SUMMARY
Inferior temporal (IT) object representations have been intensively studied in monkeys and humans, but representations of the same particular objects have never been compared between the species. Moreover, IT’s role in categorization is not well understood. Here, we presented monkeys and humans with the same images of real-world objects and measured the IT response pattern elicited by each image. In order to relate the representations between the species and to computational models, we compare response-pattern dissimilarity matrices. IT response patterns form category clusters, which match between man and monkey. The clusters correspond to animate and inanimate objects; within the animate objects, faces and bodies form subclusters. Within each category, IT distinguishes individual exemplars, and the within-category exemplar similarities also match between the species. Our findings suggest that primate IT across species may host a common code, which combines a categorical and a continuous representation of objects.
INTRODUCTION
Do monkeys and humans see the world similarly? Do monkeys categorize objects as humans do? What main distinctions between objects define their cortical representation in each species? The comparison between monkey and human brains is important from an evolutionary perspective. High-level visual object representations are of particular interest in this context, because they are at the interface between perception and cognition and have been extensively studied in each species. Moreover, the monkey brain provides the major model system for understanding primate and, in particular, human brain function. Understanding the species relationship is therefore a challenge central not only to comparative neuroscience but to systems neuroscience in general.
Great progress has been made by comparing monkey and human brains with functional magnetic resonance imaging (fMRI). Previous studies have used classical activation mapping in both species and cortical-surface-based alignment to define a spatial correspondency mapping between the species. Within the visual system, this approach has revealed coarse-scale regional homologies for early visual areas and object-sensitive inferior temporal (IT) cortex (Van Essen et al., 2001; Tsao et al., 2003; Tootell et al., 2003; Denys et al., 2004; Orban et al., 2004; Van Essen and Dierker, 2007).
These studies employed classical activation mapping in which activity patterns elicited by different particular stimuli within the same class (e.g., an object category) are averaged. Moreover, in order to increase statistical power and relate individuals and species, spatial smoothing is typically applied to the data. As a result, this classical approach reveals regions involved in the processing of particular stimulus classes. It does not reveal how those regions represent particular stimuli. In order to address the questions posed above, however, we need to understand how particular real-world object images are represented in fine-grained activity patterns within each region and how their representations are related between the species. Here, we take a first step in that direction by studying IT response patterns elicited by the same 92 object images in monkeys and humans.
One way to relate the IT representations between the species would be to compare the activity patterns on the basis of a spatial correspondency mapping between monkey and human IT. However, this approach is bound to fail at some level of spatial detail even within a species: every individual primate brain is unique by nature and nurture. A neuron-to-neuron functional correspondency cannot exist. (For proof, consider that different individuals have different numbers of neurons.) However, even if a fine-grained representation is unique in each individual, like a fingerprint, the region containing the representation may be homologous, like a finger—serving the same function in both species. For example, the region may serve the function of representing a particular kind of object information. In this study, relating the species is additionally complicated by the fact that activity was measured with single-cell recording in the monkeys and fMRI in the humans. For these reasons, we do not attempt to define a spatial correspondency mapping between monkey and human IT. Instead, we compare each response pattern elicited by a stimulus to each other response pattern in the same individual animal, so as to obtain a “representational dissimilarity matrix” (RDM) for each species. An RDM shows which distinctions between stimuli are emphasized and which are deemphasized in the representation, thus encapsulating, in an intuitive sense, the information content of the representation. Since RDMs are indexed horizontally and vertically by the stimuli, they can be directly compared between the species.
Our approach has the following key features. (1) The same particular images of real-world objects are presented to both species while measuring brain activity in IT (with electrode recording in monkeys and high-resolution fMRI in humans). (2) Stimuli are presented in random sequences; neither the experimental design nor the analysis is biased by any predefined grouping. (3) Each stimulus is treated as a separate condition, for which a response pattern is estimated without spatial smoothing or averaging (Kriegeskorte et al., 2007; Eger et al., 2008; Kay et al., 2008). (4) The analysis targets the information in distributed response patterns (Haxby et al., 2001; Cox and Savoy, 2003; Carlson et al., 2003; Kamitani and Tong, 2005; Kriegeskorte et al., 2006). (5) In order to compare the IT representations between the species and to computational models, we use the method of “representational similarity analysis” (Kriegeskorte et al., 2008), in which RDMs are visualized and quantitatively compared.
Population representations of the same stimuli have not previously been compared between monkey and human. However, our approach is deeply rooted in the similarity analyses of mathematical psychology (Shepard and Chipman, 1970). An introduction is provided by Edelman (1998), who pioneered the application of similarity analysis to fMRI activity patterns (Edelman et al., 1998) using the technique of multidimensional scaling (Torgerson, 1958; Kruskal and Wish, 1978; Shepard, 1980). Several studies have applied similarity analyses to brain activity patterns and computational models (Laakso and Cottrell, 2000; Op de Beeck et al., 2001; Haxby et al., 2001; Hanson et al., 2004; Kayaert et al., 2005; O’Toole et al., 2005; Aguirre, 2007; Lehky and Sereno, 2007; Kiani et al., 2007; Kay et al., 2008).
Beyond the species comparison, our approach allows us to address the question of categoricality. IT is thought to contain a population code of features for the representation of natural images of objects (e.g., Desimone et al., 1984; Tanaka, 1996; Grill-Spector et al., 2001; Haxby et al., 2001). Does IT simply represent the visual appearance of objects? Or are the IT features designed to distinguish categories defined independent of the visual appearance of their members?
Whether IT is optimized for the discrimination of object categories is unresolved. Human neuroimaging has investigated category-average responses for predefined conventional object categories (Puce et al., 1995; Martin et al., 1996; Kanwisher et al., 1997; Aguirre et al., 1998; Epstein and Kanwisher, 1998; Haxby et al., 2001; Downing et al., 2001; Cox and Savoy, 2003; Carlson et al., 2003; Downing et al., 2006, but see Edelman et al., 1998). This approach requires the assumption of a particular category structure and therefore cannot address whether the representation is inherently categorical. Monkey studies have reported IT responses that are correlated with categories (Vogels, 1999; Sigala and Logothetis, 2002; Baker et al., 2002; Tsao et al., 2003; Freedman et al., 2003; Kiani et al., 2005; Hung et al., 2005; Tsao et al., 2006; Afraz et al., 2006). However, more clearly categorical responses have been found in other regions (Kreiman et al., 2000; Freedman et al., 2001; Quiroga et al., 2005; Freedman and Assad, 2006), suggesting that IT has a lesser role in categorization (Freedman et al., 2003). A brief summary of the previous evidence on IT categoricality is given in the Supplemental Data.
Kiani et al. (2007) investigated monkey-IT response patterns elicited by over 1000 images of real-world objects to address whether IT is inherently categorical. The present study uses the same monkey data and a subset of the stimuli to compare the species. Cluster analysis of the monkey data revealed a detailed hierarchy of natural categories inherent to the monkey-IT representation. Will human-IT show a similar categorical structure? Our approach allows us to address the question of categoricality without the bias of predefined categories. Independent of the result, this provides a crucial piece of evidence for current theory. The question of the inherent category structure of IT is of particular interest with respect to the species comparison, because the prevalent categorical distinctions might be expected to differ between species.
Our goal is to investigate to what extent monkey and human-IT represent the same object information. In particular, we ask the following. (1) Do human-IT response patterns form category clusters as reported for monkey IT (Kiani et al., 2007)? If so, what is the categorical structure and does it match between species? (2) Is within-category exemplar information present in IT? If so, is this continuous information consistent between the species? (3) How is the representation of the objects transformed between early visual cortex and IT? (4) What computational models can account for the IT representation?
RESULTS
We presented the same 92 images of isolated real-world objects (Figure S1) to monkeys and humans while measuring IT response patterns with single-cell recording and high-resolution fMRI, respectively. Two monkeys were presented with the 92 images in rapid succession (stimulus duration, 105 ms; interstimulus interval, 0 ms) as part of a larger set while they performed a fixation task. Neuronal activity was recorded extracellularly with tungsten electrodes, one cell at a time. The cells were located in anterior IT cortex, in the right hemisphere in monkey 1 and in the left in monkey 2. The analyses are based on all cells that could be isolated and for which sufficient data were available across the stimuli. This yielded a total of 674 neurons for both monkeys combined. For each stimulus, each neuron’s response amplitude was estimated as the average spike rate within a 140 ms window starting 71 ms after stimulus onset (for details on this experiment, see Kiani et al., 2007).
Four humans were presented with the same images (stimulus duration, 300 ms; interstimulus interval, 3700 ms) while they performed a fixation task in a rapid event-related fMRI experiment. Each stimulus was presented once in each run in random order and repeated across runs within a given session. The amplitudes of the overlapping single-image responses were estimated by fitting a linear model. The task required discrimination of fixation-cross color changes occurring during image presentation. We measured brain activity with high-resolution blood-oxygen-level-dependent fMRI (3-Tesla, voxels: 1.95 × 1.95 × 2 mm3, SENSE acquisition; Prüssmann, 2004; Kriegeskorte and Bandettini, 2007; Bodurka et al., 2007) within a 5 cm thick slab including all of inferior temporal and early visual cortex bilaterally. Voxels within an anatomically defined IT-cortex mask were selected according to their visual responsiveness to the images in an independent set of experimental runs.
Representational Dissimilarity Matrices: The Same Categorical Structure May Be Inherent to IT in Both Species
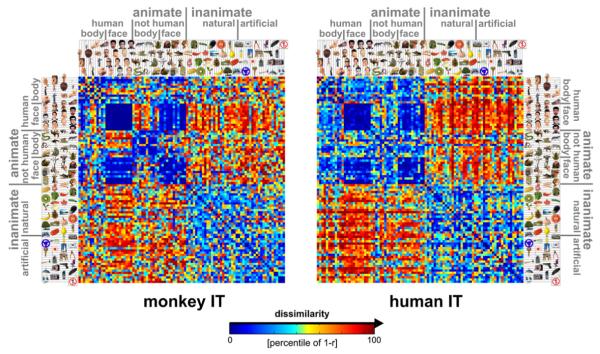
What stimulus distinctions are emphasized by IT in each species? Figure 1 shows the RDMs for monkey and human IT. Each cell of a given RDM compares the response patterns elicited by two stimuli. The dissimilarity between two response patterns is measured by correlation distance, i.e., 1 – r (Pearson correlation), where the correlation is computed across the population of neurons or voxels (Haxby et al., 2001; Kiani et al., 2007). An RDM is symmetric about a diagonal of zeros here, because we use a single set of response-pattern estimates.
Figure 1. Representational Dissimilarity Matrices for Monkey and Human IT.
For each pair of stimuli, each RDM (monkey, human) color codes the dissimilarity of the two response patterns elicited by the stimuli in IT. The dissimilarity measure is 1 – r (Pearson correlation across space). The color code reflects percentiles (see color bar) computed separately for each RDM (for 1 – r values and their histograms, see Figure 3A). The two RDMs are the product of completely separate experiments and analysis pipelines (data not selected to match). Human data are from 316 bilateral inferior temporal voxels (1.95 × 1.95 × 2mm3) with the greatest visual-object response in an independent data set. For control analyses using different definitions of the IT region of interest (size, laterality, exclusion of category-sensitive regions), see Figures S9–S11. RDMs were averaged across two sessions for each of four subjects. Monkey data are from 674 IT single cells isolated in two monkeys (left IT in one monkey, right in the other; Kiani et al., 2007).
The RDMs allow us to compare the representations between the species, although there may not be a precise correspondency of the representational features between monkey IT and human IT and although we used radically different measurement modalities (single-cell recordings and fMRI) in the two species. Our approach of representational similarity analysis requires comparisons only between response patterns within the same individual animal, obviating the need for a monkey-to-human correspondency mapping within IT.
Several important results (to be quantified in subsequent analyses) are apparent by visual inspection of the RDMs (Figure 1). First, there is a striking match between the RDMs of monkey and human IT. Two stimuli tend to be dissimilar in the human-IT representation to the extent that they are dissimilar in the monkey-IT representation, and vice versa. This is unexpected because the behaviorally relevant stimulus distinctions might be very different between the species. Moreover, single-cell recording and fMRI sample brain activity in fundamentally different ways, and it is not well understood to what extent they similarly reflect distributed representations. Second, the dissimilarity tends to be large when one of the depicted objects is animate and the other inanimate and smaller when the objects are either both animate or both inanimate. Third, dissimilarities are particularly small between faces (including human and animal faces). These observations suggest that the IT representation reflects conventional category boundaries in the same way in both species and that there may be a hierarchical structure inherent to the representation. The categorical structure of the matching dissimilarity patterns raises the question, whether the fine-scale patterns of dissimilarities within the categories also match between the species. Alternatively, the categorical structure may fully account for the apparent match. These questions cannot be decided by visual inspection and are addressed by quantitative analysis of the RDMs in the subsequent figures.
It is important to note that the two RDMs (Figure 1) which form the basis of the subsequent interspecies analyses (Figure 2-4) are the product of completely independent experiments and analysis pipelines. In particular, voxels and cells were not selected to maximize the match in any way.
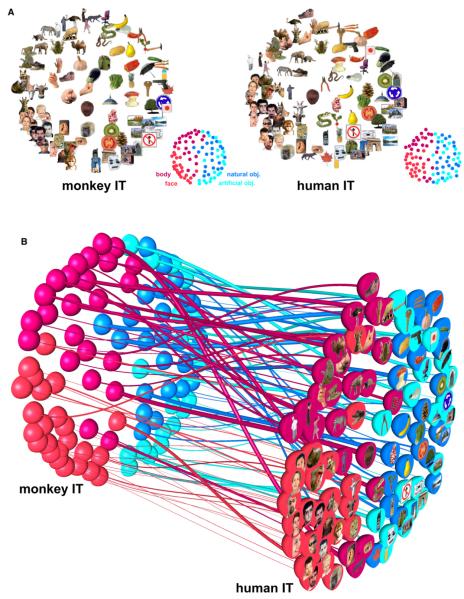
Figure 2. Stimulus Arrangements Reflecting IT Response-Pattern Similarity in Monkey and Human and the Interspecies Relationship.
(A) The experimental stimuli have been arranged such that their pairwise distances approximately reflect response-pattern similarity (multidimensional scaling, dissimilarity: 1 – Pearson r, criterion: metric stress). In each arrangement, images placed close together elicited similar response patterns. Images placed far apart elicited dissimilar response patterns. The arrangement is unsupervised: it does not presuppose any categorical structure. The two arrangements have been scaled to match the areas of their convex hulls and rigidly aligned for easier comparison (Procrustes alignment). The correlations between the high-dimensional response-pattern dissimilarities (1 – r) and the two-dimensional Euclidean distances in the figure are 0.67 (Pearson) and 0.69 (Spearman) for monkey IT and 0.78 (Pearson) and 0.78 (Spearman) for human IT.
(B) Fiber-flow visualization emphasizing the interspecies differences. This visualization combines all the information from (A) and links each pair of dots representing a stimulus in monkey and human IT by a “fiber.” The thickness of each fiber reflects to what extent the corresponding stimulus is inconsistently represented in monkey and human IT. The interspecies consistency ri of stimulus i is defined as the Pearson correlation between vectors of its 91 dissimilarities to the other stimuli in monkey and human IT. The thickness of the fiber for stimulus i is proportional to (1 – ri)2, thus emphasizing the most inconsistently represented stimuli. The analysis of single-stimulus interspecies consistency is pursued further in Figures S2 and S3.
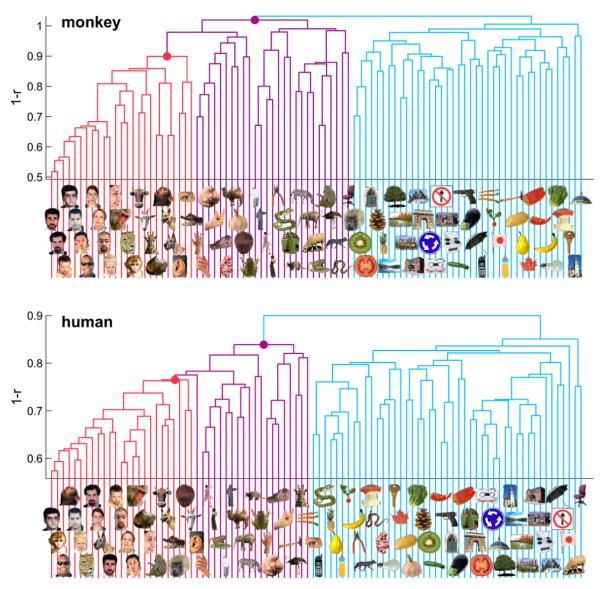
Figure 4. Hierarchical Clustering of IT Response Patterns.
In order to assess whether IT response patterns form clusters corresponding to natural categories, we performed hierarchical cluster analysis for human (top) and monkey (bottom). This analysis proceeds from single-image clusters (bottom of each panel) and successively combines the two clusters closest to each other in terms of the average response-pattern dissimilarity, so as to form a hierarchy of clusters (tree structure in each panel). The vertical height of each horizontal link indicates the average response-pattern dissimilarity (the clustering criterion) between the stimuli of the two linked subclusters (dissimilarity: 1 – r). The cluster trees for monkey and human are the result of completely independent experiments and analysis pipelines. This data-driven technique reveals natural-category clusters that are consistent between monkey and human. For easier comparison, we colored subcluster trees (faces, red; bodies, magenta; inanimate objects, light blue). Early visual cortex (Figures 5, 6, and S5) and low-level computational models (Figures S6 and S7) did not reveal such category clusters.
The human RDMs are averages of RDMs computed separately for each of two fMRI sessions in each of four subjects. Note that averaging RDMs for corresponding functional regions is a useful way of combining the data across subjects. As for the species comparison, a precise intraregional spatial correspondency mapping between human subjects is not required. In total, 7 hr, 24 min, and 16 s of fMRI data were used for these analyses. For Figures 1-6, 316 inferior temporal voxels (1.95 × 1.95 × 2 mm3) with the greatest visual response were selected bilaterally in each subject and session.
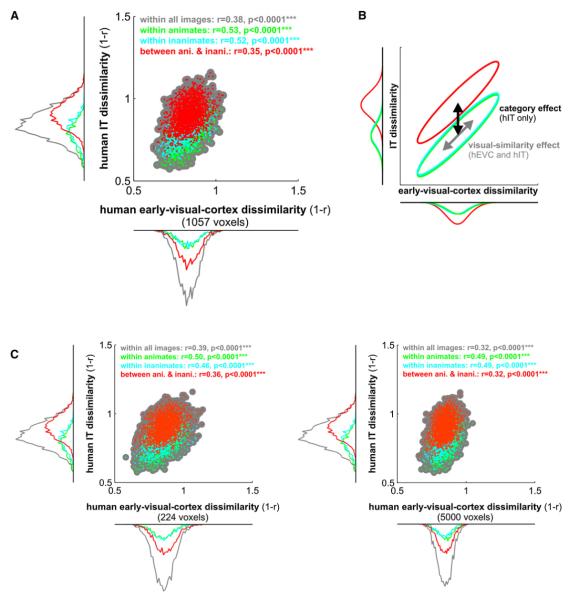
Figure 6. Representational Connectivity between Early Visual Cortex and IT in the Human.
(A) For each pair of stimuli, we plot a dot with horizontal position reflecting early visual response-pattern dissimilarity and vertical position reflecting IT response-pattern dissimilarity. Scatter plots and correlation analyses (insets) show that pairs of stimuli eliciting more dissimilar response patterns in early visual cortex also tend to elicit more dissimilar response patterns in IT. This suggests that visual similarity as reflected in the early visual representation carries over into the IT representation. However, IT additionally exhibits a strong category-boundary effect: when a stimulus pair crosses the animate-inanimate boundary (red) the two response patterns tend to be more dissimilar than when both stimuli are from the same category (green, cyan). The category-boundary effect is evident in the marginal dissimilarity histograms framing the scatter plot (for statistical analysis, see Figure 5).
(B) In this conceptual diagram, the distributions from the scatter plots are depicted as ellipsoids (iso-probability-density contours) with the same color code. The visual-similarity effect is shared between early visual and IT representations (each distribution diagonally elongated), whereas the category-boundary effect is only present in IT (red distribution vertically, but not horizontally shifted with respect to the within-category distributions).
(C) The same analyses for smaller and larger definitions of human early visual cortex (224 and 5000 voxels, respectively) show that the findings above do not depend on the size of the early visual region of interest.
Multidimensional Scaling: Category Determines Global Grouping when Stimuli Are Arranged by Representational Similarity
Figure 2A shows unsupervised arrangements of the stimuli reflecting the response-pattern similarity for monkey and human IT (multidimensional scaling, criterion: metric stress; Torgerson, 1958; Shepard, 1980; Edelman et al., 1998). In each arrangement, stimuli placed close together elicited similar response patterns; stimuli placed far apart elicited dissimilar response patterns. The arrangement is unsupervised in that it does not presuppose a categorical structure. For ease of visual comparison, the two arrangements have been scaled to equal size (matching the areas of their convex hulls) and rigidly aligned (Procrustes alignment). Stimulus arrangements computed by multidimensional scaling are data driven and serve an important exploratory function: they can reveal the properties that dominate the representation of our stimuli in the population code without any prior hypotheses. For IT in both species, the global grouping reflects the categorical distinctions between animates and inanimates, and between faces and bodies among the animates. This suggests that category is the dominant factor determining the IT response pattern in both species: if any other stimulus property were more important, it would dominate the stimulus arrangement.
Note also that neighboring stimuli within a category often differ markedly in both shape and color. The arrangements are very similar between the species. Both are characterized by a clean separation of animate and inanimate objects. Furthermore, body parts and faces occupy separate regions among the animate objects.
Note that faces appear to form a particularly tight cluster in the IT response-pattern space of both species. For the human face images this could reflect their similarity in shape and color. However, the face cluster also includes animal faces. Although human and animal faces may be somewhat separated within the face cluster (see also Figure 4), very visually dissimilar animal faces appear to group together. These and other hypotheses inspired by exploring the stimulus arrangement will need to be tested in separate experiments.
Interspecies Dissimilarity Correlation (1): Most Single Stimuli Are Consistently Represented in Both Species
Inspecting the stimulus arrangements for monkey and human separately reveals their overall similarity. However, from the arrangements alone it is not easy to see to what extent particular stimuli within a category appear in different “neighborhoods” of the representational space in the two species. The “fiber-flow” visualization of Figure 2B reproduces both stimulus arrangements and relates them by “fibers” linking dots that represent the same stimulus. This makes it easier to see how stimuli within the same category match up between species. Most fibers flow in a roughly straight line (i.e., without much displacement) from the monkey to the human representation. This reflects the within-category match of the representations, which is analyzed and tested for significance in Figure 3.
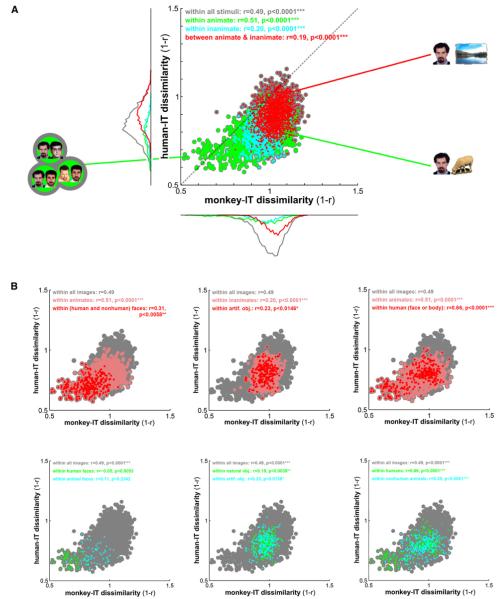
Figure 3. Correlation of Representational Dissimilarities between Monkey and Human IT.
(A) For each pair of stimuli, a dot is placed according to the IT response-pattern dissimilarity in monkey (horizontal axis) and human (vertical axis). As before, the dissimilarity between the two response patterns elicited by each stimulus pair is measured as 1 – r (Pearson correlation). Dot colors correspond to all pairs of stimuli (gray), pairs within the animate objects (green), pairs within the inanimate objects (cyan), and pairs crossing the animate-inanimate boundary (red). Marginal histograms of dissimilarities are shown for the three subsets of pairs using the same color code. For detailed exploratory analysis of the species differences, Figures S13 and S14 show the stimulus pairs corresponding to the dots for the three apical regions of the scatter plot.
(B) The same analysis as in (A), but for within-category correlations between human and monkey-IT object dissimilarities. Colored dots correspond to all pairs of stimuli (gray) or pairs within stimulus-category subsets (colors). In the top row, each panel shows the whole set (gray), a subset (pink), and a subset nested within that subset (red), as indicated in the colored legend of each panel. In the bottom row, each panel shows the whole set (gray) and two disjointed subsets (green and cyan), as indicated in the colored legend of each panel.
In both (A) and (B), each panel’s color legend (top inset) also states the correlations (r, Pearson) between monkey and human-IT dissimilarities and their significance (*p < 0.05, **p < 0.01, ***p < 0.001). The dissimilarity correlations are tested by randomization of the stimulus labels. This test correctly handles the dependency structure within each RDM. All p values < 0.0001 are stated as p < 0.0001 because the randomization test terminates after 10,000 iterations.
In order to reveal the species differences, we chose the thickness of the fibers in Figure 2B to reflect the extent to which each stimulus is inconsistently represented in monkey and human IT. For each stimulus i, its place in the high-dimensional monkey-IT response-pattern space is characterized by the vector mi of its dissimilarities to the other 91 stimuli. Its place in the human-IT representation is characterized analogously by dissimilarity vector hi. The interspecies correlation ri (Pearson) between mi and hi reflects the consistency of placement of the stimulus in the representations of both species (see Figure S2 for details). For each stimulus i, the thickness of its fiber in Figure 2B is proportional to (1 – ri)2, thus emphasizing the most inconsistently represented stimuli. The prevalence of thin fibers (which tend to be straight) reflects the overall interspecies consistency.
The single-stimulus interspecies dissimilarity correlations ri are further visualized and statistically analyzed in Figures S2 and S3. Results show significant interspecies consistency for about two-thirds of the single stimuli (p < 0.05, corrected for multiple tests). Furthermore human faces exhibit significantly higher interspecies correlations than the stimulus set as a whole, and several stimuli (including images of animate and inanimate objects) exhibit significantly lower interspecies correlations. The two stimuli with the lowest interspecies correlations (eggplant, backview of human head) were the only two stimuli described as ambiguous by human subjects during debriefing (Figure S1). This is consistent with the idea that the IT representation reflects not only the visual appearance, but also the conceptual interpretation of a stimulus.
Interspecies Dissimilarity Correlation (2): IT Emphasizes the Same Stimulus Distinctions in Both Species within and between Categories
The RDMs of Figure 1 suggest similar representations in monkey and human IT. Figure 3A quantifies this impression. The scatter plot of the monkey-IT dissimilarities (horizontal axis) and the human-IT dissimilarities (vertical axis) across pairs of stimuli reveals a substantial correlation (r = 0.49, p < 0.0001 estimated by means of 10,000 randomizations of the stimulus labels).
Does the matching categorical structure fully explain the interspecies correlation of dissimilarities? Figure 3A (colored subsets) shows that the correlation is substantial also within animates (r = 0.51, p < 0.0001) and, to a lesser extent, within inanimates (r = 0.20, p < 0.0001), as well as across pairs of stimuli crossing the animate-inanimate boundary (r = 0.19, p < 0.0001). The monkey-to-human correlation is also present (Figure 3B) within images of humans (r = 0.66, p < 0.0001), within images of nonhuman animals (r = 0.35, p < 0.0001), within images of faces (including human and animal faces, r = 0.31, p < 0.0058), within images of bodies (including human and animal bodies, r = 0.31, p < 0.0001; not shown in Figure 3), within images of human bodies (r = 0.53, p < 0.0001; not shown in Figure 3), within images of natural objects (r = 0.19, p < 0.0039), and within images of artificial objects (r = 0.23, p < 0.0139). These within-category dissimilarity correlations between the species indicate that the continuous variation of response patterns within each category cluster is not noise, but distinguishes exemplars within each category in a way that is consistent between monkey and human.
We did not find a significant monkey-to-human dissimilarity correlation either within images of human faces (r = −0.05, p < 0.605) or within images of animal faces (r = 0.12, p < 0.234; Figure 3B, bottom left). We also did not find a significant correlation within images of nonhuman bodies (r = 0.12, p < 0.21; not shown in Figure 3).
The negative findings all occurred for small subsets of images (12 images, 66 pairs) for which we have reduced power. Note, however, that the effect sizes (r) were also smaller for the insignificant correlations than for all significant correlations. That the correlation is significant within human bodies, but not within nonhuman bodies, could reflect the fact that the human-body images included whole bodies as well as body parts, whereas the nonhuman body images were all of whole bodies and 9 of the 12 images were of four-limbed animals (Figure S1), which may constitute a separate subordinate category (Kiani et al., 2007).
Regarding the absence of a significant interspecies dissimilarity correlation within human faces and within nonhuman faces, one interpretation of particular interest is that human and monkey differ in how they represent individual human faces as well as individual nonhuman faces. For example, within each species the representation of images of its own members may have a special status.
Species-Specific Face Analysis: IT Might Better Distinguish Conspecific Faces in Each Species
We observed greater dissimilarities in the human representation of human faces than in the monkey representation of human faces (Figure S8). Figure S4 explores the possibility of a species-specific face representation. We selectively analyzed the representation of monkey, ape, and human faces in monkey and human IT. The dissimilarities among human faces are significantly larger in human IT than in monkey IT (p = 0.009). The dissimilarities among monkey-and-ape faces are larger in monkey IT than in human IT in our data, but the effect is not significant (p = 0.12). The difference between the two effects is significant (p = 0.02). This analysis provides an interesting lead for future studies designed to address species-specific face representation (for details, see Figure S4).
Hierarchical Clustering: A Nested Categorical Structure Matching between Species Is Inherent to IT
The stimulus arrangements of Figure 2 suggest that the categories correspond to contiguous regions in IT response-pattern space. However, it is not apparent from Figure 2 whether the response patterns form clusters corresponding to the categories. Contiguous category regions in response-pattern space could exist for a unimodal response-pattern distribution. Category clusters would imply separate modes in response-pattern space, separated by boundaries of lower probability density. We therefore ask whether the category boundaries can be determined without knowledge of the category labels.
Figure 4 shows hierarchical cluster trees computed for the IT response patterns in monkey and human. Unlike the unsupervised stimulus arrangements (Figure 2), hierarchical cluster analysis (Johnson, 1967) assumes the existence of some categorical structure, but it does not assume any particular grouping into categories.
We find very similar cluster trees for both species. The top-level distinction is that between animate and inanimate objects. Faces and body parts form subclusters within the animate objects. Note that the clustering conforms closely, though not perfectly, to the these human-conventional categories. The deviating placements could be a consequence of inaccurate response-pattern estimation: because of the large number of conditions (92) in these experiments, our response-pattern estimates are noisier than they would be for a small number of conditions based on the same amount of data.
Results Similar between Hemispheres and Robust to Exclusion of Category-Sensitive Regions and to Varying the Number of Voxels
The results we describe for bilateral IT are similar when IT is restricted to either cortical hemisphere (for details, see Figure S9). Results are also robust to changes of the number of voxels selected. The categorical structure is present already at 100 voxels and decays only when thousands of voxels are selected (for details, see Figure S10). Finally, the categorical structure appears unaffected when the fusiform face area (FFA) (Kanwisher et al., 1997) and the parahippocampal place area (PPA) (Epstein and Kanwisher, 1998) are bilaterally excluded from the selected voxel set (for details, see Figure S11). After exclusion of FFA and PPA, the region of interest has most of its voxels in the lateral occipital complex, but also includes more anterior object-sensitive voxels within IT.
Category-Boundary Effect: Weak in Early Visual Cortex and Strong in IT
The human fMRI data allowed us to compare the representations between early visual cortex and IT (Figures 5, 6, and S5). Visual inspection of the RDMs (Figure 5) suggests a categorical representation in IT, but not in early visual cortex. The multidimensional scaling arrangements and hierarchical cluster trees also do not support that early visual cortex contains an inherently categorical representation (Figure S5). Note, however, that a subset of the human faces appears to be associated with somewhat lower dissimilarities in early visual cortex (Figure 5). This could be caused by similarities in shape and color among these stimuli.
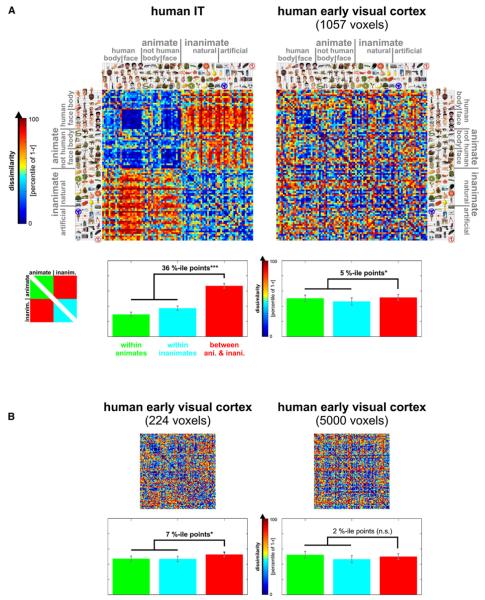
Figure 5. Early Visual Cortex and IT in the Human: Representational Dissimilarity Matrices and Category-Boundary Effects.
(A) RDMs for human IT (top left, same as in Figure 1) and human early visual cortex (top right). As in Figure 1, the color code reflects percentiles (see color bar) computed separately for each RDM (for 1 – r values and their histograms, see Figure 6). The bar graph below each RDM shows the average dissimilarity (percentile of 1 – r) within the animates (green bars), within the inanimates (cyan bars), and for pairs crossing the category boundary (red bars). Error bars indicate the standard error of the mean estimated by bootstrap resampling of the stimulus set. We define the category-boundary effect as the difference (in percentile points) between the mean dissimilarity for between-animate-and-inanimate pairs and the mean dissimilarity for within-animate and within-inanimate pairs. The zeros on the diagonal are excluded in computing these means. The category-boundary effect sizes are given above the bars in each panel with significant effects marked by stars (p ≥ 0.05 indicated by n.s. for “not significant,” *p < 0.05, **p < 0.01, ***p < 0.001). The p values are from a bootstrap test; a randomization test yields the same pattern of significant effects (see Results). Here, as in Figures 1-4, human IT has been defined at 316 voxels (for IT at 100–10,000 voxels, see Figure S10) and human early visual cortex at 1057 voxels.
(B) The same analyses for smaller and larger definitions of human early visual cortex (224 and 5000 voxels, respectively).
Although the early visual representation does not exhibit a categorical structure as observed for IT, the top-level animate-inanimate distinction might be reflected in the early visual responses in more subtle ways. To test this possibility for the top-level animate-inanimate distinction, we analyze the category-boundary effect, which we define as the difference between the mean dissimilarity for between-category pairs (i.e., one is animate, the other inanimate) and the mean dissimilarity for within category pairs (i.e., both are animate or both are inanimate). As in Figure 1, the dissimilarities are correlation distances between spatial response patterns, converted to percentiles for each RDM separately (for histograms of the original correlation distances, see Figure 6). The analysis indicates that the category-boundary effect is strong in IT and weak, but present, in early visual cortex. The category-boundary effect estimates in percentile points are 36% (p < 0.001) for IT (defined at 316 voxels as before) and 7% (0.01 < p < 0.05), 5% (0.01 < p < 0.05), and 2% (not significant) for early visual cortex, defined at 224, 1057, and 5000 voxels, respectively. We further investigated the category distinction by a linear decoding analysis (Figure S12), which suggested linear separability of animates and inanimates in IT, but not early visual cortex.
The p values for the category-boundary effect were computed by bootstrap resampling of the stimulus set, thus simulating the distributions of mean dissimilarities expected if the experiment were to be repeated with different stimuli from the same categories and with the same subjects. We also tested the category-boundary effect by a randomization test in which the stimulus labels are randomly permuted, thus simulating the null hypothesis of no difference between the response patterns elicited by the stimuli, but not generalizing to different stimuli from the same categories. This test yields the same pattern of significant results as the bootstrap test (p < 0.001 for IT and early visual cortex defined at 224 or 1057 voxels, p ≥ 0.05 for human early visual cortex defined at 5000 voxels). In addition, both bootstrap and randomization tests show a significantly larger category-boundary effect in IT than in early visual cortex (p < 0.0001 for each of the three sizes of the early visual region of interest).
Representational Connectivity Analysis: Early Visual Cortex and IT Share Visual-Similarity Information
We have compared monkey and human IT by correlating representational dissimilarities across pairs of stimuli. The same approach can serve to characterize the relationship between the representations in two brain regions of a given species. In analogy to the concept of functional connectivity, we refer to the correlation of representational dissimilarities between two brain regions as their “representational connectivity.” Like functional connectivity, representational connectivity does not imply an anatomical connection or a directed influence. Unlike functional connectivity, representational connectivity is a multivariate, nonlinear, and design-dependent connectivity measure.
Visual comparison of the RDMs for early visual cortex and IT does not suggest a strong correlation, because the categorical structure dominating IT appears absent in early visual cortex.
However, the representational-connectivity scatter plot (Figure 6) reveals a substantial correlation of the dissimilarities (r = 0.38, p < 0.0001). Pairs of stimuli eliciting more dissimilar response patterns in early visual cortex also tend to elicit more dissimilar response patterns in IT. The analysis for between- and within-category subsets of pairs reveals what drives this effect (see diagram in Figure 6B). First, the between-category distribution (red) is shifted relative to the within-category distributions, but only along the IT axis, not along the early-visual axis. This is evident in the marginal dissimilarity histograms framing the scatter plot (Figure 6) and reflects the category-boundary effect, which is strong in IT and weak in early visual cortex (Figure 5). Second, in addition to its category-boundary effect, IT reflects the dissimilarity structure of the early visual representation for within- as well as between-category pairs (diagonally elongated distributions, p < 0.0001 for all correlations, tested by randomization of the stimulus labels). These results do not depend on the size of the early-visual region of interest (Figure 6C).
Early visual response patterns are likely to reflect shape similarity in this experiment, because all stimuli were presented at the same retinal location (fovea) and size (2.9° visual angle). Shape similarity as reflected in the early visual representation (see also Kay et al., 2008) may therefore carry over to the IT representation, even if IT is more tolerant to changes of position and size.
Computational Modeling: A Range of Low- and Intermediate-Level Representations Cannot Account for the Categorical Structure Observed in IT
Can low-level feature similarity account for our results? We compared the IT representation to several low-level model representations. The low-level models included the color images themselves (in CIELAB color space), simple processed versions of the images (low-resolution color image, grayscale image, low-resolution grayscale image, spatial low- and high-pass-filtered grayscale image, binary silhouette image), CIELAB joint color histograms, and a computational model of V1 (including simple and complex cells). We also tested an intermediate-level computational model corresponding approximately to the level of V4 and posterior IT, the HMAX-C2 representation based on natural image patches. (For details on these models, see Supplemental Data.) The RDMs, multidimensional scaling arrangements, and hierarchical cluster trees for these models (Figures S6 and S7) suggest that none of them can account for the category clustering we observed in IT cortex.
DISCUSSION
Matching Information in Monkey and Human IT
IT is thought to contain a high-level representation of visual objects at the interface between perception and cognition. Our results show that monkey and human IT emphasize very similar distinctions among objects. To answer the questions posed at the end of the Introduction: (1) IT response patterns elicited by object images appear to cluster according to the same categorical structure in monkey and human. (2) Within each category, primate IT appears to represent more finegrained object information. This information as well is remarkably consistent across species and may reflect subordinate categorical distinctions as well as a high-level form of visual similarity (Op de Beeck et al., 2001; Eger et al., 2008). (3) Categoricality appears to arise in IT; it is largely absent in human early visual cortex. (4) A range of low- and intermediate-level computational models did not reproduce the categorical structure observed for human and monkey IT.
A Hierarchical Category Structure Inherent to IT
The categorical structure inherent to IT in both species appears hierarchical: animate and inanimate objects form the two major clusters; faces and bodies form subclusters within the animate cluster. The hierarchy observed for the present set of 92 stimuli has only two levels. However, the previous study by Kiani et al. (2007), using over 1000 stimuli, reported a hierarchy for monkey IT, which is consistent with our findings here, but extends into finer distinctions. This raises the question, whether finer categorical distinctions are also present in the human and, if so, if they match between the species.
Relationship between Category-Sensitive Regions and IT Pattern Information
Human neuropsychology has described category-specific deficits resulting from temporal brain damage and suggested a special status for the living/nonliving distinction (Martin, 2007; Capitani et al., 2003; Humphreys and Forde, 2001; Martin et al., 1996). Our results support the view that this distinction has a special status. We note that fruit and vegetables fall into the inanimate category in the IT cluster structure we observed (Figure 4). Our findings are also consistent with the idea that IT contains specialized features or processing mechanisms for faces and bodies (Puce et al., 1995; Kanwisher et al., 1997; Downing et al., 2001; but see also Gauthier et al., 2000).
Can the previous findings on human-IT regions sensitive to these categories explain the IT response-pattern clustering we report? Let us assume that the FFA responds with a similar overall activation to each individual face (Kriegeskorte et al., 2007). Including the FFA in the region of interest will then render IT response patterns to faces more similar, thus contributing to their clustering. More generally, a sufficiently category-sensitive feature set will exhibit categorical clustering of the response patterns.
However, the existence of the category-sensitive regions does not predict (1) that the category effects will dominate the representation such that response patterns form category clusters that are separable without prior knowledge of the categories (alternatively, the category-sensitive component could be too weak—in relation to the total response-pattern variance—to form clusters), (2) that the cluster structure will be hierarchical, or (3) what categorical distinction is at the top of the hierarchy (explaining most response-pattern variance). Moreover, our human results hardly changed when FFA and PPA were excluded (Figure S11), leaving the lateral occipital complex as the main focus within the IT region of interest. (Voxels were selected by their average response to objects versus fixation using independent data.) In the monkeys, the IT recordings did not target category-sensitive regions (Tsao et al., 2003); never-theless, the population exhibited a complex categorical clustering of its response patterns (Figure 4), and for each pair of categories, discrimination by the cell population was robust to exclusion of cells responding maximally to either category (Figure 10 of Kiani et al., 2007).
If FFA and PPA can be excluded without a qualitative change to the representational dissimilarity structure (Figure S11), the remaining portion of human IT must have similarly category-sensitive features. One interpretation is that the prominent category regions are just particularly conspicuous concentrations of related features within a larger category-sensitive feature map (Haxby et al., 2001). Such large, consistently localized foci of features may only exist for a few categories (Downing et al., 2006). Their number is limited by the available space in the brain. Beyond discovering those regions, our larger goal should be to understand the representation as a whole, including the contribution of its less prevalent—or perhaps just more scattered—features. After all, IT categoricality is inherently a population phenomenon: Step-function-like categorical responses as reported for cells in the medial temporal lobe (Kreiman et al., 2000) and prefrontal cortex (Freedman et al., 2001) are not typically observed in either single IT cells (Vogels, 1999; Freedman et al., 2003; Kiani et al., 2007; but see Tsao et al., 2006) or category-sensitive fMRI responses (Haxby et al., 2001). Categorical clustering of response patterns indicates that the categorical distinctions explain a lot of variance across the population. It does not imply that any single cell exhibits a step-function-like response.
Explaining the IT Representational Similarity Structure
Can low-level features explain the IT representational similarity structure? The categorical cluster structure observed in IT was absent in the fMRI response patterns in human early visual cortex (Figures 5 and S5) and also in several low-level model representations of the images (luminance pattern, color pattern, color histogram, silhouette pattern, V1 model representation; Figures S6 and S7). The possibility that our findings can be explained by low-level features can never be formally excluded, because the space of models to be tested is infinite. However, our results suggest that the categorical clustering in IT does not reflect only low-level features.
Can more complex natural-image features explain the IT representational similarity structure? Categorical clustering was not evident in the intermediate-complexity HMAX-C2 model based on natural image fragments (Figure S7; Serre et al., 2005). In addition, a high-level representation composed of shape-tuned units adapted to real-world object images in the HMAX framework has previously been shown not to exhibit categorical clustering (Kiani et al., 2007).
Our interpretation of the current evidence is that evolution and development leave primate IT with features optimized not only for representing natural images (as the features of the models described above), but also for discriminating between object categories. This suggests that an IT model should acquire category-discriminating features by supervised learning (Ullman, 2007). A recent study suggests that human IT responds preferentially to such category-discriminating features (Lerner et al., 2008).
Does IT categoricality arise from feedforward or feedback processing? Our tasks (in both species) minimize the top-down component by withdrawing attention from briefly presented stimuli. Although this does not abolish local recurrent processing, it minimizes feedback from higher regions, suggesting that IT categoricality is not a product of top-down influences. One interpretation is that IT categoricality arises from feedforward connectivity. Rapid feedforward animate-inanimate discrimination would explain reports that humans can perform animal detection at latencies allowing for limited recurrent processing (Thorpe et al., 1996; Kirchner and Thorpe, 2006).
Serre et al. (2007) proposed a feedforward model of rapid categorization (see also Riesenhuber and Poggio, 2002), which summarizes a wealth of neuroscientific findings. Their architecture may be able to account for our findings. However, these authors associate the category-discrimination stage with prefrontal cortex. Our results suggest that features at the stage of IT already are optimized for category discrimination.
Beyond visual features optimized for categorization, could IT represent more complex semantic information? So far we have considered the features a means to the end of categorization. Instead, we could argue, more generally, that the features serve to infer nonvisual properties from the visual input. The features, then, are the end, and category clusters may arise as a consequence of the feature set. It has been suggested, for example, that IT represents action-related properties (Mahon et al., 2007). This perspective relates our findings to the literature on semantic representations (Tyler and Moss, 2001; McClelland and Rogers, 2003; Patterson et al., 2007). In order to test semantic-feature hypotheses along with computational models, we could predict the IT representational similarity from semantic property descriptions of the stimuli.
To find a model that reproduces the empirical representational similarity structure of IT (Figure 1) would constitute a substantial theoretical advance. The reader is invited to join us in testing additional models by exposing them to our stimuli and comparing the RDMs of the model representations to our empirical RDMs from IT. If the models have parameters fitted, so as best to predict the empirical RDMs, independent stimulus sets will be needed for fitting and testing. We will provide both stimuli and RDMs of monkey and human IT upon request.
Representational Similarity Analysis
Studying a brain region’s pairwise response-pattern dissimilarities for a sizable set of stimuli reveals what distinctions are emphasized and what distinctions are abstracted from by the representation. Representational similarity analysis allows us to make comparisons between brain regions (Figure 6), between species (Figure 3), between measurement modalities (Figure 3, confounded with the species-effect here), and between biological brains and computational models (Figures S6 and S7). An RDM usefully combines the evidence across the patterns of response within a functional region (thus allowing us to see the forest), but it requires no averaging of activity across space, time, or stimuli (thus honoring the trees). The RDM has a very intricate structure ((n2 – n)/2 dissimilarities, where n is the number of stimuli), thus providing a rich characterization of the representation.
In order to understand a population code, representational similarity analysis must be complemented with a wide range of methods. For example, we need to quantify the pairwise stimulus information, address how the representation can be read out (e.g., is a given distinction explicit in the sense of linear decod-ability?; Figure S12), how it relates to other brain representations (Figure 6) and behavior, and how the activity patterns are organized in space and time.
Implications for the Relationship between fMRI and Single-Cell Data
A single voxel in blood-oxygen-level-dependent fMRI reflects the activity of tens of thousands of neurons (Logothetis et al., 2001). We therefore expect to find somewhat different stimulus information in hemodynamic and neuronal response patterns. fMRI patterns may contain more information about fine-grained neuronal activity patterns than voxel size would suggest (Kamitani and Tong, 2005). But to what extent neuronal pattern information is reflected in fMRI pattern information is not well understood, because a voxel’s signal does not provide us simply with the average activity within its boundaries, but rather reflects the complex spatiotemporal transform of the hemodynamic response. The close match we report here between the RDMs from single-cell recording and fMRI provides some hope that data from these two modalities, for all their differences, may somewhat consistently reveal neuronal representations when subjected to massively multivariate analyses of activity-pattern information (Kriegeskorte and Bandettini, 2007).
A Common Code in Primate IT
Taken together, our results suggest that evolution and individual development leave primate IT with representational features that emphasize behaviorally important categorical distinctions. The major distinctions, animate-inanimate and face-body, are so basic that their conservation across species appears plausible. However, the IT representation is not purely categorical. Within category clusters, object exemplars are represented in a continuous object space, which may reflect a form of visual similarity. The categorical and continuous aspects of the representation are both consistent between man and monkey, suggesting that a code common across species may characterize primate IT.
EXPERIMENTAL PROCEDURES
This section describes the experimental designs and brain-activity measurements in monkey and human. The monkey experiments have previously been described in detail (Kiani et al., 2007), so we only give a brief summary here. Detailed descriptions of the statistical analysis and human localizer experiments are in the Supplemental Data.
Stimuli Presented to Humans and Monkeys
The stimuli presented to monkeys and humans were 92 color photographs (175 × 175 pixels) of isolated real-world objects on a gray background (Figure S1). The objects included natural and artificial inanimate objects as well as faces and bodies of humans and nonhuman animals. No predefined stimulus grouping was implied in either the experimental design or the core analyses for either species.
Monkey Experiments
Experimental Design and Task
Two alert monkeys were presented with the 92 images in rapid succession (stimulus duration, 105 ms; interstimulus interval, 0 ms) as part of a larger set of over 1000 similar images while they performed a fixation task. Fixation was monitored with an infra-red eye-tracking system. Stimuli were presented in a pseudorandom order. The stimulus sequence started after the monkey maintained fixation for 300 ms. Stimuli spanned a visual angle of about 7°. Each stimulus lasted for 105 ms and was followed by another stimulus without intervening interstimulus interval.
Brain-Activity Measurements
Neuronal activity was recorded extracellularly with tungsten electrodes, one cell at a time. The cells were located in anterior IT cortex (anterior 13–20 mm, distributed over the ventral bank of the superior temporal sulcus and the ventral convexity up to the medial bank of the anterior middle temporal sulcus), in the right hemisphere in monkey 1 and in the left in monkey 2. On average, the stimulus set was repeated 9 ± 2 (median, 10) times for each recording site. A different random stimulus sequence was used on each repetition for each recording site in order to avoid consistent interactions between successively presented stimuli.
Human Experiments
Experimental Design and Task
We presented the 92 images to subjects in a “quick” event-related fMRI experiment, which balances the need for separable hemodynamic responses (suggesting a slow event-related design) and the need for presenting many stimuli in the limited time-span of the fMRI experiment (suggesting a rapid event-related design). The experiment included four additional images, which were excluded from the interspecies analyses because of insufficient monkey data (Figure S1). Stimuli spanned a visual angle of 2.9° and were presented foveally for a duration of 300 ms on a constantly visible uniform gray background. Stimuli were centered with respect to a fixation cross superimposed to them.
Each stimulus was presented exactly once in each run. The sequence also included 40 null trials with no stimulus presented (4 of them at the beginning, 4 of them at the end, and 32 randomly interspersed in the sequence). The trial-onset asynchrony was 4 s; the stimulus-onset asynchrony was either 4 s or a multiple of that duration when null trials occurred in the sequence. The trials (including 96 stimulus presentations and 32 interspersed null trials) occurred in random order (no sequence optimization). We used a different random sequence on each of up to 14 runs (spread over two fMRI sessions) per subject. A run lasted 9 min and 4 s (4 + 96 + 32 + 4 = 136 trials, each 4 s long).
Subjects continually fixated a fixation cross superimposed to the stimuli and performed a color-discrimination task. During stimulus presentation the fixation cross turned from white to either green or blue and the subject responded with a right-thumb button press for blue and a left-thumb button press for green. The fixation-cross changes to blue or green were chosen according to an independent random sequence.
Brain-Activity Measurements
Blood-oxygen-level-dependent fMRI was performed at high spatial resolution using a 3T GE HDx MRI scanner. For signal reception, we used a receive-only whole-brain surface-coil array (16 elements, NOVA Medical Inc., Wilmington, MA). Twenty-five 2 mm axial slices (no gap) were acquired, covering the occipital and temporal lobe, using single-shot interleaved gradient-recalled Echo Planar Imaging (EPI) with a sensitivity-encoding sequence (SENSE, acceleration factor: 2, Prüssmann 2004). Imaging parameters were as follows: EPI matrix size: 128 × 96, voxel size: 1.95 × 1.95 × 2mm3, echo time (TE): 30 ms, repetition time (TR): 2 s. Each functional run consisted of 272 volumes (9 min and 4 s per run). Four subjects were scanned in two separate sessions each, resulting in 11 to 14 runs per subject, yielding a total of 49 runs (equivalent to 7 hr, 24 min, and 16 s of fMRI data). As an anatomical reference, we acquired high-resolution T1-weighted whole-brain anatomical scans with a Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence. Imaging parameters were as follows: matrix size: 256 × 256, voxel size: 0.86 × 0.86 × 1.2 mm3, 124 slices.
Supplementary Material
ACKNOWLEDGMENTS
We thank Chris Baker, James Haxby, Alex Martin, Nancy Kanwisher, Dwight Kravitz, Leslie Ungerleider, and Vina Vo for helpful discussions. This research was supported in part by the Intramural Program of the National Institute of Mental Health.
Footnotes
SUPPLEMENTAL DATA
The Supplemental Data can be found with this article online at http://www.neuron.org/supplemental/S0896-6273(08)00943-4.
REFERENCES
- Afraz SR, Kiani R, Esteky H. Microstimulation of inferotemporal cortex influences face categorization. Nature. 2006;442:692–695. doi: 10.1038/nature04982. [DOI] [PubMed] [Google Scholar]
- Aguirre GK. Continuous carry-over designs for fMRI. Neuroimage. 2007;35:1480–1494. doi: 10.1016/j.neuroimage.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre GK, Zarahn E, D’Esposito M. An area within human ventral cortex sensitive to “building” stimuli: evidence and implications. Neuron. 1998;21:373–383. doi: 10.1016/s0896-6273(00)80546-2. [DOI] [PubMed] [Google Scholar]
- Baker CI, Behrmann M, Olson CR. Impact of learning on representation of parts and wholes in monkey inferotemporal cortex. Nat. Neurosci. 2002;5:1210–1216. doi: 10.1038/nn960. [DOI] [PubMed] [Google Scholar]
- Bodurka J, Ye F, Petridou N, Murphy K, Bandettini PA. Mapping the MRI voxel volume in which thermal noise matches physiological noise-Implications for fMRI. Neuroimage. 2007;34:542–549. doi: 10.1016/j.neuroimage.2006.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitani E, Laiacona M, Mahon B, Caramazza A. What are the facts of semantic category-specific deficits? A critical review of the clinical evidence. Cogn. Neuropsychol. 2003;20:213–261. doi: 10.1080/02643290244000266. [DOI] [PubMed] [Google Scholar]
- Carlson TA, Schrater P, He S. Patterns of activity in the categorical representations of objects. J. Cogn. Neurosci. 2003;15:704–717. doi: 10.1162/089892903322307429. [DOI] [PubMed] [Google Scholar]
- Cox DD, Savoy RL. Functional magnetic resonance imaging (fMRI) “brain reading”: detecting and classifying distributed patterns of fMRI activity in human visual cortex. Neuroimage. 2003;19:261–270. doi: 10.1016/s1053-8119(03)00049-1. [DOI] [PubMed] [Google Scholar]
- Denys K, Vanduffel W, Fize D, Nelissen K, Peuskens H, Van Essen D, Orban GA. The processing of visual shape in the cerebral cortex of human and nonhuman primates: a functional magnetic resonance imaging study. J. Neurosci. 2004;24:2551–2565. doi: 10.1523/JNEUROSCI.3569-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Albright TD, Gross CG, Bruce C. Stimulus-selective properties of inferior temporal neurons in the macaque. J. Neurosci. 1984;4:2051–2062. doi: 10.1523/JNEUROSCI.04-08-02051.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing PE, Jiang Y, Shuman M, Kanwisher N. A cortical area selective for visual processing of the human body. Science. 2001;293:2470–2473. doi: 10.1126/science.1063414. [DOI] [PubMed] [Google Scholar]
- Downing PE, Chan AW-Y, Peelen MV, Dodds CM, Kanwisher N. Domain specificity in visual cortex. Cereb. Cortex. 2006;16:1453–1461. doi: 10.1093/cercor/bhj086. [DOI] [PubMed] [Google Scholar]
- Edelman S. Representation is representation of similarities. Behav. Brain Sci. 1998;21:449–467. doi: 10.1017/s0140525x98001253. [DOI] [PubMed] [Google Scholar]
- Edelman S, Grill-Spector K, Kushnir T, Malach R. Towards direct visualization of the internal shape space by fMRI. Psychobiology. 1998;26:309–321. [Google Scholar]
- Eger E, Ashburner J, Haynes JD, Dolan RJ, Rees G. FMRI activity patterns in human LOC carry information about object exemplars within category. J. Cogn. Neurosci. 2008;20:356–370. doi: 10.1162/jocn.2008.20019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Assad JA. Experience-dependent representation of visual categories in parietal cortex. Nature. 2006;443:85–88. doi: 10.1038/nature05078. [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK. Categorical representation of visual stimuli in the primate prefrontal cortex. Science. 2001;291:312–316. doi: 10.1126/science.291.5502.312. [DOI] [PubMed] [Google Scholar]
- Freedman DJ, Riesenhuber M, Poggio T, Miller EK. A comparison of primate prefrontal and inferior temporal cortices during visual categorization. J. Neurosci. 2003;23:5235–5246. doi: 10.1523/JNEUROSCI.23-12-05235.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier I, Skudlarski P, Gore JC, Anderson AW. Expertise for cars and birds recruits brain areas involved in face recognition. Nat. Neurosci. 2000;3:191–197. doi: 10.1038/72140. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kourtzi Z, Kanwisher N. The lateral occipital complex and its role in object recognition. Vision Res. 2001;41:1409–1422. doi: 10.1016/s0042-6989(01)00073-6. [DOI] [PubMed] [Google Scholar]
- Hanson SJ, Matsuka T, Haxby JV. Combinatorial codes in ventral temporal lobe for object recognition: Haxby (2001) revisited: is there a “face” area? Neuroimage. 2004;23:156–166. doi: 10.1016/j.neuroimage.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Humphreys GW, Forde EME. Hierarchies, similarity, and interactivity in object recognition: “Category-specific” neuropsychological deficits. Behav. Brain Sci. 2001;24:453–509. [PubMed] [Google Scholar]
- Hung CP, Kreiman G, Poggio T, DiCarlo JJ. Fast readout of object identity from macaque inferior temporal cortex. Science. 2005;310:863–866. doi: 10.1126/science.1117593. [DOI] [PubMed] [Google Scholar]
- Johnson SC. Hierarchical clustering schemes. Psychometrika. 1967;32:241–254. doi: 10.1007/BF02289588. [DOI] [PubMed] [Google Scholar]
- Kamitani Y, Tong F. Decoding the visual and subjective contents of the human brain. Nat. Neurosci. 2005;8:679–685. doi: 10.1038/nn1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay KN, Naselaris T, Prenger RJ, Gallant JL. Identifying natural images from human brain activity. Nature. 2008;452:352–355. doi: 10.1038/nature06713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayaert G, Biederman I, Vogels R. Representation of regular and irregular shapes in macaque inferotemporal cortex. Cereb. Cortex. 2005;15:1308–1321. doi: 10.1093/cercor/bhi014. [DOI] [PubMed] [Google Scholar]
- Kiani R, Esteky H, Tanaka K. Differences in onset latency of macaque inferotemporal neural responses to primate and non-primate faces. J. Neurophysiol. 2005;94:1587–1596. doi: 10.1152/jn.00540.2004. [DOI] [PubMed] [Google Scholar]
- Kiani R, Esteky H, Mirpour K, Tanaka K. Object category structure in response patterns of neuronal population in monkey inferior temporal cortex. J. Neurophysiol. 2007;97:4296–4309. doi: 10.1152/jn.00024.2007. [DOI] [PubMed] [Google Scholar]
- Kirchner H, Thorpe SJ. Ultra-rapid object detection with saccadic eye-movements: visual processing speed revisited. Vision Res. 2006;46:1762–1776. doi: 10.1016/j.visres.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Kreiman G, Koch C, Fried I. Category-specific visual responses of single neurons in the human medial temporal lobe. Nat. Neurosci. 2000;3:946–953. doi: 10.1038/78868. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Bandettini P. Analyzing for information, not activation, to exploit high-resolution fMRI. Neuroimage. 2007;38:649–662. doi: 10.1016/j.neuroimage.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Goebel R, Bandettini P. Information-based functional brain mapping. Proc. Natl. Acad. Sci. USA. 2006;103:3863–3868. doi: 10.1073/pnas.0600244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Formisano E, Sorger B, Goebel R. Individual faces elicit distinct response patterns in human anterior temporal cortex. Proc. Natl. Acad. Sci. USA. 2007;104:20600–20605. doi: 10.1073/pnas.0705654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Mur M, Bandettini PA. Representational similarity analysis – connecting the branches of systems neuroscience. Front. Syst. Neurosci. 2008;2 doi: 10.3389/neuro.06.004.2008. in press. Published online November 24, 2008. 10.3389/neuro.06.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruskal JB, Wish M. Multidimensional Scaling. Sage University Series; Beverly Hills, CA: 1978. [Google Scholar]
- Laakso A, Cottrell GW. Content and cluster analysis: Assessing representational similarity in neural systems. Philos. Psychol. 2000;13:47–76. [Google Scholar]
- Lehky SR, Sereno AB. Comparison of shape encoding in primate dorsal and ventral visual pathways. J. Neurophysiol. 2007;97:307–319. doi: 10.1152/jn.00168.2006. [DOI] [PubMed] [Google Scholar]
- Lerner Y, Epshtein B, Ullman S, Malach R. Class information predicts activation by object fragments in human object areas. J. Cogn. Neurosci. 2008;20:1189–1206. doi: 10.1162/jocn.2008.20082. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Milleville SC, Negri GAL, Rumiati RI, Caramazza A, Martin A. Action-related properties shape object representations in the ventral stream. Neuron. 2007;55:507–520. doi: 10.1016/j.neuron.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. The representations of object concepts in the brain. Annu. Rev. Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV. Neural correlates of category-specific knowledge. Nature. 1996;379:649–652. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- McClelland JL, Rogers TT. The parallel distributed processing approach to semantic cognition. Nat. Rev. Neurosci. 2003;4:310–322. doi: 10.1038/nrn1076. [DOI] [PubMed] [Google Scholar]
- O’Toole A, Jiang F, Abdi H, Haxby JV. Partially distributed representation of objects and faces in ventral temporal cortex. J. Cogn. Neurosci. 2005;17:580–590. doi: 10.1162/0898929053467550. [DOI] [PubMed] [Google Scholar]
- Op de Beeck H, Wagemans J, Vogels R. Inferotemporal neurons represent low-dimensional configurations of parameterized shapes. Nat. Neurosci. 2001;4:1244–1252. doi: 10.1038/nn767. [DOI] [PubMed] [Google Scholar]
- Orban GA, Van Essen D, Vanduffel W. Comparative mapping of higher visual areas in monkeys and humans. Trends Cogn. Sci. 2004;8:315–324. doi: 10.1016/j.tics.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat. Rev. Neurosci. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Prüssmann KP. Parallel imaging at high field strength: Synergies and joint potential. Top. Magn. Reson. Imaging. 2004;15:237–244. doi: 10.1097/01.rmr.0000139297.66742.4e. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Gore JC, McCarthy G. Face-sensitive regions in human extrastriate cortex studied by functional MRI. J. Neurophysiol. 1995;74:1192–1199. doi: 10.1152/jn.1995.74.3.1192. [DOI] [PubMed] [Google Scholar]
- Quiroga RQ, Reddy L, Kreiman G, Koch C, Fried I. Invariant visual representation by single neurons in the human brain. Nature. 2005;435:1102–1107. doi: 10.1038/nature03687. [DOI] [PubMed] [Google Scholar]
- Riesenhuber M, Poggio T. Neural mechanisms of object recognition. Curr. Opin. Neurobiol. 2002;12:162–168. doi: 10.1016/s0959-4388(02)00304-5. [DOI] [PubMed] [Google Scholar]
- Serre T, Wolf L, Poggio T. Computer Vision and Pattern Recognition (CVPR 2005) San Diego, CA, USA: 2005. Object recognition with features inspired by visual cortex. [Google Scholar]
- Serre T, Oliva A, Poggio T. A feedforward architecture accounts for rapid categorization. Proc. Natl. Acad. Sci. USA. 2007;104:6424–6429. doi: 10.1073/pnas.0700622104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard RN. Multidimensional scaling, tree-fitting, and clustering. Science. 1980;210:390–398. doi: 10.1126/science.210.4468.390. [DOI] [PubMed] [Google Scholar]
- Shepard RN, Chipman S. Second-order isomorphism of internal representations: Shapes of states. Cognit. Psychol. 1970;1:1–17. [Google Scholar]
- Sigala N, Logothetis NK. Visual categorization shapes feature selectivity in the primate temporal cortex. Nature. 2002;415:318–320. doi: 10.1038/415318a. [DOI] [PubMed] [Google Scholar]
- Tanaka K. Inferotemporal cortex and object vision. Annu. Rev. Neurosci. 1996;19:109–139. doi: 10.1146/annurev.ne.19.030196.000545. [DOI] [PubMed] [Google Scholar]
- Thorpe S, Fize D, Marlot C. Speed of processing in the human visual system. Nature. 1996;381:520–522. doi: 10.1038/381520a0. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Tsao D, Vanduffel W. Neuroimaging weighs in: humans meet macaques in “primate” visual cortex. J. Neurosci. 2003;23:3981–3989. doi: 10.1523/JNEUROSCI.23-10-03981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgerson WS. Theory and Methods of Scaling. Wiley; New York: 1958. [Google Scholar]
- Tsao DY, Freiwald WA, Knutsen TA, Mandeville JB, Tootell RBH. Faces and objects in macaque cerebral cortex. Nat. Neurosci. 2003;6:989–995. doi: 10.1038/nn1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Tootell RBH, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311:670–674. doi: 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Moss HE. Towards a distributed account of conceptual knowledge. Trends Cogn. Sci. 2001;5:244–252. doi: 10.1016/s1364-6613(00)01651-x. [DOI] [PubMed] [Google Scholar]
- Ullman S. Object recognition and segmentation by a fragment-based hierarchy. Trends Cogn. Sci. 2007;11:58–64. doi: 10.1016/j.tics.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Dierker DL. Surface-based and probabilistic atlases of primate cerebral cortex. Neuron. 2007;56:209–225. doi: 10.1016/j.neuron.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Lewis JW, Drury HA, Hadjikhani N, Tootell RBH, Bakircioglu M, Miller MI. Mapping visual cortex in monkeys and humans using surface-based atlases. Vision Res. 2001;41:1359–1378. doi: 10.1016/s0042-6989(01)00045-1. [DOI] [PubMed] [Google Scholar]
- Vogels R. Categorization of complex visual images by rhesus monkeys. Part 2: single-cell study. Eur. J. Neurosci. 1999;11:1239–1255. doi: 10.1046/j.1460-9568.1999.00531.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.