Abstract
Background
C57BL/6 mice have attenuated allergic airway hyperresponsiveness (AHR) when compared with Balb/c mice but the underlying mechanisms remain unclear. SP-D, an innate immune molecule with potent immunosuppressive activities may have an important modulatory role in the allergic airway response and the consequent physiological changes. We hypothesized that an elevated SP-D production is associated with the impaired ability of C57BL/6 mice to develop allergic AHR.
Methods
SP-D mRNA and protein expression was investigated during development of allergic airway changes in a model of Aspergillus fumigatus (Af)-induced allergic inflammation. To study whether strain dependency of allergic AHR is associated with different levels of SP-D in the lung, Balb/c and C57BL/6 mice were compared.
Results
Sensitization and exposure to Af induced significant airway inflammation in both mouse strains in comparison with naïve controls. AHR to acetylcholine however was significantly attenuated in C57BL/6 mice in spite of increased eosinophilia and serum IgE when compared with Balb/c mice (p < 0.05). Af challenge of sensitized C57BL/6 mice induced a markedly increased SP-D protein expression in the SA surfactant fraction (1,894 ± 170% of naïve controls) that was 1.5 fold greater than the increase in Balb/c mice (1,234 ± 121% p < 0.01). These changes were selective since levels of the hydrophobic SP-B and SP-C and the hydrophilic SP-A were significantly decreased following sensitization and challenge with Af in both strains. Further, sensitized and exposed C57BL/6 mice had significantly lower IL-4 and IL-5 in the BAL fluid than that of Balb/c mice (p < 0.05).
Conclusions
These results suggest that enhanced SP-D production in the lung of C57BL/6 mice may contribute to an attenuated AHR in response to allergic airway sensitization. SP-D may act by inhibiting synthesis of Th2 cytokines.
Background
Airway hyperresponsiveness (AHR) is a heritable polygenic trait and together with eosinophilic airway inflammation and IgE production, is a hallmark of human allergic asthma. Demonstration of strain differences in susceptibility to develop AHR to allergic sensitization has long been intriguing and promoted the use of inbred mouse strains for the investigation of genetic determinants of allergic AHR (reviewed by Heinzmann and Daser, [1]). C57BL/6 mice are relatively hyporesponsive to non-specific airway stimuli and resistant to development of allergic AHR in comparison with a number of other inbred mouse strains [2-4]. Although the exact mechanisms that determine susceptibility or resistance to develop allergic AHR remain unclear, airway inflammation and the underlying adaptive immune responses are thought to play a major role [5-10].
The role of T cell dependent (adaptive) allergic inflammation is well established in the pathogenesis of asthma [6-8,10,11]. However, the modulatory function that the innate immune system plays during allergic sensitization remains less understood. We have recently described that expression of an innate immune molecule, surfactant protein (SP)-D was significantly increased during allergic inflammatory changes in the lung in a murine model [12]. This soluble pattern recognition receptor (also termed lung collectin that consists of a collagenous and a lectin-like motif) may play a regulatory role in the allergic airway changes although its exact mechanism of action is unknown [13].
In this study we examined the differences in allergic airway hyperresponsiveness between C57BL/6 and Balb/c mice and associated changes in surfactant component expression using age-and sex matched C57BL/6 and Balb/c mice in a model of Aspergillus fumigatus induced allergic AHR. Our results demonstrate an inverse relationship between the ability to develop allergen induced AHR and the extent of SP-D production.
Methods
Mice, sensitization and intranasal challenge with Aspergillus fumigatus (Af)-extract
To study the relationship between the ability to produce SP-D and develop AHR, a model of Af-induced allergic sensitization was characterized in two inbred mouse strains. Female BALB/c and C57BL/6 mice were housed under pathogen-free conditions. Experiments were performed between 8–12 weeks of age. All experimental animals used in this study were under a protocol approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Two groups of the mouse strains were compared: "Naive" mice received intranasal vehicle challenges with 21% glycerol in PBS. "Sensitized" mice were injected intraperitoneally (i.p.) with 20 μg of Af (Bayer Pharmaceuticals, Elkhart, IN) together with 20 mg Al(OH)3 (Imject Alum; Pierce, Rockford, IL) in PBS (100 μl) on days 1 and 14, followed by intranasal challenge (i.n.) on days 25, 26, and 27 with 25 μl of allergen extract: (12.5 μg Af in 21% glycerol/ PBS). Limulus lysate assay (Limulus Amebocyte Lysate QCL-1000; Bio-Whittaker) was used to determine the endotoxin content in the allergenic Af extract. We have found that LPS level was 1.22 pg LPS/μg protein in the Af extract we used to sensitize mice in this study. The weight range of the groups of mice were the following: Balb/c Naïve: 21–30 g (n = 15); Balb/c Sensitized: 20–30 (n = 22); C57BL/6 Naïve: 22–29 (n = 17); C57BL/6 Sensitized: 22–29 (n = 22). Intranasal treatment was carried out essentially as described previously [12,14,15]. Briefly, sensitized and control mice were anesthetized by isoflurane inhalation, and 25 μl of Af extract or vehicle was applied to the left nares respectively. The studies were performed and all mice were sacrificed 24 hour after their last intranasal treatment, when the peaks of eosinophil infiltration and airway responses were assumed to occur. Naïve mice that received intranasal glycerol treatment alone showed no difference from non sensitized, non exposed normal BALB/c and C57BL-6 mice in any of the study parameters that we investigated, including lung histology, BAL cellular content, immunoglobulin and cytokine profile and airway responses to acetylcholine (not shown).
In vivo measurement of airway responsiveness to acetylcholine (ACh)
Airway function measurements were carried out as previously described [12]. Briefly, anesthesia was provided by intra-peritoneal administration of a mixture of ketamine (100 mg/kg) and xylazine (20 mg/kg), every 20 minutes before and during all surgical procedures. Mice were canulated, and ventilated (140 breaths/min; 0.2 ml tidal volume) following administration of pancuronium bromide (1.0 mg/kg). Transduced alveolar pressure and airflow rate (Validyne DP45 and DP103, USA) was used to calculate lung resistance (RL) and dynamic compliance (Cdyn) by a computer (Buxco Electronics, Inc. NY). Baseline RL and Cdyn values were established and after administration of saline, ACh was given intravenously at concentrations ranging from 80 to 1280 μg/kg in five increments.
Serum ELISA for Immunoglobulins
To detect levels of sensitization and to compare C57BL/6 and Balb/c mice, serum samples were collected as described before [12,16,17]. Antibodies and recombinant IgE were purchased from PharMingen (San Diego CA) and antibody levels were determined according to instructions of the manufacturer. For Af-specific antibody levels plates (Dynatech, Chantilly, VA) were coated with Af (50 μg/ml in PBS, pH 7.1), incubated overnight at 4°C. Samples were diluted 1:5 for Af specific IgE and IgG2a, and 1:25 for IgG1 and for total IgE. Data were analyzed with the Microplate Manager software program for the PC version (Bio-Rad, Hercules, CA).
BAL analysis for differential cell count, cytokine and surfactant content
Lungs were lavaged using either a small volume (1 ml sterile PBS for the analysis of the BAL cytokine profile) or a large volume (5 ml of sterile saline for analysis of the surfactant protein profile). The amount of liquid retained after BAL was an average of 0.75 ml when 1 ml lavage was used and 4.5 ml when 5 ml lavage was used. Total and differential cell counts were performed as described previously [12,16,17]. Cytokine levels were determined from cell free supernatant of the small volume BAL by ELISA using antibodies and recombinant cytokines from PharMingen, (San Diego, CA). ELISA analysis was performed as previously described [12,16,17] and cytokine levels were expressed as pg/μg of total protein. Cell free supernatant of the large volume BAL was separated into large-aggregate (LA) and small-aggregate (SA) fractions by differential centrifugation as described previously [18,19]. Total protein and phospholipid contents of the LA and SA fractions were determined using standard methods. SDS-PAGE of LA and SA surfactant samples was carried out using NuPAGE 10% Bis-Tris gels (Novex, San Diego, CA) according to instructions of the manufacturer. Western blots were performed as previously described [18,19] using anti-SP-C from Byk Goulden Pharmaceuticals (Constance, Germany), monospecific, polyclonal surfactant protein antisera against SP-A, SP-B and SP-D were produced in rabbits using purified rat SP-A, bovine SP-B, and recombinant mouse SP-D as previously described [18,19].
Analysis of mRNA expression
Total RNA was isolated from lungs after BAL as described before [18,19]. Specific mRNA content was determined by Northern blot analysis. Nitrocellulose blots with total RNA were hybridized under high stringency with [α-32P]cDNA probes for rat SP-A, SP-B, SP-C and SP-D prepared from purified plasmid inserts by labeling with [α-32P]dCTP (Ready-to-Go Kit, Pharmacia, Piscataway, NJ) to specific activities of 6–8 × 106 counts/min/μg DNA as previously described (1, 2). The specific signals were normalized for loading by hybridization of each blot with an 32P end-labeled ([γ-32P]ATP) 28S rRNA oligonucleotide probe. Specific mRNA bands were quantified by Phosphoimager (Biorad, Hercules, CA).
SP-A ELISA
In order to analyze and compare the SP-A content in the SA surfactant fractions, in addition to Western blot analysis, we have developed an ELISA protocol using a commercially available kit (Vectastain 6100 kit, Vector Laboratories, Burlingame, CA). Aliquots of SA samples neat or diluted 1:2 with blocking buffer (1% FBS in Dulbecco's PBS) were applied to 96-well Nunc-Immuno MaxiSorp plates (Nalge Nunc International, Denmark). Each assay plate included a standard of human purified SP-A from the BAL fluid of patients suffering from alveolar proteinosis (1 to 2700 ng/well). Polyclonal anti-SP-A antiserum (PA3) was applied as a primary antibody (1:50,000) with goat anti-rabbit IgG (1:1000) as the secondary antibody (Vectastain kit 6101, Vector Laboratories). Colorimetric detection of antibody binding was performed according to the manufacturer's instructions using TMB (TMB Substrate Reagent Set, BD PharMingen) as substrate. Color intensity was measured at 405 nm using an automated microplate reader (Bio-Rad, Hercules, CA) and analyzed with Bio-Rad Microplate Manager software, PC version 5.0.1. Values for unknown samples falling within the linear range of the standard curve were used to obtain the total SP-A content of each sample.
Data analysis
Data were expressed as mean ± SEM. ANOVA or Student's t test assuming equal variances were performed to test differences between groups. A p value of <0.05 was considered as significant. Data were analyzed with the Sigmastat standard statistical package (Jandel Scientific).
Results and discussion
Allergen challenge of sensitized C57BL/6 mice induced attenuated airway hyperresponsiveness to ACh in comparison with Balb/c mice
To compare the effects of antigenic sensitization between C57BL/6 and Balb/c mice, we used an established model of i.p. sensitization and i.n. provocation with an extract of Af that elicited significant increases in airway responses to non-specific stimuli such as ACh [12,14-16]. Following i.p. sensitization and i.n. challenges with Af, as expected on the basis of our previous findings, significant increases in allergic AHR occurred in both the C57BL/6 and Balb/c mice. We confirmed increases in airway obstruction by measuring lung resistance (RL, Fig 1A) and by measuring changes in the dynamic compliance (Cdyn, Fig 1B) [20]. Mice sensitized and exposed to Af showed significant increases in lung resistance (RL) and decreases in dynamic compliance (Cdyn) in a dose-dependent manner in response to ACh when compared with naïve controls in both the Balb/c and C57BL/6 groups (ANOVA p < 0.001 and p < 0.01, respectively). However, the changes in both RL(655 ± 202% maximal increase over the baseline) and Cdyn (a decrease to 59.3 ± 8.8% of the original baseline) were significantly greater in sensitized Balb/c mice compared with those in C57BL/6 mice (RL: 297 ± 93% maximal increase over the baseline and Cdyn: a decrease to 79.3 ± 7.5% of the baseline) (p < 0.05, ANOVA). Baseline airway function was also examined in each mouse strains before ACh administration. There were no significant differences in baseline RL or Cdyn between mouse strains (not shown). Thus, along with a number of different models [2-4], our results demonstrated an attenuated AHR in C57BL/6 mice to non-specific airway stimuli suggesting genetically determined underlying mechanisms.
Figure 1.
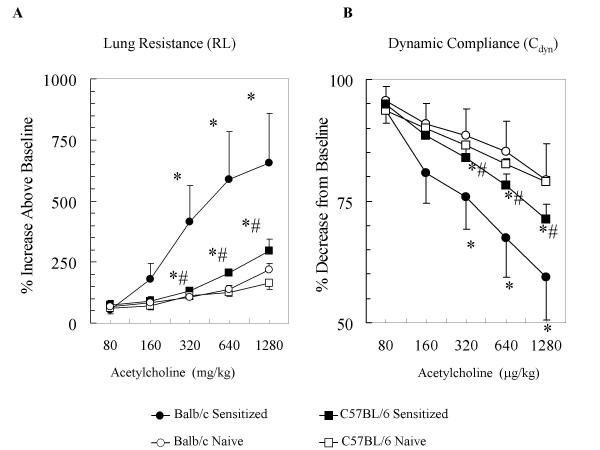
Allergen challenge of sensitized C57BL/6 mice induced a significantly attenuated airway hyperresponsiveness to ACh in comparison with Balb/c mice. Mice were sensitized and exposed to Af and their lung function was assessed by measuring Lung Resistance (RL, panel A) and Dynamic Compliance (Cdyn, panel B) by a BUXCO online system as described. RL and Cdyn values were obtained in response to increasing concentrations of intravenous (i.v.) ACh in both strains of mice. Data are expressed as % changes from baseline. The Naïve group (open square, C57BL/6 or open circle, Balb/c) received intranasal glycerol treatment alone. The Sensitized group (closed squares, C57BL/6 or closed circle, Balb/c) received intraperitoneal (i.p.) sensitization and intranasal (i.n.) challenge with Af extract as described. Baseline RL values in the Naïve and in the Sensitized groups were 0.84 ± 0.04 and 1.25 ± 0.11 Hgmm/cmH2O/min respectively, in the C57BL/6 mice and 1.26 ± 0.03 and 1.48 ± 0.06 Hgmm/cmH2O/min, respectively in the Balb/c mice. Results are expressed as Mean ± SEM of n = 6–7. ANOVA was used to compare the dose response curves followed by Student's t test for comparisons between individual data points. *p < 0.05 Sensitized vs Naive; #p < 0.05 C57BL/6 vs Balb/c.
C57BL/6 mice developed significant systemic IgE and IgG1 levels following allergenic sensitization and challenge with Af, comparable to the responses of Balb/c mice
Allergic AHR occurs through a variety of pathogenic mechanisms depending on the genetic background of the animal and the type of immunization [1,4,21]. Interestingly, the elicited airway inflammation and IgE production may dissociate from the measures of airway obstruction [1,3,4,21-25]. To investigate whether C57BL/6 mice produce total and Af specific serum IgE and IgG at a comparable level to that of Balb/c mice, we analyzed total serum IgE together with the Af-specific immunoglobulin profile (IgE, IgG1 and IgG2a). Mice were sensitized and challenged with Af and blood obtained 24 h after the last allergen challenge. Similarly to our previous findings [12,17], Af sensitization resulted in markedly increased serum IgE, IgG1 and IgG2a levels. As shown in Figure 2A, C57BL/6 mice were not deficient in producing these immunoglobulins upon systemic sensitization and challenges; in fact, levels of total serum IgE (Fig 2A; p < 0.05) and Af-specific IgG1 (Fig 2B; p < 0.01) were significantly greater in this strain than in Balb/c mice. There were no significant differences between the levels of Af-specific IgE (Fig 2C) and IgG2a (not shown) between the two mouse strains. These data suggest that C57BL/6 mice are not impaired in their capability to develop systemic allergic response upon sensitization with Af in our model. Thus, along with Zhang and colleagues [4] and Takeda and colleagues [3] we show that a decreased airway responsiveness in C57BL/6 mice is not accompanied by similar reduction in the total and antigen-specific IgE and IgG1 levels.
Figure 2.
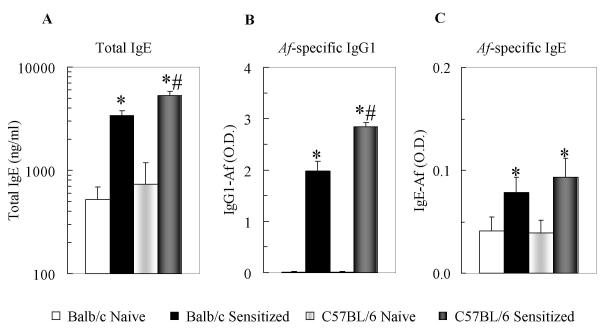
C57BL/6 mice developed significant systemic IgE and IgG1 responses following allergenic sensitization and challenge with Af comparable to the responses of Balb/c mice. Total serum IgE and Af-specific IgE and IgG1 profiles were analyzed by ELISA as described. Total serum IgE (panel A): expressed as ng/ml. Af-specific IgG1 and IgE (panel B and C) expressed as optical density (O.D.). Naïve mice (open bar: Balb/c (n = 15); light gray bar: C57BL/6 (n = 17)) received i.n. glycerol treatment alone. Sensitized mice (black bars: Balb/c (n = 22); dark gray bar: C57BL/6 (n = 22)) received i.p. sensitization and i.n. treatment with Af extract as described. Data are expressed as Mean ± SEM, *p < 0.05 Sensitized vs Naive; #p < 0.05 C57BL/6 vs Balb/c.
C57BL/6 mice develop local airway inflammatory response after i.n. challenge of sensitized mice
Although the number of eosinophils recovered from the BAL fluid of asthmatic patients (reviewed in [6]) and in mouse models of asthma [3,22,23] roughly correlates with disease severity, eosinophil numbers may not directly relate to the extent of airway responsiveness or tissue injury. Hematoxilyn eosin staining of histological lung section from both Balb/c and C57BL/6 mice that were sensitized and challenged with Af demonstrated a predominantly perivascular and peribronchial inflammatory infiltrate of mainly mononuclear cells and eosinophils, (data not shown) as previously described [3]. Analysis of the cellular fraction of BAL showed that both Balb/c and C57BL/6 mice had increased and similar total cell numbers after sensitization and challenge with Af (Figure 3A) in comparison with naïve controls. In spite of a comparable total BAL cell count between Balb/c and C57BL/6 mice however, the numbers of eosinophils were significantly higher in sensitized C57BL/6 mice than sensitized Balb/c mice (609 ± 90 × 103 vs 140 ± 42 × 103, respectively, p < 0.001), (Figure 3B).
Figure 3.

Upon allergen challenge, sensitized C57BL/6 mice developed significantly augmented airway eosinophilia when compared with Balb/c mice. The absolute number of BAL cells (panel B) was derived from the differential counts in Giemsa-stained cytospin preparations and the total cell counts (panel A) in each BAL sample as described. Naïve mice (open bar: Balb/c (n = 15); light gray bar: C57BL/6 (n = 17)) received i.n. glycerol treatment alone. Sensitized mice (black bars: Balb/c (n = 22); dark gray bar: C57BL/6 (n = 22)) received i.p. sensitization and i.n. treatment with Af extract as described. Data are expressed as Mean ± SEM, *p < 0.05 Sensitized vs Naive; #p < 0.05 C57BL/6 vs Balb/c.
In order to compare the effects of allergic sensitization and challenge on phospholipid and protein content of the BAL between Balb/c and C57BL/6 mice, we next examined the large aggregate (LA) and small aggregate (SA) surfactant fractions of the cell free BAL supernatant as described previously [26,27] (Table 1). Sensitization and challenge induced a 2-fold increase in protein levels in the SA fraction (where the majority of protein is found; p < 0.01) and a conversion of phospholipids between the LA and the SA (p < 0.05) surfactant fraction in C57BL/6 (but not Balb/c mice). Thus, analysis of the BAL cellular, protein and phospholipid profile indicated that C57BL/6 mice are capable of developing local inflammatory changes comparable and indeed more prominent than those in Balb/c mice upon allergic sensitization and challenge.
Table 1.
C57BL/6 mice showed significantly heightened BAL protein levels and inflammatory changes in the BAL phospholipid profile in comparison with Balb/c mice following allergic sensitization and challenge with Af.
| MOUSE STRAIN | Protein, μg per lung | Phospholipid, μg per lung | |||
| LA | SA | LA | SA | ||
| BALB/C | Naive | 94 ± 19 | 1,075 ± 154 | 144 ± 25 | 78 ± 11 |
| Sensitized | 99 ± 14 | 1,059 ± 108 | 146 ± 12 | 106 ± 14 | |
| C57BL/6 | Naive | 56 ± 6 | 1,283 ± 200 | 148 ± 22 | 97 ± 6 |
| Sensitized | 56 ± 7 | 1,998 ± 266*# | 100 ± 12* | 146 ± 24* | |
Protein and phospholipid content were analyzed from the large aggregate (LA) and the small aggregate (SA) surfactant fractions of naïve and sensitized mice as described. Data are expressed as amount of protein or phospholipid (micrograms) per mouse lung. Mean ± SEM of n = 16–22 mice per group were calculated after deriving the average of the results from two independent experiments. *p < 0.05 Sensitized vs Naive; #p < 0.05 C57BL/6 vs Balb/c.
C57BL/6 mice had markedly elevated SP-D levels both at baseline and after sensitization and challenge with Af in comparison with Balb/c mice
We have previously shown that allergic Th2-type inflammation induced production of SP-D, an otherwise constitutively expressed protein in the lung [12]. These studies were recently confirmed by others in murine models [28] and in human asthmatic patients [29]. Increases in SP-D expression in lung inflammation have been also described in our laboratory in a different model of inflammation induced by Pneumocystis carinii pneumonia [18,19]. In contrast to the non-specific upregulation of both hydrophilic surfactant proteins SP-A and SP-D in pneumonia however, allergic airway inflammation selectively affected SP-D production, suggesting distinct regulatory pathways and a specific role for this lung collectin [12].
We analyzed whether the changes induced by allergic sensitization and challenge with Af in pulmonary surfactant protein expression were different between C57BL/6 and Balb/c mice. Cell free supernatant of BAL was further fractionated into large (LA) and small (SA) aggregate surfactant fractions by differential centrifugation as described previously in our laboratory [18,19]. Fractionation of the BAL is important because of the differential surfactant protein profile that may be recovered from the different fractions: while the majority of the proteinaceous material and SP-D can be found in the SA, most of the phospholipids and all of the hydrophobic surfactant proteins (SP-B and C) are in the LA fraction. Analyses of the BAL cell pellet for SP-D expression showed no significant differences among the groups (not shown) suggesting that cell-associated SP-D does not represent a significant proportion of collectins in this model. Immunoblot analysis demonstrated that Af challenge of sensitized C57BL/6 mice induced increased SP-D protein expression in the LA surfactant fraction (352 ± 88% of the naïve control levels) that was 1.6 fold greater than in Balb/c mice (221 ± 36%, p < 0.05). In the SA surfactant fraction (where the majority of this protein may be found), Af challenge of sensitized C57BL/6 mice induced a 1,894 ± 170% increase in SP-D from the naïve control group, that was 1.5 fold greater than the 1,269 ± 142% increase in Balb/c mice (p < 0.01 Figure 4A and 4B). In addition, SP-D expression was also significantly greater in naïve C57BL/6 mice (SA BAL SP-D levels were 233% of naïve Balb/c SP-D levels p < 0.05). Thus, sensitization and challenge with Af markedly increased SP-D protein content in the SA BAL fraction of both Balb/c and C57BL/6 mice, however the latter show a distinctly greater capability to produce SP-D in response to allergen challenge. The fact that SP-D expression was also significantly greater in naïve C57BL/6 mice than in Balb/c mice suggests that baseline production of this collectin in the lung is genetically determined.
Figure 4.

Following allergic challenge of sensitized C57BL/6 mice there was a significantly increased production of SP-D in the lung in comparison with similarly treated Balb/c mice. Western blot of three representative SP-D samples from the SA surfactant fraction of the BAL fluid of Balb/c and C57BL/6 mice (A). Nitrocellulose blots with samples of SA surfactant from BAL naïve and sensitized mice were probed with polyclonal antisera against SP-D antibody as described. Each lane contains 10 μg total protein. The relative content of SP-D bands in each sample was determined by densitometric scanning of the 43-kD bands from multiple blots and quantified as described (B). Open bars: Naive mice; Closed bars: Sensitized mice. Data are expressed as % of naïve controls levels. N = 3–5 samples were used in each group. Mean ± SEM was calculated after deriving the average of the results from two independent experiments. *p < 0.05 Sensitized vs Naive; #p < 0.05 C57BL/6 vs Balb/c. Autoradiographs of three representative SP-D mRNA samples of Balb/c and C57BL/6 mice (C). Total RNA for northern blot analysis was prepared from the lungs of naïve and sensitized mice as described. Intensity was quantified by densitometric scanning and values were normalized to 28S mRNA (D). Intensity of 28S band was similar in each sample (data not shown). SP-D mRNA content is expressed as % of naïve Balb/c level. Open bars: Naive mice; Closed bars: Sensitized mice. N = 7–8 samples were used. Mean ± SEM was calculated after deriving the average of the results from two independent experiments.
SP-D increase following Af-challenge of sensitized mice was selective in both C57BL/6 and Balb/c mice
To study whether the increases in SP-D levels correlated with total mRNA levels, we also analyzed SP-D mRNA expression. Although there was a trend towards increases in SP-D mRNA following sensitization and challenge in C57BL/6 mice (184% of non-sensitized control SP-D mRNA), the findings were not statistically significant (Figure 4C and 4D) indicating that a possibly enhanced mRNA transcription may not be the sole requirement for allergic inflammation induced SP-D production. Since changes in mRNA expression were not proportional to those in protein expression (an approximately two fold increase in SP-D mRNA accompanied a nearly 20 fold enhancement in SP-D protein), we speculate that in addition to transcriptional regulation, metabolism of this lung collectin may also be significantly affected during allergic inflammation and a reduced recycling or an increased half life may contribute to its accumulation [30].
To confirm the selectivity of SP-D upregulation in C57BL/6 mice during allergic airway inflammation we also analyzed the LA and SA surfactant fractions for SP-A, the other member of the hydrophilic surfactant protein (lung collectin) family. SP-A protein (Fig 5A and 5B) and mRNA (Fig 5C) levels were unaffected by allergic sensitization and challenge in C57BL/6 and Balb/c mice as demonstrated by Western blot analysis of both the LA and SA surfactant fractions (Fig 5A) and Northern blot analysis of the lung tissue (Fig 5C). Further, naïve C57BL/6 mice had significantly lower levels of SP-A protein when compared with Balb/c mice by ELISA (Fig 5B) and Western blot (Fig 5A).
Figure 5.
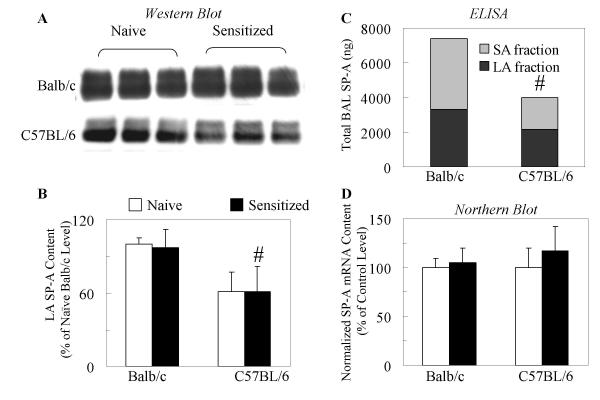
SP-A protein levels in the lung of C57BL/6 mice were not increased following allergic challenge of sensitized mice. Western blot of three representative SP-A samples from the LA surfactant fraction of the BAL fluid of Balb/c and C57BL/6 mice (A). Nitrocellulose blots with samples of LA surfactant from naïve and sensitized mice were probed with polyclonal anti-SP-A antibody as described. Each lane contains 5 μg total protein. The relative content of SP-A doublet bands in each sample was determined by densitometric scanning of the 29–35 kD bands from multiple blots and quantified as described (B). Open bars: Naive mice; Closed bars: Sensitized mice. Data are expressed as % of naïve Balb/c levels. Mean ± SEM of n = 3–5 was calculated after deriving the average of the results from two independent experiments. # p < 0.05 C57BL/6 vs naïve Balb/c mice. The SP-A content in the LA and SA fractions of naïve mice was also determined by using an ELISA protocol as described. Total BAL SP-A content (C) was expressed as total ng. Gray bars: LA fraction of BAL; Black bars: SA fractions of BAL. Data are expressed as Mean ± SEM of n = 5 samples in each group. #p < 0.05 C57BL/6 vs naïve Balb/c mice. Total RNA for northern blot analysis was prepared from the lungs of naïve and sensitized mice as described. Intensity was quantified by densitometric scanning and values were normalized to 28S mRNA (D). SP-A mRNA contents are expressed as % of naïve Balb/c level. Open bars: Naive mice; Closed bars: Sensitized mice. Mean ± SEM of n = 3–5 samples was calculated after deriving the average of the results from two independent experiments.
In addition to the hydrophilic surfactant proteins, we studied changes in the hydrophobic SP-B and SP-C. Western blot analysis of the LA aggregate fraction (where SP-B and SP-C are found) demonstrated a 50% reduction of sensitized Balb/c and C57BL/6 mice (data not shown). Although reduction of the hydrophobic surfactant proteins was similar between strains, C57BL/6 mice had slightly lower baseline levels of SP-B when compared with naïve Balb/c mice (30 ± 12% of naïve Balb/c level). Thus, the only surfactant protein that showed significant increases during allergic airway inflammation was SP-D. Regression analysis between SP-D and airway eosinophilia showed a statistically significant positive correlation (r = 0.7305, p < 0.05) indicating that greater inflammation elicits the production of more SP-D in the lung.
The mechanisms that may be responsible for enhanced production of SP-D in the lung are unknown. Positive correlations between SP-D levels and airway eosinophilia may indicate a potential common regulatory pathway. In support of that, recent studies in mice over expressing IL-4 [30,31], IL-5 [32] and IL-13 [33] in the lung tissue indicated that Th2-type inflammation induced over production of SP-D. Enhanced SP-D expression in return may exert a protective immunosuppressive function in the distal airspaces. Originally SP-D was thought to be important in stimulating clearance of pathogens and allergenic material by macrophages, providing a first line of protection from allergic sensitization [13,34]. Later this collectin was shown to inhibit lymphocyte proliferation and decrease histamine release by basophils induced by house dust mite and Af allergens [34]. In recent murine models of allergic bronchopulmonary aspergillosis (ABPA), SP-D treatment protected against mortality and inhibited the immunoglobulin, eosinophil and Th2 cytokines associated with this model of fungal infection [35,36].
Th2 cytokine levels in the BAL fluid of C57BL/6 mice were inhibited in comparison with Balb/c mice following sensitization and challenge with Af
Aside from processes such as maturation, recruitment and survival of eosinophilic granulocytes, that are ultimately reflected by the numbers of these cells observed in the lung tissue, the ability to release de novo synthesized and preformed proinflammatory mediators is also essential to mount an inflammatory response [11]. This effector function is dependent on optimal priming and activation of these cells by eosinophil active Th2-type cytokines including IL-3, IL-5, IL-9 and GM-CSF [37,38]. To that end, it is of interest that analyses of the murine IL-9 gene identified a genetic defect at the IL-9 locus in C57BL/6 mice [2]. To investigate the association of BAL SP-D levels with the cytokine profile induced by allergenic sensitization and challenges in Balb/c and C57BL/6 mice, we analyzed IFN-γ, TNF-α, IL-4 and IL-5 protein expression in the BAL supernatant. Similarly to our earlier studies, sensitization and challenge with Af markedly enhanced IL-4 and IL-5. IFN-γ decreased by approximately 50% in both strains; from 143 ± 20 to 67 ± 6 pg/ng of total protein in Balb/c mice and from 79 ± 10 to 36 ± 5 pg/ng of total protein in C57BL/6 mice. As shown in Figure 6, IL-4 and IL-5 were increased in mice sensitized and challenged with Af in comparison with naïve controls both in the Balb/c and the C57BL/6 strains (p < 0.05). The increase in cytokine levels in the C57BL/6 mice however, was significantly attenuated following sensitization and challenge with Af in comparison with Balb/c mice suggesting local inhibition of release or synthesis of these cytokines. Thus, sensitization and challenge with Af markedly enhanced IL-4 and IL-5 but not IFN-γ both in the Balb/c and the C57BL/6 strains as also shown in our previous studies [12,16,17]. The increase in the Th2 cytokine levels in the C57BL/6 mice was however, significantly attenuated suggesting inhibition of release or reduced synthesis.
Figure 6.

Allergen challenge of sensitized C57BL/6 mice induced impaired IL-4 and IL-5 release in comparison with Balb/c mice. Cytokine levels in BAL were determined by ELISA. Data are expressed as pg per μg of protein level. The Naïve group (open bar, Balb/c (n = 9) or light gray bar, C57BL/6 (n = 9)) received intranasal glycerol treatment alone. The Sensitized group (black bar, Balb/c (n = 12) or dark gray bar, C57BL/6 (n = 12)) received i.p. sensitization and i.n. treatment with Af extract. Data are expressed as Mean ± SEM, *p < 0.05 Sensitized vs Naive; #p < 0.05 C57BL/6 vs Balb/c.
Conclusions
In conclusion, SP-D production was increased in response to allergic airway inflammation in mice sensitized and challenged with Af. This process may be genetically regulated and may serve a negative feedback that would inhibit further Th2 activation. We speculate that enhanced SP-D levels may be responsible for an impaired ability of C57BL/6 mice to develop allergen induced AHR.
Authors' contributions
ENA carried out the surfactant protein analysis and analyzed the data. MFB participated in the design of the study and contributed to the manuscript writing with comments. YT carried out the animal experiments. STS carried out the protein and phospholipids measurements. SJR performed the mRNA analysis. RAP participated in the writing of the manuscript. AH conceived of, designed and coordinated the study, and participated in its writing. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
AH is a Parker B. Francis Research Fellow in Pulmonary Medicine
MFB: RO1 HL64520 & HL59867
Contributor Information
Elena N Atochina, Email: haczku@mail.med.upenn.edu.
Michael F Beers, Email: haczku@mail.med.upenn.edu.
Yaniv Tomer, Email: haczku@mail.med.upenn.edu.
Seth T Scanlon, Email: haczku@mail.med.upenn.edu.
Scott J Russo, Email: haczku@mail.med.upenn.edu.
Reynold A Panettieri, Jr, Email: haczku@mail.med.upenn.edu.
Angela Haczku, Email: haczku@mail.med.upenn.edu.
References
- Heinzmann A, Daser A. Mouse models for the genetic dissection of atopy. Int Arch Allergy Immunol. 2002;127:170–180. doi: 10.1159/000053861. [DOI] [PubMed] [Google Scholar]
- Nicolaides NC, Holroyd KJ, Ewart SL, Eleff SM, Kiser MB, Dragwa CR, Sullivan CD, Grasso L, Zhang LY, Messler CJ, Zhou T, Kleeberger SR, Buetow KH, Levitt RC. Interleukin 9: a candidate gene for asthma. Proc Natl Acad Sci U S A. 1997;94:13175–13180. doi: 10.1073/pnas.94.24.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Haczku A, Lee JJ, Irvin CG, Gelfand EW. Strain dependence of airway hyperresponsiveness reflects differences in eosinophil localization in the lung. Am J Physiol Lung Cell Mol Physiol. 2001;281:L394–L402. doi: 10.1152/ajplung.2001.281.2.L394. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lamm WJ, Albert RK, Chi EY, Henderson W.R.,Jr., Lewis DB. Influence of the route of allergen administration and genetic background on the murine allergic pulmonary response. Am J Respir Crit Care Med. 1997;155:661–669. doi: 10.1164/ajrccm.155.2.9032210. [DOI] [PubMed] [Google Scholar]
- Broide DH. Molecular and cellular mechanisms of allergic disease. J Allergy Clin Immunol. 2001;108:S65–S71. doi: 10.1067/mai.2001.116436. [DOI] [PubMed] [Google Scholar]
- Corrigan CJ, Kay AB. T cells and eosinophils in the pathogenesis of asthma. Immunol Today. 1992;13:501–507. doi: 10.1016/0167-5699(92)90026-4. [DOI] [PubMed] [Google Scholar]
- De Sanctis GT, Itoh A, Green FH, Qin S, Kimura T, Grobholz JK, Martin TR, Maki T, Drazen JM. T-lymphocytes regulate genetically determined airway hyperresponsiveness in mice. Nat Med. 1997;3:460–462. doi: 10.1038/nm0497-460. [DOI] [PubMed] [Google Scholar]
- Drazen JM, Arm JP, Austen KF. Sorting out the cytokines of asthma. J Exp Med. 1996;183:1–5. doi: 10.1084/jem.183.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt PG, Macaubas C, Stumbles PA, Sly PD. The role of allergy in the development of asthma. Nature. 1999;402:B12–B17. doi: 10.1038/35037009. [DOI] [PubMed] [Google Scholar]
- Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- Hamid Q, Tulic' MK, Liu MC, Moqbel R. Inflammatory cells in asthma: mechanisms and implications for therapy. J Allergy Clin Immunol. 2003;111:S5–S12. doi: 10.1067/mai.2003.22. [DOI] [PubMed] [Google Scholar]
- Haczku A, Atochina EN, Tomer Y, Chen H, Scanlon ST, Russo S, Xu J, Panettieri R.A.,Jr., Beers MF. Aspergillus fumigatus-induced allergic airway inflammation alters surfactant homeostasis and lung function in BALB/c mice. Am J Respir Cell Mol Biol. 2001;25:45–50. doi: 10.1165/ajrcmb.25.1.4391. [DOI] [PubMed] [Google Scholar]
- Crouch E, Wright JR. Surfactant proteins a and d and pulmonary host defense. Annu Rev Physiol. 2001;63:521–554. doi: 10.1146/annurev.physiol.63.1.521. [DOI] [PubMed] [Google Scholar]
- McLane MP, Haczku A, van de Rijn M., Weiss C, Ferrante V, MacDonald D, Renauld JC, Nicolaides NC, Holroyd KJ, Levitt RC. Interleukin-9 promotes allergen-induced eosinophilic inflammation and airway hyperresponsiveness in transgenic mice. Am J Respir Cell Mol Biol. 1998;19:713–720. doi: 10.1165/ajrcmb.19.5.3457. [DOI] [PubMed] [Google Scholar]
- Mehlhop PD, van de Rijn M., Goldberg AB, Brewer JP, Kurup VP, Martin TR, Oettgen HC. Allergen-induced bronchial hyperreactivity and eosinophilic inflammation occur in the absence of IgE in a mouse model of asthma. Proc Natl Acad Sci U S A. 1997;94:1344–1349. doi: 10.1073/pnas.94.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haczku A, Takeda K, Hamelmann E, Loader J, Joetham A, Redai I, Irvin CG, Lee JJ, Kikutani H, Conrad D, Gelfand EW. CD23 exhibits negative regulatory effects on allergic sensitization and airway hyperresponsiveness. Am J Respir Crit Care Med. 2000;161:952–960. doi: 10.1164/ajrccm.161.3.9905046. [DOI] [PubMed] [Google Scholar]
- Haczku A, Atochina EN, Tomer Y, Cao Y, Campbell C, Scanlon ST, Russo SJ, Enhorning G, Beers MF. The late asthmatic response is linked with increased surface tension and reduced surfactant protein B in mice. Am J Physiol Lung Cell Mol Physiol. 2002;283:L755–L765. doi: 10.1152/ajplung.00062.2002. [DOI] [PubMed] [Google Scholar]
- Atochina EN, Beers MF, Scanlon ST, Preston AM, Beck JM. P. carinii induces selective alterations in component expression and biophysical activity of lung surfactant. Am J Physiol Lung Cell Mol Physiol. 2000;278:L599–L609. doi: 10.1152/ajplung.2000.278.3.L599. [DOI] [PubMed] [Google Scholar]
- Atochina EN, Beck JM, Scanlon ST, Preston AM, Beers MF. Pneumocystis carinii pneumonia alters expression and distribution of lung collectins SP-A and SP-D. J Lab Clin Med. 2001;137:429–439. doi: 10.1067/mlc.2001.115220. [DOI] [PubMed] [Google Scholar]
- Drazen JM. Physiological basis and interpretation of indices of pulmonary mechanics. Environ Health Perspect. 1984;56:3–9. doi: 10.1289/ehp.84563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daser A, Daheshia M, De Sanctis GT. Genetics of allergen-induced asthma. J Allergy Clin Immunol. 2001;108:167–174. doi: 10.1067/mai.2001.116987. [DOI] [PubMed] [Google Scholar]
- Hamelmann E, Takeda K, Schwarze J, Vella AT, Irvin CG, Gelfand EW. Development of eosinophilic airway inflammation and airway hyperresponsiveness requires interleukin-5 but not immunoglobulin E or B lymphocytes. Am J Respir Cell Mol Biol. 1999;21:480–489. doi: 10.1165/ajrcmb.21.4.3659. [DOI] [PubMed] [Google Scholar]
- Hamelmann E, Takeda K, Haczku A, Cieslewicz G, Shultz L, Hamid Q, Xing Z, Gauldie J, Gelfand EW. Interleukin (IL)-5 but not immunoglobulin E reconstitutes airway inflammation and airway hyperresponsiveness in IL-4-deficient mice. Am J Respir Cell Mol Biol. 2000;23:327–334. doi: 10.1165/ajrcmb.23.3.3796. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Rabold R, Mitzner W. Differential lung mechanics are genetically determined in inbred murine strains. J Appl Physiol. 1999;86:1764–1769. doi: 10.1152/jappl.1999.86.6.1764. [DOI] [PubMed] [Google Scholar]
- Wanner A, Abraham WM, Douglas JS, Drazen JM, Richerson HB, Ram JS. NHLBI Workshop Summary. Models of airway hyperresponsiveness. Am Rev Respir Dis. 1990;141:253–257. doi: 10.1164/ajrccm/141.1.253. [DOI] [PubMed] [Google Scholar]
- Atochina EN, Beck JM, Scanlon ST, Preston AM, Beers MF. Pneumocystis carinii pneumonia alters expression and distribution of lung collectins SP-A and SP-D. J Lab Clin Med. 2001;137:429–439. doi: 10.1067/mlc.2001.115220. [DOI] [PubMed] [Google Scholar]
- Atochina EN, Beers MF, Scanlon ST, Preston AM, Beck JM. P. carinii induces selective alterations in component expression and biophysical activity of lung surfactant. Am J Physiol Lung Cell Mol Physiol. 2000;278:L599–L609. doi: 10.1152/ajplung.2000.278.3.L599. [DOI] [PubMed] [Google Scholar]
- Haley KJ, Ciota A, Contreras JP, Boothby MR, Perkins DL, Finn PW. Alterations in lung collectins in an adaptive allergic immune response. Am J Physiol Lung Cell Mol Physiol. 2002;282:L573–L584. doi: 10.1152/ajplung.00117.2001. [DOI] [PubMed] [Google Scholar]
- Cheng G, Ueda T, Numao T, Kuroki Y, Nakajima H, Fukushima Y, Motojima S, Fukuda T. Increased levels of surfactant protein A and D in bronchoalveolar lavage fluids in patients with bronchial asthma. Eur Respir J. 2000;16:831–835. doi: 10.1183/09031936.00.16583100. [DOI] [PubMed] [Google Scholar]
- Ikegami M, Whitsett JA, Chroneos ZC, Ross GF, Reed JA, Bachurski CJ, Jobe AH. IL-4 increases surfactant and regulates metabolism in vivo. Am J Physiol Lung Cell Mol Physiol. 2000;278:L75–L80. doi: 10.1152/ajplung.2000.278.1.L75. [DOI] [PubMed] [Google Scholar]
- Jain-Vora S, LeVine AM, Chroneos Z, Ross GF, Hull WM, Whitsett JA. Interleukin-4 enhances pulmonary clearance of Pseudomonas aeruginosa. Infect Immun. 1998;66:4229–4236. doi: 10.1128/iai.66.9.4229-4236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Weaver TE, Beck DC, Rothenberg ME. Interleukin-5-mediated allergic airway inflammation inhibits the human surfactant protein C promoter in transgenic mice. J Biol Chem. 2001;276:8453–8459. doi: 10.1074/jbc.M009481200. [DOI] [PubMed] [Google Scholar]
- Homer RJ, Zheng T, Chupp G, He S, Zhu Z, Chen Q, Ma B, Hite RD, Gobran LI, Rooney SA, Elias JA. Pulmonary type II cell hypertrophy and pulmonary lipoproteinosis are features of chronic IL-13 exposure. Am J Physiol Lung Cell Mol Physiol. 2002;283:L52–L59. doi: 10.1152/ajplung.00438.2001. [DOI] [PubMed] [Google Scholar]
- Madan T, Kishore U, Shah A, Eggleton P, Strong P, Wang JY, Aggrawal SS, Sarma PU, Reid KB. Lung surfactant proteins A and D can inhibit specific IgE binding to the allergens of Aspergillus fumigatus and block allergen-induced histamine release from human basophils. Clin Exp Immunol. 1997;110:241–249. doi: 10.1111/j.1365-2249.1997.tb08323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Madan T, Waters P, Parida SK, Sarma PU, Kishore U. Protective effects of a recombinant fragment of human surfactant protein D in a murine model of pulmonary hypersensitivity induced by dust mite allergens. Immunol Lett. 2003;86:299–307. doi: 10.1016/S0165-2478(03)00033-6. [DOI] [PubMed] [Google Scholar]
- Strong P, Reid KB, Clark H. Intranasal delivery of a truncated recombinant human SP-D is effective at down-regulating allergic hypersensitivity in mice sensitized to allergens of Aspergillus fumigatus. Clin Exp Immunol. 2002;130:19–24. doi: 10.1046/j.1365-2249.2002.01968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louahed J, Zhou Y, Maloy WL, Rani PU, Weiss C, Tomer Y, Vink A, Renauld J, Van Snick J, Nicolaides NC, Levitt RC, Haczku A. Interleukin 9 promotes influx and local maturation of eosinophils. Blood. 2001;97:1035–1042. doi: 10.1182/blood.V97.4.1035. [DOI] [PubMed] [Google Scholar]
- Wardlaw AJ, Moqbel R, Kay AB. Eosinophils: biology and role in disease. Adv Immunol. 1995;60:151–266. doi: 10.1016/s0065-2776(08)60586-6. [DOI] [PubMed] [Google Scholar]


