Abstract
Sensing pathogens is an essential first step in the initiation of a host response to infection, Using mouse models, Kane et al.(2011) demonstrate that Toll-like receptor 7 is required for the generation of an antibody response to infection by retroviruses.
There are two main classes of sensors that detect the presence of nucleic acid components of invading pathogens within cells(McCartney and Colonna, 2009); the cytosolic retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs), which include LGP2, MDA5, and RIG-I, all of which are activated by RNA, and the endosomal Toll-like receptors (TLRs). Of the endosomal TLRs (TLR3, 7, 8, and 9), TLR3 senses dsRNA, TLRs 7 and 8 recognize ssRNA, while TLR9 is activated by unmethylated CpG-containing DNA, which can be found in both bacterial and certain viral pathogens. Activation of either cytoplasmic or endosomal sensors results in the production of type 1 interferon (IFN) and inflammatory cytokines, with dendritic cells (DCs) being key orchestrators of this innate response (Gilliet et al., 2008). These cytokines in turn induce the expression of an array of genes, the products of which may have either direct antiviral effects or promote adaptive immune responses.
In this issue of Immunity, as Kane et al. (2011) rightly point out, the ability of the various sensors to detect the presence of certain nucleic acids in vitro is not necessarily predictive of relevance in the context of infection in vivo, and so they employed mouse models to determine what might be relevant in vivo sensors of infection by retroviruses. Mouse strains vary in their ability to control retroviral infections through innate and adaptive immune responses. Thus, beginning with the reasonable premise that mouse strains that effectively control retroviral infections are more likely to be competent for both sensing and responding to these pathogens, Kane et al. examined the viral and host requirements for mobilization of an anti-viral adaptive immune response, as measured by the production of anti-viral antibodies and control of viral replication, in mouse strains that are able to control retroviral infections.
The particular mouse strains used by Kane et al included I/LnJ mice, which are able to control the replication of two unrelated retroviruses, namely the gammaretrovirus murine leukemia virus (MuLV) and the betaretrovirus mouse mammary tumor tirus (MMTV). Another mouse strain, C57BL/6J (B6), is able to control the replication of MuLV. Both humoral and cellular immune responses likely contribute to control of retroviral infection, but in this study, Kane et al. focused on the humoral response. Importantly, by comparing mouse strains that do, or do not, effectively control MuLV and/or MMTV replication, they show that the ability to control viral replication correlates well with the ability of a given mouse strain to mount a robust humoral immune response to each virus.
To understand what component of the virus, and what aspects of viral replication might be crucial for viral sensing and immune control, Kane et al compared humoral immune responses to replicating and inactivated viruses. While MMTV replication enabled sensing of the virus, and a humoral immune response in I/LnJ hosts, heat-inactivated (and thus replication-defective) MMTV was able to elicit anti-MMTV antibodies mice only in the presence of complete Freund's adjuvant (CFA), suggesting that CFA is able to complement for signals generated by viral replication. Presumably, such signals are missing, or ineffective, when heat-inactivated virus preparations alone are used as immunogens.
Importantly, however, UV-inactivated viruses (which are physically intact, could enter cells, and were detected in endosomes, but could not replicate in vivo) were capable of eliciting anti-viral antibodies when injected into I/LnJ mice. Kane et al. were able to rule out a requirement for the endosomal dsRNA sensor TLR3 in retroviral sensing, as TLR3-deficient mice were able to generate anti-viral antibodies to both MMTV and MuLV. However a requirement for other endosomal TLRs, e.g. TLR7 or 9, was suggested by a requirement for the adaptor molecule MyD88. Both TLR7 and 9 signal via MyD88, and it has been previously demonstrated that MyD88 is required for the generation of anti-MuLV antibodies in B6 mice (Browne and Littman, 2009). Kane et al. demonstrate that this is also true for MMTV in I/LnJ mice, suggesting a general role for a MyD88-dependent pathway in the development of an anti-retroviral humoral immune response. By examining the ability of either TLR9-deficient or TLR7-deficient mice to generate anti-viral antibodies, Kane et al. were able to make the key finding, namely that while TLR9 is dispensable, TLR7 is specifically required for the development of antibodies to both MMTV and MuLV.
Activation of both endosomal TLRs and cytoplasmic nucleic acid sensors leads to the production of type I IFNs which bind the IFNα and IFNβ receptor (IFNAR), resulting in the activation of many anti-viral genes and promotion of adaptive immune responses. Somewhat surprisingly, however, Kane et al show that anti-viral antibody production in MuLV-infected B6 mice did not appear to be affected in IFNAR1-deficient mice, which lack all type I IFN-dependent signaling. Presumably some other cytokine or pathway whose expression is activated by TLR7 is more important in eliciting a humoral immune response, or there is sufficient redundancy in the system to elicit a humoral response in the absence of type 1 IFN signaling.
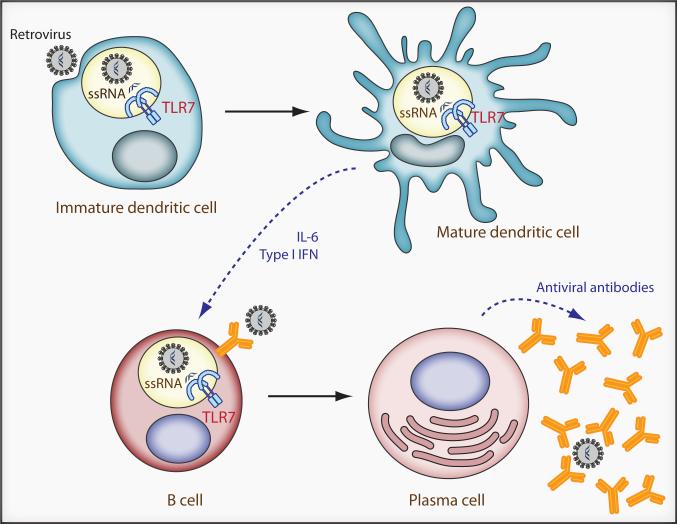
TLR7 has previously been shown to be involved in detecting a variety of viruses. Indeed, in vitro studies have suggested that HIV-1 RNA is recognized by TLR7 (Beignon et al., 2005). TLR7 is primarily expressed by DCs, and its engagement by ssRNA leads to the production of type I IFN as well as inflammatory cytokines (Figure 1). TLR7 is also expressed at lower levels in other antigen presenting cells, including B cells, and it is also possible that its presence therein could affect the humoral response to infection. In contrast to cytosolic nucleic acid sensors, which are ubiquitously expressed and activated in infected cells, endosomal TLRs such as TLR7 are capable of detecting endocytosed virus in the absence of infection. Detection leads to cytokine production as well as DC maturation and subsequent enhancement of adaptive immune responses (Stetson and Medzhitov, 2006) (Figure 1). Thus, targeted stimulation of TLR7 might be usefully employed in the context of viral vaccines. Given that the production of type 1 interferon enhances B cell function (Theofilopoulos et al., 2005), it is quite surprising that Kane et al were able to show that the generation of anti-retroviral antibodies was unaffected by the loss of type I IFN signaling. In future studies, it will be interesting to assess the contribution of other cytokines (e.g. IL-6) elicited by TLR7 engagement in the promotion of anti-retroviral humoral immune responses; again this might inform vaccine development.
Figure 1. The generation of a humoral anti-retroviral adaptive immune response requires TLR7.
TLR7 in endosomal compartments recognizes retroviral ssRNA. Engagement of TLR7 leads to DC maturation and production of type I IFN and inflammatory cytokines, including IL-6. Both type I IFN and IL-6 promote the differentiation of B cells into antibody-secreting plasma cells, although in the case of MuLV and MMTV infections, it appears that type I IFN is dispensable for the generation of anti-viral antibodies. It is also possible that TLR7 in B-cells may also affect the humoral response. For both MuLV and MMTV, virus specific antibodies play an important role in reducing the numbers of infected cells as well as reducing transmission of MMTV through milk.
While Kane et al. clearly identify TLR7 as a key immune sensor of retroviral infection, they stop short of implicating TLR7 as the defining difference between mouse strains that vary in their ability to control retroviral infection. Indeed, both retrovirus-sensitive and resistant mice express TLR7. However, given the importance of TLR7 dosage on immune responses (Deane et al., 2007), it would be interesting to know if there are any differences in TLR7 expression level between mouse strains. It is also possible, as Kane et al. point out, that the differences between resistant and susceptible strains lie downstream of TLR7 or perhaps with other viral sensors or antiviral proteins. The array of molecules known to influence the course of retroviral infections in both mice and humans is ever expanding, and while innate sensors do affect sensitivity to retroviral infection, they are unlikely to be the sole determinants of the course of infection.
The pathways involved in retroviral sensing are an area of intense current research. Retroviruses are somewhat unusual that the viral genome and replication intermediates are either present at low abundance in individual cells (as preintegrated DNA) or essentially indistinguishable in form to cellular nucleic acids (as integrated proviral DNA, or viral mRNA and genomes). Retroviral nucleic acids may also be concealed from the host sensors at certain phases of the viral life cycle by the incoming viral capsid structure or by association with the pre-integration complex. Cyclophilin A, a molecule long known to be involved in retroviral replication, has recently been suggested to be involved in sensing retroviral infections (Manel et al., 2010), as has TRIM5, a molecule that also has direct antiretroviral activity (Pertel et al., 2011). The findings of Kane et al. are an important addition to our growing understanding of how cells detect the presence of these lethal pathogens.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beignon AS, McKenna K, Skoberne M, Manches O, DaSilva I, Kavanagh DG, Larsson M, Gorelick RJ, Lifson JD, Bhardwaj N. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne EP, Littman DR. Myd88 is required for an antibody response to retroviral infection. PLoS Pathog. 2009;5:e1000298. doi: 10.1371/journal.ppat.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane JA, Pisitkun P, Barrett RS, Feigenbaum L, Town T, Ward JM, Flavell RA, Bolland S. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- Kane M. Innate immune sensing of retroviral infection occurs upon viral entry via Toll-like receptor 7. Immunity. 2011 doi: 10.1016/j.immuni.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manel N, Hogstad B, Wang Y, Levy DE, Unutmaz D, Littman DR. A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells. Nature. 2010;467:214–217. doi: 10.1038/nature09337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney SA, Colonna M. Viral sensors: diversity in pathogen recognition. Immunol Rev. 2009;227:87–94. doi: 10.1111/j.1600-065X.2008.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertel T, Hausmann S, Morger D, Zuger S, Guerra J, Lascano J, Reinhard C, Santoni FA, Uchil PD, Chatel L, et al. TRIM5 is an innate immune sensor for the retrovirus capsid lattice. Nature. 2011;472:361–365. doi: 10.1038/nature09976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity. 2006;25:373–381. doi: 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]