Abstract
Fluorides are present in the environment. Excessive systemic exposure to fluorides can lead to disturbances of bone homeostasis (skeletal fluorosis) and enamel development (dental/enamel fluorosis). The severity of dental fluorosis is also dependent upon fluoride dose and the timing and duration of fluoride exposure. Fluoride’s actions on bone cells predominate as anabolic effects both in vitro and in vivo. More recently, fluoride has been shown to induce osteoclastogenesis in mice. Fluorides appear to mediate their actions through the MAPK signaling pathway and can lead to changes in gene expression, cell stress, and cell death. Different strains of inbred mice demonstrate differential physiological responses to ingested fluoride. Genetic studies in mice are capable of identifying and characterizing fluoride-responsive genetic variations. Ultimately, this can lead to the identification of at-risk human populations who are susceptible to the unwanted or potentially adverse effects of fluoride action and to the elucidation of fundamental mechanisms by which fluoride affects biomineralization.
Keywords: bone, enamel, fluoride, fluorosis, mice
Introduction
Fluorine is a common element in the earth’s crust. Fluorides are naturally present in the soil, rocks, and water throughout the world, with higher concentrations in areas where there have been recent/past pyroclastic activities or geologic uplift. Fluorides are also widely used in many industrial processes. The major sources of systemic fluoride exposure are the diet (food and water) (USDA National Fluoride Database of Selected Beverages and Foods – 2004, http://www.nal.usda.gov/fnic/foodcomp/Data/Fluoride/Fluoride.html) and fluoride-containing dental products.
Fluoridation of community drinking water to prevent dental caries is considered as one of the ten most important public health achievements of the 20th century (Achievements in Public Health, 1900-1999: Fluoridation of Drinking Water to Prevent Dental Caries, 1999). Concurrent with the decline in dental caries has been an increase in the prevalence of dental fluorosis, a side-effect of fluoride exposure. Dental fluorosis remains highly prevalent world-wide. As recently as 2005, 23% of persons in the United States aged 6 to 39 years had very mild or greater enamel fluorosis (Beltran-Aguilar et al., 2005).
Historically, biochemists and protein chemists have used fluoride in millimolar (mM) concentrations as an enzyme inhibitor (e.g., phenylmethylsulfonyl fluoride is an inhibitor of a variety of serine proteases, whereas sodium fluoride is used as a general inhibitor of protein phosphoseryl and phosphothreonyl phosphatases). Efforts to understand additional actions of fluoride in cells, tissues, and organisms have been hampered by the use of a wide range of fluoride exposures, different fluoride salts (AlFn, BeFn, or NaF), or organic moieties of fluorine. Studies involving humans have focused on fluoride alone or in combination with other molecules (i.e., calcium). Studies involving animals vary in fluoride-dosing schedules as well as routes of administration (orally or parenterally). Finally, fluoride can demonstrate biphasic actions (i.e., mitogenic at low concentrations and toxic at higher concentrations). Collectively, this has complicated our understanding of the physiological effects of fluoride. Within this decade, the interaction of an individual’s genetic background has offered new insight into fluoride’s physiological effects. This review will focus on separate but often overlapping areas of fluoride action and genetic influences in modulating an animal’s response to fluoride.
Cell Signaling, Proliferation, Stress, And Apoptosis
As alluded to above, fluoride is part of the environment, whether one considers systemic exposure in whole animals or as part of the tissue culture milieu. Mitogen-activated protein kinases (MAPKs) signaling pathways allow cells to respond to myriad extracellular stimuli. The Jun N-terminal kinase (JNK) and p38 MAPK pathways are often activated following environmental stress (Weston and Davis, 2007; Wagner and Nebreda, 2009). Activation of these pathways can lead to diverse responses, including cell proliferation, differentiation, survival, and apoptosis. Sodium fluoride appears to act predominantly through the Jun N-terminal kinase (JNK) and p38 MAPK signaling pathways (Thrane et al., 2001; Y Zhang et al., 2007; Karube et al., 2009). An alternative MAPK / ERK1 signaling pathway involving an osteoblastic fluoride-sensitive phosphotyrosyl phosphatase (PTP) has been proposed in regard to the mitogenic effects of fluoride (Thomas et al., 1996; Wu et al., 1997; Lau and Baylink, 1998). When fluoride is associated with metals like aluminum and beryllium, more complex signaling pathways appear to be evoked (Lau et al., 1991, 2002; Caverzasio et al., 1996, 1997; Susa, 1999; Misra et al., 2002; Li, 2003). Finally, in ameloblasts there is evidence that the Rho/ROCK pathway is activated by modest fluoride exposure (Li et al., 2005).
Fluoride can have deleterious effects on cells, depending upon concentration (micromolar to millimolar), duration of exposure, and cell type (primary cultured cells or established cell lines). Exposures up to 1 mM of NaF failed to induce stress-response RNAs or initiate apoptosis in the mouse odontoblast cell line M06-G3 (Wurtz et al., 2008), whereas 1 mM NaF induced oxidative stress and apoptosis in rat primary hippocampal neurons (M Zhang et al., 2007). Exposures in the 5- to 10-mM range are required to induce apoptosis in rat thymocytes and human gingival fibroblasts, rat primary lung cells, and in the odontoblast cell line MDPC-23 (Thrane et al., 2001; Matsui et al., 2007; Lee et al., 2008; Karube et al., 2009), and micromolar NaF leads to apoptosis in neonatal rat osteoblasts and fetal human ameloblast lineage cells (Q Yan et al., 2007; X Yan et al., 2009). Rats exposed to 0, 10, 50, or 100 ppm F ions (approximately 0 to 5.0 mM F ions) in the drinking water over a period of 10 wks demonstrated increases in reactive oxygen species in the blood at 50 ppm and 100 ppm, without evidence of significant oxidative stress within the brain or liver (Chouhan and Flora, 2008). In the kidney, there was increased oxidative stress only in the 100-ppm-F group. Analysis of these data together suggests tissue-specific sensitivity to oxidative stress following fluoride exposure.
Endoplasmic reticulum (ER) stress may be a component of fluoride’s effects on amelogenesis. Depending upon the chemical form and concentration, fluoride can induce ER stress (Thrane et al., 2001; Misra et al., 2002; Kubota et al., 2005; Sharma et al., 2008; Ito et al., 2009). ER stress leading to protein misfolding can result from several insults. To alleviate the accumulation of unfolded proteins in the ER lumen, the unfolded protein response (UFP) is initiated. Two primary mechanisms are in place to deal with the accumulation of unfolded/misfolded proteins: increased transcription of genes encoding the protein chaperones and folding catalysts to up-regulate the cell’s protein-folding capacity; and decreased biosynthetic burden of the secretory pathway in the cell by down-regulating expression of genes encoding secreted proteins (Rutkowski and Kaufman, 2004; Shen et al., 2004; Schroder and Kaufman, 2005). The reduction of incoming proteins appears to be mediated at the level of translation. Additionally, the UFP response leads to transcriptional up-regulation for adaptation or for apoptosis (Kaufman, 1999; Malhotra and Kaufman, 2007). Within the ER stress cascade is the activation of c-Jun amino-terminal kinases (JNKs) via mammalian homologs of yeast IRE1, which activate chaperone genes in response to ER stress (Urano et al., 2000). As secretory cells, ameloblasts may be particularly sensitive to induction of ER stress. Recently, the predominant secreted protein amelogenin has been found to bind to calnexin (a type I integral ER membrane chaperone that facilitates protein folding) in a yeast two-hybrid assay (Wang et al., 2005).
Actions On Bone And Bone Cells
Fluoride’s actions on bone appear to be mediated at several levels. Fluoride can directly interact with the bone mineral matrix physicochemically (Grynpas, 1990; Grynpas and Rey, 1992; Pak et al., 1995; Chachra et al., 1999). In vitro fluoridation of bone with mM [F] can lead to conversion of carbonated hydroxylapatite to carbonated fluorapatite, to changes in crystallinity, and to a reduction in mechanical strength properties (Silva and Ulrich, 2000; Freeman et al., 2001; DePaula et al., 2002). Fluorapatite is more stable and resistant to acid dissolution than is hydroxyapatite (Grynpas and Cheng, 1988). Fluoride also delays mineralization and is capable of altering bone crystal structure (Grynpas, 1990; Grynpas and Rey, 1992; Mousny et al., 2008).
In addition to physicochemical actions, fluoride at 10−5 M and 10−7 M can influence matrix metalloproteinases (MMPs), affecting the composition of the remodeling matrix and subsequent mineralization in a rat in vitro mineralizing bone cell culture (Waddington and Langley, 2003).
Fluoride can act on osteoblasts and osteoclasts in vivo and in vitro. NaF is an anabolic agent capable of increasing bone mass, although the mechanism for its action remains unclear (Farley et al., 1983; Hall, 1987; Aaron et al., 1991; Lau and Baylink, 1998). While NaF may increase bone mass, the newly formed bone appears to lack normal structure and strength (Carter and Beaupre, 1990; Riggs et al., 1990; Søgaard et al., 1994). In trabecular bone, fluoride results in an increase in bone volume and trabecular thickness without a concomitant increase in trabecular connectivity (Aaron et al., 1991). It is this lack of trabecular connectivity that reduces bone quality despite the increase in bone mass. These observations in humans have been extended in rodents (Søgaard et al., 1995; Turner et al., 1995). At low local concentrations such as those that occur following fluoride modification of dental implants, there is enhanced osteoblastic differentiation and interfacial bone formation concomitant with increased expression of osteogenic markers at the implant site (Cooper et al., 2006; Monjo et al., 2008). When fluoride-substituted apatite is implanted in vivo, there is an increase in new bone formation when the percentage of fluoride is low (0.5% by weight); however, when the percentage fluoride is higher (2.23% by weight), the enhanced new bone formation is abrogated (Inoue et al., 2005). High systemic fluoride exposures can lead to skeletal fluorosis, a condition hallmarked by osteosclerosis, ligament calcifications, and often accompanying osteoporosis, osteomalacia, or osteopenia (Christie, 1980; Wang et al., 2007). Skeletal fluorosis can be complicated by malnutrition (Teotia and Teotia, 2008).
Fluoride can inhibit the function of isolated osteoclasts at modest [F] 0.5- to 1.0-mM (Okuda et al., 1990) or near-physiologic [F] 15-mg/L (approximately 0.8 mM) concentrations (Taylor et al., 1989) when examined in vitro. At a range of NaF from 0.15 mg/L to 30 mg/L (approximately 0.004 mM to 0.7 mM), biphasic effects of fluoride have been described, depending upon concentration in the culture media (Taylor et al., 1990). At 15-30 mg/L, osteoclast-resorptive activity was inhibited. However, at 1 mg/L (approximately 0.050 mM), osteoclast function was enhanced. The authors were unable to determine if the enhanced osteoclast function at 1 mg/L was a direct effect or indirect via an action on residual osteoblasts present in the culture system. Systemic fluoride treatment (50 ppm F ion in the drinking water over a period of 3 wks) results in the induction of osteoclastogenesis in the C3H/HeJ (C3H) inbred mouse strain, as evidenced by an increased number of osteoclasts along the trabecular bone surface in the tibiae (D Yan et al., 2007).
Actions On Ameloblasts
Dental fluorosis (DF) is an undesirable developmental defect of tooth enamel attributed to greater-than-optimal systemic fluoride exposure during critical periods of amelogenesis. DF is characterized by increased porosity (subsurface hypomineralization) with a loss of enamel translucency and increased opacity (Fejerskov et al., 1990). It is generally accepted that increasing DF severity correlates with increasing F exposure; however, individual variation in DF severity can exist when F exposure is relatively constant in a community (Mabelya et al., 1994; Yoder et al., 1998). Ameloblasts in the maturational phase appear to be the cellular target of chronic fluoride exposure (DenBesten and Thariani, 1992), whereas acute fluoride toxicity targets the transitional and early-secretory ameloblasts (Lyaruu et al., 2006). The mechanism(s) underlying DF remain obscure, but likely contribute to the observed retention of enamel matrix proteins and may include reduced removal of enamel matrix proteins during enamel maturation, perturbation of extracellular transport, or initiation of the ER stress-response pathway (Matsuo et al., 2000; DenBesten et al., 2002; Kubota et al., 2005; Sharma et al., 2008; Bronckers et al., 2009; Everett et al., 2009). While biological factors likely play critical roles in the pathogenesis of DF, physicochemical affects should also be considered (Robinson et al., 2004).
Effects On Hematopoietic Cells And Hematopoiesis
Fluoride’s effects on hematopoietic cells/hematopoiesis have been investigated in ex vivo and in vitro studies and in different species of mammals. Effects vary depending upon fluoride dose, duration of exposure, and species, and include anemia and leukopenia (Mehdi et al., 1978; Eren et al., 2005). At modest in vitro concentrations (< 500 µM), NaF has been shown to promote the differentiation of human promyelocytic tumor cells (HL-60) to granulocyte-like cells (Kawase et al., 1996). Similar exposures of NaF (< 500 µM) can preferentially induce differentiation of primary outbred ddY mouse bone marrow cells along the granulocytic pathway in vitro (Oguro et al., 2003). These latter studies confirmed differentiation of mouse bone marrow cells along the granulocytic lineage by demonstrating the up-regulation of granulocyte-specific markers (chloroacetate esterase, cell-surface antigens [Mac-1, Gr-1]) with no change in the monocyte-specific markers (non-specific esterase, cell-surface antigens [F4/80, MOMA-2]). The authors hypothesized that any potential shift in differentiation of bone marrow progenitor cells away from the monocytic lineage may affect osteoclast formation.
Evidence that fluoride can affect the bone marrow microenvironment comes from ex vivo studies of bone marrow cells collected from several strains of mice treated with NaF in the drinking water. Strain-dependent effects on hematopoietic colony-forming cell unit (CFU) assays were observed (D Yan et al., 2007; Chou et al., 2009). Bone marrow cells leading to the individual colonies (CFU) may have pluripotent characteristics capable of giving rise to mixed colonies containing multiple hematopoietic lineages (e.g., granulocytic, monocytic, and erythroid) or are committed, giving rise to cells from one hematopoietic lineage.
Fluoride treatment of C3H mice shifted hematopoietic differentiation along the monocyte/macrophage lineage, with dose-dependent increases in the frequencies of CFU-GEMM (colony-forming unit granulocyte, erythroid, monocyte/macrophage, and megakaryocyte), CFU-GM (colony-forming unit granulocyte and monocyte/macrophage), and CFU-M (colony-forming unit monocyte/macrophage) colonies. Fluoride’s actions on the hematopoietic compartment in the B6 mouse strain differed with a modest increase in the frequency of pluripotential/mixed CFU-GEMM colonies at the highest F dose (100 ppm). The shift in hematopoietic differentiation in the C3H strain correlates with the increase in osteoclast potential observed (D Yan et al., 2007). Important precursors for osteoclasts are the CFU-GM and CFU-M.
Mice from the C3H, C57BL/6J (B6), FVB/NJ (FVB), and BALB/cByJ (BALB) strains show strain-specific responses to fluoride in the frequencies CFU-F (colony-forming unit fibroblast) and CFU-OB/Alp+ (colony-forming unit osteoblast/alkaline phosphatase positive) derived from bone marrow cells (Chou et al., 2009). The CFU-F are expanded clonal populations that emerge from single bone marrow stromal cells/mesenchymal stem cells (MSC) and possess the potential to differentiate along multiple mesenchymal cell lineages, including osteoblast precursors (Friedenstein et al., 1976; Owen and Friedenstein, 1988).
Genetic Studies And Fluoride’s Actions
Until recently, few studies have explored the underlying genetic basis for fluoride resistance or susceptibility. Among the first, high concentrations of fluoride (400 µg/mL) have been used to isolate fluoride-resistant mutants of Caenorhabditis elegans (Katsura, 1993). Genetic studies of these mutant nematodes have led to the identification of novel fluoride-resistant (flr) genes, flr1, flr3, and flr4. The flr1 gene encodes an ion channel belonging to the degenerin/epithelial sodium channel superfamily, which regulates defecation rhythm (Katsura et al., 1994; Take-Uchi et al., 1998). The flr4 gene encodes a predicted Ser/Thr protein kinase and, like flr-1, appears to control rhythmic activities in Caenorhabditis elegans (Iwasaki et al., 1995; Iwasaki and Thomas, 1997). The flr-4 gene is closely related to the human SOK1 gene, a Ste20 protein kinase of the germinal center kinase (GCK) family. The flr-3 gene remains to be characterized.
Dental Fluorosis Studies Involving Inbred Strains of Mice
Genetic studies utilizing inbred strains of mice have focused on fluoride’s action on tooth enamel development and bone homeostasis (Everett et al., 2002, 2009; Vieira et al., 2005; Mousny et al., 2006, 2008; D Yan et al., 2007; Carvalho et al., 2009; Chou et al., 2009). Inbred mouse strains have been used for genetic studies because of the isogenicity within a strain and the genetic heterogeneity between inbred strains. The genetic diversity existing between inbred strains of mice has yielded phenotypes relevant to human health, such as cancer susceptibility, aging, obesity, susceptibility to infectious diseases, atherosclerosis, blood disorders, and neurosensory disorders (Bogue and Grubb, 2004; Bogue et al., 2007; Grubb et al., 2009). Humans and mice differ in their dental formulae, and mouse incisors continuously erupt. Despite these differences, mice have been instrumental in our understanding of the important cellular, molecular, and genetic processes controlling odontogenesis. In addition to the genetic diversity between inbred strains of mice, the continuously erupting incisors (active amelogenesis) facilitate the investigation of fluoride’s effects on tooth enamel development at any time during the animal’s life. Strain-dependent responses to fluoride in the development of dental fluorosis were first demonstrated across 12 inbred strains and the severity of dental fluorosis based upon clinical criteria (tooth enamel appearance) (Everett et al., 2002). Genetic diversity and availability were factors in the selection of these 12 strains. From that study, strains clustered into three dental fluorosis groups: resistant strains (129P3/J, FVB/NJ, CBA/J, and DBA/1J); intermediate strains (SWR/J, BALB/cByJ, C57BL/10J, and DBA/2J); and sensitive strains (A/J, SJL/J/ C3H/HeJ, and C57BL/6J). Examples of the variation in dental fluorosis severity are illustrated in Fig. 1. As in humans with DF, clinical criteria can be used to score DF in mice. Since mouse incisors are worn away as they erupt, the DF observed does not reach the deeply pitted and characteristic brown staining seen with severe DF in humans. A modified Thylstrup and Fejerskov (TF) scale can be used to score dental fluorosis in mice (Everett et al., 2002, 2009). Alternatively, a modification of quantitative light-induced fluorescence (QLF) (Everett et al., 2002; Vieira et al., 2005) can be used to provide a more objective means to score dental fluorosis (Fig. 2). Strains (129P3/J, SWR/J, and A/J) representing the three dental fluorosis groups described above were used to show that genetic factors (DF severity) and the environmental factor (fluoride concentration in tooth structure) have similar influence on tooth biomechanical properties, whereas only the environmental factor has an influence on tooth material properties (mineralization) (Vieira et al., 2005). Fluoride metabolism also differs between and among mouse strains (Carvalho et al., 2009). Whereas the A/J strain consumes more drinking water and required adjustment in [F] to maintain comparable exposure between the two strains, the 129P3/J strain retains more fluoride in the bone and has higher plasma fluoride levels. Despite this important difference, the 129P3/J strain remains resistant to the development of dental fluorosis.
Figure 1.
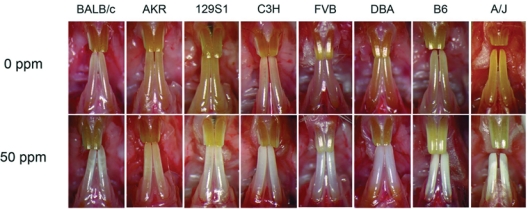
Variation in dental fluorosis severity among inbred strains of mice. Mice at 5 to 6 wks of age were treated with fluoride (0 ppm or 50 ppm [F] ion) in the drinking water for 60 days. All strains developed dental fluorosis. Dental-fluorosis-susceptible strains are on the right, with those more resistant strains on the left side of the panel.
Figure 2.
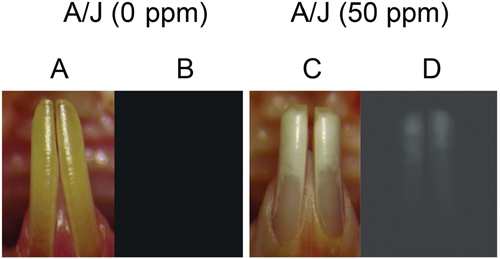
Use of quantitative fluorescence to assess dental fluorosis. Panels A and C are clinical images of the mandibular incisors from A/J mice treated with control (0 ppm [F]), Panel A; and 50 ppm [F], Panel C) for 60 days. Panels B and D demonstrate the results of quantitative fluorescence (QF), where a Nikon epifluorescence microscope equipped with a Chroma Gold 11006v2 set cube (Spectra Services Inc., Ontario, NY, USA) (exciter D360/40x, dichroic 400DCLP, and emitter E515LPv2) was used to assess the severity of dental fluorosis. Increased fluorescence is associated with increased dental fluorosis severity.
Resistance and susceptibility (risk factors), defined by host and environment interactions, as well as many quantitative phenotypes are considered complex traits. Complex traits (phenotypes) can be assessed quantitatively and are under the control of multiple genes as well as non-genetic (environmental) factors. Multiple genes that contribute to the variation in a phenotypic trait are called quantitative trait loci (QTL). QTLs can be mapped in mice by traditional genetic approaches. Typically, two strains are selected that have widely different traits or responses. The parental mice are then used in a two-generation cross. First F1 hybrid progeny are generated, then used in sister-brother mating to produce F2 mice (Fig. 3). While all F1 mice are genetically identical, each F2 mouse is unique. This is the result of re-arrangement of the parental alleles during gametogenesis (meiotic recombination) in the F1 animals. Mapping of QTLs associated with DF susceptibility was performed with a dental-fluorosis-resistant strain (129P3/J) and the dental-fluorosis-sensitive (A/J) strain in a two-generation cross to create a panel of F2 mice as described above. All F2 mice were treated with fluoride 50 ppm F in the drinking water and, after 60 days, were phenotyped for DF according to the modified TF scale. Treatment of F2 mice with 50 ppm F in the water yields a mean serum [F] of 12.366 ± 1.713 µM. The serum [F] concentrations between F2 mice with different DF severities were not significantly different and were not significantly different from serum [F] concentrations determined in comparably treated parental mice (11.296 ± 3.984 µM). To maximize the power to detect QTLs contributing to the variation in response to dental fluorosis, only the phenotypic extreme F2 animals (those with TF scores of 1 or 4) were genotyped for 354 SNP-based markers distributed throughout the mouse genome. This panel of mice was composed of equal numbers of males and females. Chi-square analysis was performed to compare the genotypic distributions in the two groups of phenotypically extreme F2 mice. Significant evidence of association was observed on chromosomes 2 and 11 for a series of consecutive markers (p < 0.0001) (Everett et al., 2009). More importantly, there was a lack of significant association on murine chromosomes X, 3, 5, 7, or 9, suggesting little role for amelogenin, ameloblastin, enamelin, amelotin, Klk-4, or Mmp20 in dental fluorosis susceptibility/resistance in this animal model. As illustrated above, the detection and mapping of QTL are straightforward in mice.
Figure 3.
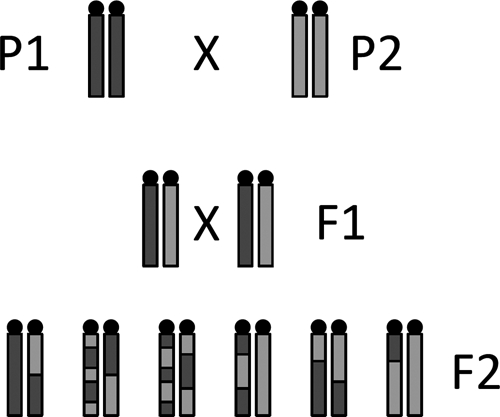
Schematic using inbred progenitor strains in a two-generation cross to produce a panel of F2 progeny. P1 and P2 are progenitor/parental strains that differ in a particular trait of response. The F1 progeny (1st generation) are all genetically identical, having inherited half their genome from P1 and half their genome from P2. The F2 progeny (2nd generation) are composed of genetically unique individuals.
Narrowing the QTL intervals to fewer genes and, ultimately, the selection of candidate genes remain the challenging aspects of complex trait dissection. This can be accomplished in mice by increasing marker densities within QTLs and using a complementary approach based upon haplotype mapping. Haplotype Association Mapping (HAM) is a phenotype-driven approach to identify genetic loci in mice. This method is similar to Genome-Wide Association Studies (GWAS) in humans. HAM looks for associations between the phenotype and the haplotypes of mouse inbred strains, treating inbred strains as individuals. Since mice within the strain are isogenic, several individuals can be phenotyped to minimize intra-strain variation. The application of haplotype association mapping in mice was first described in 2001 and has developed into a useful tool for QTL mapping (Grupe et al., 2001; Tsaih and Korstanje, 2009). Integrating haplotype-based approaches with traditional mapping tools as described above has great potential for narrowing QTL mapping intervals and prioritizing candidate genes (Pletcher and Wiltshire, 2004; Cervino et al., 2005, 2007; Arbilly et al., 2006). It is conceivable that interval-specific haplotype analysis based upon an a priori knowledge of a QTL interval can reach a resolution of less than 5 Mb (DiPetrillo et al., 2005). Recently, haplotype association mapping in mice identified a haplotype block containing the Cer1 (cerberus 1 homolog) gene that partitions inbred mice strains into high and low bone mineral density groups. The Cer1 gene is important during embryonic development and appears to play a role in bone development. Based upon the discovery in mice, the human CER1 gene was investigated, and a non-synonymous SNP (rs3747532) was identified to be associated with increased risk of low bone mineral density in pre-menopausal women (Tang et al., 2009).
QTL linkage studies in mice differ from QTL mapping in humans because many human QTL linkage studies are limited in sample size and do not have the family pedigrees that maximize the power to detect linkage (Almasy and Blangero, 2009). High-throughput genotyping and advanced computational analyses have led to the application of genome-wide association studies (GWAS) as a tool for mapping human disease genes (Hindorff et al., 2009). As of December 2009, there have been 658 published genome-wide association studies in humans (Hindorff et al., 2010).
Bone and Bone Biology Studies Involving Inbred Strains of Mice
Strain-specific responses to fluoride also include effects on bone mineral, bone micro-architecture, and bone cell biology. Bone quality as a function of mechanical properties varies among three inbred strains (A/J, SWR/J, and 129P3/J) following fluoride exposure (Mousny et al., 2006). The A/J strain demonstrated the greatest reduction in bone quality in a fluoride dose-dependent manner. The SWR/J strain demonstrated a modest reduction in bone quality, and the 129P3/J strain was refractory to the effects of fluoride. In an effort to better understand the changes in bone quality observed above, investigators assessed the effects of fluoride on bone formation and mineralization (Mousny et al., 2008). All three strains demonstrated an increase in osteoid with the vertebral bodies, which did not translate to increases in bone volume of mineralization.
Mice representing the C3H and B6 inbred strains have been particularly useful in studies of bone biology. In a landmark paper, Beamer and co-workers (1996) investigated peak bone mass in a variety of inbred strains of mice, and with respect to strain differences, the highest value for any given bone parameter was found in the C3H strain, whereas B6 values were absolutely, or statistically, the lowest. These two strains have become the foundation for many investigations contributing to more than 76 scientific articles in the literature, with the majority resulting from studies of bone cells and bone biology. As genetic tools, B6 and C3H mice have contributed to the identification of several quantitative trait loci (QTLs) implicated in determining peak bone mass (Beamer et al., 2001; Koller et al., 2003; Bouxsein et al., 2004). This formed the basis for the selection of C3H and B6 strains for studies involving fluoride. Exposure to fluoride at levels relevant to what might be experienced in humans (Srivastava et al., 1989; Riggs et al., 1990) was achieved by treatment of mice with NaF for 3 wks, achieving mean serum [F] of 8 µM (50 ppm F in the water) or 15 µM (100 ppm F in the water).
As discussed above, fluoride can have effects on the bone marrow microenvironment. The bone marrow provides a source for osteoprogenitors as well as osteoclast precursors. In addition to the increased osteoclast potential in C3H mice following fluoride treatment, there are concurrent changes in markers for markers of osteoclast activity in vivo (D Yan et al., 2007). Fluoride induces intact parathyroid hormone (iPTH), soluble receptor activator for nuclear factor kappa B ligand (RANKL), and osteoclast-specific tartrate-resistant acid phosphatase (Trap5b) in the serum of C3H mice. PTH conveys effects on osteoblast cells, which in turn produce factors like RANKL. PTH is an important early step in osteoclastogenesis. It is also worth noting that there have been repeated associations between increased fluoride intake and increases in circulating PTH and hyperparathyroidism (Sivakumar and Krishnamachari, 1976; Ream and Principato, 1981; Gupta et al., 2001; Chadha and Kumar, 2004). Stronger evidence of enhanced osteoclastogenesis is the increase in the number of osteoclasts along the bone surfaces in C3H mice. B6 mice respond quite oppositely (D Yan et al., 2007). In this strain, the anabolic actions of fluoride are favored. There is an increase in total alkaline phosphatase (ALP) in the serum, with a concomitant decrease in the pro-osteoclastogenesis markers iPTH and RANKL. Finally, there is a modest increase in trabecular bone BMD and reduction in bone quality in B6 mice. A summary of the differential actions of fluoride on B6 and C3H mice is shown in Fig. 4.
Figure 4.
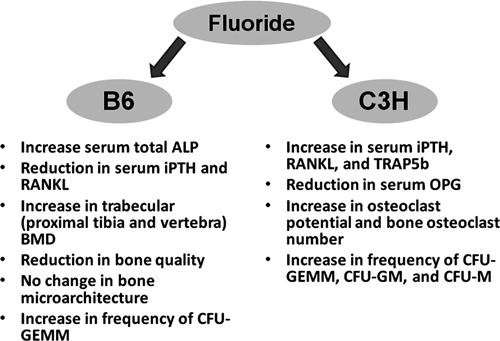
Dichotomy of fluoride responses between the high-bone-mass C3H/HeJ and low-bone-mass C57BL/6J inbred strains. The C57BL/6J strain favors the anabolic actions of fluoride, whereas the C3H/HeJ strain demonstrates enhanced osteoclastogenesis.
As mentioned earlier, C3H and B6 mice differ in their peak bone mass. Many other notable differences in bone and bone biology exist between these two strains. In particular, bone adaptation responses in B6 mice are greater than those in C3H (Akhter et al., 1998, 2002; Amblard et al., 2003), and bone loss due to disuse is also greater in B6 mice (Judex et al., 2004). C3H mice operate at a high-bone-mass production mode, where perhaps the anabolic actions of fluoride are not favored as in B6 mice. Mapping studies similar to that undertaken to identify DF-associated QTLs can be undertaken with B6 and C3H mice as progenitor strains to identify QTLs associated with fluoride’s actions on osteoclastogenesis.
Summary
Fluoride in various chemical forms, doses, and exposures has physicochemical and biologic effects on cells and tissues. A narrow therapeutic/toxicity window and biphasic actions further complicate our understanding of fluoride’s effects. Fluorides mediate their actions through MAPK signaling pathways, leading to changes in gene expression, cell stress, and even cell death. Fluorides can lead to a diverse collection of responses affecting biomineralization. The role that an individual’s genetic background plays in modulating fluoride’s actions is becoming more evident and will allow for the investigation of gene-gene and gene-environment interactions capable of modifying the function(s) of fluoride-responsive genetic variants in an animal model. This in turn will provide a better understanding of the effects of fluoride on human bone, bone cells, and tooth enamel development. Future studies will likely focus on identifying and characterizing fluoride-responsive genetic variations (e.g., polymorphisms), and on identifying those at-risk human populations who are susceptible to the unwanted or potentially adverse effects of fluoride action, and, finally, on elucidation of the fundamental mechanisms by which fluoride affects biomineralization.
Acknowledgments
This work was supported by the NIH/NIDCR R01-DE018104 and R01-DE014853.
References
- Aaron JE, de Vernejoul MC, Kanis JA. (1991). The effect of sodium fluoride on trabecular architecture. Bone 12:307-310 [DOI] [PubMed] [Google Scholar]
- Akhter MP, Cullen DM, Pedersen EA, Kimmel DB, Recker RR. (1998). Bone response to in vivo mechanical loading in two breeds of mice. Calcif Tissue Int 63:442-449 [DOI] [PubMed] [Google Scholar]
- Akhter MP, Cullen DM, Recker RR. (2002). Bone adaptation response to sham and bending stimuli in mice. J Clin Densitom 5:207-216 [DOI] [PubMed] [Google Scholar]
- Almasy L, Blangero J. (2009). Human QTL linkage mapping. Genetica 136:333-340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amblard D, Lafage-Proust MH, Laib A, Thomas T, Rüegsegger P, Alexandre C, et al. (2003). Tail suspension induces bone loss in skeletally mature mice in the C57BL/6J strain but not in the C3H/HeJ strain. J Bone Miner Res 18:561-569 [DOI] [PubMed] [Google Scholar]
- Arbilly M, Pisanté A, Devor M, Darvasi A. (2006). An integrative approach for the identification of quantitative trait loci. Anim Genet 37(Suppl 1):7-9 [DOI] [PubMed] [Google Scholar]
- Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. (1996). Genetic variability in adult bone density among inbred strains of mice. Bone 18:397-403 [DOI] [PubMed] [Google Scholar]
- Beamer WG, Shultz KL, Donahue LR, Churchill GA, Sen S, Wergedal JR, et al. (2001). Quantitative trait loci for femoral and lumbar vertebral bone mineral density in C57BL/6J and C3H/HeJ inbred strains of mice. J Bone Miner Res 16:1195-1206 [DOI] [PubMed] [Google Scholar]
- Beltran-Aguilar ED, Barker LK, Canto MT, Dye BA, Gooch BF, Griffin SO, et al. (2005). Surveillance for dental caries, dental sealants, tooth retention, edentulism, and enamel fluorosis—United States, 1988-1994 and 1999-2002. MMWR Surveill Summ 54:1-43 [PubMed] [Google Scholar]
- Bogue MA, Grubb SC. (2004). The mouse phenome project. Genetica 122:71-74 [DOI] [PubMed] [Google Scholar]
- Bogue MA, Grubb SC, Maddatu TP, Bult CJ. (2007). Mouse phenome database (MPD). Nucleic Acids Res 35(Database issue):D643-D649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouxsein ML, Uchiyama T, Rosen CJ, Shultz KL, Donahue LR, Turner CH, et al. (2004). Mapping quantitative trait loci for vertebral trabecular bone volume fraction and microarchitecture in mice. J Bone Miner Res 19:587-599 [DOI] [PubMed] [Google Scholar]
- Bronckers AL, Lyaruu DM, DenBesten PK. (2009). The impact of fluoride on ameloblasts and the mechanisms of enamel fluorosis. J Dent Res 88:877-893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter DR, Beaupre GS. (1990). Effects of fluoride treatment on bone strength. J Bone Miner Res 5(Suppl 1):177-184 [DOI] [PubMed] [Google Scholar]
- Carvalho JG, Leite AL, Yan D, Everett ET, Whitford GM, Buzalaf MA. (2009). Influence of genetic background on fluoride metabolism in mice. J Dent Res 88:1054-1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caverzasio J, Imai T, Ammann P, Burgener D, Bonjour JP. (1996). Aluminum potentiates the effect of fluoride on tyrosine phosphorylation and osteoblast replication in vitro and bone mass in vivo. J Bone Miner Res 11:46-55 [DOI] [PubMed] [Google Scholar]
- Caverzasio J, Palmer G, Suzuki A, Bonjour JP. (1997). Mechanism of the mitogenic effect of fluoride on osteoblast-like cells: evidences for a G protein-dependent tyrosine phosphorylation process. J Bone Miner Res 12:1975-1983 [DOI] [PubMed] [Google Scholar]
- Cervino AC, Li G, Edwards S, Zhu J, Laurie C, Tokiwa G, et al. (2005). Integrating QTL and high-density SNP analyses in mice to identify Insig2 as a susceptibility gene for plasma cholesterol levels. Genomics 86:505-517 [DOI] [PubMed] [Google Scholar]
- Cervino AC, Darvasi A, Fallahi M, Mader CC, Tsinoremas NF. (2007). An integrated in-silico gene mapping strategy in inbred mice. Genetics 175:321-333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chachra D, Turner CH, Dunipace AJ, Grynpas MD. (1999). The effect of fluoride treatment on bone mineral in rabbits. Calcif Tissue Int 64:345-351 [DOI] [PubMed] [Google Scholar]
- Chadha M, Kumar S. (2004). Fluorosis-induced hyperparathyroidism mimicking a giant-cell tumour of the femur. J Bone Joint Surg Br 86:594-596 [PubMed] [Google Scholar]
- Chou MY, Yan D, Jafarov T, Everett ET. (2009). Modulation of murine bone marrow-derived CFU-F and CFU-OB by in vivo bisphosphonate and fluoride treatments. Orthod Craniofac Res 12:141-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouhan S, Flora SJ. (2008). Effects of fluoride on the tissue oxidative stress and apoptosis in rats: biochemical assays supported by IR spectroscopy data. Toxicology 254:61-67 [DOI] [PubMed] [Google Scholar]
- Christie DP. (1980). The spectrum of radiographic bone changes in children with fluorosis. Radiology 136:85-90 [DOI] [PubMed] [Google Scholar]
- Cooper LF, Zhou Y, Takebe J, Guo J, Abron A, Holmen A, et al. (2006). Fluoride modification effects on osteoblast behavior and bone formation at TiO2 grit-blasted c.p. titanium endosseous implants. Biomaterials 27:926-936 [DOI] [PubMed] [Google Scholar]
- DenBesten PK, Thariani H. (1992). Biological mechanisms of fluorosis and level and timing of systemic exposure to fluoride with respect to fluorosis. J Dent Res 71:1238-1243 [DOI] [PubMed] [Google Scholar]
- DenBesten PK, Yan Y, Featherstone JD, Hilton JF, Smith CE, Li W. (2002). Effects of fluoride on rat dental enamel matrix proteinases. Arch Oral Biol 47:763-770 [DOI] [PubMed] [Google Scholar]
- DePaula CA, Abjornson C, Pan Y, Kotha SP, Koike K, Guzelsu N. (2002). Changing the structurally effective mineral content of bone with in vitro fluoride treatment. J Biomech 35:355-361 [DOI] [PubMed] [Google Scholar]
- DiPetrillo K, Wang X, Stylianou IM, Paigen B. (2005). Bioinformatics toolbox for narrowing rodent quantitative trait loci. Trends Genet 21:683-692 [DOI] [PubMed] [Google Scholar]
- Eren E, Ozturk M, Mumcu EF, Canatan D. (2005). Fluorosis and its hematological effects. Toxicol Ind Health 21:255-258 [DOI] [PubMed] [Google Scholar]
- Everett ET, McHenry MA, Reynolds N, Eggertsson H, Sullivan J, Kantmann C, et al. (2002). Dental fluorosis: variability among different inbred mouse strains. J Dent Res 81:794-798 [DOI] [PubMed] [Google Scholar]
- Everett ET, Yan D, Weaver M, Liu L, Foroud T, Martinez-Mier EA. (2009). Detection of dental fluorosis-associated quantitative trait loci on mouse chromosomes 2 and 11. Cells Tissues Organs 189:212-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley JR, Wergedal JE, Baylink DJ. (1983). Fluoride directly stimulates proliferation and alkaline phosphatase activity of bone-forming cells. Science 222:330-332 [DOI] [PubMed] [Google Scholar]
- Fejerskov O, Manji F, Baelum V. (1990). The nature and mechanisms of dental fluorosis in man. J Dent Res 69(Spec Iss):692-700 [DOI] [PubMed] [Google Scholar]
- Freeman JJ, Wopenka B, Silva MJ, Pasteris JD. (2001). Raman spectroscopic detection of changes in bioapatite in mouse femora as a function of age and in vitro fluoride treatment. Calcif Tissue Int 68:156-162 [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Gorskaja JF, Kulagina NN. (1976). Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol 4:267-274 [PubMed] [Google Scholar]
- Grubb SC, Maddatu TP, Bult CJ, Bogue MA. (2009). Mouse phenome database. Nucleic Acids Res 37(Database issue):D720-D730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe A, Germer S, Usuka J, Aud D, Belknap JK, Klein RF, et al. (2001). In silico mapping of complex disease-related traits in mice. Science 292:1915-1918 [DOI] [PubMed] [Google Scholar]
- Grynpas MD. (1990). Fluoride effects on bone crystals. J Bone Miner Res 5(Suppl 1):169-175 [DOI] [PubMed] [Google Scholar]
- Grynpas MD, Cheng PT. (1988). Fluoride reduces the rate of dissolution of bone. Bone Miner 5:1-9 [DOI] [PubMed] [Google Scholar]
- Grynpas MD, Rey C. (1992). The effect of fluoride treatment on bone mineral crystals in the rat. Bone 13:423-429 [DOI] [PubMed] [Google Scholar]
- Gupta SK, Khan TI, Gupta RC, Gupta AB, Gupta KC, Jain P, et al. (2001). Compensatory hyperparathyroidism following high fluoride ingestion - a clinico - biochemical correlation. Indian Pediatr 38:139-146 [PubMed] [Google Scholar]
- Hall BK. (1987). Sodium fluoride as an initiator of osteogenesis from embryonic mesenchyme in vitro. Bone 8:111-116 [DOI] [PubMed] [Google Scholar]
- Hindorff LA, Sethupathy P, Junkins HA, Ramos EM, Mehta JP, Collins FS, et al. (2009). Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci USA 106:9362-9367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindorff LA, Junkins HA, Mehta JP, Manolio TA. (2010). A catalog of published genome-wide association studies. Available at: www.genome.gov/gwastudies (URL accessed 08/25/2010).
- Inoue M, Nagatsuka H, Tsujigiwa H, LeGeros RZ, Yamamoto T, Nagai N. (2005). In vivo effect of fluoride-substituted apatite on rat bone. Dent Mater J 24:398-402 [DOI] [PubMed] [Google Scholar]
- Ito M, Nakagawa H, Okada T, Miyazaki S, Matsuo S. (2009). ER-stress caused by accumulated intracistanal granules activates autophagy through a different signal pathway from unfolded protein response in exocrine pancreas cells of rats exposed to fluoride. Arch Toxicol 83:151-159 [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Thomas JH. (1997). Genetics in rhythm. Trends Genet 13:111-115 [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Liu DW, Thomas JH. (1995). Genes that control a temperature-compensated ultradian clock in Caenorhabditis elegans. Proc Natl Acad Sci USA 92:10317-10321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judex S, Garman R, Squire M, Busa B, Donahue LR, Rubin C. (2004). Genetically linked site-specificity of disuse osteoporosis. J Bone Miner Res 19:607-613 [DOI] [PubMed] [Google Scholar]
- Karube H, Nishitai G, Inageda K, Kurosu H, Matsuoka M. (2009). NaF activates MAPKs and induces apoptosis in odontoblast-like cells. J Dent Res 88:461-465 [DOI] [PubMed] [Google Scholar]
- Katsura I. (1993). In search of new mutants in cell-signaling systems of the nematode Caenorhabditis elegans. Review. Genetica 88:137-146 [DOI] [PubMed] [Google Scholar]
- Katsura I, Kondo K, Amano T, Ishihara T, Kawakami M. (1994). Isolation, characterization and epistasis of fluoride-resistant mutants of Caenorhabditis elegans. Genetics 136:145-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman RJ. (1999). Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev 13:1211-1233 [DOI] [PubMed] [Google Scholar]
- Kawase T, Oguro A, Orikasa M, Burns DM. (1996). Characteristics of NaF-induced differentiation of HL-60 cells. J Bone Miner Res 11:1676-1687 [DOI] [PubMed] [Google Scholar]
- Koller DL, Schriefer J, Sun Q, Shultz KL, Donahue LR, Rosen CJ, et al. (2003). Genetic effects for femoral biomechanics, structure, and density in C57BL/6J and C3H/HeJ inbred mouse strains. J Bone Miner Res 18:1758-1765 [DOI] [PubMed] [Google Scholar]
- Kubota K, Lee DH, Tsuchiya M, Young CS, Everett ET, Martinez-Mier EA, et al. (2005). Fluoride induces endoplasmic reticulum stress in ameloblasts responsible for dental enamel formation. J Biol Chem 280:23194-23202 [DOI] [PubMed] [Google Scholar]
- Lau KH, Baylink DJ. (1998). Molecular mechanism of action of fluoride on bone cells. J Bone Miner Res 13:1660-1667 [DOI] [PubMed] [Google Scholar]
- Lau KH, Yoo A, Wang SP. (1991). Aluminum stimulates the proliferation and differentiation of osteoblasts in vitro by a mechanism that is different from fluoride. Mol Cell Biochem 105:93-105 [DOI] [PubMed] [Google Scholar]
- Lau KH, Goodwin C, Arias M, Mohan S, Baylink DJ. (2002). Bone cell mitogenic action of fluoroaluminate and aluminum fluoride but not that of sodium fluoride involves upregulation of the insulin-like growth factor system. Bone 30:705-711 [DOI] [PubMed] [Google Scholar]
- Lee JH, Jung JY, Jeong YJ, Park JH, Yang KH, Choi NK, et al. (2008). Involvement of both mitochondrial- and death receptor-dependent apoptotic pathways regulated by Bcl-2 family in sodium fluoride-induced apoptosis of the human gingival fibroblasts. Toxicology 243:340-347 [DOI] [PubMed] [Google Scholar]
- Li L. (2003). The biochemistry and physiology of metallic fluoride: action, mechanism, and implications. Crit Rev Oral Biol Med 14:100-114 [DOI] [PubMed] [Google Scholar]
- Li Y, Decker S, Yuan ZA, DenBesten PK, Aragon MA, Jordan-Sciutto K, et al. (2005). Effects of sodium fluoride on the actin cytoskeleton of murine ameloblasts. Arch Oral Biol 50:681-688 [DOI] [PubMed] [Google Scholar]
- Lyaruu DM, Bervoets TJ, Bronckers AL. (2006). Short exposure to high levels of fluoride induces stage-dependent structural changes in ameloblasts and enamel mineralization. Eur J Oral Sci 114(Suppl 1):111-115 [DOI] [PubMed] [Google Scholar]
- Mabelya L, van’t Hof MA, König KG, van Palenstein Helderman WH. (1994). Comparison of two indices of dental fluorosis in low, moderate and high fluorosis Tanzanian populations. Community Dent Oral Epidemiol 22:415-420 [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. (2007). The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol 18:716-731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H, Morimoto M, Horimoto K, Nishimura Y. (2007). Some characteristics of fluoride-induced cell death in rat thymocytes: cytotoxicity of sodium fluoride. Toxicol In Vitro 21:1113-1120 [DOI] [PubMed] [Google Scholar]
- Matsuo S, Nakagawa H, Kiyomiya K, Kurebe M. (2000). Fluoride-induced ultrastructural changes in exocrine pancreas cells of rats: fluoride disrupts the export of zymogens from the rough endoplasmic reticulum (rER). Arch Toxicol 73:611-617 [DOI] [PubMed] [Google Scholar]
- Mehdi AW, Ridha MT, Al-Kafawi AA, Injidi MH. (1978). Effect of high fluoride intake on haematological aspects of the mouse. Q J Exp Physiol Cogn Med Sci 63:83-88 [DOI] [PubMed] [Google Scholar]
- Misra UK, Gawdi G, Pizzo SV. (2002). Beryllium fluoride-induced cell proliferation: a process requiring P21(ras)-dependent activated signal transduction and NF-kappaB-dependent gene regulation. J Leukoc Biol 71:487-494 [PubMed] [Google Scholar]
- Monjo M, Lamolle SF, Lyngstadaas SP, Ronold HJ, Ellingsen JE. (2008). In vivo expression of osteogenic markers and bone mineral density at the surface of fluoride-modified titanium implants. Biomaterials 29:3771-3780 [DOI] [PubMed] [Google Scholar]
- Mousny M, Banse X, Wise L, Everett ET, Hancock R, Vieth R, et al. (2006). The genetic influence on bone susceptibility to fluoride. Bone 39:1283-1289 [DOI] [PubMed] [Google Scholar]
- Mousny M, Omelon S, Wise L, Everett ET, Dumitriu M, Holmyard DP, et al. (2008). Fluoride effects on bone formation and mineralization are influenced by genetics. Bone 43:1067-1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- No Author Listed (1999). Achievements in public health, 1900-1999: Fluoridation of drinking water to prevent dental caries. MMWR Morb Mortal Wkly Rep 48:933-940 [Google Scholar]
- Oguro A, Kawase T, Orikasa M. (2003). NaF induces early differentiation of murine bone marrow cells along the granulocytic pathway, but not the monocytic or pre-osteoclastic pathway, in vitro. In Vitro Cell Dev Biol Anim 39:243-248 [DOI] [PubMed] [Google Scholar]
- Okuda A, Kanehisa J, Heersche JN. (1990). The effects of sodium fluoride on the resorptive activity of isolated osteoclasts. J Bone Miner Res 5(Suppl 1):115-120 [DOI] [PubMed] [Google Scholar]
- Owen M, Friedenstein AJ. (1988). Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp 136:42-60 [DOI] [PubMed] [Google Scholar]
- Pak CY, Zerwekh JE, Antich P. (1995). Anabolic effects of fluoride on bone. Trends Endocrinol Metab 6:229-234 [DOI] [PubMed] [Google Scholar]
- Pletcher M, Wiltshire T. (2004). Can we find the genes involved in complex traits? Genome Biol 5:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ream LJ, Principato R. (1981). Fluoride stimulation of the rat parathyroid gland: an ultrastructural study. Am J Anat 162:233-241 [DOI] [PubMed] [Google Scholar]
- Riggs BL, Hodgson SF, O’Fallon WM, Chao EY, Wahner HW, Muhs JM, et al. (1990). Effect of fluoride treatment on the fracture rate in postmenopausal women with osteoporosis. N Engl J Med 322:802-809 [DOI] [PubMed] [Google Scholar]
- Robinson C, Connell S, Kirkham J, Brookes SJ, Shore RC, Smith AM. (2004). The effect of fluoride on the developing tooth. Caries Res 38:268-276 [DOI] [PubMed] [Google Scholar]
- Rutkowski DT, Kaufman RJ. (2004). A trip to the ER: coping with stress. Trends Cell Biol 14:20-28 [DOI] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ. (2005). ER stress and the unfolded protein response. Mutat Res 569:29-63 [DOI] [PubMed] [Google Scholar]
- Sharma R, Tsuchiya M, Bartlett JD. (2008). Fluoride induces endoplasmic reticulum stress and inhibits protein synthesis and secretion. Environ Health Perspect 116:1142-1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Zhang K, Kaufman RJ. (2004). The unfolded protein response—a stress signaling pathway of the endoplasmic reticulum. J Chem Neuroanat 28:79-92 [DOI] [PubMed] [Google Scholar]
- Silva MJ, Ulrich SR. (2000). In vitro sodium fluoride exposure decreases torsional and bending strength and increases ductility of mouse femora. J Biomech 33:231-234 [DOI] [PubMed] [Google Scholar]
- Sivakumar B, Krishnamachari KA. (1976). Circulating levels of immunoreactive parathyroid hormone in endemic genu valgum. Horm Metab Res 8:317-319 [DOI] [PubMed] [Google Scholar]
- Søgaard CH, Mosekilde L, Richards A, Mosekilde L. (1994). Marked decrease in trabecular bone quality after five years of sodium fluoride therapy—assessed by biomechanical testing of iliac crest bone biopsies in osteoporotic patients. Bone 15:393-399 [DOI] [PubMed] [Google Scholar]
- Søgaard CH, Mosekilde L, Schwartz W, Leidig G, Minne HW, Ziegler R. (1995). Effects of fluoride on rat vertebral body biomechanical competence and bone mass. Bone 16:163-169 [DOI] [PubMed] [Google Scholar]
- Srivastava RN, Gill DS, Moudgil A, Menon RK, Thomas M, Dandona P. (1989). Normal ionized calcium, parathyroid hypersecretion, and elevated osteocalcin in a family with fluorosis. Metabolism 38:120-124 [DOI] [PubMed] [Google Scholar]
- Susa M. (1999). Heterotrimeric G proteins as fluoride targets in bone (review). Int J Mol Med 3:115-126 [DOI] [PubMed] [Google Scholar]
- Take-Uchi M, Kawakami M, Ishihara T, Amano T, Kondo K, Katsura I. (1998). An ion channel of the degenerin/epithelial sodium channel superfamily controls the defecation rhythm in Caenorhabditis elegans. Proc Natl Acad Sci USA 95:11775-11780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang PL, Cheung CL, Sham PC, McClurg P, Lee B, Chan SY, et al. (2009). Genome-wide haplotype association mapping in mice identifies a genetic variant in CER1 associated with BMD and fracture in southern Chinese women. J Bone Miner Res 24:1013-1021 [DOI] [PubMed] [Google Scholar]
- Taylor ML, Boyde A, Jones SJ. (1989). The effect of fluoride on the patterns of adherence of osteoclasts cultured on and resorbing dentine: a 3-D assessment of vinculin-labelled cells using confocal optical microscopy. Anat Embryol (Berl) 180:427-435 [DOI] [PubMed] [Google Scholar]
- Taylor ML, Maconnachie E, Frank K, Boyde A, Jones SJ. (1990). The effect of fluoride on the resorption of dentine by osteoclasts in vitro. J Bone Miner Res 5(Suppl 1):121-130 [DOI] [PubMed] [Google Scholar]
- Teotia M, Teotia SP. (2008). Nutritional bone disease in Indian population. Indian J Med Res 127:219-228 [PubMed] [Google Scholar]
- Thomas AB, Hashimoto H, Baylink DJ, Lau KH. (1996). Fluoride at mitogenic concentrations increases the steady state phosphotyrosyl phosphorylation level of cellular proteins in human bone cells. J Clin Endocrinol Metab 81:2570-2578 [DOI] [PubMed] [Google Scholar]
- Thrane EV, Refsnes M, Thoresen GH, Lag M, Schwarze PE. (2001). Fluoride-induced apoptosis in epithelial lung cells involves activation of MAP kinases p38 and possibly JNK. Toxicol Sci 61:83-91 [DOI] [PubMed] [Google Scholar]
- Tsaih SW, Korstanje R. (2009). Haplotype association mapping in mice. Methods Mol Biol 573:213-222 [DOI] [PubMed] [Google Scholar]
- Turner CH, Hasegawa K, Zhang W, Wilson M, Li Y, Dunipace AJ. (1995). Fluoride reduces bone strength in older rats. J Dent Res 74:1475-1481 [DOI] [PubMed] [Google Scholar]
- Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, et al. (2000). Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287:664-666 [DOI] [PubMed] [Google Scholar]
- Vieira AP, Hanocock R, Eggertsson H, Everett ET, Grynpas MD. (2005). Tooth quality in dental fluorosis genetic and environmental factors. Calcif Tissue Int 76:17-25 [DOI] [PubMed] [Google Scholar]
- Waddington RJ, Langley MS. (2003). Altered expression of matrix metalloproteinases within mineralizing bone cells in vitro in the presence of fluoride. Connect Tissue Res 44:88-95 [PubMed] [Google Scholar]
- Wagner EF, Nebreda AR. (2009). Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer 9:537-549 [DOI] [PubMed] [Google Scholar]
- Wang H, Tannukit S, Zhu D, Snead ML, Paine ML. (2005). Enamel matrix protein interactions. J Bone Miner Res 20:1032-1040 [DOI] [PubMed] [Google Scholar]
- Wang W, Kong L, Zhao H, Dong R, Li J, Jia Z, et al. (2007). Thoracic ossification of ligamentum flavum caused by skeletal fluorosis. Eur Spine J 16:1119-1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston CR, Davis RJ. (2007). The JNK signal transduction pathway. Curr Opin Cell Biol 19:142-149 [DOI] [PubMed] [Google Scholar]
- Wu LW, Yoon HK, Baylink DJ, Graves LM, Lau KH. (1997). Fluoride at mitogenic doses induces a sustained activation of p44mapk, but not p42mapk, in human TE85 osteosarcoma cells. J Clin Endocrinol Metab 82:1126-1135 [DOI] [PubMed] [Google Scholar]
- Wurtz T, Houari S, Mauro N, MacDougall M, Peters H, Berdal A. (2008). Fluoride at non-toxic dose affects odontoblast gene expression in vitro. Toxicology 249:26-34 [DOI] [PubMed] [Google Scholar]
- Yan D, Gurumurthy A, Wright M, Pfeiler TW, Loboa EG, Everett ET. (2007). Genetic background influences fluoride’s effects on osteoclastogenesis. Bone 41:1036-1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Zhang Y, Li W, DenBesten PK. (2007). Micromolar fluoride alters ameloblast lineage cells in vitro. J Dent Res 86:336-340 [DOI] [PubMed] [Google Scholar]
- Yan X, Feng C, Chen Q, Li W, Wang H, Lv L, et al. (2009). Effects of sodium fluoride treatment in vitro on cell proliferation, apoptosis and caspase-3 and caspase-9 mRNA expression by neonatal rat osteoblasts. Arch Toxicol 83:451-458 [DOI] [PubMed] [Google Scholar]
- Yoder KM, Mabelya L, Robison VA, Dunipace AJ, Brizendine EJ, Stookey GK. (1998). Severe dental fluorosis in a Tanzanian population consuming water with negligible fluoride concentration. Community Dent Oral Epidemiol 26:382-393 [DOI] [PubMed] [Google Scholar]
- Zhang M, Wang A, He W, He P, Xu B, Xia T, et al. (2007). Effects of fluoride on the expression of NCAM, oxidative stress, and apoptosis in primary cultured hippocampal neurons. Toxicology 236:208-216 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li W, Chi HS, Chen J, DenBesten PK. (2007). JNK/c-Jun signaling pathway mediates the fluoride-induced down-regulation of MMP-20 in vitro. Matrix Biol 26:633-641 [DOI] [PMC free article] [PubMed] [Google Scholar]


