid administration of 4 antiretroviral agents, including 3 nucleoside reverse-transcriptase inhibitors, as long as there was no evidence of substantial HIV resistance to AZT. Patients continued on AZT/VGC therapy as long as there was evidence of clinical benefit. Therapy was continued several cycles beyond initial improvement of clinical symptoms with the aim of treating residual subclinical disease. The ultimate goal of therapy was complete resolution of clinical, biochemical, and radiographic abnormalities attributed to MCD with long-term remission of disease. AZT/VGC was discontinued in patients with progressive symptoms or dose-limiting toxicities, and alternative therapeutic approaches were used.
Response evaluations
Patients were monitored for clinical, biochemical, and radiographic responses (Table 2). Evaluation included structured review of symptoms, physical examination, and laboratory studies day 1 of each cycle, or at the time progression. Symptoms were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, Version 3. Clinical decisions were based on patient performance status, and review of symptoms probably or definitely attributed to KSHV-MCD (Table 1), as well as clinical laboratory parameters, including complete blood counts, blood chemistries, and CRP. Patient samples were obtained for correlative studies, including cell-associated KSHV-viral load, serum vIL-6, and serum multiplex cytokine analyses. These correlative biomarkers did not guide clinical decision-making.
Table 2.
KSHV-associated MCD response criteria
| Response category | Response and criteria |
|---|---|
| Clinical response | CR |
| Full resolution of all signs and symptoms attributable to MCD, lasting 1 cycle (3-4 wk depending on regimen); requires normalization of involved nodal areas on physical examination | |
| SFD | |
| Full resolution of all symptoms attributable to MCD | |
| PR | |
| Improvement in at least 50% of signs and symptoms by at least 1 grade (NCI-CTCAE, Version 3), with no increased MCD-related increases, lasting 1 cycle (3-4 wk depending on regimen) | |
| SD | |
| No change in signs and symptoms of MCD meeting criteria for CR, PR, or PD | |
| PD | |
| Worsening of 2 or more symptoms by at least 1 grade | |
| Biochemical response* | CR |
| Normalization of abnormalities attributed to MCD in the following laboratory values: hemoglobin, platelets, albumin, sodium, and CRP, lasting 1 cycle (3-4 wk depending on regimen) | |
| PR | |
| At least 50% improvement in all laboratory value abnormalities attributable to MCD, lasting 1 cycle (3-4 wk depending on regimen) | |
| SD | |
| No change in biochemical parameters that meet criteria for CR, PR, or PD | |
| PD | |
| ≥ 25% and 1 grade worsening of at least 2 biochemical parameters attributable to MCD OR clear deterioration in one parameter with negative impact on physiologic or health status | |
| Radiographic response | CR |
| Normalization of all lymph nodes to < 1.5 cm in greatest transverse dimension, with decrease to < 1 cm of lymph nodes that measure 1.1-1.5 cm at baseline, or 75% decrease in SPD of measured lymph nodes; spleen < 12 cm greatest dimension, no pleural effusions | |
| Cru | |
| Residual lymph node mass > 1.5 cm or spleen > 12 cm that has decrease by ≥ 75% and does not change over 1 y | |
| PR | |
| For lymph nodes, ≥ 50% decrease in SPD of 6 dominant nodes; for spleen, ≥ 50% decrease in longest transverse dimension | |
| SD | |
| Not meeting criteria for CR, CRu, PR, or PD | |
| PD | |
| For lymph nodes, ≥ 25% increase in the SPD; for spleen, increase ≥ 25% in longest dimension | |
| Overall response | CR |
| CR in all categories | |
| PR | |
| PR or better in all categories | |
| SD | |
| SD or better in all categories | |
| PD | |
| Progression in any 1 category |
Data are based on signs and symptoms probably or definitely attributed to KSHV-MCD.
CR indicates complete response; SFD, symptom-free disease; PR, partial response; NCI-CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; SD, stable disease; PD, progressive disease; SPD, sum products of the diameters; and Cru, complete response, unconfirmed.
Determination of partial or complete biochemical responses was not based on improvements in hemoglobin or platelet counts that occurred within 3 weeks (one cycle) of transfusions or growth factor support.
There are no standard MCD response criteria. One aim of this protocol was to develop MCD response criteria and gain experience with and assess the feasibility and utility of these criteria. Criteria were defined for responses in 3 categories: clinical, biochemical, and radiographic (Table 2), incorporating abnormalities commonly seen in KSHV-MCD.1 Clinical responses were considered primary. A clinical complete response required full resolution of the clinical signs and symptoms probably or definitely attributable to MCD lasting 1 cycle (3-4 weeks). Table 1 shows categories of symptoms noted at baseline and used in response evaluations. A category of “symptom-free disease” was defined as full resolution of all clinical symptoms attributed to MCD but did not require resolution of asymptomatic lymph nodes noted on physical examination or maintenance of a full cycle.
Biochemical responses were based on resolution of laboratory abnormalities generally attributable to MCD. Biochemical responses as defined in the original protocol required 50% or greater relative improvement (normalization toward the lower or upper limit of normal) in each of 12 laboratory parameters. However, as we gained experience in KSHV-MCD, it became evident that these criteria were frequently affected by conditions other than the MCD and thus did not provide a good assessment of the disease status. Before performing formal evaluation of biochemical response in any patient, criteria were revised with a study amendment to include only the 5 most commonly abnormal biochemical parameters: CRP, hemoglobin, platelets, albumin, and sodium (Table 2). In evaluating biochemical response, improvements in hemoglobin and platelets were considered only if they occurred or were sustained independent of transfusion or erythroid growth factors. All patients were assessed uniformly using revised criteria.
Radiographic responses were evaluated by computed tomography scans obtained at baseline, every 2 cycles until clinical stabilization, and then every 3 months. Radiographic response criteria were based on response criteria for malignant lymphoma established by the International Working Group.28 Modifications of radiographic criteria for KSHV-MCD included use of computed tomography imaging alone, with [18F]-fluorodeoxyglucose-positron emission tomography explored only for research purposes, specific criteria for resolution of splenomegaly, and specific requirement for resolution of pleural effusions. Overall response integrated the clinical, biochemical, and radiographic responses. Importantly, complete overall response using these criteria required resolution of all abnormalities attributed to MCD.
vIL-6 assay
KSHV-encoded vIL-6 was measured using a previously described sandwich enzyme-linked immunosorbent assay that used clone v6m 31.2.4 murine monoclonal anti–vIL-6 antibody,29 and which had been modified from an earlier vIL-6 assay.13,30,31 The lower limit of detection for serum vIL-6 was 1560 pg/mL and 95% upper limit of reactivity in serum from health blood donors was 2850 pg/mL. The assay did not cross-react with hIL-6.29 In evaluating patients with known KSHV-MCD at different time points, vIL-6 values are presented down to 1560 pg/mL.
KSHV quantitative real-time PCR
KSHV-viral load was measured using previously described methods.29,32,33 Briefly, DNA was extracted from peripheral blood mononuclear cells (PBMCs) and KSHV DNA was detected using primers for the K6 gene region.32 The number of cellular equivalents was determined using a quantitative assay for human endogenous retrovirus 3,34 and KSHV viral load was reported as viral DNA copies per million PBMCs.33
Other assays
Serum hIL-6, IL-10, and 5 other cytokines (IL-1β, IL-8, IL-12 p70, IFN γ, and TNF-α) were evaluated using the MSD 96-Well Multiarray Proinflammatory 7-plex Assay (Meso-Scale Discovery) and the Sector Imager. As previously described, the Meso-Scale hIL-6 assay did cross-react with vIL-6.29 CD4 counts were assessed by FACS. Plasma HIV-1 mRNA was measured by quantitative RNA polymerase chain reaction using Roche Amplicor HIV-1 Monitoring Kits (Roche Diagnostic Systems).
Statistical methods
As a pilot study evaluating AZT/VGC efficacy in the treatment of symptomatic KSHV-MCD, we evaluated the best clinical, biochemical, radiographic, and overall responses. The protocol used a 2-stage Simon optimal design to evaluate AZT/VGC, with α = 0.15, β = 0.15, and a targeted 25% response rate. If 1 or more of the first 8 patients had at least a partial response in 1 response parameter (clinical, biochemical, or radiographic), 14 patients were to be treated with AZT/VGC. In addition, progression-free survival (time from starting therapy to time of disease progression requiring a change in therapy or death) and overall survival were determined using Kaplan-Meier methodology. For survival analysis, patient status was assessed from the start of therapy until September 7, 2010. Clinical and research laboratory correlatives of MCD disease activity were evaluated as follows: paired nonparametric analysis was performed using an exact Wilcoxon signed-rank test comparing changes from baseline laboratories to those at a time of best clinical response for: platelets, hemoglobin, albumin, CRP, vIL-6, hIL-6, IL-10, and KSHV viral load. hIL-6, IL-10, and KSHV viral load were log10 transformed for analyses; undetectable KSHV viral loads were converted to 1 to permit valid log10 transformation. Because vIL-6 was often below the threshold of detection, vIL-6 was considered detectable or not, with changes evaluated using the McNemar test for paired categorical data. Statistical analyses of correlative parameters were exploratory, with no formal correction for multiple comparisons. However, because multiple parameters were examined, P values ≤ .01 are interpreted as statistically significant, values between .01 and ≤ .05 indicate strong trends, and values > .05 are considered not significant. All P values are 2-sided. Toxicities across all cycles possibly, probably, or definitively attributed to therapy were tabulated.
Results
Patient characteristics
Of 22 patients enrolled on the KSHV-MCD protocol between 2004 and 2009, 14 patients were treated with AZT/VGC. Of the other 8 patients, 4 were clinically asymptomatic and felt not to require therapy, 2 had advanced KS requiring chemotherapy, 1 had diffuse large B-cell lymphoma, and 1 had life-threatening MCD treated with a more aggressive regimen. Baseline characteristics of patients treated with AZT/VGC are outlined in Table 1. All had HIV infection and were on HAART, although several patients, including 4 of 5 with detectable viral loads at entry, reporting missing some doses of their antiretroviral drugs when they were ill with their MCD flare. Eight had received previous therapy for their MCD, and had received up to 7 different prior regimens. Prior therapies included cyclophosphamide, doxorubicin, vincristine and prednisone; rituximab; VGC monotherapy; and intravenous immunoglobulin.
Four patients were on glucocorticoids before starting AZT/VGC; in such cases, steroids were continued during the initial therapy and then tapered when possible. Four additional patients received at least a single dose of glucocorticoids during the first cycle (2 for thrombocytopenia, 2 for clinical symptoms). Glucocorticoids were discontinued during the first cycle in 5 of these 8 patients, whereas 3 patients remained steroid-dependent throughout therapy. Two of these 3 patients were switched to an alternate therapy for MCD during cycle 2, and 1 was maintained on physiologic dosing of hydrocortisone because of adrenal insufficiency. One additional patient who discontinued glucocorticoids during cycle 1 received methylprednisolone for sinus inflammation during cycle 7. In addition, patients sometimes required transfusions because of cytopenias attributed to MCD activity; red blood cell transfusions were required in 9 patients and platelet transfusions in 7 patients. Two patients received erythropoietin.
Clinical responses
Patients received a median of 8.5 cycles of therapy (range, 1-29 cycles). Five (36%) were symptom-free after the first cycle. With additional therapy, 12 of 14 (86%) had a major response (partial response or better) as a best clinical response (Table 3). Seven (50%) achieved a clinical complete response and 2 achieved a partial response. Three achieved symptom-free disease not classified as a complete clinical response because of either symptom-free duration of > 1 cycle (2 patients) or persistent adenopathy on physical examination with recurrence of clinical symptoms after 5 cycles (one patient). The 2 patients who did not have a clinical partial response or better had clinical stable disease at the end of cycle 1; given lack of improvement in clinical symptoms, each received an alternate regimen after 19 to 28 days of AZT/VGC. One of these patients was found to have intercurrent bacterial endocarditis. Notably, the 6 patients who never received glucocorticoids during AZT/VGC treatment all had a clinical major response; 4 had a complete response and 2 had a partial response. In 8 patients who received at least 1 dose of glucocorticoids, 3 achieved a complete response, 3 symptom-free disease, and 2 had stable disease.
Table 3.
Best response to treatment with AZT/VGC in 14 patients with symptomatic KSHV-MCD
| Response category | Best response | No. (%) |
|---|---|---|
| Clinical response | Complete response | 7 (50) |
| Symptom-free disease | 3 (21) | |
| Partial response | 2 (14) | |
| Major clinical response | 12 (86) | |
| Stable disease | 2 (14) | |
| Biochemical response | Complete response | 3 (21) |
| Partial response | 4 (29) | |
| Major biochemical response | 7 (50) | |
| Stable disease | 6 (43) | |
| Progressive disease | 1 (7) | |
| Radiographic response* | Complete response | 4 (29) |
| Complete response, unconfirmed | 1 (7) | |
| Major radiographic response | 5 (36) | |
| Stable disease | 8 (67) | |
| Overall response | Complete response | 3 (21) |
| Partial response | 1 (7) | |
| Stable disease | 9 (64) | |
| Progressive disease | 1 (7) |
Major clinical response = complete response + symptom-free disease + partial response; major biochemical response = complete response + partial response; and major radiographic response = complete response + complete response unconfirmed + partial response.
One patient was not evaluable radiographically.
Biochemical and radiographic responses
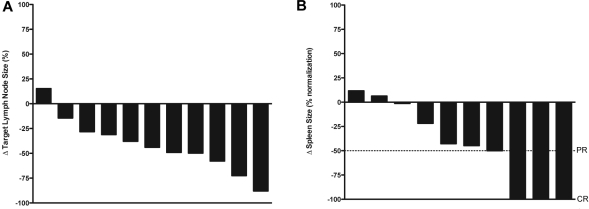
Seven patients (50%) had biochemical major response (partial response or better), with 3 (21%) achieving complete responses and 4 (29%) achieving partial responses. Six of the remaining patients met criteria for stable disease and 1 had worsening biochemical parameters when his course was complicated by bacterial endocarditis with cerebral emboli. Among 3 patients receiving steroids at the time of determination of best biochemical response, 2 were classified as stable disease and 1 as progressive disease. Thirteen patients completed the required radiographic studies and were evaluable for radiographic response. Four patients (29%) achieved a radiographic complete response as best response, which required normalization of both lymphadenopathy and splenomegaly, and 1 patient had a response unconfirmed based on residual splenomegaly (spleen 12.5 cm) that was unchanged over 12 months. Eight patients had radiographic stable disease, although decreases in spleen and lymph node size were common (Figure 1).
Figure 1.
Best radiographic responses. (A) Percentage change in sum of the products of 6 representational lymph nodes at time of best radiographic response compared with baseline, in the 11 patients with pathologic lymphadenopathy at baseline evaluable. Patients can have a radiographic complete response with < 100% decrease in sum of the products of representational lymph nodes as long as there is no pathologic adenopathy at time of evaluation. (B) Percentage change in longest dimension of spleen size (normalized to spleen upper limit of normal = 12 cm) at time of best radiographic response compared with baseline, 10 patients with splenomegaly at baseline evaluable, maximum normalization = 100%.
Overall responses
Patients were evaluated individually in each of the 3 categories at each cycle, and overall responses were conservatively defined as the lowest of the 3 categories. Overall response categorization was affected by the fact that clinical responses generally preceded biochemical and radiographic responses (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Based on stringent protocol-defined criteria, 4 of 14 patients (29%) achieved an overall partial response or better, including 3 overall complete responses and 1 overall partial response. Two of these 4 patients received limited glucocorticoids during the first cycle of therapy, but otherwise none received glucocorticoids during their treatment for up to 20 cycles. In 9 of the remaining 10 patients, best overall response was stable disease, and in 1 patient, best overall response was progressive disease. It should be noted, that of 9 patients with overall stable disease, only 1 had stable disease in all 3 categories; 8 had a partial response or better clinically, and 4 had a major response in other categories.
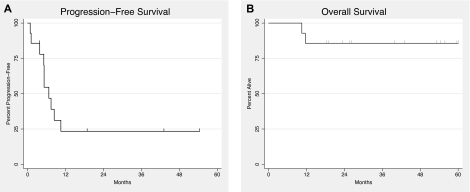
Progression-free and overall survival
With a median potential 42.8-month follow-up, median progression-free survival was 6 months (Figure 2A) and the estimated proportion of patients who had not progressed at month 12 is 23%. Three patients who obtained a lasting overall complete response were treated for 13, 20, and 29 cycles, which was 9, 10, and 22 cycles beyond resolution of clinical and biochemical abnormalities attributed to KSHV-MCD. They remain symptom-free for 10, 29, and 33 months after stopping AZT/VGC. One patient with an overall partial response when last evaluated died of untreated systemic Streptococcus pneumoniae infection with possible progressive MCD as well. In the remaining 10 patients, AZT/VGC therapy was changed for overall disease recurrence or progression (8 patients), worsening KSHV-associated extracavitary PEL (one patient), or investigator preference because of inadequate clinical improvement (one patient with overall stable disease at last evaluation). Patients who recurred or progressed went on to receive 1 or more additional therapies; subsequent therapies included a combination of rituximab and liposomal doxorubicin (7), EPOCH [infusional etoposide, vincristine, doxorubicin, with oral prednisone, and bolus cyclophosphamide] (2), IFN-α (7), subsequent additional AZT/VGC (2), and rituximab monotherapy (1).
Figure 2.
Kaplan-Meier survival function. (A) Progression-free survival. (B) Overall survival. Hatch marks represent time subjects censored.
Median overall survival has not been reached (Figure 2B). Twelve of 14 patients remain alive, and overall survival at 12 months and beyond is 86% (95% confidence interval, 60%-96%). In addition to the patient described in the preceding paragraph who died of pneumococcal infection, an additional patient died at home with febrile neutropenia while being treated with combination chemotherapy for progressive extracavitary PEL.
Changes in biochemical laboratory parameters
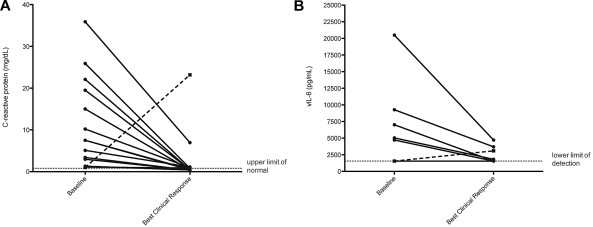
Changes in the 5 individual laboratory parameters used in the response criteria were assessed in each patient from the start of therapy to the time of best clinical response. Analyses were performed first in all 14 patients (Table 4), then repeated excluding the patient whose clinical course was complicated by bacterial endocarditis with cerebral emboli. Statistically significant changes or strong trends were observed for decreases in CRP (P = .0085; Figure 3A), increases in albumin (P = .0033), and increases in platelet counts (P = .02), whereas changes in sodium (P = .44) and hemoglobin (P = .31) were not statistically significant. Excluding the patient with bacterial endocarditis, changes in CRP (P = .0002), albumin (P = .0007), and platelets (P = .0081) were all statistically significant, whereas changes in sodium and hemoglobin were not. In addition, median CRP decreased across patients over the first 4 cycles (supplemental Figure 1).
Table 4.
Changes in biochemical parameters from baseline to time of best clinical response in patients with KSHV-MCD treated with AZT/VGC
| Parameter | n | Median baseline (range) | Median best clinical response (range) | Median change (range) | P |
|---|---|---|---|---|---|
| CRP, mg/dL | 14 | 7.5 (1, 35.9) | 0.5 (< 0.4, 23.2) | −7 (22.2, −29) | .0085 |
| Albumin, g/dL | 14 | 2.8 (1.5, 3.5) | 3.4 (1.4, 4.3) | 0.6 (−0.6, 2.4) | .0033 |
| Sodium, mEq/L | 14 | 134 (127, 142) | 136 (131, 141) | 0 (−6, 8) | .46 |
| Hemoglobin, g/dL | 14 | 10.1 (7.7, 12.7) | 11.2 (7, 14.1) | 0.85 (−2.2, 5.1) | .31 |
| Platelets, × 1000/μL | 14 | 115 (16, 347) | 206 (21, 477) | 71 (−103, 244) | .02 |
| hIL-6, pg/mL* | 13 | 17.9 (2.1, 171.5) | 5.8 (1.8, 192.2) | −5.4 (84.2, −123.7) | .027 |
| IL-10, pg/mL | 13 | 617 (5.6, 573 925) | 22.8 (1.7, 38 875) | −283 (33 000, −573,872) | .0002 |
| KSHV viral load, copies/106 PBMCs | 13 | 17 637 (0, 3.9 million) | 231 (0, 2.4 million) | −9787 (2.3 million, −2.8 million) | .021 |
Matched comparisons were performed using an exact Wilcoxon signed-rank test, using log10 transformation for hIL-6, IL-10, and KSHV viral load. Values for hIL-6, IL10, and KSHV viral load include only the 13 patients evaluable at both baseline and time of best clinical response.
Using this assay, the mean serum hIL-6 level in healthy donors is 2.3 pg/mL (SD 1.1 pg/mL).35
Figure 3.
Changes in C-reactive protein and viral IL-6 in patients with symptomatic KSHV-MCD from baseline to time of best clinical response. Wilcoxon matched-pair signed-rank test comparing changes from baseline laboratories to a time point of best clinical response is performed on all evaluable patients (14 patients evaluable for CRP, 12 patients evaluable for vIL-6) and then repeated with exclusion of 1 patient whose assessment was confounded by development endocarditis *(■----■) in (A) C-reactive protein (mg/dL), (P = .0085, *P = .0002) and (B) vIL-6 (pg/mL; not significant). In 6 patients, vIL-6 was undetectable both at baseline and time of best clinical response.
Immunologic and virologic studies
We evaluated immunologic and virologic parameters previously demonstrated to be associated with MCD activity, including vIL-6, hIL-6, IL-10, and KSHV viral load. For each patient, baseline values were compared with values from the first time the tests were obtained after the best clinical response. Thirteen patients were evaluable for changes in cytokines and KSHV viral load. Relatively large decreases that were either statistically significant or exhibiting strong trends were observed for IL-10 (P = .0002), hIL-6 (P = .027), and KSHV viral load (P = .021). Excluding the patient with bacterial endocarditis, observed differences were statistically significant for IL-10 (P = .0005), hIL-6 (P = .001), and KSHV viral load (P = .0049). Each of 5 patients with elevated vIL-6 at entry had a decrease during therapy; however, with this small number, this decrease was not statistically significant (Figure 3B).
Six of 12 patients who initially achieved partial clinical response or better subsequently had clinical progression of MCD. In all cases, clinical deterioration was accompanied by rising CRP; compared with a median CRP at the time of best clinical response of 0.49 mg/dL (range, < 0.4-0.95 mg/dL), median CRP at time of clinical progression was 9.8 mg/dL, (range, 5.3-14.2 mg/dL). vIL-6 was detectable in 3 of these 6 patients at the time of relapse (range, 2771-12 335 pg/mL) and in all cases was increased compared with the level at time of best clinical response (range, < 1560-3693 pg/mL). The other 3 patients had undetectable serum vIL-6 at both time points. Patients who relapsed all had increasing hIL-6 (median increase, 33.2 pg/mL; range, 7.3-192.2 pg/nL), IL-10 (median increase, 9324 pg/mL; range, 839-15 549 pg/mL), and KSHV viral load (median increase, 91 043 copies/106 PBMCs; range, 1600-297 830 copies/106 PBMCs).
Toxicities
Principal toxicities possibly, probably, or definitely attributable to AZT/VGC are outlined in Table 5 and were mainly hematologic. Anemia, thrombocytopenia, and neutropenia were observed, although the contribution of drugs to these abnormalities was often difficult to separate from underlying MCD. In total, 4 major infections were noted: 1 patient developed both a catheter-related urinary tract infection and Clostridium difficile colitis, 1 developed group G Streptococcus endocarditis while neutropenic and receiving high-dose dexamethasone, and 1 patient developed pneumococcal sepsis. This latter patient refused therapy for his sepsis and died of this complication, which was considered unlikely to be the result of AZT/VGC. In addition, 1 patient had neutropenic fever. No adverse effect on HIV control was noted, and HIV viral load was undetectable at the end of therapy in all patients. Fluctuations in CD4 counts were common in the setting of symptomatic MCD and were considered unlikely to be the result of AZT/VGC.
Table 5.
Select event-based toxicities possibly, probably, or definitely attributed to AZT combined with VCG, over a total of 137 cycles
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Hematologic | ||||
| Anemia | 35 (26) | 10 (7) | 11 (8) | 5 (4) |
| Thrombocytopenia | 14 (10) | 4 (3) | 4 (3) | |
| Neutropenia | 7 (5) | 17 (12) | 17 (12) | 4 (3) |
| Bleeding | 1 (< 1) | — | — | — |
| Gastrointestinal | ||||
| Abdominal pain | 7 (5) | 1 (< 1) | — | — |
| Anorexia | 11 (8) | 7 (5) | — | — |
| Constipation | 4 (3) | — | — | — |
| Diarrhea | 9 (7) | — | — | — |
| Dysguesia | 10 (7) | — | — | — |
| Mucositis | 1 (< 1) | — | — | |
| Nausea | 15 (11) | 4 (3) | 1 (< 1) | — |
| Vomit | 5 (4) | 3 (2) | — | — |
| Major infection/neutropenic fever | — | 1 (< 1) | 3 (2) | — |
| Metabolic | ||||
| Elevated alkaline phosphatase | 7 (5) | — | — | — |
| Elevated amylase | 5 (4) | — | — | — |
| Elevated transaminases | 8 (6) | 1 (< 1) | — | — |
| Elevated bilirubin | — | 1 (< 1) | — | — |
| Elevated CPK | 2 (1) | — | — | — |
| Neurologic | ||||
| Headache | 5 (4) | 2 (1) | — | — |
| Neuropathy | 5 (4) | 2 (1) | — | — |
| Insomnia | 2 (1) | — | 1 (< 1) | — |
Values are no. (%).
— indicates none observed.
Discussion
This study provides evidence that high-dose AZT/VGC has activity in patients with symptomatic KSHV-MCD. Twelve of 14 patients had a substantial clinical improvement meeting criteria for major clinical response, and 7 met criteria for a major biochemical response. Patients had improvement in circulating levels of hIL-6, vIL-6, KSHV viral load, and other parameters related to MCD pathogenesis. It is worth noting that this pilot study was not randomized and that KSHV-MCD symptoms may wax and wane spontaneously. Controlled trials would be needed to further define the efficacy of AZT/VGC and compare it with other approaches. Nonetheless, comparison with studies of the natural history of KSHV-MCD1,2,15 as well as the temporal relationship between AZT/VGC administration and symptom improvement provides evidence that responses observed in the patients in this study were largely a result of administered therapy.
The rationale for this regimen is to selectively target MCD-KSHV cells expressing KSHV lytic genes (ORF21 and ORF36) through their activation of AZT and ganciclovir to toxic moieties. However, these drugs may have other effects on the KSHV life cycle contributing to clinical activity. In particular, ganciclovir can inhibit KSHV replication,36,37 and VGC alone has been reported to reduce the frequency of flares or induce a remission in 3 patients with KSHV-MCD.17 This raises the possibility that the results seen are in part related to the ability of VGC to block KSHV replication. Through its antiviral effect, ganciclovir can reduce KSHV spread to new cells, and this may contribute to its clinical activity in KSHV-MCD. However, for cells already infected with KSHV, ganciclovir blocks a relatively late step in the KSHV lytic cycle and thus would not be expected to suppress expression of vIL-6.38,39 In addition, cidofovir, another antiviral drug with activity against KSHV, has not been found to have utility in KSHV-MCD,40 suggesting that targeting anti-KSHV replicative activity alone may not be sufficient to yield anti-MCD activity. Additional studies are needed to sort out these possibilities.
Our interest in the cytotoxic use of high-dose AZT in KSHV-associated malignancies arose after experience from the initial phase 1 trial of study of AZT raised the possibility that very high-dose AZT may have activity in KS. In this study of patients with AIDS or symptomatic HIV infection, 4 of 9 patients with KS had major responses (3 partial, 1 complete).41,42 KS patients who responded all received very high doses of AZT, 30 mg/kg per day or more. After the discovery of KSHV,3 a virally encoded thymidine kinase that efficiently phosphorylates AZT, ORF21, was described.27 AZT at a dose of 10 μm/L induces cell toxicity in PEL cells induced to lytic KSHV activation; such AZT levels are attained with an intravenous dose of 5 mg/kg.25,43 One hypothesis is that the KS responses that the original AZT trial were at least partially the result of KSHV ORF21-induced accumulation of toxic AZT triphosphate, and associated cytotoxic activity in a subset of KS tumor cells with lytic KSHV replication. However, we cannot exclude the possibility that the KS responses in that study were related to control of HIV viremia and resulting enhanced immune function, as is currently observed in some patients receiving HAART.44,45
The finding that decreases in hIL-6 and KSHV viral load paralleled clinical improvement in patients treated with AZT/VGC is consistent with the current understanding that KSHV activation, and KSHV-induced cytokine dysregulation, contributed to the pathogenesis of KSHV-MCD.14,31,46,47 Only a subset of patients had elevated vIL-6 at entry (most probably because of the relative insensitivity of the assay). Despite the inability to monitor trends in serum vIL-6 < 1560 pg/mL, in cases where serum vIL-6 was detectable, levels decreased in parallel with the improvement in clinical symptoms and increased with worsening inflammatory symptoms, consistent with an important role for vIL-6 in KSHV-MCD. Serum IL-10, which can be up-regulated by KSHV miR-K12-7,48 also paralleled symptomatology in patients receiving AZT/VGC.
Estimated overall survival of study subjects was 86% at the end of 4 years. As noted, a number of patients received additional therapies. Nonetheless, these results demonstrate that, with available therapies for KSHV-MCD, overall survival can be substantially better than the 2-year survival reported previously.1 Furthermore, in 3 of 14 patients, we attained an overall complete response with AZT/VGC, and patients subsequently remained symptom-free for up to 33 months after stopping therapy. In these 3 patients, there were incremental decreases in biochemical disease parameters over time. Interestingly, PBMC-associated KSHV was often still detectable in patients when they first attained their best clinical response, and in patients who went on to durable remission, this only became undetectable after approximately 1 year of cyclic therapy. These observations suggest that AZT/VGC, in the dose schedule used here, can diminish the burden of KSHV-infected MCD plasmablasts but that this process occurs at a relatively slow rate. AZT/VGC would only be anticipated to have direct cytotoxic effects in cells expressing KSHV lytic genes ORF21 and/or ORF36; and because many MCD plasmablastic cells that express vIL-6 do not have full lytic KSHV gene expression, we thought it unlikely to be curative for MCD when we initiated the study. Nonetheless, our results indicate that certain patients with KSHV-MCD may be able to attain lasting remissions after several months of AZT/VCG therapy and continued cART. Long-term follow-up will be important to establish whether additional KSHV-MCD directed therapy is needed.
Although this regimen of AZT/VGC appeared to have activity in KSHV-MCD, there were limitations. Patients with severe KSHV-MCD symptoms, advanced KS, or concurrent lymphoma were treated with alternative approaches, and the response rates observed cannot be extrapolated to these groups. In patients treated with AZT/VGC, median progression-free survival was 6 months, and 8 patients who initially responded had subsequent relapses. Higher doses of AZT, up to 90 mg/kg per day orally, were administered on the initial phase 1 trial of AZT,41,42,49 and regimens using higher drug doses could have greater activity. In addition, latently infected cells that do not express ORF21 and ORF36 would not be expected to respond to virus-activated cytoxic therapy, and continuous AZT/VGC might possibly have a greater cytotoxic effect by targeting KSHV-infected cells as they become lytically active. However, either of these changes in AZT/VGC administration would have to be balanced against toxicity. Alternatively, intermittent AZT/VGC could be combined with other therapies, such as rituximab, in KSHV-MCD, or used to consolidate responses after other therapies have been used in the acute flares.
Presently, there are no accepted response criteria for MCD. We developed specific response criteria both to more precisely define the responses to therapy and evaluate the utility of the proposed criteria. Initially proposed biochemical criteria were unwieldy, and based on preliminary experience, were revised to simplify the biochemical criteria (Table 2). The revised criteria could be easily implemented, rely on commonly used laboratory and imaging studies, and were consistent with our general clinical impressions. Interestingly, the radiographic responses using these criteria often lagged behind the biochemical and clinical responses. Moreover, the 3 patients who attained complete overall responses (including radiographic responses) remained relapse-free off KSHV-MCD therapy during up to 33 months of observation. Harmonization of KSHV-MCD response criteria across studies is desirable, and our observations suggest that a modified version of the proposed criteria that emphasizes clinical symptoms and select widely available biochemical measures (ie, CRP, platelets, hemoglobin, and albumin) may be useful in the assessment of KSHV-MCD in both clinical and research settings. It is not clear if persistent lympadenopathy and/or splenomegaly after clinical and biochemical responses represent residual KSHV-MCD or persistent reactive lymphoid hyperplasia, and novel imaging techniques that use radiopharmaceutical agents specific to herpesvirus-infected cells are of interest in this regard.50
In conclusion, this study provides evidence that AZT/VGC has activity in the treatment of KSHV-MCD. It supports the paradigm that lytically active KSHV-infected cells are important in disease pathophysiology and that KSHV lytic genes can be used to selectively target KSHV-infected plasmablasts in this rare lymphoproliferative disorder. Nonetheless, AZT/VGC has limitations, and it will be important to consider strategies to optimize this approach or its use in combination with other therapies for KSHV-MCD. Evaluation of additional cytotoxic approaches specifically targeting cells expressing KSHV lytic genes appears warranted in KSHV-MCD.51 As our understanding of this rare disease continues to improve, durable remissions for the majority of HIV-infected patients with KSHV-MCD obtained through the use of targeted therapy appear to be an attainable goal.
Supplementary Material
Acknowledgments
The authors thank the patients who participated in this study as well as clinical staff of the HIV and AIDS Malignancy Branch, the Medical Oncology Branch, the Metabolism Branch, the National Institutes of Health Clinical Center, and the AIDS Monitoring Laboratory.
This work was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute as well as the National Cancer Institute, National Institutes of Health (contract HHSN261200800001E).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
Authorship
Contribution: T.S.U., R.F.L., and R.Y. designed the study; T.S.U., M.N.P., K.A., D.O., K.M.W., R.F.L., and R.Y. cared for the patients; T.S.U., M.N.P., D.O., K.A., K.M.W., S.P., R.F.L., and R.Y. collected data; V.W., G.T., and R.Y. developed and performed vIL-6 assay; V.M. and D.W. developed and performed KSHV viral load assays; T.S.U., S.M.S., and R.Y. analyzed data; and T.S.U. and R.Y. wrote the manuscript.
Conflict-of-interest disclosure: G.T. is a coinventor on a patent describing the measurement of KSHV vIL-6. This invention was made when G.T. was an employee of the US Government under 45 Code of Federal Regulations Part 7. All rights, title, and interest to this patent have been assigned to the US Department of Health and Human Services. The government conveys a portion of the royalties it receives to its employee inventors under the Federal Technology Transfer Act of 1986 (PL 99-502). R.Y. is the spouse of G.T. The remaining authors declare no competing financial interests.
Correspondence: Robert Yarchoan, HIV & AIDS Malignancy Branch, Center for Cancer Research, NCI, 10 Center Dr, Rm 6N106, MSC 1868, Bethesda, MD 20892-1868; e-mail: yarchoan@helix.nih.gov.
References
- 1.Oksenhendler E, Duarte M, Soulier J, et al. Multicentric Castleman's disease in HIV infection: a clinical and pathological study of 20 patients. AIDS. 1996;10(1):61–67. [PubMed] [Google Scholar]
- 2.Waterston A, Bower M. Fifty years of multicentric Castleman's disease. Acta Oncol. 2004;43(8):698–704. doi: 10.1080/02841860410002752. [DOI] [PubMed] [Google Scholar]
- 3.Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266(5192):1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 4.Soulier J, Grollet L, Oksenhendler E, et al. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995;86(4):1276–1280. [PubMed] [Google Scholar]
- 5.Teruya-Feldstein J, Zauber P, Setsuda JE, et al. Expression of human herpesvirus-8 oncogene and cytokine homologues in an HIV-seronegative patient with multicentric Castleman's disease and primary effusion lymphoma. Lab Invest. 1998;78(12):1637–1642. [PubMed] [Google Scholar]
- 6.Cronin DM, Warnke RA. Castleman disease: an update on classification and the spectrum of associated lesions. Adv Anat Pathol. 2009;16(4):236–246. doi: 10.1097/PAP.0b013e3181a9d4d3. [DOI] [PubMed] [Google Scholar]
- 7.Du MQ, Liu H, Diss TC, et al. Kaposi sarcoma-associated herpesvirus infects monotypic (IgM lambda) but polyclonal naive B cells in Castleman disease and associated lymphoproliferative disorders. Blood. 2001;97(7):2130–2136. doi: 10.1182/blood.v97.7.2130. [DOI] [PubMed] [Google Scholar]
- 8.Chadburn A, Hyjek EM, Tam W, et al. Immunophenotypic analysis of the Kaposi sarcoma herpesvirus (KSHV; HHV-8)-infected B cells in HIV+ multicentric Castleman disease (MCD). Histopathology. 2008;53(5):513–524. doi: 10.1111/j.1365-2559.2008.03144.x. [DOI] [PubMed] [Google Scholar]
- 9.Sarid R, Flore O, Bohenzky RA, Chang Y, Moore PS. Transcription mapping of the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1). J Virol. 1998;72(2):1005–1012. doi: 10.1128/jvi.72.2.1005-1012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paulose-Murphy M, Ha NK, Xiang C, et al. Transcription program of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus). J Virol. 2001;75(10):4843–4853. doi: 10.1128/JVI.75.10.4843-4853.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staskus KA, Sun R, Miller G, et al. Cellular tropism and viral interleukin-6 expression distinguish human herpesvirus 8 involvement in Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. J Virol. 1999;73(5):4181–4187. doi: 10.1128/jvi.73.5.4181-4187.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parravicini C, Chandran B, Corbellino M, et al. Differential viral protein expression in Kaposi's sarcoma-associated herpesvirus-infected diseases: Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. Am J Pathol. 2000;156(3):743–749. doi: 10.1016/S0002-9440(10)64940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aoki Y, Tosato G, Fonville TW, Pittaluga S. Serum viral interleukin-6 in AIDS-related multicentric Castleman disease. Blood. 2001;97(8):2526–2527. doi: 10.1182/blood.v97.8.2526. [DOI] [PubMed] [Google Scholar]
- 14.Oksenhendler E, Carcelain G, Aoki Y, et al. High levels of human herpesvirus 8 viral load, human interleukin-6, interleukin-10, and C reactive protein correlate with exacerbation of multicentric castleman disease in HIV-infected patients. Blood. 2000;96(6):2069–2073. [PubMed] [Google Scholar]
- 15.Mylona EE, Baraboutis IG, Lekakis LJ, Georgiou O, Papastamopoulos V, Skoutelis A. Multicentric Castleman's disease in HIV infection: a systematic review of the literature. AIDS Rev. 2008;10(1):25–35. [PubMed] [Google Scholar]
- 16.Powles T, Stebbing J, Bazeos A, et al. The role of immune suppression and HHV-8 in the increasing incidence of HIV-associated multicentric Castleman's disease. Ann Oncol. 2009;20(4):775–779. doi: 10.1093/annonc/mdn697. [DOI] [PubMed] [Google Scholar]
- 17.Casper C, Nichols WG, Huang M-L, Corey L, Wald A. Remission of HHV-8 and HIV-associated multicentric Castleman disease with ganciclovir treatment. Blood. 2004;103(5):1632–1634. doi: 10.1182/blood-2003-05-1721. [DOI] [PubMed] [Google Scholar]
- 18.Nord JA, Karter D. Low dose interferon-alpha therapy for HIV-associated multicentric Castleman's disease. Int J STD AIDS. 2003;14(1):61–62. doi: 10.1258/095646203321043309. [DOI] [PubMed] [Google Scholar]
- 19.Kumari P, Schechter GP, Saini N, Benator DA. Successful treatment of human immunodeficiency virus-related Castleman's disease with interferon-alpha. Clin Infect Dis. 2000;31(2):602–604. doi: 10.1086/313979. [DOI] [PubMed] [Google Scholar]
- 20.Stary G, Kohrgruber N, Herneth AM, Gaiger A, Stingl G, Rieger A. Complete regression of HIV-associated multicentric Castleman disease treated with rituximab and thalidomide. AIDS. 2008;22(10):1232–1234. doi: 10.1097/QAD.0b013e3282fa75ce. [DOI] [PubMed] [Google Scholar]
- 21.Jung CP, Emmerich B, Goebel FD, Bogner JR. Successful treatment of a patient with HIV-associated multicentric Castleman disease (MCD) with thalidomide. Am J Hematol. 2004;75(3):176–177. doi: 10.1002/ajh.10467. [DOI] [PubMed] [Google Scholar]
- 22.Herrada J, Cabanillas F, Rice L, Manning J, Pugh W. The clinical behavior of localized and multicentric Castleman disease. Ann Intern Med. 1998;128(8):657–662. doi: 10.7326/0003-4819-128-8-199804150-00010. [DOI] [PubMed] [Google Scholar]
- 23.Bower M, Powles T, Williams S, et al. Brief communication: rituximab in HIV-associated multicentric Castleman disease. Ann Intern Med. 2007;147(12):836–839. doi: 10.7326/0003-4819-147-12-200712180-00003. [DOI] [PubMed] [Google Scholar]
- 24.Gerard L, Berezne A, Galicier L, et al. Prospective study of rituximab in chemotherapy-dependent human immunodeficiency virus associated multicentric Castleman's disease: ANRS 117 CastlemaB Trial. J Clin Oncol. 2007;25(22):3350–3356. doi: 10.1200/JCO.2007.10.6732. [DOI] [PubMed] [Google Scholar]
- 25.Davis DA, Singer KE, Reynolds IP, Haque M, Yarchoan R. Hypoxia enhances the phosphorylation and cytotoxicity of ganciclovir and zidovudine in Kaposi's sarcoma-associated herpesvirus infected cells. Cancer Res. 2007;67(14):7003–7010. doi: 10.1158/0008-5472.CAN-07-0939. [DOI] [PubMed] [Google Scholar]
- 26.Cannon JS, Hamzeh F, Moore S, Nicholas J, Ambinder RF. Human herpesvirus 8-encoded thymidine kinase and phosphotransferase homologues confer sensitivity to ganciclovir. J Virol. 1999;73(6):4786–4793. doi: 10.1128/jvi.73.6.4786-4793.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gustafson EA, Schinazi RF, Fingeroth JD. Human herpesvirus 8 open reading frame 21 is a thymidine and thymidylate kinase of narrow substrate specificity that efficiently phosphorylates zidovudine but not ganciclovir. J Virol. 2000;74(2):684–692. doi: 10.1128/jvi.74.2.684-692.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 29.Uldrick TS, Wang V, O'Mahony D, et al. An interleukin-6-related systemic inflammatory syndrome in patients co-infected with Kaposi sarcoma-associated herpesvirus and HIV but without multicentric Castleman disease. Clin Infect Dis. 2010;51(3):350–358. doi: 10.1086/654798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aoki Y, Yarchoan R, Braun J, Iwamoto A, Tosato G. Viral and cellular cytokines in AIDS-related malignant lymphomatous effusions. Blood. 2000;96(4):1599–1601. [PubMed] [Google Scholar]
- 31.Aoki Y, Yarchoan R, Wyvill K, Okamoto S, Little RF, Tosato G. Detection of viral interleukin-6 in Kaposi sarcoma-associated herpesvirus-linked disorders. Blood. 2001;97(7):2173–2176. doi: 10.1182/blood.v97.7.2173. [DOI] [PubMed] [Google Scholar]
- 32.de Sanjose S, Marshall V, Sola J, et al. Prevalence of Kaposi's sarcoma-associated herpesvirus infection in sex workers and women from the general population in Spain. Int J Cancer. 2002;98(1):155–158. doi: 10.1002/ijc.10190. [DOI] [PubMed] [Google Scholar]
- 33.Whitby D, Marshall VA, Bagni RK, et al. Reactivation of Kaposi's sarcoma-associated herpesvirus by natural products from Kaposi's sarcoma endemic regions. Int J Cancer. 2007;120(2):321–328. doi: 10.1002/ijc.22205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan CC, Miley W, Waters D. A quantification of human cells using an ERV-3 real time PCR assay. J Virol Methods. 2001;91(2):109–117. doi: 10.1016/s0166-0934(00)00244-5. [DOI] [PubMed] [Google Scholar]
- 35.Toedter G, Hayden K, Wagner C, Brodmerkel C. Simultaneous detection of eight analytes in human serum by two commercially available platforms for multiplex cytokine analysis. Clin Vaccine Immunol. 2008;15(1):42–48. doi: 10.1128/CVI.00211-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kedes DH, Ganem D. Sensitivity of Kaposi's sarcoma-associated herpesvirus replication to antiviral drugs: implications for potential therapy. J Clin Invest. 1997;99(9):2082–2086. doi: 10.1172/JCI119380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casper C, Krantz EM, Corey L, et al. Valganciclovir for suppression of human herpesvirus-8 replication: a randomized, double-blind, placebo-controlled, crossover trial. J Infect Dis. 2008;198(1):23–30. doi: 10.1086/588820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenner RG, Alba MM, Boshoff C, Kellam P. Kaposi's sarcoma-associated herpesvirus latent and lytic gene expression as revealed by DNA arrays. J Virol. 2001;75(2):891–902. doi: 10.1128/JVI.75.2.891-902.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu M. Dissection of the Kaposi's sarcoma-associated herpesvirus gene expression program by using the viral DNA replication inhibitor cidofovir. J Virol. 2004;78(24):13637–13652. doi: 10.1128/JVI.78.24.13637-13652.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berezne A, Agbalika F, Oksenhendler E, et al. Failure of cidofovir in HIV-associated multicentric Castleman disease. Blood. 2004;103(11):4368–4369. doi: 10.1182/blood-2004-01-0158. [DOI] [PubMed] [Google Scholar]
- 41.Yarchoan R, Broder S. Preliminary results on the use of dideoxynucleosides in the therapy of AIDS. In: Chanock RM, Lerner RA, Brown F, Ginsberg H, editors. Vaccines 87. Modern Approaches to New Vaccines: Prevention of AIDS and Other Viral, Bacterial, and Parasitic Diseases. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1987. pp. 214–224. [Google Scholar]
- 42.Yarchoan R, Klecker RW, Weinhold KJ, et al. Administration of 3′-azido-3′-deoxythymidine, an inhibitor of HTLV-III/LAV replication, to patients with AIDS or AIDS-related complex. Lancet. 1986;1(8481):575–580. doi: 10.1016/s0140-6736(86)92808-4. [DOI] [PubMed] [Google Scholar]
- 43.Klecker RW, Jr, Collins JM, Yarchoan R, et al. Plasma and cerebrospinal fluid pharmacokinetics of 3′-azido-3′-deoxythymidine: a novel pyrimidine analog with potential application for the treatment of patients with AIDS and related diseases. Clin Pharmacol Ther. 1987;41:407–412. doi: 10.1038/clpt.1987.49. [DOI] [PubMed] [Google Scholar]
- 44.Wit FW, Sol CJ, Renwick N, et al. Regression of AIDS-related Kaposi's sarcoma associated with clearance of human herpesvirus-8 from peripheral blood mononuclear cells following initiation of antiretroviral therapy. AIDS. 1998;12(2):218–219. [PubMed] [Google Scholar]
- 45.Dupont C, Vasseur E, Beauchet A, et al. Long-term efficacy on Kaposi's sarcoma of highly active antiretroviral therapy in a cohort of HIV-positive patients. CISIH 92: Centre d'information et de soins de l'immunodeficience humaine. AIDS. 2000;14(8):987–993. doi: 10.1097/00002030-200005260-00010. [DOI] [PubMed] [Google Scholar]
- 46.Gasperini P, Sakakibara S, Tosato G. Contribution of viral and cellular cytokines to Kaposi's sarcoma-associated herpesvirus pathogenesis. J Leukoc Biol. 2008;84(4):994–1000. doi: 10.1189/jlb.1107777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cannon JS, Nicholas J, Orenstein JM, et al. Heterogeneity of viral IL-6 expression in HHV-8-associated diseases. J Infect Dis. 1999;180(3):824–828. doi: 10.1086/314956. [DOI] [PubMed] [Google Scholar]
- 48.Qin Z, Kearney P, Plaisance K, Parsons CH. Pivotal advance: Kaposi's sarcoma-associated herpesvirus (KSHV)-encoded microRNA specifically induce IL-6 and IL-10 secretion by macrophages and monocytes. J Leukoc Biol. 2009 Aug 20; doi: 10.1189/jlb.0409251. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yarchoan R, Broder S. Development of antiretroviral therapy for the acquired immunodeficiency syndrome and related disorders: a progress report. N Engl J Med. 1987;316(9):557–564. doi: 10.1056/NEJM198702263160925. [DOI] [PubMed] [Google Scholar]
- 50.Fu DX, Tanhehco YC, Chen J, et al. Virus-associated tumor imaging by induction of viral gene expression. Clin Cancer Res. 2007;13(5):1453–1458. doi: 10.1158/1078-0432.CCR-06-2295. [DOI] [PubMed] [Google Scholar]
- 51.Fu DX, Tanhehco Y, Chen J, et al. Bortezomib-induced enzyme-targeted radiation therapy in herpesvirus-associated tumors. Nat Med. 2008;14(10):1118–1122. doi: 10.1038/nm.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.