Abstract
The human body displays central circadian rhythms of activity. Recent findings suggest that peripheral tissues, such as bone, possess their own circadian clocks. Studies have shown that osteocalcin protein levels oscillate over a 24-hour period, yet the specific skeletal sites involved and its transcriptional profile remain unknown. The current study aimed to test the hypothesis that peripheral circadian mechanisms regulate transcription driven by the osteocalcin promoter. Transgenic mice harboring the human osteocalcin promoter linked to a luciferase reporter gene were used. Mice of both genders and various ages were analyzed non-invasively at sequential times throughout 24-hour periods. Statistical analyses of luminescent signal intensity of osteogenic activity from multiple skeletal sites indicated a periodicity of ~ 24 hrs. The maxillomandibular complex displayed the most robust oscillatory pattern. These findings have implications for dental treatments in orthodontics and maxillofacial surgery, as well as for the mechanisms underlying bone remodeling in the maxillomandibular complex.
Keywords: maxillomandibular complex, biolu-minescence, circadian rhythm, osteocalcin, transgenic mice
Introduction
The human body displays cyclic patterns of gene expression, hormone secretion, and behavioral activity, reflecting the 24-hour light/dark cycle. Recent studies indicate that tissues peripheral to the central nervous system contain their own, independent circadian clocks (Lowrey and Takahashi, 2004). The mRNAs encoding proteins associated with the circadian clock have been detected in peripheral tissues such as adipose tissue, liver, skeletal muscle, and testis (Zylka et al., 1998; Panda et al., 2002; Storch et al., 2002; Ando et al., 2005; Ptitsyn et al., 2006; Zvonic et al., 2006). Indeed, there is now in vitro evidence that peripheral “clocks” are present even within cultured cell lines, including bone-marrow-derived mesenchymal stem cells (Balsalobre et al., 1998).
The circadian apparatus represents a self-contained transcriptional/ translational feedback loop associated with a 24-hour oscillatory expression profile (Griffin et al., 1999; Shearman et al., 2000). Using transcriptomics (i.e., expression profiling) and RT-PCR methods, we recently demonstrated the oscillatory expression profile of the mRNAs encoding the core components of the circadian apparatus in murine calvarial bone (Zvonic et al., 2007). Other investigators have reported similar observations in the murine femur (Fu et al., 2005). Recent circadian studies have tried to assess the effects of orthodontic and orthopedic forces applied to bone remodeling of the maxilloman-dibular complex. These studies showed a correlation between forces applied at rest times and accelerated bone remodeling (Lou et al., 2000; Zheng et al., 2003). Moreover, local and systemic osteocalcin expression has been shown to increase significantly when orthopedic force was applied to the mandible for 24 hrs a day vs. 12 hrs of daytime (Ye et al., 2001).
A significant body of literature examining serum protein biomarkers supports a role for circadian mechanisms in bone metabolism (Gundberg et al., 1985; Bollen et al., 1995; Heshmati et al., 1998; Srivastava et al., 2001; Shao et al., 2003; Fu et al., 2005; Patel and Elefteriou, 2007). In human and pre-clinical animal studies, serum levels of proteins associated with bone metabolism (osteocalcin and alkaline phosphatase) all oscillated in a cosinor manner over a 24-hour period (Gundberg et al., 1985; Bollen et al., 1995; Heshmati et al., 1998; Srivastava et al., 2001; Shao et al., 2003). However, the contributions of transcriptional mechanisms to the circadian control of serum protein biomarkers of bone metabolism remain relatively unexplored (Fu et al., 2005; Zvonic et al. , 2007). To date, there is no specific model or any published study that investigated the physiological circadian rhythms related to bone remodeling and osteocalcin expression in the maxillomandibular complex.
In the current manuscript, we extend our transcriptomic observations (Zvonic et al., 2007) by exploring the hypothesis that circadian mechanisms contribute to transcriptional regulation of the bone-specific gene, osteocalcin. We used a human osteocalcin promoter/luciferase reporter (hOC-Luc) transgenic murine model, previously validated in vivo for serial non-invasive bioluminescence imaging (Iris et al., 2003).
Materials & Methods
Animal Studies
Transgenic Mouse Model
FVB/N transgenic mice were generated to harbor the luciferase gene under the control of the human osteocalcin promoter, as previously described (Clemens et al., 1997). These mice express luciferase in osteogenic tissues. Wild-type FVB/N mice were used as controls. The Hebrew University Institutional Animal Care and Use Committee approved all the procedures used in this study and agreed that their care was consistent with the United States National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Time Series Analysis and in vivo Bioluminescence Imaging
Intact mice of both genders, transgenic for the human osteocalcin promoter/luciferase reporter gene, were used at ages 1, 3, and 5 mos and 1.5 yrs (N = 5 for each gender and time-point). Mice were trained for a 12-hour light/dark cycle for a minimum of 2 wks prior to each study. Each animal was sequentially analyzed at times 0, 6, 12, 18, and 24 hrs of the light/dark cycle throughout a single 24-hour period.
In vivo bioluminescence imaging was performed as previously described (Iris et al., 2003). Briefly, following anesthetization, each animal was given an intraperitoneal injection of beetle luciferin (Promega Corp., Madison, WI, USA) in phosphate-buffered saline (PBS) at 126 mg/kg body weight, and placed in a light-tight chamber. Photon emission was then integrated over a period of 2 min and recorded as pseudocolor images (Contag et al., 1997; Iris et al., 2003).
Quantitative analysis of luciferase expression was performed with the MetaImaging series 4.6 software (Molecular Devices, Downingtown, PA, USA), with a constant measurement field for all time-points. Results are presented in integrated luciferase units.
Seven skeletal sites were analyzed: calvaria, tail, maxillomandibular complex, carpals, and tarsals. (See detailed description of methods in the APPENDIX, under ‘Animal Studies’.)
Computational Methods
Computational methods included: phase assignment, spectral analysis, Fisher’s g test, permuted time test (Pt test), autocorrelation, and data analysis pipeline. A detailed description of each is presented in the APPENDIX.
We determined the phase of expression for each individual animal by cosine-based analyses using multiple algorithms. The profiles from individual animals could be subdivided into 3 distinct phase cohorts, based on the acrophase (zenith) of expression: The phase cohort 1, with an acrophase of Zeitgeber Time (ZT) 6h, accounted for 68 % of the profiles (n = 186), while phases 2 and 3, with acrophases of ZT12h and ZT18h, included 20% (n = 54) and 12% (n = 33) of the profiles, respectively. (See detailed description of methods in the APPENDIX.) Zeitgeber Time 0 (ZT0) corresponds to the point during the day when the animal is first exposed to daylight, while ZT12 corresponds to the time of day when the animal is first exposed to darkness.
Results
Osteocalcin promoter/luciferase transgenic mice were monitored individually for osteocalcin expression, based on in vivo bioluminescence, for a period of 24 hrs (a representative animal is displayed in Fig. 1). Based on previous experiments, which included the dissection of tissues followed by luciferase assay and RT-PCR, we were able to conclude that the OC-Luc transgenic mice showed osteogenic activity within the maxillomandibular complex, calvaria, tail, carpals, and tarsals (data not shown). This finding correlated with our bioluminescence imaging results (Fig. 1, Appendix Fig. 1). Because luciferase has a half-life of ~ 3 hrs in mammalian cells (Thompson et al., 1991), it was feasible to monitor circadian mechanisms dynamically in transgenic mice. Analysis of our data showed no significant ascending trend in luminescent signal, and thus no significant luciferase accumulation between time-points.
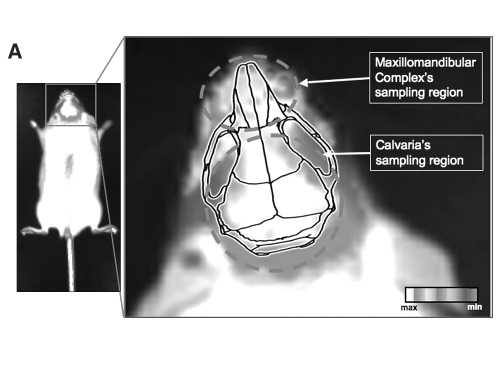
Figure 1.
(A) In vivo bioluminescence imaging was conducted based on region sampling. A sketch of mouse skull anatomy was overlaid on a magnified area of an image acquired by a non-invasive bioluminescence imaging device. Data acquisition was based on region sampling, as can be displayed for maxillomandibular and calvarial regions. A similar approach was used for the analysis of other skeletal organs. (B) In vivo bioluminescence imaging of transgenic mouse at 5 time-points within a 24-hour cycle. Intact mice of both genders for the human osteocalcin promoter/luciferase reporter gene were used at ages 1, 3, and 5 mos and 1.5 yrs. N = 5 for each gender and time-point. Each animal was sequentially analyzed at T = 0, 6, 12, 18, and 24 hrs of the light/dark cycle throughout one 24-hour period. Boxed numbers represent signal intensity measured in Integrated Light Units (ILUs) for each sampling region.
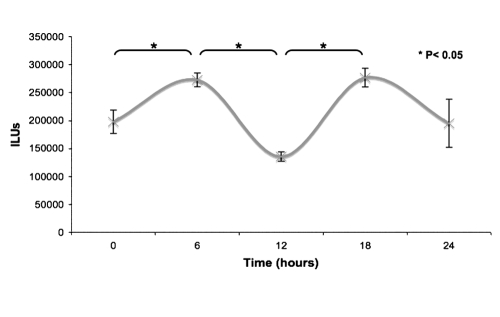
Quantified results revealed a distinct oscillatory pattern of expression in the maxillomandibular complex (a representative group of 5-month-old female transgenic mice is presented in Fig. 2). For statistical purposes, the timeline of the raw bioluminescence data from the maxillomandibular complex for the entire group of animals in Phase cohort 1 was concatenated, sorted, and normalized for amplitude relative to ZT0 for each animal (Fig. 3A). Each sequential group of 5 datapoints corresponds to the bioluminescence signal collected from an individual animal. Analysis determined that neither age nor gender significantly influenced the phase assignment (Fig. 3A) Overall, the maxillomandibular complex diaplayed the most robust oscillatory pattern. Statistical analyses indicated a periodicity of ~ 24 hrs in each of the individual skeletal sites. The magnitude of the luciferase amplitude and oscillation varied among skeletal sites in the following order: maxillomandibular complex > tarsals > carpals > calvaria > tail.
Figure 2.
Quantified in vivo bioluminescence imaging of transgenic mice at 5 time-points within a 24-hour cycle. Chart shows a representative group of 5-month-old females. Results display a circadian rhythm at the transcription level of osteocalcin within the maxillomandibular complex. Note: * indicates a p value < 0.05 by paired t test statistical examination.
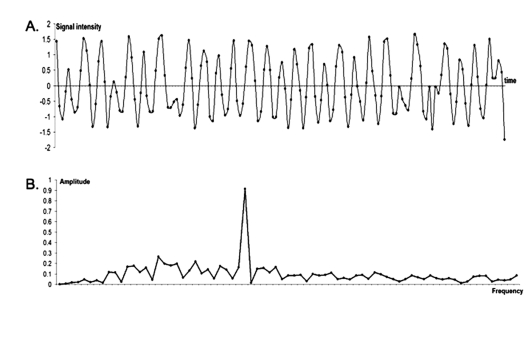
Figure 3.
Concatemer of data for individual data sites (maxillomandibular complex). Circadian oscillation of osteocalcin promoter visualized via a luciferase reporter in the maxillomandibular complex of transgenic mice in vivo. Anesthetized mice (n = 23-28) were examined in a bioimaging device for light emission at six-hour intervals over a 24-hour period immediately following injection with luciferin. (A) Data from the individual animals have been concatenated in the order of emission amplitude and frequency profile. The Y axis shows the signal intensity relative to the standard deviation of intensity (z-score). (B) The periodogram is presented based on a common scale of magnitude. Harmonics contributing to the timeline expression profile (A) are displayed along the X axis (units are numbers of complete periods in the timeline of profile A). The Y axis shows the amplitude of signal intensity (z-score). The highest peak corresponds to the most prominent frequency component with complete period in 24 hrs, i.e., circadian oscillation. Relative heights of the circadian and other peaks in periodogam also illustrate the signal-to-noise ratio (sircadian vs. stochastic variation).
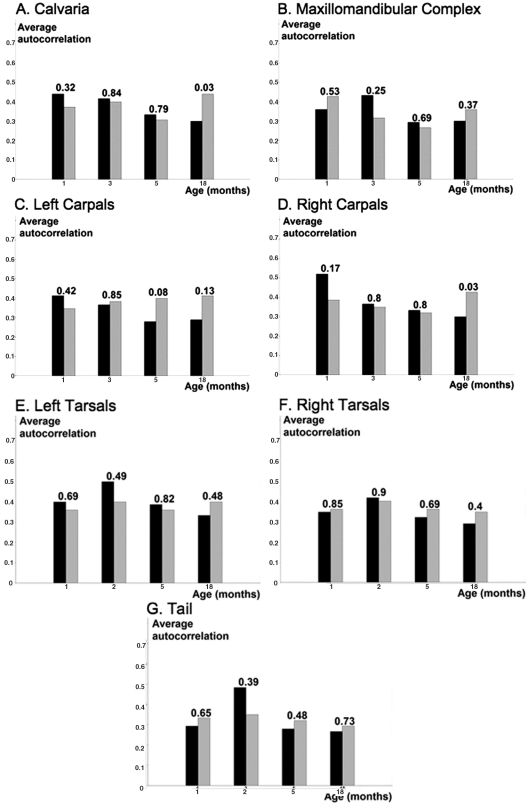
To estimate spectral density, we applied the Discrete Fourier Transform procedure (see APPENDIX), which yielded a periodogram, converting raw data from the time domain into the frequency domain, thereby highlighting the dominant rhythm. The periodograms displayed by the maxillomandibular complex of the Phase cohort 1 animals are given in Fig. 3B. The highest peak in this periodogram corresponds to the circadian frequency, i.e., one complete cycle per one day of observation. The height of this dominant peak relative to all other peaks can be used to estimate the signal-to-noise ratio, and hence statistical confidence in the detection of circadian periodicity. In the current study, the phase-synchronized cohorts displayed a 24-hour circadian profile, as determined by a panel of 3 independent statistical tests. After scaling and smoothing procedures, less than 10% of the individual profiles did not achieve statistical significance for circadian oscillation, consistent with the conclusion reached by direct visual inspection of the raw data. The expression profiles for the remaining skeletal sites in the Phase cohort 1 animals were similar (Appendix Fig. 3). We also analyzed the signals as a function of age and gender. The majority of the results failed to support a significant difference in circadian rhythm dependent upon bone location, gender, or age (Fig. 4); the only exceptions occurred between male and female 18-month cohorts for calvaria and right carpals. These data are consistent with our previously published studies regarding the skeletal growth and development of rodents (Iris et al., 2003).
Figure 4.
Circadian autocorrelation as a function of gender and age. Average circadian autocorrelation by sex and age. Each age category of 1, 3, 5, and 18 mos in consecutive order is presented by a pair of bars for male (black) and female (gray) mice. N = 5 for each gender and time-point. Significance (p-value) of difference in average autocorrelation between genders is presented on top of every age column. This study shows no significant difference in circadian rhythm dependent on the bone location, gender, or age of mice. In each panel, the Y axis shows autocorrelation with 24-hour (circadian) lag. A, calvaria; B, maxillomandibular complex; C, left carpals; D, right carpals; E, left tarsals; F, right tarsals; G, tail.
Discussion
In this study, using non-invasive in vivo bioluminescence imaging in a luciferase transgenic murine model, we tested the hypothesis that circadian mechanisms regulate transcription driven by the human osteocalcin promoter. Both genders of mice were examined at 4 different ages. Analysis of the data obtained supports the conclusion that the expression of the human osteocalcin promoter in skeletal tissues is regulated in an oscillatory manner. The luciferase reporter activity in both female and male mice between the ages of 1 and 18 mos displayed a circadian expression pattern. These results extend our previously reported cal-varial bone transcriptome findings regarding the murine osteocalcin mRNA expression profile (Zvonic et al., 2007).
A major challenge for analysis of biological periodicity is the low sampling rate. The anesthetic procedure precluded a higher frequency of data collection points in the current bioluminescence study. To address this statistical challenge, we performed a phase classification of the expression profiles before testing for periodicity. In addition, for the analysis of low-frequency sampled time series, it is essential to consider all expression profiles in separate groups classified by phase. In our data, we observed one phase cohort with peak expression (acrophase) correlating with ZT6 that included ~ 2/3 of all single-animal profiles (186). However, it remains possible that some of these profiles may have been misclassified due to stochastic noise. This distribution of individual animals to each phase cohort was consistent for each bone site examined. In general, all bones had a strong prevalence for assignment to the ZT6 phase cohort. Summarizing these observations, we conclude that, in the majority of animals, the osteocalcin promoter operates in a circadian manner. While most of the animals followed the same pattern, the phase appeared to be shifted by 6 or 12 hrs in a subpopulation of animals. The mechanism accounting for the phase differences among animal cohorts remains to be determined.
It is of interest that the most pronounced circadian oscillations were evident in the maxillomandibular complex. One explanation for this outcome could be attributed to the murine incisors, which are open-rooted and therefore grow continuously throughout the lifetime of the animal (Foster et al., 1983).
Night time corresponds to the peak serum levels of osteocalcin in humans, which has been recorded as 4 a.m. in multiple studies (Sokoll et al., 1998; Caillot-Augusseau et al., 2000). Because the mouse is a nocturnal animal, this time-point is equivalent to ZT6-12 and is consistent with the maximal luciferase activity detected in the current study. Furthermore, the peak expression for the native murine osteocalcin mRNA occurred at ZT 0-4. This correlates, in a temporally appropriate manner, with the human osteocalcin promoter-driven luciferase reporter protein in the transgenic model.
This finding could have implications with respect to dental treatment. For example, it has been suggested by orthodontists that both orthopedic and orthodontic scale forces should be routinely applied during the night-time hours, due to the fact that bone remodeling is accelerated at resting periods (Igarashi et al., 1998; Miyoshi et al., 2001; Yamada et al., 2002). Our results could provide support for this approach.
Also, one of the key factors for successful orthodontic treatment relates to patient compliance in the management of the various appliances and adjuncts that are used to move teeth. It is proposed that instructing persons to wear these removable orthodontic appliances and adjuncts only during a circadian temporal therapeutic window, i.e., the night-time period, will both achieve a higher level of compliance and offer an improved biological effect. By so doing, the overall treatment outcome will be enhanced both clinically and temporally. Another aspect is the use of distraction osteogenesis (Ilizarov et al., 1980) for maxillofacial and orthosurgical cases. The timing and magnitude of force applied are crucial for the success of treatment. To achieve the anticipated results, the person must cooperate with the activation regime, while the clinician must avoid premature consolidation of the treatment. This report links circadian mechanisms to the control of the human osteocalcin promoter in a physiological model. Future in vitro studies will be required to determine the cis-elements responsible for this phenomenon. Nevertheless, we conclude that circadian transcriptional mechanisms should be considered as contributory factors underlying common skeletal biological processes in general and in the maxillomandibular region in particular (Simmons et al., 1990; Levi and Schibler, 2007). Conventional maxillofacial surgery and orthodontic treatment procedures involve bone regeneration and force application, respectively. Understanding the circadian mechanism that underlies metabolic processes in peripheral bone could enable us to harness this effect for optimization of currently available dental procedures. We predict that our model will enable us to establish the circadian temporal therapeutic window of bone in the maxillomandibular complex, to tailor efficient clinical protocols.
Supplementary Material
Acknowledgments
The authors thank the Drs. K. Eilertsen, Z.E. Floyd, B. Kozak, R. Mynatt, and S. Zvonic, and the members of the Stem Cell Biology Laboratory (Pennington Biomedical Research Center), as well as Dr. F. Guilak (Duke University Medical Center) for discussions and comments on the final manuscript, Laura Dallam for administrative assistance, and funding support from the Pennington Biomedical Research Foundation and the National Institute of Dental and Craniofacial Research (NIDCR R21 DE016371: J.M.G, D.G.).
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This research was supported by the Dr. Izador I. Cabakoff Research Endowment Fund of the Hebrew University Hadassah School of Dental Medicine.
Dr Gimble has served as a consultant or collaborator with Artecel, Inc., Vesta Therapeutics, Cognate Bioservices, Inc., and Theradigm, Inc.
References
- Ando H, Yanagihara H, Hayashi Y, Obi Y, Tsuruoka S, Takamura T, et al. (2005). Rhythmic messenger ribonucleic acid expression of clock genes and adipocytokines in mouse visceral adipose tissue. Endocrinology 146:5631-5636 [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, Schibler U. (1998). A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93:929-937 [DOI] [PubMed] [Google Scholar]
- Bollen AM, Martin MD, Leroux BG, Eyre DR. (1995). Circadian variation in urinary excretion of bone collagen cross-links. J Bone Miner Res 10:1885-1890 [DOI] [PubMed] [Google Scholar]
- Caillot-Augusseau A, Lafage-Proust MH, Margaillan P, Vergely N, Faure S, Paillet S, et al. (2000). Weight gain reverses bone turnover and restores circadian variation of bone resorption in anorexic patients. Clin Endocrinol (Oxf) 52:113-121 [DOI] [PubMed] [Google Scholar]
- Clemens TL, Tang H, Maeda S, Kesterson RA, Demayo F, Pike JW, et al. (1997). Analysis of osteocalcin expression in transgenic mice reveals a species difference in vitamin D regulation of mouse and human osteo-calcin genes. J Bone Miner Res 12:1570-1576 [DOI] [PubMed] [Google Scholar]
- Contag CH, Spilman SD, Contag PR, Oshiro M, Eames B, Dennery P, et al. (1997). Visualizing gene expression in living mammals using a biolu-minescent reporter. Photochem Photobiol 66:523-531 [DOI] [PubMed] [Google Scholar]
- Foster H, Small DJ, Fox JG. (1983). The mouse in biomedical research. Vol. III Normative biology, immunology, and husbandry. Boston: Academic Press, Inc [Google Scholar]
- Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G. (2005). The molecular clock mediates leptin-regulated bone formation. Cell 122:803-815 [DOI] [PubMed] [Google Scholar]
- Griffin EA, Jr, Staknis D, Weitz CJ. (1999). Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science 286:768-771 [DOI] [PubMed] [Google Scholar]
- Gundberg CM, Markowitz ME, Mizruchi M, Rosen JF. (1985). Osteocalcin in human serum: a circadian rhythm. J Clin Endocrinol Metab 60:736-739 [DOI] [PubMed] [Google Scholar]
- Heshmati HM, Riggs BL, Burritt MF, McAlister CA, Wollan PC, Khosla S. (1998). Effects of the circadian variation in serum cortisol on markers of bone turnover and calcium homeostasis in normal postmenopausal women. J Clin Endocrinol Metab 83:751-756 [DOI] [PubMed] [Google Scholar]
- Igarashi K, Miyoshi K, Shinoda H, Saeki S, Mitani H. (1998). Diurnal variation in tooth movement in response to orthodontic force in rats. Am J OrthodDentofacial Orthop 114:8-14 [DOI] [PubMed] [Google Scholar]
- Ilizarov GA, Devyatov AA, Kamerin VK. (1980). Plastic reconstruction of longitudinal bone defects by means of compression and subsequent distraction. Acta Chir Plast 22:32-41 [PubMed] [Google Scholar]
- Iris B, Zilberman Y, Zeira E, Galun E, Honigman A, Turgeman G, et al. (2003). Molecular imaging of the skeleton: quantitative real-time bio-luminescence monitoring gene expression in bone repair and development. J Bone Miner Res 18:570-578 [DOI] [PubMed] [Google Scholar]
- Levi F, Schibler U. (2007). Circadian rhythms: mechanisms and therapeutic implications. Annu Rev Pharmacol Toxicol 47:593-628 [DOI] [PubMed] [Google Scholar]
- Lou X, Chen Y, Luo S, Chen G. (2000). [Circadian rhythm changes of the levels of endogenous insulin in growing rat condylar cartilage after functional mandibular protrusion]. Hua Xi Kou Qiang Yi Xue Za Zhi 18:252-254 (article in Chinese). [PubMed] [Google Scholar]
- Lowrey PL, Takahashi JS. (2004). Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet 5:407-441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi K, Igarashi K, Saeki S, Shinoda H, Mitani H. (2001). Tooth movement and changes in periodontal tissue in response to orthodontic force in rats vary depending on the time of day the force is applied. Eur J Orthod 23:329-338 [DOI] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, et al. (2002). Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109:307-320 [DOI] [PubMed] [Google Scholar]
- Patel MS, Elefteriou F. (2007). The new field of neuroskeletal biology. Calcif Tissue Int 80:337-347; erratum in Calcif Tissue Int 81:247, 2008 [DOI] [PubMed] [Google Scholar]
- Ptitsyn AA, Zvonic S, Conrad SA, Scott LK, Mynatt RL, Gimble JM. (2006). Circadian clocks are resounding in peripheral tissues. PLoS Comput Biol 2:e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao P, Ohtsuka-Isoya M, Shinoda H. (2003). Circadian rhythms in serum bone markers and their relation to the effect of etidronate in rats. Chronobiol Int 20:325-336 [DOI] [PubMed] [Google Scholar]
- Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, et al. (2000). Interacting molecular loops in the mammalian circadian clock. Science 288:1013-1019 [DOI] [PubMed] [Google Scholar]
- Simmons DJ, Yang C, Gundberg CM, Kidder L, Cornelissen G, Thomas M, et al. (1990). Calcitonin: chronotherapeutic effect on osteopenia in the ovariectomized rat. Prog Clin Biol Res 341(A):517-527 [PubMed] [Google Scholar]
- Sokoll LJ, Booth SL, Davidson KW, Dallal GE, Sadowski JA. (1998). Diurnal variation in total and undercarboxylated osteocalcin: influence of increased dietary phylloquinone. Calcif Tissue Int 62:447-452 [DOI] [PubMed] [Google Scholar]
- Srivastava AK, Bhattacharyya S, Li X, Mohan S, Baylink DJ. (2001). Circadian and longitudinal variation of serum C-telopeptide, osteocalcin, and skeletal alkaline phosphatase in C3H/HeJ mice. Bone 29:361-367 [DOI] [PubMed] [Google Scholar]
- Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, et al. (2002). Extensive and divergent circadian gene expression in liver and heart. Nature 417:78-83 [DOI] [PubMed] [Google Scholar]
- Thompson JF, Hayes LS, Lloyd DB. (1991). Modulation of firefly luciferase stability and impact on studies of gene regulation. Gene 103:171-177 [DOI] [PubMed] [Google Scholar]
- Yamada S, Saeki S, Takahashi I, Igarashi K, Shinoda H, Mitani H. (2002). Diurnal variation in the response of the mandible to orthopedic force. J Dent Res 81:711-715 [DOI] [PubMed] [Google Scholar]
- Ye L, Li J, Chen Y, Luo S. (2001). [Changes of osteocalcin in serum of young SD rats after functional protrusion]. HuaXi Kou Qiang Yi Xue Za Zhi 19:322-324 (article in Chinese). [PubMed] [Google Scholar]
- Zheng X, Tian W, Long J. (2003). [An experimental study on circadian rhythm of the proliferative index of mandibular osteoblast in goats]. Hua Xi Kou Qiang Yi Xue Za Zhi 21:10-12, 27 (article in Chinese). [PubMed] [Google Scholar]
- Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, Kilroy G, et al. (2006). Characterization of peripheral circadian clocks in adipose tissues. Diabetes 55:962-970 [DOI] [PubMed] [Google Scholar]
- Zvonic S, Ptitsyn AA, Kilroy G, Wu X, Conrad SA, Scott LK, et al. (2007). Circadian oscillation of gene expression in murine calvarial bone. J Bone Miner Res 22:357-365 [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Shearman LP, Weaver DR, Reppert SM. (1998). Three period homologs in mammals: differential light responses in the suprachias-matic circadian clock and oscillating transcripts outside of brain. Neuron 20:1103-1110 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.