Abstract
We used signal transducer and activator of transcription 4 (STAT4) and STAT6 gene knockout (–/–) mice as recipients of fully mismatched cardiac allografts to study the role of T-cell costimulatory pathways in regulating allogeneic T-helper 1 (Th1) versus Th2 responses in vivo. STAT4–/– mice have impaired Th1 responses, whereas STAT6–/– mice do not generate normal Th2 responses. Cardiac allografts from C57BL/6 mice were transplanted into normal wild-type (WT), STAT4–/–, and STAT6–/– BALB/c recipients. STAT4–/– and STAT6–/– mice rejected their grafts with the same tempo as untreated WT recipients. CD28-B7 blockade by a single injection of CTLA4Ig induced long-term engraftment and donor-specific tolerance in all three groups of recipients. CD154 blockade by a single injection of MR1 was effective in prolonging allograft survival and inducing tolerance in STAT4–/– mice but was only marginally effective in STAT6–/– recipients and WT controls. In addition, a similar protocol of MR1 was ineffective in prolonging graft survival in CD28–/– BALB/c recipients, suggesting that the lack of efficacy seen in WT and STAT6–/– mice is not due to the presence of a functional CD28-B7 pathway. Furthermore, there was a similar differential effect of CD28-B7 versus CD154-CD40 blockade in inhibiting immune responses in animals immunized with ovalbumin and complete Freund’s adjuvant. These novel data indicate that Th1 and Th2 cells are differentially regulated by CD28-B7 versus CD154-CD40 costimulation pathways in vivo and may have potential implications for the development of therapeutic strategies such as T-cell costimulatory blockade in humans.
Introduction
CD4+ T-helper (Th) cells may be divided into at least two functionally distinct subsets (1, 2). Th1 cells are important in cell-mediated immunity, whereas Th2 cells are regulators of the humoral immune response and suppress Th1 cell function (2). In transplantation models, it is traditionally thought that Th1 cells cause acute rejection and Th2 cells induce and maintain tolerance. This paradigm is supported by reports demonstrating Th2 cytokine expression in grafts of tolerant animals (3–7) and Th1 cytokine reversal of the induction of tolerance (8, 9). However, there are recent data that challenge this paradigm. For example, rejection occurs in Th1 cytokine-knockout animals (10, 11), and tolerance can be induced in Th2 cytokine knockouts (12, 13), although this is not a universal observation (14).
It has been suggested that there are several factors that determine the fate of a naive Th cell in response to antigenic challenge (15, 16). These include the strength of the T-cell receptor (TCR) signal or antigen density, the cytokine milieu, and specific costimulatory pathways. T cells require two distinct signals for full activation (17). The first signal is provided by the engagement of the TCR with the major histocompatibility complex (MHC) plus peptide complex on antigen-presenting cells (APCs), and the second “costimulatory” signal is provided by engagement of one or more T-cell surface receptors with their ligands on APCs (15–18). Among the multiple costimulatory pathways identified, increasing evidence suggests that interactions of T-cell surface receptors CD28 and CD154, with their respective ligands B7-1/B7-2 and CD40, on APCs are critical for the T-cell responses to alloantigens (17, 19). At present, the role of CD28-B7 versus CD154-CD40 in regulating Th1 versus Th2 allogeneic responses in vivo is unknown.
Members of the signal transducer and activator of transcription (STAT) gene family are critical for the differentiation of Th-cell subsets. It has been demonstrated that STAT4 knockout (–/–) mice have impaired generation of Th1 cells, whereas STAT6–/– mice do not generate normal Th2 responses (20, 21). Therefore, these gene-knockout mice may be used to study the role of Th1 and Th2 responses in rejection and tolerance induction. Using STAT4–/– and STAT6–/– mice as recipients of vascularized, fully mismatched cardiac allografts, we studied the role of T-cell costimulatory pathway blockade with cytotoxic T-lymphocyte antigen 4 (CTLA4Ig; to block CD28-B7) or anti-CD154 mAb (MR1; to block CD154-CD40) in preventing rejection and inducing tolerance in a predominant Th1 versus Th2 environment. Our data indicate that even in the absence of normal Th1 or Th2 responses, rejection proceeds with the same tempo and that alloreactive Th1 and Th2 cells are differentially regulated by CD28-B7 versus CD154-CD40 costimulatory pathways in vivo.
Methods
Mice.
C57BL/6 (H-2b) and C3H/He (H-2k) mice aged 6–8 weeks were purchased from the Jackson Laboratory (Bar Harbor, Maine, USA) and BALB/c (H-2d) mice aged 6–8 weeks were purchased from Taconic Farms (Germantown, New York, USA). CD28–/– (H-2d) mice were purchased from the Jackson Laboratory and bred in our animal facility. Mice homozygous for a targeted disruption of STAT4 gene (STAT4–/– mice) and STAT6 gene (STAT6–/– mice) have been described previously (20, 21). Briefly, IL-12–induced increases in the production of IFN-γ cellular proliferation and natural killer (NK) cell cytotoxicity are abrogated in lymphocytes from STAT4-deficient mice. These lymphocytes also demonstrate a propensity toward the development of Th2 cells. On the other hand, IL-4–induced increases in the cell-surface expression of both major histocompatibility complex (MHC) class II antigens and IL-4 receptor are completely abrogated, and lymphocytes from STAT6-deficient animals fail to differentiate into Th2 cells in response to either IL-4 or IL-13.
Fusion proteins and mAbs.
MR1 was purchased from Bioexpress Cell Culture Services (West Lebanon, New Hampshire, USA). Murine CTLA4Ig was a generous gift from Bristol Myers Squibb (Robert Peach, Princeton, New Jersey, USA).
Transplantation.
BALB/c mice (WT, STAT4–/–, STAT6–/–, and CD28–/–) were used as recipients and C57BL/6 mice as donors. The first cardiac allografts were placed in an intra-abdominal location (22), and the second heart grafts from C57BL/6 or C3H/He mice were placed in the neck using a modification of Chen’s method (23). Graft function was assessed daily by palpation. Animals received a single dose of mAb or fusion protein at a dose of 250 μg by intraperitoneal injection either on the day of transplantation (for MR1) or 2 days after engraftment (for CTLA4Ig). These administration schedules were based on our previous observations that a single injection of CTLA4Ig is most effective when administered on post-transplant day 2 , whereas MR1 is best given on the day of transplant (4, 6, 24). In some instances, animals also received an intravenous injection of 5 × 106 donor splenocytes at the time of transplantation, as described previously (6). The day of rejection was defined as the day of cessation of palpable heartbeat and was verified by autopsy and selective pathological examination. Loss of graft function within 48 hours of transplant was considered a technical failure (<10% on average), and these animals were excluded from further analysis.
Statistics.
For the graft survival, Kaplan-Meier survival graphs were constructed and log-rank comparison of the groups was used to calculate P values. For the ELISPOT results, P values were calculated using the paired t test.
Morphology.
Cardiac grafts from both the isografts and each untreated recipient group (n = 3–4) were fixed in 10% buffered formalin, embedded in paraffin, coronally sectioned, and stained with hematoxylin and eosin (H&E) for evaluation of cellular infiltrates using light microscopy. Cardiac grafts from long-term survivors (>100 days) treated with either MR1 or CTLA4Ig were similarly prepared and stained with H&E, Verhoeff’s elastin (for vessel arteriosclerosis scoring), or Masson’s trichrome (for evaluation of fibrosis).
Arteriosclerosis was assessed using light microscopy and percentage luminal occlusion by intimal thickening determined using the scoring system as described previously (25–27). In brief, a vessel score of 0 indicated less than 10% luminal occlusion, 1 indicated less than 20%, 2 indicated 20–40%, 3 indicated 40–60%, 4 indicated 60–80%, and 5 indicated 100% occlusion. Only vessels that were cut orthogonally and that displayed a clear internal elastic lamina were scored. All arteries were scored by two blinded examiners.
Matched trichrome- and H&E-stained sections were also examined for presence for fibrosis and interstitial cellular infiltration. Each of these categories was graded and the average grade calculated for each group. Fibrosis was quantified by collagen deposition highlighted by trichrome stain and scored on a 0–3 scale, where 0 indicated no fibrosis and 1, 2, and 3 indicated mild, moderate, and severe fibrosis, respectively. A 0–3 scale was also used for scoring the degree of interstitial mononuclear cell infiltration, with 0, 1, 2, and 3 indicating normal, minimal, moderate, and severe cellular infiltration, respectively.
ELISPOT cytokine measurement.
ELISPOT plates (Polyfiltronics Inc., Rockland, Massachusetts, USA) were coated with either 4 μg/mL of rat anti-mouse IFN-γ (R4-6A2) or with 5 μg/mL of rat anti-mouse IL-5 (TFRK-4) capturing mAbs in sterile PBS overnight. The plates were then blocked for 1.5 hours with sterile PBS containing 1% BSA and washed with sterile PBS. Splenocytes (1.2 × 106 in 0.2 mL AIM-V medium) were then placed in each well in the presence of 1.2 × 106 irradiated (20 Gy) syngeneic or allogeneic splenocytes (as APCs) and cultured for 48 hours at 37°C in 5% CO2. For detection of spots, 5 μg/mL of biotinylated rat anti-mouse IFN-γ mAb (XMG 1.2) or rat anti-mouse IL-5 mAb (TFRK-5) were used, followed by 2 hours of incubation with streptavidin D horseradish peroxidase (Vector Laboratories, Burlingame, California, USA) diluted at 1:2000 in PBS 0.025% Tween. All mAbs were purchased from PharMingen (San Diego, California, USA). After washing, the plates were developed using 0.8 mL of 3-amino-9-ethylcarbazole (AEC) (Pierce Chemical Co., Rockford, Illinois, USA; 10 mg dissolved in 1 mL dimethyl formamide) mixed with 24 mL of 0.1-M sodium acetate, pH 5.0, containing 12 μL H2O2. The resulting spots were counted on a computer-assisted enzyme-linked immunospot image analyzer (T Spot Image Analyzer; Cellular Technology Ltd., Cleveland, Ohio, USA) (28).
Immunization with ovalbumin and cell preparation for in vitro assay.
The animals were immunized with 100 μL of ovalbumin (OVA)/CFA subcutaneously, such that each mouse received a total of 100 μg of OVA, as described previously (29). Treatment was started with CTLA4Ig (100 μg per dose) or MR1 (100 μg per dose) on the day of immunization and given every other day for five doses. Control mice received control Ig. Lymph nodes were collected on day 10 after immunization. A single-cell suspension was prepared from draining popliteal lymph nodes for measurement of proliferation and cytokine production (29).
Proliferation and cytokine-production assays.
Proliferation and cytokine production were measured after stimulation with OVA in vitro at three doses (25, 50, and 100 μg/mL), as described previously (29). For proliferation and cytokine measurement, the cells were cultured in 96-well plates (Corning-Costar, Cambridge, Massachusetts, USA) with serum-free Ex Vivo 20 medium (BioWhittaker, Walkersville, Maryland, USA) containing 75 mM/mL L-glutamine, 100 U/mL penicillin and streptomycin, 1 mL/100 mL media of a 100× concentrated, nonessential amino acid solution, 0.1 mM HEPES/mL, 1 mM/mL sodium pyruvate (all from BioWhittaker), and 0.05 mM/mL 2-mercaptoethanol (Sigma Chemical Co., St. Louis, Missouri, USA). The cells were incubated at 37°C in humidified air containing 7% CO2.
For proliferation, lymph node cells were cultured at 2 × 106 cells/mL and 200 μL/well with various antigen concentrations. After 96 hours of culture 1 μCi 3H-thymidine (NEN Life Sciences Products, Boston, Massachusetts, USA) was added in 10 μL of media to each well for another 16 hours. Cells were harvested on filter mats using a TOMTEC harvester and incorporation of thymidine was analyzed with a Betaplate counter (Perkin-Elmer Wallac Inc., Gaithersburg, Maryland, USA).
For cytokine assays, the cells were cultured at 2 × 106 cells/mL in 200 μL media at various antigen concentrations. Supernatants for IL-4 and IFN-γ ELISA were collected after 48 hours of culture. Quantitative ELISAs for IL-4 and IFN-γ were performed using paired antibodies and recombinant cytokines from PharMingen according to the manufacturer’s recommendations.
Results
Effect of cytokine milieu on acute cardiac allograft rejection.
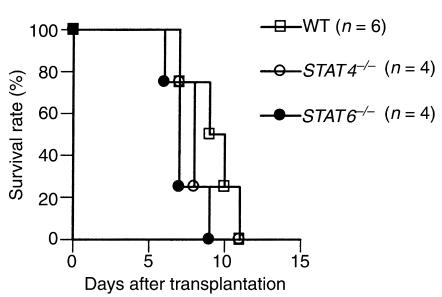
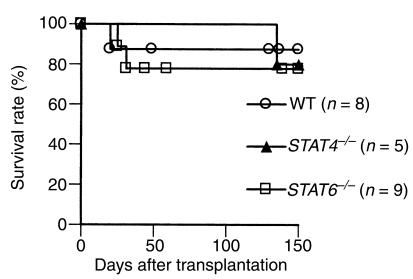
First, we investigated the effect of the absence of either the STAT4 or STAT6 signal transduction pathway on rejection of fully mismatched cardiac allografts. As shown in Figure 1, both STAT4–/– and STAT6–/– recipients were able to acutely reject their vascularized cardiac allografts; there was no significant difference in graft survival times between WT versus either STAT4–/– or STAT6–/– recipients. We then examined the graft morphology of the rejecting recipients. Sections of cardiac allografts were stained with H&E and were examined under light microscopy. Four groups of animals were studied: BALB/c into BALB/c, C57BL/6 into WT BALB/c, C57BL/6 into STAT4–/– BALB/c, and C57BL/6 into STAT6–/– BALB/c. Syngeneic functioning control grafts demonstrated preservation of myocytes with intact nuclei and absence of interstitial cellular infiltrates or vascular inflammation of cardiac vessels (Figure 2a). Rejected grafts from all three experimental recipient groups (WT, STAT4–/–, and STAT6–/–) demonstrated variable degrees of diffuse mononuclear cellular infiltration, endothelialitis, and areas of myocyte damage and interstitial infarction (Figure 2, b–d). These pathologic observations confirm the in vivo graft-survival data and clearly demonstrate that vascularized rejection occurs in the absence of normal Th1 or Th2 allogeneic responses.
Figure 1.
Allograft survival in untreated recipients. C57BL/6 hearts were transplanted into wild-type, STAT4–/–, or STAT6–/– BALB/c recipients.
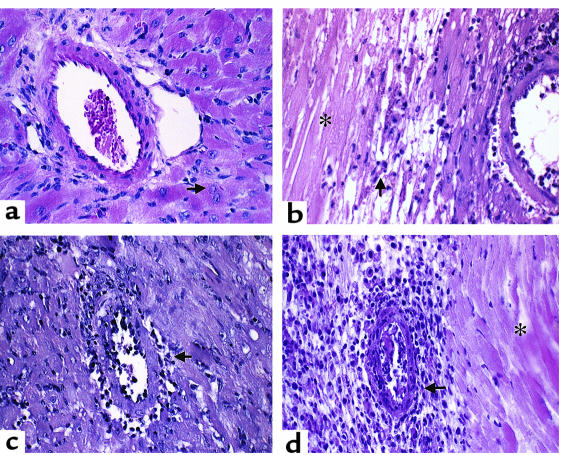
Figure 2.
Pathology of murine cardiac allograft rejection: syngeneic graft at day 8 after transplantation and allografts of WT, STAT4–/–, and STAT6–/– recipients. (a) Syngeneic graft: the artery shows no signs of inflammation and the cardiac muscle cells are intact, with viable nuclei (arrow). (b) Wild-type recipient graft: the small artery reveals inflammatory cells adherent to the endothelium. There is extensive infarction (coagulative or ischemic necrosis) of cardiac muscle cells (asterisk) and mononuclear inflammatory cell infiltration of the viable myocardium (arrow). (c) STAT4–/– recipient graft: there is active inflammation or endothelialitis of the inner layer of the small artery (arrow). The myocardium shows scattered lymphocytes. (d) STAT6–/– recipient graft: the active transmural inflammation (arrow) has resulted in extensive infarction of the myocardium (asterisk). There is also prominent inflammation of the parenchyma. Original magnification ×400 (H&E).
Cytokine profiles of untreated WT, STAT4–/–, and STAT6–/– recipients in response to donor alloantigen.
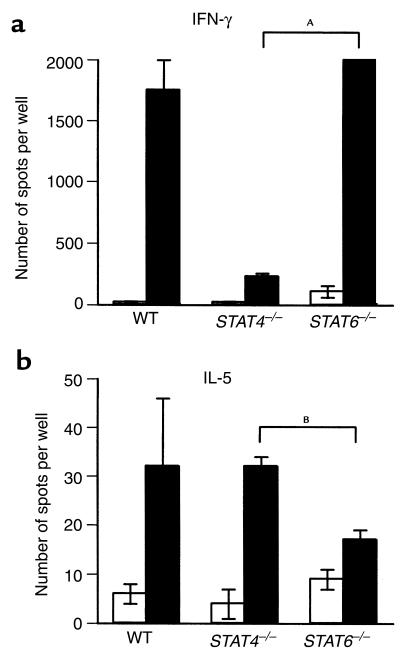
We then investigated profiles of cytokines produced by untreated recipients in response to donor alloantigen. ELISPOT assay was used to compare cytokine production (IFN-γ as a prototype Th1 cytokine and IL-5 as a prototype Th2 cytokine) by splenocytes from untreated WT, STAT4–/–, or STAT6–/– recipients that had acutely rejected heart allografts in response to donor or syngeneic (negative control) stimulator cells in vitro. As shown in Figure 3a, the frequencies of alloreactive IFN-γ–producing recipient cells were significantly higher than autoreactive frequencies in all groups, although the alloreactive frequency was significantly lower (P = 0.002; difference between the means was 1769.7) for STAT4–/– as compared with STAT6–/– mice. In comparison, the alloreactive frequency of IL-5–producing cells was only significantly higher than the autoreactive frequency in STAT4–/– recipients (Figure 3b), and that was significantly higher (P = 0.0493; difference between the means was 17) than the alloreactive frequency of STAT6–/– mice. WT recipients demonstrated some variability of IL-5 production in response to allogeneic cells (Figure 3b).
Figure 3.
Frequency of cytokine-producing donor-specific T cells in recipient WT, STAT4–/–, and STAT6–/– BALB/c mice following allogeneic heart transplantation. Mice were transplanted with C57BL/6 allogeneic hearts. Recipient spleens were removed at the time of rejection (9–10 days after transplant). Splenocytes (1.2 × 106 cells per well) were incubated with either recipient (open bars) or donor (filled bars) irradiated splenocytes. The frequencies of IFN-γ–producing T cells (a) and IL-5–producing T cells (b) were then determined using ELISPOT assay as described in Methods. The results are expressed as the mean number of spots per well ± SE obtained from three to four mice tested individually in each group. P values of syngeneic versus alloreactive responder frequencies in each animal group are as follows. IFN-γ: P = 0.0196, 0.0132, and 0.006 in WT, STAT4–/–, and STAT6–/– recipients, respectively. IL-5: P = 0.2327, 0.0092, and 0.0942 in WT, STAT4–/–, and STAT6–/– recipients, respectively. AP = 0.002; BP = 0.0493.
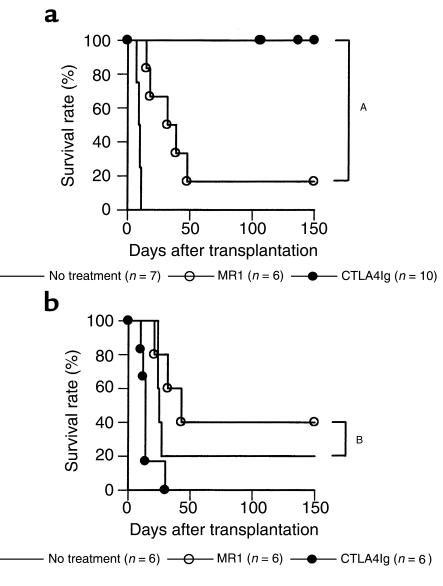
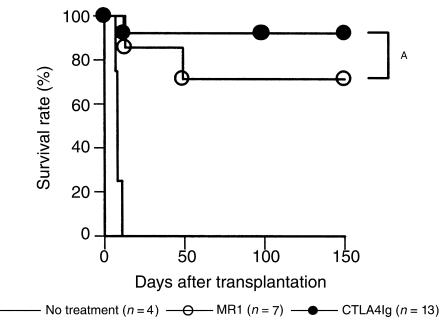
Effect of T-cell costimulatory signal blockade on cardiac allograft rejection in WT recipients.
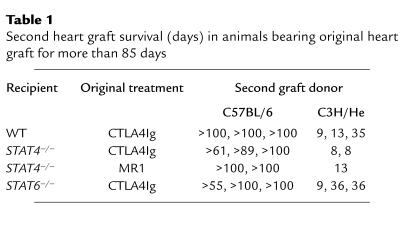
BALB/c mice were transplanted with C57BL/6 cardiac allografts and treated with CTLA4Ig or MR1. Whereas a single injection of CTLA4Ig on post-transplant day 2 markedly prolonged graft survival, a single injection of MR1 alone administered on day 0 was significantly less effective (Figure 4a). This observation with MR1 was not strain specific since a single injection of MR1 had a comparable effect in C57BL/6 recipients of BALB/c hearts (survival times: 8, 41, 41, 52, >100 × 2; P = NS) as compared with BALB/c recipients of C57BL/6 hearts. Donor-specific tolerance induction by CTLA4Ig treatment was confirmed by secondary heart-graft challenge of animals with long-term surviving grafts (>100 days). Whereas third-party (C3H/He) second grafts were rejected, grafts from the original donor strain were permanently accepted (Table 1).
Figure 4.
Effect of MR1 or CTLA4Ig treatment on cardiac allograft survival in wild-type (a) or CD28–/– mice (b). Recipients were treated with MR1 (250 μg intraperitoneally) on the day of transplantation or with CTLA4Ig (250 μg intraperitoneally) on day 2. Untreated controls are shown by solid line. AP < 0.01; BP = 0.33.
Table 1.
Second heart graft survival (days) in animals bearing original heart graft for more than 85 days
The observation that MR1 treatment was less effective than CTLA4Ig could be attributed to the continued presence of costimulation through the CD28-B7 pathway. To investigate this possibility, we administered a single injection of 250 μg MR1 to CD28–/– allograft recipients. As shown in Figure 4 b, untreated CD28–/– recipients had some prolongation of graft survival as reported previously (30), but MR1 treatment was ineffective in promoting long-term graft acceptance, suggesting that even in the absence of a functional CD28-B7 signal, CD154 blockade did not induce tolerance.
Effect of T-cell costimulatory signal blockade on cardiac allograft rejection in STAT4–/– recipients.
Similar to our findings in WT recipients, CTLA4Ig treatment prolonged survival and induced donor-specific tolerance to cardiac allografts in STAT4–/– recipients (Figure 5 and Table 1). Interestingly, unlike the case of WT recipients, MR1 promoted long-term graft survival and induced donor-specific tolerance (acceptance of second-donor strain grafts and rejection of third-party grafts; Table 1) in STAT4–/– recipients similar to CTLA4Ig therapy. These data suggest that CD154 blockade is more effective in suppressing alloimmune responses in the absence of a normal Th1 cytokine milieu.
Figure 5.
Effect of MR1 or CTLA4Ig treatment on cardiac allograft survival in STAT4–/– mice. Recipients were treated with MR1 (250 μg intraperitoneally) on the day of transplantation or with CTLA4Ig (250 μg intraperitoneally) on day 2. Untreated controls are shown by solid line. AP = 0.14.
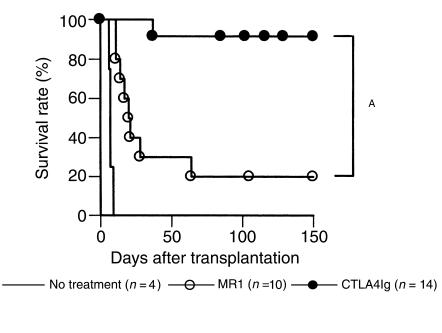
Effect of T-cell costimulatory signal blockade on cardiac allograft rejection in STAT6–/– recipients.
We then investigated the effect of CD28-B7 versus CD154-CD40 blockade on fully mismatched cardiac allograft rejection using STAT6–/– recipients. Treatment with CTLA4Ig prolonged graft survival and induced donor-specific tolerance as seen in WT and STAT4–/– recipients, whereas therapy with MR1 was significantly less effective in STAT6–/– recipients, similar to our observations in WT mice (Figure 6 and Table 1). Overall, CD154 blockade was significantly more effective in promoting long-term graft survival in STAT4–/– recipients when compared with either WT (P = 0.0438) or STAT6–/– recipients (P = 0.0461). These data indicate that CD154 blockade is only marginally effective in suppressing alloimmune responses in a predominantly Th1 environment.
Figure 6.
Effect of MR1 or CTLA4Ig treatment on cardiac allograft survival in STAT6–/– mice. Recipients were treated with MR1 (250 μg intraperitoneally) at the day of transplantation or with CTLA4Ig (250 μg intraperitoneally) on day 2. Untreated controls are shown by solid line. AP < 0.01.
Effect of CD28-B7 versus CD154-CD40 blockade on development of chronic rejection.
Grafts of WT, STAT4–/–, or STAT6–/– recipients treated with a single injection of MR1 or CTLA4Ig surviving more than 100 days were evaluated histopathologically for evidence of chronic rejection as manifested by graft arteriosclerosis, fibrosis, and interstitial inflammation. Grafts of animals treated with CTLA4Ig had minimal signs of arteriosclerosis (vessel scores were less than 0.5 in all groups), interstitial inflammation, or fibrosis. Grafts of animals treated with MR1, on the other hand, developed moderately severe arteriosclerosis (mean vessel scores of WT, STAT4–/–, and STAT6–/– recipients treated with MR1 were 3, 3.10 ± 1.07, and 3.25 ± 1.75, respectively), fibrosis, and interstitial inflammation. Although, as indicated above, the number of long-term survivors were small in the WT and STAT6–/– recipients treated with MR1 (1–2/group), there did not appear to be any difference in the pathology of chronic rejection between the different recipient groups treated with MR1.
Differential effects of CD28-B7 and CD154-CD40 blockade in priming of STAT4–/– and STAT6–/– mice to nominal antigen.
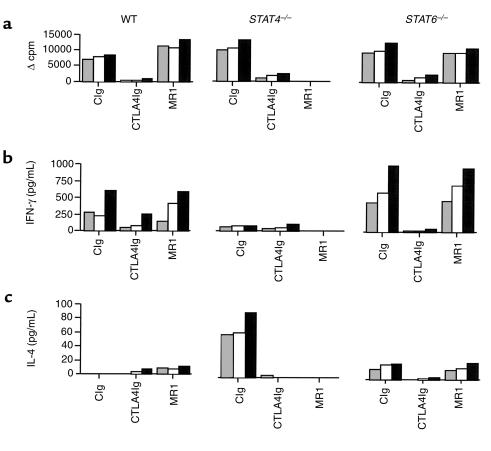
To determine if the differential effects of CD28-B7 versus CD154-CD40 blockade in regulating Th1 and Th2 responses are applicable to a nominal antigen system, we studied the immune response of WT, STAT4–/–, and STAT6–/– BALB/c mice immunized with OVA/CFA. We treated WT, STAT4–/–, and STAT6–/– BALB/c mice with CTLA4Ig or MR1 in vivo. In vitro stimulation of primed lymph node cells with OVA showed similar proliferative responses in control Ig-treated WT, STAT4–/–, and STAT6–/– mice. Whereas CTLA4Ig treatment suppressed the proliferative response in all treated mice, MR1 treatment was only effective in suppressing proliferation in STAT4–/– mice (Figure 7a). Cytokine production was also measured, and the results of both IFN-γ and IL-4 paralleled those of the proliferation assay. In STAT4–/– mice, a small amount of IFN-γ was produced, and treatment with MR1 abrogated it completely (Figure 7b). In addition, IL-4 production in STAT4–/– mice was suppressed after MR1 treatment as well as after CTLA4Ig treatment. As expected, there was minimal IL-4 detectable in the supernatants of the STAT6–/– mice cultures, but similar to IFN-γ production, it was unchanged after MR1 treatment (Figure 7c). These findings underscore the fact that the differential effects of CD154-CD40 in STAT4–/– versus STAT6–/– mice are not restricted to allogeneic stimulation but also apply to nominal antigen systems in vivo.
Figure 7.
Effect of MR1 or CTLA4Ig treatment on in vitro lymphocyte proliferation and cytokine production in mice immunized with OVA. The animals were immunized with OVA/CFA, and treatment started with CTLA4Ig or MR1 on the day of immunization and given every other day for five doses. Control mice received control Ig (CIg). Lymph nodes were collected on day 10 after immunization and proliferation (a), and cytokine production (b and c) was measured after stimulation with OVA in vitro at three doses: 25 μg/mL, shaded bar; 50 μg/mL, open bar; and 100 μg/mL, filled bar.
Strategies to optimize the effect of CD154 blockade in WT and STAT6–/– recipients.
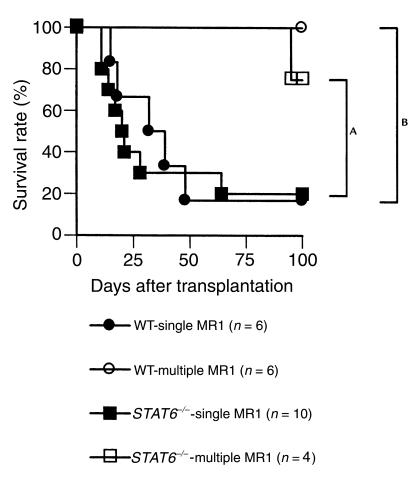
First, we investigated whether increasing the frequency of administration of MR1 could further improve graft survival in WT and STAT6–/– recipients. As shown in Figure 8, administration of four doses of MR1 (250 μg on post-transplant day 0, 2, 4, and 6) significantly improved graft survival in WT and STAT6–/– recipients as compared with the single injection protocol. In addition, we (6, 31) and others (32) have shown previously that administration of donor cells synergize with CD154 blockade to promote long-term allograft survival. Therefore, we wanted to investigate the effect of combining donor splenocytes plus CD154 blockade in WT, STAT4–/–, and STAT6–/– recipients of cardiac allografts. All recipients received an intravenous injection of 5 × 106 donor splenocytes plus a single dose of MR1 on the day of transplantation, as above. As seen in Figure 9, coadministration of donor splenocytes plus a single injection of MR1 resulted in long-term graft survival in all WT, STAT4–/–, and STAT6–/– recipients. Therefore, the addition of donor cells rendered CD154 blockade more effective in inhibiting Th1 allogeneic responses in vivo. These data, taken together with the data obtained with the multiple injection protocol, indicate that Th1 cells are not inherently resistant to CD154 blockade.
Figure 8.
Effect of administration of multiple injections of MR1 on cardiac allograft survival in WT and STAT6–/– recipients. C57BL/6 hearts were transplanted into wild-type or STAT6–/– BALB/c recipients. Recipients were treated with either a single dose of MR1 alone (250 μg intraperitoneally) on the day of transplantation or four doses of MR1 (250 μg intraperitoneally, four times) on post-transplant day 0, 2, 4, and 6. The multiple-injection protocol significantly increased graft survival in both recipient groups. AP = 0.0456; BP = 0.0043.
Figure 9.
Effect of coadministration of donor splenocytes plus a single injection of MR1 on cardiac allograft survival in WT, STAT4–/–, and STAT6–/– recipients. C57BL/6 hearts were transplanted into WT, STAT4–/–, or STAT6–/– BALB/c recipients. Recipients were treated with MR1 (250 μg intraperitoneally) plus 5 × 106 donor splenocytes intravenously on the day of transplantation. There were no significant differences between the three recipient types.
Discussion
We used STAT4–/– and STAT6–/– mice to study the role of CD28-B7 and CD154-CD40 costimulatory pathways in regulating Th1 versus Th2 alloimmune responses in vivo. Our data clearly show that clinical and morphological rejection occurs with a normal tempo in recipients lacking normal Th1 (STAT4–/–) or Th2 responses (STAT6–/–). To our knowledge, this is the first report describing in vivo studies of knockout animals targeted at the STAT 4 and 6 genes in an organ-transplant model. Our data are consistent with data published previously using single cytokine gene-knockout recipients such as IL-2–/–, IFN-γ–/–, IL-12-p40–/–, IL-12-p35–/–, or IL-4–/– animals (10–13, 33). These data, in concert with our observations, challenge the simplistic traditional Th1/Th2 paradigm in transplantation that Th1 promotes graft rejection and Th2 is permissive for graft acceptance (34–36).
The most important and novel findings of this report are the differential effects of CD28-B7 blockade by CTLA4Ig and CD154-CD40 blockade by MR1 treatment in STAT4–/– versus STAT6–/– mice. Whereas a single injection of CTLA4Ig on post-transplant day 2 was universally effective in promoting long-term graft survival and inducing donor-specific tolerance in WT, STAT4–/–, and STAT6–/– recipients, a single injection of MR1 was effective primarily in STAT4–/– mice, but not in WT and STAT6–/– mice, in promoting long-term engraftment and tolerance. Our studies with both (a) a multiple-injection protocol of MR1 and (b) a single injection of MR1 with coadministration of donor splenocytes (Figures 8 and 9), clearly indicate that Th1 cells of WT and STAT6–/– recipients are not inherently resistant to CD154 blockade.
Several studies have shown previously that CD28-B7 blockade is effective in blocking Th1 immune responses and in some models may spare Th2 cell function (4, 37, 38). However, there are also data to indicate that CD28 signaling promotes Th2 responses (39) and that CD28-B7 blockade is effective in blocking immune responses in Th2-mediated diseases (40–44). Our data clearly indicate that CTLA4Ig inhibits immune responses in both STAT4–/– and STAT6–/– recipients, suggesting that CD28-B7 blockade is effective in blocking the prevailing immune response irrespective of whether it is predominantly Th1 or Th2. In addition, CTLA4Ig was also effective in preventing development of chronic rejection, confirming our previous studies in rat models of chronic allograft rejection (26, 45, 46). These data have relevant clinical implications for several immune-mediated human diseases.
Our observations with CTLA4Ig in STAT4–/– mice are also consistent with data published previously showing that IFN-γ is necessary for tolerance induction in a fully allogeneic transplant model (10), since STAT4–/– mice produce low levels of IFN-γ that is independent of IL-12 signaling (47, 48). Our data confirm these observations in an alloantigen as well as a nominal antigen system (see Figure 3a and 7b). These levels may be sufficient for the required putative regulatory functions of IFN-γ (10).
The exact mechanisms of the interesting and somewhat surprising data of the differential effect of CD154 blockade with a single injection of MR1 in WT and STAT6–/– versus STAT4–/– animals are unknown. Our data with the CD28–/– recipients indicate that the marginal efficacy of MR1 in WT and STAT6–/– recipients is distinctly independent of a functional CD28-B7 pathway. This is also supported by the fact that a similar protocol of MR1 therapy was very effective in STAT4–/– animals. The fact that CD154 blockade is effective in blocking immune responses in STAT4–/– animals is consistent with previous studies establishing the efficacy of such an approach in Th2-mediated diseases like hapten-induced dermatitis (49), Graves’ disease (50), and collagen-induced arthritis (51).
The relative resistance of a predominantly Th1 immune response to CD154 blockade in WT and STAT6–/– animals have three potential explanations. First, it is possible that CD154-CD40 blockade requires the presence of Th2 cytokines in order to be optimally effective. This is supported by a previous observation from our group that CD154 blockade results in a Th2 switch in a cardiac transplant model (6, 31). A second, yet related, explanation is the possibility that high precursor frequency of antigen-specific T cells (such as in the transplant setting) or a high antigenic load in the presence of Th1 cytokines is less responsive to CD154 blockade alone. This hypothesis is supported by a recent study by Li et al. (52) that showed Th1 to Th2 immune deviation after anti–IL-12 therapy caused prolonged engraftment in recipients of MHC-matched but minor histocompatibility-antigen mismatched (low alloreactive T-cell mass/low antigen-density system) grafts as compared with recipients of MHC-mismatched (high alloreactive T-cell mass/high antigen-density system) allografts. Third, a predominance of Th1 cytokines may result in priming of effector cells that are resistant to costimulatory blockade. In support of this possibility are the recent findings in some models (53) and in some mouse-strain combinations (54), that T-cell costimulatory blockade was not effective in inhibiting rejection driven by CD8+ T cells. Therefore, it is possible that in WT and STAT6–/– recipients a predominance of Th1 cytokines activate CD8+ T cells, which may in turn play a critical role in graft rejection and may not be completely blocked by MR1 (see below). Indeed, the ELISPOT data presented in Figure 3a show a significantly high number of IFN-γ–producing cells in response to alloantigen in vitro in both WT and STAT6–/– recipients. Therefore, whereas a single injection of MR1 was insufficient to block this predominant Th1 immune response in vivo, increasing the frequency of administration of MR1 resulted in significant improvement in graft survival as compared with the single-injection protocol in WT and STAT6–/– recipients. Our observations are consistent with data reported by Kirk et al. in primates showing that a short course of anti-CD154 (55) was less effective in promoting long-term rejection-free survival of kidney grafts as compared with chronic anti-CD154 therapy (56). Furthermore, in our model, even in cases where MR1 did result in prolonging graft survival, it was still not as effective as CTLA4Ig in completely inhibiting the alloimmune response to prevent development of chronic rejection. These observations are important for designing clinically relevant strategies based on T-cell costimulatory blockade in organ transplantation.
In several fully allogeneic transplant models, blockade of CD154 alone was ineffective in inducing tolerance; adjunctive therapies with donor antigen in the form of donor cells or bone marrow infusion, or simultaneous blockade of CD28-B7, was necessary to achieve long-term graft acceptance and tolerance (6, 57). Our data with donor splenocytes combined with a single injection of MR1 confirm the synergy between administration of donor alloantigens and CD154 blockade in promoting long-term graft survival even in a predominantly Th1 environment that is usually less responsive to CD154 blockade alone (STAT6–/– recipients). The mechanisms of action of donor alloantigen administration in promoting tolerance remain unknown, although both microchimerism as well as donor-antigen source theories have been proposed (58, 59). However, one possibility is that administration of donor splenocytes may act by tolerizing alloreactive CD8+ T cells. Indeed, there are data to indicate that administration of donor alloantigen is effective in tolerizing recipient 2C transgenic mice (60). These mice have predominantly CD8+ T cells that are reactive to allogeneic class I MHC molecules Ld. Obviously, further studies are required to dissect the exact mechanisms of the observed differential effects of CD154 blockade in a predominantly Th1 versus Th2 environment and the role of donor cells in promoting tolerance.
In conclusion, our data show that whereas CD28-B7 blockade is effective in inhibiting Th1 as well as Th2 alloimmune responses and promoting tolerance in vivo, CD154 is quantitatively less effective in a predominantly Th1 environment. These novel observations have implications for understanding the mechanisms of allograft rejection and tolerance and may impact the development of future immunotherapeutic strategies in transplantation and other immune-mediated diseases.
Acknowledgments
This work is supported by National Institutes of Health grants RO1 AI-34965 and PO1 AI-41521 (M.H. Sayegh) and AI-43496 (S.J. Khoury). V.M. Dong is a recipient of a National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases) National Research Service Award. Review of photomicrographs by Helmut G. Rennke is greatly appreciated.
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 2.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 3.Takeuchi T, Lowry RP, Konieczny B. Heart allografts in murine systems. The differential activation of Th2-like effector cells in peripheral tolerance. Transplantation. 1992;53:1281–1294. [PubMed] [Google Scholar]
- 4.Sayegh MH, et al. CD28-B7 blockade after alloantigenic challenge in vivo inhibits Th1 cytokines but spares Th2. J Exp Med. 1995;181:1869–1874. doi: 10.1084/jem.181.5.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binder J, et al. The effects of nondepleting CD4 targeted therapy in presensitized rat recipients of cardiac allografts. Transplantation. 1996;61:804–811. doi: 10.1097/00007890-199603150-00022. [DOI] [PubMed] [Google Scholar]
- 6.Hancock WW, et al. Costimulatory function and expression of CD40 ligand, CD80, and CD86 in vascularized murine cardiac allograft rejection. Proc Natl Acad Sci USA. 1996;93:13967–13972. doi: 10.1073/pnas.93.24.13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onodera K, et al. Type 2 helper T cell-type cytokines and the development of “infectious” tolerance in rat cardiac allograft recipients. J Immunol. 1997;158:1572–1581. [PubMed] [Google Scholar]
- 8.Dallman MJ, Shiho O, Page TH, Wood KJ, Morris PJ. Peripheral tolerance to alloantigen results from altered regulation of the interleukin 2 pathway. J Exp Med. 1991;173:79–87. doi: 10.1084/jem.173.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gianello PR, et al. Mechanism of cyclosporin-induced tolerance to primarily vascularized allografts in miniature swine. Effect of administration of exogenous IL-2. J Immunol. 1994;153:4788–4797. [PubMed] [Google Scholar]
- 10.Konieczny BT, et al. IFN-gamma is critical for long-term allograft survival induced by blocking the CD28 and CD40 ligand T cell costimulation pathways. J Immunol. 1998;160:2059–2064. [PubMed] [Google Scholar]
- 11.Dai Z, Konieczny BT, Baddoura FK, Lakkis FG. Impaired alloantigen-mediated T cell apoptosis and failure to induce long-term allograft survival in IL-2-deficient mice. J Immunol. 1998;161:1659–1663. [PubMed] [Google Scholar]
- 12.Lakkis FG, et al. Blocking the CD28-B7 T cell costimulation pathway induces long term cardiac allograft acceptance in the absence of IL-4. J Immunol. 1997;158:2443–2448. [PubMed] [Google Scholar]
- 13.Mottram PL, Raisanen-Sokolowski A, Glysing-Jensen T, Stein-Oakley AN, Russell ME. Cardiac allografts from IL-4 knockout recipients: assessment of transplant arteriosclerosis and peripheral tolerance. J Immunol. 1998;161:602–609. [PubMed] [Google Scholar]
- 14.Sirak JH, Orosz CG, Roopenian DC, Wakely E, VanBuskirk AM. Cardiac allograft tolerance: failure to develop in interleukin-4-deficient mice correlates with unusual allosensitization patterns. Transplantation. 1998;65:1352–1356. doi: 10.1097/00007890-199805270-00012. [DOI] [PubMed] [Google Scholar]
- 15.Bluestone JA. New perspectives of CD28-B7-mediated T cell costimulation. Immunity. 1995;2:555–559. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 16.Thompson CB. Distinct roles for the costimulatory ligands B7-1 and B7-2 in T helper cell differentiation? Cell. 1995;81:979–982. doi: 10.1016/s0092-8674(05)80001-7. [DOI] [PubMed] [Google Scholar]
- 17.Sayegh MH, Turka LA. The role of T-cell costimulatory activation pathways in transplant rejection. N Engl J Med. 1998;338:1813–1821. doi: 10.1056/NEJM199806183382506. [DOI] [PubMed] [Google Scholar]
- 18.Linsley PS, Ledbetter JA. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- 19.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382:174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 22.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16:343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Chen ZH. A technique of cervical heterotopic heart transplantation in mice. Transplantation. 1991;52:1099–1101. doi: 10.1097/00007890-199112000-00035. [DOI] [PubMed] [Google Scholar]
- 24.Turka LA, et al. T-cell activation by the CD28 ligand B7 is required for cardiac allograft rejection in vivo. Proc Natl Acad Sci USA. 1992;89:11102–11105. doi: 10.1073/pnas.89.22.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams DH, Tilney NL, Collins JJ, Karnovsky MJ. Experimental graft arteriosclerosis. I. The Lewis-to-F344 allograft model. Transplantation. 1992;53:1115–1119. [PubMed] [Google Scholar]
- 26.Russell ME, et al. Chronic cardiac rejection in the Lewis to F344 rat model: blockade of CD28-B7 costimulation by CTLA4Ig modulates T cell and macrophage activation and attenuates arteriosclerosis. J Clin Invest. 1996;97:833–838. doi: 10.1172/JCI118483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glysing-Jensen T, Raisanen-Sokolowski A, Sayegh MH, Russell MH. Chronic blockade of CD28-B7 mediated T cell costimulation by CTLA4Ig reduces intimal thickening in MHC class I and II incompatible mouse heart allografts. Transplantation. 1997;164:1641–1645. doi: 10.1097/00007890-199712270-00002. [DOI] [PubMed] [Google Scholar]
- 28.Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999;162:352–358. [PubMed] [Google Scholar]
- 29.Chen W, Sayegh MH, Khoury SJ. Mechanisms of acquired thymic tolerance in vivo: intrathymic injection of antigen induces apoptosis of thymocytes and peripheral T cell anergy. J Immunol. 1998;160:1504–1508. [PubMed] [Google Scholar]
- 30.Lin H, et al. Cytotoxic T lymphocyte antigen 4 (CTLA4) blockade accelerates the acute rejection of cardiac allografts in CD28-deficient mice: CTLA4 can function independently of CD28. J Exp Med. 1998;188:199–204. doi: 10.1084/jem.188.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hancock WW, Buelow R, Sayegh MH, Turka LA. Antibody-induced transplant arteriosclerosis is prevented by graft expression of anti-oxidant and anti-apoptotic genes. Nat Med. 1998;4:1392–1396. doi: 10.1038/3982. [DOI] [PubMed] [Google Scholar]
- 32.Iwakoshi NN, et al. Treatment of allograft recipients with donor-specific transfusion and anti-CD154 antibody leads to deletion of alloreactive CD8+ T cells and prolonged graft survival in a CTLA4-dependent manner. J Immunol. 2000;164:512–521. doi: 10.4049/jimmunol.164.1.512. [DOI] [PubMed] [Google Scholar]
- 33.Piccotti JR, et al. Alloantigen-reactive Th1 development in IL-12-deficient mice. J Immunol. 1998;160:1132–1138. [PubMed] [Google Scholar]
- 34.Strom TB, et al. The Th1/Th2 paradigm and the allograft response. Curr Opin Immunol. 1996;8:688–693. doi: 10.1016/s0952-7915(96)80087-2. [DOI] [PubMed] [Google Scholar]
- 35.Piccotti JR, Chan SY, VanBuskirk AM, Eichwald EJ, Bishop DK. Are Th2 helper T lymphocytes beneficial, deleterious, or irrelevant in promoting allograft survival? Transplantation. 1997;63:619–624. doi: 10.1097/00007890-199703150-00001. [DOI] [PubMed] [Google Scholar]
- 36.Field EH, Gao Q, Chen NX, Rouse TM. Balancing the immune system for tolerance: a case for regulatory CD4 cells. Transplantation. 1997;64:1–7. doi: 10.1097/00007890-199707150-00002. [DOI] [PubMed] [Google Scholar]
- 37.Khoury SJ, et al. CD28-B7 costimulatory blockade by CTLA4Ig prevents actively induced experimental autoimmune encephalomyelitis and inhibits Th1 but spares Th2 cytokines in the central nervous system. J Immunol. 1995;155:4521–4524. [PubMed] [Google Scholar]
- 38.Kuchroo VK, et al. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 39.Oosterwegel MA, et al. The role of CTLA-4 in regulating Th2 differentiation. J Immunol. 1999;163:2634–2639. [PubMed] [Google Scholar]
- 40.Keane-Myers A, Gause WC, Linsley PS, Chen SJ, Wills-Karp M. B7-CD28/CTLA-4 costimulatory pathways are required for the development of T helper cell 2-mediated allergic airway responses to inhaled antigens. J Immunol. 1997;158:2042–2049. [PubMed] [Google Scholar]
- 41.Larche M, et al. Costimulation through CD86 is involved in airway antigen-presenting cell and T cell responses to allergen in atopic asthmatics. J Immunol. 1998;161:6375–6382. [PubMed] [Google Scholar]
- 42.Padrid PA, et al. CTLA4Ig inhibits airway eosinophilia and hyperresponsiveness by regulating the development of Th1/Th2 subsets in a murine model of asthma. Am J Respir Cell Mol Biol. 1998;18:453–462. doi: 10.1165/ajrcmb.18.4.3055. [DOI] [PubMed] [Google Scholar]
- 43.Harris NL, Peach RJ, Ronchese F. CTLA4-Ig inhibits optimal T helper 2 cell development but not protective immunity or memory response to Nippostrongylus brasiliensis. Eur J Immunol. 1999;29:311–316. doi: 10.1002/(SICI)1521-4141(199901)29:01<311::AID-IMMU311>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 44.Corry DB, Reiner SL, Linsley PS, Locksley RM. Differential effects of blockade of CD28-B7 on the development of Th1 or Th2 effector cells in experimental leishmaniasis. J Immunol. 1994;153:4142–4148. [PubMed] [Google Scholar]
- 45.Azuma H, et al. Blockade of T-cell costimulation prevents development of experimental chronic renal allograft rejection. Proc Natl Acad Sci USA. 1996;93:12439–12444. doi: 10.1073/pnas.93.22.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chandraker A, et al. Late blockade of T cell costimulation interrupts progression of experimental chronic allograft rejection. J Clin Invest. 1998;101:2309–2318. doi: 10.1172/JCI2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaplan MH, Wurster AL, Grusby MJ. A signal transducer and activator of transcription (Stat)4-independent pathway for the development of T helper type 1 cells. J Exp Med. 1998;188:1191–1196. doi: 10.1084/jem.188.6.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carter LL, Murphy KM. Lineage-specific requirement for signal transducer and activator of transcription (Stat)4 in interferon gamma production from CD4(+) versus CD8(+) T cells. J Exp Med. 1999;189:1355–1360. doi: 10.1084/jem.189.8.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang A, Judge TA, Turka LA. Blockade of CD40-CD40 ligand pathway induces tolerance in murine contact hypersensitivity. Eur J Immunol. 1997;27:3143–3150. doi: 10.1002/eji.1830271210. [DOI] [PubMed] [Google Scholar]
- 50.Resetkova E, et al. Antibody to gp39, the ligand for CD40 significantly inhibits the humoral response from Graves’ thyroid tissues xenografted into severe combined immunodeficient (SCID) mice. Thyroid. 1996;6:267–273. doi: 10.1089/thy.1996.6.267. [DOI] [PubMed] [Google Scholar]
- 51.Durie FH, et al. Prevention of collagen-induced arthritis with an antibody to gp39, the ligand for CD40. Science. 1993;261:1328–1330. doi: 10.1126/science.7689748. [DOI] [PubMed] [Google Scholar]
- 52.Li XC, Zand MS, Li Y, Zheng XX, Strom TB. On histocompatibility barriers, Th1 to Th2 immune deviation, and the nature of the allograft responses. J Immunol. 1998;161:2241–2247. [PMC free article] [PubMed] [Google Scholar]
- 53.Newell KA, et al. Cutting edge: blockade of the CD28/B7 costimulatory pathway inhibits intestinal allograft rejection mediated by CD4+ but not CD8+ T cells. J Immunol. 1999;163:2358–2362. [PubMed] [Google Scholar]
- 54.Trambley J, et al. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest. 1999;104:1715–1722. doi: 10.1172/JCI8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kirk AD, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci USA. 1997;94:8789–8794. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kirk AD, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999;5:686–693. doi: 10.1038/9536. [DOI] [PubMed] [Google Scholar]
- 57.Larsen CP, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 58.Wood K, Sachs DH. Chimerism and transplantation tolerance: cause and effect [discussion p. 588] Immunol Today. 1996;17:584–587. doi: 10.1016/s0167-5699(96)10069-4. [DOI] [PubMed] [Google Scholar]
- 59.Hamano K, Rawsthorne MA, Bushell AR, Morris PJ, Wood KJ. Evidence that the continued presence of the organ graft and not peripheral donor microchimerism is essential for maintenance of tolerance to alloantigen in vivo in anti-CD4 treated recipients. Transplantation. 1996;62:856–860. doi: 10.1097/00007890-199609270-00026. [DOI] [PubMed] [Google Scholar]
- 60.Yang L, DuTemple B, Gorczynski RM, Levy G, Zhang L. Evidence for epitope spreading and active suppression in skin graft tolerance after donor-specific transfusion. Transplantation. 1999;67:1404–1410. doi: 10.1097/00007890-199906150-00003. [DOI] [PubMed] [Google Scholar]